柑橘是世界上经济产值最高的果树之一,但由于童期长、具有多胚性[1](无融合生殖)的特点,阻碍了柑橘功能基因组学的研究进程,利用遗传转化鉴定基因功能通常耗时较长。笔者课题组前期开发了芸香科(Rutaceae)金柑属(Fortunella)山金柑(Fortunella hindsii)特殊材料,其特有的短童期、早花和矮化等特征缩短了试验周期,具有作为研究果树性状模式材料的潜力[2]。遗传转化是种质资源利用的基础,基于前期发掘的这一特殊材料,建立其遗传转化体系可以促进未来柑橘功能基因组的研究。
遗传转化首先要考虑转基因材料背景的一致性,比如拟南芥、番茄等模式作物的亲本及其后代均为纯合位点,从而保证了遗传背景的一致性。而对于多年生果树来说,基因组普遍具有高度杂合特性,有性后代基因位点发生遗传重组后会与亲本产生遗传背景差异,因此果树中遗传转化主要以植物组织或器官作为外植体。不同物种所适用的转化材料也存在差异,如香蕉[3]、大蕉[4]、葡萄[5]、梨[5-6]通常使用愈伤组织转化,苹果[7]、草莓[8]、可可[9]和猕猴桃[10]普遍使用叶盘转化。而柑橘一方面具有多胚性,其一粒种子可以产生多个由珠心细胞发育而来的无性胚(珠心胚),这种类型的胚不经过减数分裂和受精过程,直接由体细胞发育为种子,可以认为是母体的无性克隆[11],其遗传背景与母本可以保持一致;另一方面研究者发现以种子萌发后的上胚轴作为外植体具有较高的转化效率,因此柑橘通常以实生苗上胚轴作为遗传转化的材料。虽然柑橘具有无融合生殖特性,但子代中也会存在一定比例的有性后代(合子胚),会对转化材料的遗传背景造成干扰从而影响基因功能的鉴定。目前关于柑橘短童期种质——山金柑材料的无性克隆后代筛选方法鲜有报道。
在以往研究中,砧木繁殖过程也需要筛除有性后代来消除不良基因型,从而保证后代植株的一致性。Xiang等[12]最开始利用10个同工酶位点对12个柑橘砧木的珠心幼苗和合子幼苗进行了区分,后来不断开发出其他类型的分子标记如RAPD[13]、SSR[14-15]、ISSR[16]、EST-SSR[17]等来鉴定不同柑橘品种的合子胚。以上分子标记对多品种的鉴定有一定效果,但存在重复性差、分辨率低等缺点。随着基因组测序技术逐渐成熟,开发插入/缺失片段长度多态性(Insertion/Deletion,InDel)标记可以产生大于50 bp的差异片段[18],实现利用常规琼脂糖凝胶电泳区分带型。对于特定品系的柑橘品种,利用特异性InDel分子标记进行杂种鉴定,具有低成本,快速、遗传稳定性高、重复性好且结果准确可靠的特征。利用InDel标记来区分柑橘有性与无性后代目前还鲜有报道。
