中国是荔枝的生产和消费大国,现有荔枝种植面积55 万hm2,主要分布在广东、广西、福建、四川、云南和海南等省区[1]。由荔枝霜疫霉(Peronophythora litchii)引起的荔枝霜疫病是荔枝生产中的重要病害,该病害不仅在生长期发病,危害果实、花穗和嫩叶,也可在果实贮藏期发生,造成烂果[2],严重影响荔枝的品质、产量及鲜果外销。由于缺少农艺性状优良、高产且抗病的品种,化学防治因其防效好、见效快,是生产中防控霜疫病的主要方法。
羧酸酰胺类(carboxylic acid amides,CAAs)杀菌剂以抑菌活性高、内吸传导强、对卵菌特效等特点而受到广泛的重视。该类杀菌剂具有低至中等的抗药性风险,作用靶点与细胞壁纤维素的形成及细胞壁磷脂层的合成相关[3]。烯酰吗啉(dimethomorph)属于CAAs类杀菌剂,兼具保护和治疗的作用,在荔枝霜疫病的防控中普遍应用。据报道,40%的烯酰吗啉水分散粒剂1000~1500倍液对霜疫病的防治效果较好[4];烯酰吗啉对荔枝霜疫霉孢子囊的萌发和菌丝生长的抑制效果较显著[5],烯酰吗啉与咪鲜胺以1∶1质量比的比例混配使用可作为防控荔枝霜疫病和炭疽病的优选参考[6]。烯酰吗啉于1996年开始用于荔枝霜疫病的防控[7],随着用量及使用年限的增加,病原菌是否存在抗药性的问题愈发引起关注。Wang等[8]测定了2007年来自广东、广西和福建省的127 株荔枝霜疫霉菌株对烯酰吗啉的敏感性,结果表明,供试菌株对烯酰吗啉的敏感性高,未发现抗药性菌株。易赛等[9]利用2009—2012年间采集自广东、广西、福建和云南省区的115株荔枝霜疫霉菌株对烯酰吗啉进行敏感性测定,未发现抗药性亚群体,之后至今未有相关的抗药性研究报道。笔者在本研究中检测2019—2020年分离自中国5个省份荔枝主产区的320株荔枝霜疫霉单孢子囊菌株对烯酰吗啉的敏感性,旨在了解中国荔枝主产区的荔枝霜疫霉群体对烯酰吗啉的敏感性变化;筛选荔枝霜疫霉对烯酰吗啉的抗性突变株并对其生物学适合度进行测定;以期为荔枝霜疫霉群体的抗药性监测及抗性治理提供科学的依据。
1 材料和方法
1.1 供试菌株
采集2019—2020 年中国5 个省份(广东、广西、四川、福建和云南)荔枝主产区的病果,经组织分离、纯化获得320 株荔枝霜疫霉的单孢子囊菌株。依采集的年份:分离自2019 年的180 株,2020 年的140 株。依采集的省份:分离自广东的103 株,广西的30 株,福建的62 株,云南的30 株及四川的95株。菌株均保存于华南农业大学杀菌剂研究室,信息详见表1。
表1 荔枝霜疫霉菌株来源
Table 1 Distribution of P.litchii isolates from different provinces in China
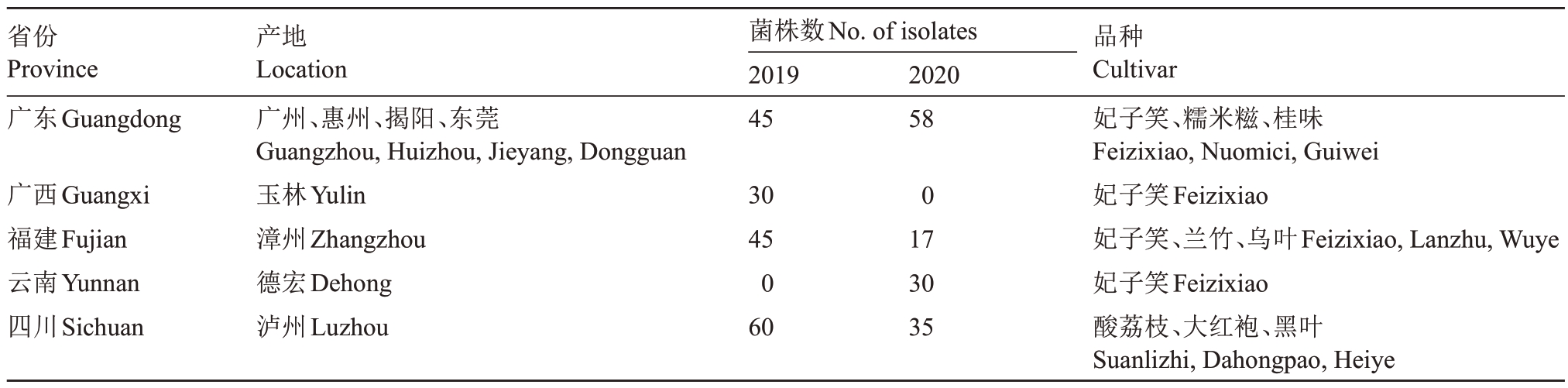
省份Province广东Guangdong菌株数No.of isolates 2019 45 2020 58广西Guangxi福建Fujian云南Yunnan四川Sichuan产地Location广州、惠州、揭阳、东莞Guangzhou,Huizhou,Jieyang,Dongguan玉林Yulin漳州Zhangzhou德宏Dehong泸州Luzhou 30 45 0 60 0 17 30 35品种Cultivar妃子笑、糯米糍、桂味Feizixiao,Nuomici,Guiwei妃子笑Feizixiao妃子笑、兰竹、乌叶Feizixiao,Lanzhu,Wuye妃子笑Feizixiao酸荔枝、大红袍、黑叶Suanlizhi,Dahongpao,Heiye
