葡萄是世界上栽培最早、分布最广的果树之一,因其富含花青素、类黄酮和维生素等成分,深受消费者喜爱。中国是世界葡萄生产大国,新郁葡萄是中国自主培育的优良鲜食葡萄品种,亲本为红地球自然杂交后代E42-6×里扎马特,该品种穗形美观,果粒大,鲜红-紫红色,肉脆味甜,贮运性能较好,栽培适应性强[1],因此栽培经济效益好,栽培面积不断扩大。在西北干旱区种植的新郁葡萄因生育期光照辐射强,常导致着色过深,降低了外观品质及商品性,急需通过栽培措施优化生育期果际光环境,改善果实色泽,提高品质。
套袋是优质葡萄生产过程中的重要环节,套袋可以防止鸟类、昆虫对果实的危害,同时阻碍病菌、灰尘和农药的侵染,保持果面的清洁和果粉的完整,使葡萄着色更均匀,也使葡萄更绿色、安全、优质[2-3]。套袋通过改变果实生长发育的微环境对品质产生影响[4]。光照条件改变是套袋引起果实品质变化的重要因素。光可作为一种重要的信号激发植物体内的光受体,调节植物的形态建成、光周期反应与生理节律,并与植物体内的脱落酸(abscisic acid,ABA)、乙烯等其他信号分子相互协作,共同调控[5-6]。不同光质通过与特定的色素互作来影响植物次生代谢物的合成,如光敏色素吸收红光、远红光,而隐花青素和向光素吸收紫外光和蓝光[7-8]。大量研究证实,果实品质中的糖、酸及花色苷含量均受到光条件的影响,如葡萄套袋后造成的弱光胁迫会降低可溶性固形物含量,导致葡萄着色不完全,延迟成熟,而去袋后果实色泽和可溶性固形物含量可迅速恢复正常[9]。Zhang 等[10]研究认为蓝光能够显著增加赤霞珠葡萄锦葵色素-3-O-葡萄糖苷和芍药花素-3-O-葡萄糖苷、醇类和酚类挥发性物质及可溶性糖中的葡萄糖、果糖含量,降低柠檬酸和苹果酸含量。光能够通过调控花色苷合成相关结构基因和调控基因的表达来调控花色苷的积累[11-13],如太阳紫外光照射能够诱导Tempranillo 葡萄花色苷合成途径中的PAL、CHS、C4H、FLS1、VvGT5、VvGT6、VvMYB24、VvMYBF1 等多个基因上调表达[14]。不同颜色果袋通过影响光信号转录因子VvHY5的表达,调控花青苷合成调控基因VvMYBA1和结构基因VvCHS、VvLDOX、VvUFGT等的表达,进而影响葡萄果皮花色苷的合成[15]。
西北干旱区葡萄园光照辐射强,容易造成新郁葡萄着色过深,关于不同颜色果袋对西北干旱区新郁葡萄果实品质及着色的调控研究未见报道。笔者在本研究中拟采用不同颜色果袋(白袋、红袋、黄袋、蓝袋和绿袋)对新郁葡萄进行套袋,以不套袋为对照,分析不同颜色果袋引起的光环境差异对新郁葡萄果实品质及着色的影响,旨在为生产优质新郁葡萄果袋的选择提供依据。
1 材料和方法
1.1 试验材料
试验于2021 年在新疆维吾尔自治区葡萄瓜果研究所葡萄栽培示范基地(90°30′ E,42°91′ N)进行。试验地海拔419 m,年降雨量25.3 mm,年蒸发量2 751.0 mm,全年日照时数3 122.8 h,10 ℃以上有效积温4525 ℃以上,无霜期192 d,属于典型的大陆性暖温带荒漠气候,为极端干旱区气候类型。土壤质地为砾石砂壤土。供试材料为长势一致的7年生欧亚种(Vitis vinifera L.)新郁葡萄,采用顺行龙干+(V+水平)叶幕模式栽培,东西行向,株行距2.0 m×3.5 m,新梢间距15 cm,单株果穗数20~24 穗,豆果期疏去穗尖和副穗,疏果至单穗果粒80粒左右。田间水肥和病虫害防治按照常规管理。
1.2 试验设计及样品采集
花后30 d(2021年6月20日)进行套袋处理。连续4 株为1 个小区,共设3 个小区,统一选取南面大小一致的果穗进行不同颜色果袋(红色、黄色、蓝色、绿色和白色)套袋,以不套袋为对照,果袋下端开口以保证各颜色果袋内温、湿度与环境基本一致。每种颜色果袋每小区随机套6穗,3次重复,共18个果穗。果实采收期(2021年8月12日)统一带袋采收,从每小区果穗的上、中、下部位随机取样,每穗取15粒,共90粒,混匀平均分成3份,2份用于常规理化指标测定,另外1份用手术刀片剥取果皮,液氮速冻后存于-80 ℃冰箱中,供花色苷单体测定。3次重复。
1.3 测定指标及方法
1.3.1 果袋内光谱测定 使用UniSpec-SC 单通道便携式光谱测定仪(PP SYSTEMS,USA)测定不同颜色果袋内的透射光谱,测定时间为上午11:30。
1.3.2 果实品质指标测定 果穗质量、果粒质量采用百分之一的电子天平称量;果粒纵径、横径及果柄粗度、果刷长度采用游标卡尺测量;果实耐压力采用GY-4 型数显果实硬度计(托普仪器,中国浙江)测定;果柄耐拉力采用NK-50 型数显推拉力计(Algol仪器,中国台湾)测定;用CR-400手持色差计(Konica Minolta,日本)测定每个果实赤道部位的色泽指标L*(亮度)、a*(红绿色差)、b*(黄蓝色差)。计算出色泽饱和度(chroma,C*)、色调角(hue angle,h°)和葡萄果实色泽指数(color index of red grape,CIRG),其中C*=[a*2+ b*2]1/2,h°=arctangent(b*/a*),CIRG=(180–h°)/(L*+C*),果实外观色泽的标准为CIRG<2 为黄绿,2≤CIRG<4 为粉红,4≤CIRG<5为红色,5≤CIRG<6为深红,CIRG≥6为蓝黑色[16]。以上果粒相关指标均读取30个数据。
新鲜葡萄榨汁,采用PAL-1 型手持数显折射仪(Atago,Tokyo,Japan)测定可溶性固形物(total soluble solid,TSS)含量;可滴定酸(titratable acidity,TA)含量采用0.05 mol·L-1 NaOH 滴定法[17]测定;维生素C含量采用钼蓝比色法[18]测定。以上指标每个样品平行测定3 次。固酸比(TSS/TA)为可溶性固形物与可滴定酸含量的比值。
1.3.3 花色苷单体测定 样品前处理。果皮液氮速冻后真空冷冻干燥,利用球磨仪研磨(30 Hz,1.5 min)至粉末状,称取50 mg 的粉末溶解于500 μL 提取液[50%(φ,后同)的甲醇水溶液,含0.1%盐酸]中,涡旋10 min,超声10 min,离心(转速12 000 r·min-1,30 min),吸取上清液,重复操作1 次,合并2 次上清液,用微孔滤膜(0.22 μm pore size)过滤样品,并保存于进样瓶中,用于超高效液相色谱(ultra performance liqiud chromatography,UPLC)和串联质谱(tandem mass spectrometry,MS/MS)分析。
色谱条件。色谱柱:ACQUITY BEH C18 1.7 μm,2.1 mm×100 mm;流动相:A 相为超纯水(加入0.1%甲酸),B 相为甲醇(加入0.1%甲酸);洗脱梯度:0.00 min B 相比例为5%,6.00 min 增至50%,12.00 min 增至95%,保持2.00 min,14.00 min 降至5%,并平衡2 min;流速0.35 mL·min-1;柱温40 ℃;进样量2 μL。
质谱条件。电喷雾离子源(electospray Ionization,ESI)温度550 ℃,正离子模式下质谱电压5500 V,气帘气(curtain gas,CUR)35 psi。在QTrap 6500+中,每个离子对是根据优化的去簇电压(declustering potential,DP)和碰撞能(collision energy,CE)进行扫描检测。