笔者在本研究中以野生山金柑多胚类型DB02系为材料,通过重测序数据进行InDel 标记的挖掘,利用琼脂糖凝胶电泳实验筛选无性后代,以期为山金柑遗传转化体系的优化奠定基础。
1 材料和方法
1.1 材料
多胚型野生山金柑DB02系作为母本以及其种子产生的子代植株。
1.2 基因组DNA的提取与检测
采集山金柑DB02及其子代成熟叶片并提取基因组DNA,提取方法采用改良CTAB法,具体步骤参照卢素文[19]博士学位论文,使用NanoDrop1000超微量分光光度计对DNA质量和含量检测,将质量合格的DNA初提液稀释至工作质量浓度(约100 ng·μL-1),保存于-20 ℃冰箱备用。
1.3 InDel引物的筛选
利用母本DB02的重测序数据以山金柑基因组为参考基因组进行比对,提取InDel 变异位点,筛选含有50 bp 左右差异的(0/1)杂合类型位点。在In-Del变异位点上下游600 bp范围内设计引物(表1),以母本DNA 为模板进行PCR 扩增,PCR 扩增反应体系20 μL,其中包括10 μL PCR-Mix,1 μL DNA,InDel 上下游引物各1 μL,加入ddH2O 补足体积。PCR扩增产物使用3%琼脂糖凝胶检测,选择清晰稳定的杂合带型标记。
表1 InDel 标记引物序列
Table 1 The primer sequences of InDel
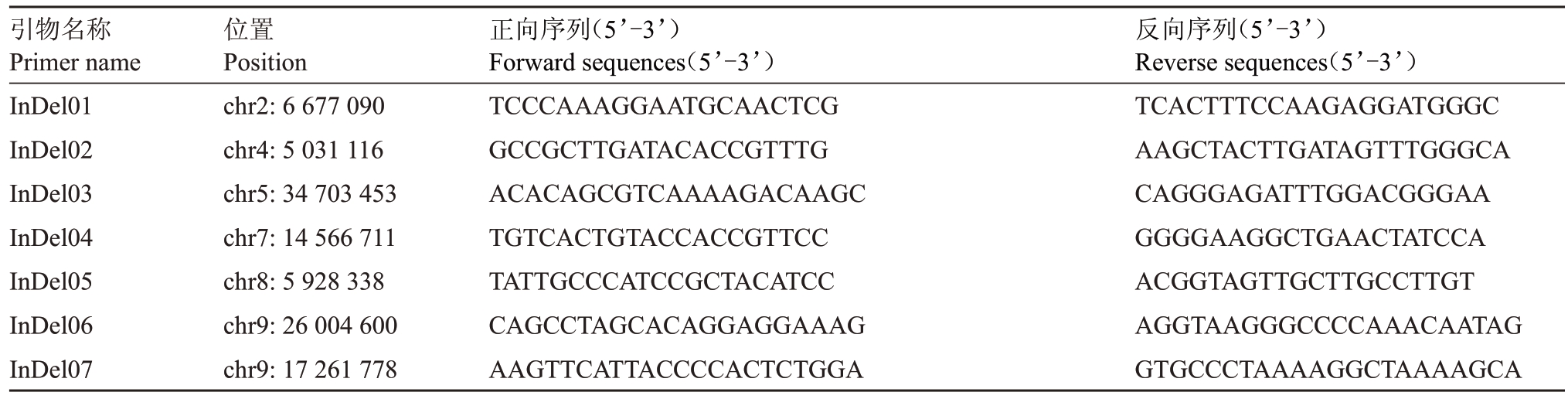
引物名称Primer name InDel01 InDel02 InDel03 InDel04 InDel05 InDel06 InDel07位置Position chr2:6 677 090 chr4:5 031 116 chr5:34 703 453 chr7:14 566 711 chr8:5 928 338 chr9:26 004 600 chr9:17 261 778正向序列(5’-3’)Forward sequences(5’-3’)TCCCAAAGGAATGCAACTCG GCCGCTTGATACACCGTTTG ACACAGCGTCAAAAGACAAGC TGTCACTGTACCACCGTTCC