1.2 供试药剂
烯酰吗啉原药(99%)购自沈阳化工研究院。二甲基亚砜(dimethyl sulfoxide,99%)购自上海昂一生物科技有限公司。将烯酰吗啉原药溶于二甲基亚砜,配置成质量浓度为0.05、0.10、0.15、0.20、0.25、0.30 mg·mL-1的药液备用。
1.3 荔枝霜疫霉对烯酰吗啉的敏感性测定
采用菌丝生长速率法测定荔枝霜疫霉对烯酰吗啉的有效抑制中浓度值(the median effective concentration,EC50),配置不同浓度的含药胡萝卜琼脂培养基(carrots agar,CA)平板,从新鲜菌落边缘打取直径5.0 mm菌饼置于含药平板中央,25 ℃黑暗培养5 d,以加入等体积量二甲基亚砜的处理为对照,每个处理3次重复。采用十字交叉法测量各处理的菌落生长直径,计算其菌丝生长抑制率。

以杀菌剂浓度的对数和抑制率的概率值建立毒力回归方程,计算抑制菌落生长的有效抑制中浓度(EC50)。采用Kolmogorov-Smirnov 检验(K-S 检验),就供试菌株对杀菌剂的lg(EC50)值进行正态性检验(p>0.05 符合正态分布),利用SPSS 23.0 软件进行异常值检测,以剔除异常值后符合正态分布的EC50均值作为敏感性基线。以lg(EC50)值为横坐标、供试菌株的分布频率为纵坐标,绘制敏感性频率分布图。通过计算抗性指数进行供试菌株的抗性评价。根据Food and Agriculture Organization(FAO)的抗药性评价标准[10],抗性突变株的EC50值比野生型菌株高5倍为敏感菌株(S);>5~10倍为低抗菌株(low resistance,LR);>10~100倍为中抗菌株(medium resistance,MR);100 倍以上的为高抗菌株(high resistance,HR)。
抗性指数(resistance factor,RF)=供试菌株EC50值/敏感性基线EC50值。
1.4 药剂驯化诱导抗性突变株及抗药稳定性测定
将荔枝霜疫霉(野生型)单孢子囊菌株接种于质量浓度依次为0.25、0.5、1、2、5、10、20 μg·mL-1的含药平板进行多代驯化诱导,将能在含5 μg·mL-1烯酰吗啉的CA培养基上正常生长的视为抗性突变株进行编号保存。将获得的抗性突变株在不含药的CA平板上继代培养9 代,测定第1、3、5、7 和9 代的抗性突变株对烯酰吗啉的敏感性并进行抗药稳定性的分析。
1.5 抗性突变株的生物学适合度测定
1.5.1 孢子囊的产生、萌发及形态变化 参考任德春[11]的方法,进行孢子囊产生及萌发的测定。孢子囊产量的测定:将亲本及其抗性突变株在无药CA平板上25 ℃培养4 d,配制孢子囊悬浮液,用血球计数板进行孢子囊产量的计数,每个处理3次重复。孢子囊萌发率的测定:吸取孢子囊悬浮液涂布于水琼脂(water agar,WA)平板上于28 ℃培养,观察孢子囊的萌发并计算萌发率,每个处理3 次重复(300 个孢子囊·菌株-1)。休止孢萌发率:吸取适量的孢子囊悬浮液,待孢子囊释放游动孢子,取游动孢子悬浮液振荡,获得休止孢的悬浮液,观察休止孢的萌发并计算萌发率,每个处理3 次重复(300 个休止孢·菌株-1)。以水为浮载剂,对各菌株的孢子囊制片,用显微镜观察其形态并测量其长和宽(100个孢子囊·菌株-1)。
