卵形家族蛋白(ovate family proteins,OFPs)是具有保守OVATE 结构域的转录因子,为植物所特有,因番茄果实形态由卵形变为梨形而发现[1]。研究发现,OFPs 参与植物次生细胞壁形成,水稻维管束发育,拟南芥胚珠发育,辣椒和番茄果实的形状、香蕉果实的成熟及品质形成等过程[2];在拟南芥中过表达AtOFP1 发现花粉活力受到影响,植物发育迟缓[3];此外,OFPs 参与植物逆境抵御和赤霉素(gibberellin,GA)、脱落酸(abscisic acid,ABA)、乙烯(ethylene)及油菜素内酯(brassinolide,BR)等激素的信号传递[4];OsOFP22 通过抑制GA 和BR 信号传导,调节水稻形态发育[5];外源施加GA3 抑制SlOFP20 的表达,低温、干旱、盐胁迫和外源施加吲哚-3-乙酸(indole-3-acetic acid,IAA)和ABA能促进SlOFP20的表达[6]。ABA是一种在植物生长发育和各种非生物胁迫耐受过程中起重要调节作用的激素,参与调节种子成熟、种子发芽、幼苗生长和气孔运动等[7],外源施加ABA 可以有效缓解植物受到的冷害、盐胁迫和干旱胁迫等[8-10]。
杧果(Mangifera indica L.)是漆树科杧果属的热带水果,是世界五大热带水果之一,适时开花和对非生物胁迫的抵御能力影响杧果的产量和品质。OFPs 参与植物的成花调控且与逆境胁迫应答有关[11],目前杧果尚无OFP 基因的研究报道,在前期研究中,笔者从杧果成花酵母文库中筛选获得了一个OFP 基因,命名为MiOFP1。笔者在本研究中对杧果MiOFP1基因的表达模式和转基因功能进行研究,为揭示MiOFP1 参与杧果成花和杧果逆境应答的分子机制提供参考。
1 材料和方法
1.1 植物材料及取样
供试杧果品种为四季蜜杧,栽培于广西大学农学院果树标本园,拟南芥(Arabidopsis thaliana)为哥伦比亚野生型,种子由广西大学农学院杧果课题组保存。组织表达特性样品采集:2022-02-15 采集6株长势一致的童期实生杧果树(3年生)冬梢的成熟叶、芽和茎(韧皮部组织),6株长势一致的成年期嫁接杧果树(12 年生)冬梢的成熟叶、茎(韧皮部组织)、花,以及盛花后20 d和100 d的植物果实胚和果肉。时间表达特性样品为6株长势一致的嫁接杧果树的成熟叶,样品采集时间为2021年9月至2022年5月。采样时间统一定为17:00—18:00,样品采集后立即处理,放入-80 ℃冰箱冷冻保存备用。
1.2 试验方法
1.2.1 MiOFP1 启动子序列分析 利用NEW PLACE(https://www.dna.affrc.go.jp/PLACE/?action=newplace)在线软件预测四季蜜杧MiOFP1 启动子区域顺式作用元件,利用TBtools绘制启动子顺式作用元件位置信息图。
1.2.2 MiOFP1 组织特性和时间表达特性分析 提取杧果组织表达特性样品和时间表达特性样品的RNA,逆转录为cDNA 待用,根据四季蜜杧MiOFP1基因序列设计荧光定量PCR引物qOFP1 F(5’CTGTACCTGCCGTGCTACAA 3’)、qOFP1 R(5’CTGTACCTGCCGTGCTACAA 3’)。以杧果MiActin1为内 参 基 因,引 物 为qActin F(5’CCGAGACATGAAGGAGAAGC 3’)、qActin R(5’GTGGTCTCATGGATACGAGCA 3’)。实时荧光定量PCR 仪器为ABI7500。扩增反应体系和程序参照试剂盒SYBR Premix Dimer Eraser(TaKaRa)说明书进行[12]。
1.2.3 拟南芥转化与成花表型分析 实验室前期已构建好四季蜜杧MiOFP1 的超量表达载体,命名为pBI121-MiOFP1 载体,转化EHA105 感受态。通过花序侵染法转化哥伦比亚野生型拟南芥[13]。利用抗生素筛选和PCR 技术鉴定阳性植株。以T3 代纯合植株为试验材料进行相关试验。成花表型观察:利用半定量技术鉴定杧果MiOFP1在转基因拟南芥中的表达水平,并观察转基因拟南芥的抽薹时间和第一朵花开放的时间。通过荧光定量技术,采集抽薹期拟南芥叶片,检测内源成花基因的表达水平,以拟南芥AtActin2 为内参基因,引物为AtActin2 F(5’-GCAGAGCGGGAAATTGTAAG-3’)、AtActin2 R(5’-GGATATCAGGAAGGATCTGTAC-3’),AtFLC F(5’-ATCATCATGTGGGAGCAGAAG-3’)、AtFLC R(5’-TTCAACCGCCGATTTAAGG-3’),AtFT F(5’-CTTGGCAGGCAAACAGTGTATGCAC-3’)、AtFT R(5’-GCCACTCTCCCTCTGACAATTGTAGA-3’),检测方法参考课题组研究报道[14]。
