中国是世界上苹果属植物最丰富的国家,作为苹果起源地之一,苹果种植面积不断扩大,目前已成为全球最大的苹果生产国[1]。苹果绵蚜[Eriosoma lanigerum(Hausmann)],属半翅目瘿绵蚜科,是以苹果属植物为主要寄主的入侵害虫,对北方苹果产业构成巨大危害[2-3],多在新梢芽腋、枝干裂缝、根蘖基部处危害,其典型危害特征是寄主受害部位覆盖白色棉絮状物、形成肿瘤,难以防治[4]。研究显示,生产中主栽品种红富士高感苹果绵蚜,新红星、小国光具有一定抗性[5-8]。Zhou等[9]基于EPG刺吸电位技术进一步研究发现,苹果绵蚜取食韧皮部阶段,在抗蚜品种新红星和小国光上需要多量、多次分泌唾液才可保证其顺利吸食;非韧皮部取食阶段,在新红星和小国光上第一次刺探开始时间和非穿透波的总时间显著多于红富士,推测两抗蚜品种中可能存在影响苹果绵蚜口针刺探的物理或化学抗性因子。
次生代谢产物木质素在被子植物中主要由愈创木基木质素(G)、紫丁香基木质素(S)和对羟基木质素(H)组成,参与细胞壁的形成,并与纤维素一起增加细胞硬度[10],是近年来研究的热点问题[11]。研究证明木质素与植物抗病虫有密切联系,An等[12]研究发现菊花木质素通过促进细胞壁增厚、木质化而提高了机械强度,从而阻止蚜虫取食。漆酶EC.1.10.3.2(laccase,LAC)是一种含铜的糖蛋白氧化酶,一般具有4 个铜离子,均形成其催化位点,并包含3 个铜氧化酶蛋白结构域(Cu-oxidase、Cu-oxidase 2、Cu-oxidase 3)[13-14]。在高等植物中,漆酶参与木质素合成通路中催化单分子醇聚合的最后一步[15]。此外,漆酶还参与植物的防御过程,研究发现小麦漆酶基因TaLAC4 能够通过增加G-木质素合成,使细胞壁增厚而抵抗小麦赤霉病菌的侵染[16];GhLac1 在棉花中过表达,促进了木质素的合成,从而抵御棉铃虫和棉蚜的取食[17],可见漆酶基因在植物抗病虫中起着重要作用。
目前苹果漆酶的研究未见报道,笔者通过转录组分析苹果漆酶基因在苹果绵蚜胁迫压力下的表达模式,基于漆酶基因的保守结构域对不同苹果品种漆酶差异基因进行鉴定,初步筛选出抗苹果绵蚜漆酶候选基因,并对原核表达所用载体及表达条件进行研究,为深入了解苹果漆酶基因的抗蚜功能提供理论依据。
1 材料和方法
1.1 试验材料
标准蛋白质分子质量Marker、Escherichia coli BL21(DE3)菌株购自北京全式金生物科技有限公司。原核表达载体pET-28a(+)、pET-32a(+)、pGEXTEV、pHAT2 由本实验室保存。PUC19-EGFP 质粒干粉购自淼灵质粒平台。酵母提取物、胰蛋白胨购自OXOID。漆酶检测活性试剂盒、琼脂粉购自北京索莱宝科技有限公司。测序由北京擎科生物科技有限公司完成。E.coli DH5α、DNA聚合酶、DNA连接酶(ClonExpress®ⅡOne Step Cloning Kit)均购自南京诺唯赞生物科技股份有限公司。多糖多酚植物总RNA 提取试剂盒、反转录试剂、质粒小提试剂盒、DNA 凝胶回收试剂盒、DNA Marker、各种限制性内切酶购自宝生物工程(大连)有限公司。DNA合成、异丙基硫代β-D 半乳糖苷(IPTG)、抗His 标签单克隆抗体、HRP 标记兔抗小鼠IgG 抗体购自生工生物工程(上海)股份有限公司,SDS-PAGE 蛋白上样缓冲液、ECL 化学发光试剂盒购自山东思科捷生物技术有限公司,其他常规试剂为分析纯。
1.2 不同苹果品种被苹果绵蚜危害前后的转录组测序
苹果绵蚜在(23±2)℃、14 h光照、10 h黑暗、相对湿度60%~80%的温室中用剥离成熟苹果种子内外种皮法[18]获得的苹果幼苗进行繁殖。苹果试验苗木(Malus domestica)栽植于青岛农业大学胶州实验农场果树基地(120.11°E,36.44°N),其砧木为海棠(M.spectabilis)。于2019 年5 月随机选取1 年生健康植株新红星(Starkrimson,SM)、小国光(Ralls Genet,RG)、红富士(Red Fuji,RF)3 种苹果品种,在距顶芽8~10 cm 处每株接种苹果绵蚜40 头,在植物对苹果绵蚜的应激反应早期(12 h)[19]和苹果绵蚜在苹果苗木上的种群定殖期(5 d)[20]采集被苹果绵蚜为害6 cm左右的苹果枝条样本,取未接种苹果绵蚜的抗蚜、感蚜苹果品种作为对照,每组处理均3次生物学重复,共采集27个样本。样本采集后迅速在液氮中冷冻,于-80 ℃保存备用。由上海欧易生物医学科技有限公司进行有参转录组测序。
1.3 苹果LAC基因的筛选与鉴定
以蛋白质数据库Uniprot(https://www.uniprot.org/)下载的拟南芥(Arabidopsis thaliana)漆酶蛋白为检索序列,通过Blastp 在苹果转录组测序序列中比对匹配的蛋白序列,筛选出E-value<1e-5的候选基因,删除冗余序列后将其蛋白序列通过NCBI CDD(https://www.ncbi.nlm.nih.gov/cdd)数 据 库 和Pfam(http://pfam.xfam.org/)数据库进行比对,通过漆酶蛋白结构域鉴定基因;基于转录组测序,分析新红星、小国光及红富士被苹果绵蚜为害0 h(对照,ctr)、12 h、5 d漆酶差异表达基因及其表达模式。组间差异表达分析,以p-value<0.05、Fold Change>2作为判断基因表达差异显著的标准。使用TBtools软件制作结构域直观图与基因表达模式热图[21]。
