分枝(蘖)角度是形成“理想树(株)型”的重要组成部分,其与产量形成、环境适应和竞争能力密切相关。遗传因素、植物激素、重力向地性和环境因素等在植物分枝(蘖)角度形成中发挥重要作用[1]。
热激转录因子(heat shock transcription factor,HSFs)是植物中研究最广泛的转录因子家族之一,根据蛋白质结构的差异,植物HSFs 可分为A、B、C三类[2],其中只有A 类HSFs 成员C 末端含有具有转录激活功能的AHA基序,因此现有的对植物热激转录因子的研究主要集中在A 类,B 类和C 类研究相对较少[3]。A 类热激转录因子作为热胁迫的重要调控因子,通过调控热激蛋白(heat shock protein,HSPs)等多种类型基因的表达来参与植物热胁迫响应[4-5];此外,A 类热激转录因子还参与了干旱、盐等非生物胁迫[6-7]。近年来对A类热激转录因子的研究发现其在植物树(株)型相关性状的形成中也发挥了重要的作用。Zhang 等[8]研究发现水稻hsfa2d 突变体植株表现分蘖角度增大,hsfa2d/lazy1双突变体表现出比二者单突变体更大的分蘖角度,且OsLAZY1基因过表达能够挽救hsfa2d 突变体表型,而LAZY1基因属于IGT 基因家族,在分蘖(枝)角度形成中具有非常重要的作用。过表达AtHsfA3、AtHsfA2 和OsHsfA2e 的转基因拟南芥植株均出现(极)矮化表型[9-10];过表达苣苔(Boea hygrometrica)A 类BhHsf1的转基因拟南芥和烟草植株生长受阻,均表现矮化表型,同时转基因拟南芥植株的叶片、花序、果荚均变小[11]。
桃(Prunus persica L.)是世界最重要的果树之一,原产中国,种质资源丰富。依据树高和分枝角度的不同,桃树型可分为普通型、矮化型、半矮化型、柱型、直立型、垂枝型和曲枝型[12-13]。目前桃生产上主栽品种主要为普通型,其树型开张,分枝角度过大,随着结果年限延长,树冠内枝叶密集,中下部透光差,是限制桃园产量的主要因素,也在一定程度上影响了果实品质提升。柱型桃是桃资源中的一类特异种质,与普通型桃相比,柱型桃树冠较小,具有分枝角度小、侧枝数量少等特点[14-15]。发掘控制桃分枝角度相关基因,通过分子育种手段对现有主栽桃品种树型进行改良具有重要意义。笔者在本研究中以普通型桃大久保和柱型桃洒红龙柱为试验材料,分析2个品种一年生植株分枝角度差异,通过2个品种茎尖转录组数据,筛选出与水稻中调控分蘖角度的关键转录因子OsHSFA2D 基因[8]的同源基因桃A 类HSF 转录因子PpHSF18,克隆PpHSF18 基因并对其功能进行研究,为后续桃分枝角度分子机制研究提供理论支撑。
1 材料和方法
1.1 试验材料
一年生普通型桃大久保(Okubo,O)和柱型桃洒红龙柱(Sahonglongzhu,S)于2019年12月栽植于河南农业大学三区果树试验站,采取茎尖进行转录组测序分析,采取嫩叶用于RNA的提取和后续基因克隆等试验,所有样品采集后迅速置于液氮中速冻,带回实验室后放于-80 ℃超低温冰箱保存;并以大久保和洒红龙柱一年生植株为试材进行分枝角度测定。采用一年生大久保和洒红龙柱分枝连接处进行PpHSFA18 基因相对表达量测定。采用野生型拟南芥(Arabidopsis thaliana ecotype Columbia)进行PpHSFA18基因功能验证。
1.2 一年生桃植株分枝角度测量
从5 月5 日开始到5 月30 日,每隔5 d 用SC-K1原位活体植物分枝角自动测量仪系统(杭州万深)分别测量大久保和洒红龙柱一年生枝条的基角(一年生枝与主干的夹角),每处理选取3棵长势一致的植株,从每棵植株随机选取3 个枝条测定其分枝角度并记录,3次重复。
1.3 转录组测序和表达分析
大久保(O)和洒红龙柱(S)茎尖转录组数据参考谭彬等[16]的研究,基于转录组测序数据分析桃中11 个HSF 家族A 类基因[17](包含PpHSF18 基因)、PpLAZY1 基因和PpTAC1 基因在大久保和洒红龙柱茎尖中的表达量,并利用TBtools v0.6733 软件[18]绘制表达量热图。利用实时荧光定量PCR 分析候选基因PpHSF18 在大久保和洒红龙柱一年生枝条分枝连接处的表达量。
1.4 PpHSF18基因克隆及过表达载体构建