数据分析。基于标准品构建MWDB(metware database)数据库,对质谱检测的数据进行定性分析;利用三重四级杆质谱的多反应监测模式(multiple reaction monitoring,MRM)完成定量分析;利用软件Analyst 1.6.3处理质谱数据。
1.4 数据处理
用Microsoft Excel 2007 软件整理数据及绘制光谱分布特征图,用Origin 8.0 软件绘制相关性热图;用SPSS 25.0 软件进行统计分析,单因素方差分析(ANOVA)采用Duncan’s 法,差异显著性定义为p<0.05。
2 结果与分析
2.1 不同颜色果袋内光谱特征
不同颜色果袋内及对照光谱分布存在明显差异,果袋均不同程度降低了各波段辐射强度。相比对照,果袋对700~930 nm 波段辐射强度降幅最大,白袋、红袋和黄袋主、次峰值波长与对照基本一致,蓝袋和绿袋次峰不明显,绿袋主峰值波长出现明显偏移,主峰值(辐射强度)大小顺序为对照>白袋>红袋>黄袋>蓝袋>绿袋(图1)。
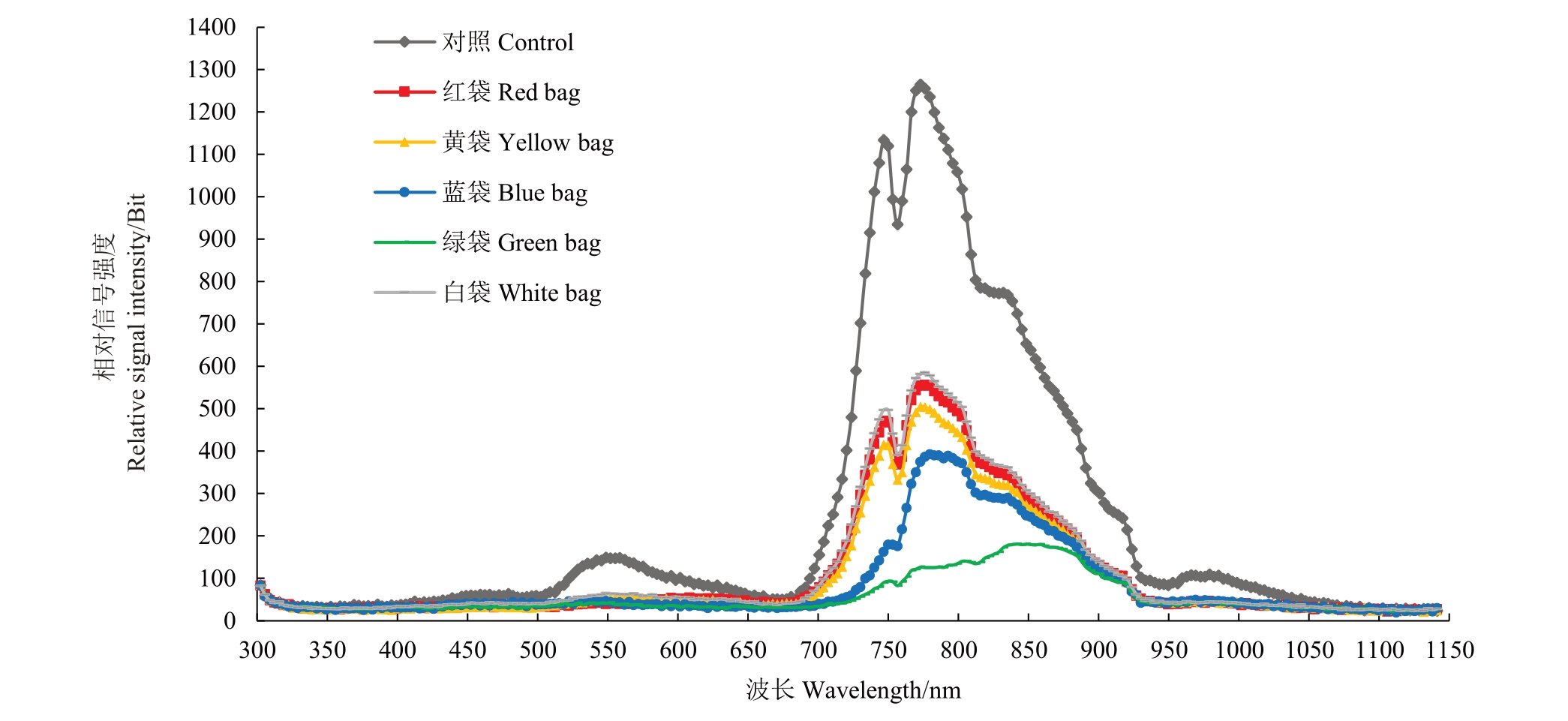
图1 不同颜色果袋内光谱分布特征
Fig.1 Spectral distribution characteristics of light within different color fruit bags
不同颜色果袋内总辐射及透光率相比对照均明显降低,大小顺序均为对照>白袋>红袋>黄袋>蓝袋>绿袋,且不同颜色果袋内各波段光辐射强度较对照均降低,红光、红外光降幅最大,降幅均在50%以上。各颜色果袋紫外光辐射强度差异不大,蓝袋和白袋紫光、蓝光辐射强度大于其他果袋,黄袋和白袋绿光辐射强度大于其他果袋,蓝袋和绿袋橙光、红光辐射强度小于其他果袋,绿袋红外光辐射强度最小。5种颜色果袋均增大了紫外光、紫光、蓝光和橙光的比例,其中绿袋和蓝袋紫外光、紫光、蓝光增幅均较大,绿袋橙光增幅最大;红袋降低了绿光比例,绿袋则增大了绿光比例;蓝袋和绿袋大幅降低了红光比例,仅为19.75%和13.99%,提高了红外光的比例。不同颜色果袋均降低了蓝光/紫外光值、红光/蓝光值和红光/红外光值,蓝袋和白袋蓝光/紫外光值较大,蓝袋和绿袋红光/蓝光值和红光/红外光值较小(表1~表2)。可见,不同颜色果袋内光照度和光质分布有较大差异,果袋均不同程度降低了袋内总辐射,蓝袋和绿袋对橙光、红光和红外光透过性较差,大幅降低了红光的比例。
表1 不同颜色果袋内光质分布特征
Table 1 The light quality in different color fruit bags×1000 Bit
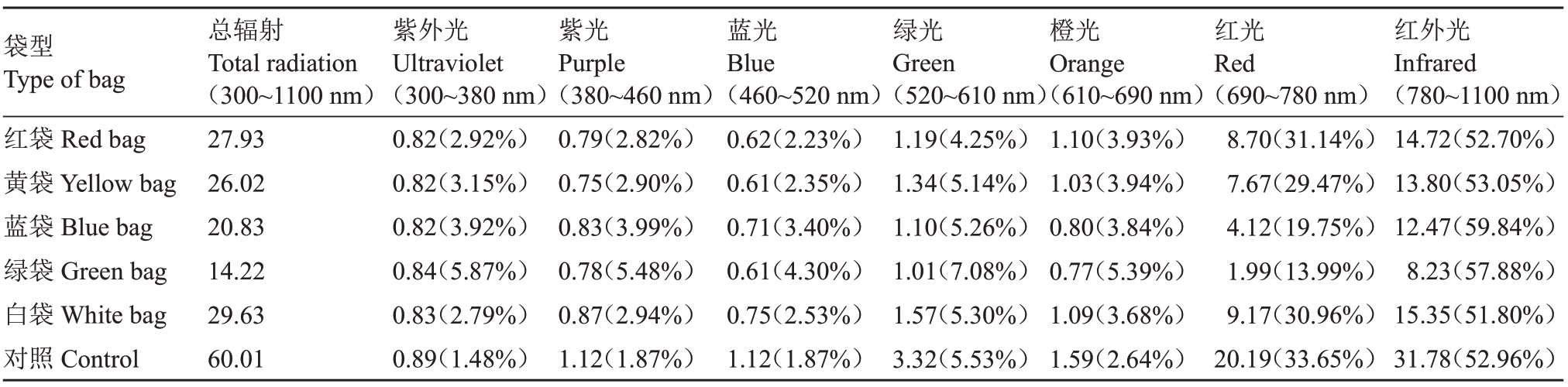
注:括号内数字为相应波段辐射占总辐射的百分率。
Note:The number in parentheses is the ratio of the corresponding waveband to the total radiation.
不同颜色果袋内总辐射及透光率相比对照均明显降低,大小顺序均为对照>白袋>红袋>黄袋>蓝袋>绿袋,且不同颜色果袋内各波段光辐射强度较对照均降低,红光、红外光降幅最大,降幅均在50%以上。各颜色果袋紫外光辐射强度差异不大,蓝袋和白袋紫光、蓝光辐射强度大于其他果袋,黄袋和白袋绿光辐射强度大于其他果袋,蓝袋和绿袋橙光、红光辐射强度小于其他果袋,绿袋红外光辐射强度最小。5 种颜色果袋均增大了紫外光、紫光、袋型Type of bag红袋Red bag黄袋Yellow bag蓝袋Blue bag绿袋Green bag白袋White bag对照Control总辐射Total radiation(300~1100 nm)27.93 26.02 20.83 14.22 29.63 60.01紫外光Ultraviolet(300~380 nm)0.82(2.92%)0.82(3.15%)0.82(3.92%)0.84(5.87%)0.83(2.79%)0.89(1.48%)紫光Purple(380~460 nm)0.79(2.82%)0.75(2.90%)0.83(3.99%)0.78(5.48%)0.87(2.94%)1.12(1.87%)蓝光Blue(460~520 nm)0.62(2.23%)0.61(2.35%)0.71(3.40%)0.61(4.30%)0.75(2.53%)1.12(1.87%)绿光Green(520~610 nm)1.19(4.25%)1.34(5.14%)1.10(5.26%)1.01(7.08%)1.57(5.30%)3.32(5.53%)橙光Orange(610~690 nm)1.10(3.93%)1.03(3.94%)0.80(3.84%)0.77(5.39%)1.09(3.68%)1.59(2.64%)红光Red(690~780 nm)8.70(31.14%)7.67(29.47%)4.12(19.75%)1.99(13.99%)9.17(30.96%)20.19(33.65%)红外光Infrared(780~1100 nm)14.72(52.70%)13.80(53.05%)12.47(59.84%)8.23(57.88%)15.35(51.80%)31.78(52.96%)
表2 不同颜色果袋的透光率及蓝光/紫外光、红光/蓝光、红光/红外光比值
Table 2 The transmittance and ratio of blue/Ultraviolet,red/blue,and red/infrared in different color fruit bags
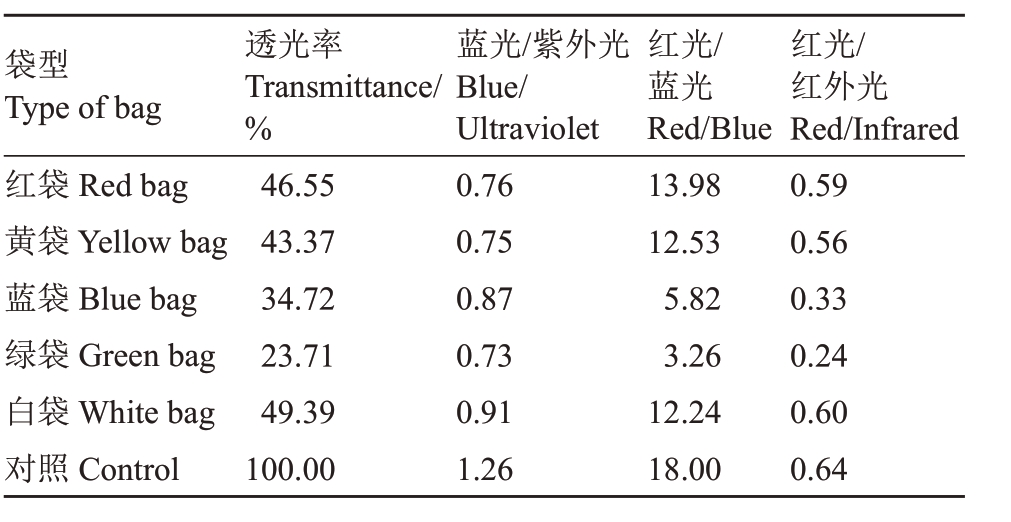
袋型Type of bag红袋Red bag黄袋Yellow bag蓝袋Blue bag绿袋Green bag白袋White bag对照Control袋型Type of透光率Transmittance/%46.55 43.37 34.72 23.71 49.39 100.00总辐射蓝光/紫外光Blue/Ultraviolet 0.76 0.75 0.87 0.73 0.91 1.26 Total radiation 300~380 nm红光/蓝光Red/Blue 13.98 12.53 5.82 3.26 12.24 18.00 380~460 nm红光/红外光Red/Infrared 0.59 0.56 0.33 0.24 0.60 0.64
2.2 不同颜色果袋对新郁葡萄果实品质的影响
2.2.1 果穗、果粒性状 绿袋果粒质量及纵、横径最小,显著小于其他颜色果袋及对照,其果粒质量比对照(13.92 g)减小15.59%,且果穗质量显著小于红袋和蓝袋;黄袋果粒纵径显著小于对照,降幅3.61%;果形指数以黄袋和绿袋最小,显著小于其他颜色果袋及对照。不同颜色果袋及对照的葡萄果柄直径无明显差异,绿袋果刷长度显著大于白袋(表3)。可见,绿袋减小新郁果粒质量及果形指数,略增大了果刷长度,黄袋减小了果形指数,白袋略减小了果刷长度。
表3 不同颜色果袋对新郁葡萄果穗、果粒性状的影响
Table 3 Effect of different color fruit bags on cluster and berry characters of Xinyu grape
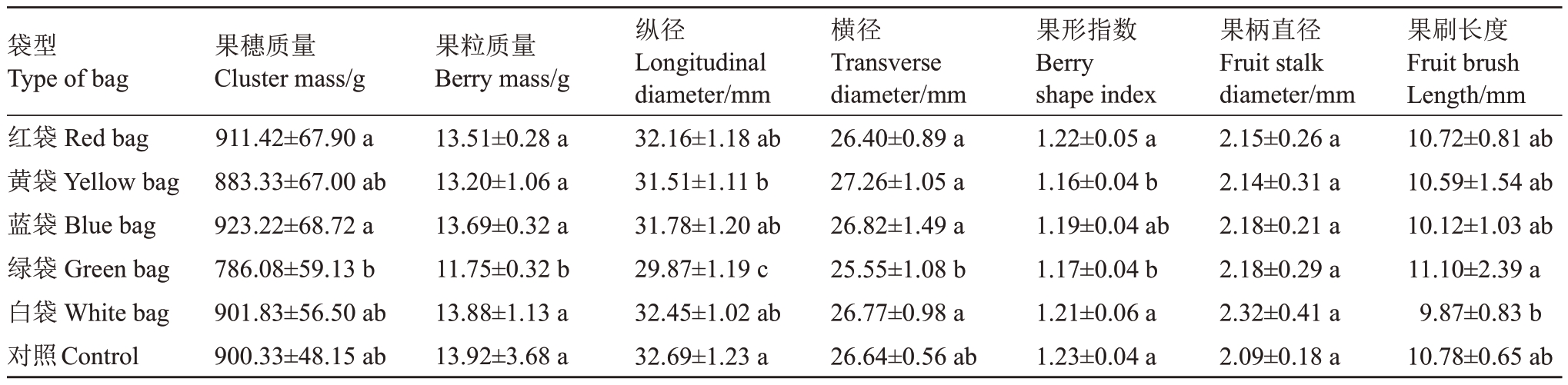
注:同列不同小写字母表示差异显著(p<0.05)。下同。
Note:Different lowercase letters in the same column mean significant differences at p<0.05.The same below.