1.5.2 卵孢子的产生 参考Flier 等[12]方法,将亲本及其抗性突变株的菌饼接种于CA 平板上,25 ℃黑暗培养14 d 至卵孢子产生,配制卵孢子的悬浮液进行观测计数,每个处理3次重复。
1.5.3 致病力的测定 将亲本及其抗性突变株在CA 平板上培养活化,配制浓度为1×104 个·mL-1的孢子囊悬浮液,采集生长度和大小较一致的嫩叶(妃子笑),用解剖针在叶片背部主叶脉两侧刺伤叶表皮,吸取孢子囊悬浮液10 μL 滴于刺伤处,每个菌株接种6 枚叶片,设置3 次重复。以等量无菌水接种为空白对照。对叶片的发病情况进行逐日观察并记录,利用ImageJ软件测量并计算病斑面积占比及发病率。
发病率(%)=发病叶片数/总叶片数×100。
1.6 数据统计与分析
采用SPSS 23.0、Duncan新复极差法(DMRT)及独立样本t检验进行差异显著性分析(α=0.05)。
2 结果与分析
2.1 荔枝霜疫霉对烯酰吗啉的敏感性测定
利用2019—2020年分离自广东、广西、福建、四川和云南等5个荔枝主产区获得的荔枝霜疫霉单孢子囊菌株(320 株)对烯酰吗啉进行敏感性测定,结果表明,敏感性频率分布不呈连续单峰曲线。经正态性检验表明,供试菌株对烯酰吗啉的敏感性频率分布不符合正态分布(p<0.05),检测到3 个敏感性升高的异常值。剔除异常值后的317株荔枝霜疫霉的敏感性频率分布呈连续单峰曲线(图1)。317 株供试菌株对烯酰吗啉的EC50 值范围为0.086 3~0.173 3 μg·mL-1,最值之间相差2.01 倍,平均EC50值为(0.122 2±0.000 9)μg·mL-1,以此作为荔枝霜疫霉对烯酰吗啉的敏感性基线。
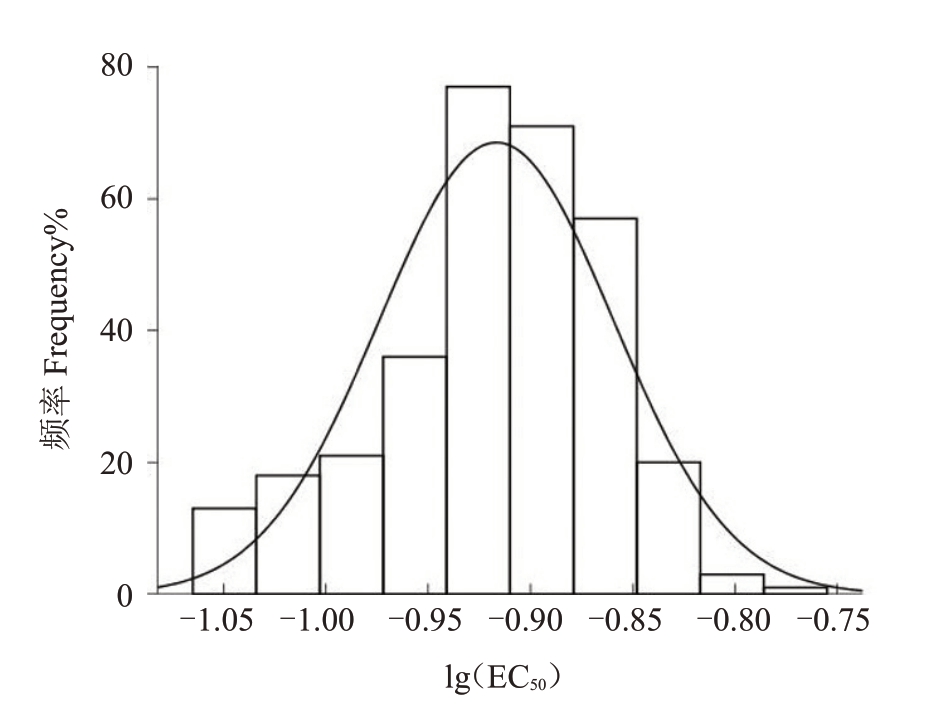
图1 荔枝霜疫霉(317 株)对烯酰吗啉的敏感性频率分布
Fig.1 Frequency distribution of sensitivity of P.litchii(317 isolates)to dimethomorph
以317 株荔枝霜疫霉的EC50值与敏感性基线的比值计算抗性倍数。317 株菌株的抗性倍数均小于5,未发现荔枝霜疫霉对烯酰吗啉的田间抗性菌株。
2.2 2019—2020 两年间荔枝霜疫霉对烯酰吗啉的敏感性比较
利用2019—2020 两年间获得的荔枝霜疫霉菌株对烯酰吗啉的敏感性进行比较,结果表明,2019年180 株荔枝霜疫霉菌株对烯酰吗啉的EC50值范围为0.080 8~0.144 3 μg·mL-1,最值之间相差1.79倍,平均EC50 值为(0.117 5±0.001 2)μg·mL- 1。2020 年140 株荔枝霜疫霉菌株对烯酰吗啉的EC50值范围为0.0974~0.1733 μg·mL-1,最值之间相差1.78 倍,平均EC50值为(0.127 3±0.001 1)μg·mL-1。经独立样本t 检验表明,分离自2020 年的荔枝霜疫霉对烯酰吗啉的EC50均值较2019 年显著提高了8.34%(p<0.05,图2)。与2019年的相比较,2020年荔枝霜疫霉对烯酰吗啉的抗药性风险有所增加。
