苹果(Malus domestica)是世界上种植最广泛的经济果树之一。作为典型的多年生木本植物,在其生长过程中极易受到病毒或类病毒的侵染[1]。目前常见的侵染苹果的病毒包括苹果茎沟病毒(apple stem grooving virus,ASGV)[3]、苹果花叶病毒(apple mosaic virus,ApMV)[4]、苹果褪绿叶斑病毒(apple chlorotic leaf spot virus,ACLSV)、苹果锈果类病毒(apple scar skin viroid,ASSVd)[5-6]、苹果凹果类病毒(apple dimple fruit viroid,ADFVd)[7-9],以及新近鉴定的与中国苹果花叶病紧密相关的苹果坏死花叶病毒(apple necrotic mosaic virus,ApNMV)[4,10-11]。
由类病毒引发的苹果锈果病是苹果生产中最具破坏性的病害之一。目前,该病害在世界各苹果主产区均有发生,特别是在中国、日本、韩国,以及美国、印度和伊朗[12-15]。在苹果生产中,该病害根据在不同品种上的表型可分为3种症状:锈果型、花脸型和锈果-花脸混合型症状[16]。锈果型的典型症状是在果实上生有与心室顶部对应的五条规则的木栓化斑纹,主要发生在金冠等品种上。花脸型症状主要表现在果皮表面形成圆形的黄绿色或红色斑点,主要发生在着色品种上,如富士系[16]。而锈果-花脸混合型是上述两种症状的复合症状[1]。该病害主要影响果实的品质,包括硬度、风味等内在品质和果实着色等外观品质,使果实失去商业价值,造成严重经济损失[1]。
诱导苹果锈果病发生的主要病原是ASSVd,其属于马铃薯纺锤形块茎类病毒科(Pospiviroidae),苹果锈果类病毒属(Apscarviroid)[17-18]。苹果植株被ASSVd侵染后将终生带毒,严重影响苹果果实的产量和品质。ASSVd的基因组为环状单链RNA,长度约为330个核苷酸[17]。其基因组RNA不编码任何蛋白质,完全依靠寄主的转录机制进行复制和增殖[17]。
尽管苹果锈果病已有30多年的研究历史,但目前主要集中于其病原物ASSVd 的检测及分离方面。关于该病害发生的内在机制研究尚不多见。笔者在本研究中利用转录组测序技术对山东烟台地区的表现花脸症状富士苹果检测,通过分析差异表达基因的功能,探究与花脸症状形成的可能代谢途径,为后续深入探究苹果花脸症状形成的内在机制提供重要依据。
1 材料和方法
1.1 试验材料
对照和感病呈现花脸症状的富士果实样品(图1)于2018年9月采自山东省烟台市烟台农业科学研究院果树基地(121.39°E,37.52°N),样本分别来自树龄11年、长势相对一致的健康且无花脸症状和有花脸症状的弘前富士苹果树,随机取样后削取果皮,混样后设置3次生物学重复,液氮冷冻后置于-80 ℃用于后续RNA的提取及转录组测序分析。

图1 健康和花脸症状的富士果实
Fig.1 Healthy and dapple Fuji apple samples
1.2 ASSVd检测及克隆
参照GenBank中已公布的ASSVd(AY972082.1)序列设计引物ASSVd-F1/R1 用以检测富士苹果中的ASSVd。根据扩增到的序列设计基因特异性引物ASSVd-F2/R2进行第二轮PCR扩增,通过序列组装获得ASSVd全基因序列。
1.3 转录组测序
1.3.1 cDNA文库构建及测序质量控制 委托青岛欧易生物公司提取果皮总RNA,经过Agilent 2100 Bioanalyzer 质量检测后进行样本转录组测序分析。在原始数据Raw reads 中去接头(Adaptor),去除低质量Reads,从3'端及5′端以不同方式去除低质量碱基,得到clean data用于后续数据分析。
1.3.2 测序数据比对和表达分析 利用Hisat2以金冠苹果基因组(GDDH13)为参考基因组对clean data进行序列比对。采取序列相似性比对的方法鉴定出各蛋白编码基因在各样本中的表达丰度。使用htseq-count软件获取每个样本中比对到蛋白编码基因上的reads 数,cufflinks 软件来计算蛋白编码基因的表达量FPKM值。
1.3.3 差异基因筛选及功能分析 利用DESeq 软件进行样本间的差异基因分析,对各个样本基因的counts 数目进行标准化处理(采用basemean 值来估算表达量),计算差异倍数,并采用NB(负二项分布检验的方式)对reads 数进行差异显著性检验,最终根据差异倍数及差异显著性检验结果来筛选差异蛋白编码基因。筛选标准为|log2 FoldChange|>1 且p<0.05。利用基因本体数据库(GO)和京都基因与基因组百科全书(KEGG)对基因进行功能注释、分析以及统计。
1.4 总RNA的提取及实时定量PCR(qRT-PCR)验证
采用天根生化公司的植物RNA 提取试剂盒进行RNA 提取;通过Clontech SMARTTM Library 试剂盒进行反转录,合成用于定量分析的cDNA 链。康为世纪公司的Ultra SYBR Mixture(Low ROX)试剂盒用于实时荧光定量PCR,以18S核糖体RNA基因作为内参基因[17],引物序列见表1。
