桃(Prunus persica)原产中国,自1993年起中国桃的栽培面积和产量全面超过意大利和美国,成为世界第一产桃大国,并一直保持至今[1]。桃因皮薄肉厚、味道鲜美而广受消费者喜爱[2]。与柑橘、苹果、梨等水果不同,大多数桃果实成熟后迅速软化,留树时间和货架期短,若提前采收又会导致果实品质下降,影响消费体验。此外,随着桃产业向集约化方向的发展,采后远距离运输成为常态,软溶质桃不耐贮运的短板很大程度上限制了该种肉质类型桃的发展。
质地影响桃果实留树期和贮运性。目前桃果肉质地性状主要包括:溶质(melting flesh,MF)、不溶质(non-melting flesh,NMF)、硬 质(stony hard)等[3]。不溶质桃成熟后果实有韧性,留树时间较长,传统上多用于加工罐头,当前也有部分品种进入鲜食市场。硬质桃果实成熟后不释放乙烯,果实保持硬脆,留树时间2 周以上,具备优异的采后贮运特性[4],但该种类型果实成熟后不变软,伴随着没有果实成熟期桃特征性香气,因此也有一些局限性。此外,国外最早于2011 年报道了油桃品种Big Top 独特的软化特征,这种肉质虽然最终会释放乙烯使果实变软,但果实在成熟前期能保持较高的硬度,具有较长的留树期和货架期[5-6],并将这一质地类型分类为慢溶质桃(slow-melting flesh,SMF)。Chen等[7]对慢溶质单株S11-8 与非慢溶质单株M12-10 成熟过程硬度变化规律进行比较,发现S11-8 软化速度比M12-10 慢得多,而且S11-8 完全软化的发生相对较晚。Ghiani等[6]通过比较慢溶质型桃Big Top和溶质型桃Bolero 采收后硬度和乙烯的变化,发现果实成熟期的Big Top果实硬度比Bolero明显更硬,采后室温放置5 d 后果实变软,最终果实硬度与Bolero 类似。在果实成熟期采收后,Bolero桃迅速释放乙烯,而Big Top桃果实采收后4 d才开始释放乙烯。对于慢溶质性状定位的研究也有一些报道,但由于肉质判别标准的缺乏导致基因定位困难,已发表的定位结果表现出较大的差异,相关分子标记仍未能实践应用[7-9]。
