百香果属于西番莲科(Passifloraceae)西番莲属(Passiflora Linn.)藤本植物[1],生长迅速,具有当年种植当年投产的优点,因此,近年来全国百香果种植面积不断增加,但是由于受到气温、降雨等气候因素的影响常常存在坐果难、果实可食率低、果实空囊等问题[2],成为制约百香果产业可持续发展的重要原因。
果实坐果依赖于双受精过程的完成,花粉是植物的雄配子体,柱头为花粉黏附及水合萌发、花粉管伸长提供物质基础[3],大量有活性的花粉传授到具有良好可授性的柱头上是确保顺利完成受精过程的必要条件。植物花粉、柱头的活性影响双受精过程的成功率,最终影响坐果率[4],因此花粉及柱头的发育及其活性一直是研究者们关注的重要内容[5-6]。花粉及柱头的寿命因物种的不同有很大的差异,从花后数小时到数天不等,如甘蔗的散粉时间为1 h,在约3 h 后花粉即丧失活性[7],而狭瓣辣木的柱头和花粉的活性则可维持数天[8],同一物种不同品种间也有很大的差异,不同品种烟草花粉活力及柱头的可授性有显著的差异,且不同品种的最佳授粉时期不同[9]。扫描电镜观察是研究植物生殖系统的重要手段,扫描电镜观察研究表明花粉及柱头形态、活力不仅在不同品种之间存在差异,同一品种花粉、柱头在花发育的不同时期也存在差异[10-11]。而花粉及柱头活性常常受到发育阶段、气候等外界因素的影响[12-13],从而影响结实及坐果[14-15]。因此,研究花粉及柱头的活性具有重要的实践意义。
前人对百香果的双受精过程[16]及其花粉和花粉-柱头互作效应[17]等做了一些研究,但在不同发育时期花粉、柱头活力的变化及其对坐果率、果实特性的影响还鲜有报道。笔者在本研究中以桂百一号百香果为研究材料,测定花不同发育时期花粉及柱头活力和不同时间授粉对坐果率及果实特性的影响,为百香果生产及杂交育种提供理论指导。
1 材料和方法
1.1 材料
试验材料为桂百一号,于2020 年9 月采用限根栽培的方法将桂百一号嫁接苗栽种于广西壮族自治区农业科学院园艺研究所试验基地(108.24°E,22.85°N),试验于2022年4月15日至2022年7月20日进行,试验期间当地的最高气温为33 ℃,最低气温为25 ℃,株行距为2 m×3 m,田间正常管理。
1.2 方法
1.2.1 花粉活力测定 在试验开始前1 d 观察并记录桂百一号的开花时间及雌雄蕊的发育状况,并选择旺盛生长的植株20 株,每株选择5 朵第2 天将要开放的花蕾,做好标记。分别于开花前5 h(-5 h)、开花前3 h(-3 h)、开花前1 h(-1 h)、开花时(0 h)、开花后1 h(1 h)、开花后3 h(3 h)、开花后5 h(5 h)、开花后7 h(7 h)、开花后9 h(9 h)、开花后20 h(20 h)采花药,每个时间段的花药分别在10 株植株上采集,每株采1朵,共计10朵。连续3 d同一时间点的3次观测,作为3 次重复。将采集的花朵带回实验室后立即用毛笔轻轻扫下花粉,花粉混合均匀采用液体培养基离体培养法测定花粉的萌发率及花粉管长度,百香果花粉活力的测定采用液体培养基离体培养法,培养基成分为150 g·L-1蔗糖+150 g·L-1 PEG-4000+25 mg·L-1 H3BO3+1 200 mg·L-1 Ca(NO3)2·4H2O,具体操作参考蔡昭艳等[18]的方法。
1.2.2 柱头可授性测定方法 柱头可授性测定采用联苯胺-过氧化氢法,测定的时间段及取样的花朵与1.2.1 方法相同,取不同时间点花朵的柱头置于含联苯胺-过氧化氢反应液(1%联苯胺∶3%过氧化氢∶水体积比为4∶11∶22)的培养皿中,使柱头完全淹没在反应液中,持续5 min 后,置于OLYMPUS SZX10 体视显微镜下观察,并用佳能750D 相机拍照。柱头可授性的强弱等级依照柱头周围的反应液产生气泡的数量判断,柱头可授性强则在柱头周围反应液会产生大量的气泡,反之,柱头可授性弱则产生的气泡少。分级标准参考孙建等[19]的方法,即1级:有少量的气泡,柱头可授性较弱,标记为+,计1分;2 级:有较多气泡,即柱头可授性中等,标记为++,计2 分;3 级:有大量气泡,即柱头可授性强,标记为++,计3 分。柱头的采样时间点与花粉的采样时间点相同。