表1 RT-PCR 克隆ASSVd 及qRT-PCR 检测转录因子表达量所用引物
Table 1 Primers for cloning ASSVd by RT-PCR and for testing the expression levels of transcription factors using qRT-PCR

引物名称Primer name ASSVd-F1(RT-PCR)ASSVd-R1(RT-PCR)ASSVd-F2(RT-PCR)ASSVd-R2(RT-PCR)18s-F(qRT-PCR)18s-R(qRT-PCR)MdWRKY18-like-F(qRT-PCR)MdWRKY18-like-R(qRT-PCR)MdWRKY71-F(qRT-PCR)MdWRKY71-R(qRT-PCR)MdNAC29-F(qRT-PCR)MdNAC29-R(qRT-PCR)MdWRKY70-F(qRT-PCR)MdWRKY70-R(qRT-PCR)MdERF61-F(qRT-PCR)MdERF61-R(qRT-PCR)MdMYB34-like-F(qRT-PCR)MdMYB34-like-R(qRT-PCR)MdWRKY72-F(qRT-PCR)MdWRKY72-R(qRT-PCR)序列(5’→3’)Sequence(5’→3’)CCGGTGAGAAAGGAGCTGCCAGCA CCTTCGTCGACGACGACAGGTGAGT CCGGACGGCGCCCTCGCACCAGTTCCGCTGTGG CTTAGTGCTGGCAGCTCCTTTCTCACCGGCCTTCG AGGCGCGAAATTACCAATCC GCCCTCCAATTGTTCCTCGTTAAG CTCGTACGCCTAACAAACAAAAGGTTGTAGA CTTTAGAGTCGGTTTTTACTAGGAACTGTG GAAAAATCCTTACCAGTACGATCCTTTCGA CTTCATTAGATGAAGAAGATATTGAGGAAT TCAGCTTCCTCGCAGTCATCTTCAGCGTCCT CAGCATTGTGTTTGAAGTCACCATTCGGCAG GACTGATCGGAGAGCTACATGAAGGCCAGA TTTTGGCCATATTTTCTCCAAGCCTGTCCAT ATGCATGAAAACAACCTTGCTTTTGGGTTC CTCGGCGACCCATTTGCCCCAATGCCTCTG GATCACCGTGCTGTGACAAGGTGGGTTTGA CGTATTCCAGTAGTTTTTTATCTCATTGTC GGGAGTGAACTGGATGATGCCAAAGCTGAA TTCCAGCTTCTGCACGATCCTTATTAGGTTC
1.5 数据分析
所有试验设置3 次生物学重复,使用SPSS 19.0软件进行差异显著性检验。
2 结果与分析
2.1 ASSVd检测及序列克隆
利用引物ASSVd-F1/R1进行PCR检测,从表现花脸的苹果果皮中扩增得到约300 bp 左右的条带,而在健康果皮中则没有该条带(图2-A)。经过DNA测序并将所得序列在NCBI数据库(https://www.ncbi.nlm.nih.gov/)进 行BLASTn 比 对,发 现 该 序 列 与ASSVd山东烟台苹果分离物SDYT-1(MW302328.1)高度相似。为进一步获得ASSVd基因组全序列,根据已经获得的序列设计基因特异性引物ASSVd-F2/R2。用该引物进行PCR扩增,获得第二轮ASSVd序列(图2-B)。最终,通过序列比对和组装获得2 条ASSVd全长序列,全长分别是331 nt和330 nt。通过比对,发现2 条序列与GenBank 数据库中ASSVd 山东烟台苹果分离物SDYT-1(MW302328.1)和SDYT-3(MW315909.1)序列完全一致。

图2 表现花脸症状果皮中ASSVd 的RT-PCR 检测及序列扩增
Fig.2 RT-PCR detection and sequence amplification of ASSVd
A.利用引物ASSVd-F1/R1 检测ASSVd;B.利用引物ASSVd-F2/R2 扩增ASSVd 全长序列;泳道1.RT-PCR 检测的健康富士苹果果皮样品;泳道2.RT-PCR 检测表现花脸富士苹果果皮样品;泳道3.水作为阴性对照;泳道4.阳性对照;M.DNA Marker.
A.Using primer ASSVd-F1/R1 to detect ASSVd;B.Using prime ASSVd-F2/R2 to amplify ASSVd sequence.Lane 1.The RT-PCR product amplified using the peel of healthy Fuji apple sample;Lane 2.The RT-PCR product amplified using the peel of dapple Fuji apple sample;Lane 3.Negative control by using ddH2O as template;Lane 4.Positive control;M.DNA Marker.
