葡萄(Vitis vinifera L.)为葡萄科葡萄属木质藤本植物,是世界范围内重要的经济类果树之一,在我国栽培历史悠久,因其营养丰富,受到消费者的广泛喜爱[1]。类黄酮是葡萄中最丰富的次生代谢物,在植株生长发育、果实色泽和品质等方面起着重要作用[2]。葡萄中的类黄酮主要包括花色苷、黄酮醇和黄烷醇3 类,主要以糖基结合态的形式存在于葡萄各组织中[3]。花色苷是一种天然植物色素,主要存在有色品种的果皮中,对葡萄和葡萄酒的颜色起决定性作用,但染色品种烟73 的果肉中也含有花色苷[4]。
近年来,随着气温逐年升高,干旱一直是困扰农业生产的主要问题,我国葡萄种植面积和产量持续增长,西北地区面临的干旱问题也日益突出[5]。水分是影响葡萄长势和品质的重要因素,水分胁迫能诱导植物产生各种生理生化反应,过度水分胁迫会导致植株生长停止,光合受到抑制,呼吸和代谢紊乱,功能和蛋白变性等[6],而适度的水分胁迫则对酿酒葡萄的糖酸、单宁、花色苷等果实品质有一定促进作用。因此,在我国西北地区葡萄节水栽培已成为主要栽培模式。
植物黎明前的叶片水势可以直观地反映植株的水分亏损状况,是监测植株体内水分含量的传统指标之一[7]。马瑟兰葡萄属于喜水植物,在葡萄栽培的水分管理中,适宜的灌溉时间和灌溉量对葡萄生长十分重要[8],同时本课题组之前研究表明,黎明前叶片水势在中度范围内可显著提升酿酒葡萄果实品质[9-10],适度水分胁迫不仅可以保证酿酒葡萄正常生长,而且可以达到节水和提质增效的效果,为西北地区马瑟兰葡萄的栽培管理和节水灌溉体系奠定实践基础[11-12]。
1 材料和方法
1.1 试验材料
本试验于2020年5—10月在宁夏玉泉营农场国家葡萄产业技术体系水分生理与节水栽培岗位(CARS-29-zp-3)试验点进行(38.28°N,106.24°E)。本试验选用3 年生马瑟兰(Vitis Vinifera L.‘Marselan’)葡萄为材料,东西行向定植,“厂”字整形,株行距为1.5 m×3 m,冬季埋土防寒,灌溉方式为滴灌,除灌水量不同外,其他管理指标均一致,试验设3个生物学重复,各重复10 株葡萄树,水分胁迫处理模型如图1所示,不同处理之间用保护行间隔,绿色表示无水分胁迫,黄色代表水分胁迫,颜色越深水分胁迫程度越大。
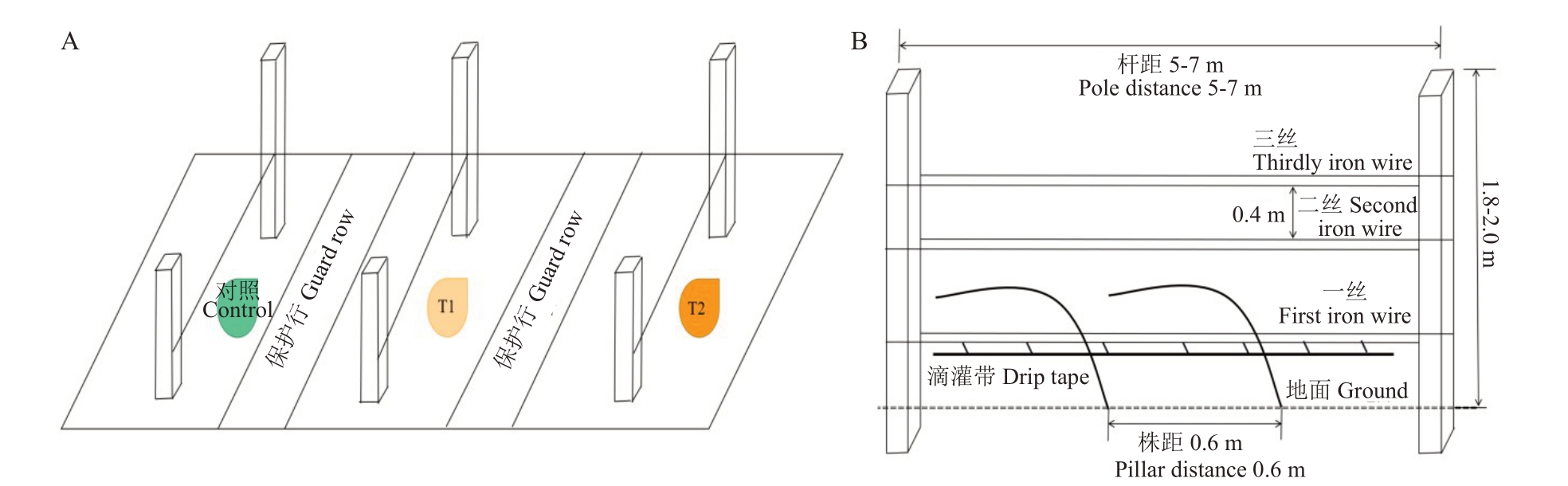
图1 试验设计模型
Fig.1 The model of test design
A. 主视图;B. 侧视图。
A in the figure is front view;B is side view.
1.2 试验设计
从葡萄萌芽期至盛花期(5月20日)对所有葡萄树进行正常灌溉处理,于6 月10 日即花后20 d(20 days after anthesis,20 DAA)开始水分胁迫处理。根据黎明前叶片水势(Ψb)变化趋势分别设置无水分胁迫(对照,0 MPa ≥Ψb ≥-0.2 MPa)、轻度水分胁迫(T1,-0.2 MPa >Ψb ≥-0.4 MPa)、中度水分胁迫(T2,-0.4 MPa >Ψb ≥-0.6 MPa)。试验采用滴灌管带两头安装控水阀门,控制滴灌管流速为0.6 L·h-1,每10 d 监测1 次Ψb值,根据测定Ψb值及试验期间葡萄园降雨量和气温(表1),确定各处理是否灌水和所需灌水量(表2)。试验分别于40 DAA(膨大期)、60 DAA(转色期)、70 DAA、90 DAA、110 DAA、120 DAA(成熟采收期,10 月31 日)进行采样,选取植株阴阳面的果穗,随机选取果穗上、中、下、内侧和外侧各部位果粒共100粒,液氮速冻后置于-80 ℃冰箱保存。
表1 试验期间气温和降雨量
Table 1 Temperature and monthly rainfall during the experiment
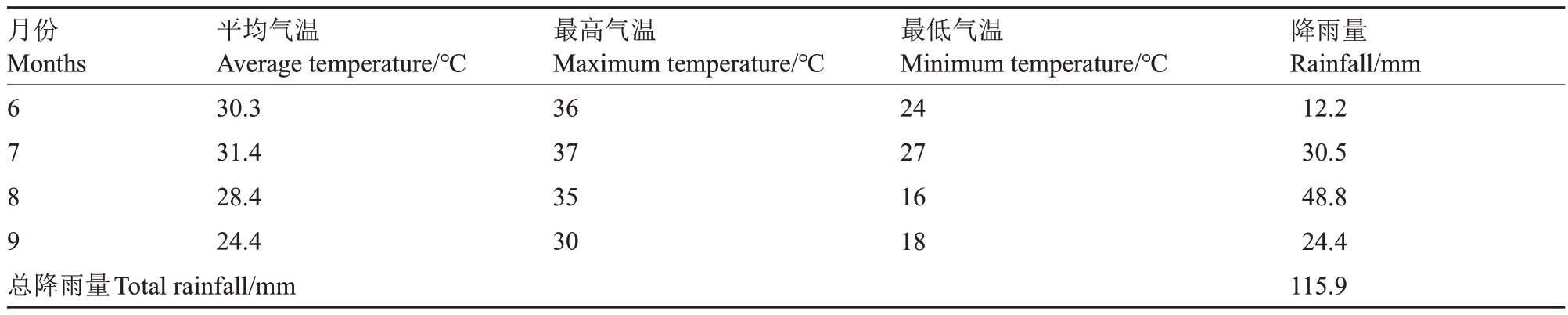
月份Months 6789总降雨量Total rainfall/mm平均气温Average temperature/℃30.3 31.4 28.4 24.4最高气温Maximum temperature/℃36 37 35 30最低气温Minimum temperature/℃24 27 16 18降雨量Rainfall/mm 12.2 30.5 48.8 24.4 115.9
表2 不同处理灌水量
Table 2 Irrigation quantity of different treatment groups(L·plant-1)
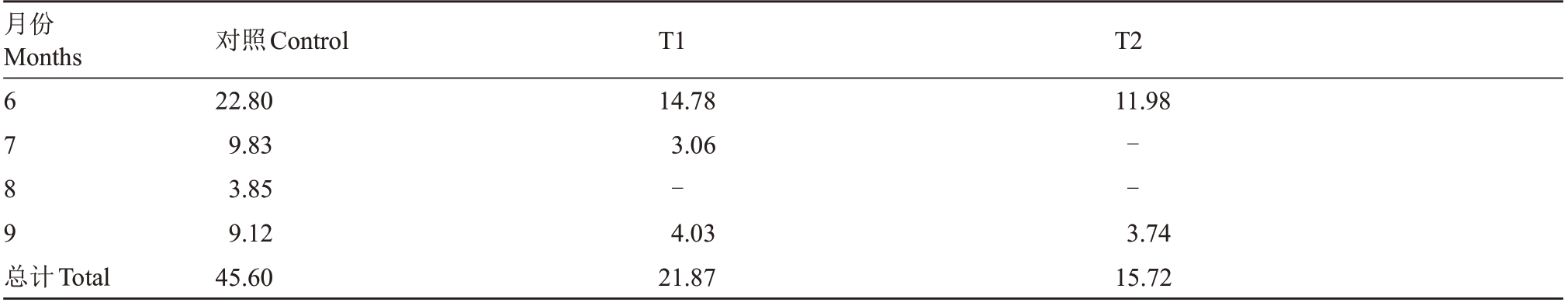
注:“-”表示没有灌水。
Note:“-”means no irrigation.
