石榴(Punica granatum L.)属石榴科(Punicaceae)石榴属(Punica L.)植物[1],是集生态作用与经济价值为一体的特色果树[2]。石榴产业是新疆特色林果业之一,目前已成为南疆乡村振兴、农民致富的支柱产业。在生产中,新疆当地种植的硬籽石榴品种尽管有很强的适应性,但存在品种较为单一、口味和宜食性等品质指标无法满足消费者需求等问题[3]。近年来,突尼斯软籽石榴因籽粒柔软、粒大多汁、品质好和可食率较高等特性具有巨大的市场潜力[4-5],已成为新疆石榴主产区更新换代的主流品种。软籽石榴喜温畏寒,低温是限制其引种和分布的主要因素。在新疆南疆的石榴主产区,软籽石榴可露地栽培,但冬季需采取防冻保护措施。早春的低温和倒春寒往往会对软籽石榴造成不同程度的冷害和冻害,而其中幼树的抗寒性更弱,容易遭受低温危害,进而抑制石榴的生长和发育,影响后期的产量和品质[6-7]。因此,在新疆寒冷地区引进软籽石榴新品种时,研究软籽石榴对低温胁迫的生理响应是评价其在该地区生长适应性的主要依据之一。
光合作用是植物最基本的生理现象,是干物质积累的唯一途径[8]。光合能力强弱与植物产量和品质密切相关[9]。低温胁迫破坏叶绿体结构使叶绿素合成受阻,导致叶绿素含量显著下降[10],植株黄化,叶片净光合速率(Pn)下降[11]。强耐寒型品种植株的Pn相对较高[12];低温胁迫会影响光能的吸收、转换与光电子传递,致使过剩激发能大量积累于光合系统Ⅱ(PSⅡ)反应中心[13],进而损伤光合机构,降低最大光化学效率(Fv/Fm)、实际光化学效率(ΦPSⅡ)、电子传递速率(electron transport rate,ETR)和光化学淬灭系数(qP),导致叶片光合能力减弱[14]。低温胁迫还会改变细胞膜透性,丙二醛(malondialdehyde,MDA)及渗透调节物质如脯氨酸、可溶性糖和可溶性蛋白含量增加[15],以维持细胞渗透平衡,减缓低温带来的损伤[16]。
目前,已有低温胁迫对秋菊[17]、砂糖橘[18]、脐橙[19]、小麦[20]、棉花[21]等影响的相关生理研究。石榴生理方面的相关研究主要集中在枝条忍耐低温的极限温度上,而对于突尼斯软籽石榴幼苗能忍受的低温胁迫时间却鲜见报道。在早春低温的生产中,短暂低温下植物可以恢复生长能力,但持续低温会对植物造成不可逆的影响。在新疆众多引种的软籽石榴品种中,突尼斯软籽石榴栽培最广泛。为解决生产上遇到的实际问题,本研究以新疆本地的喀什酸石榴和引进的突尼斯软籽石榴幼苗作为研究对象,采用人工模拟早春低温方法,探究不同低温胁迫下2个石榴品种的色素含量、光合气体交换和叶绿素荧光参数、叶片细胞相对电导率、MDA含量及渗透调节物质含量等指标的变化差异,以期为软籽石榴在新疆地区的合理引种和抗逆栽培管理提供理论依据。
1 材料和方法
1.1 试验材料
供试材料为1年生春季扦插的喀什酸石榴和突尼斯软籽石榴幼苗(分别引自新疆喀什和河南荥阳)。试验于石河子大学农学院试验站温室和兵团特色果蔬栽培生理与种质资源利用重点实验室进行。选取生长健壮、无病虫害和机械损伤且整齐一致的幼苗,移栽于直径12 cm、高18.5 cm的营养袋中,袋内盛装草炭、蛭石与珍珠岩复配基质的体积比为1∶1∶1。幼苗统一修剪,保留苗高35~40 cm,正常养护管理,待缓苗结束后转移至人工气候箱中进行低温处理。
1.2 试验设计
试验共设置5 个温度处理,即(1)CK:以25 ℃(昼)/20 ℃(夜)为对照;(2)T1:6 ℃(轻度低温胁迫);(3)T2:3 ℃(中度低温胁迫);(4)T3:0 ℃(重度低温胁迫);(5)T4:-3 ℃(极重度低温胁迫)。处理前,幼苗均在人工气候箱(Percival,美国)中进行预培养,培养环境条件均为温度25 ℃(昼)/20 ℃(夜)、光照周期16 h/8 h、光照度100 μmol·m-2·s-1、相对湿度70%~80%。然后将4个低温胁迫处理按3 ℃·h-1连续降温的方式进行降温处理,直至降至目标低温。每个处理3次重复。于处理2、4、6 d后测定各项指标。
1.3 测定项目及方法
1.3.1 叶绿素及类胡萝卜素含量的测定 用95%乙醇浸泡叶片48 h,置于黑暗条件下浸提,至叶片组织完全变白,即为浸提完全,得到色素提取溶液,参照严衍禄等[22]方法测定并计算叶绿素a、叶绿素b、类胡萝卜素及叶绿素总量。
1.3.2 光合参数的测定 试验以正常25 ℃温度处理为对照(CK)处理2、4、6 d,于低温处理相同天数后,使用美国LI-COR公司便携式光合仪LI-6400在上午10∶00—12∶00测定2个石榴品种幼苗功能叶片(从枝条顶部倒数第7~8枚完全叶)的净光合速率(Pn)、气孔导度(Gs)、胞间CO2浓度(Ci)和蒸腾速率(Tr),并计算水分利用效率(water use efficiency,WUE)=光合速率/蒸腾速率。测定光照度为400 mol·m-2s-1,CO2浓度为400 μmol·mol-1,流速为500 μmol·s-1。每个处理3个重复,每个重复选取1株幼苗的2片功能叶。
1.3.3 叶绿素荧光参数测定 利用脉冲调制式叶绿素荧光成像系统MAX-Imaging-PAM测定叶绿素荧光动力学参数,测定时选取生长一致且受光方向相同的叶片,暗适应30 min 后测定慢速动力学曲线。石榴幼苗叶片的测定参数包括PSⅡ最大光化学效率(Fv/Fm)、实际光化学效率[Y(Ⅱ)]、光化学淬灭系数(qP)、非光化学淬灭系数(non-photochemical quenching,NPQ)和光系统Ⅱ的绝对电子传递速率(Electron transport rate,ETR)。
1.3.4 相对电导率、膜脂过氧化程度和渗透调节物质含量的测定 叶片相对电导率采用电解质外渗量法[23]测定。MDA 含量参考硫代巴比妥酸(TBA)显色法[24]测定。脯氨酸含量参考磺基水杨酸比色法测定,可溶性蛋白质含量采用考马斯亮蓝法测定,可溶性糖含量采用蒽酮比色法[25]测定。
1.4 统计分析
采用Excel 2010 软件进行数据统计与制图,采用R 语言(Mango,英国)软件进行数据分析(p<0.05),采用ANOVA作方差分析和采用Duncan多重比较法进行比较分析。
2 结果与分析
2.1 低温胁迫对石榴叶片叶绿素含量的影响
由表1 可知,随低温胁迫程度的加强和胁迫时间的延长,2 个石榴品种叶片的色素含量均呈不同程度降低的趋势。T1 处理对在整个处理期间喀什酸石榴的叶绿素a、叶绿素b、类胡萝卜素及叶绿素总量均无显著性影响,但显著降低了突尼斯软籽石榴的叶绿素a(除持续2 d 处理)、叶绿素b 及叶绿素总量。此外,T2、T3 和T4 处理均显著降低了2 个石榴品种的叶绿素a、叶绿素b和叶绿素总量。在同一低温胁迫时间下,突尼斯软籽石榴的叶绿素总量下降幅度高于喀什酸石榴,且随胁迫程度的加剧,叶绿素总量下降幅度越高于喀什酸石榴。这说明低温影响了叶绿素的合成与降解,使叶绿素总量降低。突尼斯软籽石榴的色素含量对低温的响应较喀什酸石榴敏感。
表1 低温胁迫对2 个石榴品种的叶绿素a、叶绿素b、类胡萝卜素及叶绿素总量的影响
Table 1 Effects of low temperature stress on chlorophyll a,chlorophyll b,carotenoids and total chlorophyll for seedlings of two pomegranate varieties

注:每个值代表3 次重复的(平均值±标准差)。同一品种相同胁迫时间不同的胁迫温度,通过Duncan 检验,不同的字母表示差异显著(p<0.05)。下同。
Note:Each value represents(the average±standard deviation)of three repetitions.Through Duncan test,different letters of the same variety with different stress temperatures at the same stress time showed significant differences(p<0.05).The same below.