1.4 苹果LAC基因的克隆
基于漆酶基因表达量及其在抗蚜、感蚜苹果品种的表达模式,选择了3类漆酶基因,即在抗蚜品种新红星、小国光中均上调表达的MdLac23 基因,新红星特有的上调表达基因MdLac6、MdLac7,小国光特有的上调表达基因MdLac2 进行序列克隆。利用4 种原核表达质粒pET-28a(+)、pET-32a(+)、pGEXTEV、pHAT2 构建目的蛋白表达载体。根据苹果漆酶mRNA序列,去除信号肽序列后,利用Primer Premier 5.0 软件设计引物(下划线部分为酶切位点序列,下划线前的碱基为线性化载体两末端同源序列)。为方便构建目的DNA 质粒,插入片段扩增引物设计方式为:上游/下游载体末端同源序列+酶切位点+基因特异性正向/反向扩增引物序列(表1,表2)。以苹果苗木中获得的cDNA 为模板,采用PCR扩增基因的全长编码序列(CDS)。PCR 扩增程序:95 ℃预变性5 min;95 ℃变性15 s,各基因退火温度分别为MdLac23 59.7 ℃、MdLac6 53 ℃、MdLac7 及MdLac2 56.7 ℃,退火20 s,72 ℃延伸3 min,35个循环;72 ℃再延伸5 min。PCR 反应结束后,产物用1%的琼脂糖凝胶进行电泳,并用DNA 凝胶回收试剂盒纯化回收目的条带。
表1 同源臂及酶切位点序列
Table 1 Homologous arm and enzyme cut site sequence
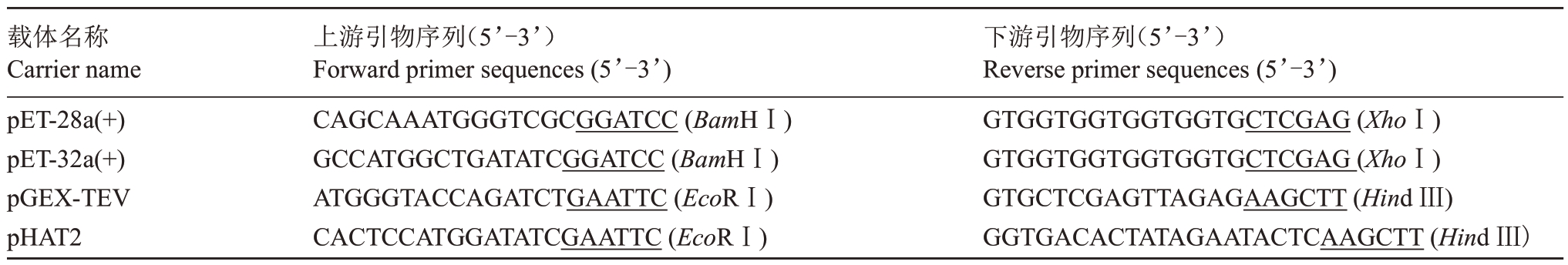
载体名称Carrier name pET-28a(+)pET-32a(+)pGEX-TEV pHAT2上游引物序列(5’-3’)Forward primer sequences(5’-3’)CAGCAAATGGGTCGCGGATCC(BamHⅠ)下游引物序列(5’-3’)Reverse primer sequences(5’-3’)GTGGTGGTGGTGGTGCTCGAG(XhoⅠ)GCCATGGCTGATATCGGATCC(BamHⅠ)GTGGTGGTGGTGGTGCTCGAG(XhoⅠ)ATGGGTACCAGATCTGAATTC(EcoRⅠ)GTGCTCGAGTTAGAGAAGCTT(Hind Ⅲ)CACTCCATGGATATCGAATTC(EcoRⅠ)GGTGACACTATAGAATACTCAAGCTT(Hind Ⅲ)
表2 基因特异性引物序列
Table 2 Gene-specific primer sequences
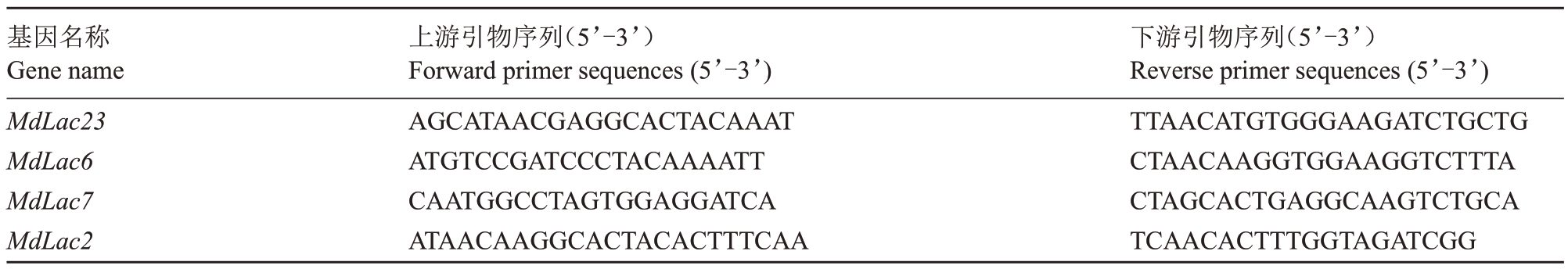
基因名称Gene name MdLac23 MdLac6 MdLac7 MdLac2上游引物序列(5’-3’)Forward primer sequences(5’-3’)AGCATAACGAGGCACTACAAAT ATGTCCGATCCCTACAAAATT CAATGGCCTAGTGGAGGATCA ATAACAAGGCACTACACTTTCAA下游引物序列(5’-3’)Reverse primer sequences(5’-3’)TTAACATGTGGGAAGATCTGCTG CTAACAAGGTGGAAGGTCTTTA CTAGCACTGAGGCAAGTCTGCA TCAACACTTTGGTAGATCGG
1.5 重组蛋白原核表达载体的构建
利 用 内 切 酶 消 化pET-28a(+)、pET-32a(+)、pGEX-TEV、pHAT2,双酶切使载体线性化,凝胶回收后,参照南京诺唯赞一步克隆试剂盒说明书进行同源重组反应。线性化载体和插入的目的片段按照0.03 pmol∶0.06 pmol比例,37 ℃条件下连接2 h。连接产物用热激法导入感受态DH5α,获得阳性克隆后继续培养提取质粒,将其转至E.coli BL21(DE3)感受态细胞,同时将未连接目的片段的pET-28a(+)、pET-32a(+)、pGEX-TEV、pHAT2 质粒也转入E.coli BL21(DE3),挑取阳性克隆进行测序,获得重组表达菌株和对照表达菌株。
1.6 重组蛋白的诱导表达、Western-Blot检测
将20 个重组蛋白表达菌株按1∶50 比例转接至15 mL LB 液体培养基中,37 ℃、200 r·min-1 震荡培养至OD600 为0.7~0.8,加入IPTG 至终浓度为0.5 mmol·L-1,16 ℃、220 r·min-1诱导24 h,以不加IPTG的菌液作为阴性对照。收集菌体,加入PBS重悬后进行超声波破碎,分别取IPTG诱导和未诱导的上清液、沉淀,进行SDS-PAGE凝胶电泳(5%浓缩胶和10%分离胶),Western-Blot 检测蛋白表达情况。Western-Blot后续步骤如下:将蛋白条带转至PVDF膜上,经5%脱脂奶粉4 ℃封闭过夜,使用His标签单克隆抗体摇床孵育1 h,1×TBST缓冲液洗涤5次,每次5 min;加入HRP标记兔抗小鼠IgG抗体室温孵育50 min,1×TBST 缓冲液洗涤5 次,最后使用ECL 发光试剂显影。