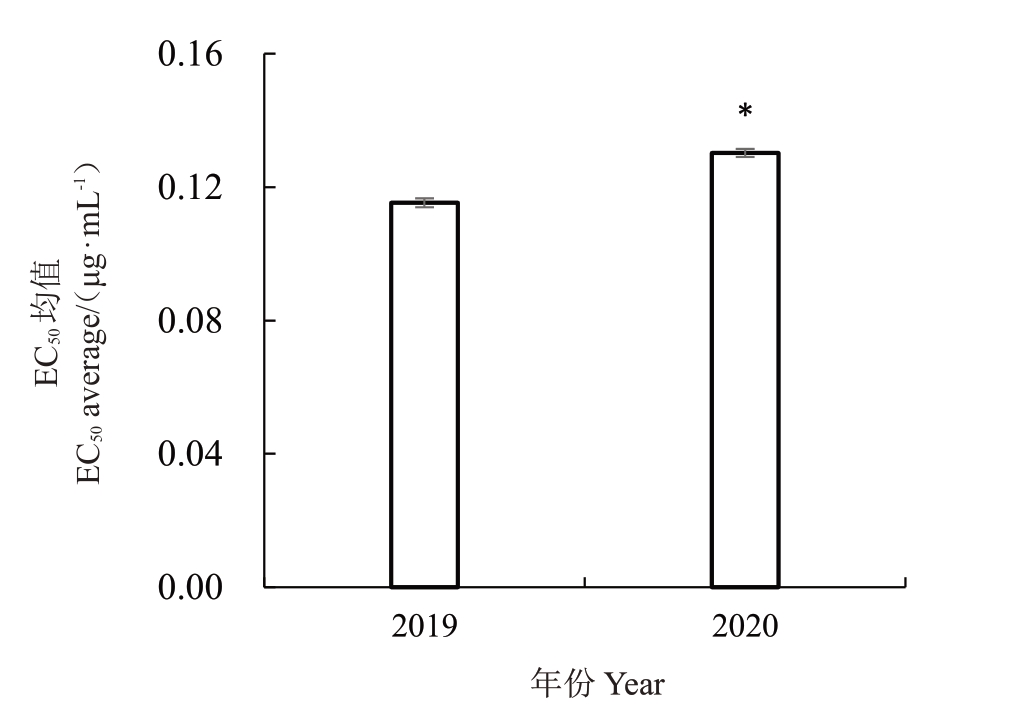
图2 2019 和2020 两年间荔枝霜疫霉对烯酰吗啉的敏感性比较
Fig.2 The comparation of sensitivity of P.litchii to dimethomorph between 2019 and 2020
2.3 不同省区荔枝霜疫霉对烯酰吗啉的敏感性差异
将来自不同省份的荔枝霜疫霉对烯酰吗啉的敏感性进行比较,结果表明,来自广东和广西的荔枝霜疫霉菌株群体对烯酰吗啉的敏感性较低,而来自福建、云南和四川的荔枝霜疫霉菌株群体的敏感性相对较高,两个群体之间存在显著性差异(表2)。其中,来自四川的荔枝霜疫霉菌株EC50 均值最低(0.115 4±0.001 7 μg·mL-1),对烯酰吗啉的敏感性相对较高。而来自广东的荔枝霜疫霉菌株EC50均值最高(0.128 9±0.001 5 μg·mL-1),对烯酰吗啉的敏感性相对低。
表2 不同省份的荔枝霜疫霉对烯酰吗啉的敏感性
Table 2 Sensitivity of P.litchii to dimethomorph in different provinces
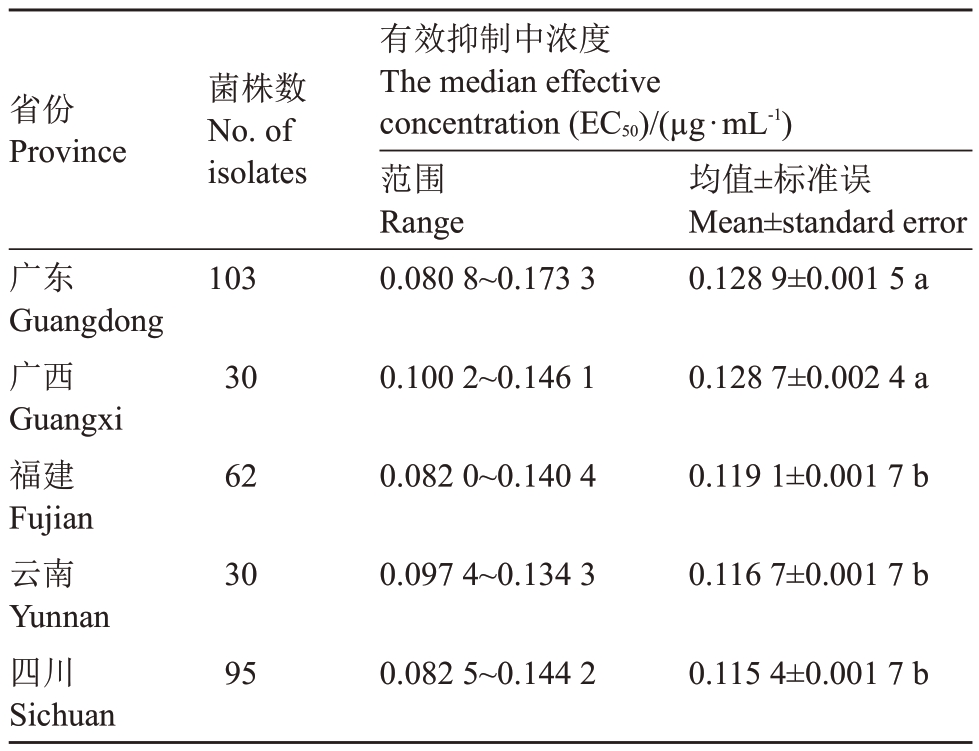
注:同列数据后不同小写字母表示在p<0.05 差异显著(Duncan新复极差法检验)。
Note:The different small letters in a column indicate significant difference at p<0.05(Duncan’s multiple range test).
省份Province菌株数No.of isolates有效抑制中浓度The median effective concentration(EC50)/(μg·mL-1)范围Range 0.080 8~0.173 3均值±标准误Mean±standard error 0.128 9±0.001 5 a 0.100 2~0.146 1 30 103 0.128 7±0.002 4 a 62 0.082 0~0.140 4 0.119 1±0.001 7 b 30 0.097 4~0.134 3 0.116 7±0.001 7 b广东Guangdong广西Guan gxi福建Fujia n云南Yunn an四川Sich uan 95 0.082 5~0.144 2 0.115 4±0.001 7 b
2.4 烯酰吗啉抗性突变株的抗性水平及抗药稳定性
经药剂驯化获得3株荔枝霜疫霉的抗烯酰吗啉突变株(GZ3-3R、DH1-9R和LZ4-10R),经敏感性测定表明,抗性突变株对烯酰吗啉的抗性指数范围为77.17~360.11,第9代较第1代的抗性指数均升高(表3),其中GZ3-3R和DH1-9R在第1 代和第9 代均为高抗突变株;LZ4-10R在第1代为中抗突变株,在第9代为高抗突变株。3株抗性突变株的抗药性均能够稳定遗传。
表3 抗性突变株的抗性指数及抗性水平
Table 3 Resistance factor and resistance level of resistant mutants

注:MR.中等抗性;HR.高等抗性。
Note:MR.Medium resistance;HR.High resistance.