利用Primer Premier 5.0设计PpHSF18基因CDS(Coding sequence,编码区)全长引物PpHSF18-F1和PpHSF18-R1(引物序列见表1),以普通型桃大久保叶片cDNA为模板进行PCR扩增,PCR反应体系和反应程序、PCR产物回收、连接及转化大肠杆菌步骤参照谭彬等[19]的方法进行。将PCR检测呈阳性的单菌落活化后送至武汉擎科生物科技有限公司进行测序,使用MEGA7.0软件将测序结果与参考序列进行比对。
表1 PpHSF18 基因克隆、表达及功能分析所用引物
Table 1 Primers used for cloning,expression and functional analysis of PpHSF18

引物名称Primer name PpHSF18-F1 PpHSF18-R1 PpHSF18-F2 PpHSF18-R2 PpHSF18-F3 PpHSF18-R3 AtUBC-F AtUBC-R引物序列(5’-3’)Primer sequence(5’-3’)ATGAACTATCTGTACCCAGTGAAGG TCACTACTTTGGGCATGAACCTAAGTAA AGAATTCAAAAAGCTTATGAACTATCTGTACCCAGTGAAGG AAGCAGGACTCTAGATCACTACTTTGGGCATGAACCTAAGTAA GGCAAGAAAGGAACAGGATGAA CCAAAATCCCTCATCGAACTCT CTGCGACTCAGGGAATCTTCTAA TTGTGCCATTGAATTGAACCC用途Application基因克隆Gene cloning表达载体Expression vector表达分析Expression analysis拟南芥内参基因Constitutive control in Arabidopsis
利用Primer premier 5.0设计含Hind Ⅲ和Xba Ⅰ酶切位点的引物PpHSF18-F2 和PpHSF18-R2(引物序列见表1),PCR 扩增和载体构建参照谭彬等[19]的方法进行。
1.5 PpHSF18遗传转化拟南芥及其鉴定分析
将构建好的pSAK277-35S::PpHSF18 过表达载体转化农杆菌GV3101 菌株,使用蘸花法侵染拟南芥[20],待果荚成熟时收取T0代种子。T0代拟南芥植株培养、叶片DNA和RNA提取、PCR扩增和实时荧光定量PCR 方法参照谭彬等[19]的方法进行,以拟南芥AtUBC 基因为内参基因,引物序列见表1。对T2代阳性拟南芥植株分枝角度进行测定,每株系选取3株,每株选取3个枝条,3次重复。
1.6 数据统计与分析
利用SPSS 17.0软件对数据进行显著性分析,采用Excel 2010进行数据统计和作图。
2 结果与分析
2.1 两种树型桃枝条不同发育期分枝角度变化
对一年生普通型桃大久保和柱型桃洒红龙柱植株枝条不同发育阶段分枝角度进行测量(图1-A)。普通型桃大久保分枝角度显著大于柱型桃品种洒红龙柱分枝角度(图1-B)。随着枝条发育,大久保桃分枝角度逐渐增加,从62°显著增加到76°,而洒红龙柱桃枝条分枝角度在整个发育时期没有显著变化(31°~34°),但是均显著低于大久保各个时期分枝角度。综上分析,2 个品种间分枝角度及其分枝角度变化规律均存在差异。
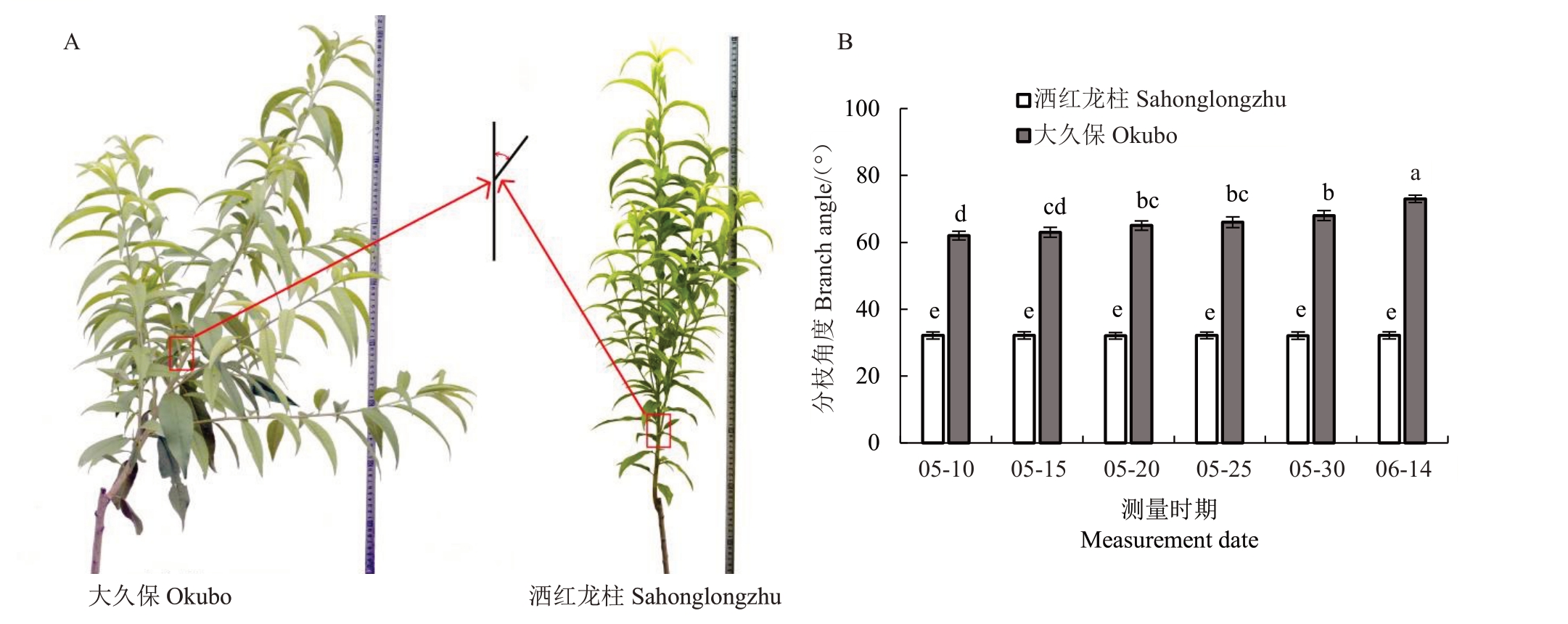
图1 2 种树型桃品种表型与不同发育时期分枝角度差异分析
Fig.1 The phenotype and analysis of branch angle in different stages of two tree architectures in Okubo and Sahonglongzhu
A.两种树型表型;B.两种树型不同发育时期分枝角度测量。不同小写字母表示差异显著(p<0.05)。下同。
A.Phenotype of two tree architectures;B.Branch angle in different stages of two tree architectures.Different small letters mean significant differences(p<0.05).The same below.