品种Varieties喀什酸石榴Kashi acid pomegranate突尼斯软籽石榴Tunisia soft seed pomegranate持续时间Duration time/d 2 4 6 2 4 6处理Treatment对照Control T1 T2 T3 T4对照Control T1 T2 T3 T4对照Control T1 T2 T3 T4对照Control T1 T2 T3 T4对照Control T1 T2 T3 T4对照Control T1 T2 T3 T4 w(叶绿素a)Chlorophyll a content/(mg·g-1)1.051±0.204 a 0.928±0.090 ab 0.660±0.023 bc 0.329±0.034 cd 0.223±0.041 d 1.425±0.214 a 1.058±0.181 a 0.514±0.012 b 0.266±0.019 b 0.137±0.028 b 0.857±0.010 a 0.838±0.017 a 0.527±0.051 b 0.263±0.080 c 0.100±0.008 d 0.769±0.017 a 0.631±0.097 ab 0.584±0.010 b 0.144±0.025 c 0.079±0.023 c 0.823±0.039 a 0.588±0.035 b 0.356±0.046 c 0.222±0.014 d 0.138±0.024 d 0.849±0.122 a 0.416±0.037 b 0.353±0.095 b 0.100±0.011 c 0.113±0.035 c w(叶绿素b)Chlorophyll b content/(mg·g-1)0.309±0.078 a 0.271±0.022 a 0.276±0.090 a 0.121±0.042 a 0.139±0.030 a 0.142±0.142 a 0.307±0.229 a 0.185±0.109 a 0.164±0.003 a 0.083±0.030 a 0.467±0.033 a 0.351±0.060 ab 0.305±0.059 b 0.087±0.013 c 0.077±0.007 c 0.521±0.023 a 0.218±0.021 b 0.191±0.004 b 0.224±0.013 b 0.134±0.013 c 0.503±0.052 a 0.200±0.004 b 0.120±0.020 bc 0.024±0.012 d 0.037±0.007 cd 0.420±0.072 a 0.147±0.015 b 0.114±0.043 bc 0.028+0.013 bc 0.015±0.004 c w(类胡萝卜素)Carotenoid content/(mg·g-1)0.205±0.069 a 0.197±0.050 a 0.098±0.012 ab 0.049±0.013 b 0.013±0.008 b 0.272±0.051 a 0.189±0.108 ab 0.090±0.058 ab 0.011±0.004 b 0.017±0.012 b 0.172±0.031 a 0.116±0.046 ab 0.029±0.018 c 0.033±0.002 bc 0.005±0.004 c 0.036±0.009 b 0.137±0.009 a 0.119±0.005 a 0.009±0.009 b 0.018±0.008 b 0.103±0.015 ab 0.125±0.004 a 0.076±0.008 b 0.043±0.006 c 0.017±0.010 c 0.158±0.082 a 0.097±0.006 ab 0.032±0.022 ab 0.027±0.007 b 0.012±0.008 b叶绿素总量Total chlorophyll content/(mg·g-1)1.360±0.126 a 1.199±0.076 a 0.936±0.101 ab 0.450±0.033 b 0.362±0.069 b 1.567±0.216 a 1.365±0.204 a 0.700±0.121 b 0.430±0.015 bc 0.220±0.049 c 1.324±0.023 a 1.190±0.044 a 0.832±0.099 b 0.350±0.093 c 0.178±0.015 c 1.291±0.008 a 0.849±0.118 b 0.775±0.011 b 0.368±0.014 c 0.213±0.019 c 1.326±0.090 a 0.788±0.039 b 0.477±0.063 c 0.246±0.011 d 0.174±0.017 d 1.269±0.051 a 0.562±0.048 b 0.467±0.052 b 0.127±0.006 c 0.128±0.036 c
2.2 低温胁迫对石榴光合特性的影响
如表2所示,与对照相比,相同处理时间的4个低温处理均导致2个品种石榴叶片的Pn显著降低,且温度越低,Pn的降幅越大。同一低温胁迫下,随胁迫天数的增加,2个石榴品种的Pn亦呈下降趋势。其中,突尼斯软籽石榴在极重度胁迫(T4处理)6 d后未检测到光合参数,说明极重度低温处理下时间过长会导致叶片光合功能受到破坏,无法进行光合作用;同一低温胁迫处理下随低温处理时间的延长,不同低温处理下,2个石榴品种Tr的变化趋势与Pn相同,且各处理间差异显著。突尼斯软籽石榴Tr的下降幅度大于喀什酸石榴;Ci总体结果上显示随温度下降先升高后降低,同一低温条件下随胁迫时间的延长呈升高趋势;同一胁迫时间不同低温处理下2个品种石榴叶片的胞间CO2浓度与对照的差异随处理温度的降低而呈显著负相关。WUE 是植物生产中单位水分的能量转化率指标[26],同一胁迫时间随温度降低喀什酸石榴的水分利用效率显著下降,突尼斯软籽石榴各处理之间变化不显著。这说明2个石榴品种的光合气体交换参数都受温度和时间互作的影响,且对突尼斯软籽石榴的抑制效应更大。
表2 低温胁迫下2 个石榴品种光合特性参数的变化
Table 2 Changes of low temperature stress on photosynthetic characteristics parameters for seedlings of two pomegranate varieties

注:“-”表示没有测出数值,表示无法进行光合作用。
Note:“-”means that no value is measured,which means that photosynthesis cannot be carried out.
