中国是世界上最大的苹果生产和消费国,苹果种植面积和产量均超过世界总量的50%[1]。中国的自然地理、生态环境和气候条件复杂多样,且苹果种植产区分布广泛,每年都可能在不同地域发生霜冻、寒潮、低温冻害、冰雹、雨涝、大风、干旱等自然灾害,因此中国苹果生产经常面临各种自然灾害的威胁[2]。新疆野苹果(Malus sieversii)又称塞威士苹果,是现代栽培苹果的祖先,种群遗传多样性丰富,具有抗寒、抗旱、抗盐碱等优良特性[3]。因此,研究新疆野苹果抗寒性以及挖掘抗寒性相关的基因,对苹果的抗寒育种和产业的健康发展具有重要的现实意义。
谷胱甘肽(glutathione,GSH)在1929 年由Hopkins 最早发现并予以命名[4-5],为谷氨酸、半胱氨酸和甘氨酸组成的三肽[6]。植物细胞内GSH 的合成通常发生于细胞质、叶绿体和线粒体中[7-8]。谷胱甘肽是一种重要的抗氧化剂,参与AsA-GSH 循环[9]。在AsA-GSH 循环中,抗坏血酸过氧化物酶(APX)以抗坏血酸(AsA)为电子供体催化过氧化氢(H2O2)还原为水,脱氢抗坏血酸还原酶(DHAR)利用谷胱甘肽提供的电子将脱氢抗坏血酸(DHA)还原为AsA[10-11],其次是GSH 调控酶促抗氧化剂,间接参与ROS 清除[12]。当植物受到胁迫时,还原型谷胱甘肽与蛋白质的半胱氨酸巯基形成二硫化物使得蛋白质自身谷胱甘肽化[13]。蛋白质谷胱甘肽化可保护蛋白免受其活性氧诱导的不可逆的氧化,从而调节蛋白的活性[14],植物电子信号传输过程中GSH/GSSG 可以作为氧化形式和还原形式的电子载体以达到氧化还原平衡状态,对水杨酸[15](SA)、茉莉酸[16](JA)、脱落酸[17](ABA)和乙烯[18](ET)信号分子参与的植物防御反应进行调控。谷胱甘肽代谢中的3 个重要的酶类,即谷胱甘肽还原酶(glutathione reductase,GR)、谷胱甘肽过氧化物酶(glutathione peroxidase,GPX)和谷胱甘肽转硫酶(glutathione S-transferase,GST)活性的变化与植物对环境胁迫的抗性密切相关[19],植物中的谷胱甘肽还原酶(GR)催化氧化型谷胱甘肽(oxidized glutathione disulfide,GSSG)生成还原型谷胱甘肽(reduced glutathione,GSH);对维持细胞内高GSH/GSSG 比率有重要作用[20]。在低温、高温、干旱、高盐胁迫过程中,油菜谷胱甘肽还原酶发挥重要作用。其中GR1和GR2 基因的转录以及GR 活性水平明显上升[21]。谷胱甘肽过氧化物酶(GPX)是生物机体内重要的抗氧化酶之一,它可以消除机体内的过氧化氢及脂质过氧化物,阻断活性氧自由基对机体的进一步损伤,是生物体内重要的活性氧自由基清除剂[22],在毛竹抗低温和强光中,毛竹叶片中PeGPXs 表达量发生明显变化[23]。