2.2 PpHSFAs在2种不同树型桃中的表达分析
PpTAC1 和PpLAZY1 是同属于IGT 家族的一对作用相反的控制植物分枝角度的关键基因[21]。对普通型桃大久保和洒红龙柱茎尖转录组数据中HSF家族中11 个A 类基因、PpTAC1 和PpLAZY1 表达趋势进行分析(图2-A)。结果发现,PpTAC1 和PpLAZY1表达趋势相反,PpTAC1在大久保中表达量高于洒红龙柱中表达量,而PpLAZY1 则相反。PpHSF16在2种树型中均不表达,其余PpHSFs基因呈现2种表达趋势,PpHSF2、PpHSF3、PpHSF10、PpHSF11 和PpHSF14 与PpTAC1 表达趋势一致,表现为在大久保中表达量高于洒红龙柱(图2-A);而PpHSF1、PpHSF4、PpHSF7、PpHSF9、PpHSF18与PpLAZY1表达趋势一致,表现为在洒红龙柱中表达量高于大久保(图2-A),其中,PpHSF18与水稻OsHSFA2D高度同源(E-value=1e-120)(图2-B),OsHSFA2D 是Os-LAZY1 上游正调节因子[8],故推测PpHSF18 可能参与调控桃分枝角度。
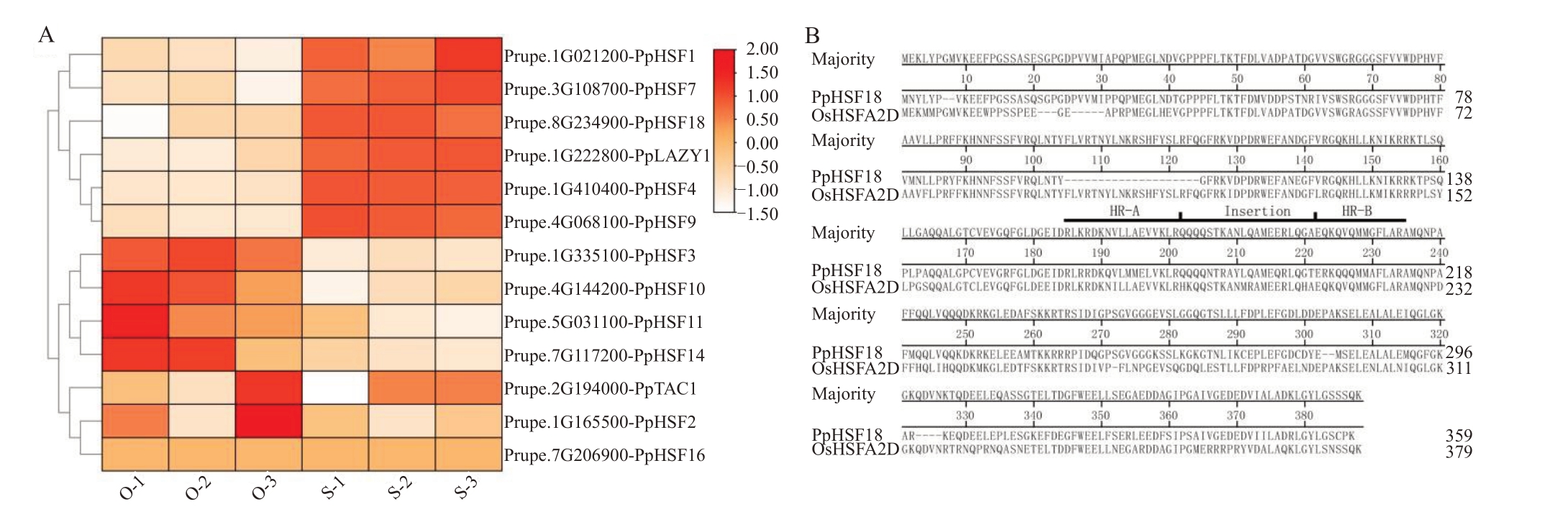
图2 PpHSFAs 在两种树型中表达量分析与PpHSF18 基因结构域比较
Fig.2 Expression pattern analysis of PpHSFAs in two tree architectures of peach and conserved domains of PpHSF18
A.PpHSFAs 表达量分析;B.PpHSF18 中HSFA 保守结构域分析。O.大久保;S.洒红龙柱。
A.Expression analysis of PpHSFAs;B.Conserved domains of HSFA in PpHSF18.O.Okubo;S.Sahonglongzhu.