慢溶质桃成熟后既有较长的留树时间和货架期,又能够保持桃果实的特殊风味,已成为欧美国家桃肉质改良和发展的主要方向,但是,慢溶质桃在中国相对较晚才被利用,遗传资源匮乏,相关机制研究甚少。笔者在本研究中以春雪、春瑞等慢溶质桃品种为材料,通过构建分离群体,对后代表型进行分析,明确慢溶质性状的遗传倾向,利用BSA 分析对该性状进行初步定位。研究结果初步揭示了桃果实慢溶质肉质类型的生理特征、遗传倾向及初步定位,为今后慢溶质候选基因的挖掘以及调控机制的研究奠定基础。
1 材料和方法
1.1 试验材料
以SMF品种春雪、春瑞为主要亲本构建遗传群体,具体信息见表1。对杂交单株的肉质鉴定于2021年5—8月和2022年5—8月在中国农业科学院郑州果树研究所新乡试验基地的杂种苗定植圃内进行,株行距为1.0 m×4.0 m,2017 年定植。慢溶质桃软化特点分析所用品种为春丽、春瑞,非慢溶质分析所用品种为春美、春蜜,均来自中国农业科学院郑州果树研究所桃育种圃。
表1 遗传群体信息
Table 1 The information of crosses used in this study
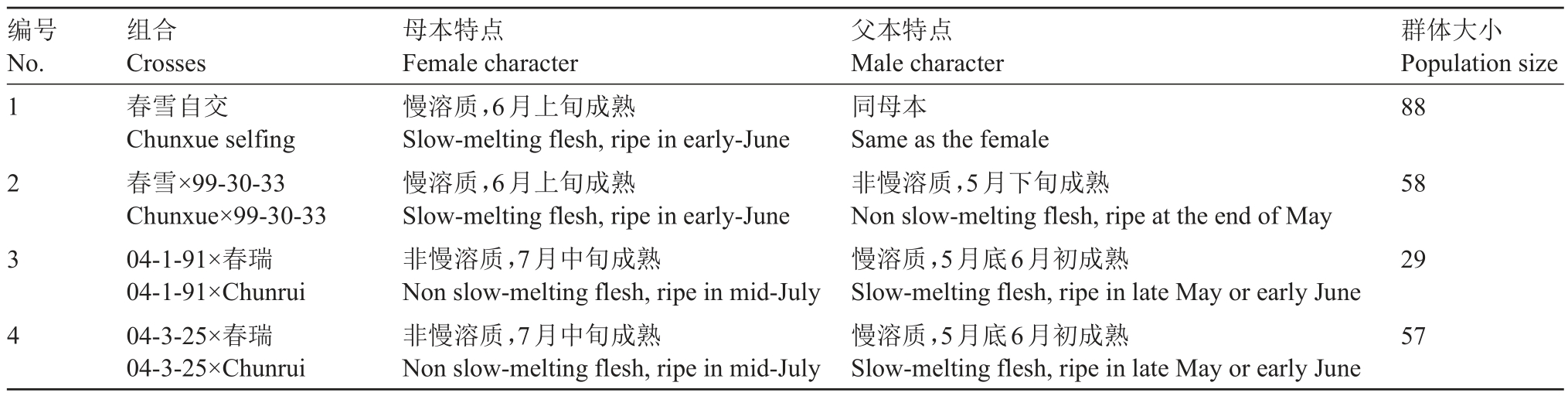
编号No.1 2 3 4组合Crosses春雪自交Chunxue selfing春雪×99-30-33 Chunxue×99-30-33 04-1-91×春瑞04-1-91×Chunrui 04-3-25×春瑞04-3-25×Chunrui母本特点Female character慢溶质,6月上旬成熟Slow-melting flesh,ripe in early-June慢溶质,6月上旬成熟Slow-melting flesh,ripe in early-June非慢溶质,7月中旬成熟Non slow-melting flesh,ripe in mid-July非慢溶质,7月中旬成熟Non slow-melting flesh,ripe in mid-July父本特点Male character同母本Same as the female非慢溶质,5月下旬成熟Non slow-melting flesh,ripe at the end of May慢溶质,5月底6月初成熟Slow-melting flesh,ripe in late May or early June慢溶质,5月底6月初成熟Slow-melting flesh,ripe in late May or early June群体大小Population size 88 58 29 57