1.2.4 转基因拟南芥响应植物激素ABA 试验 萌发率测定:将转基因拟南芥T3代纯合株系种子播种于含0、2、5 μmol·L-1ABA 的1/2 MS 固体培养基上,每个处理3次重复,以胚根伸出为萌发依据,每12 h记录一次萌发率,10 d 后拍照记录。根长测定:将T3 代纯合株系种子播种于1/2 MS 固体培养基上培养,5 d 后移至含0、5、10 μmol·L-1ABA 的1/2 MS 固体培养基上继续培养,每个处理3 次重复,移栽5 d后拍照并统计根长。生理指标测定:播种9 d后移栽幼苗到穴盆中,培养12 d后进行20 μmol·L-1ABA喷施处理,以清水处理为对照,处理1 d后采集拟南芥叶片,每个处理3次重复,采用试剂盒检测脯氨酸含量和过氧化物酶活性(北京索莱宝科技有限公司);提取ABA 处理组及对照组拟南芥叶片的RNA,逆转录后通过荧光定量技术检测参与拟南芥ABA响应的内源基因表达量,引物为AtABI1 F(5’-GTTTTCCCGTCTCACATCTTCGT-3’)、AtABI1 R(5’-CTTCATCCGTCA TTACATCCCAA-3’),At-MFT F(5’-CGAGCCGAACATGAGAGAAT-3’)、At-MFT R(5’- AAGTATCTCTTTTCCTCTTGAGGG-3’)、AtDREB2B F(5’-CATCAGAGCCAAGACCAAAACC-3’)、AtDREB2B R(5’-TGTAGGACCATTGCCTCAGAAC-3’)[15]。
1.3 数据分析
将所得数据采用Excel 进行数据处理及图片制作,利用IBM SPSS 22.0 软件进行方差分析。
2 结果与分析
2.1 MiOFP1基因启动子序列分析
对四季蜜杧MiOFP1的启动子序列(2000 bp)进行顺式作用元件预测,发现MiOFP1启动子中存在的激素响应元件有:1个乙烯响应元件,5个ABA响应元件,7个SA响应元件,9个GA响应元件;逆境响应元件有:1个盐响应元件,4个脱水响应元件,9个MYC转录因子和10个MYB转录因子结合位点(图1)。
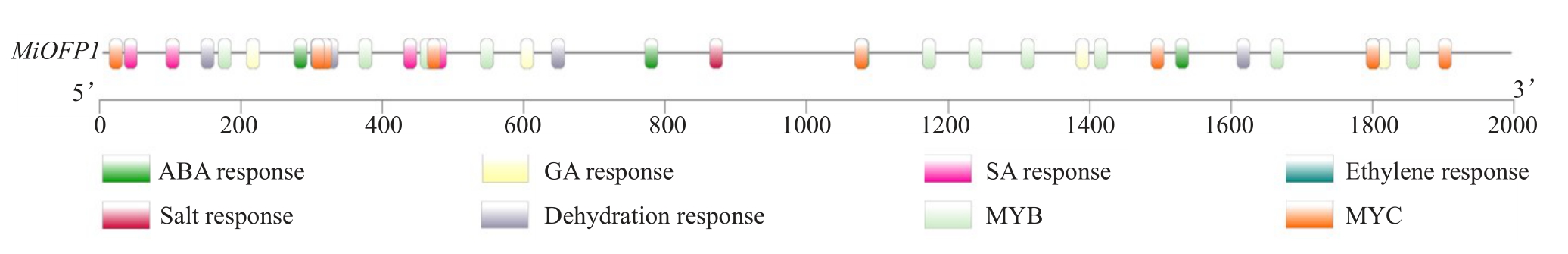
图1 四季蜜杧MiOFP1 启动子主要顺式作用元件
Fig.1 Cis-elements analysis of MiOFP1 promoter in SiJiMi mango
2.2 MiOFP1表达模式分析
对MiOFP1在杧果不同生长发育周期的不同组织器官中的表达模式进行实时荧光定量分析。结果如图2-A 所示,MiOFP1 存在组织表达差异性。MiOFP1在童期树组织中的表达水平高于成年期树。MiOFP1在茎的韧皮部中表达水平较高,在叶、花和幼果中表达水平最低,在成熟果实中表达水平最低。不同成花发育时期叶片中的表达模式分析显示,MiOFP1在营养生长期的叶片中表达水平较高,在成花诱导期和花发育期的叶片中表达水平较低,而后在果实发育期的叶片中表达水平逐步上升(图2-B)。

图2 四季蜜杧MiOFP1 基因的表达模式分析
Fig.2 Expression pattern analysis of MiOFP1 gene of SiJiMi
A.四季蜜杧童期和成年期不同组织中MiOFP1 基因表达模式;B.四季蜜杧不同发育时期叶片中MiOFP1 基因表达模式。字母表示差异显著水平(p<0.05)。
A.Expression pattern of MiOFP1 in different tissues of SiJiMi;B.Expression pattern of MiOFP1 in leaf of SiJiMi at different developmental stages.Letters indicate the level of significant difference(p<0.05).