为进一步明确PpHSF18基因的表达模式,以一年生大久保和洒红龙柱的分枝连接处(O 和S),分枝连接处上(O-Upper 和S-Upper)和分枝连接处下(O-Lower 和S-Lower)为材料检测PpHSF18的相对表达量,分析发现PpHSF18 基因在洒红龙柱分枝连接处及分枝连接处上下部相对表达量均显著高于大久保。PpHSF18 基因相对表达量与2 种树型桃枝条分枝角度大小呈负相关关系(图3)。
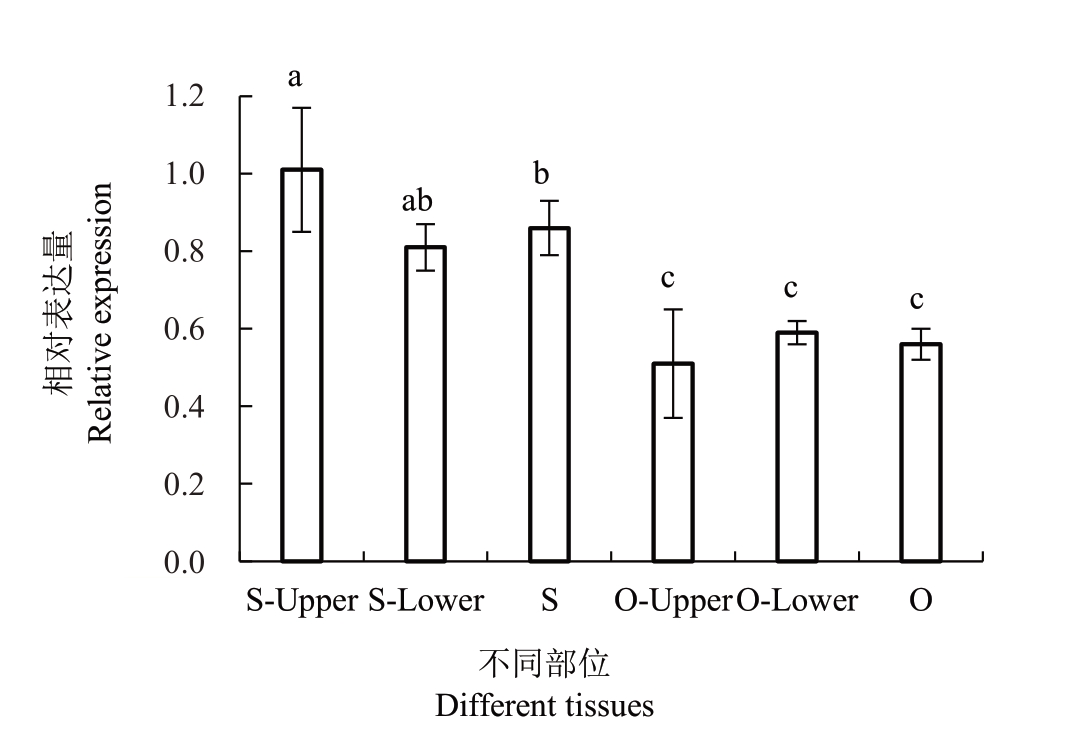
图3 PpHSF18 在一年生洒红龙柱和大久保分枝连接处表达分析
Fig.3 Expression analysis of PpHSF18 in Sahonglongzhu and Okubo peach
O.大久保;S.洒红龙柱;O/S-Upper.大久保或洒红龙柱分枝连接处上;O/S-Lower.大久保或洒红龙柱分枝连接处下。
O.Okubo;S.Sahonglongzhu;O/S-Upper.Upper sides of the shoot in O or S;O/S-Lower.Lower sides of the shoot in O or S.