1.2 试验方法
1.2.1 自交 选择生长健壮的成年树作母本树,于蕾期对全株进行套袋,防止异花授粉,2周后去除纸袋。其他管理按常规。
1.2.2 杂交 选择生长健壮的成年树作母本,于大蕾期去雄,点授父本花粉,套袋,10 d后去袋,其他管理按常规。
1.2.3 果实生理指标测定 果实成熟阶段的样品,果实达到八成熟开始采集,每3 d 取样1 次,每次20个果实。采样时间点设为R、R+3、R+6、R+9、R+12、R+15,代表八成熟后天数。果实采收后,立即带回实验室,选取大小均匀、成熟度一致、无病虫害和机械损伤的果实,用于软化生理特点分析。随机选取9个果实,在果实赤道处,削去缝合线两侧及对面部位的果皮,用水果硬度计GY-4 测定果实去皮硬度,每个果实的三个部位的平均值作为每个果实的果肉硬度,单位为牛顿(N)。乙烯测定参考Zeng 等[10]的方法:随机选取9 个果实,3 个为一组,置于密封的2.0 L保鲜罐中,密闭2 h,取1 mL气体,用气相色谱仪GC-2010(岛津,日本)测定乙烯释放量,单位为nL·g-1·h-1。气相色谱仪条件为:AI2O3/S 色谱柱(30 m×0.53 mm,0.25 μm);柱温50 ℃,SPL1检测室温度为200 ℃,FID1 检测室温度为200 ℃;载气氮气流速为32 mL·min-1,氢气流速为40 mL·min-1,空气流速为400 mL·min-1。
1.2.4 杂交群体后代果实性状调查 通过判断果实着色完全、果个已膨大到商品果大小、果皮底色已完成褪绿、品质风味已可食用,即果实成熟,之后每2 d调查1 次果实硬度,参考王力荣等[11]的方法将硬度划分为“很硬、硬、中、软、很软”5 个等级,统计杂种单株70%果实在成熟后变软之前的挂树时间,大于等于10 d为慢溶质,小于10 d为非慢溶质。