抗性突变株Resistant mutants GZ3-3R DH1-9R LZ4-10R有效抑制中浓度The median effective concentration(EC50)/(μg·mL-1)第1代The 1stgeneration 44.01 12.31 9.43抗性指数Resistance factor第1代The 1stgeneration 360.11 100.75 77.17抗性水平Resistance level第1代The 1stgeneration HR HR MR第9代The 9thgeneration HR HR HR第9代The 9thgeneration 49.32 17.83 12.40 2019年与2020年之间供试菌株的EC50均值第9代The 9thgeneration 403.59 145.94 101.48平均值 标准误
2.5 烯酰吗啉抗性突变株的生物学适合度
2.5.1 孢子囊的产量、萌发率及形态变化 对3 株荔枝霜疫霉的抗烯酰吗啉突变株(GZ3-3R、DH1-9R和LZ4-10R)及其亲本菌株(GZ3-3S、DH1-9S和LZ4-10S)进行孢子囊的产量、萌发率及形态变化的测定,结果(表4)表明,抗性突变株与其亲本相比较,孢子囊的产量不具有显著性差异(p>0.05);抗性突变株的孢子囊萌发率均较其亲本下降,其中,抗性突变株GZ3-3R和DH1-9R的孢子囊萌发率比其亲本分别降低了32%和41%。3 株抗性突变株的孢子囊大小均比其亲本大,其中,抗性突变株GZ3-3R和LZ4-10R的孢子囊的大小(长和宽)均较其亲本菌株显著增大(p<0.05)。抗性突变株GZ3-3R休止孢的萌发率较其亲本显著下降(p<0.05)。
表4 抗性突变株及其亲本孢子囊产量、萌发率及大小变化
Table 4 Production and germination rate of sporangia and resting spore of resistant mutants and their parental isolates

注:表中数据为(平均值±标准误);*表示抗性突变株较其亲本菌株在p<0.05 差异显著(t 检验)。
Note: Data are presented as (mean ± standard error); * indicates that the resistant mutant is significantly different from its parental isolates at p<0.05(t-test).
亲本/突变株Parental isolates/Mutants GZ3-3S GZ3-3R DH1-9S DH1-9R LZ4-10S LZ4-10R孢子囊产量Sporangial production/(×104 mL-1)1.22±0.59 0.89±0.11 1.33±0.00 1.44±0.11 1.33±0.19 2.00±0.39孢子囊平均宽度Average width of sporangia/μm 18.15±1.27*21.07±2.08*17.70±1.56*18.67±1.78*18.32±1.52*18.78±1.57*孢子囊萌发率Sporangial germination rate/%22.33±1.20*15.18±0.88*45.88±1.03*27.22±2.03*41.67±1.00 36.67±0.75休止孢萌发率Resting spore germination rate/%85.00±1.53*75.53±2.54*80.49±2.63 92.33±1.20 91.73±2.89 92.36±0.88 0.16*孢子囊平均长度Average length of sporangia/μm 34.81±2.56*38.09±3.51*33.21±3.37 33.23±3.56 30.97±2.93*31.94±3.24*0.12 0.04 0.08
2.5.2 卵孢子的产量 对3 株荔枝霜疫霉的抗烯酰吗啉突变株及其亲本菌株进行有性卵孢子产量的测定表明,与亲本相比较,3 株抗性突变株的卵孢子产量均下降,其中,抗性突变株DH1-9R的卵孢子产量比其亲本降低了40%,经独立样本t检验显示,抗性突变株与其亲本的卵孢子产量之间差异不显著(p>0.05,图3)。
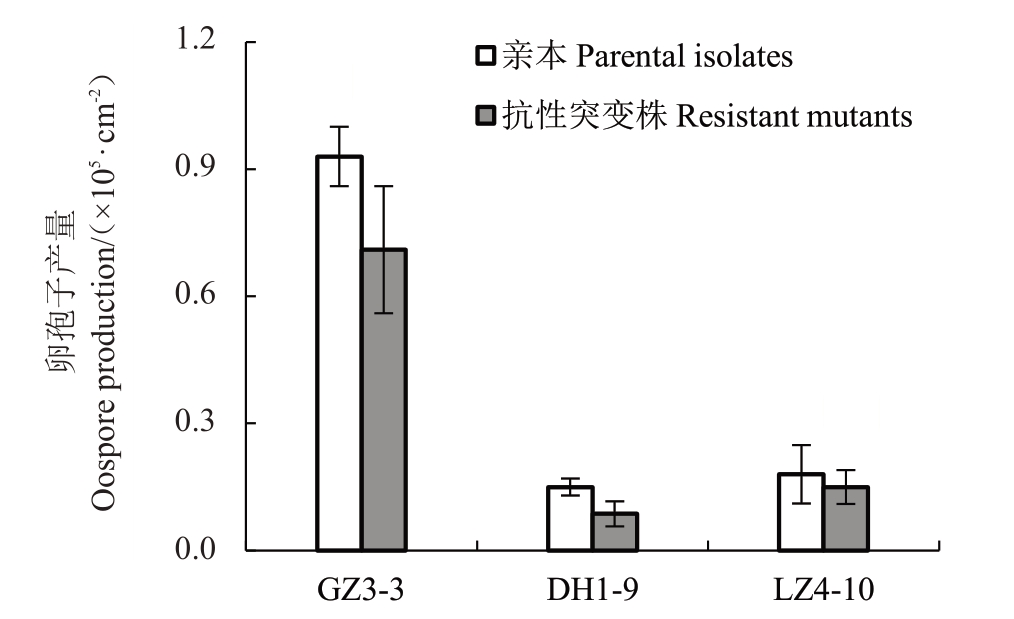
图3 抗性突变株及其亲本的卵孢子产量
Fig.3 Oospore production of resistant mutants and their parental isolates