植物谷胱甘肽转硫酶(GST)的主要功能是解除外界以及内源代谢有毒产物的伤害[24],GST 过表达能提高烟草对ROS 以及重金属Cd2+的清除能力,从而增强烟草对干旱、镉、NaCl 的耐受能力[25],在植物耐受不同环境胁迫中发挥重要保护作用,这表明它们在植物应对胁迫过程中具有重要的作用。关于冻害条件下新疆野苹果谷胱甘肽代谢机制的研究较少。笔者在本研究中以单株系的新疆野苹果组培苗为材料,在笔者课题组前期研究的基础上,筛选冻害与新疆野苹果组培苗谷胱甘肽代谢相关的基因,以期为解析新疆野苹果组培苗响应冻害的分子机制提供参考。
1 材料和方法
试验于2021年1月至2022年3月在石河子大学农学院园艺系特色果蔬栽培生理与种质资源利用兵团重点实验室进行。
1.1 试验材料及处理
新疆野苹果种子来自新疆伊犁哈萨克自治州霍城县。种子经过层积处理,种植在5 cm×10 cm的穴盘中,当实生苗长至6~8 片叶时,以顶端2~3 cm 茎段为外植体进行组织培养。参考何晨晨等[26]的方法进行培养基的配制,每隔1个月继代1次,选取增殖较好的单株系丛生芽进行生根培养,培养60 d 后选择生长一致的组培苗进行处理,组培苗均在人工气候培养箱(RXZ 智能型,宁波江南仪器厂)中培养,培养条件均为:光照度5 000 lx、昼25 ℃/14 h、夜23 ℃/10 h,相对湿度75%。
-3 ℃模拟冻害试验处理参考范宗民等[27]的方法,在人工改造的冰箱(容声BD/BC-310MS)中进行处理,冰箱内的条件为:光照度5 000 lx、昼25 ℃/14 h、夜23 ℃/10 h,相对湿度75%。以25 ℃下培养的材料为对照(CK),温度从25 ℃开始以4 ℃·h-1降温至-3 ℃,在-3 ℃模拟冻害条件下处理6 h、12 h、36 h(分别记为T6h、T12h、T36h),-3 ℃处理36 h 后立即放到25 ℃培养箱恢复24 h(HF24h),共5 个处理,每个处理6株苗,3次重复,观察不同处理的形态变化及测定生理指标,利用R语言与Excel处理数据以及绘图。
1.2 生理指标的测定
采用慢速荧光成像系统(MAX-Imaging-PAM,WALZ,德国)测定光系统Ⅱ的最大量子产量(Fv/Fm);相对电导率参照李合生[28]的方法测定;丙二醛含量采用硫代巴比妥酸法测定;过氧化氢含量采用二甲基橙法测定;谷胱甘肽含量采用DTNB显色法测定。
1.3 谷胱甘肽相关差异基因筛选
