澳 洲 青 苹(Malus domestica Borkh.‘Granny Smith’)作为世界上著名的绿色苹果品种,果实具有优良的品质和良好的耐贮性,备受广大消费者的喜爱[1]。该品种果实除可直接食用外,更是加工苹果汁的首选品种[2-3]。然而该品种在贮藏后期和货架期会出现典型的虎皮病症状[4]。
虎皮病是苹果采后主要生理性病害之一,表现为果皮上出现棕色或黑色斑点。随着病程的加重,由开始的斑点逐渐向外扩展成片,颜色也会逐渐呈现褐色至深褐色,甚至果实出现凹陷、皱缩,最终腐烂变质[5]。虽然通常不会影响到果实的内部质量,但伴随着该病的发展,会严重影响果实的外观品质和商品价值[6-7]。因此,如果能够将澳洲青苹苹果在虎皮病发生前提前出库,则可以在保持果实品质的同时,降低经济损失。因此,建立苹果虎皮病的早期预测模型在苹果产业发展上具有重要意义。
目前关于苹果虎皮病的研究多集中在其病因、发生条件以及利用不同采后处理方式延缓和控制苹果虎皮病发生等方面[8]。大量研究表明,果实中α-法尼烯的氧化是虎皮病发生的主要因素,α-法尼烯合酶基因MdAFS 的表达量会先于虎皮病的发生而增加[9-12]。乙烯能通过调控AFS基因的表达,进而调节α-法尼烯的合成,对苹果果实虎皮病的发展起到控制作用[13]。此外,在冷藏过程中,伴随着H2O2和丙二醛(malondialdehyde,MDA)的积累,果实开始出现虎皮病[14]。前人的研究也表明,虎皮病敏感的品种中过氧化物酶(peroxidase,POD)和抗坏血酸过氧化物酶(APX)的活性明显低于抗病性品种,进一步表明抗氧化系统与虎皮病的发病率之间存在密切关系[15]。POD 可将过氧化物还原为H2O2,并进一步清除体内过量的过氧化氢,从而保护果实免受损害,过氧化氢酶(hydrogen peroxide,CAT)的作用与POD类似[13]。此外,Busatto 等[16]研究发现绿原酸含量的增加与苯丙氨酸解氨酶(phenylalanine ammonia lyase,PAL)、对香豆酸3 羟化酶(P-coumarate 3 hydroxylase,C3H)和多酚氧化酶(polyphenol oxidase,PPO)的基因表达增强一致,表明虎皮病的发生与绿原酸途径存在密切关系。另外Gong 等[17]认为发生虎皮病的苹果果皮组织褐变的形成除了受多酚氧化酶的影响外,还有可能是由漆酶(laccase,LAC)活性变化引起的。
关于苹果虎皮病发生预警的研究主要集中在叶绿素荧光参数及挥发性物质检测方面,其中叶绿素荧光参数作为一种无损检测指标,被广泛地运用到采后贮藏相关领域。有研究初步证明,叶绿素荧光参数,即Fv/Fm的值可以预测澳洲青苹、红富士等多种果实虎皮病的发展状况[18-19]。此外,有研究表明利用紫外吸收光谱评估α-法尼烯和共轭三烯(conjugated trienes)的含量变化,可作为冷库中长期贮藏苹果的虎皮病预警标记物[20-21]。然而,目前在分子层面还没有可靠的方法预测苹果虎皮病的发生。在苹果采后贮藏期间,基因表达通常先于或伴随着果实的成熟和衰老而变化,基于目前苹果虎皮病的相关研究基础,可通过结合转录组测序以及qPCR 进一步验证的方法确定相应品种虎皮病发生的关键基因[22-23]。
笔者在本研究中以澳洲青苹苹果果实为试材,于商业采收期采收并对其进行低温贮藏[(0±0.5)℃],90%湿度,贮藏期监测果实色泽和乙烯释放量;同时统计冷库贮藏期间及出库后货架期的虎皮病发病情况;并采用qPCR 技术分析与苹果虎皮病发生密切相关的基因的表达水平;以筛选虎皮病发生的关键预警基因,从而在果实发病前出库,降低虎皮病造成的经济损失。
1 材料和方法
1.1 试验材料及处理
1.1.1 植物材料 澳洲青苹于商业采收期(即花后150 d)采摘自宝鸡千阳苹果试验站,选取大小一致,成熟度相对均匀、色泽相近、无机械损伤且无病虫害的950个果实,采收后立即运往实验室,常温下放置于西北农林科技大学园艺学院采后生物实验室12 h,去除田间热后转入冷库[温度:(0±0.5)℃,相对湿度:90%]中贮藏备用。
1.1.2 处理方法 该试验分为取样组(150 个果实)和调查组(800 个果实),调查组分为冷库调查组(200个果实)及货架期调查组(600个果实)。