2.3 PpHSF18基因克隆与过表达载体构建
以大久保嫩叶cDNA为模板进行PpHSF18基因CDS 区全长(1080 bp)扩增,结果显示,PpHSF18 基因CDS 区大小为1000 bp 左右(图4-A);测序结果显示,PpHSF18 基因CDS 区序列与参考基因组无差异,编码359 个氨基酸,与OsHSFA2D蛋白序列比对显示,均包含HSF家族基因保守结构域(图2-B)。将PpHSF18基因CDS区序列与酶切后的pSAK277过表达载体进行连接和转化(图4-B),挑取单菌落进行PCR 验证,结果显示,PCR 产物条带位于1000 bp 左右的位置,说明目的基因成功连接至pSAK277载体,PpHSF18基因过表达载体构建成功。
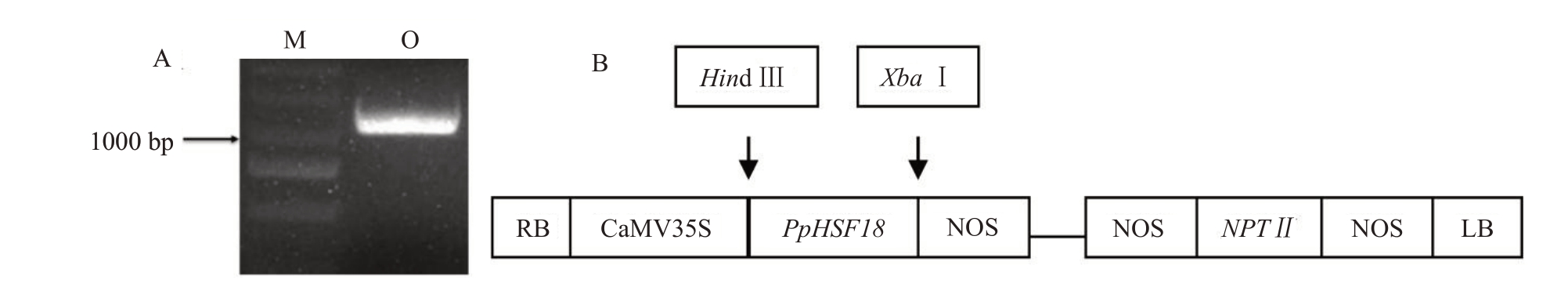
图4 PpHSF18 基因CDS 全长扩增与过表达载体构建
Fig.4 The amplification of full length with PpHSF18 and detection of overexpression vector colony
A.PpHSF18 CDS 区PCR 扩增;B.pSAK277-35S::PpHSF18 载体结构图。O.大久保;M.DNA marker DL2000。
A.PCR amplification of PpHSF18;B.Vector map of pSAK277-35S::PpHSF18.O.Okubo;M.DNA marker DL2000.
2.4 转基因拟南芥筛选与鉴定
用包含pSAK277-35S::PpHSF18 过表达载体的农杆菌进行拟南芥侵染,在含有卡那霉素的抗性培养基上筛选拟南芥种子,获得4 株T0代抗性苗。进一步通过PCR 验证,4 株T0代抗性苗均能扩出目的基因条带(图5-A),说明4 株抗性苗均为PpHSF18转基因阳性苗。
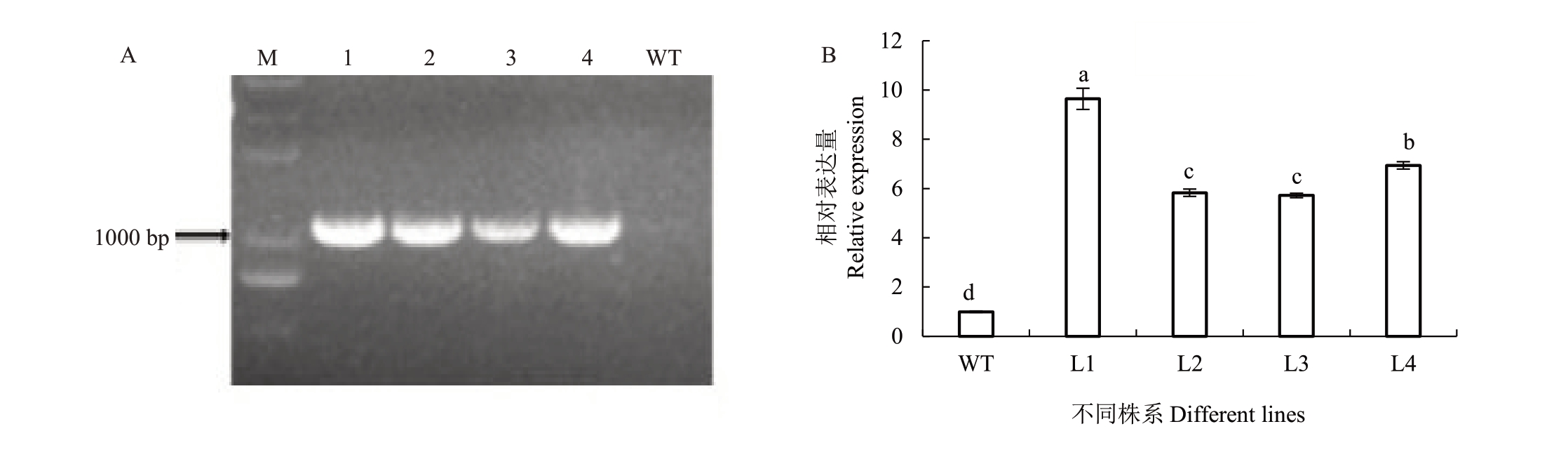
图5 PpHSF18 T0代转基因拟南芥PCR 鉴定与相对表达量分析
Fig.5 PCR identification of Arabidopsis thaliana and relative expression of PpHSF18 in transgenic plants
A.转基因拟南芥PCR 鉴定;B.阳性苗PpHSF18 表达量分析。M.DNA marker DL2000;1~4.4 个转基因植株;WT.阴性对照。
A. PCR identification of transgenic Arabidopsis thaliana; B. Relative expression of PpHSF18. M. DNA marker DL2000; 1-4. Four transgenic plants;WT as negative control.