1.2.3 花粉、柱头扫描电镜观察 采样时间点与1.2.1相同,每个时间点选择不同植株上健壮花朵的花药及柱头,分别放置在不同的EP 管中,迅速投入到2.5%戊二醛电镜固定液中固定2 h,后转移至4 ℃保存。固定好的样品经0.1 mol·L-1磷酸缓冲液PB(pH=7.4)漂洗3 次,每次15 min,后依次经30%、50%、70%、80%、90%、95%乙醇逐级脱水1次,100%乙醇脱水2 次,每次15 min,后用QuorumK850 临界点干燥仪进行干燥,使用裕隆时代LJ-16离子溅射仪喷金镀膜30 s,用卡尔蔡司EVOLS10扫描电镜进行扫描观察,选择有代表性的视野观察花粉的赤道观、极轴观、花粉萌发沟、萌发孔等,并用分析软件Image-pro plus 6.0随机测量15粒花粉的赤道轴长、极轴长,并统计平均数,观察柱头的乳突细胞的形态。
1.2.4 不同授粉时间的坐果率及果实性状测定 授粉时间点与花粉活力及柱头可授性测定的时间点相同,选择盛花期晴好天气,在授粉前1 d傍晚选择10株生长旺盛的植株,将次日即将开放的花蕾去雄并挂吊牌做好标记,授粉时用同期5 朵以上异花的雄蕊,毛笔轻轻扫下收集花粉,混匀后用软毛笔均匀地涂抹到柱头上完成授粉,每个时间点授粉20 朵,重复3 d作为3次重复。于授粉10 d后统计坐果率,坐果率/%=(坐果数/总授粉花朵数)×100。并在果实自然成熟后统计果实的单果质量、果实横径、果实纵径、可食率、出汁率、单个果实的种子数量等性状。1.2.5 数据处理 试验数据采用Excel 软件进行整理,用SPSS 20.0进行方差分析、Bonferroni多重比较和Pearson相关性分析。
2 结果与分析
2.1 百香果花不同发育阶段花粉活力及柱头可授性变化
桂百一号百香果在开花前5 h(-5 h)处于花蕾期,花药未开裂,从表1 可知,此时的花粉已经有25.21%的萌发率,此后花开放时间越接近花粉萌发率越高,在花前1 h(-1 h)的花粉活力与开花时(0 h)的花粉活力无显著差异,但花粉活力并没有在花开时达到最高,而是在开花后3 h 花粉活力最高,此后随时间推移花粉活力在每个检测时间点均比上一个时间点显著降低,花后9 h花粉活力只有10.63%,花后20 h花粉活力为0%。相关性分析表明,花粉萌发率与花粉管长度之间呈极显著相关,相关系数为0.935。柱头可授性在开花前5 h 较弱,开花时间越接近可授性越强(表1,图1),在花前1 h(-1 h)柱头可授性达到了开花时(0 h)的柱头可授性强度,均较强。与花粉活力的变化不同,柱头可授性在开花7 h后均保持较强,在开花9 h 后柱头可授性降低,可授性由较强降低为中等,在花后20 h 柱头可授性降低到较弱水平。相关性分析表明,百香果花粉活力与柱头可授性呈极显著相关,相关系数为0.711,表明百香果花粉与柱头为同期成熟。

图1 百香果柱头可授性强弱等级划分
Fig.1 Classification of stigma receptivity of passionfruit
A.1 级,有少量的气泡,柱头可授性较弱,标记为+,记1 分;B.2 级,有较多气泡,柱头可授性中等,标记为++,记2 分;C.3 级,有大量气泡产生,柱头可授性较强,标记为+++,记3 分。
A.Grade 1,there are a few bubbles and the stigma receptivity is weak,marked as+,1 point;B.Grade 2,with more bubbles and the stigma receptivity is medium,marked as++,2 points;C.Grade 3,with a lot of bubbles and the stigma receptivity is strong,marked as+++,3 points.
表1 百香果花不同时间花粉萌发率与柱头可授性
Table 1 pollen vitality and stigma receptivity of passionfruit at different periods

注:数据为(平均值±标准差),不同小写字母表示差异显著(p<0.05)。下同。
Note:The datas are(mean±standard),with different small letters indicate significant difference at(p<0.05).The same below.