月份Months 6789总计Total对照Control 22.80 9.83 3.85 9.12 45.60 T1 14.78 3.06-4.03 21.87 T2 11.98--3.74 15.72
1.3 试验方法
1.3.1 黎明前叶片水势测定 黎明前(05:30—06:00)迅速摘取葡萄枝条中部节位的健康叶片,置于塑封袋中,带回实验室,用刀片在叶柄末端切出斜面,迅速装入3005 型植物水分压力室(美国Soil Moisture Equipment 公司)中,使叶柄末端切口从压力室密封圈中部的小孔露出,拧紧钢塞,关闭控制阀,缓慢转动加压阀,当叶柄末端出现小水珠时,立即关闭加压阀,此时读数表盘示数,即为叶片水势的绝对值,记录数值,每个处理9次重复。
1.3.2 果实生长及基本品质指标测定 果实纵横径用游标卡尺测定,试验处理开始后每10 d进行1次;随机选取100 粒果实,百粒质量用分析电子天平称质量;可溶性固形物(TSS)含量用WYT-32 型手持糖量折光仪测定;可滴定酸含量采用NaOH 滴定法测定。
1.3.3 果实单糖、有机酸的提取与测定 葡萄果实用液氮研磨至粉末状态,去除果梗和种子,准确称取粉末0.2 g于2 mL离心管中,加入1.8 mL超纯水,超声波提取25 min,12 000 r·min-1离心10 min,取上清液于新的离心管中,之后用0.22 μm 的水相滤膜过滤至进样瓶,每个样品设置3 个重复,利用UltiMate 3000 型高效液相色谱仪测定(ThermoFisher 科技公司)。
单糖测定色谱条件:示差检测器,色谱柱:Hypersil GOLDTM Amino色谱柱(250 mm×4.6 mm,5 μm);流动相为乙腈∶水(V/V)=75∶25;检测波长:245 nm;流速0.5 mL·min-1;柱温:30 ℃;进样量10 μL。
有机酸测定色谱条件:紫外检测器,色谱柱:C18 色谱柱(250 mm×4.6 mm,5 μm);流动相为甲醇∶KH2PO4(V/V)= 3∶97,磷酸调节pH 至2.8;检测波长:215 nm;流速0.8 mL·min-1;柱温:35 ℃;进样量10 μL。
1.3.4 果实总酚、单宁含量的测定 葡萄果实用液氮研磨至粉末状态,去除果梗和种子,备用。
总酚含量:采用福林酚法[13]测定,准确称取粉末1.0 g,加入20 mL 70%的乙醇研磨浸提,90 ℃水浴15 min,4000×g 离心15 min,收集提取液并用70%乙醇定容至100 mL,然后吸取1 mL稀释液,依次加60 mL 蒸馏水、5 mL 福林酚、15 mL 20%碳酸钠,用蒸馏水定容至100 mL。20 ℃避光显色2 h,在765 nm下测定吸光值。
单宁含量:采用福林丹尼斯法[13]测定,准确称取粉末1.0 g,加20 mL 20%乙醇研磨浸提,70 ℃水浴30 min,4000×g离心15 min,残渣用20%乙醇溶液洗涤,重复浸提2次,取上清液用蒸馏水定容至100 mL容量瓶中。吸取1 mL 提取液,依次加70 mL 蒸馏水、5 mL福林丹尼斯、10 mL饱和碳酸钠,用蒸馏水定容至100 mL,20 ℃避光显色30 min,在760 nm下测定吸光值。
1.3.5 总花色苷和单体花色苷含量的测定 样品提取方法参照李栋梅等[14]的方法有所改动。挑选大小均匀、无病虫害的果实,冷冻状态下剥皮,液氮研磨成粉末,去除种子和果梗;称取果皮粉末0.1 g 于2 mL离心管中,每个处理3次重复;加入1 mL的1%甲酸甲醇溶液;恒温摇床20 ℃避光浸提10 min;8000×g、4 ℃离心5 min;收集上清液于10 mL离心管中,重复浸提3次,合并上清,20 ℃旋转蒸发去除甲酸和甲醇,并用1.5 mL 甲醇洗涤溶液于2 mL 离心管中,放入4 ℃避光保存,该提取液用于测定总花色苷和单体花色苷含量。
总花色苷含量:采用pH示差法等[15]测定。
单体花色苷色谱条件:紫外检测器,Infinity Poroshell 120 SB-C18 色谱柱(150 mm × 4.6 mm,2.7 μm),流动相A为甲酸∶乙腈∶水(V/V/V)=7.5∶30∶240;流动相B 为甲酸∶乙腈∶水(V/V/V)=7.5∶150∶120;流速1.0 mL·min-1,柱温:35 ℃,检测波长:520 nm,进样量:30 μL,样品测定前用0.22 μm 有机膜过滤,定量分析以二甲花翠素-3-O-葡萄糖苷为标准品,采用外标法进行定量分析,根据花色苷保留时间、最大吸收波长和本实验花色苷质谱数据对花色苷进行定性分析。
1.3.6 总RNA 的提取和实时荧光定量PCR 总RNA 提取用RNAprep Pure 多糖多酚植物总RNA提取试剂盒流程进行操作;以总RNA 为模板,参照PrimeScriptTM RT reagent Kit with gDNA Eraser反转录试剂盒说明书进行反转录;选取VvActin 为内参基因,VvPAL、VvF3′5′H、VvLAR、VvUFGT 基因的引物用Primer 5.0 设计,引物由生工生物工程(上海)公司合成(表3)。RT-qPCR 反应体系为20 μL:cDNA(200 ng·μL-1)1 μL,上游引物和下游引物各0.4 μL,2 × Perfecstar 10 μL,ddH2O 8.2 μL;RT-qPCR 扩增程序为94 ℃2 min、94 ℃10 s、60 ℃30 s,2~3 步循环40 次。每个模板设3次生物学重复,取其平均值,目的基因的相对表达量用2-ΔΔCt计算[16]。
表3 实时荧光定量PCR 引物序列
Table 3 Primer sequences for real-time quantitative PCR

基因名称Gene name VvPAL VvF3′5′H VvLAR VvUFGT VvActin引物序列(5’-3’)Sequence of primer上游引物Forward TTAAAATGGCTGGGATCGAG AAGCGTGCTCACGAAGAAAT CTACGGTGATGGCTCTGTCA TGCAGGGCCTAACTCACTCT CTTGCATCCCTCAGCACCTT下游引物Reverse CCTGCATCACTTCAGCAAAA TTCCCAGACATCAGGGTCTC GCAGCGGCTAGTAGGTCATC GCAGTCGCCTTAGGTAGCAC TCCTGTGGACAATGGATGGA
1.4 数据处理及分析
采用Microsoft office excel 2019 和Origin8.0 进行数据记录和作图,用DPS V9.01 进行统计分析,LSD多重检验样本间的差异显著性(p<0.05)。
2 结果与分析
2.1 水分胁迫下葡萄植株黎明前的叶片水势
黎明前植株叶片水势是表征植株体内含水量和水分亏缺状况的最佳指标,如图2所示,各处理在20 DAA水势基本控制在-0.15 MPa左右,30 DAA后对照、T1和T2的水势分别控制在-0.17、-0.36、-0.51 MPa,根据玉泉营试验基地2020年日降雨量和每日气温变化及时调整灌水时间及灌水量,在40 DAA至120 DAA期间各处理Ψb值稳定于变化范围之内,说明试验处理符合设计要求。
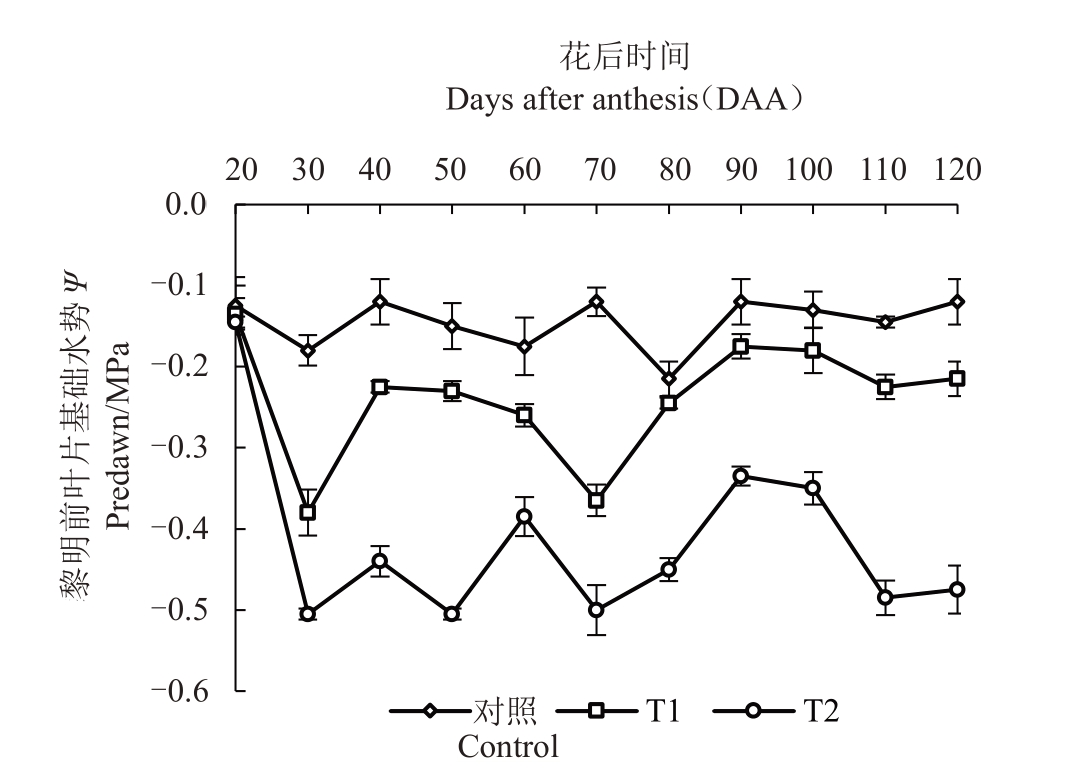
图2 葡萄植株黎明前叶片水势
Fig.2 Leaf water potential values of grapevine before dawn
2.2 水分胁迫处理对马瑟兰葡萄果实生长和基本品质指标的影响
不同水分胁迫处理对马瑟兰葡萄果实生长和基本品质指标的影响如图3所示。图3-A表示葡萄生长过程中的表型变化。随着果实生育期的推进,T1和T2处理后果实的纵横径显著低于对照,各处理果实在70 DAA 至80 DAA 快速增大,在110 DAA 至120 DAA 期间纵横径几乎不变,趋于稳定(图3-B、图3-C);T1和T2处理后,果实百粒质量显著低于对照,在120 DAA 时,与对照相比,T1 和T2 分别降低12.58%和17.49%(图3-D);T1 和T2 的可滴定酸含量显著低于对照,在120 DAA时,与对照相比,T1和T2分别降低9.81%和15.96%(图3-E);T1和T2的可溶性固形物含量显著高于对照,在120 DAA 时,与对照相比,T1 和T2 分别高于4.22%和6.13%(图3-F)。说明随水分胁迫程度的增加,果实纵横径、百粒质量和TA含量均下降,TSS含量提高。

图3 不同水分胁迫处理对马瑟兰葡萄生长及基本品质的影响
Fig.3 Effects of water stress on Grape growth and basic quality in Marselan
A.3 种处理下葡萄果实不同生长发育过程的表型图;B. 果实纵径;C. 果实横径;D. 百粒质量;E. 可滴定酸含量;F. 可溶性固形物含量。
A. The process of grape berry development under three treatments; B. Vertical diameter of grape berry;C. Horizontal diameter of grape berry;D. Hundred grain mass;E.Titratable acid content;F.Soluble solids content.