1.2.5 BSA 分析 在群体后代中分别选取非慢溶质(留树时间<6 d)或慢溶质(留树时间>14 d)各20株,组成2个极端性状混池(非慢溶质混池为NSMF pool,慢溶质混池为SMF pool),提取基因组DNA,委托北京百迈客生物科技有限公司进行重测序,全部样品的测序深度为30倍。参考基因组为桃Lovell(https://www.rosaceae.org/gb/gbrowse/prunus_persica_v2.0.a1/)。利用两混池间基因型存在差异的SNP、InDel 位点[12],统计差异位点在不同混池中的深度,并计算每个位点ED(欧氏距离,Euclidean Distance)值。为消除背景噪音,对原始ED 值进行乘方处理,笔者在本试验中取原始ED的5次方作为关联值以达到消除背景噪音的功能,然后采用DISTANCE方法[13]对ED值进行拟合。取所有位点拟合值的median+3SD 作为分析的关联阈值,计算得0.10,设定为筛选的阈值,阈值外的区域为潜在候选调控基因定位区域。
1.2.6 数据统计分析与作图 数据统计分析与作图采用微软Excel 2019。
2 结果与分析
2.1 慢溶质品种成熟过程中硬度变化与乙烯释放
为了显示慢溶质桃成熟过程中的软化特征,选择成熟期相近的两组慢溶质桃vs 非慢溶质桃—春丽vs春美(图1)和春瑞vs春蜜(图2)进行比较。结果显示,非慢溶质品种春美、春蜜果实硬度在成熟后9 d已降至最低,慢溶质品种春丽、春瑞果实在成熟后15 d才完全软化。非慢溶质桃春美和春蜜成熟阶段硬度保持在15 N以上的时间仅6 d;慢溶质桃春丽和春瑞至少12 d。因此,将果实成熟后硬度保持在15 N以上水平的时期定为果实留树时间,慢溶质桃留树时间(天数)是非慢溶质桃的2倍左右。慢溶质桃品种春丽在R+12 阶段之前乙烯释放量很低,春瑞在R+12阶段之前没有乙烯释放,慢溶质桃直到最终软化发生时才伴随乙烯大量释放;而非慢溶质桃品种在R+6阶段开始就产生很高的乙烯释放量。综上两组数据,慢溶质桃春瑞、春丽留树时间至少12 d,相较非慢溶质桃春美、春蜜长6 d,乙烯也相应延迟释放。
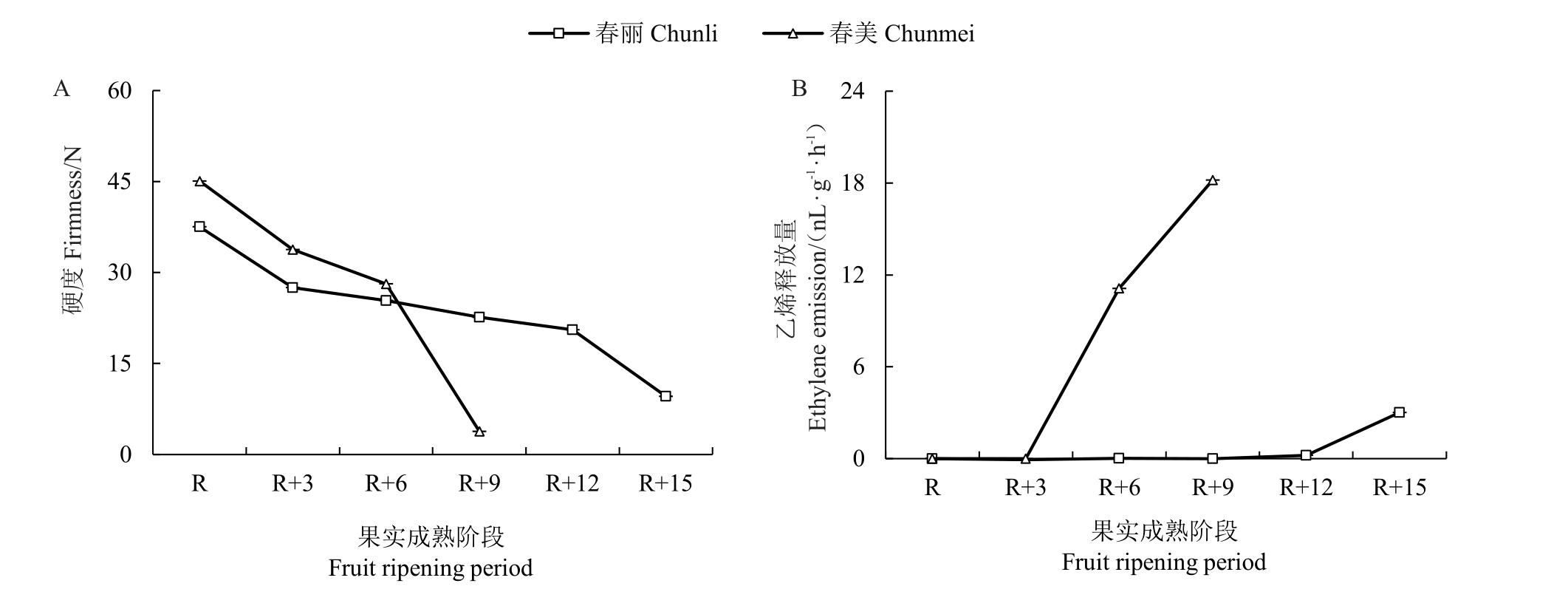
图1 慢溶质品种春丽和非慢溶质品种春美硬度、乙烯释放量的变化
Fig.1 Changes in firmness and ethylene emission in Chunli and Chunmei during fruit ripening
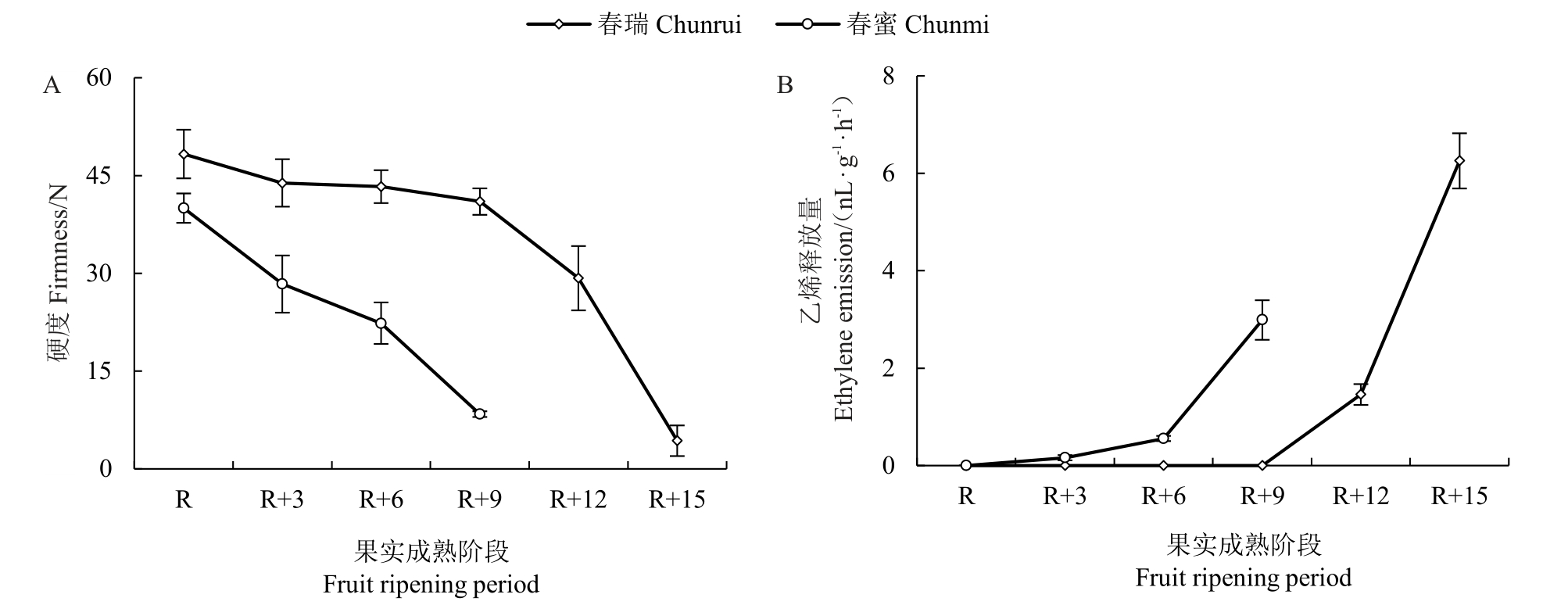