袋型Type of bag红袋Red bag黄袋Yellow bag蓝袋Blue bag绿袋Green bag白袋White bag对照Control果穗质量Cluster mass/g 911.42±67.90 a 883.33±67.00 ab 923.22±68.72 a 786.08±59.13 b 901.83±56.50 ab 900.33±48.15 ab果粒质量Berry mass/g 13.51±0.28 a 13.20±1.06 a 13.69±0.32 a 11.75±0.32 b 13.88±1.13 a 13.92±3.68 a纵径Longitudinal diameter/mm 32.16±1.18 ab 31.51±1.11 b 31.78±1.20 ab 29.87±1.19 c 32.45±1.02 ab 32.69±1.23 a横径Transverse diameter/mm 26.40±0.89 a 27.26±1.05 a 26.82±1.49 a 25.55±1.08 b 26.77±0.98 a 26.64±0.56 ab果形指数Berry shape index 1.22±0.05 a 1.16±0.04 b 1.19±0.04 ab 1.17±0.04 b 1.21±0.06 a 1.23±0.04 a果柄直径Fruit stalk diameter/mm 2.15±0.26 a 2.14±0.31 a 2.18±0.21 a 2.18±0.29 a 2.32±0.41 a 2.09±0.18 a果刷长度Fruit brush Length/mm 10.72±0.81 ab 10.59±1.54 ab 10.12±1.03 ab 11.10±2.39 a 9.87±0.83 b 10.78±0.65 ab
2.2.2 果实理化指标 白袋相比对照果柄耐拉力显著减小,降幅22.37%,黄袋、红袋和白袋相比对照果实耐压力显著减小,降幅分别为21.07%、9.92%和9.92%。不同颜色果袋果实可溶性固形物含量较对照均显著减少,以蓝袋和绿袋最少,较对照分别减少19.25%和19.06%;黄袋、红袋和白袋较对照分别减少13.80%、11.53%和9.79%。绿袋可滴定酸含量相比对照显著升高,升幅13.95%,其他颜色果袋与对照差异均不显著。白袋固酸比与对照差异不显著,其他颜色果袋固酸比均显著小于对照,以绿袋和蓝袋最小,相比对照降幅29.55%和23.54%,红袋和黄袋降幅16.21%和13.79%。绿袋相比对照维生素C含量显著升高,而红袋则显著降低,其他颜色果袋与对照差异均不显著(表4)。可见,白袋对新郁葡萄理化指标的影响最小,其次是红袋和黄袋,蓝袋和绿袋对新郁葡萄内在品质产生了较大的负面影响。
表4 不同颜色果袋对新郁葡萄果实理化指标的影响
Table 4 Effects of different color fruit bags on physical and chemical indexes of Xinyu grape
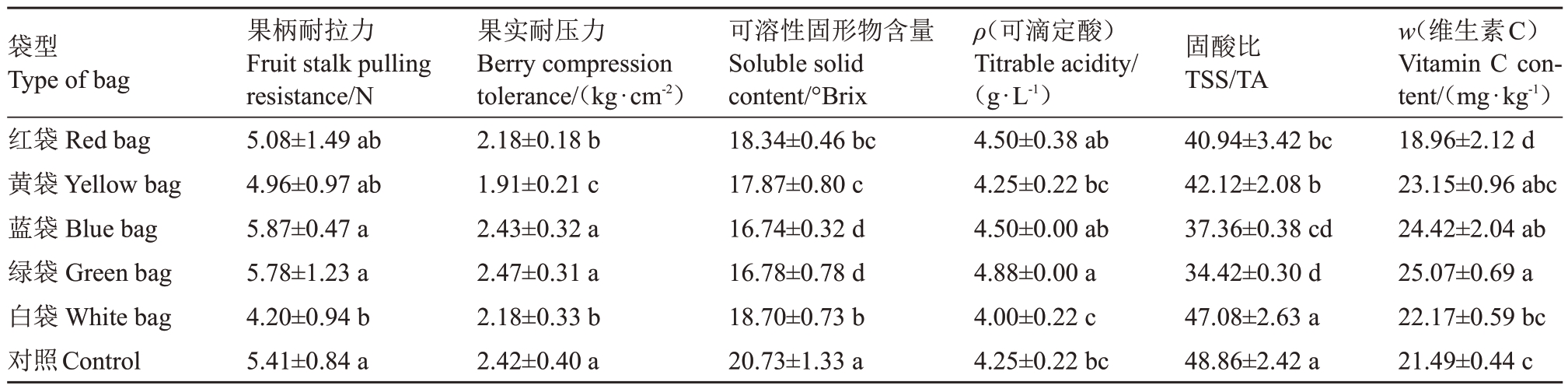
袋型Type of bag红袋Red bag黄袋Yellow bag蓝袋Blue bag绿袋Green bag白袋White bag对照Control果柄耐拉力Fruit stalk pulling resistance/N 5.08±1.49 ab 4.96±0.97 ab 5.87±0.47 a 5.78±1.23 a 4.20±0.94 b 5.41±0.84 a果实耐压力Berry compression tolerance/(kg·cm-2)2.18±0.18 b 1.91±0.21 c 2.43±0.32 a 2.47±0.31 a 2.18±0.33 b 2.42±0.40 a可溶性固形物含量Soluble solid content/°Brix 18.34±0.46 bc 17.87±0.80 c 16.74±0.32 d 16.78±0.78 d 18.70±0.73 b 20.73±1.33 a ρ(可滴定酸)Titrable acidity/(g·L-1)4.50±0.38 ab 4.25±0.22 bc 4.50±0.00 ab 4.88±0.00 a 4.00±0.22 c 4.25±0.22 bc固酸比TSS/TA 40.94±3.42 bc 42.12±2.08 b 37.36±0.38 cd 34.42±0.30 d 47.08±2.63 a 48.86±2.42 a w(维生素C)Vitamin C content/(mg·kg-1)18.96±2.12 d 23.15±0.96 abc 24.42±2.04 ab 25.07±0.69 a 22.17±0.59 bc 21.49±0.44 c
2.2.3 果实色泽 L*代表色泽的亮度,不同颜色果袋均显著增大了新郁葡萄L*值,增大了葡萄亮度,其中绿袋最大,其次是蓝袋,白袋与对照L*值差异最小,亮度最接近。a*值代表红绿色差,5种颜色果袋的a*值显著大于对照,且红袋、黄袋、蓝袋和绿袋的a*值显著大于白袋和对照,说明这4 种果袋的葡萄红色调较深,而白袋和对照红色较浅。b*值代表黄蓝色差,b*为负值时表示蓝色,数值越小表示蓝色越深,表明葡萄着色越深,白袋和对照b*值最小,显著小于其他果袋,表明二者着色最深,绿袋和蓝袋b*为正值,表明二者着色最浅。C*代表色泽饱和度(彩度),对照C*值最小,饱和度最小,其次是白袋,其他颜色果袋葡萄具有较高的饱和度,说明套袋后的果实更加鲜艳。对照CIRG值显著大于不同颜色果袋,葡萄着色过深,白袋CIRG>6,葡萄也存在着色过深现象,绿袋葡萄为粉红色,蓝袋、红袋和黄袋葡萄为红色,但红袋和黄袋CIRG值显著大于蓝袋,着色最好(表5)。可见,白袋存在着色过深的问题,而蓝袋和绿袋着色不良,从色泽指标上看,红袋和黄袋着色最好。
表5 不同颜色果袋对新郁葡萄果实色泽的影响
Table 5 Effects of different color fruit bags on berry color of Xinyu grape
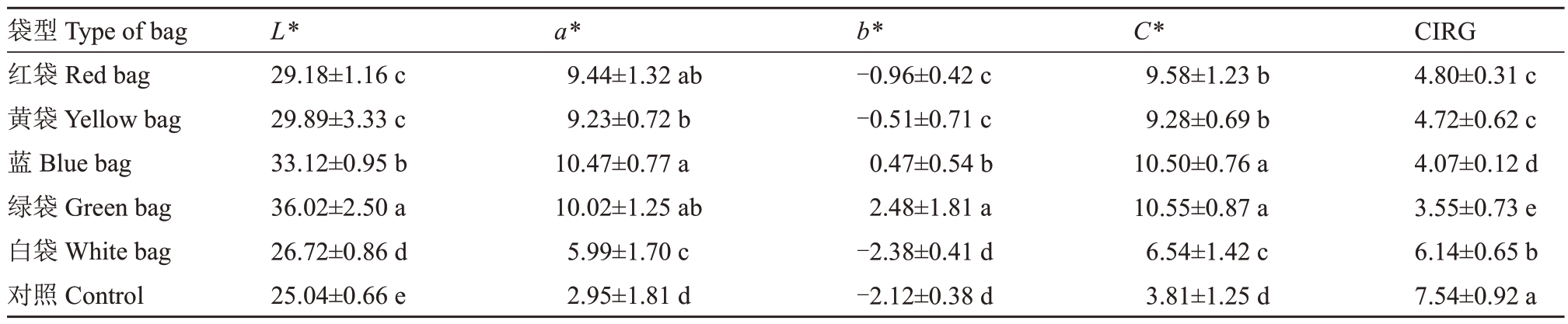
袋型Type of bag红袋Red bag黄袋Yellow bag蓝Blue bag绿袋Green bag白袋White bag对照Control L*29.18±1.16 c 29.89±3.33 c 33.12±0.95 b 36.02±2.50 a 26.72±0.86 d 25.04±0.66 e a*9.44±1.32 ab 9.23±0.72 b 10.47±0.77 a 10.02±1.25 ab 5.99±1.70 c 2.95±1.81 d b*-0.96±0.42 c-0.51±0.71 c 0.47±0.54 b 2.48±1.81 a-2.38±0.41 d-2.12±0.38 d C*9.58±1.23 b 9.28±0.69 b 10.50±0.76 a 10.55±0.87 a 6.54±1.42 c 3.81±1.25 d CIRG 4.80±0.31 c 4.72±0.62 c 4.07±0.12 d 3.55±0.73 e 6.14±0.65 b 7.54±0.92 a