检测时间Testing time-5 h-3 h-1 h 0 h 1 h 3 h 5 h 7 h 9 h 20 h花粉萌发率Pollen germination rate/%25.21±0.96 e 33.93±4.66 d 43.38±1.07 c 49.58±0.90 bc 51.72±4.44 b 60.83±3.81 a 33.70±2.11 d 21.86±1.64 e 10.63±1.05 f 0.00±0.00 g花粉管长度Pollen tube length/μm 146.23±8.39 f 160.10±6.66 e 170.67±13.19 d 201.43±3.55 c 229.07±8.62 b 247.04±14.39 a 150.43±6.03 f 135.52±5.74 g 120.69±7.08 h 0.00±0.00 i柱头可授性Stigma receptivity+(可授性较弱Weak stigma receptivity)++(可授性中等Medium stigma receptivity)+++(可授性较强High stigma receptivity)+++(可授性较强High stigma receptivity)+++(可授性较强High stigma receptivity)+++(可授性较强High stigma receptivity)+++(可授性较强High stigma receptivity)+++(可授性较强High stigma receptivity)++(可授性中等Medium stigma receptivity)+(可授性较弱Poor stigma receptivity)
2.2 不同时间百香果花粉、柱头电镜扫描观察
桂百一号百香果花具有5 枚雄蕊,对百香果开花前后不同时间点的花粉进行电镜扫描观察,根据扫描结果,桂百一号百香果花粉大小较均匀,为单粒花粉,呈扁球形,具3条环状的萌发沟,长至近两极,但3个环形的萌发沟不相交。花粉外壁具有清晰的网状纹饰,网眼为不规则形状,大小不一,网眼内具有大小不均匀的乳突状突起(图2)。百香果花粉大小较均匀,花粉的赤道轴长51.18~60.81 μm,平均57.38 μm,极轴长为49.42~54.74 μm,平均52.04 μm,通过电镜扫描,对比观察了桂百一号百香果花前后不同时间的花粉,发现不同时间花粉大小及外观无明显变化,由此可见百香果花粉活力不能通过外观判断。
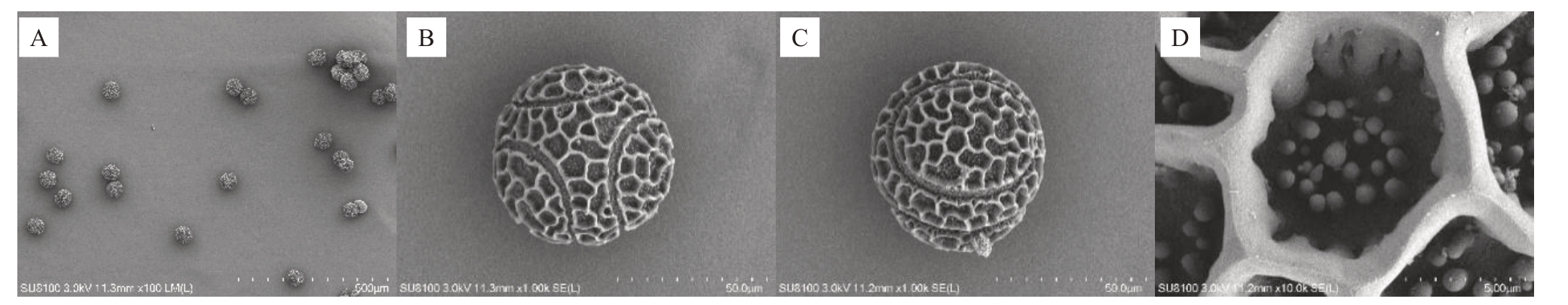
图2 桂百一号百香果花粉电镜扫描图
Fig.2 Scanning electron micrographs of pollen grains of passionfruit Guibai No.1
A. 花粉群体观;B. 花粉群单粒花粉赤道面观;C. 单粒花粉极面观;D. 花粉表面纹饰。
A.Pollen grain population view;B.Single pollen grain equatorial plane view;C.Single pollen grain polar view;D.Pollen surface decoration.

图3 开花前后不同时间百香果柱头表面扫描电镜图
Fig.3 Scanning electron micrographs of stigma surface of passionfruit at different times before and after flowering
A~J 分别为-5 h、-3 h、-1 h、0 h、1 h、3 h、5 h、7 h、9 h、20 h 柱头表面扫描电镜图。
A-J were the scanning electron microscope micrographs of stigma surface at-5 h,-3 h,-1 h,1 h,3 h,5 h,7 h,9 h and 20 h respectively.