笔者课题组前期将温度从25 ℃开始以4 ℃·h-1降温至-3 ℃,在-3 ℃模拟冻害条件下处理6 h、12 h的新疆野苹果组培苗和对照进行转录组测序[29],基于负二项分布原理的DEseq2 方法进行DEGs 的检测,将Q-value≤0.05(adjusted P-value≤0.05)的基因定义为显著差异表达基因,将每个处理相对CK差异倍数绝对值为1.5 以上的DEGs 定义为极显著差异表达基因。登录KEGG 数据库,获取谷胱甘肽代谢通路基因,然后将在KEGG 数据库获得的谷胱甘肽代谢相关基因与转录组数据KEGG注释文件进行比对,从而筛选出转录组数据中有显著表达谷胱甘肽代谢的相关基因进行分析。
1.4 谷胱甘肽相关差异表达基因qRT-PCR验证
根据笔者课题组前期试验选择UBQ 为内参基因[26],利用Primer 3设计qRT-PCR引物,引物合成由上海生物工程公司完成(表1)。以不同处理的新疆野苹果组培苗叶片RNA 为模板,参照ABM 公司反转试剂盒说明书合成cDNA。qRT-PCR 按照Green Real time PCR Master MIX 试剂盒(TOYOBO,日本)进行,基因表达量采用相对定量2-ΔΔCT法,即log2(T/CK)分析。
表1 谷胱甘肽代谢相关差异表达基因qRT-PCR 引物序列
Table 1 qRT-PCR primer sequences for differentially expressed genes related to glutathione metabolism
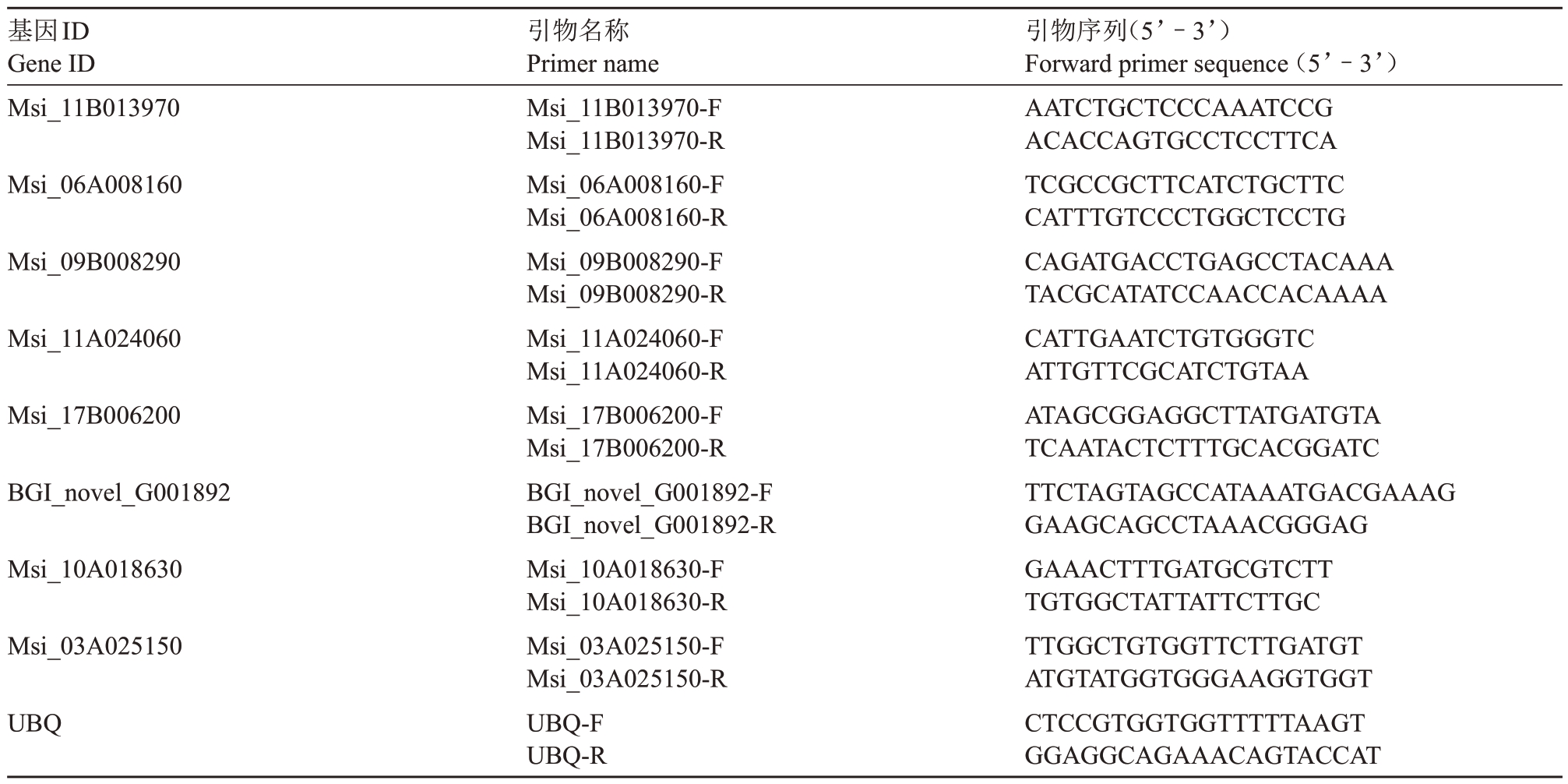
基因ID Gene ID Msi_11B013970 Msi_06A008160 Msi_09B008290 Msi_11A024060 Msi_17B006200 BGI_novel_G001892 Msi_10A018630 Msi_03A025150 UBQ引物名称Primer name Msi_11B013970-F Msi_11B013970-R Msi_06A008160-F Msi_06A008160-R Msi_09B008290-F Msi_09B008290-R Msi_11A024060-F Msi_11A024060-R Msi_17B006200-F Msi_17B006200-R BGI_novel_G001892-F BGI_novel_G001892-R Msi_10A018630-F Msi_10A018630-R Msi_03A025150-F Msi_03A025150-R UBQ-F UBQ-R引物序列(5’–3’)Forward primer sequence(5’–3’)AATCTGCTCCCAAATCCG ACACCAGTGCCTCCTTCA TCGCCGCTTCATCTGCTTC CATTTGTCCCTGGCTCCTG CAGATGACCTGAGCCTACAAA TACGCATATCCAACCACAAAA CATTGAATCTGTGGGTC ATTGTTCGCATCTGTAA