2.3 水分胁迫处理对马瑟兰葡萄果实葡萄糖和果糖含量的影响
不同水分胁迫处理对马瑟兰葡萄果实单糖含量的影响如图4所示。采用HPLC法检测不同处理条件下果实糖含量,其中以葡萄糖和果糖含量为主,蔗糖为辅。随着果实生育期的推进,葡萄糖和果糖含量均呈上升趋势,且从60 DAA 至70 DAA增速最大,在不同时期,T1 和T2 处理下葡萄糖和果糖含量均显著高于对照。在120 DAA时,各处理果糖含量最大值分别为97.16、104.59、105.83 mg·g-1,与对照相比,T1 和T2 果糖含量分别提高7.10%和8.19%(图4-A)。T2在110 DAA葡萄糖含量最大为93.61 mg·g-1,而对照和T1在120 DAA 时,葡萄糖含量最大值为79.23、94.73 mg·g-1,与对照相比,T1 和T2 葡萄糖含量分别提高16.36%和15.36%(图4-B)。表明T1 和T2 处理均可提高马瑟兰葡萄果实单糖含量。
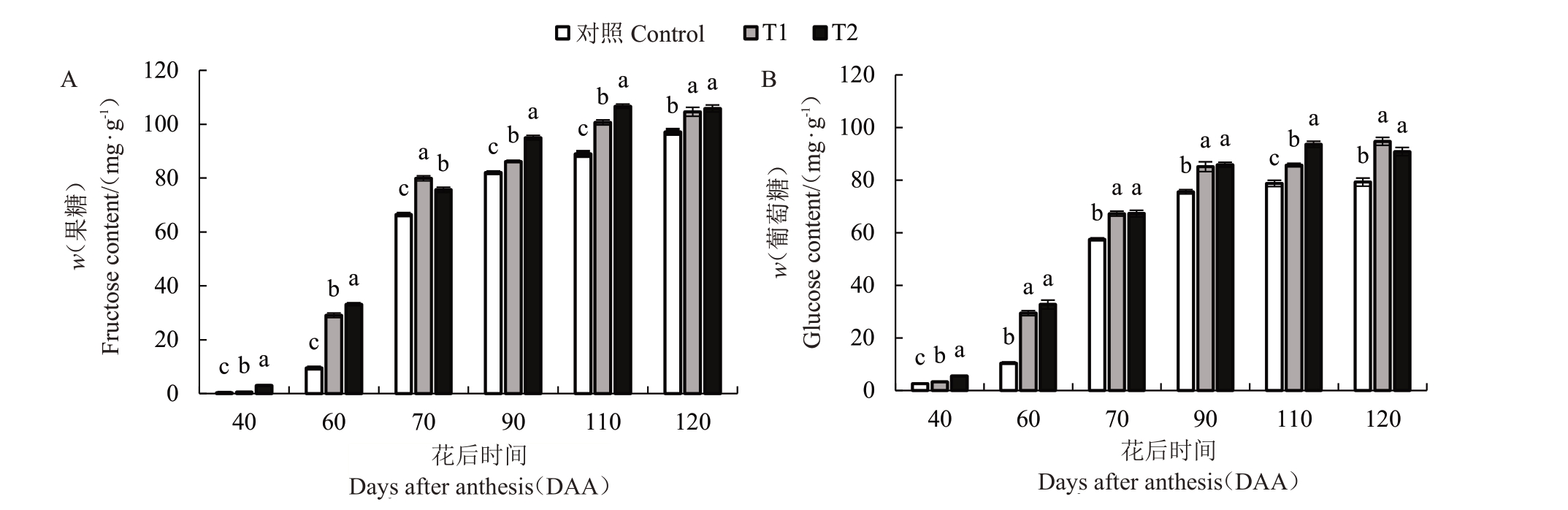
图4 不同水分胁迫处理对马瑟兰葡萄单糖含量的影响
Fig.4 Effects of water stress on the individual sugar content in Marselan
2.4 水分胁迫处理对马瑟兰葡萄有机酸含量的影响
不同水分胁迫处理对马瑟兰葡萄果实有机酸含量的影响如图5所示。采用HPLC法对不同处理的果实有机酸含量进行测定,随着果实生育期的变化,有机酸含量均处于下降趋势,以酒石酸和苹果酸含量为主,柠檬酸含量几乎检测不出。对于酒石酸含量而言,T1和T2在60 DAA至70 DAA降速最大,在60 DAA 后各时期T1 和T2 均显著低于对照,在120 DAA 时,各处理酒石酸含量达到最低11.29、9.62、10.56 mg·g-1,与对照相比,T1和T2分别降低17.35%和6.91%(图5-A)。对于苹果酸含量而言,对照在70 DAA至90 DAA降速最大,T1和T2在60 DAA至70 DAA 降速最大,在60 DAA 后各时期T1 和T2 均显著低于对照,在120 DAA 时,各处理分别达到最低8.47、5.95、4.52 mg·g-1,与对照相比,T1和T2分别降低45.08%和48.16%(图5-B)。说明T1和T2均可降低葡萄有机酸含量。
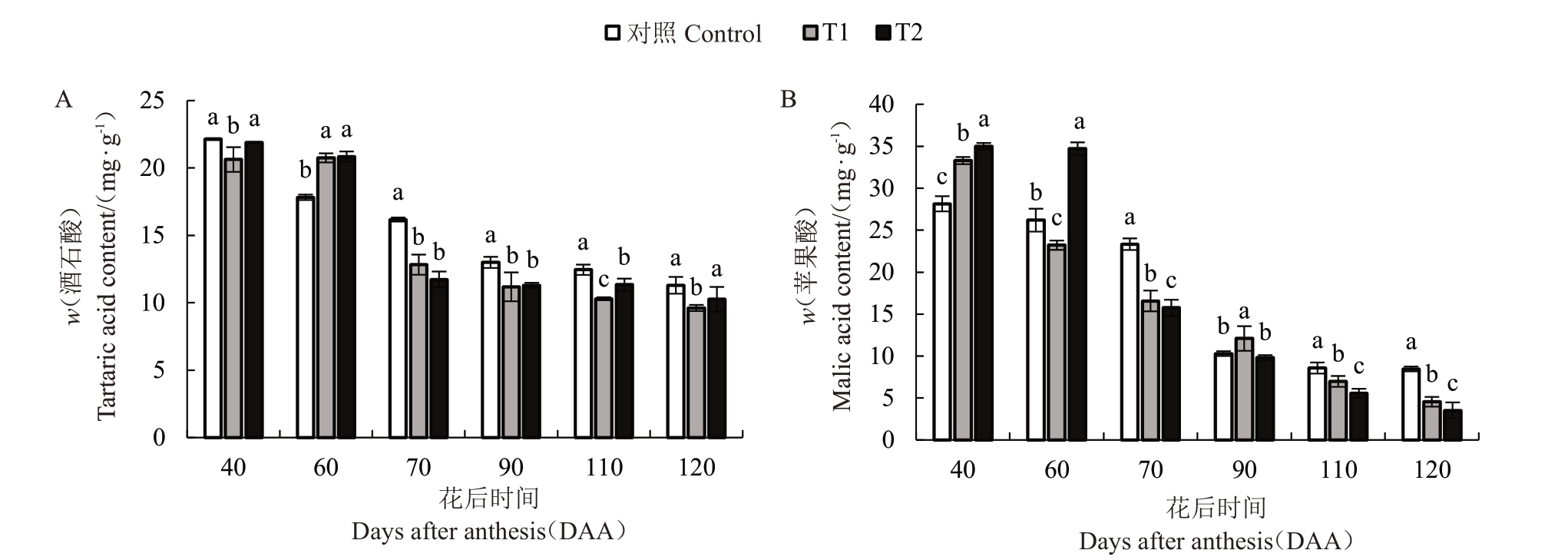
图5 不同水分胁迫处理对马瑟兰葡萄有机酸含量的影响
Fig.5 Effects of water stress on the organic acid content in Matheran
2.5 水分胁迫处理对马瑟兰葡萄总酚和单宁含量的影响
不同水分胁迫处理对马瑟兰葡萄果实单宁和总酚含量的影响如图6 所示。随果实的成熟,单宁和总酚含量逐渐降低,在120 DAA 时,各处理的总酚含量为T2>T1>对照,相比于对照,T1和T2处理下总酚含量提高了20.00%和30.30%(图6-A);各处理的单宁含量为T1>T2>对照,相比于对照,T1和T2处理下单宁含量显著提高了14.58%和6.87%(图6-B),表明T1 和T2 处理均可促进马瑟兰葡萄总酚含量的积累,同时减缓单宁的分解,使成熟期单宁含量积累增加。

图6 不同水分胁迫处理对马瑟兰葡萄果实总酚和单宁含量的影响
Fig.6 Effects of different water stress treatments on tannins and total phenols in Marselan