1.7 重组蛋白的纯化及活性鉴定
将构建的pET-28a(+)-MdLac23、pET-28a(+)-MdLac2 原核表达载体按上述诱导条件进行大量诱导(1200 mL),以pET-28a(+)-EGFP重组载体作为对照,通过超声波破碎,离心后取上清液,参照康为世纪的His-Tagged Protein Purification Kit(Soluble Protein)说明书,过Ni2+亲和柱对所表达蛋白进行纯化。纯化的蛋白洗脱液,使用截留分子质量为30 ku的超滤管进行适当浓缩和脱盐,用SDS-PAGE 电泳检测各样品纯度。采用Bradford 法测定蛋白浓度,以牛血清白蛋白(BSA)作为标准。按北京索莱宝漆酶检测活性试剂盒测定表达蛋白的活性,以ABTS[2,2′-联氮-二(3-乙基-苯并噻唑-6-磺酸)二铵盐]为底物,测定420 nm下42 ℃反应3 min前后的吸光度值。酶活定义为:每毫克蛋白每分钟氧化1 nmol 底物ABTS 所需的酶量为1 个酶活力单位。漆酶酶活性(U·mg-1)=ΔA÷(ε × d)×V 反总× 109÷(V 样× cpr)÷T。其中ΔA 测定管= A2 测定-A1 测定,ΔA 空白管=A2 空白-A1 空白,ΔA=ΔA 测定管-ΔA 空白管;A1为反应3 min前初始吸光值;A2 为反应3 min 后吸光值;ε 为ABTS 摩尔消光系数,36 000 L·mol-1·cm-1;d 为96 孔板光径,0.6 cm;V 反总为反应总体积,2×10-4 L;V 样为反应中样本体积,0.03 mL;cpr为样本蛋白质量浓度,mg·mL-1;T 为反应时间,3 min;109为单位换算系数,1 mol=109 nmol。
2 结果与分析
2.1 苹果LAC差异表达基因的鉴定
对LAC 差异表达基因保守结构域的分析结果如图1所示。共鉴定出27个LAC差异表达基因,其中MD10G1042700(MdLac25)仅含有Cu-oxidase 3结构域,MD10G1042600(MdLac24)仅含有Cu-oxidase 2 结构域,其他25 个基因均具有典型漆酶的3个特征结构域(Cu-oxidase、Cu-oxidase 2、Cu-oxidase 3)。27个漆酶基因的简称如表3所示。
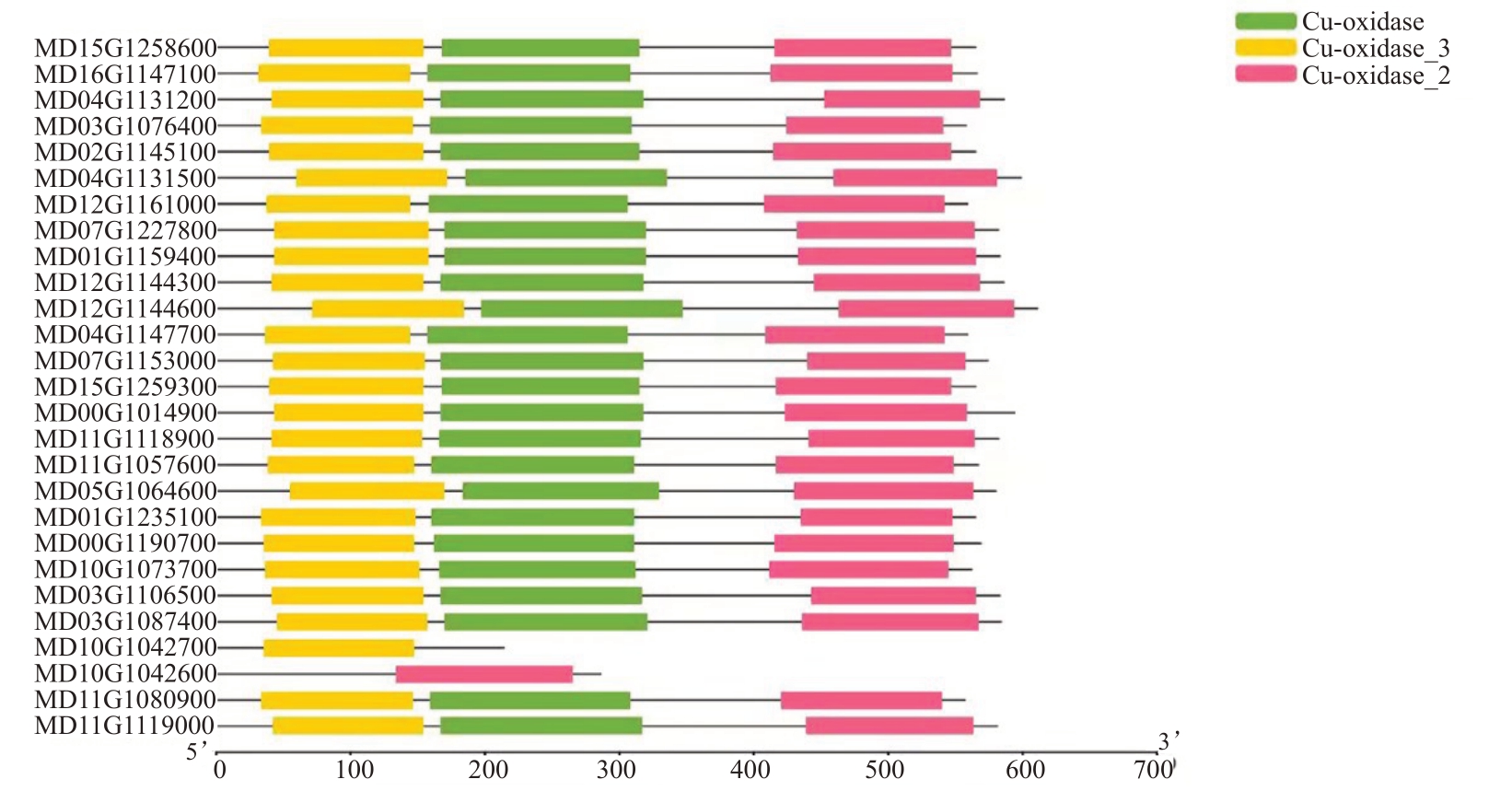
图1 LAC 保守结构域
Fig.1 Conserved structural domain of LAC
表3 苹果漆酶基因简称
Table 3 Abbreviations of apple laccase genes
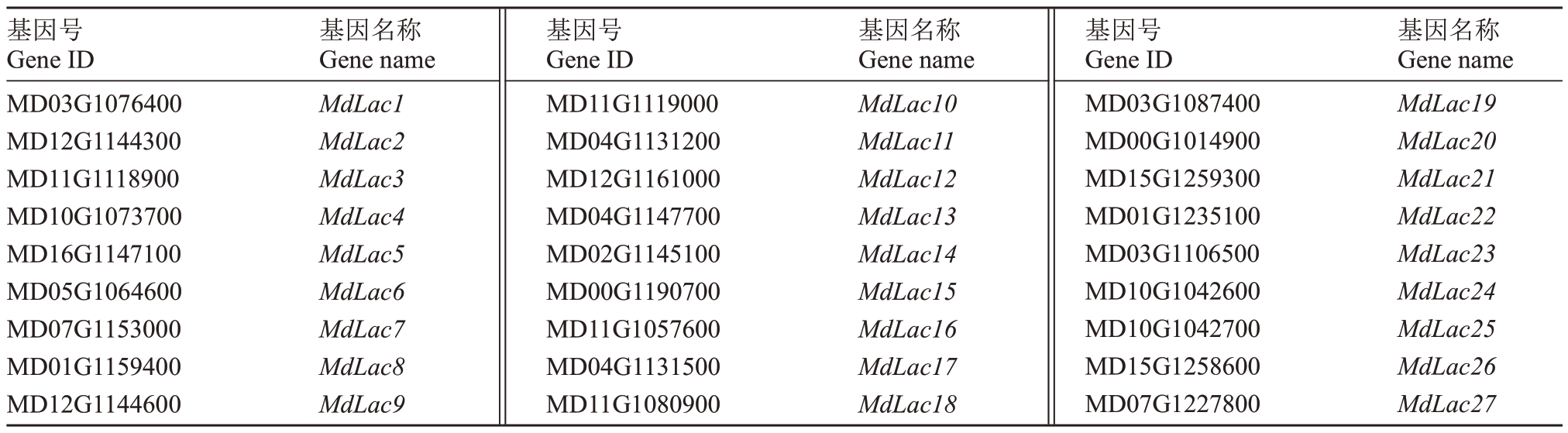
基因号Gene ID MD03G1076400 MD12G1144300 MD11G1118900 MD10G1073700 MD16G1147100 MD05G1064600 MD07G1153000 MD01G1159400 MD12G1144600基因名称Gene name MdLac1 MdLac2 MdLac3 MdLac4 MdLac5 MdLac6 MdLac7 MdLac8 MdLac9基因号Gene ID MD11G1119000 MD04G1131200 MD12G1161000 MD04G1147700 MD02G1145100 MD00G1190700 MD11G1057600 MD04G1131500 MD11G1080900基因名称Gene name MdLac10 MdLac11 MdLac12 MdLac13 MdLac14 MdLac15 MdLac16 MdLac17 MdLac18基因号Gene ID MD03G1087400 MD00G1014900 MD15G1259300 MD01G1235100 MD03G1106500 MD10G1042600 MD10G1042700 MD15G1258600 MD07G1227800基因名称Gene name MdLac19 MdLac20 MdLac21 MdLac22 MdLac23 MdLac24 MdLac25 MdLac26 MdLac27
2.2 LAC基因在不同苹果品种差异中的表达
同一品种不同时间点分别与对照相比,抗蚜品种新红星(SM)、小国光(RG)和感蚜品种红富士(RF)被苹果绵蚜为害前后(ctr、12 h、5 d)漆酶差异表达基因数量为27个,其表达模式如图2所示。根据漆酶基因在不同苹果品种中的差异表达情况,统计结果(表4)显示,新红星12个上调表达基因,无下调表达基因;小国光9个上调表达基因,3个下调表达基因;红富士10个下调表达基因,4个上调表达基因。其中,MdLac20、MdLac21、MdLac22 在3 个苹果品种均上调表达;MdLac23 仅在新红星、小国光中上调表达;MdLac24、MdLac25在新红星中上调表达,小国光中下调表达;MdLac26在小国光和红富士中上调表达;MdLac27在小国光和红富士中下调表达,其余差异表达基因均在各品种中表现出特有的上下调表达。