2.3 拟南芥转化及成花表型分析
将包含pBI121-MiOFP1 超量表达载体和空载体的农杆菌通过花序侵染法分别转化模式植物拟南芥。半定量PCR 检测结果显示,转基因株系MiOFP1#7、MiOFP1#8 和MiOFP1#9 中MiOFP1 正常表达,而在对照植株中没有表达(图3-B)。超量表达MiOFP1 转基因株系表现出晚花表型,其抽薹时间和第一朵花开放时间均比WT和转空载体植株(pBI121)晚2~4 d(图3-A、C)。转基因拟南芥内源成花基因表达水平检测显示,超量表达MiOFP1 显著提高了拟南芥AtFLC的表达水平,显著下调了At-FT的表达水平(图3-D)。

图3 MiOFP1 转基因对拟南芥成花的影响
Fig.3 Effect of MiOFP1 transgene on flowering of Arabidopsis thaliana
A.超量表达MiOFP1 转基因拟南芥植株表型;B.半定量检测外源基因MiOFP1 在转基因和对照植物叶片中的表达;C.超量表达MiOFP1转基因拟南芥抽薹时间和开花时间;D.拟南芥叶片中内源AtFLC 和AtFT 基因表达水平检测。字母表示差异显著水平(p<0.05)。
A.Overexpression of MiOFP1 transgenic Arabidopsis plant phenotypes;B.The expression of exogenous genes MiOFP1 verified by semi-quantitative PCR in the leaf of transgenic plant;C.The bolting time and flowering time of MiOFP1 transgenic Arabidopsis;D.The expression of endogenous genes about AtFLC and AtFT were verified by quantitative RT-PCR in the leaf of transgenic plant.Letters indicate the level of significant difference(p<0.05).
2.4 外源ABA 处理对MiOFP1 转基因拟南芥的影响
2.4.1 外源ABA 处理后MiOFP1 转基因拟南芥种子萌发表型分析 T3 代纯合拟南芥种子在0、2、5 μmol·L-1 ABA的1/2 MS固体培养基上培养,记录发芽情况。ABA 处理10 d 后拍照记录如图4-A 所示,在未添加ABA 的培养基上,拟南芥幼苗长势基本一致;在添加ABA 的培养基中,所有种子发芽均受抑制,种子在含5 μmol·L-1ABA 的1/2 MS 固体培养基上的长势较在含2 μmol·L-1ABA的1/2 MS固体培养基上更弱。萌发率统计分析显示(图4-B),未添加ABA时,前60 h转基因拟南芥种子的萌发率略高于WT种子,但差异不显著;在2 μmol·L-1 ABA 处理时,所有种子萌发均受到抑制,转基因拟南芥种子萌发速度明显快于WT 种子;在5 μmol·L-1 ABA 处理时,种子萌发受到的抑制更强,转基因拟南芥种子的萌发率极显著高于对照植株,最终的转基因拟南芥种子发芽率分别为84%、88%、77%,WT种子发芽率为65%。

图4 ABA 处理对MiOFP1 转基因拟南芥种子萌发率的影响
Fig.4 Effect of ABA treatment on seed germination rate of MiOFP1 transgenic Arabidopsis thaliana
A.超量表达MiOFP1 的转基因拟南芥种子在0、2、5 μmol·L-1 ABA 处理下的萌发表型;B.超量表达MiOFP1 的转基因拟南芥种子在0、2、5 μmol·L-1ABA 处理下的萌发率统计数据。
A.The germination phenotype of transgenic Arabidopsis seeds overexpressing MiOFP1 under ABA treatment;B.The germination rate of transgenic Arabidopsis seeds overexpressing MiOFP1 under ABA treatment.