2.5.3 致病力测定 对3 株荔枝霜疫霉的抗烯酰吗啉突变株及其亲本菌株进行致病力测定表明,抗性突变株及其亲本均能侵染荔枝新鲜嫩叶引起发病。接种48 h 之后,以接种点为中心形成褐色病斑,并向外扩展(图4)。抗性突变株GZ3-3R及LZ4-10R接种叶片后发病的病斑面积占比分别为(11.52±2.32)%和(19.75±3.03)%,较其各自亲本菌株[分别为(13.88±3.38)%和(20.59±3.87)%]略低;而抗性突变株DH1-9R接种发病的病斑面积占比(21.28±2.52)%与其亲本接种发病的(21.18±2.62)%相当。独立样本t 检验表明3 株抗性突变株与其亲本的致病力之间差异不显著(p>0.05)。
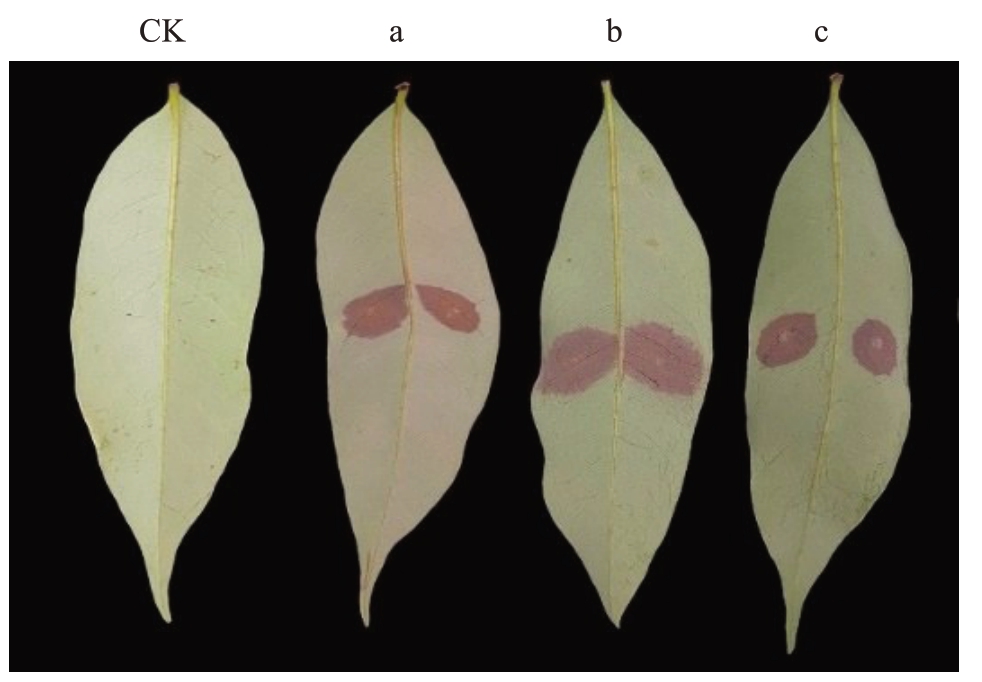
图4 抗性突变株及其亲本的致病力
Fig.4 Pathogenicity of resistant mutants and their parental isolates
CK.对照(两侧均接种无菌水);a.GZ3-3R;b.DH1-9R;c.LZ4-10R(a~c,左侧接种亲本菌株,右侧接种抗性突变株)。
CK.Control (both sides of leave inoculated with sterile water); a.GZ3-3R;b.DH1-9R;c.LZ4-10R(a-c,the left side of leave was inoculated with parental isolate, while the right side was inoculated with resistant mutant).
3 讨 论
病原菌对CAAs类杀菌剂的抗性遗传由一对隐性基因控制[13],靶标基因发生突变后经有性杂交形成纯合体表现抗药性[14]。已有卵菌(如霜霉菌)对烯酰吗啉产生田间抗药性的相关报道。Gisi等[13]检测到分离自法国、德国果园的葡萄霜霉菌(Plasmopara viticola)对烯酰吗啉具有抗性,抗性菌株的频率为42%。路粉等[15]测定了2011—2016 年来自河北和山东的1821 个黄瓜霜霉菌(Pseudoperonospora cubensis)菌株对烯酰吗啉的敏感性,表明抗性频率为88.5%。有关荔枝霜疫霉对烯酰吗啉抗性风险的研究报道甚少。易赛等[9]测定了2009—2012 年来自广东、广西、福建和云南4 个省份的115 株荔枝霜疫霉菌株对烯酰吗啉的敏感性,表明EC50均值为(0.170 2±0.002 6)μg·mL-1,无抗药性亚群体产生。笔者测定了2019—2020 年来自广东、广西、福建、四川和云南5 个省份的320 株荔枝霜疫霉菌株对烯酰吗啉的敏感性,EC50 均值为(0.122 2±0.000 9)μg·mL-1,未发现田间抗药性菌株。与易赛等[9]的结果相比较,2019—2020 年来自中国荔枝主产区的荔枝霜疫霉对烯酰吗啉的敏感性有所增加;且来自2020 年的荔枝霜疫霉菌株对烯酰吗啉的敏感性较2019年的显著升高,抗药性风险相应有所增加。烯酰吗啉仍可作为防治荔枝霜疫病的有效杀菌剂继续使用,但应加强荔枝霜疫霉对烯酰吗啉的连年抗药性动态监测,为田间荔枝霜疫病的防控及科学用药提供重要的指导。
易赛等[9]对不同地理来源的荔枝霜疫霉对烯酰吗啉的敏感性分析表明,4 个不同省份的荔枝霜疫霉对烯酰吗啉的敏感性差异不显著。本研究结果表明,来自广东、广西两省份的荔枝霜疫霉群体与来自福建、云南和四川三省的菌株群体对烯酰吗啉的敏感性差异显著,其中来自四川省的荔枝霜疫霉EC50均值最低,而来自广东省的EC50均值最高,则敏感性最低。不同地域的施药水平、年限及栽培管理等因素均会影响病原菌对杀菌剂抗药性风险水平。在荔枝生产中应注意合理使用药剂,选择与烯酰吗啉无交互抗性的杀菌剂交替或混合使用,从而延缓抗药性的发生。
生物学适合度是抗性突变株及其亲本菌株单独存在时的生存力或致病力的反映[16]。Stein等[17]的研究表明致病疫霉(Phytophthora infestans)抗烯酰吗啉的突变株与亲本相比较,生长速率和致病性降低。袁善奎等[18]通过紫外诱变获得3 株P.infestans对烯酰吗啉的抗性突变株,突变株的生长速率和产孢子囊的能力显著下降。Zhu 等[19]利用紫外诱导获得的黄瓜霜霉菌抗烯酰吗啉突变株的孢子囊产率及致病力均下降。崔晓岚等[20]通过紫外诱变获得抗烯酰吗啉的辣椒疫霉突变株,抗性指数介于16.38~132.15 之间,致病力与亲本菌株相当。笔者在本研究中通过药剂驯化获得荔枝霜疫霉抗烯酰吗啉的突变株,抗性指数介于77.17~360.11之间,抗药性可稳定遗传,突变株的孢子囊萌发率及卵孢子产量均下降。较低的孢子囊萌发率及卵孢子产量不利于荔枝霜疫霉群体在田间形成优势菌群,这与Stein等[17]研究结果较一致。笔者在本研究中荔枝霜疫霉抗烯酰吗啉突变株的致病力与其亲本菌株略低或相当,有必要在后续研究中获得更多的抗性突变株,扩大群体评估荔枝霜疫霉对CAAs 类杀菌剂的抗药性风险。卵菌对CAA 类杀菌剂抗药性的产生可能与编码纤维素合成酶复合体的CesA1、CesA2、CesA3 和CesA4 等基因突变有关[21-24]。笔者在本研究中获得的荔枝霜疫霉抗烯酰吗啉突变株的抗性机制有待深入研究,结合田间抗药性风险的持续监测,以期为烯酰吗啉等CAAs类杀菌剂持久发挥良好防效提供重要的依据。