2.2 花脸症状果皮中差异表达基因分析
将对照组(CK_1,CK_2,and CK_3)和表现花脸症状果皮样品(Diseased_1, Diseased_2, and Diseased_3)经Illumina测序后共获得43.47 G原始测序数据(表2)。质控检测结果表明,clean data覆盖度超过89%,各样本的Q30 均超过92%,GC 含量约为48%,说明测序质量较高,符合后续的数据分析要求。
表2 转录组测序质控后的质量统计
Table 2 Quality statistics of filtered transcript group sequencing data
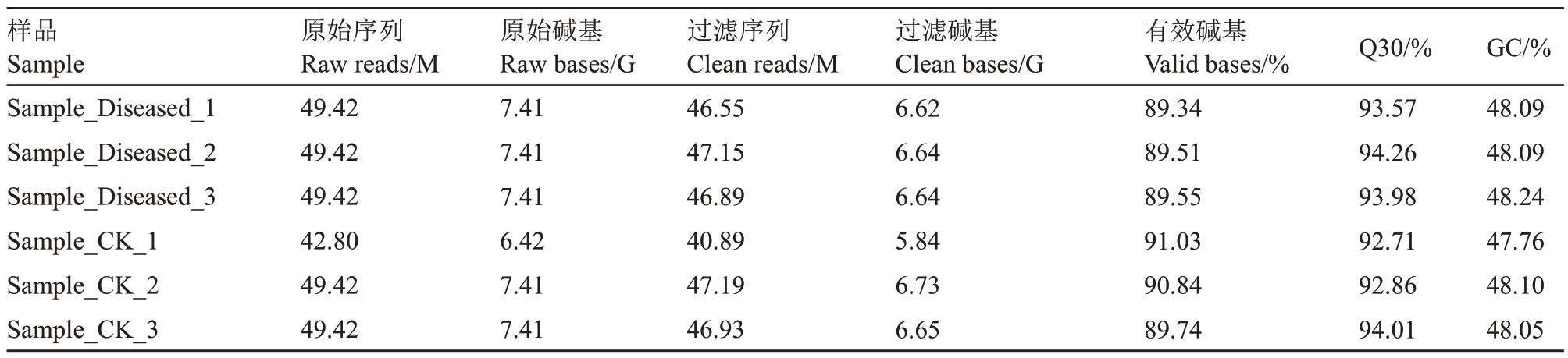
样品Sample Sample_Diseased_1 Sample_Diseased_2 Sample_Diseased_3 Sample_CK_1 Sample_CK_2 Sample_CK_3原始序列Raw reads/M 49.42 49.42 49.42 42.80 49.42 49.42原始碱基Raw bases/G 7.41 7.41 7.41 6.42 7.41 7.41过滤序列Clean reads/M 46.55 47.15 46.89 40.89 47.19 46.93过滤碱基Clean bases/G 6.62 6.64 6.64 5.84 6.73 6.65有效碱基Valid bases/%89.34 89.51 89.55 91.03 90.84 89.74 Q30/%93.57 94.26 93.98 92.71 92.86 94.01 GC/%48.09 48.09 48.24 47.76 48.10 48.05
为探究感染苹果花脸病后果皮中的基因表达情况,对差异表达基因(DEGs)进行分析。以|log2 Fold Change|>1&p<0.05为筛选标准,两组样品中共筛选出6938个DEGs,其中上调DEGs共有3331个,下调DEGs共有3607个。
2.3 ASSVd侵染苹果果实DEGs的GO富集分析
为探究上述差异表达基因的功能,笔者对DEGs进行GO富集分析,结果表明,上述DEGS涉及生物进程(biological process,BP)、细胞组分(cellular component,CC)和分子功能(molecular function,MF)3个大类。在DEGs富集数目的前30个亚类中(图3),生物进程注释到17个亚类,其中主要集中在细胞过程(cellular process)、代谢过程(metabolic process)、单有机体过程(single-organism process)和刺激反应(response to stimulus)等过程(图3)。富集在细胞组分的DEGs主要分为9个亚类,集中在细胞(cell)、细胞部分(cell part)、细胞器(organelle)和膜(membrane)等组分(图3)。在分子功能的4个亚类中,DEGs 主要涉及结合(binding)和催化活性(catalytic activity)(图3)。

图3 GO 分析中DEGs 在不同功能分类中的数目及比例
Fig.3 The amount and ratio of DEGs in different categories of GO analysis
2.4 表现花脸症状苹果果皮中DEGs 的KEGG 富集分析
为了进一步研究DEGs 的生物学功能,笔者对筛选出的DEGs 进行KEGG 富集分析。结果表明,共有2432 个DEGs 富集到了191 条通路中(图4)。其中富集显著的前20 条代谢通路包括植物激素信号转导(plant hormone signal transduction)、苯丙烷生物合成(phenylpropanoid biosynthesis)、光合作用-触角蛋白(photosynthesis-antenna proteins)、光合作用(photosynthesis)、脂肪酸降解(fatty acid degradation)、不饱和脂肪酸的生物合成(biosynthesis of unsaturated fatty acids)(图4)。

图4 表现花脸症状苹果果皮中DEGs 的KEGG 富集分析
Fig.4 Analysis of the KEGG enrichment of DEGs in dapple apple fruit
2.5 表现花脸症状苹果果皮的激素相关DEGs分析
植物激素是调控植物生长发育和应对环境变化的重要调节因子。在KEGG 富集分析中,植物激素信号转导是DEGs 富集数目最多的代谢通路之一,共有90 个DEGs 得到富集(图5)。上述与植物激素信号转导相关的90个DEGs中,有51个生长素(auxin,IAA)相关的DEGs,10 个细胞分裂素(cytokinin,CK)相关的DEGs,7 个赤霉素(gibberellins,GAs)相关的DEGs,6 个脱落酸(abscisic acid,ABA)相关的DEGs,2 个乙烯(ethylene,ET)相关的DEGs,8 个油菜素内酯(brassinosteroid,BR)相关的DEGs,5 个茉莉酸(jasmonic acid,JA)相关的DEGs 和1 个水杨酸(salicylic acid,SA)相关的DEGs。

图5 植物激素相关DEGs 的表达水平热图
Fig.5 Heat map of the expression level of plant hormone-related DEGs
图中红色、黄色和蓝色的圆点表示茉莉酸、水杨酸和乙烯相关DEGs。
The red,yellow and blue dots in the figure represent DEGs related to JA,SA and ET.