2.4.2 外源ABA 处理对超量表达MiOFP1 拟南芥根长、生理指标与内源基因表达的影响 不同浓度的ABA处理对T3代纯合拟南芥幼苗根长的影响如图5-A,在未施加ABA 时,转基因拟南芥与对照拟南芥的根长无显著差异,随着ABA 浓度的增加,转基因拟南芥与对照拟南芥幼苗的根长均表现为受抑制,但在相同处理下,对照植株根长显著短于转基因植株的根长(图5-B)。
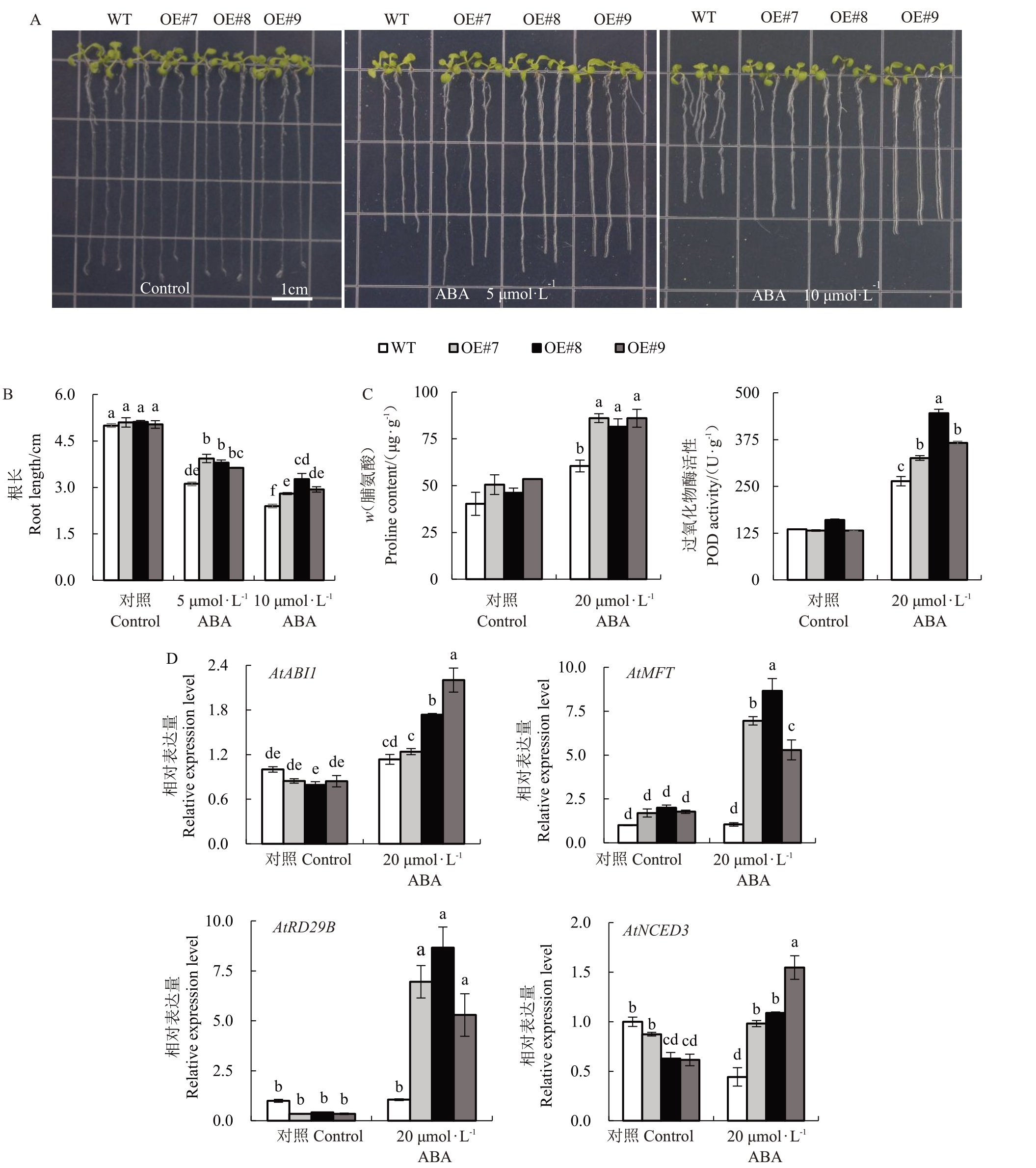
图5 ABA 处理对MiOFP1 转基因拟南芥的影响
Fig.5 Effect of ABA treatment on MiOFP1 transgenic Arabidopsis thaliana
A.WT 和OE 植株在0、5、10 μmol·L-1ABA 处理下的根长表型;B.WT 和OE 植株在0、5、10 μmol·L-1ABA 处理下的根长统计数据;C.WT和OE 植株经0、20 μmol·L-1ABA 喷施处理后的脯氨酸含量及过氧化物酶活性;D.WT 和OE 植株经0、20 μmol·L-1ABA 喷施处理后的内源基因AtABI1、AtMFT、AtRD29B 和AtNCED3 表达水平。字母表示差异显著水平(p<0.05)。
A.The root length phenotype of transgenic Arabidopsis overexpressing MiOFP1 under ABA treatment;B.The root length of transgenic Arabidopsis seeds overexpressing MiOFP1 under ABA treatment;C.The proline content and POD activity of MiOFP1 overexpression Arabidopsis;D.Expression levels of the ABA stress-related genes AtABI1,AtMFT,AtRD29B and AtNCED3 in 30 d old WT and transgenic plants.Letters indicate the level of significant difference(p<0.05).