冷库调查组:分别在贮藏60、70、73、76、79、82、85 d统计200个果实的虎皮病发病情况。
货架期调查组:分别在冷库贮藏0、15、30、40、50、60、70、80 d 随机取40 个果实测定色差,之后将果实转移至常温贮藏(温度:25±0.5 ℃,相对湿度:85%),模拟货架期,并统计0~10 d 货架期期间果实虎皮病的发病情况(图1)。
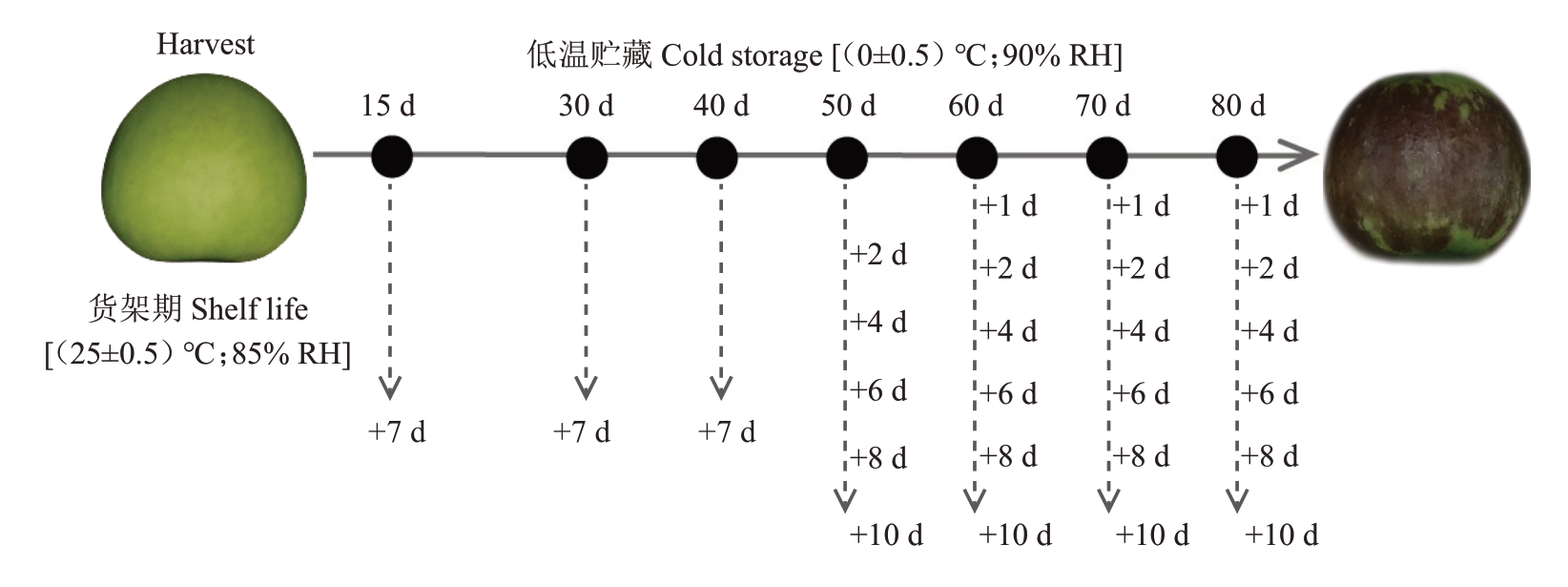
图1 发病率统计方案
Fig.1 Incidence statistics scheme
取样组:分别在冷库贮藏0、15、30、40、50、60、70、80 d随机取9个果实,将其分为3组,每组为1个重复,共设置3个生物学重复,于冷库中测定乙烯释放速率(图1),之后立即对果皮组织进行存样;当澳洲青苹果实出现虎皮病后,对果皮健康组织与发病组织分别取样。
1.2 测定指标及方法
1.2.1 果实基础生理指标的测定 乙烯释放速率的测定,在冷库中随机选取9个果实,分为3组,每组3个,放入8 L 玻璃干燥器中密封,1 h 后抽取1 mL 气体并使用气相色谱仪(岛津GC-14A)进行测定。色谱条件:GDX-502 色谱柱,载气为氮气(99.999%),柱温为70 ℃,进样口温度为100 ℃,检测室温度110 ℃,乙烯释放速率的单位为μL·kg-1·h-1。
色差的测定:使用手持式色差仪(日本美能达CR-400 型色差计)进行测量,每个果实沿果实赤道面取3 个点进行测量。用L*、a*、b*表示。其中,L*表示光泽明亮度,由0~100表示由黑到白;a*表示红绿值,由负到正表示由绿到红;b*表示黄蓝值,由负到正表示由蓝到黄。
1.2.2 虎皮病发病情况的统计 分别在0、15、30 d以及之后的每10 d统计冷库调查组中200个果实的发病情况,同时在货架期调查组中取出40个果实转移至常温贮藏,统计7 d 或10 d 后该40 个果实的发病情况。发病率及发病指数计算公式如下:
虎皮病发病率/%=(发病果数/总果数)×100,
虎皮病的病情指数=∑(病果数×病果级数)/(检查总数×最高级数)[19]。
其中病果级数是根据苹果虎皮病发病面积进行划分,分为5个等级,分别是:0级未发病的正常果;1级病果病斑面积小于等于10%;2级病果病斑面积大于10%、小于等于30%;3级病果病斑面积大于30%、小于等于50%;4级病果病斑面积大于50%(图2)。
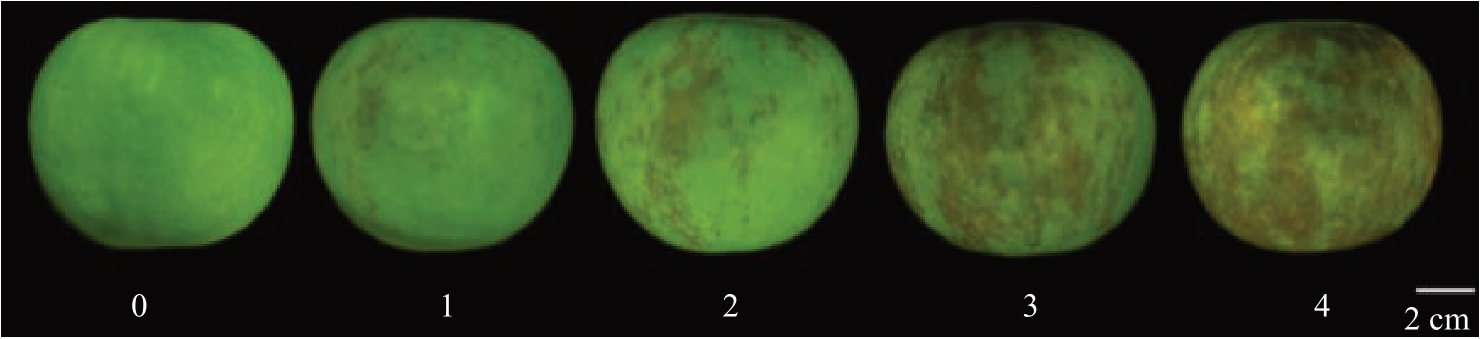
图2 虎皮病等级图
Fig.2 Superficial scald grade chart
1.2.3 虎皮病相关基因qRT-PCR 验证 总RNA 提取:对不同时期存样的果皮采用CTAB 法进行总RNA 提取。称取1 g 果皮组织,在液氮中将果皮研磨成粉末并放入离心管中;加入65 ℃下预热的CTAB 溶液700 μL 和40 μL 的巯基乙醇,充分涡旋混匀;放置于65 ℃水浴锅中水浴10 min,期间每隔几分钟进行轻微的上下翻转,使其充分混合;加入700 μL 的氯仿∶异戊醇(24∶1),充分混匀至乳白色,10 000 r·min-1 离心10 min;吸取上清液500 μL 于2 mL的离心管中,再加入等体积的氯仿∶异戊醇(24∶1),充分混匀后,10 000 r·min-1离心10 min;吸取上清液500 μL 于1.5 mL 的离心管中,加入125 μL 的LiCl溶液,轻微翻转数次;于-20 ℃过夜沉降;沉降后于4 ℃下融化,12 000 r·min-1离心20 min,小心吸掉上清;加入4 ℃预冷的75%乙醇500 μL,离心1 min,倒掉乙醇;加入4 ℃预冷的无水乙醇400 μL,离心1 min,倒掉无水乙醇,此步骤重复1次;之后吸掉无水乙醇,置于通风橱,打开离心管盖子自然晾干;20 min 后加入30 μL 的DEPC 水,吸打混匀使RNA 溶解;使用Nanadrop 2000 测量RNA 浓度,并通过在1.2%琼脂溴化乙锭染色凝胶电泳检测其质量;经检验合格的RNA 样品存于-80 ℃备用。
cDNA 合成:使用PrimeScript RT 试剂盒(中国大连Takara)从1.0 μg DNA 游离RNA 合成第1 链cDNA,操作方法详见说明书。