4 结 论
建立了荔枝霜疫霉对烯酰吗啉的敏感性基线,荔枝霜疫霉对烯酰吗啉仍保持较高的敏感性,未检测到田间抗性菌株,烯酰吗啉仍可作为防治荔枝霜疫病的有效药剂。通过驯化测定表明,荔枝霜疫霉抗烯酰吗啉的突变株孢子囊的萌发率及卵孢子产量均下降,致病力相当,但存在一定风险。笔者将继续加强荔枝霜疫霉群体对烯酰吗啉的抗药性监测及风险评估。
致谢:国家荔枝龙眼产业技术体系各综合试验站提供病样采集支持,谨此致谢。
[1]郭栋梁,黄石连,向旭.2022 年广东荔枝生产形势分析[J].广东农业科学,2022,49(6):130-137.GUO Dongliang,HUANG Shilian,XIANG Xu.Analysis of Guangdong Litchi production situation in 2022[J].Guangdong Agricultural Sciences,2022,49(6):130-137.
[2]郑重禄.荔枝霜疫霉病菌及其生物学特性研究概述[J].中国南方果树,2015,44(6):157-168.ZHENG Zhonglu.Summary of studies on Phytophthora litchii and its biological characteristics[J].South China Fruits,2015,44(6):157-168.
[3]BLUM M,BOEHLER M,RANDALL E,YOUNG V,CSUKAI M,KRAUS S,MOULIN F,SCALLIET G,AVROVA A O,WHISSON S C,FONNE-PFISTER R.Mandipropamid targets the cellulose synthase-like PiCesA3 to inhibit cell wall biosynthesis in the oomycete plant pathogen,Phytophthora infestans[J].Molecular Plant Pathology,2010,11(2):227-243.
[4]周振标,李畅方,冼金曹,张晓华.40%烯酰吗啉水分散粒剂防治荔枝霜疫霉病田间药效试验[J].农药科学与管理,2005,26(8):17-18.ZHOU Zhenbiao,LI Changfang,XIAN Jincao,ZHANG Xiaohua.Effect of dimethomoph 40% WG for controlling Peronophythora litchii Chen ex Ko et al [J].Pesticide Science and Administration,2005,26(8):17-18.
[5]蔡学清,林娜,陈炜,胡方平.荔枝霜疫霉的生防菌株与化学制剂的筛选[J].福建农林大学学报(自然科学版),2008,37(5):463-468.CAI Xueqing,LIN Na,CHEN Wei,HU Fangping.Selection of the biological agents and fungicides against Phytophthora litchii[J].Journal of Fujian Agriculture and Forestry University(Natural Science Edition),2008,37(5):463-468.
[6]凌金锋,彭埃天,宋晓兵,陈霞,习平根,姜子德.烯酰吗啉与咪鲜胺混配对荔枝霜疫霉病菌和炭疽病菌的联合作用研究[J].广东农业科学,2010,37(8):146-148.LING Jinfeng,PENG Aitian,SONG Xiaobing,CHEN Xia,XI Pinggen,JIANG Zide.Study on the combined effect of dimethomorph and prochloraz against two pathogens of litchi downy blight and litchi anthracnose[J].Guangdong Agricultural Sciences,2010,37(8):146-148.
[7]王璧生,彭埃天,黄华,刘朝桢.安克锰锌防治荔枝霜疫霉病试验初报[J].广东农业科学,1999,26(3):40-41.WANG Bisheng,PENG Aitian,HUANG Hua,LIU Chaozhen.Preliminary report of Anke manganese zinc against litchi downy blight[J].Guangdong Agricultural Science,1999,26(3):40-41.