图2 慢溶质品种春瑞和非慢溶质品种春蜜硬度、乙烯释放量的变化
Fig.2 Changes in firmness and ethylene emission in Chunrui and Chunmi during fruit ripening
2.2 慢溶质群体后代肉质鉴定及遗传倾向
为了更高效快速鉴定群体后代表型,笔者对不同组合后代果实留树时间进行评价,判别果实肉质。对春雪自交群体进行表型评价,发现后代留树时间呈双峰分布,集中在<5 d 和≥10 d,中间类型6~9 d几乎没有(图3-A),慢溶质桃∶非慢溶质桃=67∶21。对春雪×99-30-33群体进行表型评价,发现后代留树时间呈双峰分布,集中在<5 d和≥10 d,中间类型6~9 d只有5个单株(图3-B),慢溶质桃∶非慢溶质桃=28∶33。对04-1-91×春瑞和04-3-25×春瑞进行表型评价,后代留树时间只有≥10 d一种类型,后代没有出现<10 d 的类型,群体后代全部为慢溶质桃类型(图3-C~D)。
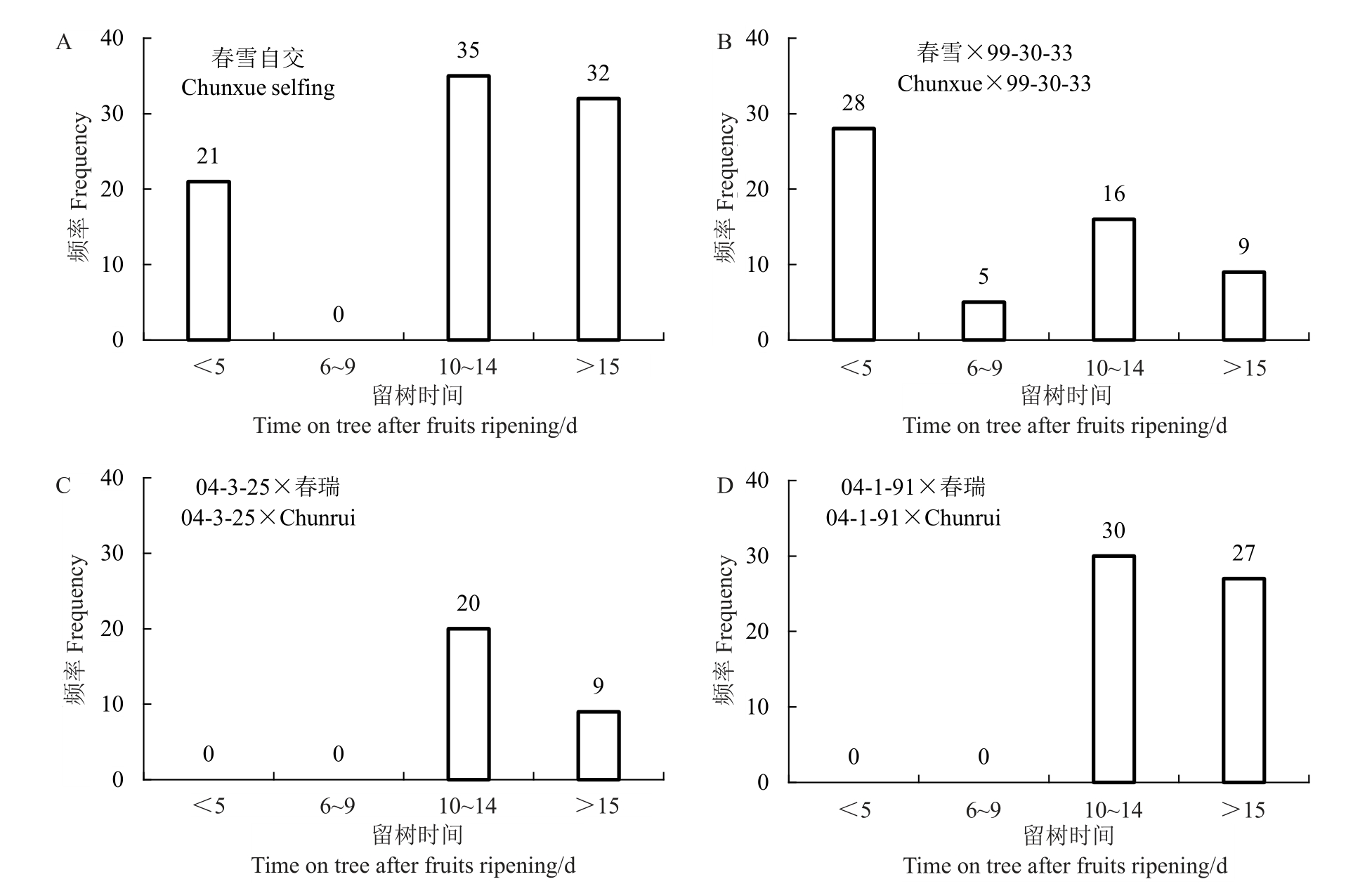
图3 不同杂交组合后代留树时间分布
Fig.3 Distribution of time on tree after fruits ripening within different crosses
春雪组合后代群体中肉质分离,春瑞组合后代中肉质不发生分离。因此,推测亲本慢溶质桃基因型春雪为杂合,春瑞为纯合。春雪自交群体慢溶质桃∶非慢溶质桃分离数据经卡方检验符合3∶1 孟德尔理论分离比例,春雪×99-30-33 群体慢溶质桃∶非慢溶质桃分离数据符合1∶1 的分离比例(表2),说明慢溶质桃性状的分离比例与显性单基因或主效基因调控性状的分离比例一致。
表2 慢溶质性状的遗传分析
Table 2 Inheritance of slow-melting flesh
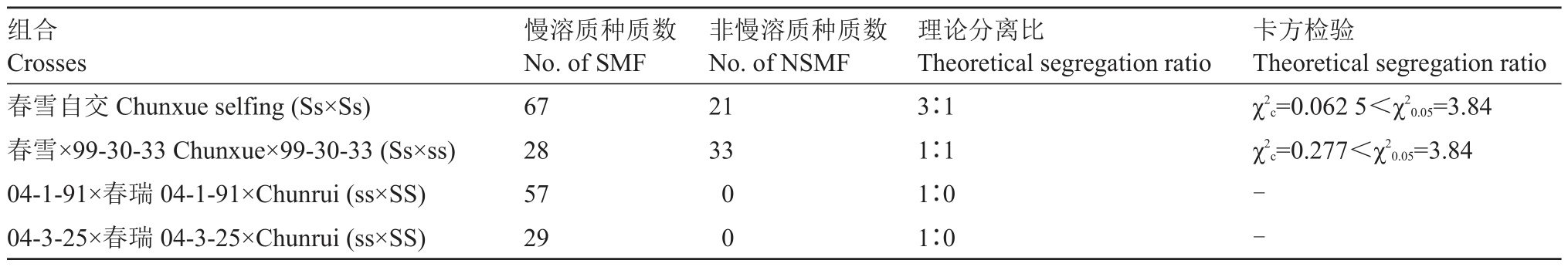