对上述90 个植物激素信号转导相关DEGs 的表达量分析发现,其中24 个DEGs 相比于对照组表达水平是上调的,66 个DEGs 是下调的(图5),笔者选取表达倍数差异显著的前10 个基因进行分析,发现其多数为生长素信号相关基因。通过设计基因特异性引物进行qRT-PCR 验证,发现其与转录组测序结果一致,所有基因表达量均显著下调(图6,表3)。
表3 植物激素相关DEGs 信息及实时定量PCR 引物
Table 3 Plant hormone related DEGs information and qRT-PCR primers

基因ID Gene ID MD16G1127400 MD10G1059600 MD17G1198100 MD10G1060500 MD16G1014900 MD02G1100000 MD10G1303700 MD15G1077100 MD10G1059800 MD10G1192900注释Annotation茉莉酸-锌-结构域蛋白10 Jasmonate-zim-domain protein 10 SAUR-like 生长素响应蛋白家族SAUR-like auxin-responsive protein family吲哚-3-乙酸诱导19 Indole-3-acetic acid inducible 19 SAUR-like 生长素响应蛋白家族SAUR-like auxin-responsive protein family包含组氨酸激酶蛋白的CHASE结构域CHASE domain containing histidine kinase protein SAUR-like 生长素响应蛋白家族SAUR-like auxin-responsive protein family F-box/RNI-like 亚家族蛋白F-box/RNI-like superfamily protein细胞周期-D3-1-like Cyclin-D3-1-like SAUR-like 生长素响应蛋白家族SAUR-like auxin-responsive protein family吲哚-3-乙酸7 Indole-3-acetic acid 7引物序列(5’→3’)Primer sequence(5’→3’)F:ACCGTTGAGCTTGATTTTTTCGGCA R:CTGGCATTGCCGGAAGCGATGACGG F:CCTGGTATTTTACTTGCCAAGAAAA R:CTACTTATACACCAAAGCTGAGGTGA F:CTTGAAATTACAGAGCTGAGGTTGG R:GCCATAATAACCAAATAACTTCTCC F:ATGGGTTTCCGTCGTCCAAGTGTAA R:CTGGCAGAGGAAAGATATGGGAACCA F:CAAGAAGAAGAATCAAAGAAATTGG R:GGGTTCTTGTAGTAATGGAAGGTGGA F:AAGTTGACCGGAATCAGGCAGATTG R:CCCTTGGGGGACATCAGGTGGCGGT F:CGGGTTGCACGACCCGGAAGACGAA R:CCGCGAACCGGGGCTTCCCCTTCAG F:TAGAATCAAGTGAAGAACAAAACCC R:GCACCGCCGTGAGGGCGGAGAAAGAG F:ATGGGGTTCCGTCTGCCTTCTGTAA R:TCACTGACACAAATTTTCAAGAATAGT F:GCCGGGTGGTGGTGGCGGCGGCGTC R:TTGGCTAGGGCATCAGAGAGCTCAG