品种Varieties喀什酸石榴Kashi acid pomegranate突尼斯软籽石榴Tunisia soft seed pomegranate持续时间Duration time/d 2 4 6 2 4 6处理Treatment对照Control T1 T2 T3 T4对照Control T1 T2 T3 T4对照Control T1 T2 T3 T4对照Control T1 T2 T3 T4对照Control T1 T2 T3 T4对照Control T1 T2 T3 T4净光合速率Net photosynthetic rate,Pn/(μmol·m-2·s-1)15.640±0.309 a 13.011±0.151 b 8.164±0.166 c 5.207±0.148 d 2.313±0.100 e 16.168±0.171 a 10.978±0.222 b 7.246±0.102 c 4.169±0.271 d 1.520±0.198 e 16.310±0.051 a 10.072±0.546 b 6.309±0.246 c 2.447±0.115 d 0.808±0.139 e 12.391±0.524 a 8.317±0.564 b 4.593±0.284 c 1.711±0.306 d 0.422±0.020 d 11.012±2.074 a 4.163±0.564 b 3.284±0.233 bc 0.792±0.207 cd 0.106±0.022 d 12.806±2.145 a 3.392±0.172 b 3.109±0.133 b 0.086±0.039 b-蒸腾速率Transpiration rate,Tr/(mmol·m-2·s-1)4.947±0.084 a 5.483±0.080 b 3.567±0.270 c 2.588±0.113 d 1.640±0.096 e 5.253±0.101 a 4.362±0.061 b 3.141±0.146 c 2.281±0.103 d 1.154±0.126 e 4.992±0.161 a 3.492±0.186 b 2.347±0.091 c 1.550±0.037 d 0.657±0.065 e 5.263±0.061 a 3.994±0.198 b 2.770±0.372 c 1.574±0.073 d 0.429±0.174 e 4.982±0.537 a 1.663±0.303 b 1.694±0.067 b 0.725±0.256 bc 0.225±0.052 c 4.481±0.361 a 2.111±0.027 b 1.207±0.148 c 0.201±0.022 d-气孔导度Stomatal conductance,Gs/(mmol·m-2·s-1)0.546±0.019 a 0.473±0.006 b 0.358±0.018 c 0.249±0.012 d 0.170±0.011 e 0.515±0.009 a 0.416±0.007 b 0.306±0.004 c 0.240±0.029 d 0.141±0.014 e 0.516±0.015 a 0.385±0.006 b 0.264±0.011 c 0.189±0.008 d 0.105±0.012 e 0.351±0.036 a 0.222±0.005 b 0.194±0.009 b 0.081±0.008 c 0.135±0.009 c 0.339±0.032 a 0.259±0.003 b 0.164±0.005 c 0.042±0.012 d 0.066±0.018 d 0.351±0.003 a 0.157±0.023 b 0.179±0.006 b 0.079±0.017 c-胞间CO2浓度Intercellular CO2 concentration,Ci/(μmol·m-2·s-1)240.590±1.601 d 285.753±7.634 c 345.703±3.293 b 373.063±7.976 a 383.937±9.534 a 307.957±3.461 b 313.433±14.643 b 374.970±9.643 a 326.880±4.270 b 375.037±8.486 a 296.563±3.806 c 305.890±4.099 bc 393.437±5.226 a 376.663±3.554 a 317.277±10.167 b 272.413±4.564 d 205.707±3.340 e 328.626±6.967 c 392.040±3.596 a 367.090±11.796 b 298.131±2.461 c 217.275±6.150 d 358.251±2.095 a 327.922±11.955 b 373.190±12.852 a 277.196±5.773 c 317.806±6.771 b 386.968±7.867 a 380.987±7.535 a-水分利用效率Water use efficiency,WUE/(μmol·mmol-1)3.163±0.091 a 2.373±0.028 b 2.300±0.155 bc 2.018±0.145 c 1.413±0.053 d 3.080±0.088 a 2.518±0.086 b 2.312±0.106 b 1.836±0.192 c 1.314±0.032 d 3.271±0.105 a 2.887±0.111 a 2.691±0.121 a 1.579±0.075 b 1.262±0.336 b 2.354±0.077 a 2.093±0.230 a 1.676±0.137 ab 1.097±0.236 b 1.169±0.492 b 2.206±0.283 a 2.557±0.348 a 1.937±0.065 a 1.127±0.097 b 0.472±0.018 b 2.862±0.442 a 1.606±0.061 b 2.631±0.458 ab 0.427±0.194 c-
2.3 低温胁迫对叶绿素荧光参数的影响