桂百一号百香果为复雌蕊,子房上位,柱头由3 枚心皮构成,柱头扫描电镜观察发现,柱头的乳突细胞密集,呈圆柱形,直径相近,顶端突起,在整个观察期间柱头未见分泌物,表明百香果柱头为干性柱头。在开花前5 h(-5 h)柱头乳突细胞表面皱褶较多,此时柱头的可授性较弱,越接近开花柱头越饱满,可授性较强;在开花后9 h 柱头表面皱褶增多,此时柱头的可授性降低为中等;开花后20 h 柱头出现明显的皱缩,乳突细胞间的缝隙变大,此时柱头的可授性弱(见2.1 结果)。可见,百香果花粉柱头的形态与其可授性一致,说明可通过观察柱头乳突组织的形态来判断其可授性。同时,从开花前5 h 到开花后20 h 的扫描电镜观察均未在柱头上发现花粉,说明桂百一号百香果在无媒介的情况下不能完成授粉。
2.3 不同时间点授粉对百香果坐果率及果实特性的影响
如表2 所示,百香果花前后不同时间授粉的坐果率具有显著差异,而单果质量、果实横径、果实纵径、可食率、单果种子数无显著差异。对不同时间点的花粉萌发率、柱头可授性、坐果率、单果质量、果实横径、果实纵径、可食率及单果种子数的相关性分析(表3)表明,坐果率与花粉萌发率呈极显著相关,相关系数为0.806,与柱头可授性呈显著相关,相关系数为0.716,单果质量、果实横径、果实纵径、可食率、单果种子数与花粉萌发率及柱头可授性均无显著相关性。表明雌雄蕊活力只对百香果的坐果率产生影响,而对果实的特性并无显著影响。
表2 不同时间授粉对百香果坐果率及果实特性的影响
Table 2 Effect of pollination at different times on fruit setting rate and fruit characteristics of passionfruit
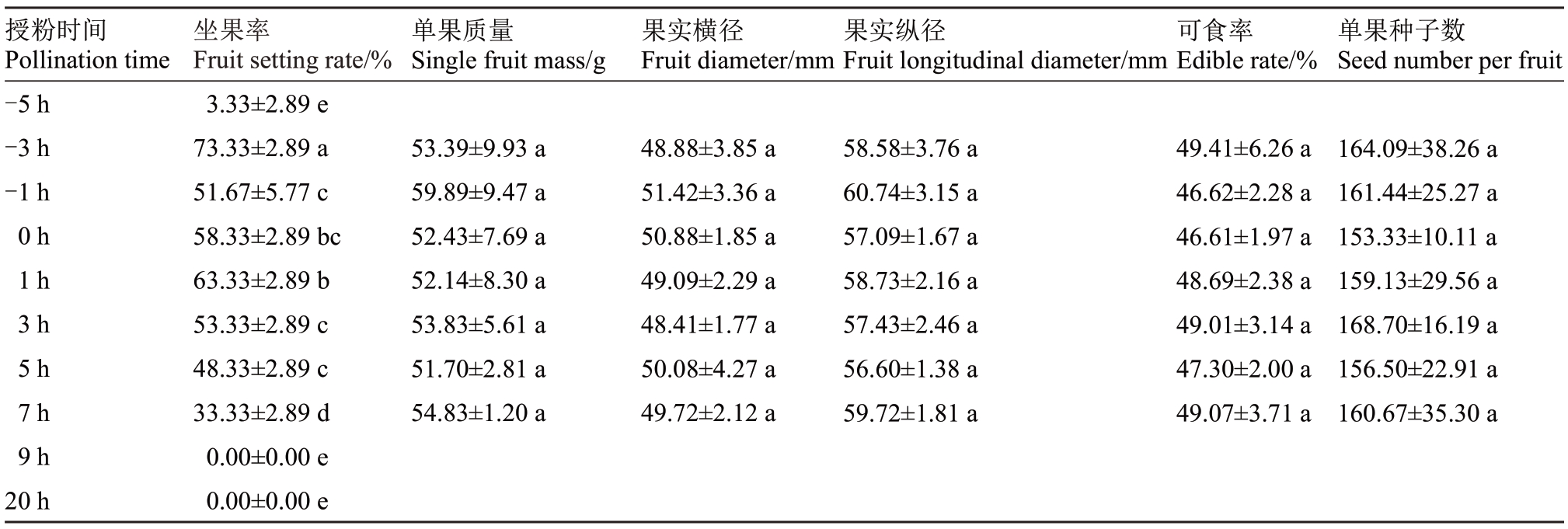
授粉时间Pollination time-5 h-3 h-1 h 0 h 1 h 3 h 5 h 7 h 9 h 20 h坐果率Fruit setting rate/%3.33±2.89 e 73.33±2.89 a 51.67±5.77 c 58.33±2.89 bc 63.33±2.89 b 53.33±2.89 c 48.33±2.89 c 33.33±2.89 d 0.00±0.00 e 0.00±0.00 e单果质量Single fruit mass/g 53.39±9.93 a 59.89±9.47 a 52.43±7.69 a 52.14±8.30 a 53.83±5.61 a 51.70±2.81 a 54.83±1.20 a果实横径Fruit diameter/mm 48.88±3.85 a 51.42±3.36 a 50.88±1.85 a 49.09±2.29 a 48.41±1.77 a 50.08±4.27 a 49.72±2.12 a果实纵径Fruit longitudinal diameter/mm 58.58±3.76 a 60.74±3.15 a 57.09±1.67 a 58.73±2.16 a 57.43±2.46 a 56.60±1.38 a 59.72±1.81 a可食率Edible rate/%49.41±6.26 a 46.62±2.28 a 46.61±1.97 a 48.69±2.38 a 49.01±3.14 a 47.30±2.00 a 49.07±3.71 a单果种子数Seed number per fruit 164.09±38.26 a 161.44±25.27 a 153.33±10.11 a 159.13±29.56 a 168.70±16.19 a 156.50±22.91 a 160.67±35.30 a
表3 花粉活力、柱头可授性与坐果率及果实特性的相关性分析
Table 3 Correlation analysis between pollen viability,stigma receptivity,fruit setting and fruit characteristics

注:*表示差异显著水平为0.05,**表示差异显著水平为0.01。
Note:*stands for significant difference at p<0.05 and**at p<0.01.