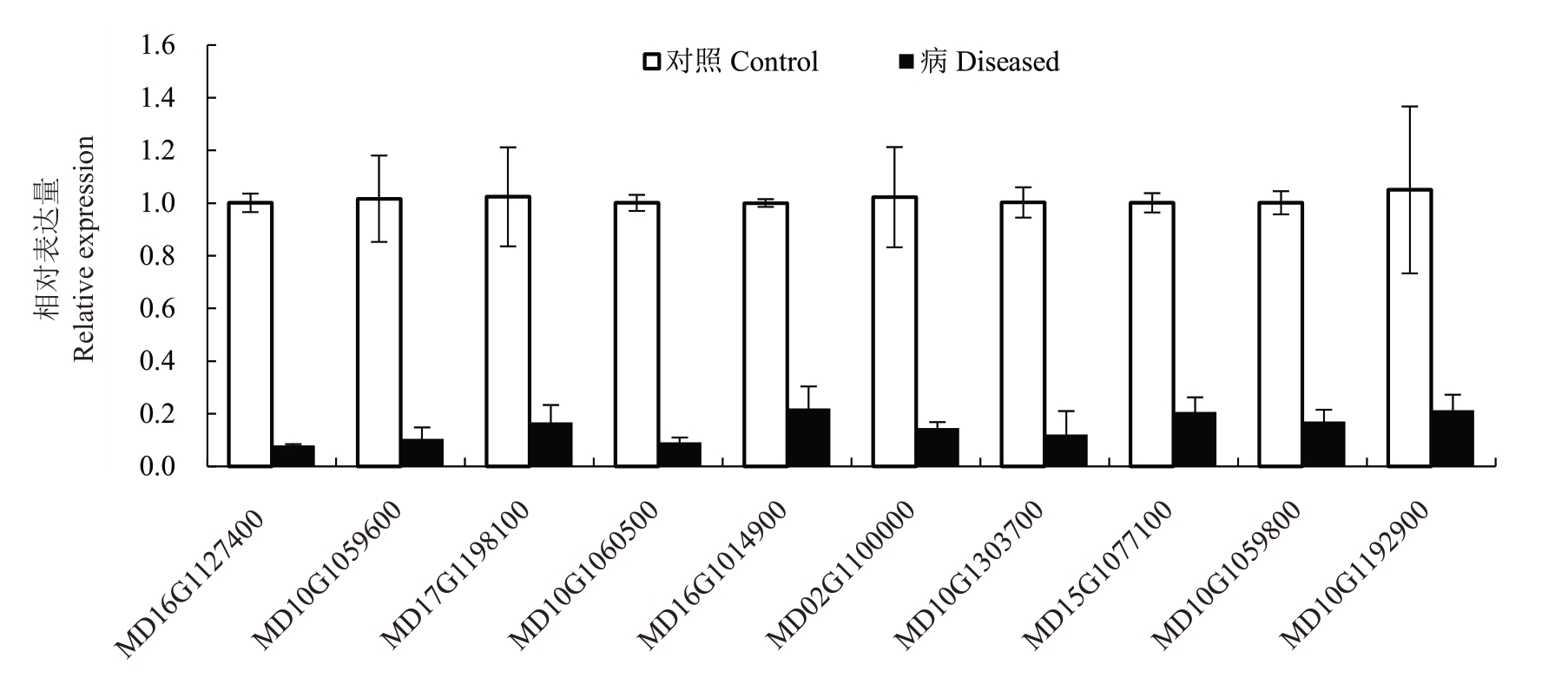
图6 实时定量PCR 检测植物激素相关DEGs 基因表达量
Fig.6 Determination for the expression of plant hormonal-related DEGs using qRT-PCR
2.6 表现花脸症状苹果果皮中的转录因子表达分析
转录因子在调控植物生长发育及抗病反应中发挥重要作用。对DEGs中编码转录因子的基因进行分析,发现共有194 个转录因子的编码基因被显著诱导表达,并筛选出其中与植物抗病相关的转录因子家族编码基因:14 个WRKY 家族蛋白编码基因、14 个MYB 家族蛋白编码基因、12 个ERF 家族蛋白编码基因和5个NAC家族蛋白编码基因。与对照组相比,25个转录因子表达上调,20个转录因子表达下调(图7)。为验证转录组数据的可靠性,笔者利用qRT-PCR 验证发现,MdWRKY70(MD01G1168600)、MdWRKY71(MD17G1138100)、MdWRKY18- like(MD15G1039600)、MdNAC29(MD13G1063900)基因表达量显著上调,而MdERF61(MD14G1127700)、MdMYB34-like(MD17G1051700)、MdWRKY7(MD1-7G10-48400)基因的表达量显著下调,与转录组检测结果一致(图8)。

图7 转录因子相关DEGs 的表达水平热图
Fig.7 Heat map of the expression level of transcription factor-related DEGs
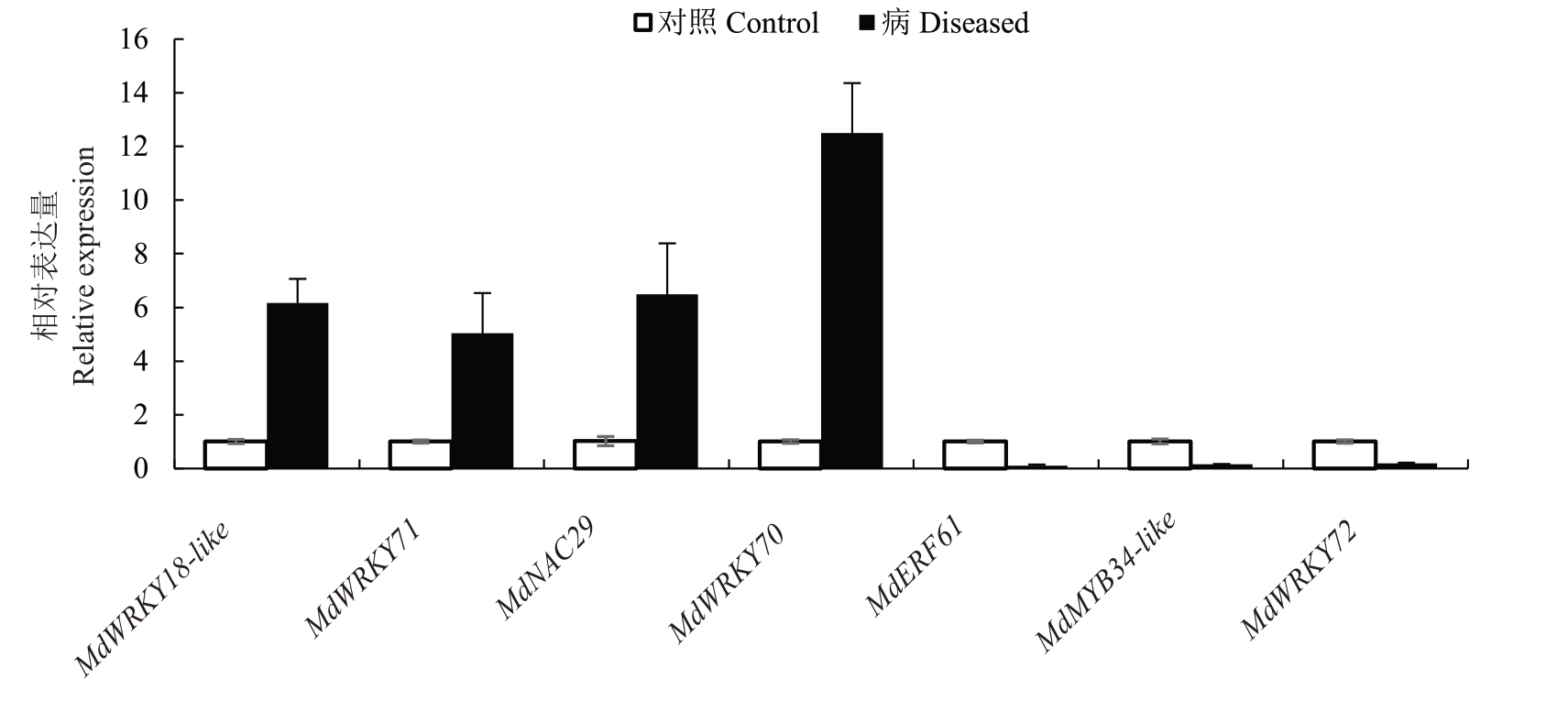
图8 实时荧光定量PCR 检测转录因子编码基因的表达量差异
Fig.8 Determination of transcription factor-encoding genes using qRT-PCR
3 讨 论
近年来,在中国苹果主产区,如山东、辽宁、陕西、甘肃、新疆、河北等地,苹果锈果类病毒引发的病害逐年加重,已成为限制苹果产业健康发展的重要因素。但目前关于该病害的研究主要集中于ASSVd的检测及序列分析,关于病害发生的机制等问题尚未见报道。笔者在本研究中借助RT-PCR和转录组测序技术,对表现花脸症状的苹果果皮进行分析,共获得了6938 个差异表达基因。并对差异表达基因的功能注释进行分析总结,为后续研究苹果花脸病害发生的机制及相关代谢途径提供了参考依据。
笔者在本研究中获得的2 条ASSVd 序列与GenBank 中已收录的来自烟台地区富士苹果上的ASSVd 分离物SDYT-1(MW302328.1)和SDYT-3(MW315909.1)完全一致,未发现碱基变异。有研究 表 明ADFVd 与ASSVd 同 为Pospiviroidae 科Apscarviroid属成员,其基因组RNA长度为306~310 nt,且与ASSVd 序列高度相似,含有ASSVd 序列的整个保守区域。
GO 富集分析结果表明,表现花脸症状的苹果果皮中的DEGs主要集中在细胞过程、代谢过程、细胞、细胞组分、结合和催化活性等功能;同时,KEGG分析发现DEGs富集程度最高的代谢通路是植物激素信号转导,其次是苯丙烷生物合成和光合作用等通路,这说明花脸病对上述调控及反应途径有重要影响。