对转基因植株与对照WT植株进行20 μmol·L-1 ABA喷施处理,以清水喷施处理为对照,处理后1 d采集叶片为样品,对处理组与对照组植株进行生理指标测定和拟南芥内源基因表达水平检测。如图5-C所示,清水对照处理下,转基因植株与WT植株的脯氨酸含量和过氧化物酶活性无明显差异,但在ABA 处理后,转基因植株及WT 植株的脯氨酸含量均有增加,且转基因植株脯氨酸含量显著高于WT植株,是WT 植株的1.4 倍;在ABA 处理后,转基因植株的过氧化物酶活性显著高于对照WT植株。
内源基因检测显示,清水处理时,MiOFP1转基因株系中AtABI1、AtRD29B和AtNCED3的表达水平略低于WT 株系,而AtMFT 的表达水平略高于WT植株。在ABA处理后,上述4个基因的表达水平均显著高于WT植株(图5-D)。
3 讨 论
启动子顺式作用元件关系着植物对激素、低温、盐和干旱等非生物胁迫作出反应,非生物胁迫与激素均可诱导相关转录因子结合到下游基因启动子顺式作用元件上,从而诱导其表达[16-17],MiOFP1 前2000 bp 启动子中包含大量激素响应元件和逆境胁迫响应元件,推测MiOFP1可能受到激素、非生物胁迫以及上游MYB、MYC 转录因子的调控和诱导。组织表达分析表明,MiOFP1具有组织表达特异性,主要在童期树和成年期树的茎中表达,在花、叶、果实和胚中表达量相对较低。此前的研究发现,水稻OsOFP在种子发育时期表达量较高[18],葡萄VvOFP在开花前表达量较高,开花一段时间后,表达量显著下降[19],小麦TaOFP 在根和穗中表达量最高[20]。对比本研究结果,可以推测,OFP 参与多个植物生长发育的过程,但是不同物种OFP主要发挥的功能和表达的位置可能不同。此外,对过表达MiOFP1 拟南芥进行表型观察,发现抽薹时间和开花时间有显著延迟,这与拟南芥OFP1 功能类似,AtOFP1 在拟南芥中过表达出现延迟开花现象[21]。内源基因表达分析结果表明,在过量表达MiOFP1 的拟南芥株系中,成花抑制基因FLC 的表达得到促进,成花促进基因FT 的表达受到抑制,从而使植物开花时间延迟,这证明了MiOFP1 对转基因拟南芥的开花起到抑制作用。研究发现,过量表达SlOFP20 的番茄开花时间明显推迟,SlOFP20 转基因植株的SlFT 表达水平显著低于WT[22]。目前尚未见有OFP在杧果中是否同样具有延迟开花的功能报道,但过量表达MiOFP1的拟南芥植株与过量表达番茄SlOFP20的植株均出现延迟开花现象,且植株内FT的表达量均显著降低,表明二者可能通过相同或相似的途径调控FT 的表达,从而实现对植株开花时间的调控,综上所述,推测MiOFP1通过影响FLC和FT的表达水平来调节杧果开花时间。
已有研究表明,除参与植物生长发育外,OVATE 家族的多数基因还参与植物激素调节及逆境胁迫响应过程。外源施加GA3可以减缓AtOFP1超表达株系的矮化现象;水稻OFP1 通过与GSK2、OsBZR1 和DLT 的相互作用来正调控BR 响应;Os-OFP6-RNAi 转基因株系表现出正常生长条件下侧根变短和IAA 处理后侧根密度增加[22];过表达AtOFP8 显著增强了拟南芥的抗旱性,其种子的发芽率和绿叶率更高,叶片中脯氨酸含量提高,丙二醇含量降低,可溶性蛋白含量高,叶片抗氧化酶的活性较强[23];对去除水稻OsOFP6 的植株进行干旱和低温处理,明显发现植株缺水且相对电导率升高的现象,说明OsOFP6 参与水稻对干旱和低温胁迫的防御过程[24];苹果OFP 家族基因在盐、干旱和高低温等非生物逆境下呈现出不同的响应差异,尤其是在低温下MdOFP 基因家族中多个基因的表达量均有不同程度的上升[25];同样的,低温条件下,油桐幼苗VfOFP3/7/10/12 等基因的表达量上升,推测VfOFP 可能在油桐低温胁迫响应方面发挥积极的调节作用[26]。ABA 是种子萌发和大多数常见非生物胁迫反应的主要介质之一,包括对盐、干旱和寒冷的反应[27-28]。与野生型植物相比,MiOFP1的超量表达减轻了ABA 对种子萌发的抑制作用和根长敏感性,提高了外源ABA 情况下植株的脯氨酸含量和过氧化物酶活性,促进了ABA 响应基因ABI1、MFT 和RD29B 以 及ABA 合 成 基 因NCED3 的 表达。植物在受到胁迫时,细胞的渗透压发生变化,脯氨酸含量会升高以维持细胞渗透压;同时,细胞内产生大量的过氧化氢及酚类、胺类等物质,对细胞造成损害,过氧化物酶可催化过氧化氢及酚类、胺类等物质,达到减轻上述物质对细胞的毒害作用,通过外源喷施ABA,提高植物体内的脯氨酸含量和过氧化物酶活性,从而增强植物抵御非生物胁迫的能力[29-30];ABI1 在ABA 的信号转导过程中起负调控作用[31],MFT 直接抑制ABI5的表达,对ABA 信号通路起负反馈调节作用[32],外源ABA 处理明显诱导RD29B 表达量升高[33]。这些发现表明,MiOFP1 在杧果开花和外源ABA 响应中可能具有关键的作用,推测杧果MiOFP1 可能是通过介导ABA 的信号通路参与种子萌发调控过程和逆境防御过程,但是MiOFP1 是如何参与ABA 信号通路的、MiOFP1 是否影响杧果开花、能否通过提高ABA 含量来增强杧果逆境抵御能力还不清楚,需要进一步研究。