由图1 可知,2 个品种石榴叶片的Fv/Fm随温度处理的降低和胁迫时间的延长均呈下降趋势,其中以突尼斯软籽石榴的降幅较大。此外,图2的Fv/Fm荧光图像结果显示,低温处理下2 个品种石榴叶片蓝色面积减少,绿色和红棕的面积增加的趋势与图1 中Fv/Fm值的变化基本一致。在低温胁迫6 d 后的T3 和T4 处理下,可以明显看到突尼斯软籽石榴的芽头萎缩,叶片外围失水萎蔫、出现斑块,颜色变黄。这说明低温胁迫损害了2 个品种石榴叶片的光系统Ⅱ(PSⅡ),导致光抑制发生,尤其对突尼斯软籽石榴的光合能力抑制更大。在极重度低温和胁迫时间过长下,该品种受到了严重且不可恢复的损伤。

图1 低温胁迫对2 个石榴品种幼苗叶绿素荧光参数的影响
Fig.1 Effects of low temperature stress on chlorophyll fluorescence parameters for seedlings of two pomegranate varieties

图2 低温胁迫对2 个石榴品种幼苗Fv/Fm荧光图像的影响
Fig.2 Effects of low temperature stress on Fv/Fm fluorescence images for seedlings of two pomegranate varieties
随着温度的降低和胁迫时间的延长,2 个石榴品种的Y(Ⅱ)和qP均呈下降趋势,说明该情况下石榴叶片的光化学效率及电子传递速率下降;此外,由图1 可知,2 个石榴品种叶片的NPQ 与低温胁迫持续时间关系不大,但随着低温强度加剧呈上升趋势;温度越低,NPQ下降幅度越大,说明低温胁迫使2个品种石榴叶片PSⅡ反应中心潜在热耗能力增强,从而避免植物因吸收过多的光能而造成伤害,NPQ的升高也有可能源于类囊体质子梯度差的增加和叶黄素氧化程度的增强。
2.4 低温胁迫对2个石榴品种幼苗渗透调节物质含量的影响
2.4.1 脯氨酸含量 如图3 所示,与CK 相比,处理2 d 后,T3 和T4 处理下喀什酸石榴的脯氨酸含量分别显著增加了107.71%和215.75%,而T1 和T2 处理下的脯氨酸含量无显著性变化。胁迫处理4 d 后,4个低温胁迫处理的脯氨酸含量均较对照显著增加,分别增加了40.77%、117.8%、156.55%和201.66%。胁迫处理6 d 后,T2、T3 和T4 处理的脯氨酸含量较对照分别显著增加了126.57%、201.1%、226.49%;4个低温胁迫处理2、4、6 d后,突尼斯软籽石榴叶片的脯氨酸含量均较对照显著提高,分别增加了127.84%~173.27% 、212.3%~324.79% 、252.71%~373.19%和329.92%~398.75%。2 个品种相比,低温处理过程中脯氨酸含量均随胁迫时间延长和胁迫程度加剧而呈现上升趋势。其中,突尼斯软籽石榴品种脯氨酸含量的增幅大于喀什酸石榴品种。
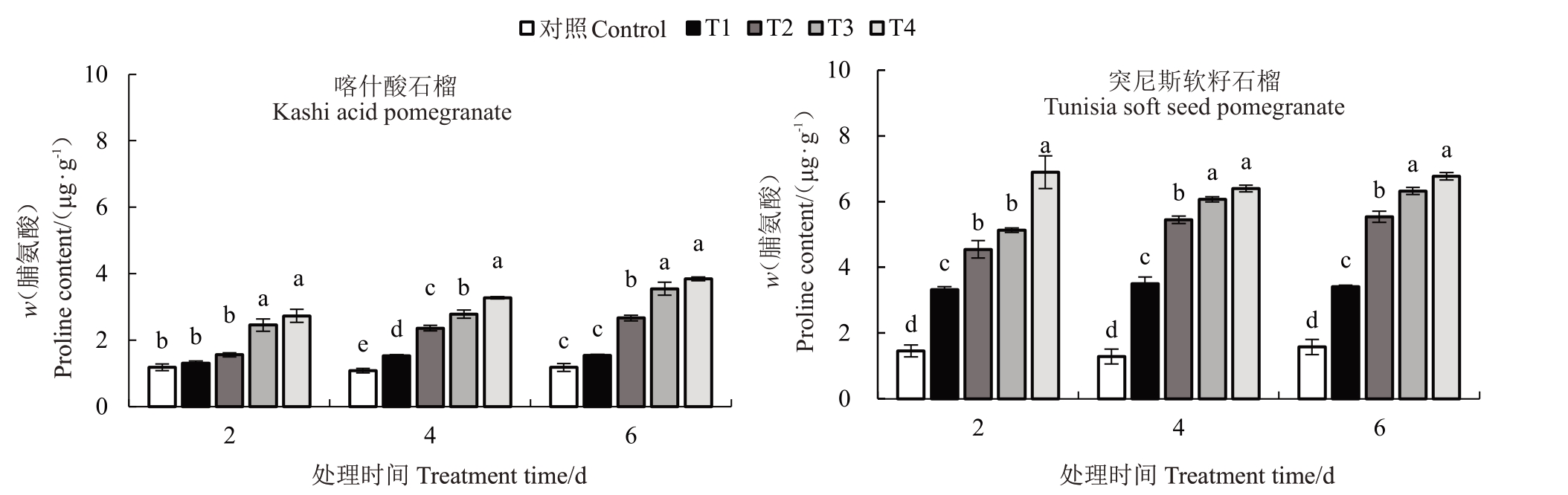
图3 低温胁迫对2 个石榴品种幼苗叶片脯氨酸含量的影响
Fig.3 Effects of low temperature stress on proline content in leaves for seedlings of two pomegranate varieties
2.4.2 可溶性糖含量 由图4可知,喀什酸石榴在处理2、4、6 d 后各处理的可溶性糖含量较对照分别显著增加21.6%~57.93%、34.05%~132.56%、17.65%~47.28%,虽然胁迫6 d后的T3、T4处理可溶性糖含量与T2处理相比有下降趋势,但与对照相比可溶性糖含量仍有所增加;同一处理时间突尼斯软籽石榴随低温胁迫程度提高呈先升高后降低的趋势,胁迫处理2、4、6 d 后4 个低温胁迫处理的可溶性糖含量较对照分别显著升高了14.72%~48.7%、39.65%~93.02%、46.92%~71.74%。从整体来看,2 个石榴品种叶片中可溶性糖含量在不同低温胁迫处理4 d 时的增幅均表现最大。
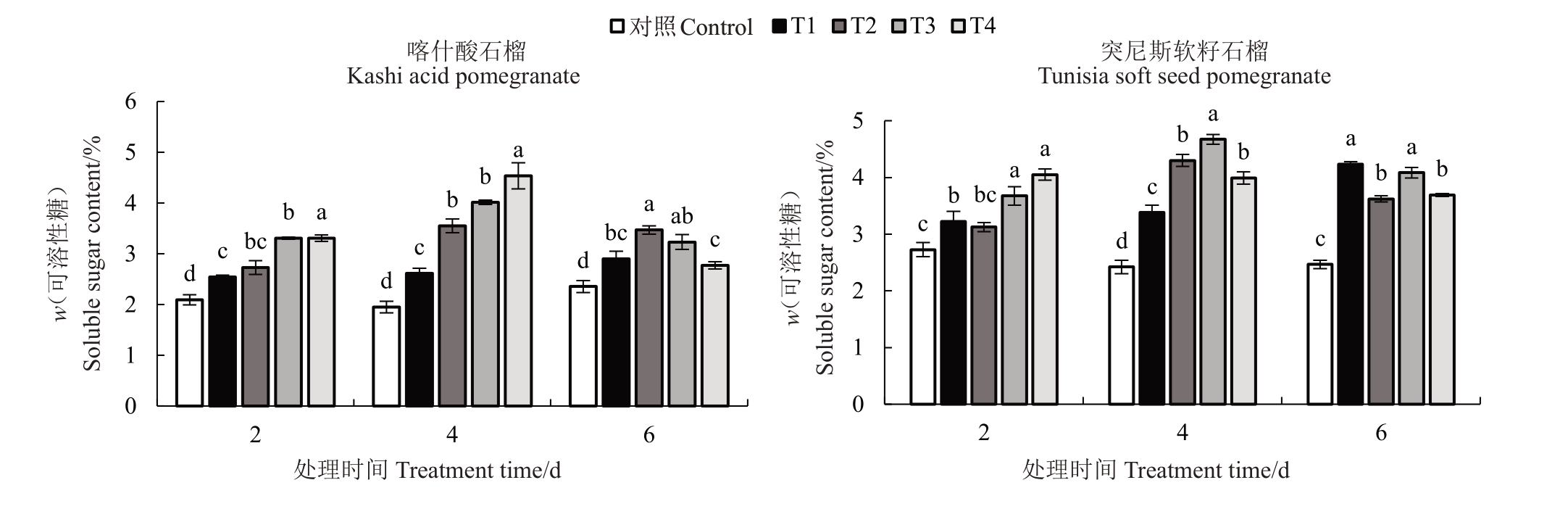
图4 低温胁迫对2 个石榴品种幼苗叶片可溶性糖含量的影响
Fig.4 Effects of low temperature stress on soluble sugar content in leaves for seedlings of two pomegranate varieties
2.4.3 可溶性蛋白质含量 如图5 所示,随着处理温度的下降,2 个石榴品种叶片的可溶性蛋白质含量均表现出升高的趋势。喀什酸石榴在胁迫2、4、6 d后,可溶性蛋白质含量(w,后同)分别在1.887~3.425、2.485~4.486 和2.734~4.167 mg·g-1,其中各处理较对照分别增加56.21%~183.53%、110.95%~280.81%和125.96%~244.38%,均与对照呈显著差异。突尼斯软籽石榴在低温胁迫2、4、6 d 后可溶性蛋白质含量在2.826~6.120、4.649~6.762 和3.292~6.568 mg·g-1之间,分别较对比组显著增加72.74%~274.08%、180.74%~308.33%和92.51%~284.09%。从胁迫时间看,2 个品种石榴叶片可溶性蛋白质含量随胁迫时间的延长,大致表现为先上升再下降的趋势。其中,突尼斯软籽石榴品种可溶性蛋白质含量的增幅明显大于喀什酸石榴品种,且突尼斯软籽石榴在低温胁迫后整体可溶性蛋白质含量比喀什酸石榴高。
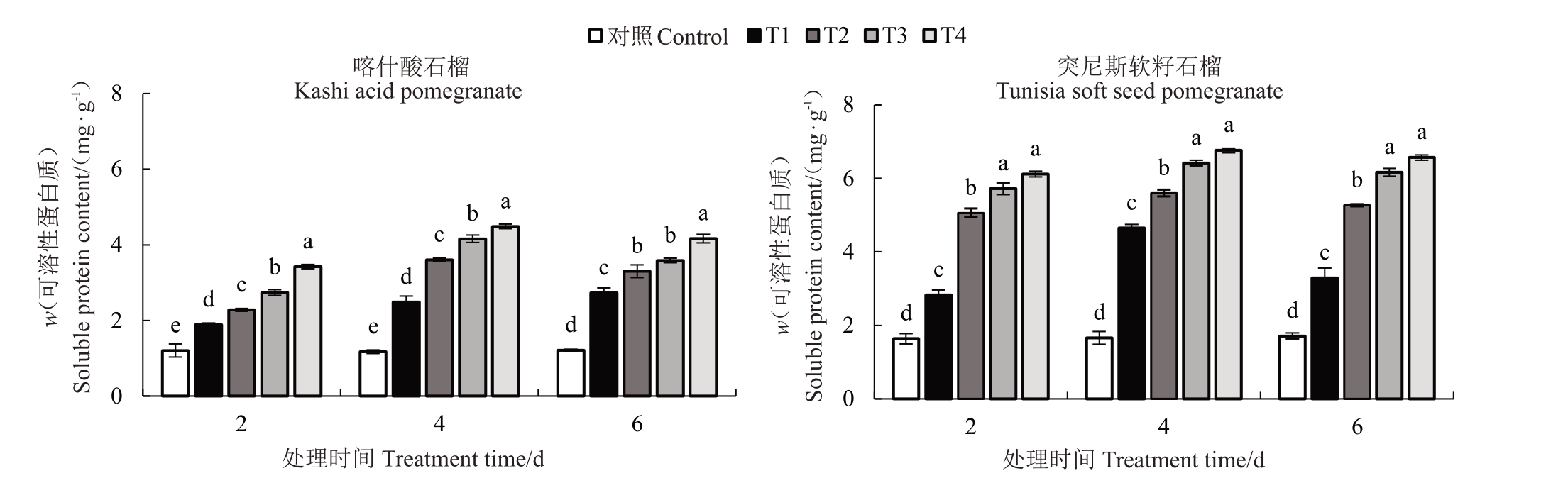
图5 低温胁迫对2 个石榴品种幼苗叶片可溶性蛋白质含量的影响
Fig.5 Effects of low temperature stress on soluble protein content in leaves for seedlings of two pomegranate varieties
2.5 低温胁迫对2个石榴品种幼苗电解质渗透率和MDA含量的影响