2.6 水分胁迫处理对马瑟兰葡萄果皮花色苷含量的影响
不同水分胁迫处理对马瑟兰葡萄果皮花色苷含量的影响如图7 所示,图7-A 表示果皮花色苷提取液颜色的变化,图7-B 表示不同处理下马瑟兰葡萄总花色苷含量的变化,随着葡萄果实成熟,总花色苷含量呈上升趋势。于40 DAA 时,3 种处理条件下均未检测到花色苷,而在60 DAA,即果实转色期,3 种处理条件下均可检测到花色苷,且T1 和T2处理条件下总花色苷含量显著高于对照,在120 DAA(成熟采收期)时,与对照相比,T1 和T2 处理条件下分别提高了18.57%和21.05%。图7-C 表示不同处理下马瑟兰葡萄单体花色苷含量的变化,葡萄果皮中共检测出17 种单体花色苷,其中包括5 种基本花色苷,5 种乙酰化花色苷,5 种香豆酰化花色苷,2 种咖啡酰化花色苷。在120 DAA 时,单体花色苷含量最高的是二甲花翠素-3-O-葡萄糖苷,与对照相比,T1 和T2 分别显著提高52.85%和43.11%,其次是二甲花翠素-3-O-反式香豆酰化葡萄糖苷,与对照相比,T1 和T2 分别显著提高26.98%和25.08%,甲基花青素-3-O-咖啡酰化葡萄糖苷含量最低,表明T1 和T2 均能增加果皮总花色苷含量,促进二甲花翠素-3-O-葡萄糖苷等单体花色苷的积累,增强果实颜色。
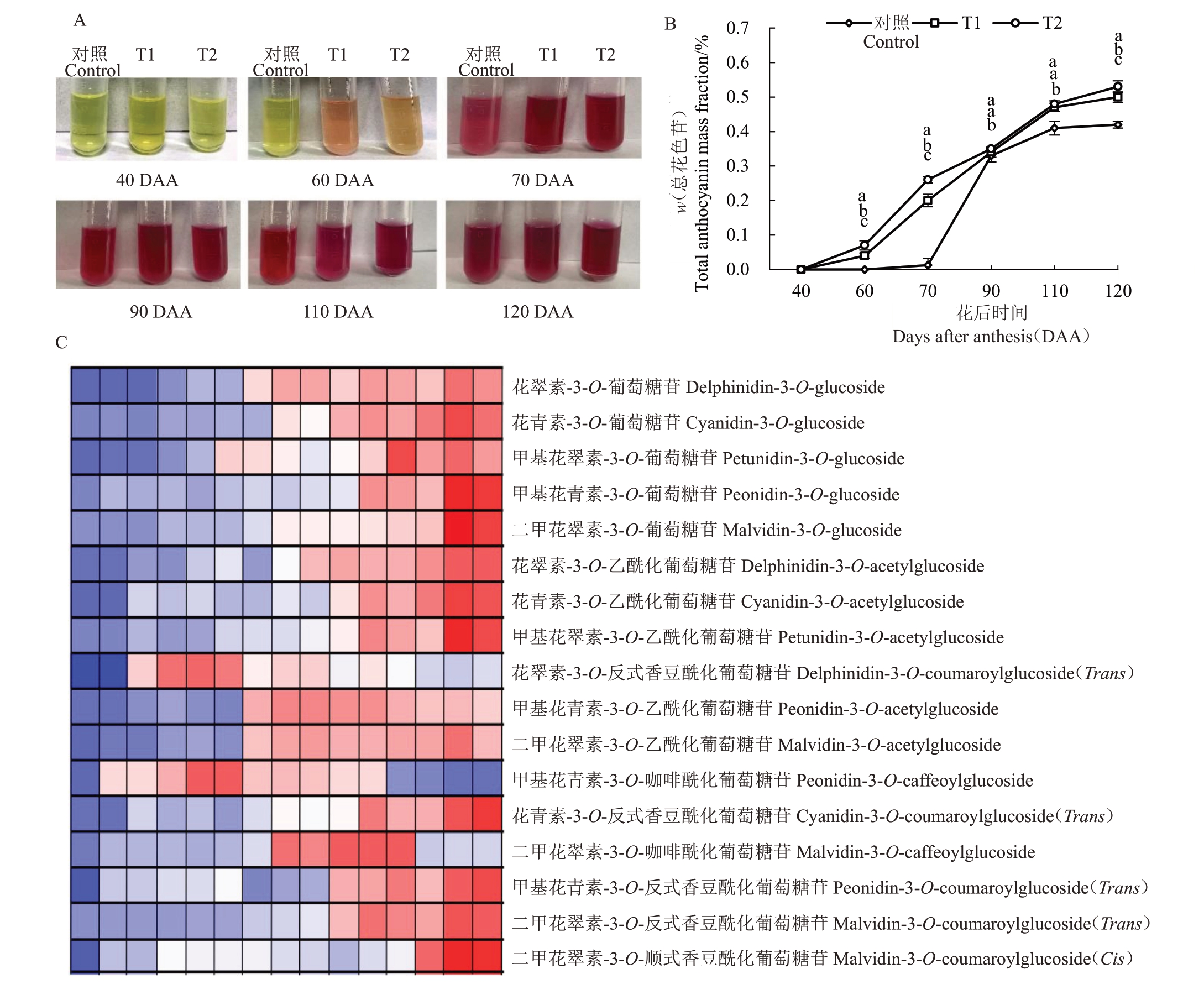
图7 不同水分胁迫处理对马瑟兰葡萄花色苷含量的影响
Fig.7 Effects of water stress on anthocyanin content in Marselan
A.果皮花色苷提取液颜色图;B.总花色苷含量变化;C.不同处理下马瑟兰葡萄果皮单体花色苷含量变化热图,数据用Z-score 进行标准化。
A.Color diagram of anthocyanin;B.The change of total anthocyanin content;C.The heatmap of the variation of anthocyanin content in Matheran grape under different treatments,the data were standardized by Z-score.
2.7 水分胁迫处理对类黄酮代谢相关基因表达的影响
VvPAL 是类黄酮代谢途径中上游的关键基因,由图8 可知,在果实发育后期,T1 和T2 处理条件下VvPAL的表达量显著高于对照,其中在110 DAA时,与对照相比,T1 和T2 表达量分别提高了6.65 倍和3.78倍,在120 DAA时,T1和T2表达量分别提高了3.82 倍和2.83 倍(图8-A)。VvLAR 是原花青素合成途径中的关键基因,其中在60 DAA 至90 DAA 期间,T2 处理条件下VvLAR显著高于对照和T1,与对照相比,T2 的表达量分别提高了1.90 倍、1.38 倍、1.37 倍,在果实发育前期和后期,对照的表达量与T2无差异(图8-B)。
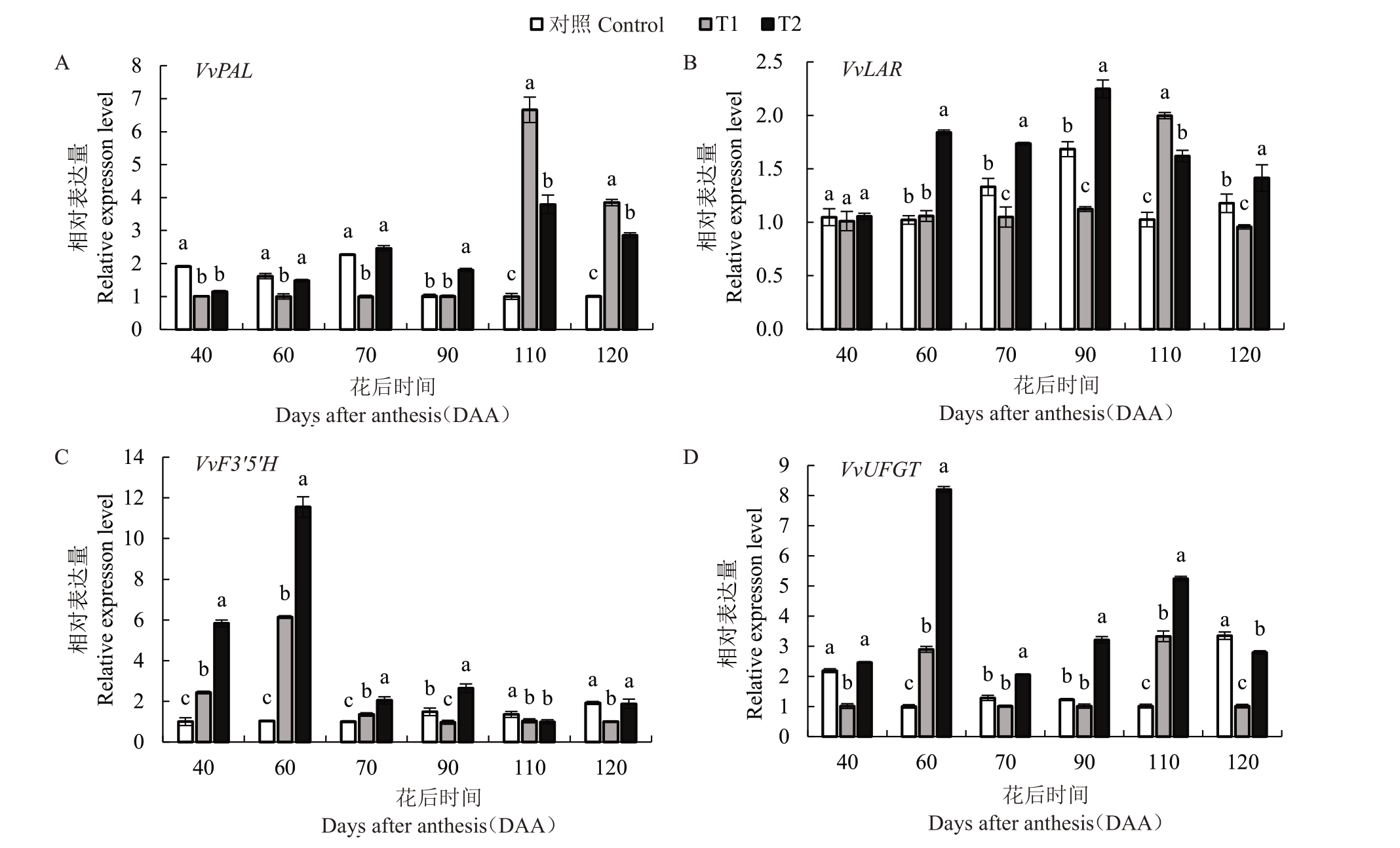
图8 不同水分胁迫处理对马瑟兰葡萄花色苷合成途径相关基因表达的影响
Fig.8 Effects of water stress on expression of anthocyanin metabolity-related genes in Marselan
A.苯丙氨酸解氨酶;B.原花青素还原酶;C.类黄酮-3′5′-羟化酶;D,类黄酮葡萄糖基转移酶。