实时荧光定量PCR:以苹果组织中稳定表达的MdActin、MdUBI 作为内参基因;从NCBI 中下载基因序列,利用Primer3 设计RT-qPCR 引物,引物合成由北京擎科生物科技有限公司完成,引物序列见表1。
表1 实时荧光定量PCR 引物序列
Table 1 Real-time fluorescence quantitative PCR primer sequences
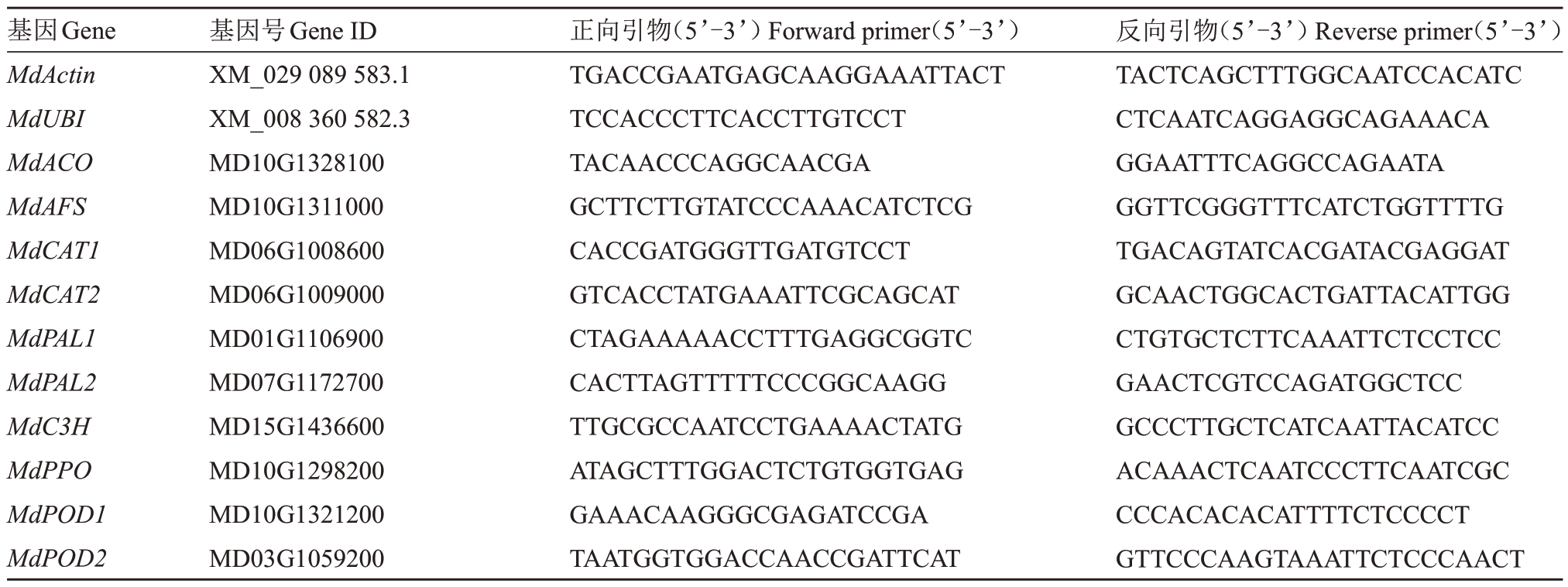
基因Gene MdActin MdUBI MdACO MdAFS MdCAT1 MdCAT2 MdPAL1 MdPAL2 MdC3H MdPPO MdPOD1 MdPOD2基因号Gene ID XM_029 089 583.1 XM_008 360 582.3 MD10G1328100 MD10G1311000 MD06G1008600 MD06G1009000 MD01G1106900 MD07G1172700 MD15G1436600 MD10G1298200 MD10G1321200 MD03G1059200正向引物(5’-3’)Forward primer(5’-3’)TGACCGAATGAGCAAGGAAATTACT TCCACCCTTCACCTTGTCCT TACAACCCAGGCAACGA GCTTCTTGTATCCCAAACATCTCG CACCGATGGGTTGATGTCCT GTCACCTATGAAATTCGCAGCAT CTAGAAAAACCTTTGAGGCGGTC CACTTAGTTTTTCCCGGCAAGG TTGCGCCAATCCTGAAAACTATG ATAGCTTTGGACTCTGTGGTGAG GAAACAAGGGCGAGATCCGA TAATGGTGGACCAACCGATTCAT反向引物(5’-3’)Reverse primer(5’-3’)TACTCAGCTTTGGCAATCCACATC CTCAATCAGGAGGCAGAAACA GGAATTTCAGGCCAGAATA GGTTCGGGTTTCATCTGGTTTTG TGACAGTATCACGATACGAGGAT GCAACTGGCACTGATTACATTGG CTGTGCTCTTCAAATTCTCCTCC GAACTCGTCCAGATGGCTCC GCCCTTGCTCATCAATTACATCC ACAAACTCAATCCCTTCAATCGC CCCACACACATTTTCTCCCCT GTTCCCAAGTAAATTCTCCCAACT
1.2.4 转录组测序 将0、30、50、80 d 健康组织和80 d发病组织的果皮进行转录组测序,分别记为:0、30、50、80、80 d-B,每个样品3次重复,共15个样品进行转录组测序,转录组测序工作由北京百迈客生物科技有限公司完成。使用NovaSeq 6000测序得到的原始图像数据文件,经碱基识别(Base calling)分析转化为原始测序序列Raw reads。通过Fast QC软件对Raw reads 进行测序的质量控制(QC)来评估测序数据,得到Clean reads之后,将产生的RNA-seq数据比对到参考基因组Malus×domestica GDDH13 V1.1 genome上,对统计结果进行统计分析。
1.2.5 差异表达基因的筛选 根据FPKM 值>5的基因作聚类热图。将错误发生率(false discovery rate)<0.05 且差异倍数(Fold change)>2 的基因定义为差异表达基因,进而筛选与虎皮病相关且30 d和50 d存在明显差异的基因做进一步分析。
1.3 数据分析
采用Excel 2016、SPSS 和Origin 2022 软件进行数据统计并绘制图表,利用TBtools进行数据归一化热图绘制[24]。
2 结果与分析
2.1 澳洲青苹在贮藏过程中虎皮病的发生情况
2.1.1 澳洲青苹在低温贮藏过程中虎皮病的发生情况 澳洲青苹在低温贮藏过程中,贮藏前60 d均未发生虎皮病;在贮藏70 d 后,果实表面出现轻微的虎皮病症状,发病率和病情指数分别为12.5%和0.031;贮藏85 d时,发病率提高到93.5%,病情指数从3.13升高到0.439(图3)。综上,澳洲青苹苹果虎皮病在冷库贮藏中主要发生在低温贮藏中后期。随着贮藏期的延长,发病率、病情指数急剧升高。