指标Index花粉萌发率Pollen germination rate柱头可授性Stigma recetivity坐果率Fruit setting rate单果质量Single fruit weight果实横径Fruit diameter果实纵径Fruit longitudinal diameter可食率Edible rate种子数Seed number花粉萌发率Pollen germination rate 0.714*0.806**-0.080-0.184-0.296-0.144 0.224柱头可授性Stigma receptivity 0.716*0.101 0.365-0.050-0.474-0.312坐果率Fruit setting rate-0.255 0.273 0.188 0.128 0.098单果质量Single fruit mass 0.505 0.822-0.283 0.299果实横径Fruit diameter 0.279-0.903**-0.643果实纵径Fruit longitudinal diameter 0.070 0.252可食率Edible rate 0.657种子数Seed number
3 讨 论
花粉及柱头是参与有性生殖过程的重要元件,研究花粉及柱头的活力,探明可授期不仅能提高坐果率,对授粉效率也具有重要的影响,在实际生产及杂交育种中具有重要的意义。花粉活力及柱头可授性的持续时间与物种及品种紧密相关,同一品种不同发育阶段也有所差异[20],黄连[21]在开花后2 h即失去活性,可可[22]、木地肤[23]的花粉活力只有数小时,葛[24]、肋柱[25]的花粉活力可持续数天,而大花蕙兰[26]的花粉活力可持续35 d。本研究中百香果花粉在开花前5 h 就有一定的萌发率(25.21%),之后萌发率不断提升,在花后3 h 达到最高,到花后9 h 花粉活力只有10.63%,可见花粉寿命比较短,电镜扫描观察发现在本研究试验时段内花粉的外观没有明显的变化,表明不能仅通过观察外观判断花粉的活力。值得注意的是前人在对花粉活力的研究中使用的检测方法不同[21-26],主要有离体培养法及染色法,检测方法的不同可能会对花粉寿命的结果产生较大的差别[27-28]。
柱头是识别花粉及花粉发生水合、萌发的场所,对坐果产生重要的影响,甚至是制约坐果的重要因素[12]。柱头可授性持续时间的长短也有较大的差异,乌丹蒿[29]柱头可授性持续到花后第6天,大花铁线莲[30]保持到开花第9天,大花蕙兰[26]的柱头可授性可持续到花后第45天,而兰州百合[31]的柱头在开花当天即失去可授性。本研究中百香果的柱头在开花前3 h已具有可授性,这与高文杰等[32]在野生绵枣儿中的研究相同,花前1 h到花后7 h为最适可授期,在开花后9 h 柱头可授性开始降低,为弱-强-弱的过程。亚显微观察柱头表面的乳突细胞经历了从皱缩到饱满,再至严重皱缩的过程,与柱头可授性从弱到强再到弱过程相关,可见由柱头乳突细胞的形态可判断柱头的可授性,这与邵凤侠等[20]的研究结果一致。电镜扫描没有观察到柱头有分泌物,说明百香果的柱头为干性柱头。由此可见百香果柱头的寿命也较短,最佳可授期为花前1 h到花后7 h,即约8 h。
除寿命长短外,花粉及柱头的成熟时序也是影响坐果的重要因素,存在雌雄异熟及雌雄同熟之分,本研究结果表明,百香果花属于雌雄同熟,花朵较大,颜色艳丽,较易吸引昆虫传粉,另外花粉多,柱头的面积较大等特点使得百香果在有昆虫媒介的情况下较易完成授粉。
百香果坐果率与花粉活力呈极显著相关,与柱头显著相关,表明花粉及柱头的活力均对坐果率有显著的影响,这与前人的研究结果相同[31-33]。有效授粉期为开花前3 h到开花后7 h,即约10 h,值得注意的是坐果率最高的时间点并不是花粉萌发率及柱头可授性最高的时间点(花后3 h),而是在开花前3 h,此时的花粉活力及柱头的可授性均相对较低,表明百香果坐果除了受到花粉柱头活力的影响外还有其他因素产生了重要的影响,例如温度、湿度等均会影响坐果率[34]。此外,不同时间点授粉所得的百香果果实在单果质量、果实横径、果实纵径、可食率、种子数等特性上没有显著的差异。说明百香果果实的特性可能主要受肥水及栽培环境的影响,而与雌雄蕊活性无关。
4 结 论
百香果花属于雌雄同熟,花粉及柱头的寿命均较短,在开花当天即失去活性。花粉的活力不能仅通过观察外观进行判断,但可由柱头乳突细胞的形态可判断柱头的可授性。花粉及柱头的活力均对坐果率产生显著的影响,有效授粉期仅10 h,因此,在生产及杂交育种中要及时安排授粉,以便提高坐果率。
[1] 中国科学院中国植物志编辑委员会.中国植物志(第25 卷第1 分册)[M].北京:科学出版社,1999:113.Sinicae Agendae Academiae Sinicae Edita. Flora reipublicae popularis sinicae (Vol. 52,No. 1)[M]. Beijing:Science Press,1999:113.