利用qRT-PCR 检测PpHSF18 基因在4 个T0代转基因植株中的相对表达量,结果(图5-B)显示,相较于WT 植株,L1~L4 四个转基因植株中PpHSF18表达量均显著高于WT 植株,其中L1 表达量最高,L4次之,L2和L3之间没有明显差异,表明PpHSF18基因已成功整合到4个转基因植株的基因组中并发挥作用。
2.5 过表达PpHSF18转基因拟南芥表型观察与分析
对3个T2代阳性转基因拟南芥株系进行分枝角度观测和统计,结果显示,与WT 植株相比,PpHSF18 过表达株系的分枝角度显著减小(图6-A),且WT 拟南芥的“冠层”显著大于3 个PpHSF18转基因株系(图6-B)。对WT 和过表达株系分枝角度进行测量,WT 植株分枝角度在60°左右;而过表达株系的分枝角度为40°左右。进一步的方差分析发现,过表达株系植株分枝角度极显著低于WT(p<0.01)(图6-C),表明过表达PpHSF18 基因导致拟南芥分枝角度变小。

图6 PpHSF18 T2代转基因拟南芥植株表型与分枝角度统计
Fig.6 Phenotype and branch angle of T2 generation of transgenic Arabidopsis thaliana for PpHSF18
A 和B.转基因拟南芥表型;C.不同株系分枝角度统计。L1,L2,L4 分别为3 个转基因株系;WT 为野生型对照。**表示差异极显著(p<0.01),ns 表示无差异。
A and B.Phenotype of transgenic Arabidopsis thaliana;C.Branch angle of different lines in transgenic Arabidopsis thaliana.L1,L2,L4 represent the transgenic lines of PpHSF18;WT represents the wild type control.**indicates extremely significant difference at p<0.01,ns indicates on difference.
3 讨 论
桃树型是影响果实产量和品质形成、栽培管理措施确定的基础[13]。高而窄的树型是适于目前果树生产提出的“宽行密植”栽培理念的理想树型,不仅有利于行间作业,通过逐步实现机械化,可以大大降低劳动强度和劳动力支出,而且改善了果园群体的通风透光条件,对减少病虫发生、提升果实品质均具有重要意义[22]。前人研究发现柱型桃枝条接近直立,而普通型桃分枝角度大、鲜有近直立枝,且柱型桃的花梗与枝条夹角也显著小于普通型桃[21,23]。笔者在本试验中分析了一年生普通型桃大久保和柱型桃洒红龙柱生长季枝条分枝角度的变化,发现柱型桃枝条在整个5 月份变化不大,且显著低于普通型桃,研究结果与前人一致。
热激转录因子HSFs 在植物的生长发育中发挥重要作用[2]。Wang等[24]利用水稻幼苗响应重力诱导试验的转录组数据筛选出一个具有时空特异性表达模式的差异表达基因OsHSFA2D,OsHSFA2D 基因响应重力信号后表达量升高并激活OsLAZY1 的表达,进而引发了生长素的不对称分布导致水稻分蘖角度变小。LAZY1是目前研究较多的一个控制植物分枝(蘖)角度的重要基因[24-27],笔者团队前期研究发现PpLAZY1 基因的异位表达导致转基因拟南芥和烟草分枝角度(叶角)变小;Dardick等[21]研究发现与PpLAZY1 同属于IGT 家族的作用相反的PpTAC1 基因的突变是柱型桃Crimson Rocket分枝角度小的主要原因。基于PpLAZY1基因和PpTAC1基因的表达模式,笔者在本研究中发现11个PpHSFAs基因在普通型桃大久保和柱型桃洒红龙柱茎尖中呈现3种表达模式,即普通型桃中表达量高于柱型桃、低于柱型桃和在两种树型中无差异。其中的PpHSF18 基因在2 种树型中的表达模式与PpLAZY1 相同,这与水稻中OsHSFA2D 和OsLAZY1 的表达模式一致[8],且PpHSF18 与水稻中的OsHSFA2D 高度同源(50.13%),推测PpHSF18可能参与调控桃分枝角度形成。
过表达PpHSF18基因的3个转基因拟南芥株系的分枝角度显著小于野生型拟南芥。前人研究发现柱型桃侧枝中和腋芽中生长素的含量明显高于普通型桃[28]。水稻hsfs2d突变体植株分蘖角度显著大于野生型植株,OsHSFA2D 和OsLAZY1 过表达均可恢复hsfa2d 突变体表型,推测OsHSFA2D 是OsLAZY1上游正调节因子,通过激活OsLAZY1的表达调节生长素的不对称分布进而影响水稻分蘖角度[8]。本试验研究结果与前人研究结果一致。探究桃中PpHSF18转录因子在2种树型桃中的表达特性及功能,可为HSF家族在桃树型相关性状形成中的作用研究奠定基础。笔者在本研究中也发现PpLAZY1基因与PpHSF18具有相同的表达趋势,但二者之间的关系还需要进一步明确,研究与分枝角度形成的关键基因并开发标记基因,为利用分子育种手段进行桃树型遗传改良提供参考。
4 结 论
笔者在本研究中成功克隆了桃热激转录因子PpHSF18基因,该基因在普通型桃一年生植株茎尖中表达量明显低于柱型桃;异源转化拟南芥后导致拟南芥分枝角度明显变小,其调控的分子机制有待进一步研究。
[1] HOLLENDER C A,HILL J L,WAITE J,DARDICK C.Opposing influences of TAC1 and LAZY1 on lateral shoot orientation in Arabidopsis[J].Scientific Reports,2020,10(1):6051.