2.3 不同颜色果袋对新郁葡萄果皮花色苷组成的影响
2.3.1 果皮花色苷组分及含量 白袋和对照样品均检测到27种花色苷,红袋检测到25种花色苷(未检测到花翠素-3-O-鼠李糖苷和甲基花翠素-3-O-阿拉伯糖苷),黄袋检测到25种花色苷(未检测到二甲花翠素-3-O-(6”-乙酰基葡萄糖苷)-5-葡萄糖苷和甲基花翠素-3-O-阿拉伯糖苷),蓝袋检测到24种花色苷[未检测到花翠素-3-O-鼠李糖苷、二甲花翠素-3-O-(6”-乙酰基葡萄糖苷)-5-葡萄糖苷和甲基花翠素-3-O-阿拉伯糖苷],绿袋检测到23 种花色苷[未检测到花翠素-3-O-鼠李糖苷、二甲花翠素-3-O-(6”-乙酰基葡萄糖苷)-5-葡萄糖苷、二甲花翠素-3-O-(6-O-丙二酰-β-D-葡萄糖苷)和甲基花翠素-3-O-阿拉伯糖苷]。不同颜色果袋及对照新郁葡萄果皮含量较大的花色苷均为二甲花翠素-3-O-葡萄糖苷、甲基花青素-3-O-葡萄糖苷、花青素-3-O-葡萄糖苷、花翠素-3-O-葡萄糖苷、花青素-3-(6-O-p-对香豆酰)-葡萄糖苷、甲基花青素-3-(6-O-p-对香豆酰)-葡萄糖苷、甲基花翠素-3-O-葡萄糖苷等7种。其中,含量最大的二甲花翠素-3-O-葡萄糖苷以对照和白袋最大,显著大于其他颜色果袋,且二者无显著差异,红袋、黄袋、蓝袋和绿袋相比对照分别降低56.03%、73.07%、75.46%和89.22%;含量较大的甲基花青素-3-O-葡萄糖苷以对照最大,其次是红袋,白袋次之,红袋、白袋、黄袋、蓝袋和绿袋较对照分别降低15.74%、21.14%、40.74%、48.46%和55.78%;其他5种含量较大的花色苷基本呈对照>白袋>红袋>黄袋>蓝袋>绿袋的趋势;总花色苷含量大小顺序为对照>白袋>红袋>黄袋=蓝袋>绿袋,白袋、红袋、黄袋、蓝袋和绿袋总花色苷含量较对照分别降低16.49%、46.96%、64.27%、68.59%和79.05%(表6)。可见,套袋均不同程度降低了新郁葡萄果皮中总花色苷及各主要花色苷单体含量,同时也减少了花色苷单体种类。白袋影响相对较小,绿袋影响最大,而黄袋和蓝袋的影响效果较一致。
表6 不同颜色果袋对新郁葡萄果皮花色苷组分及含量的影响
Table 6 Effects of different color fruit bags on the anthocyanin components and their contents in Xinyu grape skins

注:同行不同小写字母表示差异显著(p<0.05);Tr 表示痕量,Nd 表示未检测到。下同。
Note: Different lowercase letters in the same row mean significant differences at p<0.05; Tr means trace, Nd means not detected. The same below.
Control照0.16±0.01c 0.11±0.04 a 4.52±0.84 a 0.04±0.02 a 0.02±0.01 a 0.05±0.01 b 3.82±1.58 a 0.39±0.30 a 0.07±0.01 a 0.13±0.03 a 0.09±0.01 a 12.32±2.16 b 0.55±0.14 a 1.40±0.10 a 1.22±0.34 a 0.06±0.01 a 79.28±14.60 a 0.14±0.02 a 0.15±0.02 a 7.35±0.87 a Tr对239.85±18.40 a 109.68±11.33 a 127.39±10.75 a 1 291.28±120.09 a 670.77±16.43 a 123.96±7.37 a 2 674.78±181.16 a White bag袋0.16±0.01c 60.12±5.05 b 0.06±0.01 a 1.91±0.24 b 0.01±0.00 b 0.01±0.00 a 0.06±0.01 ab 1.23±0.28 b 0.16±0.03 ab 0.07±0.02 a 0.06±0.02 b 0.07±0.01 b 16.03±2.61 a 0.65±0.06 a 1.17±0.09 b 0.71±0.06 b 0.04±0.00 b 36.04±8.04 b 0.08±0.01 b 0.08±0.01 b 5.57±0.73 b Tr白117.24±10.51 b 101.08±24.06 b 1 193.05±104.32 a 528.95±44.98 c 104.63±6.98 b 2 233.61±276.89 b Green bag袋0.17±0.00 bc 0.03±0.00 c 1.12±0.00 c 0.01±0.00 b 8.37±1.22 d 0.03±0.00 b 0.22±0.03 ab 3.05±0.18 e 0.09±0.00 d 0.79±0.03 e 0.36±0.03 c 0.02±0.00 d 5.76±1.16 d 0.04±0.00 0.02±0.00 a绿67.19±4.40 d 24.62±1.18 d Nd0.06±0.00 a 139.15±13.67 d NdNd0.01±0.00 e 296.60±13.02 e 10.63±1.96 e Nd1.91±0.11 d 560.26±30.90 e Blue bag 0.19±0.01 a苷)Anthocyanin content/(mg·kg-1)0.03±0.00 c 1.10±0.00 c 0.01±0.00 b 0.10±0.02 b 0.27±0.03 ab 0.02±0.00 d 5.38±0.53 de 0.23±0.04 c 0.97±0.10 cd 0.48±0.04 bc 0.02±0.00 d 0.04±0.00 c 0.01±0.00 b蓝72.29±4.11 d 25.65±1.90 d 26.49±3.18 cd Nd0.06±0.01 ab 316.89±18.31 c Nd0.01±0.00 c 345.74±14.65 d 10.57±0.93 c 30.62±1.84 d Nd2.67±0.31 cd 840.17±46.45 d Yellow bag袋80.56±4.45 cd 0.18±0.01 ab 35.74±0.00 c 0.04±0.00 bc 1.49±0.17 bc 0.02±0.00 b 29.77±3.15 c 0.01±0.00 a 0.05±0.01 ab 0.15±0.03 b 0.31±0.01 ab Nd0.01±0.00 c 0.02±0.00 d 7.88±1.10 cd 0.18±0.04 cd 0.85±0.03 de 0.51±0.02 bc 0.03±0.00 c 13.17±0.95 c 0.06±0.01b 36.37±4.61 d Nd3.07±0.27 c 0.01±0.00 b黄347.78±39.48 c 397.49±16.03 d 955.76±69.69 d色Red bag袋91.61±3.95 c 0.17±0.01 bc 28.28±1.95 cd 0.04±0.00 bc 1.47±0.19 bc 