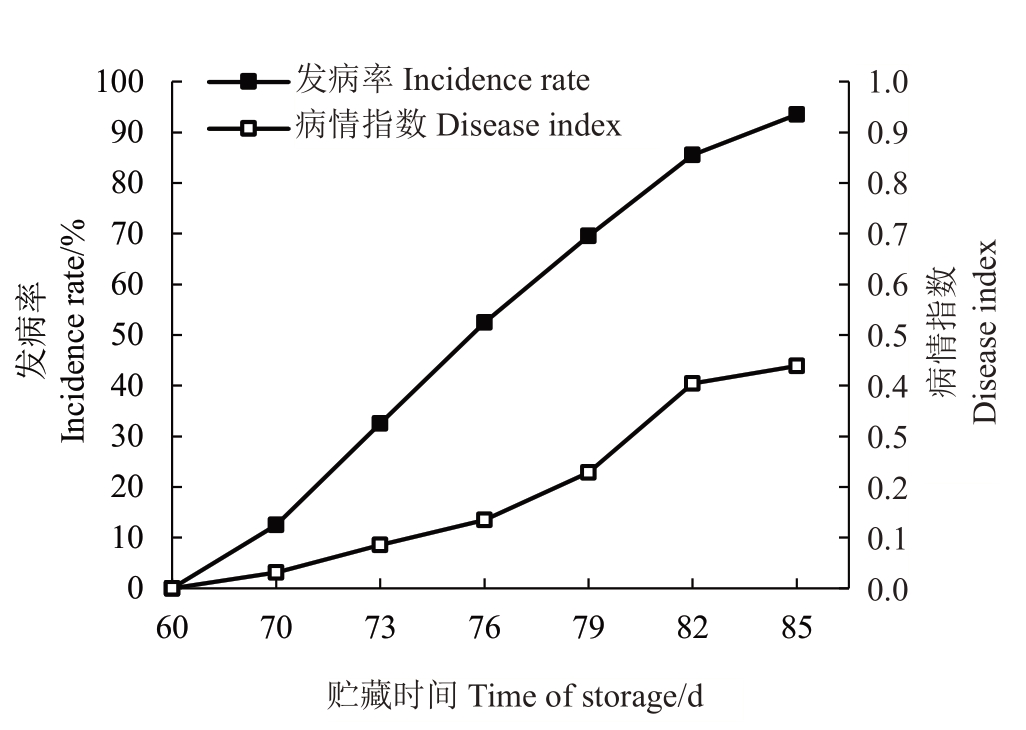
图3 澳洲青苹在低温贮藏期间虎皮病发病率和病情指数的变化
Fig.3 Changes of scald incidence and disease index in Granny Smith apples during low-temperature storage
2.1.2 澳洲青苹在冷库贮藏后货架期虎皮病的发生情况 澳洲青苹在低温贮藏前期(0~40 d),果实长期冷藏后置于货架期10 d 无虎皮病发生。低温贮藏至50 d 时,果实置于货架期第2 天开始出现轻微发病;发病率和病情指数分别为55%和0.163,货架期贮藏10 d 后,发病率升至70%,病情指数从0.163 增加到0.213;低温贮藏至60 d 时,果实置于货架期第1 天开始出现轻微发病;发病率和病情指数分别为55%和0.156,货架期贮藏10 d 后,发病率升至100%,病情指数从0.156 增加到0.681(图4,图5);低温贮藏至70 d 时,冷库中的果实开始出现轻微的虎皮病症状,发病率和病情指数分别为20%和0.05(图3),货架期贮藏10 d 后,发病率升至100%,病情指数从0.05 增加到0.881;低温贮藏至80 d 时,冷库中的果实出现大量的虎皮病症状,发病率和病情指数分别为82.5%和0.325,货架期贮藏10 d 后,发病率升至100%,病情指数从0.325 增加到0.975(图4,图5);综上,在低温贮藏后期(50~80 d),果实冷藏后放置货架期2 d 内,虎皮病的发病率及病情指数都呈现急剧上升趋势,2 d 后上升较为缓慢。由此可见,对于冷藏后期货架期的苹果果实虎皮病的发生速度很快,在货架期前2 d就大量发生。

图4 澳洲青苹在冷藏后货架期虎皮病发病率(A)和病情指数(B)的变化
Fig.4 Changes of scald incidence(A)and disease index(B)in Granny Smith apples during shelf life after cold storage

图5 冷藏后货架期跟踪澳洲青苹苹果虎皮病发病变化图
Fig.5 Tracking the change map of superficial scald of Granny Smith apples during shelf life after cold storage
2.2 澳洲青苹在低温贮藏过程中乙烯释放量的变化
澳洲青苹在低温贮藏期间,乙烯释放速率在前50 d 内随着贮藏时间的延长呈缓慢上升的趋势;冷藏50 d后,乙烯释放速率急剧上升。此时果实冷藏后转入货架期也开始出现虎皮病(图5,图6)。
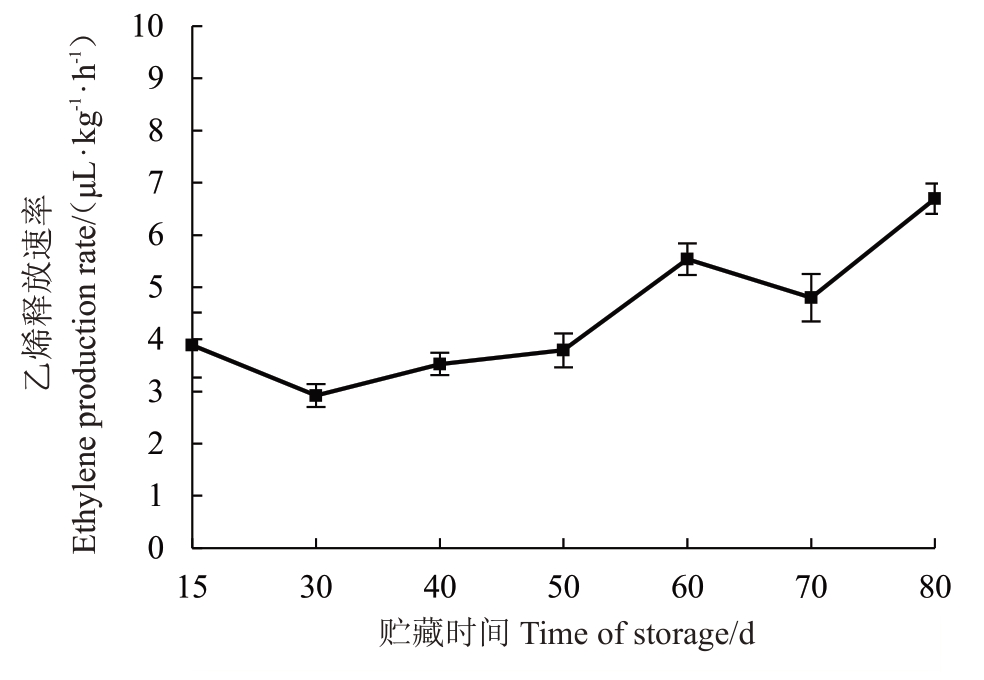
图6 澳洲青苹在低温贮藏期间乙烯释放速率的变化
Fig.6 Changes of ethylene production rate in Granny Smith apples during low-temperature storage
2.3 澳洲青苹在低温贮藏过程中果皮色泽和色度的变化
澳洲青苹在低温贮藏期间,贮藏15 d 时,L*值为78.46,第40天后开始明显降低。贮藏50 d后,L*值呈现轻微上升趋势,基本保持在65左右。a*值在贮藏期间变化相对稳定。b*值在贮藏40 d时相比冷藏30 d 出现明显上升趋势,贮藏40 d 后变化较为稳定,基本保持在35左右(图7)。

图7 澳洲青苹在低温贮藏期间果皮色泽和色度的变化
Fig.7 Changes of color and luster of Granny Smith apples during low-temperature storage
A.L*值与a*值的变化;B.为b*值的变化。不同小写字母代表差异显著性(p<0.05)。
A.Changes of L*value and a*value;B.Changes of b*value.Different small letters represent significant difference(p<0.05).