ATAGCGGAGGCTTATGATGTA TCAATACTCTTTGCACGGATC TTCTAGTAGCCATAAATGACGAAAG GAAGCAGCCTAAACGGGAG GAAACTTTGATGCGTCTT TGTGGCTATTATTCTTGC TTGGCTGTGGTTCTTGATGT ATGTATGGTGGGAAGGTGGT CTCCGTGGTGGTTTTTAAGT GGAGGCAGAAACAGTACCAT
2 结果与分析
2.1 冻害对新疆野苹果组培苗形态的影响
如图1 所示,对-3 ℃低温胁迫下新疆野苹果组培苗的形态进行观察,发现与CK相比,在T6h,植株的叶形、叶色未见明显变化;在T12h,新疆野苹果组培苗顶部叶片边缘出现反卷,叶色变暗,顶梢嫩叶可见萎蔫;在T36h,新疆野苹果组培苗叶片萎蔫下垂,叶色进一步变暗;在HF24h,萎蔫卷曲的叶片舒展,叶色也由暗变亮,恢复后叶片出现褐色小斑点。

图1 冻害胁迫后新疆野苹果组培苗形态
Fig.1 Changes of morphological of Malus sieversii seedlings in vitro under freezing stress
2.2 冻害对新疆野苹果组培苗叶绿素荧光的影响
叶绿素荧光技术能够快速无损地检测作物叶片对环境胁迫的敏感性。其颜色依次从粉红、蓝、绿、橙、黑色表示植物受到的胁迫越重,对应的Fv/Fm数值范围为1~0(图2-A),当Fv/Fm下降时,代表植物受到了胁迫。由图2-A 可见,与CK 相比,新疆野苹果组培苗在-3 ℃模拟冻害条件下处理不同时间段,再到室温恢复24 h,幼苗叶片的荧光从深蓝色逐渐变为黄绿色又恢复到深蓝色,表明随着处理时间的延长,新疆野苹果组培苗受到的胁迫逐渐加深,室温恢复24 h,新疆野苹果组培苗荧光颜色恢复到与CK 相同。同时Fv/Fm数值的变化趋势与叶绿素荧光颜色变化一致。与CK 相比,-3 ℃处理的Fv/Fm均发生显著变化,T6h、T12h、T36h 的Fv/Fm分别下降20%、30%、53%,HF24h 与CK 的Fv/Fm无显著差异(图2-B)。

图2 冻害胁迫后新疆野苹果组培苗叶绿素荧光的变化
Fig.2 Changes of chlorophyll fluorescence of M.sieversii seedlings in vitro under freezing stress
2.3 冻害对新疆野苹果组培苗生理指标的影响