0.01±0.00 b 44.50±2.02 c Nd0.05±0.00 ab 0.15±0.00 b 0.12±0.01 b 601.39±22.45 b 0.03±0.00 b 0.02±0.00 c 0.03±0.00 c 10.44±1.47 bc 0.37±0.04 b 565.18±20.20 b 1.05±0.06 bc 0.69±0.02 b 0.04±0.00 b 14.50±1.34 c 0.08±0.01 b 52.88±4.19 c Nd5.36±0.44 b Tr w(花红1 418.67±57.06 c Cyanidin-3-O-(6-O-p-coumaroyl)-glucoside Cyanidin-3-O-glucoside 萄)-5-O-葡Delphinidin-3-O-glucoside Delphinidin-3-O-(6-O-acetyl)-glucoside Delphinidin-3-O-arabinoside Cyanidin-3-O-rutinoside Malvidin-3-O-glucoside)Malvidin-3-O-(6-O-malonyl-β-D-glucoside苷Cyanidin-3-O-sophoroside )Peonidin-3-O-(6-O-malonyl-β-D-glucoside Malvidin-3-O-(6-O-p-coumaroyl)-glucoside Cyanidin-3-O-xyloside Peonidin-3-O-(6-O-p-coumaroyl)-glucoside苷糖苷苷糖Malvidin-3-O-arabinoside Pelargonidin-3-O-(6-O-p-coumaroyl)-glucoside糖萄萄苷苷萄糖萄苷苷苷糖糖糖糖)-葡萄萄Malvidin-3,5-O-diglucoside Cyanidin-3-O-arabinoside糖Peonidin-3,5-O-diglucoside)-葡萄-β-D-葡萄萄)-葡酰-β-D-葡酰苷苷Peonidin-3-O-arabinoside Petunidin-3-O-arabinoside Peonidin-3-O-glucoside)-葡Peonidin-3-O-rutinoside苷)-葡豆酰酰豆糖糖苷Petunidin-3-O-glucoside糖基苷)-葡苷苷苷酰二Delphinidin-3-O-rhamnoside酰葡二香香糖苷糖酰豆苷苷豆酰基糖萄-3-(6-O-p-对伯苷萄糖糖伯伯Pelargonidin-3-O-glucoside豆苷香苷苷糖萄苷糖拉葡酰萄香葡拉萄拉苷香糖伯糖糖糖伯糖苷苷香糖萄拉萄李拉萄-3-O-木-3-O-葡-3-O-芸糖-3-O-阿-3-(6-O-p-对omponent-3-O-槐-3-O-葡-3-O-鼠-3-O-阿糖-3-(6-O-p-对-3-O-(6-O-乙香-3-O-葡-3-O-阿-3-O-(6”-乙-3-O-(6-O-丙-3,5-O-二-3-O-葡-3-O-芸-3,5-O-二-3-O-(6-O-丙-3-(6-O-p-对-3-O-阿-3-O-葡-3-O-阿素素素素素素素素素素素素素素Total anthocyanins翠翠翠翠翠翠青青青青青青翠翠-3-O-葡-3-(6-O-p-对苷素物素素素素素素素素素素花花花花花花花花花花花花花花素素色青合青青青糖青青翠翠翠翠翠甲甲甲甲甲甲基基基基基基基基葵葵花elphinidin-3-O-(6-O-p-coumaroyl)-glucoside alvidin-3-O-(6”-acetylglucoside)-5-O-glucoside化C 花花花花花花花花花花D 花二二M 二二二二甲甲甲甲甲甲甲甲花花总
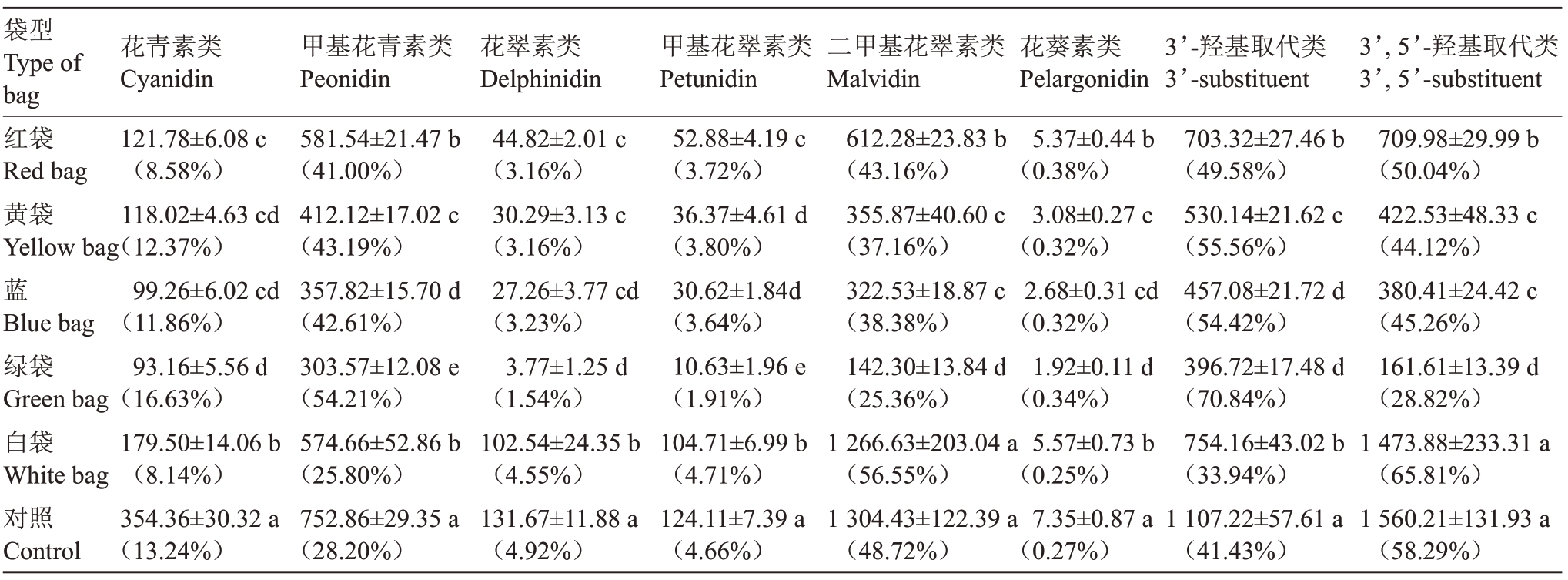
2.3.2 各类花色苷组成比例 成熟新郁葡萄果皮中主要包括二甲基花翠素类、甲基花青素类、花青素类、花翠素类和甲基花翠素类花色苷,花葵素类花色苷含量极少。除白袋与对照二甲基花翠素类花色苷含量无显著差异外,套袋均不同程度降低了各类型花色苷含量,不同果袋各类型花色苷含量大小顺序整体呈对照>白袋>红袋>黄袋>蓝袋>绿袋的趋势。红袋、黄袋和蓝袋间花青素类花色苷和花翠素类花色苷含量差异均较小,未表现出明显差异;黄袋和蓝袋间甲基花翠素类花色苷、二甲基花翠素类花色苷和花葵素类花色苷含量差异均较小,未表现出明显差异;蓝袋和绿袋间花青素类花色苷、花翠素类花色苷及花葵素类花色苷差异均较小,未表现出明显差异,说明不同颜色果袋对某些类型花色苷含量的影响结果一致。白袋、红袋、黄袋和蓝袋相比对照均降低了花青素类花色苷的比例,以白袋和红袋降幅最大,分别降低5.10%和4.66%,而绿袋花青素类花色苷比例则提高了3.39%;白袋相比对照甲基花青素类花色苷比例降低2.40%,其他颜色果袋则提高12.80%~26.01%;不同颜色果袋相比对照花翠素类花色苷比例降低了0.37%~3.88%;白袋相比对照甲基花翠素类花色苷比例提高0.05%,其他颜色果袋则降低0.86%~2.75%;白袋相比对照二甲基花翠素类花色苷比例提高7.83%,其他颜色果袋则降低5.56%~23.36%;白袋比相比对照花葵素类花色苷比例降低0.02%,其他颜色果袋则提高0.05%~0.11%(表7)。除白袋3’,5’-羟基取代类花色苷含量与对照未表现出明显差异外,套袋相比对照均显著降低了3’-羟基取代类和3’,5’-羟基取代类花色苷含量,蓝袋和绿袋的3’-羟基取代类花色苷含量之间无显著差异,黄袋与蓝袋的3’,5’-羟基取代类花色苷含量之间无显著差异,说明不同颜色果袋对某些类型花色苷含量的影响结果一致。白袋相比对照3’-羟基取代类花色苷比例降低了7.49%,而3’,5’-羟基取代类花色苷比例提高7.25%;红袋、黄袋、蓝袋和绿袋3’-羟基取代类花色苷比例分别提高8.15%、14.13%、12.99%和29.41%,而3’,5’-羟基取代类花色苷比例分别降低8.25%、14.17%、13.03%和29.47%。可见,套袋不仅改变了新郁葡萄果皮中各类型花色苷含量,还会改变各类型花色苷比例。白袋能够提高花翠素类(3’, 5’-羟基取代)花色苷比例,降低花青素类(3’-羟基取代)及花葵素类花色苷比例;其他颜色果袋则会提高花青素类(3’-羟基取代)及花葵素类花色苷比例,降低花翠素类(3’,5’-羟基取代)花色苷比例。白袋及对照总花色苷含量高及花翠素类(3’,5’-羟基取代)花色苷比例大是引起新郁葡萄着色过深的主要原因。
表7 不同颜色果袋对新郁葡萄果皮中不同类型花色苷含量与所占总量比例的影响
Table 7 Effects of different color fruit bags on the contents and their proportions of different anthocyanins in Xinyu grape skins
w/(mg·kg-1)
注:括号内数字为相应类型花色苷含量占总花色苷含量的百分率。下同。
Note:The number in parentheses is the ratio of the corresponding anthocyanin to the total anthocyanin.The same below.