A.Phenylalanine ammonialyase;B.Proanthocyanin reductase;C.Flavonoid-3′5′-hydroxylase;D.Flavonoid glucosyltransferase.
VvF3′5′H和VvUFGT是花色苷合成途径中的关键基因。对于VvF3′5′H而言,在60 DAA时,与对照相比,T1 和T2 表达量分别提高了5.93 倍和11.15倍,其他时期(除120 DAA),T2 处理下显著高于对照和T1(图8-C);对于VvUFGT 而言,在60 DAA 至120 DAA,T2 均显著高于对照和T1,在60 DAA 时,与对照相比,T1 和T2 表达量分别提高了2.89 倍和8.19 倍,在120 DAA 时,与对照相比,T1 和T2 表达量分别提高了3.29 倍和5.19 倍,此外在40 DAA 和120 DAA 时,对照显著高于T1 且与T2 无极显著差异(图8-D)。
3 讨 论
3.1 水分胁迫对葡萄单糖和有机酸含量的影响
葡萄果实含糖量的高低主要受果实成熟过程中糖分积累的影响,适度的水分亏缺可抑制果实鲜质量增长而提升果实含糖量,改善果实品质[16-17]。高彦婷等[18]的研究结果表明,轻度水分胁迫条件下葡萄糖和果糖含量最高,这与本研究结果不一致,可能原因是在温室进行水分胁迫处理和大田试验有误差。杨昌钰等[19]的研究结果表明,水分胁迫会抑制果树光合作用,减少碳水化合物的消耗,提高蔗糖转化酶、蔗糖合成酶和蔗糖磷酸合成酶的活性,增强蔗糖的代谢能力,利于果实糖分的代谢转化,促进糖分积累,这与本试验研究结果基本一致。李红燕[20]的研究表明,随着灌溉调亏程度的加强,葡萄的还原糖含量呈增加趋势,适当的水分胁迫能促进葡萄浆果糖分含量的增加,严重亏缺则会抑制,这与本研究结果一致。
果实有机酸含量与果实的风味密切相关[21]。有机酸的代谢过程极为复杂,其含量的高低主要受果实内在特性、外在环境和栽培措施等因素的影响[22-23]。龚成宇等[24]对黄果柑果实酸代谢的研究结果表明,轻度干旱胁迫可通过提升细胞质顺-乌头酸酶(ACO)和异柠檬酸脱氢酶(NAD-IDH)的活性来增强果实中有机酸的降解,这与本试验有机酸变化的趋势一致。李航等[25]对樱桃果实研究结果表明,果实在成熟过程中苹果酸含量最高,且呈现先升高后降低的变化趋势。本试验结果表明,葡萄果实在成熟前期以苹果酸为主,在成熟后期苹果酸含量明显低于酒石酸含量,可能原因是果实在受水分胁迫处理后可增强苹果酸酶(NADP-ME)的活性,催化苹果酸向丙酮酸转化,或可抑制苹果酸脱氢酶(NADMDH)和磷酸烯醇式丙酮酸羧化酶(PEPC)的活性,进而抑制磷酸烯醇式丙酮酸(PEP)向苹果酸转化,此猜测仍有待进一步研究证明。邓浩亮等[26]的研究结果表明,在美乐葡萄果实膨大期进行亏水处理,亏水处理后的果实含酸量高于对照,而在葡萄果实着色期进行中度亏水处理,可以降低有机酸含量。研究结果也表明T2 处理下酒石酸和苹果酸含量在40 DAA至60 DAA变化不明显且高于对照,转色之后才出现明显的下降趋势,两者有机酸含量变化趋势基本一致,但不同品种之间可能存在差异。研究表明,酒石酸在葡萄果实成熟过程中具有较好的代谢稳定性,而苹果酸含量极易受环境胁迫影响而下降,研究结果表明,水分胁迫处理之后,在果实成熟期T2 酒石酸含量高于T1,但T2 苹果酸含量低于T1。曹慧玲等[27]的研究表明,抗坏血酸(Asc)作为酒石酸合成的前提物质,多集中于果实和叶片,其生物合成与光合作用中碳的流向改变有关,目前Asc 主要由L-半乳糖途径和D-半乳糖醛酸途径合成,但抗坏血酸合成酒石酸途径中部分相关的酶及基因还未证实,水分胁迫处理如何影响酒石酸代谢进而影响葡萄果实酒石酸含量变化还需大量研究,因此研究结果显示T2 处理后酒石酸含量低于对照但未达显著水平,还需进一步研究。