图2 漆酶差异基因表达热图
Fig.2 Differential gene expression heat map of laccase
热图提供了不同色标,以指示差异表达水平。红色表示表达上调,蓝色表示表达下调。
A colour scale is provided with the heat map to indicate the levels of differential expression.Red shades indicate higher expression and blue shades indicate lower expression.
表4 不同苹果品种中差异基因表达量上下调统计
Table 4 Statistics of up-and down-regulation of differential gene expression in different apple varieties

基因名称Gene name小国光(上调/下调)Ralls Genet(up/down)红富士(上调/下调)Red Fuji(up/down)MdLac5 MdLac6 MdLac7 MdLac8 MdLac9 MdLac10 MdLac23 MdLac20 MdLac21 MdLac22 MdLac24 MdLac25 MdLac1 MdLac2 MdLac3 MdLac4 MdLac26 MdLac27 MdLac11 MdLac12 MdLac13 MdLac14 MdLac15 MdLac16 MdLac17 MdLac18 MdLac19新红星(上调/下调)Starkrimson(up/down)up up up up up up up up up up up up up up up up up up up down down up up up up up up down down down down down down down down down down down
综上可见,不同苹果品种漆酶基因表现出明显的差异表达模式。抗蚜品种新红星及小国光漆酶差异表达基因上调表达居多,感蚜品种红富士下调表达居多。
2.3 原核表达载体的构建
MdLac23、MdLac6、MdLac7、MdLac2 4 个LAC基因全长分别为1659、1743、1644、1665 bp,PCR产物经1%琼脂糖凝胶电泳检测,在靠近2000 bp处出现特异性扩增条带,与预期目的片段大小一致(图3)。原核表达载体pET-28a(+)、pET-32a(+)的质粒使用BamHⅠ和XhoⅠ双酶切,pGEX-TEV、pHAT2 质粒使用EcoRⅠ和Hind Ⅲ双酶切,各质粒双酶切线性化长度分别为5322、5854、4386、5027 bp(图4)。将PCR 产物及线性化质粒纯化后经DNA连接酶进行连接,最终获得测序正确的大肠杆菌BL21(DE3)表达菌株单菌落,用于后续试验。

图3 苹果LAC 基因克隆
Fig.3 The electrophoresis results of LAC gene
M.DL2000 Marker;1~2.MdLac2 PCR 产物;3~4.MdLac7 PCR 产物;5~6.MdLac6 PCR 产物;7~8.MdLac23 PCR 产物。
M.DL2000 Marker; 1-2.PCR products of MdLac2; 3-4.PCR products of MdLac7; 5-6.PCR products of MdLac6; 7-8.PCR products of MdLac23.

图4 原核表达质粒双酶切线性化
Fig.4 Linearization of prokaryotic expression plasmids by double digestion
M.DL5000 Marker;1.pET-28a(+)质粒;2.pET-28a(+)酶切产物;3.pET-32a(+)质粒;4.pET-32a(+)酶切产物;5.pGEX-TEV 质粒;6.pGEX-TEV 酶切产物;7.pHAT2 质粒;8.pHAT2 酶切产物。
M.DL5000 Marker; 1.Plasmids of pET-28a(+); 2.Digested production of pET-28a(+); 3.Plasmids of pET-32a(+); 4.Digested production of pET-32a(+); 5.Plasmids of pGEX-TEV; 6.Digested production of pGEX-TEV;7.Plasmids of pHAT2;8.Digested production of pHAT2.