[2] NOVER L,SCHARF K D,GAGLIARDI D,VERGNE P,CZARNECKA-VERNER E,GURLEY W B. The Hsf world:Classification and properties of plant heat stress transcription factors[J].Cell Stress&Chaperones,1996,1(4):215-223.
[3] 焦淑珍,姚文孔,张宁波,徐伟荣.园艺植物热激转录因子研究进展[J].果树学报,2020,37(3):419-430.JIAO Shuzhen,YAO Wenkong,ZHANG Ningbo,XU Weirong.Research progress of heat stress transcription factors (Hsfs) in horticultural plants[J]. Journal of Fruit Science,2020,37(3):419-430.
[4] NISHIZAWA A,YABUTA Y,YOSHIDA E,MARUTA T,YOSHIMURA K,SHIGEOKA S.Arabidopsis heat shock transcrip tion factor A2 as a key regulator in response to several types of environmental stress[J].The Plant Journal,2006,48(4):535-547.
[5] CHAN- SCHAMINET K Y,BANIWAL S K,BUBLAK D,NOVER L,SCHARF K D. Specific interaction between tomato HsfA1 and HsfA2 creates hetero-oligomeric superactivator complexes for synergistic activation of heat stress gene expression[J].The Journal of Biological Chemistry,2009,284(31):20848-20857.
[6] YU X Y,YAO Y,HONG Y H,HOU P Y,LI C X,XIA Z Q,GENG M T,CHEN Y H.Differential expression of the Hsf family in cassava under biotic and abiotic stresses[J]. Genome,2019,62(8):563-569.
[7] CAI S Y,ZHANG Y,XU Y P,QI Z Y,LI M Q,AHAMMED G J,XIA X J,SHI K,ZHOU Y H,REITER R J,YU J Q,ZHOU J.HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants[J]. Journal of Pineal Research,2017,62(2):e12387.
[8] ZHANG N,YU H,YU H,CAI Y Y,HUANG L Z,XU C,XIONG G S,MENG X B,WANG J Y,CHEN H F,LIU G F,JING Y H,YUAN Y D,LIANG Y,LI S J,SMITH S M,LI J Y,WANG Y H.A core regulatory pathway controlling rice tiller angle mediated by the LAZY1-Dependent asymmetric distribution of auxin[J].The Plant Cell,2018,30(7):1461-1475.
[9] OGAWA D,YAMAGUCHI K,NISHIUCHI T. High-level overexpression of the Arabidopsis HsfA2 gene confers not only increased themotolerance but also salt/osmotic stress tolerance and enhanced callus growth[J].Journal of Experimental Botany,2007,58(12):3373-3383.
[10] YOKOTANI N,ICHIKAWA T,KONDOU Y,MATSUI M,HIROCHIKA H,IWABUCHI M,ODA K. Expression of rice heat stress transcription factor OsHsfA2e enhances tolerance to environmental stresses in transgenic Arabidopsis[J]. Planta,2008,227(5):957-967.
[11] ZHU Y,WANG Z,JING Y J,WANG L L,LIU X,LIU Y X,DENG X.Ectopic over-expression of BhHsf1,a heat shock factor from the resurrection plant Boea hygrometrica,leads to increased thermotolerance and retarded growth in transgenic Arabidopsis and tobacco[J].Plant Molecular Biology,2009,71(4):451.
[12] 王力荣,朱更瑞,方伟超.中国桃遗传资源[M].北京:中国农业出版社,2012.WANG Lirong,ZHU Gengrui,FANG Weichao. Peach genetic resource in China[M].Beijing:China Agriculture Press,2012.
[13] 谭彬,程钧,郑先波,王志强,冯建灿.桃树型及其调控关键基因研究进展[J].果树学报,2020,37(4):599-605.TAN Bin,CHENG Jun,ZHENG Xianbo,WANG Zhiqiang,FENG Jiancan. Progress on tree architecture and key genes of its regulation in peach[J]. Journal of Fruit Science,2020,37(4):599-605.