[2] 田青兰,吴艳艳,黄伟华,刘洁云,韦绍龙,牟海飞,韦弟,黄永才,熊晓兰,张英俊.‘台农1 号’西番莲的成花坐果特性及与气象因子的关系[J].果树学报,2020,37(9):1358-1370.TIAN Qinglan,WU Yanyan,HUANG Weihua,LIU Jieyun,WEI Shaolong,MOU Haifei,WEI Di,HUANG Yongcai,XIONG Xiaolan,ZHANG Yingjun. Flower formation and fruit setting in‘Tainong No. 1’passion fruit andits relationship with meteorological factors[J].Journal of Fruit Science,2020,37(9):1358-1370.
[3] 杨小丹,李枝林,王京,刘一鸣,陈贵春,刘丹,王玉英.花粉活力与柱头可授性对兰花杂交结荚率的影响[J].江苏农业科学,2019,47(10):166-172.YANG Xiaodan,LI Zhilin,WANG Jing,LIU Yiming,CHEN Guichun,LIU Dan,WANG Yuying. Effects of pollen viability and stigma receptivity on interbreeding rate of orchid[J]. Jiangsu Agricultural Sciences,2019,47(10):166-172.
[4] COWAN A A,MARSHALL A H,MICHAELSON-YEATES T P T. Effect of pollen competition and stigmatic receptivity on seed set in white clover (Trifolium repens L.)[J]. Sexual Plant Reproduction,2000,13(1):37-42.
[5] 欧行奇,李新华,欧阳娟,乔红,王紫娟,刘源海.不同小麦品种柱头活力及花粉活性模拟研究[J].中国农学通报,2019,35(8):1-4.OU Xingqi,LI Xinhua,OUYANG Juan,QIAO Hong,WANG Zijuan,LIU Yuanhai.Modeling study on stigma vitality and pollen activity of wheat varieties[J]. Chines Agricultural Sicence Bulletin,2019,35(8):1-4.
[6] LORA J,GARCIA-LOR A,ALEZA P. Pollen development and viability in diploid and doubled diploid citrus species[J]. Frontiers in Plant Science,2022,13:862813.
[7] 常海龙,张垂明,张伟,陈俊吕,邱永生,周峰,吴其卫,王勤南.甘蔗花粉活力和柱头可授性日变化研究[J].广东农业科学,2017,44(4):14-18.CHANG Hailong,ZHANG Chuiming,ZHANG Wei,CHEN Junlü,QIU Yongsheng,ZHOU Feng,WU Qiwei,WANG Qinnan. Study on diumal variation of sugarcane’s pollen vitality and stigma energy[J]. Guangdong Agricultural Sciences,2017,44(4):14-18.
[8] 吕亚,王愣,马志亮,杨焱,李海泉,张祖兵.狭瓣辣木的开花动态及花粉活力和柱头可授性[J]. 分子植物育种,2020,18(7):2371-2375.LÜ Ya,WANG Leng,MA Zhiliang,YANG Yan,LI Haiquan,ZHANG Zubing. Floral dynamics,pollen viability and stigma receptivity of Moringa stenopetala[J]. Molecular Plant Breeding,2020,18(7):2371-2375.
[9] 姚志敏,刘艳华,戴培刚,向德虎,张兴伟,赵韬智,王志德.野生烟草花粉活力与柱头可授性及繁育特性研究[J].西北植物学报,2015,35(3):614-621.YAO Zhimin,LIU Yanhua,DAI Peigang,XIANG Dehu,ZHANG Xingwei,ZHAO Taozhi,WANG Zhide. Wild tobacco pollen viabilitu,stigma receptivity and reproductive characteristecs[J].Acta Botanica Boreali-Occidentalia Sinica,2015,35(3):614-621.
[10] 李洪池,吴天彧,罗建.西藏色季拉山区龙胆科30 种植物的花粉形态特征[J].园艺学报,2021,48(12):2427-2442.LI Hongchi,WU Tianyu,LUO Jian. Pollen morphological characteristics of 30 species of gentianaceae in shergyla mountain area,Tibet[J].Acta Horticulturae Sinica,2021,48(12):2427-2442.
[11] 陈相洁,毛礼米,潘昱安,王英浩.部分十字花科植物花粉形态特征比较[J].植物资源与环境学报,2022,31(1):13-20.CHEN Xiangjie,MAO Limi,PAN Yu’an,WANG Yinghao.Comparison on pollen morphological characteristics of some species of Brassicaceae[J].Journal of Plant Resources and Environment,2022,31(1):13-20.
[12] CARPENEDO S,RASEIRA M,FRANZON R C,BYRNE D H,DA SILVA J B.Stigmatic receptivity of peach flowers submitted to heat stress[J].Acta Scientiarum-Agronomy,2020,42:e42450.