2.4 重组蛋白Western-Blot检测
Western-Blot结果显示,经IPTG诱导的pET-28a(+)-MdLac23、pET-28a(+)-MdLac2 重组蛋白表达菌株各抽提液(上清液、沉淀)在大约70 ku(含标签大小)处出现了目的重组蛋白,与理论预测的蛋白质相对分子质量大小相符合,而pET-28a(+)对照组的各抽提液均没有条带,表明其重组蛋白在pET-28a(+)16 ℃诱导系统成功表达,且有可溶性蛋白的表达,重组表达菌株在沉淀抽提液明显亮于上清抽提液(图5-A);pET-32a(+)-MdLac7、pET-32a(+)-MdLac2大约在70 ku处也出现了清晰的目的蛋白表达条带,与理论预测值相符合,以包涵体的形式存在,而pET-32a(+)对照组没有出现目的蛋白表达条带(图5-B);pGEX-TEV、pHAT2原核表达载体中均无目的蛋白表达条带(图5-C~D)。

图5 LAC 重组蛋白的Western-Blot 分析
Fig.5 Western-Blot analysis of LAC recombinant protein
方框所指为目标产物条带;A.pET-28a(+)载体上重组蛋白的表达;B.pET-32a(+)载体上重组蛋白的表达;C.pGEX-TEV 载体上重组蛋白的表达;D.pHAT2 载体上重组蛋白的表达;1~4.诱导的上清液、未诱导的上清液、诱导的沉淀、未诱导的沉淀;M.蛋白质Marker。
The box refers to the target product protein;A.Expression of recombinant protein on pET-28a(+)vector;B.Expression of recombinant protein on pET-32a(+) vector; C.Expression of recombinant protein on pGEX-TEV vector; D.Expression of recombinant protein on pHAT2 vector; 1-4.Induced supernatant,uninduced supernatant,induced precipitation,uninduced precipitate;M.Protein marker.

图5 (续) Fig.5 (Continued)
2.5 重组蛋白的纯化及其体外催化活性测定
SDS-PAGE 电 泳 显 示pET-28a(+)-MdLac23、pET-28a(+)-MdLac2、pET-28a(+)-EGFP 成功纯化出重组蛋白(图6-A~B)。BSA 蛋白标准曲线见图7,获得标准方程y=21.704x+0.511 4,R²=0.993 4,计算获得MdLac23、MdLac2纯化后蛋白质量浓度分别为0.011 2 μg·μL-1、0.012 6 μg·μL-1,EGFP蛋白质量浓 度 为0.013 8 μg·μL- 1。以ABTS 为 底 物 测 定EGFP、MdLac23、MdLac2 纯化蛋白的活性,分别为408 U·mg-1、427 U·mg-1、433 U·mg-1 ,与对照EGFP相比,MdLac23、MdLac2蛋白均无活性(图8)。
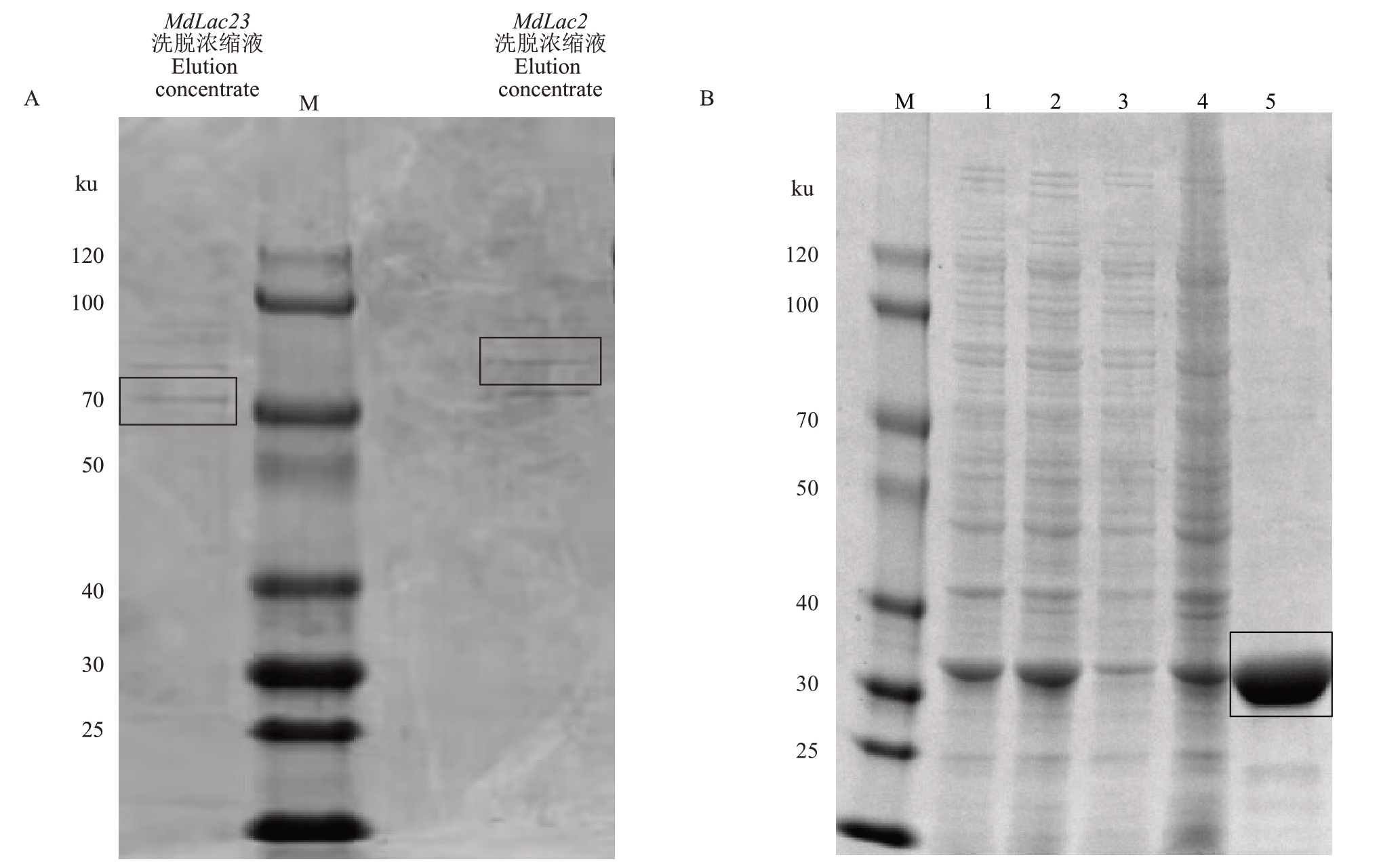
图6 纯化目的重组蛋白SDS-PAGE 分析
Fig.6 Purification of target recombinant protein SDS-PAGE analysis
方框所指纯化为目的蛋白;A.MdLac23、MdLac2 纯化蛋白;B.EGFP 纯化蛋白;1~5.诱导的上清液、诱导的沉淀、未诱导上清液、未诱导沉淀、洗脱浓缩液;M.蛋白质Marker。
Boxes refer to purified target proteins;A.MdLac23,MdLac2 purified protein;B.EGFP purified protein;1-5.Induced supernatant,induced precipitate,uninduced supernatant,uninduced precipitate,elution concentrate;M.Protein marker.