TATTGCCCATCCGCTACATCC CAGCCTAGCACAGGAGGAAAG AAGTTCATTACCCCACTCTGGA反向序列(5’-3’)Reverse sequences(5’-3’)TCACTTTCCAAGAGGATGGGC AAGCTACTTGATAGTTTGGGCA CAGGGAGATTTGGACGGGAA GGGGAAGGCTGAACTATCCA ACGGTAGTTGCTTGCCTTGT AGGTAAGGGCCCCAAACAATAG GTGCCCTAAAAGGCTAAAAGCA
1.4 子代合子胚和珠心胚鉴定
以山金柑DB02 系子代DNA 为模板进行PCR扩增,只含有一条带的后代为合子幼苗,珠心幼苗在所有的InDel标记扩增产物都与母本的两条带一致。
2 结果与分析
2.1 InDel引物的筛选
利用选取的7 对InDel 标记组对山金柑DB02系母本进行扩增,经琼脂糖凝胶电泳检测后,所有标记在母本DNA 中均包含两条带型(图1),记为Aa,便于后续子代合子胚和珠心胚筛选。后代若均与母本条带一致,认为该植株为珠心胚,若含有一个标记表现为AA 或aa,则认为该植株为合子胚。
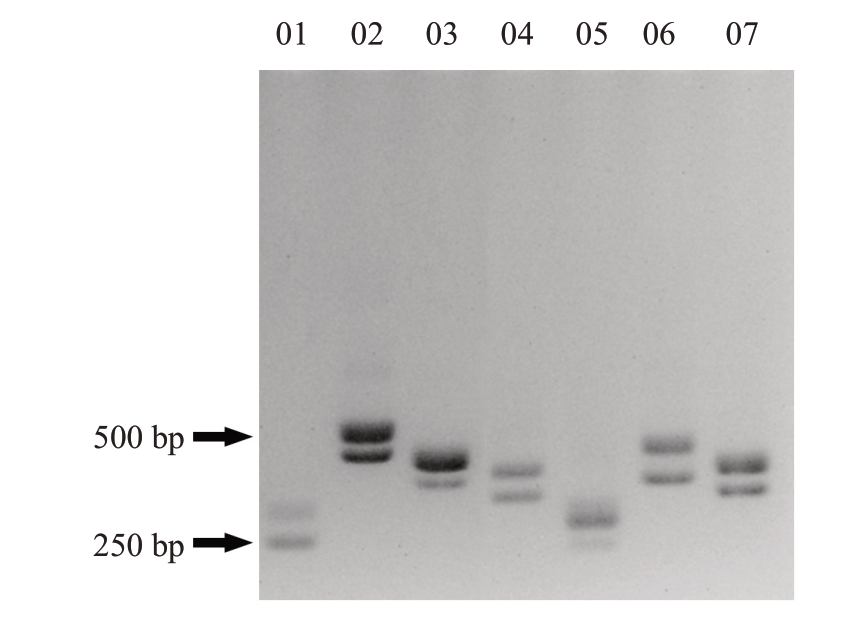
图1 InDel 标记组在DB02 系山金柑中的琼脂糖凝胶检测
Fig.1 Agarose gel detection of InDel primer group in DB02 kumquat
2.2 土播子代珠心胚苗与合子胚苗鉴定
将DB02 种子进行基质播种,统计萌发后代共计获得56 株幼苗。根据7 对InDel 标记的扩增产物鉴定结果(图2),发现在InDel01 标记结果中,子代34、41、56 号幼苗为单条带,则这3 株植株被认定为合子幼苗;同样地,利用InDel02 标记鉴定出1、3、23、27、41 号为合子幼苗;InDel03 标记结果鉴定1、27、34 号为合子幼苗;InDel04 标记结果鉴定出1、3号为合子幼苗;InDel05 标记结果鉴定出1、3、27 号为合子幼苗;InDel06及InDel 07标记结果鉴定出1、3、23、27、41、56号为合子幼苗。综合所有标记结果可以得出结论,在56 株DB02 子代中共鉴定出1、3、23、27、34、41、56号7株合子幼苗,其余48株珠心幼苗在7 个标记中带型均与母本一致,珠心胚率为87.5%。