[13] 郭媛,邵有全,郭宝贝,张旭凤.梨花粉和柱头发育与温度关系研究[J].中国生态农业学报,2014,22(12):1446-1452.GUO Yuan,SHAO Youquan,GUO Baobei,ZHANG Xufeng.Relationship of pear pollen and stigma development with temperature[J]. Chinese Journal of Eco-Agriculture,2014,22(12):1446-1452.
[14] SANZOL J,RALLO P,HERRERO M. Stigmatic receptivity limits the effective pollination period in‘Agua de Aranjuez’pear[J]. Journal of the American Society for Horticultural Science,2003,128(4):458-462.
[15] GUPTA R,SUTRADHAR H,CHAKRABARTY S K,ANSARI M W,SINGH Y.Stigmatic receptivity determines the seed set in Indian mustard,rice and wheat crops[J].Communicative&Integrative Biology,2015,8(5):e1042630.
[16] 曾华金,秦云霞,刘志昕,彭存智.西番莲双受精过程的细胞学观察[J].植物研究,2003,223(4):407-409.ZENG Huajin,QIN Yunxia,LIU Zhixin,PENG Cunzhi.Abservation of double fertilition of Passiflora edulis f.flavicarpa Deg.[J].Bulletin of Botanical Research,2003,223(4):407-409.
[17] MEZZONATO-PIRES A C,MILWARD-DE-AZEVEDO M A,MENDONÇA C B F,GONÇALVES-ESTEVES V. Pollen morphology and detailed sexine of Passiflora subgenus Astrophea(Passifloraceae)[J]. Plant Systematics and Evolution,2015,301(9):2189-2202.
[18] 蔡昭艳,苏伟强,董龙,邱文武,施平丽,刘业强,黄辉晔,黄章保,任惠,王小媚.钙、镁、钾浓度及光照、温度对西番莲花粉离体萌发的影响[J].果树学报,2022,39(1):86-94.CAI Zhaoyan,SU Weiqiang,DONG Long,QIU Wenwu,SHI Pingli,LIU Yeqiang,HUANG Huiye,HUANG Zhangbao,REN Hui,WANG Xiaomei. Effects of calcium,magnesium,potassium,light and temperature on pollen germination and pollen tube elongation of passion fruit in vitro[J]. Journal of Fruit Science,2022,39(1):86-94.
[19] 孙建,魏星,乐美旺,饶月亮,颜廷献,颜小文,梁俊超,周红英.芝麻繁育特性研究Ⅴ:花粉活力与柱头可授性[J].西北农业学报,2020,29(10):1537-1546.SUN Jian,WEI Xing,LE Meiwang,RAO Yueliang,YAN Tingxian,YAN Xiaowen,LIANG Junchao,ZHOU Hongying.Studies on breeding characteristics of Sesamum indicum L. Ⅴ:Pollen viability and stigma receptivity[J]. Acta Agriculturae Borali-occidentalis Sinica,2020,29(10):1537-1546.
[20] 邵凤侠,王森,陈建华,陈娟,洪荣艳,唐艳,王佳.‘中秋酥脆枣’柱头形态发育进程与可授性[J].园艺学报,2019,46(12):2309-2322.SHAO Fengxia,WANG Sen,CHEN Jianhua,CHEN Juan,HONG Rongyan,TANG Yan,WANG Jia. Stigma shape development and receptivity of‘Zhongqiu Sucui’Chinese jujube[J].Acta Horticulturae Sinica,2019,46(12):2309-2322.
[21] 雷蕾,李芊夏,岳莉然,张彦妮.黄连花花期动态及其授粉特性研究[J].北京林业大学学报,2019,41(6):129-137.LEI Lei,LI Qianxia,YUE Liran,ZHANG Yanni. Flowering dynamics and pollination characteristics of Lysimachia davurica[J].Journal of Beijing Forestry University,2019,41(6):129-137.
[22] ARENAS-DE-SOUZA M D,ROSSI A A B,VARELLA T L,DA SILVEIRA G F,SOUZA S A M. Stigmatic receptivity and pollen viability of Theobroma subincanum Mart.:Fruit species from the Amazon region[J]. Revista Brasileira de Fruticultura,2016,38(4):e-757.
[23] 郭红超,严成,魏岩.木地肤的开花动态与花粉活力及柱头可授性研究[J].草业学报,2014,23(4):87-93.GUO Hongchao,YAN Cheng,WEI Yan.Study on the flowering dynamic,pollen viability and stigma receptivity of Kochia prostrate[J].Acta Prataculturae Sinica,2014,23(4):87-93.
[24] 陈清健,朱校奇,龙世平,彭斯文.葛花粉活力及柱头可授性检测[J].分子植物育种,2021,19(1):299-304.CHEN Qingjian,ZHU Xiaoqi,LONG Shiping,PENG Siwen.Detection of pollen viability and stigma receptivity of Pueraria montana (Willd.) Ohwi[J]. Molecular Plant Breeding,2021,19(1):299-304.