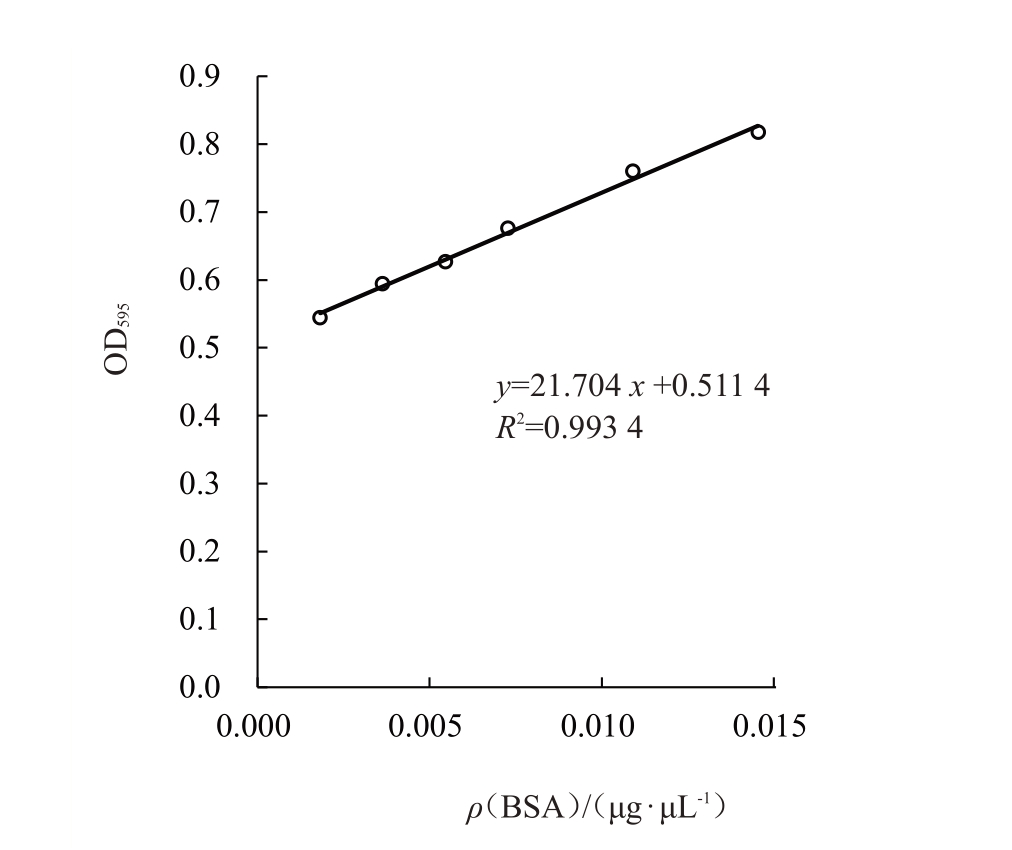
图7 BSA 浓度标准曲线
Fig.7 Standard curve of BSA concentration
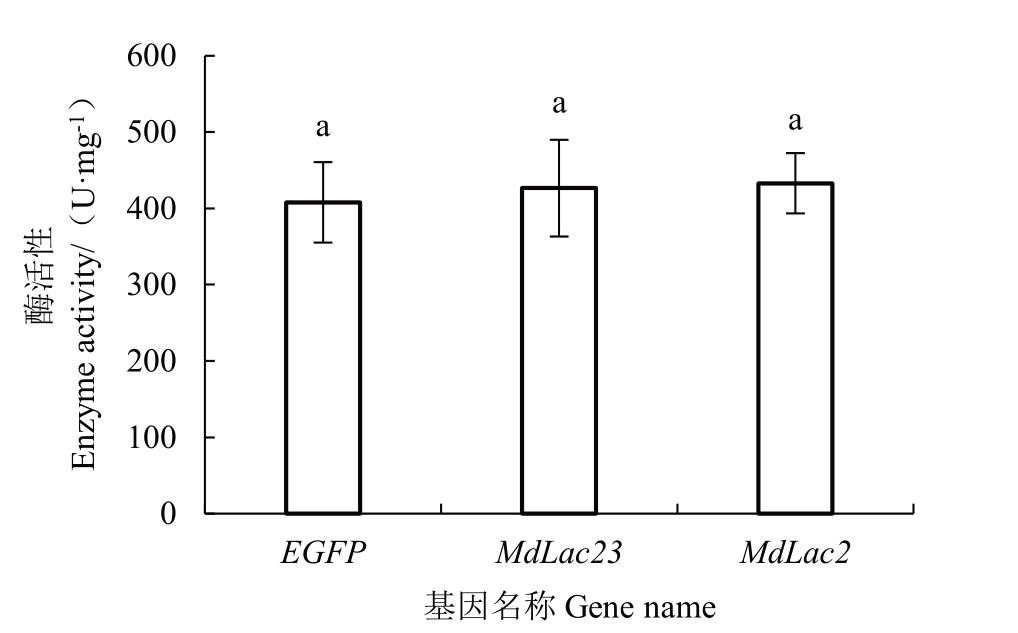
图8 漆酶活性测定结果
Fig.8 Determination result of laccase activity
数据为(平均值±标准差),不同小写字母表示差异显著(Tukey,p<0.05)。
Data are (mean ± standard deviation), and different small letters indicate significant differences(Tukey,p<0.05).
3 讨 论
漆酶作为植物次生细胞壁形成过程中木质素生物合成的关键酶,是催化木质素单体聚合的最后一步。含有3个保守结构域Cu-oxidase、Cu-oxidase 2、Cu-oxidase 3,这是大多数植物漆酶基因的典型特征,例如梨(Pyrus bretschneideri)含有41 个漆酶基因[22]、高粱(Sorghum bicolor)含有27 个漆酶基因[23],这些漆酶基因均包含3 个特征结构域,属于典型的多铜氧化酶。但也有一些漆酶基因含有一个或两个保守结构域,例如柑橘(Citrus sinensis)漆酶基因Cs-LAC1-02 仅含有Cu-oxidase 和Cu-oxidase 2 结构域,CsLAC13 和CsLAC19 仅 含 有Cu-oxidas 3 结 构域[15]。笔者在本研究共鉴定出的27 个苹果漆酶抗蚜候选基因中,MdLac25、MdLac24基因分别只含有1个Cu-oxidase 3结构域、Cu-oxidase 2结构域,其余25 个漆酶基因均具有Cu-oxidase、Cu-oxidase 2、Cuoxidase 3 保守结构域,这与上述柑橘漆酶基因的鉴定结果一致。
在胁迫压力下,基因表达变化是植物防御反应的根本[24]。大豆(Glycine max)感染疫霉后,9 个漆酶基因表达量上调,12 个漆酶基因表达水平被抑制[25];当茶树(Camellia sinensis)受到食草昆虫为害后,同样也引起CsLACs 基因表达量的变化[13]。近年来,许多研究数据表明植物漆酶基因在生物胁迫反应中发挥重要作用。在白杨(Populus tomentosa)中,PtoLAC14 的过表达促进了杨树的木质化,证实了漆酶参与木质素的生物合成[26]。棉花(Gossypium hirsutum)GhLac1 的过表达加速了细胞壁的再生,木质素含量增加,增强了对棉蚜的抗性;而RNAi 后植株木质素减少,更易感染棉蚜[17]。愈创木基木质素(G)作为木质素的一种,其交联性强,耐解聚,当棉花被大丽轮枝菌侵染后,GhLAC15 在抗性棉花品种Jimian20 中的表达水平高于敏感品种Han208;同样通过表达或抑制GhLAC15 的转录水平,能够导致愈创木基木质素(G)含量的增加和降低,进而调节了对黄萎病的抗性[27],可见漆酶基因的上调表达,有利于增强植株对病虫害的抵御能力。本研究中,不同苹果品种受到苹果绵蚜刺吸为害后,抗蚜品种新红星、小国光中漆酶基因呈现相似的表达模式,基因上调表达居多。新红星中12个上调表达基因,无下调表达基因;小国光中9 个上调表达基因,3 个下调表达基因,众多的上调表达基因调控了相应的代谢通路,增强了对苹果绵蚜的抗性,这可能是苹果品种抗性增强的关键因素。感蚜品种红富士中的漆酶基因,仅4 个基因上调表达,10 个基因下调表达,与抗性苹果品种相比整体表现出相反的表达模式,因而红富士品种中的漆酶基因易感苹果绵蚜。漆酶基因在不同苹果品种中表现出不同的表达模式,这表明漆酶基因在植物抗蚜反应中发挥重要作用。
基因体外表达是研究其功能的重要方法,目前对植物漆酶基因体外表达的研究不多。有研究显示,梨漆酶基因PbLAC4-like 在pET-28a(+) 28 ℃诱导系统成功诱导出62.6 ku 的可溶性蛋白[28];水稻漆酶基因OsLAC10 通过pET-30a 原核表达载体,37 ℃诱导条件下,在沉淀中产生了66 ku 的重组蛋白条带[29]。原核表达系统为研究蛋白表达和功能提供了一条有效途径,但外源基因的表达还受诸多因素影响,如目的蛋白特性、原核表达载体类型、诱导条件等。较低的诱导温度有利于产生折叠的可溶性蛋白质[30],因此,笔者在本研究中用较低温度16 ℃诱导,且从4 种不同的原核表达载体出发尝试表达。结果显示,不同的原核表达载体对漆酶蛋白的表达影响显著,漆酶蛋白的表达主要以不溶性包涵体形式存在,pET-28a(+)载体诱导出了MdLac23、MdLac2 的可溶性蛋白;pET-32a(+)中,诱导产生的MdLac7、MdLac2 蛋白只在沉淀中存在;pGEX-TEV、pHAT2 原核表达载体中,Md-Lac23、MdLac6、MdLac7、MdLac2 蛋 白 均 无 法 表达。本研究中苹果漆酶活性检测结果显示无活性,在遗传背景方面,原核表达系统因缺少稀有密码子,可能导致较低的表达水平,且蛋白分子质量越大,大肠杆菌中可溶性表达的概率随之降低,特别是对于大于60 ku 的蛋白质[30],加之不具备活性所需的真核翻译后修饰[31],而漆酶作为一种高糖基化修饰的酶[32],重组蛋白未经糖基化或空间修饰,推测以上情况可能是导致漆酶活性丧失的原因。
综上所述,笔者在本研究中分析了不同苹果品种漆酶基因抗苹果绵蚜的表达模式,克隆了苹果LAC 基因序列,通过原核表达获得了漆酶蛋白,进一步检测了漆酶活性。由于原核表达系统不足以获得大量可溶性蛋白,表达的蛋白无活性,下一步还可尝试在酵母细胞、丝状真菌、植物、昆虫细胞中等进行漆酶表达纯化的研究,还需大量试验去探索漆酶蛋白的功能、作用机制等一系列问题。