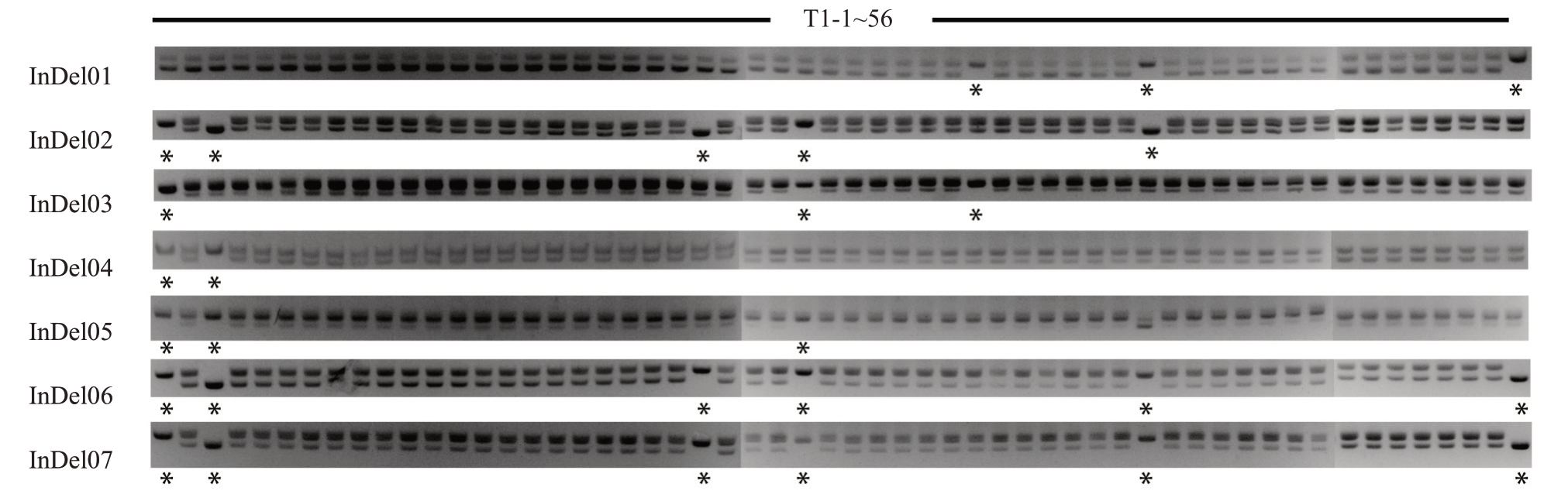
图2 InDel 标记组在DB02 系山金柑土播子代中的琼脂糖凝胶检测
Fig.2 Agarose gel detection of InDel primer group in soil sowing progeny of DB02 kumquat
*表示该植株为合子幼苗。下同。
*indicates that the plant is zygotic seedling.The same below.
2.3 试管播种子代珠心胚苗与合子胚苗鉴定
柑橘遗传转化过程中所需要的上胚轴材料一般来自于无菌播种的试管苗,为了研究该播种条件下珠心胚的比例,将DB02种子进行试管播种,统计萌发后代共计获得62 株幼苗。根据7 对InDel 标记的扩增产物鉴定结果(图3),在62株DB02子代中共鉴定出2、3、8、9、11、13、15、17、18、23、36、39、45、48、49、52 号16 株合子幼苗,其余46 株为珠心幼苗,合子胚率为25.8%,珠心胚率为74.2%。
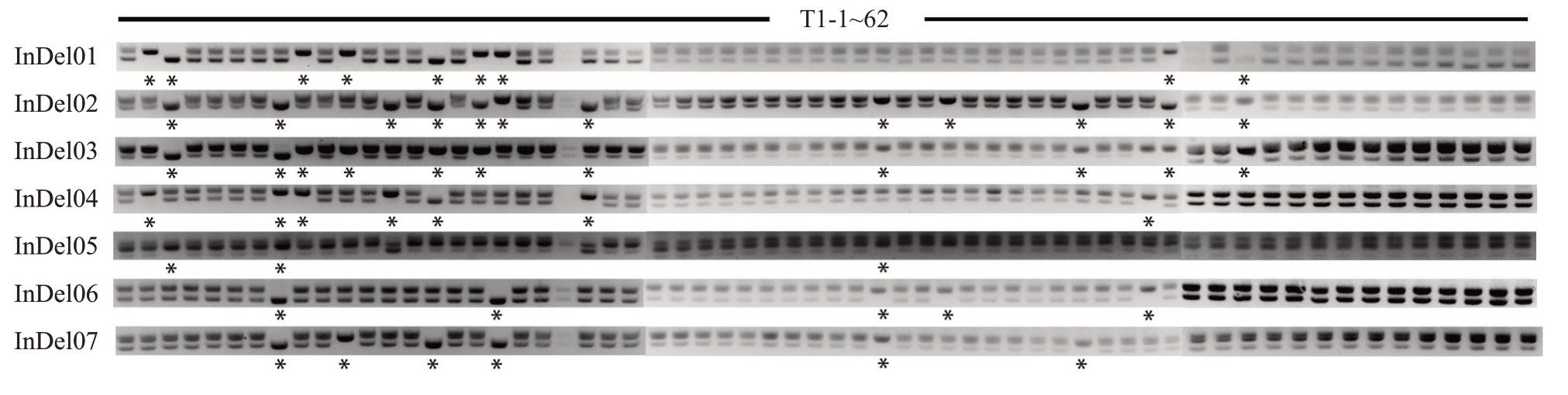
图3 InDel 标记组在DB02 系山金柑试管播种子代中的琼脂糖凝胶检测
Fig.3 Agarose gel detection of InDel primer group in medium seeding progeny of DB02 kumquat
3 讨 论
珠心胚对柑橘果实的进化、繁殖和育种具有重要意义,由珠心胚发育而来的无性后代在遗传上与亲本相同,这有助于保存和保持柑橘砧木品种间的遗传一致性。柑橘的遗传转化同样要求转基因材料与母本之间保持遗传一致性,Gill 等[20]最初以珠心苗为外植体,对不同的组织如叶片、上胚轴、子叶和根段进行再生诱导,发现上胚轴的诱导再生效率最高,因此柑橘后来的遗传转化主要以上胚轴作为外植体。相对于其他外植体,如苹果[21]、凤梨[22]等物种需要长期通过组织培养来继代母本组织材料,对实验条件有较为严格的要求,而柑橘只需要播种多胚种子即可长出完全无菌的茎段,降低了操作难度。然而,目前在柑橘基因功能验证研究中,虽然获得了转基因植株,但不同的材料间并没有统一筛选珠心胚的方法,大多数研究并未对转基因材料进行遗传背景的鉴定[23-24],但不同的遗传背景可能会对表型产生无法判断的影响。