[25] 李旭新,巴根那,杨恒山,张淑娟.肋柱花开花动态及繁育系统的研究[J].园艺学报,2018,45(7):1393-1401.LI Xuxin,Bagenna,YANG Hengshan,ZHANG Shujuan. Studies on flowering dynamics and breeding system of mongolian medicine Lomatogonium rotatum[J]. Acta Horticulturae Sinica,2018,45(7):1393-1401.
[26] 褚怡,范义荣,张韶伊,孙玉芬,宁惠娟.大花蕙兰与国兰花粉活力及柱头可授性分析[J].浙江农林大学学报,2013,30(6):950-954.CHU Yi,FAN Yirong,ZHANG Shaoyi,SUN Yufen,NING Huijuan. Pollen vitality and stigma receptivity of Cymbidium hybridum and Chinese orchid[J].Journal of Zhejiang A&F University,2013,30(6):950-954.
[27] 贾聖凤,孙德玺,孙守如,邓云,朱迎春,安国林,李卫华,刘君璞. 瓜菜花粉细胞学观察及活力测定概述[J]. 中国瓜菜,2019,32(3):1-7.JIA Shengfeng,SUN Dexi,SUN Shouru,DENG Yun,ZHU Yingchun,AN Guolin,LI Weihua,LIU Junpu. Summary of pollen cytological observation and vigor determination of melon and vegetable crops[J]. China Cucurbits and Vegetables,2019,32(3):1-7.
[28] 郭元元,张力,蒋月喜,陈振东,黄如葵,宋焕忠,车江旅,吴永升,陈琴,零活,张云芸.细香葱种质资源花粉活力及测定方法的比较[J].中国瓜菜,2020,33(8):45-48.GUO Yuanyuan,ZHANG Li,JIANG Yuexi,CHEN Zhendong,HUANG Rukui,SONG Huanzhong,CHE Jianglü,WU Yongsheng,CHEN Qin,LING Huo,ZHANG Yunyun. Comparison of pollen viability and determination methods of the germplasm resources of Allium schoenoprasum[J].China Cucurbits and Vegetables,2020,33(8):45-48.
[29] 刘辉,蔡萍,宛涛,伊卫东,张佳琦.乌丹蒿开花动态、花粉活力及柱头可授性研究[J].中国草地学报,2020,42(1):43-47.LIU Hui,CAI Ping,WAN Tao,YI Weidong,ZHANG Jiaqi.Observation on the flowering dynamic,pollen viability and stigma receptivity of Artemisia wudanica[J]. Chinese Journal of Grassland,2020,42(1):43-47.
[30] 王非,王园园,张永胜,郭艳平,王竞红.铁线莲2 个野生种花粉活力和柱头可授性[J].东北林业大学学报,2019,47(7):75-78.WANG Fei,WANG Yuanyuan,ZHANG Yongsheng,GUO Yanping,WANG Jinghong. Pollen viability and stigma receptivity of two wild clematis species[J]. Journal of Northeast Forestry University,2019,47(7):75-78.
[31] 孙鸿强,师桂英,冉昪,张丽娜,贾喜霞.兰州百合(Lilium davidii var.unicolor)的花粉活力、柱头可授性及繁育系统[J].中国沙漠,2019,39(2):62-69.SUN Hongqiang,SHI Guiying,RAN Bian,ZHANG Lina,JIA Xixia. Pollen viability,stigma receptivity and breeding system of Lanzhou Lily[J].Journal of Desert Research,2019,39(2):62-69.
[32] 高文杰,王欢,王想,王霁佳,宫明雪,崔兰明,何淼.野生绵枣儿的花粉活力与柱头可授性[J].东北林业大学学报,2017,45(9):45-48.GAO Wenjie,WANG Huan,WANG Xiang,WANG Jijia,GONG Mingxue,CUI Lanming,HE Miao. Pollen viability and stigma receptivity of Scilla scillodes[J]. Journal of Northeast Forestry Universty,2017,45(9):45-48.
[33] 王丽娟,刘林德,张莉,王艳杰,连玮,姜中武,张福兴.烟台甜樱桃柱头的可授性、形态特征与坐果率[J].植物学报,2011,46(1):44-49.WANG Lijuan,LIU Linde,ZHANG Li,WANG Yanjie,LIAN Wei,JIANG Zhongwu,ZHANG Fuxing. Stigma receptivity,stigma morphology and fruit set rate of Yantai sweet cherry(Cerasus avium)[J].Chinese Bulletin of Botany,2011,46(1):44-49.
[34] 纠松涛,徐岩,付朝斌,刘勋菊,王世平,张才喜.花发育阶段以及温度对不同品种甜樱桃柱头可授性的影响[J].西北植物学报,2020,40(10):1698-1705.JIU Songtao,XU Yan,FU Chaobin,LIU Xunju,WANG Shiping,ZHANG Caixi. Effect of flower phenology and temperature on stigma receptivity in sweet cherries[J]. Acta Botanica Boreali-Occidentalia Sinica,2020,40(10):1698-1705.