3.2 水分胁迫对葡萄花色苷含量及其代谢途径的影响
葡萄果皮花色苷含量直接决定葡萄着色度,使葡萄果实呈现出红色、蓝色和紫色,果实花色苷的合成主要从转色期开始,在整个成熟过程中逐渐积累,种植技术、栽培环境等都会影响花色苷的含量和单体花色苷的比例[28]。王新[29]和Poulsen等[30]的试验表明,对赤霞珠葡萄转色后进行持续干旱(转色后对植物不灌水处理),随果实的成熟可显著增加果皮花色苷的含量,同时在果皮花色苷单体中二甲花翠素类及其衍生物所占比例最高,本试验结果与之基本一致,但是其他花色苷单体占比可能存在葡萄品种之间的差异变化。在葡萄果皮中,花色苷以3-O-葡萄糖苷的形式在液泡中积累,花色苷糖基可在A 环的6位发生酰基化修饰,增加花色苷的稳定性。Castellarin等[31]的试验表明,对葡萄品种进行干旱处理后,非乙酰化花色苷单体含量高于乙酰化花色苷单体含量,本研究结果与之一致,同时笔者在本研究中发现水分胁迫处理下,酰基化单体花色苷中含量高低依次是乙酰化花色苷>香豆酰化花色苷>咖啡酰化花色苷。赵裴等[32]试验表明,“干化”处理后马瑟兰葡萄果皮中共检测出19种花色苷,从B环结构形式来看,二甲花翠素类葡萄糖苷占比最高,甲基花青素类占比最低,从3′和5′位C的取代形式来看,基本花色苷的占比最大,这与本试验结果一致。
花色苷是决定葡萄和葡萄酒质量的重要因素之一,植物花青素生物合成需要一系列的结构基因和转录因子协同作用[33]。Robinson 等[34]的研究表明,在转基因葡萄中,随着VvF3′5′H 的表达量降低,果皮的花青素、单宁及酚类物质含量都显著降低且葡萄酒的颜色变浅。Martínez-Lüscher 等[35]的结果表明,在水分亏缺条件下,VvF3′5′H、VvUFGT 2个关键类黄酮生物合成基因的上调会引起果皮花色苷含量的升高,这与本研究结果一致。Wang等[36]的试验结果表明,在干旱胁迫处理下,VvMYBF1 过表达可上调类黄酮生物合成中VvPAL 的表达量,这与本试验VvPAL 的前期表达结果不一致,可能原因是水分胁迫处理下存在某些转录因子调节其生物合成,还需进一步研究。
4 结 论
轻度和中度水分胁迫处理下酿酒葡萄在转色期后可明显提升果糖和葡萄糖含量,降低酒石酸和苹果酸含量,增加成熟期总酚、单宁含量,进而提升果实品质。
轻度水分胁迫显著增加二甲花翠素-3-O-葡萄糖苷和二甲花翠素-3-O-反式香豆酰化葡萄糖苷含量,降低甲基花青素-3-O-咖啡酰化葡萄糖苷、二甲花翠素-3-O-咖啡酰化葡萄糖苷和花翠素-3-O-反式香豆酰化葡萄糖苷含量。
中度水分胁迫处理,可明显提高果实转色期VvF3′5′H 和VvUFGT 的表达量,进而促进后期果皮花色苷的合成。
[1] YANG B H,HE S,LIU Y,LIU B C,JU Y L,KANG D Z,SUN X Y,FANG Y L. Transcriptomics integrated with metabolomics reveals the effect of regulated deficit irrigation on anthocyanin biosynthesis in cabernet sauvignon grape berries[J].Food Chemistry,2020,314:126-170.