2.4 澳洲青苹在低温贮藏过程中与虎皮病相关基因的表达量分析
基于前人关于虎皮病发生机制的大量研究结果发现,LAC、ACC 氧化酶(ACC oxidase,ACO)、α-法尼烯合酶(alfa-farnesene synthase,AFS)、CAT、绿原酸途径相关基因,即PAL、C3H 和PPO 与苹果虎皮病具有相关性[9-17]。基于苹果基因组数据库,提取鉴定以上与虎皮病发生相关酶的基因,共计247 个基因(表2)。
表2 澳洲青苹苹果虎皮病发生相关酶的编码基因
Table 2 Coding genes of enzymes related to the occurrence of superficial scald in Granny Smith apples

基因Gene MdACO MdAFS MdCAT MdPAL MdC3H MdPPO MdLAC MdPOD基因号Gene ID MD10G1328100,MD15G1205100 MD10G1310900,MD10G1311000 MD06G1008600,MD06G1008700,MD06G1009000 MD01G1106900,MD04G1096200,MD07G1172700,MD12G1116700,MD03G1121400,MD03G1121500 MD08G1242900,MD08G1243000,MD14G1198300,MD15G1218200,MD15G1436500,MD15G1436600 MD05G1318900,MD05G1319100,MD05G1319300,MD05G1319400,MD05G1319500,MD05G1319800,MD05G1320100,MD05G1320200,MD05G1320500,MD05G1320800,MD10G1298200,MD10G1298300,MD10G1298400,MD10G1298500,MD10G1298700,MD10G1299100,MD10G1299300,MD10G1299400 MD00G1014800,MD00G1014900,MD00G1015000,MD00G1190600,MD00G1190700,MD01G1146500,MD01G1098800,MD01G1158900,MD01G1159400,MD01G1234800,MD01G1234900,MD01G1235100,MD01G1235200,MD02G1145100,MD02G1163600,MD02G1264400,MD03G1000200,MD03G1000500,MD03G1056400,MD03G1076400,MD03G1087400,MD03G1106500,MD03G1106600,MD04G1083900,MD04G1131200,MD04G1131500,MD04G1142300,MD04G1142400,MD04G1142500,MD04G1142600,MD04G1142900,MD04G1147700,MD05G1064600,MD05G1128800,MD05G1158300,MD05G1229100,MD05G1348500,MD06G1066000,MD06G1230100,MD07G1153000,MD07G1165500,MD07G1165800,MD07G1213300,MD07G1227800,MD07G1227900,MD07G1254600,MD07G1307400,MD07G1308000,MD08G1005000,MD09G1110500,MD10G1042300,MD10G1042400,MD10G1042500,MD10G1042600,MD10G1042700,MD10G1073700,MD10G1132000,MD10G1151300,MD10G1323500,MD11G1000300,MD11G1000400,MD11G1057600,MD11G1058200,MD11G1080900,MD11G1096500,MD11G1118900,MD11G1119000,MD11G1182500,MD11G1195700,MD12G1144300,MD12G1144600,MD12G1156900,MD12G1157000,MD12G1157100,MD12G1157900,MD12G1161000,MD13G1026500,MD13G1026600,MD14G1234800,MD15G1004400,MD15G1258600,MD15G1259300,MD15G1276600,MD15G1276700,MD15G1328900,MD15G1412600,MD16G1029700,MD16G1147100,MD17G1098300 MD00G1088400,MD00G1112500,MD00G1190800,MD01G1034400,MD01G1034500,MD01G1034900,MD01G1162100,MD01G1162400,MD01G1162700,MD01G1193400,MD01G1193700,MD01G1194000,MD02G1031200,MD02G1118700,MD02G1124700,MD02G1138900,MD02G1139300,MD02G1167700,MD03G1012700,MD03G1012800,MD03G1012900,MD03G1013200,MD03G1013300,MD03G1013600,MD03G1013700,MD03G1014000,MD03G1014200,MD03G1014300,MD03G1014400,MD03G1014500,MD03G1059200,MD03G1084400,MD03G1147900,MD03G1223300,MD03G1274400,MD03G1274500,MD04G1046400,MD04G1101700,MD04G1171700,MD04G1171900,MD04G1211800,MD05G1075100,MD05G1224400,MD05G1240100,MD05G1243700,MD05G1306500,MD05G1345800,MD06G1038800,MD06G1180500,MD06G1180700,MD07G1230500,MD07G1230700,MD07G1230800,MD07G1230900,MD07G1301400,MD08G1027800,MD08G1051200,MD08G1222600,MD08G1230700,MD08G1230900,MD09G1009900,MD09G1010000,MD09G1038600,MD09G1039100,MD09G1105900,MD09G1106000,MD09G1211000,MD10G1033300,MD10G1206100,MD10G1218200,MD10G1225600,MD10G1321200,MD11G1004100,MD11G1015300,MD11G1015400,MD11G1015500,MD11G1015700,MD11G1061000,MD11G1061100,MD11G1061200,MD11G1061400,MD11G1241100,MD12G1012500,MD12G1056000,MD12G1121300,MD12G1201400,MD13G1086200,MD13G1097600,MD13G1097700,MD13G1150600,MD13G1152800,MD13G1175300,MD13G1246700,MD14G1010300,MD14G1056000,MD14G1185700,MD15G1022200,MD15G1108300,MD15G1172800,MD15G1238800,MD15G1252300,MD15G1279900,MD15G1294200,MD15G1321200,MD15G1321300,MD15G1321400,MD16G1052000,MD16G1085600,MD16G1098700,MD16G1098800,MD16G1150800,MD16G1150900,MD16G1153200,MD16G1177000,MD17G1040100,MD17G1092400,MD17G1093600,MD17G1093700,MD17G1193700,MD17G1265200,MD17G1265300
以澳洲青苹低温贮藏阶段果皮组织为材料进行转录组测序分析,对以上基因做出进一步筛选,其中对FPKM>5的基因(28个)进行重点关注,并绘制热图同时进行聚类分析。结果表明,28个基因根据表达趋势可聚为3组,Group Ⅰ的基因在健康果皮组织中,表达量随贮藏时间延长呈下降趋势且在常温贮藏条件下的表达量显著高于低温贮藏。其中,基因MD17G1098300、MD06G1008700、MD10G1321200在健康果皮组织中表达量显著高于病皮组织;相反,基因MD00G1112500、MD08G1242900、MD08G1243000在病皮组织中表达量显著高于低温贮藏条件下健康果皮组织。值得注意的是,基因MD10G1321200 在低温贮藏30 d时的表达量高于低温贮藏50 d时表达量3 倍以上。Group Ⅱ的基因在低温贮藏期间,健康果皮组织中表达量较高,且在低温贮藏30 d与50 d的差异较为显著。Group Ⅲ的基因在低温贮藏期间,大部分基因的病皮组织中表达量显著高于健康果皮组织。其中,基因MD10G1298500 在低温贮藏期间,表达量呈逐渐下降的趋势且健康果皮组织高于病皮组织。值得注意的是,基因MD03G1059200在低温贮藏30 d时的表达量高于低温贮藏50 d时表达量44倍以上。综上,MdAFS(MD10G1311000)、MdACO(MD10G1328100)、MdCAT1(MD06G1008600)、Md-CAT2(MD06G1009000)、MdPAL1(MD01G1106900)、MdPAL2(MD07G1172700)、MdC3H(MD15G143660-0)、MdPPO(MD10G1298200)以及MdPOD1(MD10-G1321200)、MdPOD2(MD03G1059200)可能与澳洲青苹苹果虎皮病预警存在相关性(图8)。

图8 澳洲青苹在低温贮藏期间与虎皮病相关基因的表达量热图
Fig.8 Heat map of expression of genes related to superficial scald of Granny Smith apples during low-temperature storage
80 d-B 表示低温贮藏80 d 病皮的基因表达情况。颜色从蓝到红表示基因的表达量从低到高。
80 d-B indicates the gene expression of diseased skin after 80 d cold storage.Colors from blue to red indicate gene expression levels from low to high.