病原菌入侵会触发植物体内激素信号途径而激活植物免疫反应,提高植物抵御病原菌侵染的能力[19]。一般认为,SA、JA 和ET 是植物抗病反应中重要防御激素,SA 通常参与植物对活体营养型和半活体营养型病菌防卫反应的激活,JA 和ET 则负责激活对死体营养型病菌的抗性,其他激素如IAA、ABA、GA 等通过相互作用直接或间接地参与调节植物的抗病性。笔者在本研究中发现表现花脸症状的苹果果皮中SA 信号途径相关基因NPR4a(MD05G1256300)、JA 信号途径相关基因TIFYs(MD13G1127100、MD09G1178600、MD02G1096100、MD16G1127400、MD17G1164400)和ET 信号途径相关基因ERS1(MD03G1292200)表达水平下降,而EIN3(MD08G1245800)表达水平上调。然而,不同于典型的抗病反应激素,表现花脸症状的苹果果皮中IAA 途径相关DEGs 数目最多,且多为编码IAA 信号途径中的关键酶和受体蛋白。IAA 信号途径的相关基因的表达模式与对照组相比多呈现下调趋势,表明IAA 信号可能参与调控果实抗病性。同时基于IAA 对植物生长发育的影响,笔者推测花脸病的发生可能通过影响IAA 途径相关基因,减缓了IAA 的生物合成,这可能是果实变小的原因之一。
当植物受到外界胁迫刺激时,转录因子对于传递胁迫信号及启动特定基因的表达具有重要作用[20]。研究表明,WRKY、MYB、ERF和NAC类转录因子广泛参与植物生长发育及防御过程,特别是WRKY 类转录因子[19-20]。当植物受到细菌、病毒等病原物侵染时,WRKY 基因的转录水平、转录后翻译水平以及结合活性都会发生明显变化[21],从而调节植物抗性反应。杨树PsnWRKY70 基因与MARK级联成员结合增强植物对叶枯病的抗性[22];过表达苹果MdWRKY100基因正调控苹果植株对炭疽病菌(Colletotrichum)的抗性[23]。MYB 类转录因子主要通过调节植物的过敏反应(hypersensitive response,HR)和系统性获得抗性(systemic acquired resistance,SAR)来增强植物对病原菌的抗性[24]。此外,ERF 和NAC 家族蛋白也在植物抗病反应中发挥重要作用,如过表达MdERF11 增强苹果对轮纹病菌(Botryosphaeria dothidea)的抗性[25]。水稻OsNAC6的表达受稻瘟菌所诱导,过量表达水稻OsNAC6 基因提高了水稻对稻瘟病的抗性。笔者在本研究中发现,表现花脸病的果皮中大量WRKY、MYB、ERF类转录因子的编码基因表达水平发生显著变化。其中,MdWRKY70、MdWRKY71、MdWRKY18-like 和MdNAC29 表达受到显著诱导,而MdERF61、Md-MYB34-like 和MdWRKY72 表达则受到显著抑制。推测转录因子可能通过调控下游结构基因的表达,参与寄主对病原菌的抗病反应。但关于其准确的内在机制还有待进一步验证。
4 结 论
笔者在本研究中对表现花脸症状的富士苹果果皮进行分析,分离得到2个ASSVd序列。转录组测序分析共获得6938 个差异表达基因。利用基因功能注释分析发现这些差异表达基因多参与植物激素信号途径及苯丙烷代谢途径。此外,与抗病相关的WRKY、MYB、ERF 类转录因子可能参与到苹果对花脸病的防御过程。
[1]胡国君,张尊平,范旭东,任芳,李正男,董雅凤.我国主要苹果病毒及其研究进展[J].中国果树,2017(3):71-74.HU Guojun,ZHANG Zunping,FAN Xudong,REN Fang,LI Zhengnan,DONG Yafeng.Main apple viruses in China and their research progress[J].China Fruits,2017(3):71-74.