[2] 卢素文,郑暄昂,王佳洋,房经贵.葡萄类黄酮代谢研究进展[J].园艺学报,2021,48(12):2506-2524.LU Suwen,ZHENG Xuan’ang,WANG Jiayang,FANG Jinggui.Research progress on the metabolism of flavonoids in grape[J].Acta Horticulturae Sinica,2021,48(12):2506-2524.
[3] GOUOT J C,SMITH J P,HOLZAPFEL B P,WALKER A R,BARRIL C.Grape berry flavonoids:A review of their biochemicalresponses to high and extreme high temperatures[J]. Journal of Experimental Botany,2019,70(2):397-423.
[4] GUAN L,LI J H,FAN P G,CHEN S,FANG J B,LI S H,WU B H. Anthocyanin accumulation in various organs of a teinturier cultivar(Vitis vinifera L.)during the growing season[J]. American Journal of Enology and Viticulture,2012,63(2):177-184.
[5] LIANG Z C,OWENS C L,ZHONG G Y,CHENG L L. Polyphenolic profiles detected in the ripe berries of Vitis vinifera germplasm[J].Food Chemistry,2011,129(3):940-950.
[6] 胡宏远,李双岑,马丹阳,王振平.水分胁迫对赤霞珠葡萄果实品质的影响研究[J].节水灌溉,2016(12):36-41.HU Hongyuan,LI Shuangcen,MA Danyang,WANG Zhenping.Effect of water stress on grape fruit quality of Cabernet Sauvignon[J].Water Saving Irrigation,2016(12):36-41.
[7] 房玉林,惠竹梅,陈洁,何建林,张振文.水分胁迫对葡萄光合特性的影响[J].干旱地区农业研究,2006,24(2):135-138.FANG Yulin,HUI Zhumei,CHEN Jie,HE Jianlin,ZHANG Zhenwen. Effects of water stress on photosynthetic characteristics of grapevine[J]. Agricultural Research in the Arid Areas,2006,24(2):135-138.
[8] 段长青,刘崇怀,刘凤之,王忠跃,刘延琳,徐丽明.新中国果树科学研究70 年:葡萄[J]. 果树学报,2019,36(10):1292-1301.DUAN Changqing,LIU Chonghuai,LIU Fengzhi,WANG Zhongyue,LIU Yanlin,XU Liming. Fruit scientific research in new China in the past 70 years:Grape[J]. Journal of Fruit Science,2019,36(10):1292-1301.
[9] JU Y L,YANG B H,HE S,TU T Y,MIN Z,FANG Y L,SUN X Y.Anthocyanin accumulation and biosynthesis are modulated by regulated deficit irrigation in Cabernet Sauvignon(Vitis Vinifera L.)grapesand wines[J].Plant Physiology and Biochemistry,2019,135:469-479.
[10] PINASSEAU L,VALLVERDÚ-QUERALT A,VERBAERE A,ROQUES M,MEUDEC E,LE C L,PÉROS J P,AGEORGES A,SOMMERER N,BOULET J C,TERRIER N,CHEYNIER V. Cultivar diversity of grape skin polyphenol composition and changes in response to drought investigated by LC-MS based metabolomics[J].Frontiers in Plant Science,2017,8:1826.
[11] 张钥,王呈阳,周嘉玲,李有梅,谢兆森,冷锋.不同水分调亏处理对葡萄果皮酚类物质的影响[J].果树学报,2021,38(8):1296-1307.ZHANG Yue,WANG Chengyang,ZHOU Jialing,LI Youmei,XIE Zhaosen,LENG Feng. Effects of different regulated deficit irrigation treatments on phenols in grape berries[J]. Journal of Fruit Science,2021,38(8):1296-1307.
[12] 李雅善,赵现华,王华,李华.葡萄调亏灌溉技术的研究现状与展望[J].干旱地区农业研究,2013,31(1):236-241.LI Yashan,ZHAO Xianhua,WANG Hua,LI Hua. Research advance and prospect of regulated deficit irrigation on grapevines[J]. Agricultural Research in the Arid Areas,2013,31(1):236-241.
[13] WILSON M F. Comparison of tannin levels in developing fruit buds of two orchard pear varieties using two techniques,Folin-Denis and protein precipitation assays[J]. Journal of Chemical Ecology,1984,10(3):493-498.
[14] 李栋梅,王振平,李相怡,孙思捷,刘博洋,李嘉佳,王磊,王世平.根域限制对玫瑰香葡萄果实糖酸及酚类物质和内源激素的影响[J].果树学报,2022,39(3):376-387.LI Dongmei,WANG Zhenping,LI Xiangyi,SUN Sijie,LIU Boyang,LI Jiajia,WANG Lei,WANG Shiping.Effect of root restriction on the quality and endogenic hormone of grape berry(Vitis vinifera L.‘Muscat Hamburg’)[J]. Journal of Fruit Science,2022,39(3):376-387.
[15] 吕丹桂,谢岳,徐伟荣,王振平.水分胁迫对赤霞珠葡萄果实花色苷生物合成的影响[J]. 西北农业学报,2019,28(8):1274-1281.LÜ Dangui,XIE Yue,XU Weirong,WANG Zhenping. Effects of water stress on anthocyanin biosynthesis in Cabernet Sauvignon grapes[J]. Acta Agriculturae Boreali-Occidentalis Sinica,2019,28(8):1274-1281.
[16] 席奔,柳巧禛,吕丹桂,徐伟荣,王振平,代红军.水分胁迫对葡萄果实白藜芦醇合成相关基因表达的影响[J]. 核农学报,2019,33(8):1490-1500.XI Ben,LIU Qiaozhen,LÜ Dangui,XU Weirong,WANG Zhenping,DAI Hongjun. Effects of water stress on expression of genes related to resveratrol biosynthesis in grape berries[J].Journal of Nuclear Agricultural Sciences,2019,33(8):1490-1500.