在此基础上,对澳洲青苹苹果进行更加细致的取样,并采用实时荧光定量PCR技术对以上候选基因进行趋势富集分析以及表达量分析。结果表明,该类基因大致可分为四种趋势。在澳洲青苹苹果低温贮藏期间,A组基因与D组中MdPPO基因的表达量一致,呈先下降后上升的趋势,其中,MdPPO在贮藏60 d 后开始上升,MdPAL1 在贮藏50 d 后开始上升;MdPPO 在贮藏80 d 时健康组织的表达量显著高于病皮组织,而MdPAL1呈相反趋势。B组中MdPOD1和MdPOD2的表达量在低温贮藏期间始终呈下降趋势,且在果实贮藏第40天时急剧下降,较30 d分别下降了2.41倍和8.5倍。相反,C组呈明显上升趋势;其中MdACO、MdPAL2在贮藏第80天时,病皮组织的表达量显著高于健康组织。MdCAT2表达量在贮藏前期呈上升趋势,在贮藏70 d时达到顶峰,贮藏80 d时急剧下降;相比病皮组织,健康组织中MdCAT2的表达量较高。因此以上基因并不能作为澳洲青苹苹果贮藏期虎皮病发生的关键预警基因。D组中MdAFS和MdC3H的表达量在贮藏40 d时显著上升,随后呈下降趋势;其中MdAFS 在40 d 的表达量是30 d 的1.94倍,MdC3H在40 d的表达量是30 d的5.29倍;且两个基因在70 d和80 d健康组织的表达量均显著高于病皮组织。MdCAT1表达量在贮藏40 d 时急剧上升,贮藏50 d 时急剧下降,随后又呈逐渐上升的趋势,相比病皮组织,健康组织中的MdCAT1的表达量较高。由此可见,该类基因可作为澳洲青苹苹果贮藏期虎皮病发生的关键预警基因(图9)。

图9 澳洲青苹在低温贮藏期间不同基因的趋势富集分析及相对表达量
Fig.9 Trend enrichment analysis and relative expression of different genes of Granny Smith apples during low-temperature storage
不同小写字母代表差异显著(p<0.05)。
Different small letters represent significant differenc(ep<0.05).
3 讨 论
澳洲青苹在低温贮藏期间随着果实的成熟和衰老,果皮颜色也会发生相应的变化,由绿色转变为黄绿色。色差中L*值和b*值分别代表果皮的亮度及由绿转黄的程度[25]。在本研究中,伴随着虎皮病的出现,澳洲青苹果实色差L*值呈现下降的趋势,表明果皮亮度随着贮藏时间的延长,逐渐变暗;直到贮藏50 d 后,L*值保持稳定,此时果实在冷库中未发生虎皮病,但冷藏后货架期果实开始出现虎皮病。b*值在冷藏40 d 时相比冷藏30 d 显著上升,40 d 后变化较为稳定,这与田雪婷等[26]关于澳洲青苹贮藏特性的研究结果一致。b*值的由低转高,表明澳洲青苹苹果果皮由绿到黄的转变,出现该现象的原因可能是果皮中叶绿素发生降解,使得果皮逐渐显现出黄色[27]。
乙烯作为调控苹果果实成熟衰老的重要激素,可以调控MdAFS基因表达,进而控制α-法尼烯的合成;而α-法尼烯进一步氧化为共轭三烯以及其他产物,三者之间相互作用,共同影响着虎皮病的发展[28-29]。邵淑君[11]研究发现,乙烯处理能够促进国光苹果虎皮病的发生,同时提升α-法尼烯及α-法尼烯合成酶MdAFS1 的相对表达量,相反1-MCP 作为乙烯作用抑制剂,对虎皮病的发生起到了抑制作用。Zhou 等[30]证实了1-MCP 处理可以抑制五九香(Wujiuxiang)梨的乙烯释放和α-法尼烯代谢,以及Martins等[31]对澳洲青苹进行辐射处理,同样抑制了乙烯释放和α-法尼烯生物合成相关酶的基因表达,进而减缓了虎皮病的发生。Liu等[32]用芦荟凝胶对Starking 苹果进行处理,乙烯释放量在贮藏后期显著增加;MdACS1的表达量在冷藏期间上调,促进了α-法尼烯的释放和氧化;从而加重了Starking 苹果虎皮病的风险。Lindo-garcía等[33]的研究中也提到,乙烯和PcAFS1基因具有标记Blanquilla梨虎皮病易感性的潜力。在本研究中,随着贮藏期延长,MdACO 的表达量逐渐上升,尤其在贮藏后期(70~80 d),在病皮组织的表达量显著高于健康组织。然而,由于该基因在40 d与50 d表达量相比无显著差异,所以不能对苹果虎皮病起到预警的作用。MdAFS 在40 d时表达量急剧升高,随之10 d后即贮藏50 d时,澳洲青苹苹果拿到货架期2 d时便开始出现虎皮病;由此证明,MdAFS可作为低温贮藏期澳洲青苹苹果虎皮病发生的关键预警基因。
有研究表明,多酚氧化酶与虎皮病的发生具有密切相关性[34]。然而,Lu等[14]进一步证实,在冷藏过程中,虎皮病症状的发展高度依赖于H2O2和MDA的积累,而PPO活性则不太重要。在本研究中,PPO相关基因多在病皮中高表达,进一步证实了PPO与苹果虎皮病存在明显的相关性。但由于该基因40 d和50 d 的表达量差异不显著,所以并不能作为虎皮病预警的关键基因。此外,胡小松等[35]早期的研究发现,H2O2能够诱导苹果果实虎皮病的发生,过氧化氢酶能够使虎皮病明显减少。Rao 等[15]认为,虎皮病的发生与H2O2和脂质过氧化产物的增加密切相关。刘少华[13]在研究中提到,过氧化物酶可将过氧化物还原为H2O2从而清除果实组织内过量的过氧化氢,进而保护果实免受损伤,过氧化氢酶的作用与过氧化物酶类似。因此,对以上基因也进行了重点关注。其中MdPOD1 和MdPOD2 表达量在低温贮藏期间始终呈下降趋势,且后期差别较小,可能是由于伴随着虎皮病的出现,果实抗氧化酶活性显著降低。虽然在预警时期点(即40 d)两个基因的表达量下降较为显著,均达到2倍以上,但能否作为预警基因,仍然存在争议,后期将针对这两个基因进一步验证。然而MdCAT1在低温贮藏40 d时,相比10 d前即贮藏30 d 的表达量有明显的上升趋势,因此,Md-CAT1 可以作为低温贮藏期澳洲青苹苹果虎皮病发生的关键预警基因。
也有研究结果显示,由于MdPAL 和MdC3H 的激活上调表达,绿原酸的积累同样会引起虎皮病的发生[36-37]。Busatto 等[16]研究指出绿原酸途径以及随后被多酚氧化酶氧化的途径,是导致虎皮病发生的主要因素。在本研究中,MdPAL1和MdPAL2在低温贮藏期间,尤其在病皮组织中表达量较高,在健康果皮组织中无显著差异;MdC3H 在低温贮藏40 d 时,较10 d 前即贮藏30 d 的表达量有明显上升趋势,达到5 倍以上。因此,MdC3H 可以作为低温贮藏期澳洲青苹苹果虎皮病发生的关键预警基因。
近年来,漆酶已在许多植物中被发现,在苹果贮藏过程中,该酶可以通过催化表儿茶素的氧化反应,在果皮中呈棕色,可能与虎皮病的发生有关[17]。Fang等[38]研究发现漆酶参与荔枝果皮组织褐变的形成。Wei等[39]发现漆酶介导的类黄酮聚合物在果皮褐变过程中起着重要作用,并通过对Red Delicious和Cortland 苹果中的漆酶基因的表达分析发现,经过DPA 和1-MCP 处理可以显著抑制漆酶基因的表达;而对照苹果果皮漆酶蛋白的酶活性随贮藏时间的延长而升高,进而推测苹果虎皮病的发生可能是由漆酶引起的;并提出为进一步证明漆酶在虎皮病发生中发挥作用,鼓励未来的研究可以对虎皮病高度敏感的品种如澳洲青苹或不同的超低氧贮藏条件进行更多的研究。杨阳等[40]首次对苹果MdLAC 基因家族进行鉴定,共得到77 个家族成员。综上,基于前人的期望,笔者在本研究中对漆酶相关基因表达进行重点关注。研究结果显示,澳洲青苹苹果在低温贮藏条件下,漆酶相关基因在虎皮病发病初期表达量较低,而在病皮中的表达量较高,该结果与多酚氧化酶基因的表达分析结果类似。由此可以初步证明,漆酶与苹果虎皮病存在一定相关性,但对于澳洲青苹苹果虎皮病预警并不能发挥较好的作用。
综上所述,MdAFS、MdC3H 和MdCAT1 可作为澳洲青苹苹果贮藏期虎皮病发生的关键预警基因。但是,该结果仍需进一步研究,以验证该类预警基因的稳定性即评估该类基因在其他苹果品种的适用性,如易发生虎皮病的富士、红星、国光、元帅等苹果品种[5]。此外,澳洲青苹苹果多被用作榨汁原料,在今后的实际生活中,可尝试调查已发病的澳洲青苹苹果果实口味仍可继续食用的接受范围,进而减少采后损失,进一步促进苹果产业的发展。
4 结 论
澳洲青苹苹果果实虎皮病主要发生在低温贮藏中后期,即贮藏至50 d时,放置货架期果实开始出现虎皮病;贮藏至70 d时,果实在冷库中开始发病。基于前人的研究,笔者在本研究中对可能参与虎皮病发生的相关酶基因(MdACO、MdAFS、MdCAT1、Md-CAT2、MdPAL1、MdPAL2、MdC3H、MdPPO、Md-POD1、MdPOD2)进行了基因组鉴定及表达分析。其中MdAFS、MdC3H和MdCAT1的表达量在虎皮病发生前10 d,即贮藏第40 天急剧上升,随后呈现下降的趋势。综上所述,MdAFS、MdC3H 和MdCAT1可作为澳洲青苹苹果贮藏期虎皮病发生的关键预警基因。以上基因具有很好的预测能力,可用于预测澳洲青苹苹果贮藏期间虎皮病的发生,从而指导苹果采后贮藏,旨在苹果果实虎皮病出现之前能出库销售。该研究为澳洲青苹苹果虎皮病发生预警研究提供了理论依据。