袋型Type of bag红袋Red bag黄袋Yellow bag蓝Blue bag绿袋Green bag白袋White bag对照Control花青素类Cyanidin 121.78±6.08 c(8.58%)118.02±4.63 cd(12.37%)99.26±6.02 cd(11.86%)93.16±5.56 d(16.63%)179.50±14.06 b(8.14%)354.36±30.32 a(13.24%)甲基花青素类Peonidin 581.54±21.47 b(41.00%)412.12±17.02 c(43.19%)357.82±15.70 d(42.61%)303.57±12.08 e(54.21%)574.66±52.86 b(25.80%)752.86±29.35 a(28.20%)花翠素类Delphinidin 44.82±2.01 c(3.16%)30.29±3.13 c(3.16%)27.26±3.77 cd(3.23%)3.77±1.25 d(1.54%)102.54±24.35 b(4.55%)131.67±11.88 a(4.92%)甲基花翠素类Petunidin 52.88±4.19 c(3.72%)36.37±4.61 d(3.80%)30.62±1.84d(3.64%)10.63±1.96 e(1.91%)104.71±6.99 b(4.71%)124.11±7.39 a(4.66%)二甲基花翠素类Malvidin 612.28±23.83 b(43.16%)355.87±40.60 c(37.16%)322.53±18.87 c(38.38%)142.30±13.84 d(25.36%)1 266.63±203.04 a(56.55%)1 304.43±122.39 a(48.72%)花葵素类Pelargonidin 5.37±0.44 b(0.38%)3.08±0.27 c(0.32%)2.68±0.31 cd(0.32%)1.92±0.11 d(0.34%)5.57±0.73 b(0.25%)7.35±0.87 a(0.27%)3’-羟基取代类3’-substituent 703.32±27.46 b(49.58%)530.14±21.62 c(55.56%)457.08±21.72 d(54.42%)396.72±17.48 d(70.84%)754.16±43.02 b(33.94%)1 107.22±57.61 a(41.43%)3’,5’-羟基取代类3’,5’-substituent 709.98±29.99 b(50.04%)422.53±48.33 c(44.12%)380.41±24.42 c(45.26%)161.61±13.39 d(28.82%)1 473.88±233.31 a(65.81%)1 560.21±131.93 a(58.29%)
如表8 所示,白袋酰化修饰花色苷含量与对照未表现出明显差异,红袋、黄袋、蓝袋和绿袋均显著降低酰化修饰类型花色苷含量,且4 种颜色果袋间差异均未达显著水平,红袋、黄袋、蓝袋和绿袋酰化修饰类花色苷比例相比对照分别降低3.88%、1.66%、2.64%和1.64%;套袋相比对照显著降低甲基化修饰类型花色苷含量,大小顺序为对照>白袋>红袋>黄袋=蓝袋>绿袋,白袋、红袋、黄袋和蓝袋甲基化修饰花色苷比例相比对照提高5.47%、6.31%、2.57%和3.06%,绿袋则与对照差异不大;白袋与对照总修饰类花色苷含量无显著差异,其他果袋均显著降低总修饰类花色苷含量,整体呈红袋>黄袋>蓝袋>绿袋的趋势,蓝袋与对照总修饰类花色苷比例差异不大,绿袋则降低1.71%,白袋、红袋和黄袋则分别提高5.17%、2.44%和0.92%。可见,白袋对新郁葡萄果皮中酰化、总修饰类花色苷含量无明显影响,但会降低甲基化修饰类花色苷含量,而红袋、黄袋、蓝袋和绿袋均会显著降低酰化及甲基化花色苷含量。5种颜色果袋均降低了酰化修饰类花色苷比例,白袋、红袋、黄袋和蓝袋提高了甲基化修饰花色苷比例;绿袋降低了总修饰类花色苷比例,白袋、红袋和黄袋则提高了总修饰类花色苷比例。
表8 不同颜色果袋对新郁葡萄果皮中不同修饰类型花色苷含量与所占总量比例的影响
Table 8 Effects of different color fruit bags on the contents and proportions of anthocyanins of different modification types in Xinyu grape skins
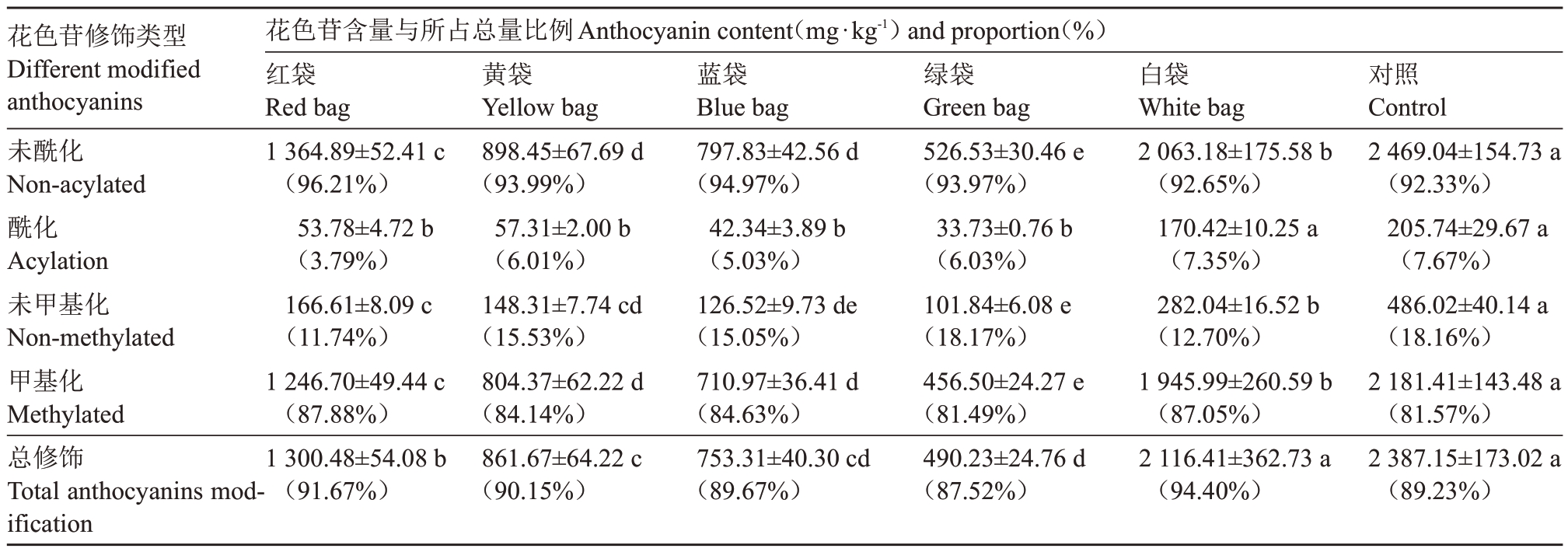
花色苷修饰类型Different modified anthocyanins未酰化Non-acylated酰化Acylation未甲基化Non-methylated甲基化Methylated总修饰Total anthocyanins modification花色苷含量与所占总量比例Anthocyanin content(mg·kg-1)and proportion(%)红袋Red bag 1 364.89±52.41 c(96.21%)53.78±4.72 b(3.79%)166.61±8.09 c(11.74%)1 246.70±49.44 c(87.88%)1 300.48±54.08 b(91.67%)黄袋Yellow bag 898.45±67.69 d(93.99%)57.31±2.00 b(6.01%)148.31±7.74 cd(15.53%)804.37±62.22 d(84.14%)861.67±64.22 c(90.15%)蓝袋Blue bag 797.83±42.56 d(94.97%)42.34±3.89 b(5.03%)126.52±9.73 de(15.05%)710.97±36.41 d(84.63%)753.31±40.30 cd(89.67%)绿袋Green bag 526.53±30.46 e(93.97%)33.73±0.76 b(6.03%)101.84±6.08 e(18.17%)456.50±24.27 e(81.49%)490.23±24.76 d(87.52%)白袋White bag 2 063.18±175.58 b(92.65%)170.42±10.25 a(7.35%)282.04±16.52 b(12.70%)1 945.99±260.59 b(87.05%)2 116.41±362.73 a(94.40%)对照Control 2 469.04±154.73 a(92.33%)205.74±29.67 a(7.67%)486.02±40.14 a(18.16%)2 181.41±143.48 a(81.57%)2 387.15±173.02 a(89.23%)
2.4 基于光环境指标和色泽指标的相关性分析
光环境指标与果实色泽指标L*、a*、b*、C*值之间基本呈负相关,而与果实色泽指标CIRG值、果皮各类型花色苷含量、总花色苷含量之间呈正相关。L*值与红光/蓝光值、红光/红外光值呈极显著负相关,a*值与绿光辐射强度呈极显著负相关,b*值与红光/红外光值呈极显著负相关,C*值与绿光、橙光、红光辐射强度及总辐射呈极显著负相关。CIRG值与橙光、红光、红外光辐射强度及总辐射呈极显著正相关;花青素类花色苷含量与绿光辐射强度和总辐射呈极其显著正相关,与紫外光、紫光、蓝光、橙光、红光、红外光辐射强度及蓝光/紫外光值呈极显著正相关;甲基花青素类花色苷含量与橙光、红光辐射强度及红光/蓝光值呈极显著正相关;花葵素类花色苷含量与橙光呈极显著正相关;3’-羟基取代类花色苷含量与橙光、红光辐射强度呈极其显著正相关,与绿光、红外光辐射强度及总辐射呈极显著正相关(图2)。可见,随着总辐射及各波段光辐射强度的增强,果实色泽指标L*、a*、b*、C*值均减小,而色泽指标CIRG值及各类型花色苷含量均增高,果实着色加深;绿光辐射强度和总辐射的增大会使花青素类花色苷含量极其显著增高,橙光和红光辐射强度的增大会导致3’-羟基取代类花色苷含量的极其显著增高,3’-羟基取代类花色苷含量的增加有利于葡萄着色向红色发展。

图2 基于光环境指标和色泽指标的相关性分析
Fig.2 Correlation analysis based on light environment index and berry color index
3 讨 论
3.1 光环境对新郁葡萄果实发育及品质的影响
光照是影响植物生长和果实品质形成的重要因子之一。不同的光照度、光质量和光周期均会影响植物的生长和生理代谢[19]。研究表明,较短波长的蓝光(420~490 nm)、UV-A(315~400 nm)、UV-B(280~315 nm)和UV-C(200~280 nm)可以通过上调类黄酮途径基因的表达来促进果实中类黄酮积累[20-21],而更长波长的红光(600~700 nm)和远红光(700~800 nm)则会提高水果中可溶性固形物含量[22]。不同颜色果袋改变了果实生长发育的光环境(光照度和光质),进而影响果实品质。Pisciotta等[23]研究认为套袋能够增大皇家秋天(Autumn Royal)和帝王无核(Regal Seedless)葡萄果穗和果粒质量,但对意大利(Italia)葡萄果粒质量无显著影响,套袋后无核葡萄果实硬度增大,有核葡萄果实硬度减小且成熟延迟。Zha 等[9]研究认为套袋能够降低成熟期申华和申丰葡萄葡萄糖及果糖的积累,造成果实成熟延迟。本研究中,绿袋果粒质量相比对照显著减小,这可能与绿袋透光率较低,受到较强的弱光胁迫导致向果实运输养分减少,果实碳同化物积累减少有关[24-25]。Zhang 等[10]研究认为红光能够通过上调光合作用、固碳等途径基因,促进葡萄果实可溶性固形物含量的增加。程建徽等[26]认为蓝色滤光膜套袋可以增加蓝紫光的比例,调控葡萄光合产物分配,诱导苯丙氨酸解氨酶活性,促进欧亚种红地球葡萄果实糖的积累和着色。本研究中,不同颜色果袋均不同程度降低了袋内总辐射及透光率,且葡萄可溶性固形物含量随总辐射及透光率的降低而减少,蓝袋相比其他果袋蓝紫光的增幅远小于红光的降幅,所以总辐射和红光强度的降低是造成蓝袋和绿袋可溶性固形物含量降低的主要原因,这与前人在苹果[27]、葡萄[9,15,28]上的研究认为套袋造成的弱光或光抑制会延迟果实成熟的结论相一致。
3.2 新郁葡萄果皮花色苷组成
果皮颜色是葡萄重要的经济性状,由果皮花色苷的组成和浓度决定[29]。一般认为欧亚种葡萄花色苷主要为花青素类、甲基花青素类、花翠素类、甲基花翠素类、二甲基花翠素类等5大类,一般不含花葵素类花色苷[30],且欧亚种葡萄中由于5GT 基因发生了2 个位点的突变,导致其不能合成双糖苷化的花色苷[31]。但近年研究发现,欧亚种葡萄赤霞珠、黑比诺和烟73能够检测到痕量的花葵素类花色苷[32];美乐、西拉、桑娇维塞、黑比诺、赤霞珠和品丽珠等欧亚种葡萄均能检测到二甲花翠素-3,5-O-二葡萄糖苷[33]。Xing等[34]通过质谱和酶学手段进一步证实了欧亚种葡萄赤霞珠中花青素3,5-O-二葡萄糖苷的存在,并推测一种新的基因Vv5GT3 可能对花青素和黄酮醇的3-O和5-O位置进行了糖基化形成双糖苷的花色苷。笔者本研究的欧亚种新郁葡萄果皮中共检测到了27 种花色苷,主要包括花青素类、甲基花青素类、花翠素类、甲基花翠素类、二甲基花翠素类等5大类花色苷,还包括低含量的2种花葵素类花色苷及3 种双糖苷花色苷,这与上述研究结果[32-34]一致。其中,含量较高的为二甲基花翠素类花色苷和甲基花青素类花色苷,这与Liang等[35]认为甲基花青素及其衍生物和二甲基花翠素-3-O-葡萄糖苷是欧亚种葡萄主要花色苷的研究结论一致。
3.3 光环境对新郁葡萄花色苷的影响
光可以调节与花青素合成相关的结构基因和调节基因的表达,从而调控花色苷的积累[11,36-37]。多项研究证实,光照可以增加葡萄果皮花色苷浓度,弱光或者光抑制可以显著减少葡萄浆果中花色苷的积累[27,38-39];光照度的改变还可以引起葡萄花色苷组分的改变,如遮阴后的黑比诺葡萄甲基花青素类花色苷比例会显著增高,而花翠素、甲基花翠素及二甲基花翠素类花色苷比例则显著降低[40];佳美(Gamay)和福优佳美(Gamay Freáux)葡萄在进行光抑制处理后二羟基化和三羟基化花青素的比例增大[12];在绝对的遮光条件下马瑟兰葡萄不能合成光诱导型花色苷Malvidin-3-O-coumaroylglucoside(trans)[41]。本研究中套袋造成的光抑制均不同程度降低了新郁葡萄果皮中大部分类型花色苷及总花色苷的含量,且红、黄、蓝、绿4种颜色果袋提高了花青素类(3’-羟基取代)花色苷比例,降低了花翠素类(3’,5’-羟基取代)花色苷比例,这与前人研究结论基本一致。