2.5.1 电解质渗透率 由图6 可知,喀什酸石榴在胁迫2 d 后T1 与对照相比升高7.19%,无显著差异(p>0.05),在T2、T3、T4 处理后与对照相比分别升高287.96%、364.33%、446.72%,与对照差异显著;胁迫4 d 后,与对照相比分别显著升高102.23%、238.25%、298.48%、468.72%;胁迫6 d后与对照相比分别增加42.85%、78.62%、259.08%、469.86,除T1处理无显著性变化外,与其余处理均呈显著差异。突尼斯软籽石榴随低温胁迫相对电导率也随之上升,胁迫2、4、6 d 后分别较对照上升87.83%~406.93%、51.43%~388.62%、102.23%~560.14%,在T3 处理的相对电导率较前一处理有所下降,但与对照相比仍是上升的,且除胁迫6 d 后T3 处理外各个处理均与对照相比达到显著差异。随温度的降低,2 个品种石榴叶片相对电导率呈升高趋势。其中,突尼斯软籽石榴品种相对电导率在T4处理时胁迫4、6 d后的升幅激增,明显大于喀什酸石榴品种。
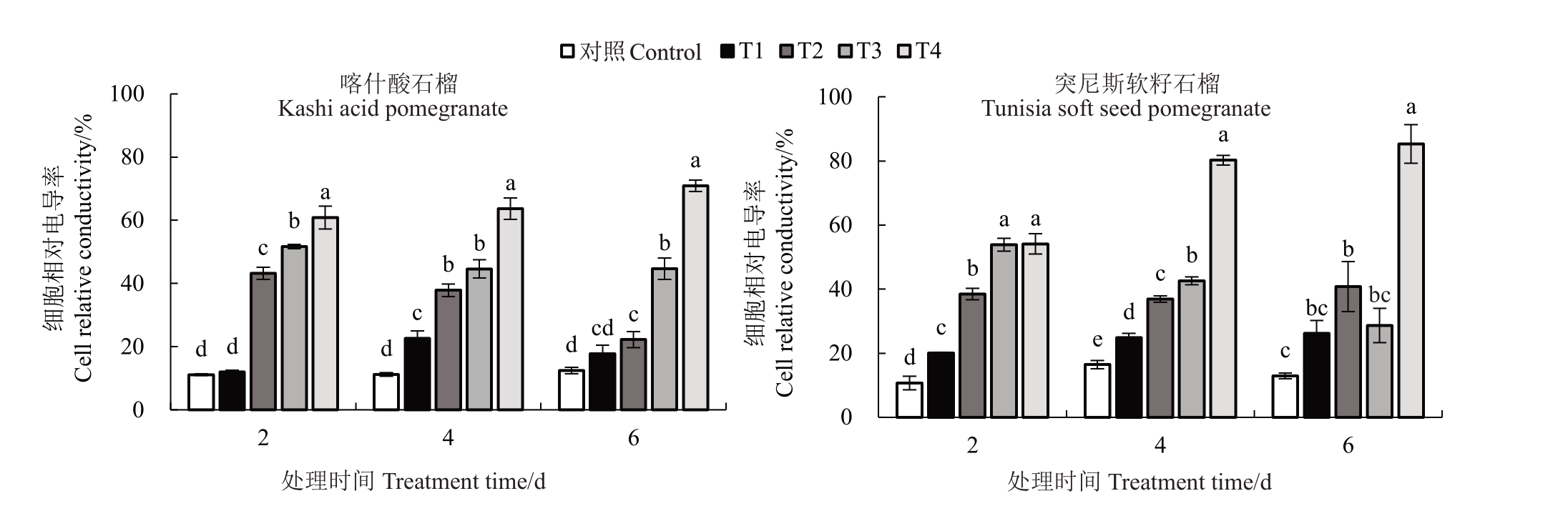
图6 低温胁迫对2 个石榴品种幼苗叶片相对电导率的影响
Fig.6 Effects of low temperature stress on relative electrical conductivity in leaves for seedlings of two pomegranate varieties
2.5.2 MDA含量 如图7所示,喀什酸石榴在胁迫2 d 后MDA 含量(w,后同)在0.496~0.855 mg·g-1之间,T1、T2 处理时较对照上升9.27%和9.07%,无显著性差异(p>0.05),T3、T4处理的分别上升75.2%和72.37%,与对照相比差异显著;胁迫4、6 d后MDA含量分别在0.669~0.973、0.637~1.04 mg·g-1之间,增幅分别为45.43%~111.52%和23.93%~102.33%,除T1处理的胁迫6 d 外其余各处理均与对照差异显著。突尼斯软籽石榴与对照相比,处理2、4、6 d 后MDA含量分别在0.552~1.073、0.539~1.403 和1.229~1.198 4 mg·g-1之间,在胁迫2 d 后的T3、T4 处理、胁迫4 d后的T2、T3、T4处理和胁迫6 d后的各处理均与对照呈显著差异。低温处理过程中,2 个石榴品种处理T1、T2 和T3 胁迫2 d 的MDA 含量无显著变化,在低温各处理胁迫4、6 d后均随胁迫时间延长和胁迫程度加剧而呈现出上升趋势。其中,突尼斯软籽石榴品种MDA 含量的增幅大于喀什酸石榴品种,尤其在胁迫4 d时T2、T3和T4处理和胁迫6 d时的各处理,MDA含量急剧升高。
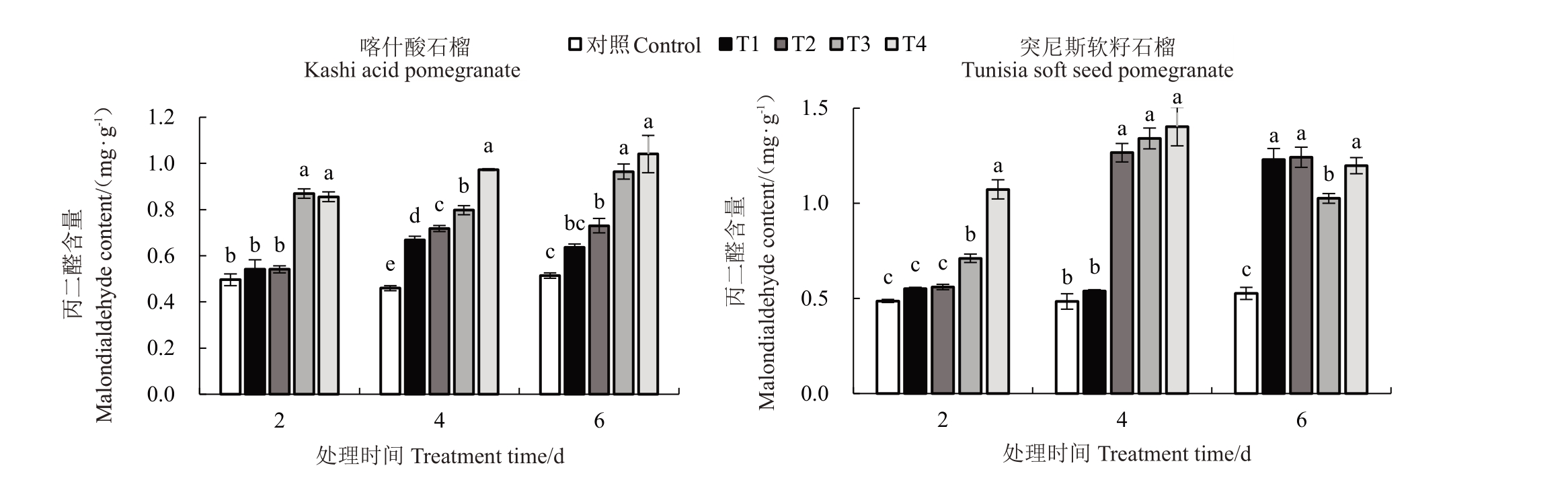
图7 低温胁迫对2 个石榴品种幼苗叶片MDA 含量的影响
Fig.7 Effects of low temperature stress on malondialdehyde content in leaves for seedlings of two pomegranate varieties
3 讨 论
低温是植物生长、发育和生存的环境胁迫因子之一,影响植物的光合活性和生理生化过程。光合作用是植物生长最根本的基础,可为植物提供所需的能量和有机物质。其中,光合参数可直接反映植株长势和抗性强弱,与生理指标一样都是评估低温胁迫程度和植物耐寒能力的可靠依据[27-28]。本研究中,低温导致2 个石榴品种叶片光合参数受到显著抑制,随着低温胁迫时间的延长Pn、Gs以及Tr持续下降,说明低温抑制了植物的光合作用,且随着胁迫时间的延长和胁迫程度的加强而呈加重趋势。此外,不同低温处理对突尼斯软籽石榴的抑制效应更大。突尼斯软籽石榴在极重度低温胁迫6 d 后光合参数测不出数值,同时重度低温胁迫6 d和极重度低温胁迫4 d净光合速率趋近于0,说明突尼斯软籽石榴品种较喀什酸石榴品种的低温耐性弱,在重度低温和极重度低温胁迫环境下突尼斯软籽石榴品种几乎无光合产物积累。大多数研究学者认为,低温导致光合速率下降的因素既有气孔因素也有非气孔因素。如果Pn、Gs及Tr同时下降,Ci也相应下降,即气孔因素占主导;如果Pn、Gs及Tr下降的同时Ci升高,则是由非气孔因素阻碍CO2的利用,造成Ci积累所致。本试验中,低温条件下Ci随温度降低呈先上升后下降趋势,表明在低温胁迫下,石榴叶片Pn下降主要是由非气孔限制为主作用的结果。这与冯立娟等[29]等在设施条件下测得突尼斯软籽石榴光合参数的结论一致。
光合色素具有吸收、传递和转化光能的作用,叶绿素含量常作为叶绿体发育、光合能力的指标[30],与光合速率呈正相关,类胡萝卜素可以起到光能捕获和光破坏防御的作用[31]。本研究中随着处理温度的下降和处理时间的延长,2个品种叶片叶绿素a和叶绿素总量较CK 相比均显著降低,喀什酸石榴在轻度胁迫时与对照相比无显著变化,突尼斯软籽石榴的叶绿素含量对低温更加敏感。一方面可能是叶绿素合成酶活性降低使叶绿素合成减少导致光合性能的降低,另一方面可能是过度低温导致叶绿体超微结构受到破坏,PSⅡ的电子传递受阻,位于类囊体上的色素复合体受到影响从而导致叶绿素含量的降低[32]。类胡萝卜素含量降低表明低温胁迫下石榴光破坏防御能力的降低,进一步损伤光合机构导致光合能力下降,影响植株生长速度。叶绿素a、叶绿素b和叶绿素总量与低温胁迫程度和低温胁迫时间呈负相关,这与李琦瑶等[33]在低温胁迫条件下烤烟叶绿素含量变化趋势的研究结论一致。