[8]WANG H C,SUN H Y,STAMMLER G,MA J X,ZHOU M G.Baseline and differential sensitivity of Peronophythora litchii(lychee downy blight) to three carboxylic acid amide fungicides[J].Plant Pathology,2009,58(3):571-576.
[9]易赛,潘汝谦,徐大高,凌金锋,彭埃天,习平根,姜子德.荔枝霜疫霉菌对烯酰吗啉的敏感性测定[J].广东农业科学,2014,41(2):87-91.YI Sai,PAN Ruqian,XU Dagao,LING Jinfeng,PENG Aitian,XI Pinggen,JIANG Zide.Sensitivity of Peronophythora litchii to dimethomorph[J].Guangdong Agricultural Sciences,2014,41(2):87-91.
[10]FAO.Recommended methods for the detection and measurement of resistance of agricultural pests to pesticides[J].FAO Plant Protection Bulletin,1982,30(2):39-42.
[11]任德春.荔枝霜疫霉菌对甲霜灵和精甲霜灵的抗药性研究[D].广州:华南农业大学,2016.REN Dechun.The resistance of Peronophythora litchii to Metalaxyl and Metalaxyl-M[D].Guangzhou:South China Agricultural University,2016.
[12]FLIER W G,GRÜNWALD N J,FRY W E,TURKENSTEEN L J.Formation,production and viability of oospores of Phytophthora infestans from potato and Solanum demissum in the Toluca Valley,central Mexico[J].Mycological Research,2001,105(8):998-1006.
[13]GISI U,WALDNER M,KRAUS N,DUBUIS P H,SIEROTZKI H.Inheritance of resistance to carboxylic acid amide(CAA)fungicides in Plasmopara viticola[J].Plant Pathology,2007,56(2):199-208.
[14]COHEN Y,RUBIN E,HADAD T,GOTLIEB D,SIEROTZKI H,GISI U.Sensitivity of Phytophthora infestans to mandipropamid and the effect of enforced selection pressure in the field[J].Plant Pathology,2007,56(5):836-842.
[15]路粉,吴杰,王会君,孟润杰,赵建江,韩秀英,马志强,王文桥.山东省和河北省黄瓜霜霉病菌对烯酰吗啉及双炔酰菌胺的抗性监测[J].植物病理学报,2018,48(5):666-674.LU Fen,WU Jie,WANG Huijun,MENG Runjie,ZHAO Jianjiang,HAN Xiuying,MA Zhiqiang,WANG Wenqiao.Resistance monitoring of Pseudoperonospora cubensis to dimethomorph and mandipropamid in Hebei and Shandong provinces of China[J].Acta Phytopathologica Sinica,2018,48(5):666-674.
[16]翟明涛,王开运,许辉,唐剑锋,刘杰.抗氟吡菌胺辣椒疫霉菌株的诱导及其生物学特性的研究[J].植物病理学报,2014,44(1):88-96.ZHAI Mingtao,WANG Kaiyun,XU Hui,TANG Jianfeng,LIU Jie.Induction and characteristics of Phytophthora capsici isolates resistant to fluopicolide[J].Acta Phytopathologica Sinica,2014,44(1):88-96.
[17]STEIN J M,KIRK W W.The generation and quantification of resistance to dimethomorph in Phytophthora infestans[J].Plant Disease,2004,88(9):930-934.
[18]袁善奎,刘西莉,刘亮,王恒,姜辉,陈隆智.马铃薯晚疫病菌对烯酰吗啉的敏感性基线及其室内抗药突变体的研究[J].植物病理学报,2005,35(6):545-551.YUAN Shankui,LIU Xili,LIU Liang,WANG Heng,JIANG Hui,CHEN Longzhi.Study on baseline sensitivity and laboratory resistant mutants of Phytophthora infestans to dimethomorph[J].Acta Phytopathologica Sinica,2005,35(6):545-551.
[19]ZHU S,LIU P,LIU X,LI J,YUAN S,SI N.Assessing the risk of resistance in Pseudoperonospora cubensis to the fungicide flumorph in vitro[J].Pest Management Science,2008,64(3):255-261.
[20]崔晓岚,孟庆晓,毕扬,吴鹏飞,刘西莉.辣椒疫霉对烯酰吗啉的敏感性基线及室内抗药突变体研究[J].植物病理学报,2009,39(6):630-637.CUI Xiaolan,MENG Qingxiao,BI Yang,LIU Pengfei,LIU Xili.Baseline sensitivity and laboratory mutants of Phytophthora capsici resistant to dimethomorph[J].Acta Phytopathologica Sinica,2009,39(6):630-637.
[21]BLUM M,WALDNER M,GISI U.A single point mutation in the novel PvCesA3 gene confers resistance to the carboxylic acid amide fungicide mandipropamid in Plasmopara viticola[J].Fungal Genetics and Biology,2010,47(6):499-510.
[22]BLUM M,GAMPER H A,WALDNER M,SIEROTZKI H,GISI U.The cellulose synthase 3 (CesA3) gene of oomycetes:Structure,phylogeny and influence on sensitivity to carboxylic acid amide (CAA) fungicides[J].Fungal Biology,2012,116(4):529-542.
[23]CHEN L,ZHU S S,LU X H,PANG Z L,CAI M,LIU X L.Assessing the risk that Phytophthora melonis can develop a point mutation(V1109L)in CesA3 conferring resistance to carboxylic acid amide fungicides[J].PLoS One,2012,7(7):e42069.
[24]PANG Z L,SHAO J P,CHEN L,LU X H,HU J,QIN Z H,LIU X L.Resistance to the novel fungicide pyrimorph in Phytophthora capsici:risk assessment and detection of point mutations in CesA3 that confer resistance[J].PLoS One,2013,8(2):e56513.