[1]李林光,杨建明,隋从义.加工鲜食兼用型绿色苹果优良品种:澳洲青苹[J].落叶果树,2001,33(1):25-26.LI Linguang,YANG Jianming,SUI Congyi.An excellent green apple variety for processing fresh and edible:Australian green apple[J].Deciduous Fruits,2001,33(1):25-26.
[2]张顺妮,阎振立.苹果汁加工首选品种:澳洲青苹[J].现代农业,2001(5):6.ZHANG Shunni,YAN Zhenli.The preferred variety of apple juice processing:Australian green apple[J].Journal of Modern Agricultute,2001(5):6.
[3]韩林.我国高酸榨汁苹果的栽培现状及发展前景[J].山西果树,2007(3):38-39.HAN Lin.Cultivation status and development prospect of apple with high acid juice in China[J].Shanxi Fruits,2007(3):38-39.
[4]WATKINS C B,BRAMLAGE W J,CREGOE B A.Superficial scald of‘Granny Smith’apples is expressed as a typical chilling injury[J].Journal of the American Society for Horticultural Science,1995,120(1):88-94.
[5]蒋帅,周会玲,刘焕,贺军花,马利菁.苹果冷藏期间虎皮病发病原因及防治方法[J].北方园艺,2017(19):179-183.JIANG Shuai,ZHOU Huiling,LIU Huan,HE Junhua,MA Lijing.Causes and prevention of superficial scald disease during cold storage of apple[J].Northern Horticulture,2017(19):179-183.
[6]LURIE S,WATKINS C B.Superficial scald,its etiology and control[J].Postharvest Biology and Technology,2012,65:44-60.
[7]MDITSHWA A,FAWOLE O A,VRIES F,VAN DER MERWE K,CROUCH E,OPARA U L.Classification of‘Granny Smith’apples with different levels of superficial scald severity based on targeted metabolites and discriminant analysis[J].Journal of Applied Botany and Food Quality,2016,89:49-55.
[8]刘丹丹,南学平,曹格妮,郑爱英,景姗.苹果虎皮病的研究现状探析:基于CNKI 40 年间学术论文的内容分析[J].河南农业,2021(10):43-45.LIU Dandan,NAN Xueping,CAO Geni,ZHENG Aiying,JING Shan.Research status of apple tiger skin disease:Based on the content analysis of academic papers of CNKI in the past 40 years[J].Agriculture of Henan,2021(10):43-45.
[9]ROWAN D D,ALLEN J M,FIELDER S,SPICER J A,BRIMBLE M A.Identification of conjugated triene oxidation products of α-farnesene in apple skin[J].Journal of Agricultural and Food Chemistry,1995,43(8):2040-2045.
[10]龚意辉,宋雅慧,熊子璇,肖靖,李鹏,曾永贤.采后苹果虎皮病研究进展[J].安徽农业科学,2021,49(19):13-15.GONG Yihui,SONG Yahui,XIONG Zixuan,XIAO Jing,LIPeng,ZENG Yongxian.Research progress of superficial scald in postharvest apple fruits[J].Journal of Anhui Agricultural Sciences,2021,49(19):13-15.
[11]邵淑君.苹果虎皮病致病因子筛选及代谢规律研究[D].淄博:山东理工大学,2017.SHAO Shujun.Research on screening and metabolism of pathogenic factors of the apple superficial scald[D].Zibo:Shandong University of Technology,2017.
[12]PECHOUS S W,WHITAKER B D.Cloning and functional expression of an (E,E)-α-farnesene synthase cDNA from peel tissue of apple fruit[J].Planta,2004,219(1):84-94.
[13]刘少华.AVG 和CTM 处理对红星苹果虎皮病的影响[D].泰安:山东农业大学,2020.LIU Shaohua.Effects of AVG and CTM treatments on superficial scald in‘Starking’apple[D].Taian:Shandong Agricultural University,2020.
[14]LU X G,MA Y P,LIU X H.Effects of hydrogen peroxide accumulation,lipid peroxidation,and polyphenol oxidation during superficial scald development in‘Fuji’apples[J].Horticulture,Environment,and Biotechnology,2014,55(4):299-307.
[15]RAO M V,WATKINS C B,BROWN S K,WEEDEN N F.Active oxygen species metabolism in‘White Angel’בRome Beauty’apple selections resistant and susceptible to superficial scald[J].Journal of the American Society for Horticultural Science,1998,123(2):299-304.
[16]BUSATTO N,FARNETI B,TADIELLO A,VRHOVSEK U,CAPPELLIN L,BIASIOLI F,VELASCO R,COSTA G,COSTA F.Target metabolite and gene transcription profiling during the development of superficial scald in apple (Malus × domestica Borkh.)[J].BMC Plant Biology,2014,14:193.
[17]GONG Y H,SONG J,DU L N,VINQVIST M,PALMER L C,FILLMORE S,PANG X Q,ZHANG Z Q.Characterization of laccase from apple fruit during postharvest storage and its response to diphenylamine and 1-methylcyclopropene treatments[J].Food Chemistry,2018,253:314-321.
[18]徐盼盼.红富士苹果虎皮病发生与叶绿素荧光参数关系的研究[J].现代农业科技,2018(19):267-269.XU Panpan.Study on the relationship between the occurrence of tiger skin disease of red Fuji apple and chlorophyll fluorescence parameters[J].Modern Agricultural Science and Technology,2018(19):267-269.