InDel标记作为一种共显性标记,虽然可以区分杂合与纯合,但是利用杂合标记进行子代杂种鉴定时,如果父本基因型与母本相同,后代存在有性后代带型和母本一致的情况,因此需要多个标记共同鉴定才能达到筛选无性后代的要求。Shimada 等[25]在研究中利用3 个CAPS 标记进行合子胚鉴定,当标记数量过少时结果的可靠性不高,标记数量过多又耗时耗力不经济,最佳的标记对数理论上是1/27,即7 对标记可以有99%以上的可靠性[14]。因此,张斯淇[26]在多胚调控相关基因CitRWP 启动子区域开发的MITE 杂合分子标记,虽然也可以用于多胚山金柑无性克隆后代的筛选,但想精确筛选出无性后代,仍需要多个标记共同验证。笔者在本研究中利用7个InDel 杂合标记可以实现对野生山金柑DB02 系无性后代的精确筛选。
山金柑作为未来柑橘遗传转化的模式材料,较高的珠心胚比例符合遗传转化的需求。以往研究发现不同柑橘品种的多胚性也不同,其产生珠心胚的能力和无性后代数量也存在很大差异,Xiang等[12]对12种类型柑橘砧木(柚,柠檬,枳杂种,甜橙)进行合子胚率统计,结果发现合子胚率在5.5%~50.6%之间;对酸橙砧木的合子胚率也进行了鉴定,发现存在22.2%~26.64%的合子胚。本研究在成功筛选山金柑无性后代的同时,对不同生长条件下的珠心胚率也进行了统计,发现DB02 山金柑系在基质播种条件下,珠心胚率约为87.5%,与其他柑橘品种相比处于适中的水平,而培养基播种条件下的珠心胚率为74.2%。这可能是由于在组培条件下,一些发育不良的合子胚得到了较好的培养条件,再生机会增加,而在土播条件下,发育不良的合子胚往往难以成苗,进而导致组培的合子苗比例上升。但总体来说,该品系绝大多数子代仍为无性后代,可以作为山金柑遗传转化的备选材料。
4 结 论
选用7对InDel杂合标记组对野生山金柑DB02系子代在试管播种和土播两种条件下的合子胚率进行统计,试管播种子代的合子胚率高于土播子代,但均表现出较高的珠心胚率。同时利用该InDel 标记组可以快速准确地鉴定出合子胚与珠心胚,进一步完善了山金柑材料实生苗上胚轴遗传转化体系。
[1] KOLTUNOW A M. Apomixis:Embryo sacs and embryos formed without meiosis or fertilization in ovules[J]. The Plant Cell,1993,5(10):1425-1437.