[14] SCORZA R,BASSI D,DIMA A,RIZZO M. Developing new peach tree growth habits for higher density plantings[J]. Compact Fruit Tree,2000,33(1):19-21
[15] WANG X B,WANG Q P,YAN L X,HAO Y H,LIAN X D,ZHANG H P,ZHENG X B,CHENG J,WANG W,ZHANG L L,YE X,LI J D,TAN B,FENG J C. PpTCP18 is upregulated by lncRNA5 and controls branch number in peach(Prunus persica)through positive feedback regulation of strigolactone biosynthesis[J].Horticulture Research,2023,10(1):224.
[16] 谭彬,杨丽萍,陈立川,冯玫侨,连晓东,魏鹏程,程钧,王小贝,冯建灿.桃PIN 蛋白基因家族鉴定及其在不同树型桃中的表达分析[J].农业生物技术学报,2020,28(10):1747-1760.TAN Bin,YANG Liping,CHEN Lichuan,FENG Meiqiao,LIAN Xiaodong,WEI Pengcheng,CHENG Jun,WANG Xiaobei,FENG Jiancan. Identification and expression analysis of PIN gene family in different tree architectures of peach (Prunus persica)[J]. Journal of Agricultural Biotechnology,2020,28(10):1747-1760.
[17] TAN B,YAN L,LI H N,LIAN X D,CHENG J,WANG W,ZHENG X B,WANG X B,LI J D,YE X,ZHANG L L,LI Z Q,FENG J C. Genome-wide identification of HSF family in peach and functional analysis of PpHSF5 involvement in root and aerial organ development[J].PeerJ,2021,9:e10961.
[18] CHEN C J,CHEN H,ZHANG Y,THOMAS H R,FRANK M H,HE Y H,XIA R. TBtools:An integrative toolkit developed for interactive analyses of big biological data[J]. Molecular Plant,2020,13(8):1194-1202.
[19] 谭彬,魏鹏程,栗焕楠,连晓东,陈谭星,郑先波,程钧,王伟,王小贝,冯建灿.桃PEBP 基因家族全基因组鉴定及桃TFL1 基因功能分析[J].果树学报,2020,37(10):1443-1454.TAN Bin,WEI Pengcheng,LI Huannan,LIAN Xiaodong,CHEN Tanxing,ZHENG Xianbo,CHENG Jun,WANG Wei,WANG Xiaobei,FENG Jiancan. Genome-wide identification of PEBP gene family and functional analysis of TFL1 gene in peach (Prunus persica)[J]. Journal of Fruit Science,2020,37(10):1443-1454.
[20] CLOUGH S J,BENT A F. Floral dip:A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana[J]. The Plant Journal,1998,16(6):735-743.
[21] DARDICK C,CALLAHAN A,HORN R,RUIZ K B,ZHEBENTYAYEVA T,HOLLENDER C,WHITAKER M,ABBOTT A,SCORZA R. PpeTAC1 promotes the horizontal growth of branches in peach trees and is a member of a functionally conserved gene family found in diverse plants species[J].The Plant Journal,2013,75(4):618-630.
[22] 王志强,牛良,崔国朝,鲁振华,曾文芳.我国桃栽培模式现状与发展建议[J].果农之友,2015(9):3-4.WANG Zhiqiang,NIU Liang,CUI Guochao,LU Zhenhua,ZENG Wenfang. Present situation and development suggestions of peach cultivation mode in China[J]. Fruit Growers’Friend,2015(9):3-4.
[23] TWORKOSKI T,MILLER S,SCORZA R.Relationship of pruning and growth morphology with hormone ratios in shoots of pillar and standard peach trees[J]. Journal of Plant Growth Regulation,2006,25(2):145-155.
[24] WANG H,TU R R,SUN L P,WANG D F,RUAN Z Y,ZHANG Y E,PENG Z Q,ZHOU X P,FU J L,LIU Q N,WU W X,ZHAN X D,SHEN X H,ZHANG Y X,CAO L Y,CHENG S H. Tiller angle control 1 is essential for the dynamic changes in plant architecture in rice[J]. International Journal of Molecular Sciences,2022,23(9):4997.
[25] DONG Z B,JIANG C,CHEN X Y,ZHANG T,DING L,SONG W B,LUO H B,LAI J S,CHEN H B,LIU R Y,ZHANG X L,JIN W W.Maize LAZY1 mediates shoot gravitropism and inflorescence development through regulating auxin transport,auxin signaling,and light response[J].Plant Physiology,2013,163(3):1306-1322.
[26] FURUTANI M,MORITA M T. LAZY1-LIKE-mediated gravity signaling pathway in root gravitropic set-point angle control[J].Plant Physiology,2021,187(3):1087-1095.
[27] CHE X M,SPLITT B L,ECKHOLM M T,MILLER N D,SPALDING E P.BRXL4-LAZY1 interaction at the plasma membrane controls Arabidopsis branch angle and gravitropism[J].The Plant Journal,2023,113(2):211-224.
[28] TWORKOSKI T,WEBB K,CALLAHAN A. Auxin levels and MAX1-4 and TAC1 gene expression in different growth habits of peach[J].Plant Growth Regulation,2015,77(3):279-288.