组合Crosses春雪自交Chunxue selfing(Ss×Ss)春雪×99-30-33 Chunxue×99-30-33(Ss×ss)04-1-91×春瑞04-1-91×Chunrui(ss×SS)04-3-25×春瑞04-3-25×Chunrui(ss×SS)慢溶质种质数No.of SMF 67 28 57 29非慢溶质种质数No.of NSMF 21 33卡方检验Theoretical segregation ratio χ2 c=0.062 5<χ2 0.05=3.84 χ2 c=0.277<χ2 0.05=3.84 0 0理论分离比Theoretical segregation ratio 3∶1 1∶1 1∶0 1∶0--
2.3 慢溶质性状初步定位
以春雪为亲本自交构建的遗传分离群体为试材,根据分离单株肉质表型的鉴定结果,选取极端非慢溶质单株和极端慢溶质单株各20株,构建非慢溶质、慢溶质基因池,进行BSA定位分析。为了鉴定慢溶质桃调控位点,对两个混池进行高通量特异性片段测序,非慢溶质池和慢溶质池的测序深度分别为33 倍和37 倍。在混池之间共获得79 304 个SNP 和21 715 个InDel,采用欧氏距离法(Euclidean Distance,ED算法)定位分析方法,将该性状的调控位点关联到了第4号染色体(图4)。关联分析共得到4个与慢溶质桃性状相关的候选区域,总长度为7.87 Mb(表3)。

图4 桃慢溶质性状的BSA 定位分析
Fig.4 BSA mapping analysis of slow-melting flesh character
横坐标为染色体名称,彩色的点代表每个SNP 位点的ED 值,黑色实线为拟合后的ED 值,红色虚线代表显著性关联阈值,超出红色虚线的染色体部分即为候选区段。
The abscissa is the name of the chromosome,the colored dot represents the ED value of each SNP site,the black solid line represents the fitted ED value,the red dotted line represents the significance association threshold,and the chromosome part beyond the red dotted line is the candidate section.