不同类型光质也会引起葡萄花色苷含量及组分的变化,多项研究表明,紫外光、蓝光和红光均会增加葡萄花色苷含量[42-43],且蓝光效果比红光更有效[44]。Kondo等[36]研究认为夜间LED蓝光补光能够促进巨峰葡萄二甲基花翠素类花色苷含量的增加,且补蓝光和红光均能提高二甲基花翠素花色苷比例,降低甲基花青素类花色苷比例。本研究中5 种颜色果袋均提高了紫外光、紫光和蓝光比例,降低了红光比例,且以绿袋和蓝袋变化最大,红光/蓝光值最小。红、黄、蓝和绿袋紫外光、蓝光比例增大,促进二甲基花翠素类花色苷比例增大,但红光辐射强度及比例的大幅度降低则会导致二甲基花翠素类花色苷含量及比例大幅度减小,最终导致花翠素类(3’,5’-羟基取代)花色苷比例降低,花青素类(3’-羟基取代)花色苷比例提高;白袋紫外光、蓝光比例的提高促进了二甲基花翠素类花色苷比例的提高,而红光比例变化不大,导致花翠素类(3’,5’-羟基取代)花色苷比例提高,花青素类(3’-羟基取代)花色苷比例降低。可见,葡萄果皮花色苷组成受光照度及光质组成等多因素调控。
相关性分析还发现橙光和红光辐射强度与花青素类(3’-羟基取代)花色苷含量呈极显著正相关,可能原因是橙光和红光可以通过影响光响应因子VvHY5 和VvCOP1 来促进葡萄中F3’H 基因上调表达,进而合成更多的花青素类(3’-羟基取代)花色苷[45-46],具体影响机制还有待从分子水平进行深入研究。Tarara等[47]研究认为,直接暴露在太阳辐射下的美乐葡萄酰化花色苷浓度和比例会降低,在高温条件下花色苷会转向酰基化以应对高温胁迫,导致酰化花色苷比例升高。Zhang等[10]研究认为采用LED进行补光,蓝光、红光和绿光相比白光乙酰化花色苷种类减少。此外,花色苷乙酰化、甲基化水平也会随着UV-B辐射的增加而增加[48-49]。本研究中黄、蓝和绿袋乙酰化花色苷种类相比对照减少,且5 种颜色果袋均降低了酰化花色苷比例,可见,吐鲁番地区高温气候条件下高光照辐射可积累更多酰化花色苷。研究中白、红、黄和蓝袋提高了甲基化修饰花色苷比例,绿袋降低了总修饰类花色苷比例,这一结果与光照度和光质(红光比例减小、紫外光比例增大)的改变有关,花色苷的修饰受到温度、光照、水分、糖含量和内源ABA 含量等内外因素复杂的相互作用控制[50]。二羟基化花青素呈鲜红色,而三羟基化花青素则呈深蓝色和紫色[51];甲基化程度的增加,红色色调会得到增强[52];花色苷酰化程度的增加,颜色的稳定性会得到提高,且颜色逐渐呈现蓝色[53]。采用红袋套袋的新郁葡萄果皮甲基化、花青素类(3’-羟基取代)花色苷比例增高,酰化修饰类花色苷比例及总花色苷含量降低,有效解决了产区新郁葡萄着色过深问题,果实色泽鲜红。
4 结 论
新疆吐鲁番地区高温强光照条件下,不同颜色果袋套袋的新郁葡萄果际光环境和果实品质及果皮花色苷组分存在明显差异,果袋均不同程度降低了果际总辐射和果实中可溶性固形物含量及果皮中总花色苷含量。采用红袋套袋的新郁葡萄果皮具有适度的总花色苷含量,有效解决了新郁葡萄着色过深问题,果皮色泽鲜红,内在品质较优。综合而言,西北干旱区新郁葡萄宜采用红色果袋进行套袋。
[1]骆强伟,孙锋,蔡军社,耿新丽,热比古丽. 葡萄新品种‘新郁’[J].园艺学报,2007,34(3):797.LUO Qiangwei,SUN Feng,CAI Junshe,GENG Xinli,REBI Guli.A new grape cultivar‘Xinyu’[J].Acta Horticulturae Sinica,2007,34(3):797.
[2]KARAJEH M R.Pre-harvest bagging of grape clusters as a nonchemical physical control measure against certain pests and diseases of grapevines[J].Organic Agriculture,2018,8(3):259-264.
[3]SIGNES A J,BURLÓ F,MARTINEZ-SÁNCHEZ F,CARBONELL-BARRACHINA Á A.Effects of preharvest bagging on quality of black table grapes[J].World Journal of Agricultural Sciences,2007,3(1):32-38.
[4]张建光,王惠英,王梅,孙建设,刘玉芳,LARRY S.套袋对苹果果实微域生态环境的影响[J]. 生态学报,2005,25(5):1082-1087.ZHANG Jianguang,WANG Huiying,WANG Mei,SUN Jianshe,LIU Yufang,LARRY S.Effect of bagging on microenvironments of apple fruits[J]. Acta Ecologica Sinica,2005,25(5):1082-1087.
[5]PIERIK R,KEUSKAMP D H,SASIDHARAN R,DJAKOVICPETROVIC T,DE WIT M,VOESENEK L A C J. Light quality controls shoot elongation through regulation of multiple hormones[J].Plant Signaling&Behavior,2009,4(8):755-756.
[6]HEIJDE M,ULM R. UV-B photoreceptor-mediated signalling in plants[J].Trends in Plant Science,2012,17(4):230-237.
[7]SMITH H. Phytochromes and light signal perception by plants:An emerging synthesis[J].Nature,2000,407(6804):585-591.
[8]LIN C T. Blue light receptors and signal transduction[J]. The Plant Cell,2002,14(S1):207-225.
[9]ZHA Q,XI X J,HE Y N,JIANG A L. Bagging affecting sugar and anthocyanin metabolism in the ripening period of grape berries[J]. Notulae Botanicae Horti Agrobotanici Cluj- Napoca,2019,47(4):1194-1205.
[10] ZHANG P A,LU S W,LIU Z J,ZHENG T,DONG T Y,JIN H C,JIA H F,FANG J G.Transcriptomic and metabolomic profiling reveals the effect of LED light quality on fruit ripening and anthocyanin accumulation in Cabernet Sauvignon grape[J].Frontiers in Nutrition,2021,8:790697.
[11] KOYAMA K,IKEDA H,POUDEL P R,GOTO-YAMAMOTO N. Light quality affects flavonoid biosynthesis in young berries of Cabernet Sauvignon grape[J]. Phytochemistry,2012,78:54-64.
[12] GUAN L,DAI Z W,WU B H,WU J,MERLIN I,HILBERT G,RENAUD C,GOMÉS E,EDWARDS E,LI S H,DELROT S.Anthocyanin biosynthesis is differentially regulated by light in the skin and flesh of white-fleshed and teinturier grape berries[J].Planta,2016,243(1):23-41.
[13] SUN R Z,CHENG G,LI Q,HE Y N,WANG Y,LAN Y B,LI S Y,ZHU Y R,SONG W F,ZHANG X,CUI X D,CHEN W,WANG J. Light-induced variation in phenolic compounds in Cabernet Sauvignon grapes(Vitis vinifera L.)involves extensive transcriptome reprogramming of biosynthetic enzymes,transcription factors,and phytohormonal regulators[J]. Frontiers in Plant Science,2017,8:547.
[14] CARBONELL- BEJERANO P,DIAGO M P,MARTÍNEZABAIGAR J,MARTÍNEZ- ZAPATER J M,TARDÁGUILA J,NÚÑEZ-OLIVERA E.Solar ultraviolet radiation is necessary to enhance grapevine fruit ripening transcriptional and phenolic responses[J].BMC Plant Biology,2014,14:183.
[15] 冀晓昊,王海波,张克坤,王孝娣,史祥宾,王宝亮,郑晓翠,王志强,刘凤之.不同颜色果袋对葡萄花青苷合成的调控[J].中国农业科学,2016,49(22):4460-4468.JI Xiaohao,WANG Haibo,ZHANG Kekun,WANG Xiaodi,SHI Xiangbin,WANG Baoliang,ZHENG Xiaocui,WANG Zhiqiang,LIU Fengzhi.The grape anthocyanin biosynthesis regulation by different color fruit bags[J].Scientia Agricultura Sinica,2016,49(22):4460-4468.
[16] AMIRI M E,FALLAHI E,PARSEH S.Application of ethephon and ABA at 40% veraison advanced maturity and quality of‘Beidaneh Ghermez’grape[J]. Acta Horticulturae,2010,884:371-377.
[17] 中华人民共和国国家健康委员会,国家市场监督管理总局.食品安全国家标准食品中总酸的测定:GB 12456—2021[S].北京:中国标准出版社,2021.National Health Commission of the People Republic of China,State Administration of Market Supervision and Administration.Food safety national standard determination of total acid in foods:GB 12456—2021[S].Beijing:China Standards Press,2021.
[18] 高俊凤.植物生理学实验指导[M].北京:高等教育出版社,2006:203-204.GAO Junfeng. Experimental guidance for plant physiology[M].Beijing:High Education Press,2006:203-204.
[19] ZORATTI L,KARPPINEN K,ESCOBAR A L,HELY HAGGMANET H,JAAKOLA L.Light-controlled flavonoid biosynthesis in fruits[J].Frontiers in Plant Science,2014,5:534.