[2] 朱晨桥. 柑橘模式材料的开发与金柑属植物系统发育学研究[D].武汉:华中农业大学,2020.ZHU Chenqiao.Development of Citrus model material and phylogenetic study of genus Fortunella[D].Wuhan:Huazhong Agricultural University,2020.
[3] NAIM F,DUGDALE B,KLEIDON J,BRININ A,SHAND K,WATERHOUSE P,DALE J. Gene editing the phytoene desaturase alleles of Cavendish banana using CRISPR/Cas9[J]. Transgenic Research,2018,27(5):451-460.
[4] YE S W,CHEN G,KOHNEN M V,WANG W J,CAI C Y,DING W S,WU C,GU L F,ZHENG Y S,MA X Q,LIN C T,ZHU Q. Robust CRISPR/Cas9 mediated genome editing and its application in manipulating plant height in the first generation of hexaploid Ma bamboo (Dendrocalamus latiflorus Munro)[J].Plant Biotechnology Journal,2020,18(7):1501-1503.
[5] NAKAJIMA I,BAN Y,AZUMA A,ONOUE N,MORIGUCHI T,YAMAMOTO T,TOKI S,ENDO M. CRISPR/Cas9-mediated targeted mutagenesis in grape[J]. PLoS One,2017,12(5):e0177966.
[6] PANG H G,YAN Q,ZHAO S L,HE F,XU J F,QI B X,ZHANG Y X. Knockout of the S-acyltransferase gene,Pb-PAT14,confers the dwarf yellowing phenotype in first generation pear by ABA accumulation[J]. International Journal of Molecular Sciences,2019,20(24):6347.
[7] NISHITANI C,HIRAI N,KOMORI S,WADA M,OKADA K,OSAKABE K,YAMAMOTO T,OSAKABE Y. Efficient genome editing in apple using a CRISPR/Cas9 system[J].Scientific Reports,2016,6:31481.
[8] WILSON F M,HARRISON K,ARMITAGE A D,SIMKIN A J,HARRISON R J. CRISPR/Cas9-mediated mutagenesis of phytoene desaturase in diploid and octoploid strawberry[J]. Plant Methods,2019,15:45.
[9] FISTER A S,LANDHERR L,MAXIMOVA S N,GUILTINAN M J. Transient expression of CRISPR/Cas9 machinery targeting TcNPR3 enhances defense response in Theobroma cacao[J].Frontiers in Plant Science,2018,9:268.
[10] VARKONYI-GASIC E,WANG T C,VOOGD C,JEON S,DRUMMOND R S M,GLEAVE A P,ALLAN A C. Mutagenesis of kiwifruit CENTRORADIALIS-like genes transforms a climbing woody perennial with long juvenility and axillary flowering into a compact plant with rapid terminal flowering[J].Plant Biotechnology Journal,2019,17(5):869-880.
[11] 谭志友.《中国果树志·柑橘卷》正式出版发行[J].中国南方果树,2010,39(3):11.TAN Zhiyou.Chinese Fruit Trees&Citrus Rolls official publication[J].South China Fruits,2010,39(3):11.
[12] XIANG C,ROOSE M L.Frequency and characteristics of nucellar and zygotic seedlings in 12 citrus rootstocks[J].Scientia Horticulturae,1988,37(1/2):47-59.
[13] ANDRADE-RODRÍGUEZ M,VILLEGAS-MONTER A,CARRILLO-CASTAÑEDA G,GARCÍA-VELÁZQUEZ A. Polyembryony and identification of Volkamerian lemon zygotic and nucellar seedlings using RAPD[J].Pesquisa Agropecuária Brasileira,2004,39(6):551-559.
[14] RUIZ C,BRETO M P,ASÍNS M J. A quick methodology to identify sexual seedlings in citrus breeding programs using SSR markers[J].Euphytica,2000,112(1):89-94.