表3 慢溶质性状关联区域信息
Table 3 Related regional information statistics
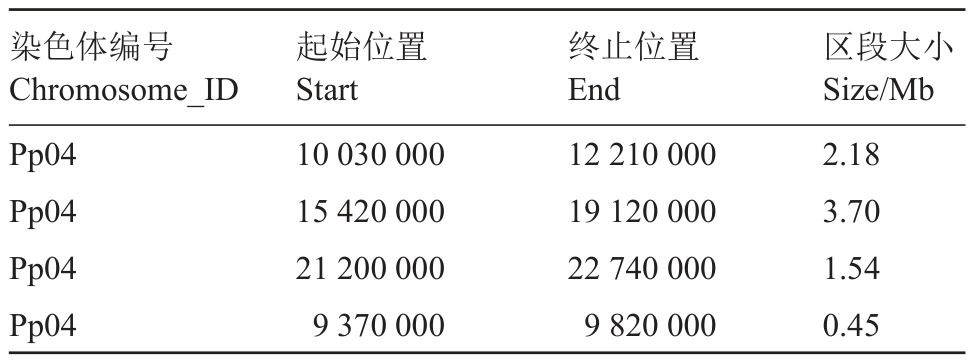
染色体编号Chromosome_ID Pp04 Pp04 Pp04 Pp04起始位置Start 10 030 000 15 420 000 21 200 000 9 370 000终止位置End 12 210 000 19 120 000 22 740 000 9 820 000区段大小Size/Mb 2.18 3.70 1.54 0.45
3 讨 论
桃是典型的呼吸跃变型果实,在成熟后期出现很高的呼吸高峰,果实迅速软化[14]。笔者对慢溶质桃品种春丽、春瑞成熟软化的生理特点进行分析,留树时间长达12 d以上,在成熟前期保持较高的硬度,这与前人[7]对慢溶质桃杂交单株S11-8 的生理特征观察结果一致。慢溶质桃与MF 相比,前者果实乙烯释放延迟发生,果实软化相应延迟,这可能是慢溶质桃硬熟期长的原因。除此之外,对慢溶质桃遗传倾向的分析也与前期春雪×春美杂交后代的研究结果一致[15]。此外,Chen等[7]通过对春雪和红不软的F1群体分析,发现后代慢溶质桃∶非慢溶质桃接近1∶1,推测春雪慢溶质桃位点也为杂合,可能受显性单基因控制。
慢溶质性状的基因定位研究相对匮乏,并且没有获得准确的定位区间。Serra等[8]以慢溶质型桃品种Big Top 为父本,两种溶质型(Armking 和Nectaross)分别为母本,构建杂交群体,通过高密度SNP芯片构建遗传图谱,定位了与慢溶质桃性状相关联的3个区段,分别位于第4、5、6三条染色体上。Ciacciulli 等[9]通过建立力学模型,对慢溶质型BRebus028和MF型BMax10杂交后代进行表型评价,进行连锁作图,将慢溶质桃定位到第8 号染色体上的一个QTL(qSwS8.1),并利用全基因组关联分析(genomewide association study,GWAS)进行了验证。Chen等[7]通过BSA-seq鉴定到了第4号染色体两个QTL,并结合转录组分析,在5.6 Mb定位区间内鉴定到了29 个可能的慢溶质基因。笔者在本试验中通过对春雪自交后代的连续调查,结合遗传定位分析将慢溶质桃定位在了桃第4 号染色体上,与Serra 等[8]和Chen等[7]的定位结果一致,且有一定的重叠,未来需要进一步研究缩小慢溶质桃定位区间,挖掘慢溶质桃性状的候选基因。
此外,桃果实成熟期也可能对肉质有影响,在对春雪分离群体的调查发现,亲本春雪含有极早熟的基因,5 月底之前成熟的极早熟单株中没有发现慢溶质桃,之后全部为慢溶质桃。由于控制成熟期的遗传位点被定位在第4号染色体[15-17],与慢溶质桃在同一连锁群上,后代极早熟单株中没有慢溶质型,可能是慢溶质桃与熟期性状存在不完全连锁。笔者在本试验中进行BSA 定位的两个混池之间除了肉质的显著差别外,也无法规避熟期的差异,熟期会影响对肉质的判定。因此,定位结果中包括了与熟期相关的基因,这也为慢溶质候选基因的筛选增加了一定难度。后续笔者将利用基因组重测序数据,在定位区间开发更多标记,通过更多群体进行验证,更精细地对慢溶质性状进行定位,为慢溶质分子标记辅助育种提供帮助。
4 结 论
通过对慢溶质和非慢溶质桃成熟软化的生理差异进行比较,发现慢溶质桃春丽、春瑞成熟期留树时间长达12 d,是非慢溶质桃春美、春蜜的2 倍左右,乙烯延迟释放,果实最终变软。遗传倾向分析表明,慢溶质性状为显性单基因或主效基因控制遗传,且调控基因定位在第4号染色体上。
[1]俞明亮,王力荣,王志强,彭福田,张帆,叶正文.新中国果树科学研究70 年:桃[J].果树学报,2019,36(10):1283-1291.YU Mingliang,WANG Lirong,WANG Zhiqiang,PENG Futian,ZHANG Fan,YE Zhengwen.Fruit scientific research in New China in the past 70 years:Peach[J].Journal of Fruit Science,2019,36(10):1283-1291.
[2]徐磊,陈超.中国桃产业经济分析与发展趋势[J].果树学报,2023,40(1):133-143.XU Lei,CHEN Chao.Economic situation and development countermeasures of Chinese peach industry[J].Journal of Fruit Science,2023,40(1):133-143.