4 结 论
杧果MiOFP1在童期茎的韧皮部组织中高度表达,而在成熟果实的果肉中表达水平较低;在营养生长期叶片中表达水平较高,而在成花期表达水平较低。超量表达的MiOFP1 抑制拟南芥开花,但提高了种子在ABA 处理下的萌发率,减弱了拟南芥对ABA 胁迫的敏感性。说明MiOFP1 可能与杧果的成花和ABA胁迫有关。
[1]LIU J P,VAN ECK J,CONG B,TANKSLEY S D.番茄OVATE基因调控果实梨形发育[J].杨莉,张岚岚,徐昌杰,译.植物学通报,2003,38(1):127.LIU J P,VAN Eck J,CONG B,TANKSLEY S D. OVATE gene regulates pear shape development of tomato fruit[J].,YANG Li,ZHANG Lanlan,XU Changjie,Translated.Chinese Bulletin of Botany,2003,38(1):127.
[2]张静,谢碧玉,徐碧玉,刘菊华.卵形家族蛋白对植物生长发育的调控作用[J].分子植物育种,2019,17(10):3283-3288.ZHANG Jing,XIE Biyu,XU Biyu,LIU Juhua.Regulatory roles of ovate family proteins on plant growth and development[J].Molecular Plant Breeding,2019,17(10):3283-3288.
[3]HACKBUSCH J,RICHTER K,MÜLLER J,SALAMINI F,UHRIG J F.A central role of Arabidopsis thaliana ovate family proteins in networking and subcellular localization of 3-aa loop extension homeodomain proteins[J].Proceedings of the National Academy of Sciences of the United States of America,2005,102(13):4908-4912.
[4]李晓.水稻OsOFP22 转录因子功能分析[D].长春:吉林大学,2017.LI Xiao.The functional analysis of OsOFP22 transcription factor[D].Changchun:Jilin University,2017.
[5]陈浩源.OsOFP22 通过赤霉素和油菜素甾醇调控水稻株型和粒型的机制分析[D].长春:吉林大学,2021.CHEN Haoyuan.Mechanism analysis of OsOFP22 regulating plant morphology and grain shape by gibberellin and brassinosteroid in rice (Oryza sativa L.)[D].Changchun:Jilin University,2021.
[6]ZHOU S G,CHENG X,LI F F,FENG P P,HU G L,CHEN G P,XIE Q L,HU Z L.Overexpression of SlOFP20 in tomato affects plant growth,chlorophyll accumulation,and leaf senescence[J].Frontiers in Plant Science,2019,10:1510.
[7]SHAN Y J,LI D,CAO J J,ZHANG L,HAN L Q,ZHANG M P,SHEN Z G.Over-expression of Arabidopsis ORANGE gene enhances drought stress tolerance through ABA-dependent pathway in Arabidopsis thaliana[J].Plant Growth Regulation,2022,96(1):91-101.
[8]梁潇,刘丹梅,姜兆彤,刁玉峰,刘文璐,袁珺.‘红颜’草莓栽培种FaNCED4 基因克隆与胁迫表达[J/OL].分子植物育种,2022:1-10[2022-07-10].http://kns.cnki.net/kcms/detail/46.1068.S.20220613.1150.010.html.LIANG Xiao,LIU Danmei,JIANG Zhaotong,DIAO Yufeng,LIU Wenlu,YUAN Jun.Cloning and expression of FaNCED4 gene in‘Benihoppe’strawberry cultivars under stress[J/OL].Molecular Plant Breeding,2022:1-10[2022-07-10].http://kns.cnki.net/kcms/detail/46.1068.S.20220613.1150.010.html.