叶绿素荧光分析技术是光合作用的灵敏探针,能检测植物光合生理状况,反映光能的吸收、传递与分配情况,是阐明植物光合机制的重要手段[34]。可变荧光与最大荧光的比值Fv/Fm反映了PSⅡ反应中心的最大光化学效率,Y(Ⅱ)表示实际光化学效率,常用来表示植物光合作用中电子传递的量子产额。低温胁迫下Fv/Fm明显下降,可作为低温胁迫程度的指针和探针[35]。本研究中随着温度的降低,2个品种叶片Fv/Fm和Y(Ⅱ)的数值均呈下降趋势,不耐寒品种突尼斯软籽石榴在低温下叶绿素荧光数值变化更大,可能是低温胁迫下叶片同化CO2的能力下降,致使叶绿体中ATP 和NADPH 的含量下降,引发对PSⅡ反馈氧化还原作用的发生,进而导致光能过剩,引起Fv/Fm下降,同时说明PSⅡ的结构受到低温胁迫的伤害,产生了光抑制。光化学猝灭系数qP是光化学反应所引起的荧光产额的下降,反映PSⅡ原初电子受体QA的还原状态及开放的反应中心占总PSⅡ反应中心的比例[36]。本研究中石榴叶片qP 随低温持续时间的延长和温度的降低均呈下降趋势,这与杨柳青等[37]和王兆等[38]对南美天胡荽和彩叶草叶的研究结论一致,说明PSⅡ开放的反应中心比例降低和固定CO2的电子减少。NPQ 是指PSⅡ天线色素吸收的光能不能用于光合电子传递而以热能的形式耗散掉的光能部分,是一种自我保护机制,对光合系统起一定的保护作用[21]。本研究中,石榴叶片的NPQ与低温持续时间长短关系不大,但随着低温强度加剧NPQ呈较为显著的上升趋势,表明植物在受到低温胁迫时开启自我保护机制,植物可能通过NPQ的升高来抵御过量光能的伤害,同时部分失活的PSⅡ可能仍在启动热耗散的保护机制来抵御过量光能的伤害,这一研究结果与刘蕾蕾等[39]对小麦的研究结果一致。
在植物受到低温胁迫时体内积累大量渗透调节物质来使细胞膜渗透势下降从而减轻伤害,可溶性糖、可溶性蛋白和脯氨酸是具有提供能量、提高细胞质浓度和信号转导的3种重要调节物质[40]。本研究中,脯氨酸含量随低温胁迫时间和低温的降低均逐渐增加,而可溶性糖和可溶性蛋白含量呈先升后降的趋势,与谢小玉等[41]的研究结果一致,说明石榴叶片可在一定程度上通过积累渗透调节物质来缓解低温胁迫。
在低温胁迫下,植物需要维持细胞的行为和活性,特别是维持具有生物活性的细胞膜和蛋白质结构的稳定性,才能在不利的环境中生存[42]。因此,在细胞水平上的低温应激往往伴随着脱水应激,从而导致电解质泄漏的增加和膜脂相的变化。在极端情况下,冰晶可以穿透植物细胞,导致细胞质流出,最终植物死亡。本研究中,随着低温时间的延长和胁迫程度的加强,2 个品种石榴的相对电导率均显著升高,说明低温导致细胞质膜的稳定性受到一定的破坏,突尼斯软籽石榴在极重度胁迫4、6 d后急剧上升,说明低温持续时间对相对电导率的影响更大。MDA 也被认为是植物氧化应激和细胞膜对低温反应的有机统一性的可靠标志[43]。本研究中,2个石榴的MDA 含量都随低温加剧而升高,但胁迫2 d 时,轻度胁迫和重度胁迫对喀什酸石榴无显著影响,之后随胁迫时间的延长喀什酸石榴表现出升高趋势,而突尼斯软籽石榴是先升高后降低,表明膜脂过氧化程度较严重。
4 结 论
突尼斯软籽石榴的低温耐性显著低于新疆当地主栽品种喀什酸石榴,具体表现突尼斯软籽石榴在4个低温胁迫处理下电解质渗透率及MDA、可溶性糖、可溶性蛋白和脯氨酸含量均不同程度高于喀什酸石榴。此外,光合色素含量、光合参数和叶绿素荧光参数的降幅亦不同程度高于喀什酸石榴。且在该试验条件下,突尼斯软籽石榴不耐较长低温持续时间的胁迫,重度低温(0 ℃持续6 d)和极重度低温(-3 ℃持续4 d)均导致光合作用严重受阻,引起低温冻害,研究结果可为实际生产提供参考。
[1] JALIKOP S H,VENUGOPALAN R,KUMAR R. Association of fruit traits and aril browning in pomegranate (Punica granatum L.)[J].Euphytica,2010,174(1):137-141.
[2] TEIXEIRA DA SILVA J A,RANA T S,NARZARY D,VERMA N,MESHRAM D T,RANADE S A. Pomegranate biology and biotechnology:A review[J]. Scientia Horticulturae,2013,160:85-107.
[3] 卓热木·塔西,木合塔尔·扎热,卢明艳,帕丽旦·阿布什,吴正保.自然干旱条件下2 个石榴品种生长和光合等生理特性的变化[J].干旱地区农业研究,2018,36(6):77-85.Zhuoremu·Tashi,Muhertar·Zhare,LU Mingyan,Palidan·Abbosh,WU Zhengbao. Changes in growth and photosynthetic and other physiological characteristics of two pomegranate cultivars under drought condition[J].Agricultural Research in the Arid Areas,2018,36(6):77-85.
[4] 胡青霞,冯梦晨,陈延惠,郭强,简在海,史江莉,万然,郑先波.突尼斯软籽石榴果实生长发育及其品质形成规律研究[J].果树学报,2022,39(3):426-438.HU Qingxia,FENG Mengchen,CHEN Yanhui,GUO Qiang,JIAN Zaihai,SHI Jiangli,WAN Ran,ZHENG Xianbo. Growth and development of Tunisia soft seed pomegranate and its quality formation regulation[J].Journal of Fruit Science,2022,39(3):426-438.
[5] 柴亚倩,关思慧,崔洪鑫,许静,朱晓玲,刁明,孔秋生.水氮互作对石榴幼苗光合荧光及生理特性的影响[J]. 果树学报,2022,39(12):2352-2364.CHAI Yaqian,GUAN Sihui,CUI Hongxin,XU Jing,ZHU Xiaoling,DIAO Ming,KONG Qiusheng. Effects of water and nitrogen interaction on photosynthetic fluorescence and physiological characteristics of pomegranate seedlings[J]. Journal of Fruit Science,2022,39(12):2352-2364.