[3]曾文芳,王志强,牛良,潘磊,丁义峰,鲁振华,崔国朝.桃果实肉质研究进展[J].果树学报,2017,34(11):1475-1482.ZENG Wenfang,WANG Zhiqiang,NIU Liang,PAN Lei,DING Yifeng,LU Zhenhua,CUI Guochao.Research process on peach fruit flesh texture[J].Journal of Fruit Science,2017,34(11):1475-1482.
[4]牛良,曾文芳,潘磊,孟君仁,鲁振华,崔国朝,王志强.硬质桃研究现状及展望[J].果树学报,2020,37(8):1227-1235.NIU Liang,ZENG Wenfang,PAN Lei,MENG Junren,LU Zhenhua,CUI Guochao,WANG Zhiqiang.Research status and perspective for stony-hard peach[J].Journal of Fruit Science,2020,37(8):1227-1235.
[5]LAYNE D R,BASSI D.The peach:Botany,production and uses[M].UK:CABI,2008:1-36.
[6]GHIANI A,NEGRINI N,MORGUTTI S,BALDIN F,NOCITO F F,SPINARDI A,MIGNANI I,BASSI D,COCUCCI M.Melting of‘Big Top’nectarine fruit:Some physiological,biochemical,and molecular aspects[J].Journal of the American Societyfor Horticultural Science,2011,136(1):61-68.
[7]CHEN C W,GUO J,CAO K,ZHU G R,FANG W C,WANG X W,LI Y,WU J L,XU Q,WANG L R.Identification of candidate genes associated with slow-melting flesh trait in peach using bulked segregant analysis and RNA-seq[J].Scientia Horticulturae,2021,286:110208.
[8]SERRA O,GINÉ-BORDONABA J,EDUARDO I,BONANY J,ECHEVERRIA G,LARRIGAUDIÈRE C,ARÚS P.Genetic analysis of the slow-melting flesh character in peach[J].Tree Genetics&Genomes,2017,13(4):77.
[9]CIACCIULLI A,CIRILLI M,CHIOZZOTTO R,ATTANASIO G,DA SILVA LINGE C,PACHECO I,ROSSINI L,BASSI D.Linkage and association mapping for the slow softening (SwS)trait in peach [P.persica (L.) Batsch]fruit[J].Tree Genetics &Genomes,2018,14(6):93.
[10]ZENG W F,PAN L,LIU H,NIU L,LU Z H,CUI G C,WANG Z Q.Characterization of 1-aminocyclopropane-1-carboxylic acid synthase (ACS) genes during nectarine fruit development and ripening[J].Tree Genetics&Genomes,2015,11(2):18.
[11]王力荣,朱更瑞,方伟超.桃(Prunus persica L.)种质资源果实数量性状评价指标探讨[J].园艺学报,2005,32(1):1-5.WANG Lirong,ZHU Gengrui,FANG Weichao.The evaluating criteria of some fruit quantitative characters of peach (Prunus persica L.) genetic resources[J].Acta Horticulturae Sinica,2005,32(1):1-5.
[12]CINGOLANI P,PLATTS A,WANG L L,COON M,NGUYEN T,WANG L,LAND S J,LU X Y,RUDEN D M.A program for annotating and predicting the effects of single nucleotide polymorphisms,SnpEff[J].Fly,2012,6(2):80-92.
[13]HILL J T,DEMAREST B L,BISGROVE B W,GORSI B,SU Y C,YOST H J.MMAPPR:Mutation mapping analysis pipeline for pooled RNA-seq[J].Genome Research,2013,23(4):687-697.
[14]TONUTTI P,CASSON P,RAMINA A.Ethylene biosynthesis during peach fruit development[J].Journal of the American Society for Horticultural Science,1991,116(2):274-279.
[15]孟君仁.桃慢溶质性状调控机制初步研究[D].北京:中国农业科学院,2021.MENG Junren.Preliminary study on regulation mechanism of slow melting flesh characteristics in peach[D].Beijing:Chinese Academy of Agricultural Sciences,2021.
[16]EDUARDO I,PACHECO I,CHIETERA G,BASSI D,POZZI C,VECCHIETTI A,ROSSINI L.QTL analysis of fruit quality traits in two peach intraspecific populations and importance of maturity date pleiotropic effect[J].Tree Genetics & Genomes,2011,7(2):323-335.
[17]PIRONA R,EDUARDO I,PACHECO I,DA SILVA LINGE C,MICULAN M,VERDE I,TARTARINI S,DONDINI L,PEA G,BASSI D,ROSSINI L.Fine mapping and identification of a candidate gene for a major locus controlling maturity date in peach[J].BMC Plant Biology,2013,13:166.