[9]罗忍忍,王瑞丹,曹磊,李丽丽,李翔,袁烨,晏家茹,侯娟,胡建斌.植物生长调节剂对冷胁迫下甜瓜幼苗生理特性及相关基因表达的影响[J].河南农业大学学报,2022,56(3):411-419.LUO Renren,WANG Ruidan,CAO Lei,LI Lili,LI Xiang,YUAN Ye,YAN Jiaru,HOU Juan,HU Jianbin.Effects of plant growth regulators on physiological characteristics and related gene expression in melon seedlings under cold stress[J].Journal of Henan Agricultural University,2022,56(3):411-419.
[10]李振华,刘容,张馨馨,赵心笛,刘敏婷,柴琦.外源脱落酸增强高羊茅耐盐性的作用[J].北方园艺,2022(7):66-75.LI Zhenhua,LIU Rong,ZHANG Xinxin,ZHAO Xindi,LIU Minting,CHAI Qi.Effects of exogenous abscisic acid on enhancing salt tolerance of Festuca arundinacea[J].Northern Horticulture,2022(7):66-75.
[11]卢琳.OsOFP19 过表达转基因水稻农艺性状与干旱响应的鉴定与分析[D].长春:吉林大学,2022.LU Lin.Identification and analysis of agronomic traits and drought response of OsOFP19-overexpressing transgenic rice[D].Changchun:Jilin University,2022.
[12]范志毅,罗聪,余海霞,曾学梅,王金英,谢小杰,何新华.芒果MiFY 基因克隆和表达模式分析[J].热带作物学报,2021,42(2):297-302.FAN Zhiyi,LUO Cong,YU Haixia,ZENG Xuemei,WANG Jinying,XIE Xiaojie,HE Xinhua.Cloning and expression analysis of a MiFY gene in mango[J].Chinese Journal of Tropical Crops,2021,42(2):297-302.
[13]GUO Y H,LUO C,LIU Y,LIANG R Z,YU H X,LU X X,MO X,CHEN S Q,HE X H.Isolation and functional analysis of two CONSTANS-like 1 genes from mango[J].Plant Physiology and Biochemistry,2022,172:125-135.
[14]MO X,LUO C,YU H X,CHEN J W,LIU Y,XIE X J,FAN Z Y,HE X H.Isolation and functional characterization of two SHORT VEGETATIVE PHASE homologous genes from mango[J].International Journal of Molecular Sciences,2021,22(18):9802.
[15]ARIF M,LI Z T,LUO Q,LI L H,SHEN Y Q,MEN S Z.The BAG2 and BAG6 genes are involved in multiple abiotic stress tolerances in Arabidopsis thaliana[J].International Journal of Molecular Sciences,2021,22(11):5856.
[16]杨天宸,陈晓童,吕可,张荻.百子莲脱水素基因ApSK3对逆境与激素信号的应答模式与调控机制[J].园艺学报,2021,48(8):1565-1578.YANG Tianchen,CHEN Xiaotong,LÜ Ke,ZHANG Di.Expression pattern and regulation mechanism of ApSK3 dehydrin(Agapanthus praecox) response to abiotic stress and hormone signals[J].Acta Horticulturae Sinica,2021,48(8):1565-1578.
[17]李世琦.巨球百合细胞壁转化酶基因及其启动子功能初探[D].杭州:浙江大学,2021.LI Shiqi.A preliminary study on the function research of cell wall invertase gene and its promoter of Lilium brownii var. giganteum[D].Hangzhou:Zhejiang University,2021.
[18]于慧.水稻OsOFP 转录因子家族基因克隆与功能分析[D].长春:吉林大学,2015.YU Hui.The genes cloning and functional analysis of OsOFP transcription factor family in rice (Oryza sativa L.)[D].Changchun:Jilin University,2015.
[19]袁月,张亚光,高世敏,陶建敏.葡萄OVATE 基因家族生物信息学及表达[J].中国农业科学,2016,49(19):3786-3797.YUAN Yue,ZHANG Yaguang,GAO Shimin,TAO Jianmin.Bioinformatics and expression of the OVATE gene family in grape[J].Scientia Agricultura Sinica,2016,49(19):3786-3797.
[20]徐渴,王伟伟,武宁静,张树华,赵勇,杨学举.小麦OFP 基因家族鉴定及其低温胁迫的表达分析[J].农业生物技术学报,2021,29(9):1665-1677.XU Ke,WANG Weiwei,WU Ningjing,ZHANG Shuhua,ZHAO Yong,YANG Xueju.Identification of OFP gene family and their expression analysis under low-temperature stress in wheat (Triticum aestivum)[J].Journal of Agricultural Biotechnology,2021,29(9):1665-1677.