[2]李正男,张双纳,张尊平,范旭东,任芳,胡国君,董雅凤.苹果茎沟病毒吉林沙果分离物全基因组序列分析[J].园艺学报,2018,45(4):641-649.LI Zhengnan,ZHANG Shuangna,ZHANG Zunping,FAN Xudong,REN Fang,HU Guojun,DONG Yafeng.Analysis of the complete genome of apple stem grooving virus Isolate Jilin-Shaguo[J].Acta Horticulturae Sinica,2018,45(4):641-649.
[3]邢飞,王红清,李世访.中国苹果花叶病病原研究现状分析[J].果树学报,2020,37(12):1953-1963.XING Fei,WANG Hongqing,LI Shifang.Advances in the identification of pathogens associated with apple mosaic disease of apple trees in China[J].Journal of Fruit Science,2020,37(12):1953-1963.
[4]查富蓉,王振华,徐勤耕,陈姣,殷玉梦,谌茂秋,王国平,洪霓,徐文兴.苹果花脸和锈果症状伴随的苹果锈果类病毒分离物的分子特性分析[J/OL].植物病理学报,2022.[2022-05-13].https://doi.org/10.13926/j.cnki.apps.000655.ZHA Furong,WANG Zhenhua,XU Qingeng,CHEN Jiao,YIN Yumeng,SHEN Maoqiu,WANG Guoping,HONG Ni,XU Wenxing.Molecular characteristics analysis of apple scar skin viroid isolates associated with dapple and scar symptoms of apple fruits[J/OL].Acta Phytopathologica Sinica,2022.[2022-05-13].https://doi.org/10.13926/j.cnki.apps.000655.
[5]郗娜娜,李紫腾,张静怡,孟祥龙,王亚南,曹克强.苹果锈果类病毒实时荧光定量反转录PCR 检测及其在苹果树体内的扩散转移规律[J].植物保护学报,2020,47(6):1304-1312.XI Nana,LI Ziteng,ZHANG Jingyi,MENG Xianglong,WANG Yanan,CAO Keqiang.Detection of apple scar skin viroid by real-time fluorescence quantitative reverse transcription PCR and its movement in an apple tree[J].Journal of Plant Protection,2020,47(6):1304-1312.
[6]赵英,牛建新.苹果凹果类病毒(ADFVd)的检测与序列分析[J].果树学报,2008,25(5):682-685.ZHAO Ying,NIU Jianxin.Detection and sequencing of apple dimple fruit viroid in apple trees[J].Journal of Fruit Science,2008,25(5):682-685.