[20] ÁVILA-SOSA R,ÁVILA-CRISÓSTOMO E,REYES-ARCOS E A,CID-PEREZ T S,NAVARRO- CRUZ A R,OCHOAVELASCO C E.Effect of blue and UV-C irradiation on antioxidant compounds during storage of hawthorn (Crataegus mexicana)[J].Scientia Horticulturae,2017,217:102-106.
[21] SIIPOLA S M,KOTILAINEN T,SIPARI N,MORALES L O,LINDFORS A V,ROBSON T M,APHALO P J.Epidermal UVa absorbance and whole-leaf flavonoid composition in pea respond more to solar blue light than to solar UV radiation[J].Plant,Cell&Environment,2015,38(5):941-952.
[22] MIAO L X,ZHANG Y C,YANG X F,XIAO J P,ZHANG H Q,ZHANG Z F,WANG Y Z,JIANG G H.Colored light-quality selective plastic films affect anthocyanin content,enzyme activities,and the expression of flavonoid genes in strawberry(Fragaria × ananassa) fruit[J]. Food Chemistry,2016,207:93-100.
[23] PISCIOTTA A,PLANETA D,GIACOSA S,PAISSONI M A,ROLLE L. Quality of grapes grown inside paper bags in Mediterranean area[J].Agronomy,2020,10(6):792.
[24] 郝燕燕,任宏伟,郭平毅.苹果果实套袋对光合同化物积累与转化的影响[J].园艺学报,2011,38(2):233-239.HAO Yanyan,REN Hongwei,GUO Pingyi. Effects of bagging on the accumulation and transformation of photosynthates in apple fruits[J].Acta Horticulturae Sinica,2011,38(2):233-239.
[25] HIRATSUKA S,YOKOYAMA Y,NISHIMURA H,MIYAZAKI T,NADA K. Fruit photosynthesis and phosphoenolpyruvate carboxylase activity as affected by lightproof fruit bagging in Satsuma mandarin[J].Journal of the American Society for Horticultural Science,2012,137(4):215-220.
[26] 程建徽,魏灵珠,雷鸣,郑婷,吴江.不同滤光膜袋对‘红地球’葡萄果实品质的影响[J].果树学报,2015,32(1):87-93.CHENG Jianhui,WEI Lingzhu,LEI Ming,ZHENG Ting,WU Jiang.Influences of different light filter film bags on berry quality in‘Red Globe’[J].Journal of Fruit Science,2015,32(1):87-93.
[27] FENG F J,LI M J,MA F W,CHENG L L. The effects of bagging and debagging on external fruit quality,metabolites,and the expression of anthocyanin biosynthetic genes in‘Jonagold’apple (Malus domestica Borkh.) [J]. Scientia Horticulturae,2014,165:123-131.
[28] MARTÍNEZ-LÜSCHER J,BRILLANTE L,KURTURAL S K.Flavonol profile is a reliable indicator to assess canopy architecture and the exposure of red wine grapes to solar radiation[J].Frontiers in Plant Science,2019,10:10.
[29] CASTELLARIN S D,DI GASPERO G,MARCONI R,NONIS A,PETERLUNGER E,PAILLARD S,ADAM-BLONDON A F,TESTOLIN R. Colour variation in red grapevines (Vitis vinifera L.):Genomic organisation,expression of flavonoid 3’-hydroxylase,flavonoid 3’,5’-hydroxylase genes and related metabolite profiling of red cyanidin-/blue delphinidin-based anthocyanins in berry skin[J].BMC Genomics,2006,7(1):12.
[30] AGEORGES A,FERNANDEZ L,VIALET S,MERDINOGLU D,TERRIER N,ROMIEU C. Four specific isogenes of the anthocyanin metabolic pathway are systematically co-expressed with the red colour of grape berries[J]. Plant Science,2006,170(2):372-383.
[31] JÁNVÁRY L,HOFFMANN T,PFEIFFER J,HAUSMANN L,TÖPFER R,FISCHER T C,SCHWAB W.A double mutation in the anthocyanin 5-O-glucosyltransferase gene disrupts enzymatic activity in Vitis vinifera L.[J]. Journal of Agricultural and Food Chemistry,2009,57(9):3512-3518.
[32] XIE S,ZHAO T,ZHANG Z W,MENG J F.Reduction of dihydrokaempferol by Vitis vinfera dihydroflavonol 4-reductase to produce orange pelargonidin-type anthocyanins[J]. Journal of Agricultural and Food Chemistry,2018,66(13):3524-3532.
[33] PANTELIĆ M,DABIĆ ZAGORAC D,NATIĆ M,GAŠIĆ U,JOVIĆ S,VUJOVIĆ D,DJORDJEVIĆ J P. Impact of clonal variability on phenolics and radical scavenging activity of grapes and wines:A study on the recently developed merlot and Cabernet Franc clones(Vitis vinifera L.)[J].PLoS One,2016,11(10):e0163823.
[34] XING R R,LI S Y,HE F,YANG Z,DUAN C Q,LI Z,WANG J,PAN Q H. Mass spectrometric and enzymatic evidence confirm the existence of anthocyanidin 3,5-O-diglucosides in Cabernet Sauvignon (Vitis vinifera L.) grape berries[J]. Journal of Agricultural and Food Chemistry,2015,63(12):3251-3260.
[35] LIANG Z C,WU B H,FAN P G,YANG C X,DUAN W,ZHENG X B,LIU C Y,LI S H.Anthocyanin composition and content in grape berry skin in Vitis germplasm[J].Food Chemistry,2008,111(4):837-844.
[36] KONDO S,TOMIYAMA H,RODYOUNG A,OKAWA K,OHARA H,SUGAYA S,TERAHARA N,HIRAI N. Abscisic acid metabolism and anthocyanin synthesis in grape skin are affected by light emitting diode(LED)irradiation at night[J].Journal of Plant Physiology,2014,171(10):823-829.
[37] SUN L,LI S C,TANG X P,FAN X C,ZHANG Y,JIANG J F,LIU J H,LIU C H. Transcriptome analysis reveal the putative genes involved in light-induced anthocyanin accumulation in grape‘Red Globe’(V.vinifera L.)[J].Gene,2020,728:144284.
[38] LEE J. Light exclusion influence on grape anthocyanin[J]. Heliyon,2017,3(2):e00243.
[39] AZUMA A,YAKUSHIJI H,KOSHITA Y,KOBAYASHI S.Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions[J]. Planta,2012,236(4):1067-1080.
[40] CORTELL J M,KENNEDY J A.Effect of shading on accumulation of flavonoid compounds in (Vitis vinifera L.) Pinot Noir fruit and extraction in a model system[J].Journal of Agricultural and Food Chemistry,2006,54(22):8510-8520.
[41] MA Z H,LI W F,MAO J,LI W,ZUO C W,ZHAO X,DAWUDA M M,SHI X Y,CHEN B H. Synthesis of light-inducible and light-independent anthocyanins regulated by specific genes in grape‘Marselan’(V.vinifera L.)[J].PeerJ,2019,7:e6521.
[42] GONZÁLEZ C V,FANZONE M L,CORTÉS L E,BOTTINI R,LIJAVETZKY D C,BALLARÉ C L,BOCCALANDRO H E. Fruit-localized photoreceptors increase phenolic compounds in berry skins of field-grown Vitis vinifera L.cv.Malbec[J].Phytochemistry,2015,110:46-57.
[43] RODYOUNG A,MASUDA Y,TOMIYAMA H,SAITO T,OKAWA K,OHARA H,KONDO S.Effects of light emitting diode irradiation at night on abscisic acid metabolism and anthocyanin synthesis in grapes in different growing seasons[J]. Plant Growth Regulation,2016,79(1):39-46.
[44] AZUMA A,ITO A,MORIGUCHI T,YAKUSHIJI H,KOBAYASHI S. Light emitting diode irradiation at night accelerates anthocyanin accumulation in grape skin[J]. Acta Horticulturae,2012,956:341-348.
[45] SAMKUMAR A,JONES D,KARPPINEN K,DARE A P,SIPARI N,ESPLEY R V,MARTINUSSEN I,JAAKOLA L. Red and blue light treatments of ripening bilberry fruits reveal differences in signalling through abscisic acid-regulated anthocyanin biosynthesis[J]. Plant,Cell & Environment,2021,44(10):3227-3245.
[46] 成果,陈国品,郭荣荣,黄秋凤,王博,白先进.补光处理对巨峰葡萄春果花色苷组分的影响[J].南方园艺,2017,28(5):1-8.CHENG Guo,CHEN Guopin,GUO Rongrong,HUANG Qiufeng,WANG Bo,BAI Xianjin.Effect of supplementary light treatment on anthocyanin components in spring fruit of Kyoho grape[J].Southern Horticulture,2017,28(5):1-8.
[47] TARARA J M,LEE J,SPAYD S E,SCAGEL C F.Berry temperature and solar radiation alter acylation proportion and concentration of anthocyanin in Merlot grapes[J].American Journal of Enology and Viticulture,2008,59(3):235-247.
[48] DE OLIVEIRA A F,NIEDDU G.Accumulation and partitioning of anthocyanins in two red grape cultivars under natural and reduced UV solar radiation[J]. Australian Journal of Grape and Wine Research,2016,22(1):96-104.
[49] MARTÍNEZ-LÜSCHER J,SÁNCHEZ-DÍAZ M,DELROT S,AGUIRREOLEA J,PASCUAL I,GOMÈS E. Ultraviolet-B radiation and water deficit interact to alter flavonol and anthocyanin profiles in grapevine berries through transcriptomic regulation[J].Plant and Cell Physiology,2014,55(11):1925-1936.
[50] AZUMA A. Genetic and environmental impacts on the biosynthesis of anthocyanins in grapes[J]. The Horticulture Journal,2018,87(1):1-17.
[51] JAAKOLA L,MÄÄTTÄ K,PIRTTILÄ A M,TÖRRÖNEN R,KÄRENLAMPI S,HOHTOLA A. Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin,proanthocyanidin,and flavonol levels during bilberry fruit development[J].Plant Physiology,2002,130(2):729-739.
[52] FOURNIER-LEVEL A,HUGUENEY P,VERRIÈS C,THIS P,AGEORGES A. Genetic mechanisms underlying the methylation level of anthocyanins in grape (Vitis vinifera L.)[J]. BMC Plant Biology,2011,11:179.
[53] GOMEZ C,TERRIER N,TORREGROSA L,VIALET S,FOURNIER-LEVEL A,VERRIÉS C,SOUQUET J M,MAZAURIC J P,KLEIN M,CHEYNIER V,AGEORGES A. Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters[J]. Plant Physiology,2009,150(1):402-415.