[15] 谭李梅,符红艳,徐静,朱志媚,胡哲,肖顺元,马先锋,邓子牛.柠檬合子胚自交实生苗的鉴定及其对柑橘溃疡病的抗性初步评价[J].分子植物育种,2020,18(7):2258-2267.TAN Limei,FU Hongyan,XU Jing,ZHU Zhimei,HU Zhe,XIAO Shunyuan,MA Xianfeng,DENG Ziniu. Identification of lemon zygotic self-crossed seedlings and the primary evaluation of the resistance to Citrus canker disease[J]. Molecular Plant Breeding,2020,18(7):2258-2267.
[16] AWAD M,EL-ALAKMY H,ABDALLA M,ELDEEP M. Distinguishing of zygotic and nucellar seedlings in citrus rootstocks using ISSR technique[J]. Sinai Journal of Applied Sciences,2019,8(1):1-8.
[17] RAO M N,SONEJI J R,CHEN C X,HUANG S,GMITTER J F G. Characterization of zygotic and nucellar seedlings from sour orange-like Citrus rootstock candidates using RAPD and EST-SSR markers[J]. Tree Genetics & Genomes,2008,4(1):113-124.
[18] ALBERS C A,LUNTER G,MACARTHUR D G,MCVEAN G,OUWEHAND W H,DURBIN R. Dindel:Accurate indel calls from short-read data[J].Genome Research,2011,21(6):961-973.
[19] 卢素文.柑橘番茄红素β-环化酶基因功能分析及其转录调控研究[D].武汉:华中农业大学,2017.LU Suwen.Functional identification and transcriptional regulatory analysis of Citrus lycopene β-cyclase genes[D].Wuhan:Huazhong Agricultural University,2017.
[20] GILL M I S,SINGH Z,DHILLON B S,GOSAL S S.Somatic embryogenesis and plantlet regeneration in mandarin(Citrus reticulata Blanco)[J].Scientia Horticulturae,1995,63(3/4):167-174.
[21] 邹春好.基于体细胞胚胎发生的苹果遗传转化体系的建立[D].泰安:山东农业大学,2021.ZOU Chunhao. Establishment of a genetic transformation system for Malus domestica based on somatic embryogenesis[D].Taian:Shandong Agricultural University,2021.
[22] 张国芳.观赏凤梨高频再生以及遗传转化研究[D].杭州:浙江大学,2011.ZHANG Guofang. Studies on high frequency regeneration and genetic transformation of ornamental pineapple[D]. Hangzhou:Zhejiang University,2011.
[23] WANG L J,CHEN S C,PENG A H,XIE Z,HE Y R,ZOU X P.CRISPR/Cas9-mediated editing of CsWRKY22 reduces susceptibility to Xanthomonas citri subsp. citri in Wanjincheng orange[Citrus sinensis (L.) Osbeck][J]. Plant Biotechnology Reports,2019,13(5):501-510.
[24] ZHU C Q,ZHENG X J,HUANG Y,YE J L,CHEN P,ZHANG C L,ZHAO F,XIE Z Z,ZHANG S Q,WANG N,LI H,WANG L,TANG X M,CHAI L J,XU Q,DENG X X.Genome sequencing and CRISPR/Cas9 gene editing of an early flowering Mini-Citrus (Fortunella hindsii) [J]. Plant Biotechnology Journal,2019,17(11):2199-2210.
[25] SHIMADA T,ENDO T,FUJII H,NAKANO M,SUGIYAMAA,DAIDO G,OHTA S,YOSHIOKA T,OMURA M. MITE insertion-dependent expression of CitRKD1 with a RWP-RK domain regulates somatic embryogenesis in citrus nucellar tissues[J].BMC Plant Biology,2018,18(1):166.
[26] 张斯淇.柑橘无融合生殖的遗传分析和相关基因挖掘[D].武汉:华中农业大学,2017.ZHANG Siqi. Genetic analysis of citrus apomixis and its related genes discovery[D]. Wuhan:Huazhong Agricultural University,2017.