4 结 论
该研究分别从转录组及不同原核表达载体分析不同苹果品种潜在的抗苹果绵蚜基因,筛选漆酶表达载体。不同苹果品种被苹果绵蚜为害0 h、12 h、5 d的转录组分析中,共鉴定获得27个漆酶差异表达基因,为潜在的抗苹果绵蚜基因,各品种漆酶基因的表达模式为抗蚜品种新红星、小国光,上调表达居多,感蚜品种红富士下调表达居多。克隆的MdLac23、Md-Lac6、MdLac7、MdLac2 4个漆酶基因,在pET-28a(+)、pET-32a(+)、pGEX-TEV、pHAT2 原核表达载体,16 ℃,220 r·min-1,IPTG 终浓度0.5 mmol·L-1,诱导24 h 的条件下,16 个原核表达重组构建体,仅Md-Lac23、MdLac2 在pET-28a(+)载体中成功表达出可溶性蛋白,经检测无酶催化活性。本研究为进一步探究苹果漆酶基因在抗苹果绵蚜中的作用、研究其功能蛋白奠定了基础。
[1]ZHANG Q Q,SHI F J,ABDULLAHI N M,SHAO L Q,HUO X X.An empirical study on spatial-temporal dynamics and influencing factors of apple production in China[J].PLoS One,2020,15(10):e0240140.
[2]ZHOU H X,ZHANG R M,TAN X M,TAO Y L,WAN F H,WU Q,CHU D.Invasion genetics of woolly apple aphid (Hemiptera:Aphididae) in China[J].Journal of Economic Entomology,2015,108(3):1040-1046.
[3]ZHOU H,TAN X M,TENG Z W,DU L J,ZHOU H X.EPG analysis of stylet penetration preference of woolly apple aphid on different parts of apple trees[J].PLoS One,2021,16(8):e0256641.
[4]STOKWE N F,MALAN A P.Woolly apple aphid,Eriosoma lanigerum (Hausmann),in South Africa:Biology and management practices,with focus on the potential use of entomopathogenic nematodes and fungi:review article[J].African Entomology,2016,24(2):267-278.
[5]TAN X M,YANG Z S,ZHOU H,YANG Q M,ZHOU H X.Resistance performance of four principal apple cultivars to woolly apple aphid,Eriosoma lanigerum (Hemiptera:Pemphigidae),by simulated seasonal temperature in northern China[J].Arthropod-Plant Interactions,2021,15(1):59-69.
[6]ZHOU H X,WANG X C,YU Y,TAN X M,CHENG Z Q,ZHANG A S,MEN X Y,LI L L.Chemical characteristics of normal,woolly apple aphid-damaged,and mechanically damaged twigs of six apple cultivars,measured in autumn wood[J].Journal of Economic Entomology,2013,106(2):1011-1017.
[7]尹学伟,李向永,张龙,陈斌.苹果品种对苹果绵蚜的抗性测定[J].云南农业大学学报,2010,25(1):29-33.YIN Xuewei,LI Xiangyong,ZHANG Long,CHEN Bin.Resistance determination of the apple varieties against the woolly apple aphid Eriosoma lanigerum[J].Journal of Yunnan Agricultural University,2010,25(1):29-33.
[8]王西存,周洪旭,郭建英,万方浩,于毅.苹果绵蚜在不同寄主果园中的种群数量动态比较[J].生物安全学报,2011,20(3):220-226.WANG Xicun,ZHOU Hongxu,GUO Jianying,WAN Fanghao,YU Yi.Comparison of population dynamics of Eriosoma lanigerum Hausmann in different orchards[J].Journal of Biosafety,2011,20(3):220-226.
[9]ZHOU H,DU L J,WAN F H,ZHOU H X.Comparative analysis of stylet penetration behaviors of Eriosoma lanigerum (Hemiptera:Aphididae)on main apple cultivars in China[J].Journal of Economic Entomology,2020,113(4):1761-1767.