[17] HUBBARD N L,PHARR D M,HUBER S C. Sucrose phosphate synthase and other sucrose metabolizing enzymes in fruits of various species[J]. Physiologia Plantarum,1991,82(2):191-196.
[18] 高彦婷,张芮,李红霞,魏鹏程.水分胁迫对葡萄糖分及其蔗糖代谢酶活性的影响[J].干旱区研究,2021,38(6):1713-1721.GAO Yanting,ZHANG Rui,LI Hongxia,WEI Pengcheng. Effect of water stress on sugar accumulation and sucrose metabolism enzyme activities of greenhouse grape fruit[J]. Arid Zone Research,2021,38(6):1713-1721.
[19] 杨昌钰,张芮,蔺宝军,王腾飞,王春宏.水分胁迫对鲜食葡萄果实品质影响的研究进展[J].农业工程,2020,10(1):86-91.YANG Changyu,ZHANG Rui,LIN Baojun,WANG Tengfei,WANG Chunhong. Research progress on the effect of water stress on fruit quality of table grapes[J]. Agricultural Engineering,2020,10(1):86-91.
[20] 李红燕.不同补光措施、调亏灌溉及有机肥对酿酒葡萄生长及品质的影响[D].杨凌:西北农林科技大学,2016.LI Hongyan.Effects of different light supplement measures,regulat irrigation and organic fertilizer on the growth and quality of wine grapes[D]. Yangling:Northwest Agriculture and Forestry University,2016.
[21] CHEN M,XIE X L,LIN Q,CHEN J Y,GRIERSON D,YIN X R,SUN C D,CHEN K S. Differential expression of organic acid degradation-related genes during fruit development of navel oranges (Citrus sinensis) in two habitats[J]. Plant Molecular Biology Reporter,2013,31:1131-1140.
[22] 张规富,卢晓鹏,谢深喜.不同时期水分胁迫对椪柑果实柠檬酸代谢相关基因表达的影响[J].果树学报,2015,32(4):525-535.ZHANG Guifu,LU Xiaopeng,XIE Shenxi. Influence of water stress in different development stage on the citric acid metabolism-related genes expression in the Ponkan fruits[J]. Journal of Fruit Science,2015,32(4):525-535.
[23] WANG Q J,QI W W,WANG Y,SUN F,QIAN X Y,LUO X J,YANG J S. Isolation and identification of an AP2/ERF factor that binds an allelic cis-element of rice gene LRK6[J]. Genetics Research,2011,93(5):319-332.
[24] 龚成宇,王毅,宋海岩,杨科,陶海青,刘俊宏,龚荣高.干旱胁迫对黄果柑果实品质及糖酸代谢酶活性的影响[J].西南农业学报,2021,34(2):272-278.GONG Chengyu,WANG Yi,SONG Haiyan,YANG Ke,TAO Haiqing,LIU Junhong,GONG Ronggao. Effects of drought stress on fruit quality and enzyme activity of glycolic acid metabolism in Huangguogan fruit[J]. Southwest China Journal of Agricultural Sciences,2021,34(2):272-278.
[25] 李航,陶海青,陈益香,龚成宇,杨科,龚荣高.2 种中国樱桃果实有机酸积累及代谢相关酶活性的研究[J].西北农业学报,2019,28(12):2019-2026.LI Hang,TAO Haiqing,CHEN Yixiang,GONG Chengyu,YANG Ke,GONG Ronggao. Evaluation of organic acid accumulation and metabolism related enzymes activities in two Chinese cherry fruits[J].Acta Agriculturae Boreali-Occidentalis Sinica,2019,28(12):2019-2026.
[26] 邓浩亮,孔维萍,张恒嘉,李福强.不同生育期调亏灌溉对酿酒葡萄耗水及果实品质的影响[J].中国生态农业学报,2016,24(9):1196-1205.DENG Haoliang,KONG Weiping,ZHANG Hengjia,LI Fuqiang.Effect of regulated deficit irrigation at different growth stages on water consumption and fruit quality of wine grape[J]. Chinese Journal of Eco-Agriculture,2016,24(9):1196-1205.
[27] 曹慧玲,舒河霖,邵建辉,张海明,马春花.葡萄果实酒石酸生物合成研究进展[J].中国果树,2021(4):8-13.CAO Huiling,SHU Helin,SHAO Jianhui,ZHANG Haiming,MA Chunhua.Research progress on biosynthesis of tartaric acid in grape berries[J].China Fruits,2021(4):8-13.
[28] 赵权,刘广娜,孔令瑶.干旱胁迫对山葡萄花色苷合成及相关品质的影响[J].北方园艺,2012(24):44-46.ZHAO Quan,LIU Guangna,KONG Lingyao. Effect of drought stress on the anthocyanins synthesis and related quality of Vitis amurensis[J].Northern Horticulture,2012(24):44-46.
[29] 王新.转色后持续干旱对赤霞珠葡萄植株生理及果实类黄酮化合物的影响[D].杨凌:西北农林科技大学,2021.WANG Xin. Effects of continuous drought stress on plant physiology characteristics and flavonoids of Cabernet Sauvignon grape[D].Yangling:Northwest Agriculture and Forestry University,2021.
[30] POULSEN M,JORGENSEN J O L,JESSEN N,RICHELSEN B,PEDERSEN S B. Resveratrol in metabolic health:An overview of the current evidence and perspectives[J]. Annals of the New York Academy of Sciences,2013,1290(1):74-82.
[31] CASTELLARIN S D,MATTHEWS M A,DI GASPERO G,GAMBETTA G A.Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries[J].Planta,2007,227(1):101-112.
[32] 赵裴,成甜甜,王开贤,韩富亮.干化处理对‘马瑟兰’葡萄有机酸、花色苷和单宁组分的影响[J]. 食品与发酵工业,2021,47(18):194-200.ZHAO Pei,CHENG Tiantian,WANG Kaixian,HAN Fuliang.Effects of postharvest dehydration on the organic acids,anthocyanins and tannin fractions of‘Marselan’grapes[J]. Food and Fermentation Industries,2021,47(18):194-200.
[33] 赖呈纯,潘红,黄贤贵,范丽华,赖钟雄,段长青,刘文慧.刺葡萄愈伤组织UFGT 基因克隆及表达分析[J].核农学报,2019,33(9):1677-1685.LAI Chengchun,PAN Hong,HUANG Xiangui,FAN Lihua,LAI Zhongxiong,DUAN Changqing,LIU Wenhui. Cloning and expression analysis of UFGT gene in callus of grapevine SPP[J].Journal of Nuclear Agricultural Sciences,2019,33(9):1677-1685.
[34] ROBINSON S P,PEZHMANMEHR M,SPERIS J,MCDAVID D A J,HOOPER L C,RINALDO A R,BOGE J,EBADI A,WALKER A R. Grape and wine flavonoid composition in transgenic grapevines with altered expression of flavonoid hydroxylase genes[J]. Australian Journal of Grape and Wine Research,2019,25(3):293-306.
[35] MARTÍNEZ-LÜSCHER J,SÁNCHEZ-DÍAZ M,DELROT S,AGUIRREOLEA J,PASCUAL I,GOMḖS E. Ultraviolet-B radiation and water deficit interact to alter flavonol and anthocyanin profiles in grapevine berries through transcriptomic regulation[J].Plant and Cell Physiology,2014,55(11):1925-1936.
[36] WANG J Z,WANG F B,JIN C,TONG Y,WANG T.A R2R3-MYB transcription factor VvMYBF1 from grapevine (Vitis vinifera L.) regulates flavonoids accumulation and abiotic stress tolerance in transgenic Arabidopsis[J].The Journal of Horticultural Science and Biotechnology,2020,95(2):147-161.