[6] 沈晓燕,揭波,李爽,王幸,李文杰.倒春寒危害软籽石榴树的温度及受害情况研究[J].落叶果树,2021,53(2):22-25.SHEN Xiaoyan,JIE Bo,LI Shuang,WANG Xing,LI Wenjie.Investigation and research on the effect of late spring coldness on the growth of soft seed pomegranate trees[J]. Deciduous Fruits,2021,53(2):22-25.
[7] MORADI S,BANINASAB B,GHOLAMI M,GHOBADI C.Paclobutrazol application enhances antioxidant enzyme activities in pomegranate plants affected by cold stress[J].The Journal of Horticultural Science and Biotechnology,2017,92(1):65-71.
[8] ZHANG C J,CHEN G X,GAO X X,CHU C J. Photosynthetic decline in flag leaves of two field-grown spring wheat cultivars with different senescence properties[J]. South African Journal of Botany,2006,72(1):15-23.
[9] 杜祥备,王家宝,刘小平,夏家平,韩杨.减氮运筹对甘薯光合作用和叶绿素荧光特性的影响[J]. 应用生态学报,2019,30(4):1253-1260.DU Xiangbei,WANG Jiabao,LIU Xiaoping,XIA Jiaping,HAN Yang. Effects of nitrogen fertilizer reduction management on photosynthesis and chlorophyll fluorescence characteristics of sweetpotato[J]. Chinese Journal of Applied Ecology,2019,30(4):1253-1260.
[10] 许永安.低温胁迫对甜瓜幼苗光合能力及叶绿素荧光参数的影响[J].中国瓜菜,2020,33(2):22-26.XU Yong’an.Effects of low-temperature stress on photosynthetic capacity and chlorophyll fluorescence parameters in melon[J].China Cucurbits and Vegetables,2020,33(2):22-26.
[11] 郑春芳,刘伟成,魏龙,陈继浓,张呈念,仇建标,丁文勇,郑青松.外施褪黑素对低温胁迫下红树植物秋茄光合作用和抗坏血酸-谷胱甘肽循环的调控[J]. 植物生理学报,2019,55(8):1211-1221.ZHENG Chunfang,LIU Weicheng,WEI Long,CHEN Jinong,ZHANG Chengnian,QIU Jianbiao,DING Wenyong,ZHENG Qingsong. Melatonin regulates photosynthesis and ascorbateglutathione cycle in a mangrove Kandelia obovata under low temperature stress[J]. Plant Physiology Journal,2019,55(8):1211-1221.
[12] 邹娅,米文博,徐明霞,徐春梅,刘自刚,赵彩霞,米超.低温胁迫下北方强冬性区甘蓝型冬油菜的低温光合生理特征[J].甘肃农业大学学报,2021,56(2):105-113.ZOU Ya,MI Wenbo,XU Mingxia,XU Chunmei,LIU Zigang,ZHAO Caixia,MI Chao. Physiological and photosynthetic characteristics of winter rapeseeds under low temperature stress in northern strong winterness region[J]. Journal of Gansu Agricultural University,2021,56(2):105-113.
[13] 罗鑫辉,刘明月,黄科,刘玉兵,彭淼,王军伟.不同品种番茄幼苗低温适应性评价及光合特性分析[J]. 中国瓜菜,2021,34(8):49-55.LUO Xinhui,LIU Mingyue,HUANG Ke,LIU Yubing,PENG Miao,WANG Junwei.Evaluation of low temperature adaptability and analysis of photosynthetic characteristics in different tomato varieties[J]. China Cucurbits and Vegetables,2021,34(8):49-55.
[14] 胡晨曦,李子恒,张云虹,张林巧,祁建波,张瑛,周如美,张永泰,张永吉.低温弱光对不同品种辣椒幼苗生长和光合特性的影响[J].福建农业学报,2022,37(5):617-625.HU Chenxi,LI Ziheng,ZHANG Yunhong,ZHANG Linqiao,QI Jianbo,ZHANG Ying,ZHOU Rumei,ZHANG Yongtai,ZHANG Yongji. Effects of low temperature and reduced light on growth and photosynthesis of seedlings of different pepper cultivars[J]. Fujian Journal of Agricultural Sciences,2022,37(5):617-625.
[15] 杨鸿基,徐诚,高亚宁,杨建超,杨平,轩正英,阿依买木·沙吾提. 新疆哈密瓜苗期耐冷性评价[J]. 中国瓜菜,2022,35(2):90-94.YANG Hongji,XU Cheng,GAO Yaning,YANG Jianchao,YANG Ping,XUAN Zhengying,Ayimaimu·Shawuti. Cold tolerance evaluation of Hami melon seedlings in Xinjiang[J]. China Cucurbits and Vegetables,2022,35(2):90-94.
[16] BAN Q Y,WANG X W,PAN C,WANG Y W,KONG L,JIANG H G,XU Y Q,WANG W Z,PAN Y T,LI Y Y,JIANG C J. Comparative analysis of the response and gene regulation in cold resistant and susceptible tea plants[J]. PLoS One,2017,12(12):e0188514.
[17] 逯久幸,苗润田,王司琦,赵鹏飞,张开明,李永华.低温胁迫下秋菊叶片光系统特性分析[J].植物生理学报,2022,58(2):425-434.LU Jiuxing,MIAO Runtian,WANG Siqi,ZHAO Pengfei,ZHANG Kaiming,LI Yonghua. Analysis of photosystem features in autumn chrysanthemum leaves under low temperature stress[J].Plant Physiology Journal,2022,58(2):425-434.
[18] 郭雁君,吉前华,杜鹏飞,黄火金,许冰玲.低温胁迫对2 种主要砧穗组合砂糖橘幼树抗寒性的影响[J].果树学报,2022,39(5):784-799.GUO Yanjun,JI Qianhua,DU Pengfei,HUANG Huojin,XU Bingling. Effect of two main rootstock species on cold resistance of Shatangju mandarin saplings under low temperature stress[J].Journal of Fruit Science,2022,39(5):784-799.
[19] 杨军,黄淑娥,郭建平,李翔翔,李亚靖,丁美萍.不同夜间低温条件对脐橙光合参数和形态特征的影响[J]. 果树学报,2022,39(11):2088-2098.YANG Jun,HUANG Shu’e,GUO Jianping,LI Xiangxiang,LI Yajing,DING Meiping. Effects of different low night temperature conditions on the photosynthetic parameters and morphological characteristics in navel orange[J]. Journal of Fruit Science,2022,39(11):2088-2098.
[20] 王洋洋,贺利,任德超,段剑钊,胡新,刘万代,郭天财,王永华,冯伟.基于主成分-聚类分析的不同水分冬小麦晚霜冻害评价[J].作物学报,2022,48(2):448-462.WANG Yangyang,HE Li,REN Dechao,DUAN Jianzhao,HU Xin,LIU Wandai,GUO Tiancai,WANG Yonghua,FENG Wei.Evaluations of winter wheat late frost damage under different water based on principal component-cluster analysis[J].Acta Agronomica Sinica,2022,48(2):448-462.
[21] 张陇艳,程功敏,魏恒玲,王寒涛,芦建华,马峙英,喻树迅.陆地棉种子萌发期对低温胁迫的响应及耐冷性鉴定[J].中国农业科学,2021,54(1):19-33.ZHANG Longyan,CHENG Gongmin,WEI Hengling,WANG Hantao,LU Jianhua,MA Zhiying,YU Shuxun. Chilling tolerance identification and response to cold stress of Gossypium hirsutum varieties (lines) during germination stage[J]. Scientia Agricultura Sinica,2021,54(1):19-33.
[22] 严衍禄,刘心生.叶绿素测定方法的研究[J].北京农业大学学报,1982,8(2):53-67.YAN Yanlu,LIU Xinsheng. Studies on determination chlorophylls a and b[J]. Journal of China Agricultural University,1982,8(2):53-67.
[23] 邹琦.植物生理学实验指导[M].北京:中国农业出版社,2000.ZOU Qi. Experimental instruction of plant physiology[M]. Beijing:China Agriculture Press,2003.
[24] 李合生.植物生理生化实验原理和技术[M].北京:高等教育出版社,2000.LI Hesheng.Principles and techniques of plant physiological biochemical experiment[M].Beijing:Higher Education Press,2000.