[10]PING X K,WANG T Y,LIN N,DI F F,LI Y Y,JIAN H J,WANG H,LU K,LI J N,XU X F,LIU L Z.Genome-wide identification of the LAC gene family and its expression analysis under stress in Brassica napus[J].Molecules Online,2019,24(10):1985.
[11]WANG X,ZHOU C L,XIAO X R,WANG X Q,DOCAMPOPALACIOS M,CHEN F,DIXON R A.Substrate specificity of LACCASE8 facilitates polymerization of caffeyl alcohol for clignin biosynthesis in the seed coat of Cleome hassleriana[J].The Plant Cell,2020,32(12):3825-3845.
[12]AN C,SHENG L P,DU X P,WANG Y J,ZHANG Y,SONG A P,JIANG J F,GUAN Z Y,FANG W M,CHEN F D,CHEN S M.Overexpression of CmMYB15 provides chrysanthemum resistance to aphids by regulating the biosynthesis of lignin[J].Horticulture Research,2019,6(1):1-10.
[13]YU Y C,XING Y X,LIU F J,ZHANG X,LI X W,ZHANG J,SUN X L.The laccase gene family mediate multi-perspective trade-offs during tea plant (Camellia sinensis) development and defense processes[J].International Journal of Molecular Sciences,2021,22(22):12554.
[14]马双新,刘宁,贾慧,戴冬青,许苗苗,曹志艳,董金皋.玉米大斑病菌漆酶基因Stlac2 结构分析及原核表达[J].中国农业科学,2016,49(21):4130-4139.MA Shuangxin,LIU Ning,JIA Hui,DAI Dongqing,XU Miaomiao,CAO Zhiyan,DONG Jingao.Analysis and expression of laccase gene Stlac2 in Setosphaeria turcica[J].Scientia Agricultura Sinica,2016,49(21):4130-4139.
[15]XU X Y,ZHOU Y P,WANG B,DING L,WANG Y,LUO L,ZHANG Y L,KONG W W.Genome-wide identification and characterization of laccase gene family in Citrus sinensis[J].Gene,2019,689:114-123.
[16]SONI N,HEGDE N,DHARIWAL A,KUSHALAPPA A C.Role of laccase gene in wheat NILs differing at QTL-Fhb1 for resistance against Fusarium head blight[J].Plant Science,2020,298:110574.
[17]HU Q,MIN L,YANG X Y,JIN S X,ZHANG L,LI Y Y,MA Y Z,QI X W,LI D Q,LIU H B,LINDSEY K,ZHU L F,ZHANG X L.Laccase GhLac1 modulates broad-spectrum biotic stress tolerance via manipulating phenylpropanoid pathway and jasmonic acid synthesis[J].Plant Physiology,2018,176(2):1808-1823.
[18]高相福.促进苹果种子快速发芽的新方法[J].中国农学通报,1987,3(2):30.GAO Xiangfu.A new method to promote rapid germination of apple seeds[J].Chinese Agricultural Science Bulletin,1987,3(2):30.
[19]JIN S,REN Q Q,LIAN L L,CAI X M,BIAN L,LUO Z X,LI Z Q,YE N X,WEI R F,HE W Y,LIU W,CHEN Z M.Comparative transcriptomic analysis of resistant and susceptible tea cultivars in response to Empoasca onukii (Matsuda) damage[J].Planta,2020,252(1):10.
[20]SANDANAYAKA W R M,BUS V G M,CONNOLLY P.Mechanisms of woolly aphid [Eriosoma lanigerum (Hausm.)]resistance in apple[J].Journal of Applied Entomology,2005,129(9/10):534-541.
[21]CHEN C J,CHEN H,ZHANG Y,THOMAS H R,FRANK MH,HE Y H,XIA R.TBtools:An integrative toolkit developed for interactive analyses of big biological data[J].Molecular Plant,2020,13(8):1194-1202.
[22]CHENG X,LI G H,MA C H,ABDULLAH M,ZHANG J Y,ZHAO H,JIN Q,CAI Y P,LIN Y.Comprehensive genomewide analysis of the pear (Pyrus bretschneideri) laccase gene(PbLAC) family and functional identification of PbLAC1 involved in lignin biosynthesis[J].PLoS One,2019,14(2):e0210892.
[23]WANG J H,FENG J J,JIA W T,FAN P X,BAO H,LI S Z,LI Y X.Genome-wide identification of Sorghum bicolor laccases reveals potential targets for lignin modification[J].Frontiers in Plant Science,2017,8:714.
[24]WAR A R,PAULRAJ M G,AHMAD T,BUHROO A A,HUSSAIN B,LGNACIMUTHU S,SHARMA H C.Mechanisms of plant defense against insect herbivores[J].Plant Signaling&Behavior,2012,7(10):1306-1320.
[25]WANG Q,LI G,ZHENG K J,ZHU X B,MA J J,WANG D M,TANG K Q,FENG X X,LENG J T,YU H,YANG S X,FENG X Z.The soybean laccase gene family:Evolution and possible roles in plant defense and stem strength selection[J].Genes,2019,10(9):701.
[26]QIN S F,FAN C F,LI Y H,LI Y,HU J,LI C F,LUO K M.LACCASE14 is required for the deposition of guaiacyl lignin and affects cell wall digestibility in poplar[J].Biotechnology for Biofuels,2020,13(1):197.
[27]ZHANG Y,WU L Z,WANG X F,CHEN B,ZHAO J,CUI J,LI Z K,YANG J,WU L Q,WU J H,ZHANG G Y,MA Z Y.The cotton laccase gene GhLAC15 enhances Verticillium wilt resistance via an increase in defence-induced lignification and lignin components in the cell walls of plants[J].Molecular Plant Pathology,2019,20(3):309-322.
[28]ZHAO G P,XIANG F X,ZHANG S C,SONG J X,LI X Y,SONG L,ZHAI R,YANG C Q,WANG Z G,MA F M,XU L F.PbLAC4-like,activated by PbMYB26,related to the degradation of anthocyanin during color fading in pear[J].BMC Plant Biology,2021,21(1):469.
[29]LIU Q Q,LUO L,WANG X X,SHEN Z G,ZHENG L Q.Comprehensive analysis of rice laccase gene(OsLAC)family and ectopic expression of OsLAC10 enhances tolerance to copper stress in Arabidopsis[J].International Journal of Molecular Sciences,2017,18(2):209.
[30]FRANCIS D M,PAGE R.Strategies to optimize protein expression in E.coli[J].Current Protocols in Protein Science,2010,5(1):1-29.
[31]RAY M,MISHRA P,DAS P,SABAT S C.Expression and purification of soluble bio-active rice plant catalase:A from recombinant Escherichia coli[J].Journal of Biotechnology,2012,157(1):12-19.
[32]宁娜,谭慧军,孙新新,倪金凤.真核生物来源漆酶的异源表达研究进展[J].生物工程学报,2017,33(4):565-577.NING Na,TAN Huijun,SUN Xinxin,NI Jinfeng.Advance of heterologous expression study of eukaryote-origin laccases[J].Chinese Journal of Biotechnology,2017,33(4):565-577.