[21]蔡玲.卵形蛋白家族转录因子AtOFP15 对拟南芥荚果形态的调控[D].长春:东北师范大学,2017.CAI Ling.Regulation of silique morphology by ovate family protein AtOFP15 in Arabidopsis[D].Changchun:Northeast Normal University,2017.
[22]周升恩.两个番茄转录因子SlOFP20 和SlGT16 在生长发育中的功能研究[D].重庆:重庆大学,2020.ZHOU Sheng’en.Functional study of two tomato transcription factors SlOFP20 and SlGT16 genes in growth and development[D].Chongqing:Chongqing University,2020.
[23]唐尧.拟南芥转录因子AtOFP8 在干旱胁迫下的功能分析[D].哈尔滨:东北农业大学,2018.TANG Yao.Functional analysis of Arabidopsis transcriptional factor AtOFP8 under drought stress[D].Harbin:Northeast Agricultural University,2018.
[24]MA Y M,YANG C,HE Y,TIAN Z H,LI J X.Rice OVATE family protein 6 regulates plant development and confers resistance to drought and cold stresses[J].Journal of Experimental Botany,2017,68(17):4885-4898.
[25]许瑞瑞,李睿,王小非,郝玉金.苹果OFP 基因家族的全基因组鉴定与非生物逆境表达分析[J].中国农业科学,2018,51(10):1948-1959.XU Ruirui,LI Rui,WANG Xiaofei,HAO Yujin.Identification and expression analysis under abiotic stresses of OFP gene family in apple[J].Scientia Agricultura Sinica,2018,51(10):1948-1959.
[26]王静雅,刘文娟,吕祎馨,尚海,米小琴,张琳,刘美兰.油桐OFP 基因家族的全基因组鉴定及低温响应分析[J].植物生理学报,2020,56(5):949-960.WANG Jingya,LIU Wenjuan,LÜ Yixin,SHANG Hai,MI Xiaoqin,ZHANG Lin,LIU Meilan.Genome-wide identification and low temperature response analysis of OFP gene family in tung tree (Vernicia fordii)[J].Plant Physiology Journal,2020,56(5):949-960.
[27]FUJII H,VERSLUES P E,ZHU J K. Arabidopsis decuple mutant reveals the importance of SnRK2 kinases in osmotic stress responses in vivo[J].Proceedings of the National Academy of Sciences of the United States of America,2011,108(4):1717-1722.
[28]NISHIMURA N,YOSHIDA T,KITAHATA N,ASAMI T,SHINOZAKI K,HIRAYAMA T.ABA- Hypersensitive Germination1 encodes a protein phosphatase 2C,an essential component of abscisic acid signaling in Arabidopsis seed[J].The Plant Journal,2007,50(6):935-949.
[29]符杨磊,魏志园,王宇,刘潇阳,王冰冰,乔亚科,李桂兰,张锴.冀东野生大豆(Glycine soja)耐盐碱性鉴定及耐性生理指标测定[J].核农学报,2020,34(10):2316-2325.FU Yanglei,WEI Zhiyuan,WANG Yu,LIU Xiaoyang,WANG Bingbing,QIAO Yake,LI Guilan,ZHANG Kai.Identification of saline-alkali stress and determination of physiological index of wild soybean (Glycine soja) in eastern Hebei Province[J].Journal of Nuclear Agricultural Sciences,2020,34(10):2316-2325.
[30]汤日圣,唐现洪,钟雨,张大栋,余永柱,童红玉.微生物源ABA 对茄苗抗冷性和某些生理指标的影响[J].园艺学报,2006,33(1):149-151.TANG Risheng,TANG Xianhong,ZHONG Yu,ZHANG Dadong,YU Yongzhu,TONG Hongyu.Effect of microorganismsourced ABA on chilling resistance and some physiological indexes in eggplant seedling[J].Acta Horticulturae Sinica,2006,33(1):149-151.
[31]SEO Y J,PARK J B,CHO Y J,JUNG C,SEO H S,PARK S K,NAHM B H,SONG J T.Overexpression of the ethylene-responsive factor gene BrERF4 from Brassica rapa increases tolerance to salt and drought in Arabidopsis plants[J].Molecules and Cells,2010,30(3):271-277.
[32]韩萍萍.藜麦PEBP 基因家族及种子休眠相关基因功能分析[D].济南:山东师范大学,2021.HAN Pingping.Function analysis of quinoa PEBP family gene and seed dormancy related genes[D].Jinan:Shandong Normal University,2021.
[33]MSANNE J,LIN J S,STONE J M,AWADA T.Characterization of abiotic stress-responsive Arabidopsis thaliana RD29A and RD29B genes and evaluation of transgenes[J].Planta,2011,234(1):97-107.