[25] 高俊凤. 植物生理学实验指导[M]. 北京:高等教育出版社,2006.GAO Junfeng. Experimental guidance for plant physiology[M].Beijing:Higher Education Press,2006.
[26] 钟信念,梁其干,李有忠,赵建华,马海霞,李志博.蕾期低温胁迫对棉花光合特性的影响[J]. 新疆农业科学,2022,59(7):1573-1581.ZHONG Xinnian,LIANG Qigan,LI Youzhong,ZHAO Jianhua,MA Haixia,LI Zhibo. Effects of low temperature stress in bud stage on photosynthetic characteristics of cotton[J].Xinjiang Agricultural Sciences,2022,59(7):1573-1581.
[27] 项洪涛,郑殿峰,何宁,李琬,王曼力,王诗雅.植物对低温胁迫的生理响应及外源脱落酸缓解胁迫效应的研究进展[J].草业学报,2021,30(1):208-219.XIANG Hongtao,ZHENG Dianfeng,HE Ning,LI Wan,WANG Manli,WANG Shiya.Research progress on the physiological response of plants to low temperature and the amelioration effcectiveness of exogenous ABA[J].Acta Prataculturae Sinica,2021,30(1):208-219.
[28] GOHARRIZI K J,MERU G,KERMANI S G,HEIDARINEZHAD A. Short-term cold stress affects physiological and biochemical traits of pistachio rootstocks[J]. South African Journal of Botany,2021,141:90-98.
[29] 冯立娟,杨雪梅,王嘉艳,史作亚,尹燕雷.设施条件下两个石榴品种光合荧光特征参数差异与变化[J/OL].分子植物育种,2021:1-13[2022-09-28].http://kns.cnki.net/kcms/detail/46.1068.s.20210918.1620.022.html.FENG Lijuan,YANG Xuemei,WANG Jiayan,SHI Zuoya,YIN Yanlei. Differences and changes of photosynthetic fluorescence characteristic parameters of two pomegranate varieties under protected conditions[J/OL]. Molecular Plant Breeding,2021:1-13[2022-09-28]. http://kns.cnki.net/kcms/detail/46.1068.s.20210918.1620.022.html.
[30] LING Q H,HUANG W H,JARVIS P.Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana[J].Photosynthesis Research,2011,107(2):209-214.
[31] 张振花,孙胜,刘洋,李靖,袁宏霞,郑金英,邢国明.增施CO2对温室番茄结果期叶片光合特性的影响[J]. 生态学杂志,2018,37(5):1398-1402.ZHANG Zhenhua,SUN Sheng,LIU Yang,LI Jing,YUAN Hongxia,ZHENG Jinying,XING Guoming. Effects of CO2 enrichment on photosynthetic characteristics of greenhouse tomato during fruiting stage[J]. Chinese Journal of Ecology,2018,37(5):1398-1402.
[32] 岳海,李国华,李国伟,陈丽兰,孔广红,梁国平.澳洲坚果不同品种耐寒特性的研究[J].园艺学报,2010,37(1):31-38.YUE Hai,LI Guohua,LI Guowei,CHEN Lilan,KONG Guanghong,LIANG Guoping. Studies on cold resistance of different Macadamia cultivars[J].Acta Horticulturae Sinica,2010,37(1):31-38.
[33] 李琦瑶,陈爱国,王程栋,刘光亮,周培禄,郑璇,曾文龙,陈志厚,王树声.低温胁迫对烤烟幼苗光合荧光特性及叶片结构的影响[J].中国烟草学报,2018,24(2):30-38.LI Qiyao,CHEN Aiguo,WANG Chengdong,LIU Guangliang,ZHOU Peilu,ZHENG Xuan,ZENG Wenlong,CHEN Zhihou,WANG Shusheng. Effects of low temperature stress on photosynthetic fluorescence characteristics and leaf tissue structure of flue-cured tobacco seedlings[J].Acta Tabacaria Sinica,2018,24(2):30-38.
[34] 张利霞,常青山,薛娴,刘伟,张巧明,陈苏丹,郑轶琦,李景林,陈婉东,李大钊.酸胁迫对夏枯草叶绿素荧光特性和根系抗氧化酶活性的影响[J].草业学报,2020,29(8):134-142.ZHANG Lixia,CHANG Qingshan,XUE Xian,LIU Wei,ZHANG Qiaoming,CHEN Sudan,ZHENG Yiqi,LI Jinglin,CHEN Wandong,LI Dazhao. Effects of acid stress on chlorophyll fluorescence characteristics and root antioxidant activity of Prunella vulgaris[J].Acta Prataculturae Sinica,2020,29(8):134-142.
[35] 邵怡若,许建新,薛立,张柔,吴彩琼,卢广超.低温胁迫时间对4 种幼苗生理生化及光合特性的影响[J].生态学报,2013,33(14):4237-4247.SHAO Yiruo,XU Jianxin,XUE Li,ZHANG Rou,WU Caiqiong,LU Guangchao.Effects of low temperature stress on physiological-biochemical indexes and photosynthetic characteristics of seedlings of four plant species[J].Acta Ecologica Sinica,2013,33(14):4237-4247.
[36] CONSTANT S,PEREWOSKA I,ALFONSO M,KIRILOVSKY D. Expression of the psbA gene during photoinhibition and recovery in Synechocystis PCC 6714:Inhibition and damage of transcriptional and translational machinery prevent the restoration of photosystem Ⅱactivity[J]. Plant Molecular Biology,1997,34(1):1-13.
[37] 杨柳青,张柳,廖飞勇,吴红强,刘志昂,曾红,朱小青.低温胁迫对南美天胡荽光合作用和叶绿素荧光的影响[J].经济林研究,2014,32(4):99-102.YANG Liuqing,ZHANG Liu,LIAO Feiyong,WU Hongqiang,LIU Zhiang,ZENG Hong,ZHU Xiaoqing. Effects of low temperature stress on photosynthesis and chlorophyll fluorescence parameters in Hydrocotyle vulgaris[J]. Nonwood Forest Research,2014,32(4):99-102.
[38] 王兆,刘晓曦,郑国华.低温胁迫对彩叶草光合作用及叶绿素荧光的影响[J].浙江农业学报,2015,27(1):49-56.WANG Zhao,LIU Xiaoxi,ZHENG Guohua.Effects of low temperature stress on the photosynthesis and chlorophyll fluorescence of Solenostemon scutellarioides[J].Acta Agriculturae Zhejiangensis,2015,27(1):49-56.
[39] 刘蕾蕾,纪洪亭,刘兵,马吉锋,肖浏骏,汤亮,曹卫星,朱艳.拔节期和孕穗期低温处理对小麦叶片光合及叶绿素荧光特性的影响[J].中国农业科学,2018,51(23):4434-4448.LIU Leilei,JI Hongting,LIU Bing,MA Jifeng,XIAO Liujun,TANG Liang,CAO Weixing,ZHU Yan. Effects of jointing and booting low temperature treatments on photosynthetic and chlorophyll fluorescence characteristics in wheat leaf[J].Scientia Agricultura Sinica,2018,51(23):4434-4448.
[40] DING Y L,SHI Y T,YANG S H. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants[J].New Phytologist,2019,222(4):1690-1704.
[41] 谢小玉,侯爽,郭金格,汪芬芬,崔凯东,杨奇,陈锦芬,顾开元.烤烟伸根期对低温胁迫的生理响应[J].中国烟草学报,2022,28(5):47-55.XIE Xiaoyu,HOU Shuang,GUO Jinge,WANG Fenfen,CUI Kaidong,YANG Qi,CHEN Jinfen,GU Kaiyuan. Physiological responses of flue-cured tobacco to low temperature stress at rootextending stage[J].Acta Tabacaria Sinica,2022,28(5):47-55.
[42] BAYSAL F. Effects of low-temperature acclimation on morphological and physiological indices of banana seedlings[J]. Emirates Journal of Food and Agriculture,2022,34(5):446-454.
[43] HE W D,GAO J,DOU T X,SHAO X H,BI F C,SHENG O,DENG G M,LI C Y,HU C H,LIU J H,ZHANG S,YANG Q S,YI G J.Early cold-induced peroxidases and aquaporins are associated with high cold tolerance in dajiao (Musa spp.‘Dajiao’)[J].Frontiers in Plant Science,2018,9:282.