[19]周虹燕,任小林,田建文.澳洲青苹果实叶绿素荧光参数与虎皮病相关性[J].食品科学,2014,35(10):258-262.ZHOU Hongyan,REN Xiaolin,TIAN Jianwen.Correlation of chlorophyll fluorescence parameters with superficial scald development in Granny Smith apple[J].Food Science,2014,35(10):258-262.
[20]DU Z Y,BRAMLAGE W J.A modified hypothesis on the role of conjugated trienes in superficial scald development on stored apples[J].Journal of the American Society for Horticultural Science,1993,118(6):807-813.
[21]GINÉ-BORDONABA J,MATTHIEU-HURTIGER V,WESTERCAMPP,COUREAU C,DUPILLE E,LARRIGAUDIÈRE C.Dynamic changes in conjugated trienols during storage may be employed to predict superficial scald in‘Granny Smith’apples[J].LWT-Food Science and Technology,2013,54(2):535-541.
[22]HARGARTEN H,WALIULLAH S,KALCSITS L,HONAAS L A.Leveraging transcriptome data for enhanced gene expression analysis in apple[J].Journal of the American Society for Horticultural Science,2018,143(5):333-346.
[23]LEISSO R S,GAPPER N E,MATTHEIS J P,SULLIVAN N L,WATKINS C B,GIOVANNONI J J,SCHAFFER R J,JOHNSTON J W,HANRAHAN I,HERTOG M L A T M,NICOLAÏ B M,RUDELL D R.Gene expression and metabolism preceding soft scald,a chilling injury of‘Honeycrisp’apple fruit[J].BMC Genomics,2016,17(1):798.
[24]CHEN C J,CHEN H,ZHANG Y,THOMAS H R,FRANK M H,HE Y H,XIA R.TBtools:an integrative toolkit developed for interactive analyses of big biological data[J].Molecular Plant,2020,13(8):1194-1202.
[25]贾晓辉,王文辉,姜云斌,杜艳民,王志华,佟伟.不同贮藏温度对‘玉露香’梨果实保绿效果和品质维持的影响[J].果树学报,2016,33(S1):166-174.JIA Xiaohui,WANG Wenhui,JIANG Yunbin,DU Yanmin,WANG Zhihua,TONG Wei.Effects of storage temperature on green keeping and quality of‘Yuluxiang’pear[J].Journal of Fruit Science,2016,33(S1):166-174.
[26]田雪婷,吴晗笑,王雷,田晓宁,李瑞,戚英伟,任小林.1-MCP处理对采后‘澳洲青苹’苹果叶绿素降解的影响[J].果树学报,2020,37(5):734-742.TIAN Xueting,WU Hanxiao,WANG Lei,TIAN Xiaoning,LI Rui,QI Yingwei,REN Xiaolin.Effect of 1-MCP treatment on chlorophyll degradation in postharvest‘Granny Smith’apple fruit[J].Journal of Fruit Science,2020,37(5):734-742.
[27]王阳光.采后青梅果实叶绿素降解机制及保绿措施的研究[D].杭州:浙江大学,2003.WANG Yangguang.Study on chlorophyll degradation mechanism and green-keeping measures of postharvest plum fruit[D].Hangzhou:Zhejiang University,2003.
[28]王晴.苹果转录因子MdMYC2 与MdERF3 对α-法尼烯生物合成的转录调控[D].泰安:山东农业大学,2019.WANG Qing.Transcriptional regulation of α-farnesene synthesis by MdMYC2 and MdERF3 in apple fruit[D].Tai’an:Shandong Agricultural University,2019.
[29]KARAGIANNIS E,MICHAILIDIS M,TANOU G,SAMIOTAKI M,KARAMANOLI K,AVRAMIDOU E,GANOPOULOS I,MADESIS P,MOLASSIOTIS A.Ethylene-dependent andindependent superficial scald resistance mechanisms in‘Granny Smith’apple fruit[J].Scientific Reports,2018,8(1):1-16.
[30]ZHOU S,CHENG Y D,GUAN J F.The molecular basis of su-perficial scald development related to ethylene perception and αfarnesene metabolism in‘Wujiuxiang’pear[J].Scientia Horticulturae,2017,216:76-82.
[31]MARTINS MELO A A,OLABODE P N,ATAMIAN H S,NYAKUNDI B,PACIOLES C T,PRAKASH A.Irradiation reduces superficial scald by downregulating ethylene and α-farnesene biosynthetic enzymes in‘Granny Smith’apples[J].Radiation Physics and Chemistry,2021,189:109650.
[32]LIU H,LIU S H,DU B Y,DONG K T,WANG Y,ZHANG Y H. Aloe vera gel coating aggravates superficial scald incidence in‘Starking’apples during low-temperature storage[J].Food Chemistry,2021,339:128151.
[33]LINDO-GARCÍA V,GINÉ-BORDONABA J,LECLERC C,UBACH D,LARRIGAUDIÈRE C.The relationship between ethylene-and oxidative-related markers at harvest with the susceptibility of pears to develop superficial scald[J].Postharvest Biology and Technology,2020,163:111135.
[34]BOSS P K,GARDNER R C,JANSSEN B J,ROSS G S.An apple polyphenol oxidase cDNA is up-regulated in wounded tissues[J].Plant Molecular Biology,1995,27(2):429-433.
[35]胡小松,赵晨霞.过氧化氢对苹果虎皮病的致病探讨[J].中国食品学报,1999,3(1):64-68.HU Xiaosong,ZHAO Chenxia.Research on the mechanism of desease of H2O2 on AppleSuperficial scald[J].Journal of Chinese Institute of Food Science and Technology,1999,3(1):64-68.
[36]CEBULJ A,HALBWIRTH H,MIKULIC-PETKOVSEK M,VEBERIC R,SLATNAR A.The impact of scald development on phenylpropanoid metabolism based on phenol content,enzyme activity,and gene expression analysis[J].Horticulture,Environment,and Biotechnology,2020,61(5):849-858.
[37]GONG Y H,SONG J,PALMER L C,VINQVIST-TYMCHUK M,FILLMORE S,TOIVONEN P,ZHANG Z Q.Tracking the development of the superficial scald disorder and effects of treatments with diphenylamine and 1-MCP using an untargeted metabolomic approach in apple fruit[J].Food Chemistry:Molecular Sciences,2021,2:100022.
[38]FANG F,ZHANG X L,LUO H H,ZHOU J J,GONG Y H,LI W J,SHI Z W,HE Q,WU Q,LI L,JIANG L L,CAI Z G,OREN-SHAMIR M,ZHANG Z Q,PANG X Q.An intracellular laccase is responsible for epicatechin-mediated anthocyanin degradation in Litchi fruit pericarp[J].Plant Physiology,2015,169(4):2391-2408.
[39]WEI J B,ZHANG X,ZHONG R H,LIU B,ZHANG X L,FANG F,ZHANG Z Q,PANG X Q.Laccase-mediated flavonoid polymerization leads to the pericarp browning of Litchi fruit[J].Journal of Agricultural and Food Chemistry,2021,69(50):15218-15230.
[40]杨阳,刘媛媛.苹果MdLAC 基因家族成员的鉴定与序列分析[J].河南农业科学,2021,50(7):125-135.YANG Yang,LIU Yuanyuan.Identification and sequence analysis of the MdLAC gene family members in apple[J].Journal of Henan Agricultural Sciences,2021,50(7):125-135.