[7]YE T,CHEN S,WANG R H,HAO L,CHEN H,WANG N,GUO L,FAN Z F,LI S F,ZHOU T M.Identification and molecular characterization of apple dimple fruit viroid in China[J].Journal of Plant Pathology,2013,95(3):637-641.
[8]KASAI H,ITO T,SANO T.Symptoms and molecular characterization of apple dimple fruit viroid isolates from apples in Japan[J].Journal of General Plant Pathology,2017,83(4):268-272.
[9]NODA H,YAMAGISHI N,YAEGASHI H,XING F,XIE J P,LI S F,ZHOU T,ITO T,YOSHIKAWA N.Apple necrotic mosaic virus,a novel ilarvirus from mosaic-diseased apple trees in Japan and China[J].Journal of General Plant Pathology,2017,83(2):83-90.
[10]弟豆豆,宋来庆,曹晓敏,胡慧,张硕,张学勇,姜中武,赵玲玲.苹果坏死花叶病毒烟台分离物的鉴定与序列分析[J].中国果树,2021(9):42-47.DI Doudou,SONG Laiqing,CAO Xiaomin,HU Hui,ZHANG Shuo,ZHANG Xueyong,JIANG Zhongwu,ZHAO Lingling.Identification and sequence analysis of Apple necrosis mosaic virus in Yantai isolates[J].China Fruits,2021(9):42-47.
[11]HADIDIA,BARBAM.CHAPTER 12:Apple scar skin viroid[M]//HADIDI A,BARBA M,CANDRESSE T,JELKMANN W.Vi-rus and virus-like diseases of pome and stone fruits.St.Paul,MN:The American Phytopathological Society,2011:57-62.
[12]KIM N,LEE H J,KIM N K,OH J,LEE S H,KIM H,MOON J,JEONG R.Occurrence pattern of viral infection on pear in Korea and genetic characterization of apple scar skin viroid isolates[J].Horticultural Science&Technology,2019,37(6):767-778.
[13]YAZARLOU A,JAFARPOUR B,HABILI N,RANDLES J W.First detection and molecular characterization of new apple scar skin viroid variants from apple and pear in Iran[J].Australasian Plant Disease Notes,2012,7(1):99-102.
[14]WALIA Y,KUMAR Y,RANA T,BHARDWAJ P,RAM R,DAS THAKUR P,SHARMA U,HALLAN V,ZAIDI A A.Molecular characterization and variability analysis of apple scar skin viroid in India[J].Journal of General Plant Pathology,2009,75(4):307-311.
[15]HADIDI A,BARBA M,HONG N,HALLAN V.Chapter 21:Apple scar skin viroid[M]//HADIDI A.Viroids and Satellites.Amsterdam:Elsevier,2017:217-228.
[16]HASHIMOTO J,KOGANEZAWA H.Nucleotide sequence and secondary structure of apple scar skin viroid[J].Nucleic Acids Research,1987,15(17):7045-7052.
[17]DI SERIO F,FLORES R,Verhoeven J T J,LI S F,PALLÁS V,RANDLES J W,SANO T,VIDALAKIS G,OWENS R A.Current status of viroid taxonomy[J].Archives of Virology,2014,159(12):3467-3478.
[18]VERMA V,RAVINDRAN P,KUMAR P P.Plant hormone-mediated regulation of stress responses[J].BMC Plant Biology,2016,16(1):86.
[19]SINGH K B,FOLEY R C,OÑATE-SÁNCHEZ L.Transcription factors in plant defense and stress responses[J].Current Opinion in Plant Biology,2002,5(5):430-436.
[20]YANG B,JIANG Y Q,RAHMAN M,DEYHOLOS M,KAV N.Identification and expression analysis of WRKY transcription factor in canola(Brassica napus L.)in response to fungal pathogens and hormone treatments[J].BMC Plant Biology,2009,9(1):68.
[21]ZHAO H,JIANG J,LI K L,LIU G F. Populus simonii × Populus nigra WRKY70 is involved in salt stress and leaf blight disease responses[J].Tree Physiology,2017,37(6):827-844.
[22]ZHANG F,WANG F,YANG S,ZHANG Y Y,XUE H,WANG Y S,YAN S P,WANG Y,ZHANG Z H,MA Y.MdWRKY100 encodes a group I WRKY transcription factor in Malus domestica that positively regulates resistance to Colletotrichum gloeosporioides infection[J].Plant Science,2019,286:68-77.
[23]RAFFAELE S,RIVAS S,ROBY D.An essential role for salicylic acid in AtMYB30-mediated control of the hypersensitive cell death program in Arabidopsis[J].FEBS Letters,2006,580(14):3498-3504.
[24]WANG J H,GU K D,HAN P L,YU J Q,WANG C K,ZHANG Q Y,YOU C X,HU D G,HAO Y J.Apple ethylene response factor MdERF11 confers resistance to fungal pathogen Botryosphaeria dothidea[J].Plant Science,2020,291:110351.
[25]NAKASHIMA K,TRAN L S P,VAN NGUYEN D,FUJITA M,MARUYAMA K,TODAKA D,ITO Y,HAYASHI N,SHINOZAKI K,YAMAGUCHI-SHINOZAKI K.Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice[J].The Plant Journal,2007,51(4):617-630.