由图3 可见,与CK 相比,新疆野苹果组培苗叶片相对电导率在T6h 无显著差异,在T12h 和T36h相对电导率分别显著上升50%和175%,HF24h 与T36h 相比,新疆野苹果组培苗叶片相对电导率显著下降48%;与CK 相比,新疆野苹果组培苗叶片MDA 含量在T6h 没有显著变化,在T12h 和T36h,新疆野苹果组培苗叶片MDA 含量分别显著上升57%和86%,HF24h 相比T36h,MDA 含量显著下降23%;与CK 相比,新疆野苹果组培苗叶片过氧化氢含量在T6h 没有显著变化,在T12h 和T36h 新疆野苹果组培苗叶片过氧化氢含量分别上升171%和291%,HF24h与T36h相比下降30%;与CK相比,新疆野苹果组培苗叶片还原性谷胱甘肽含量在T6h、T12h 和T36h 分别显著上升40%、89%和110%,HF24h 与T36h 相比,新疆野苹果组培苗叶片差异不显著。
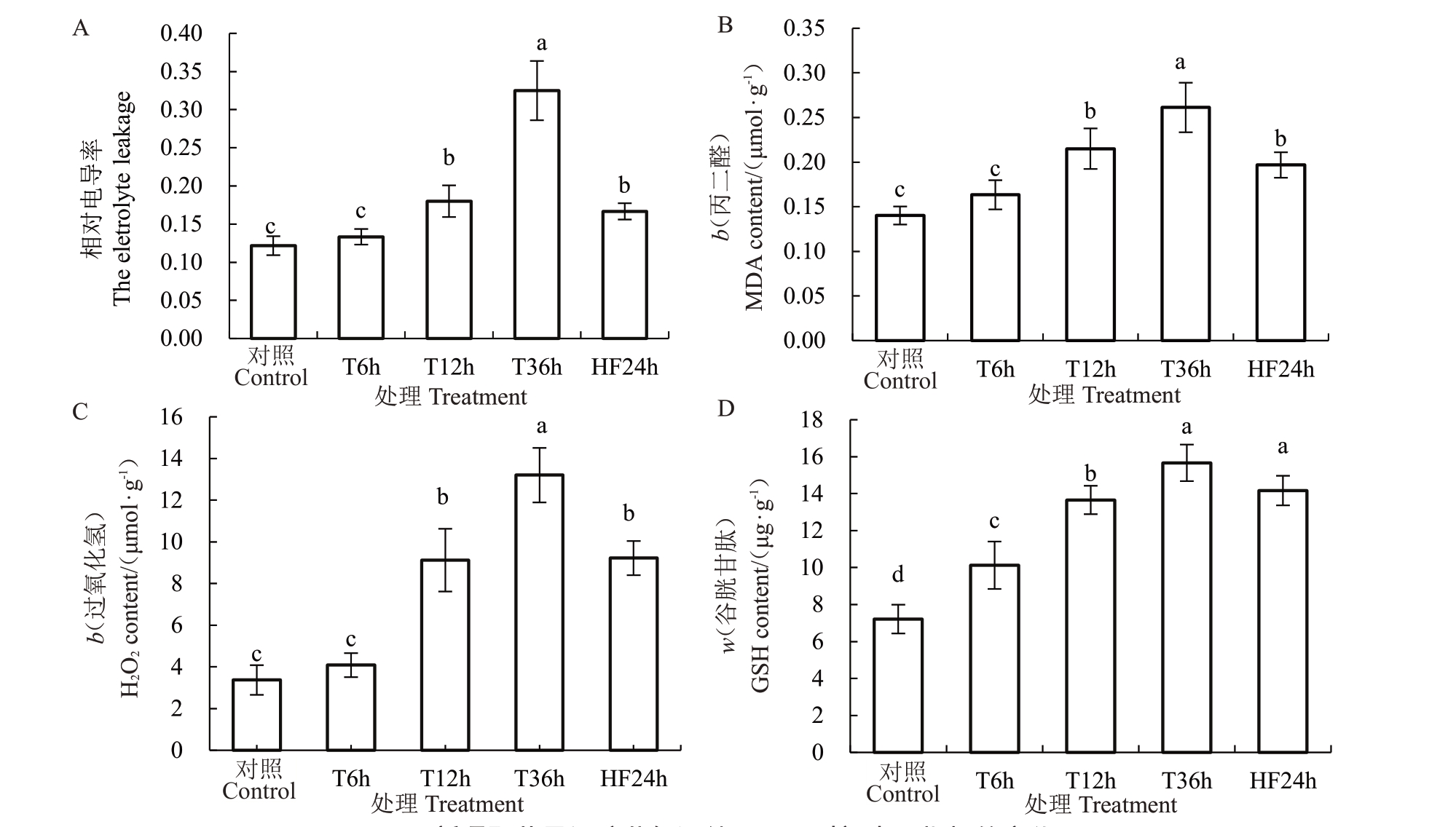
图3 新疆野苹果组培苗低温处理不同时间生理指标的变化
Fig.3 Changes of physiological indexes of M.sieversii seedlings in vitro under different low temperature treatments
2.4 新疆野苹果响应冻害的谷胱甘肽代谢差异表达基因筛选
利用KEGG数据库对转录组数据中差异表达基因注释分析,筛选到谷胱甘肽代谢途径的差异基因。由图4-A 可见,与CK 相比在T6h 和T12h,BGI_novel_G000969、Msi_11B024060、Msi_11B002610、Msi_15B018350、Msi_11A024060 下调表达,在T6h 和T12h 处理,Msi_08B010370、Msi_11B013970、Msi_17B006200、Msi_12A020080、Msi_14A011470、Msi_14B010870、Msi_09B008290、Msi_06A008160、Msi_03A025150、Msi_10A018630、BGI_novel_G001892、Msi_09A009290、Msi_07A023660 上调表达。通过KEGG 数据库对18 个差异基因进行注释,1 个基因被注释为γ-谷氨酰环转移酶[EC:4.3.2.9]GGCT,1个基因被注释为谷胱甘肽合酶[EC:6.3.2.3]GSS;1 个基因被注释为谷胱甘肽过氧化物酶[EC:1.11.1.9]GPX;1 个基因被注释γ-谷氨酰转肽酶[EC:2.3.2.2、3.4.19.13、3.4.19.14]K18592 GGT1_5;4 个基因注释为亮氨肽酶K01255 CARP;4 个基因注释为谷胱甘肽S-转移酶[EC:2.5.1.18] K00799 GST;1 个基因注释为亚精胺合酶[EC:2.5.1.16] K00797 speE;3 个基因注释为6-磷酸葡萄糖酸脱氢酶[EC:1.1.1.44 1.1.1.343] K00033 PGD;2 个基因注释为异柠檬酸脱氢酶[EC:1.1.1.42]K00031 IDH1。
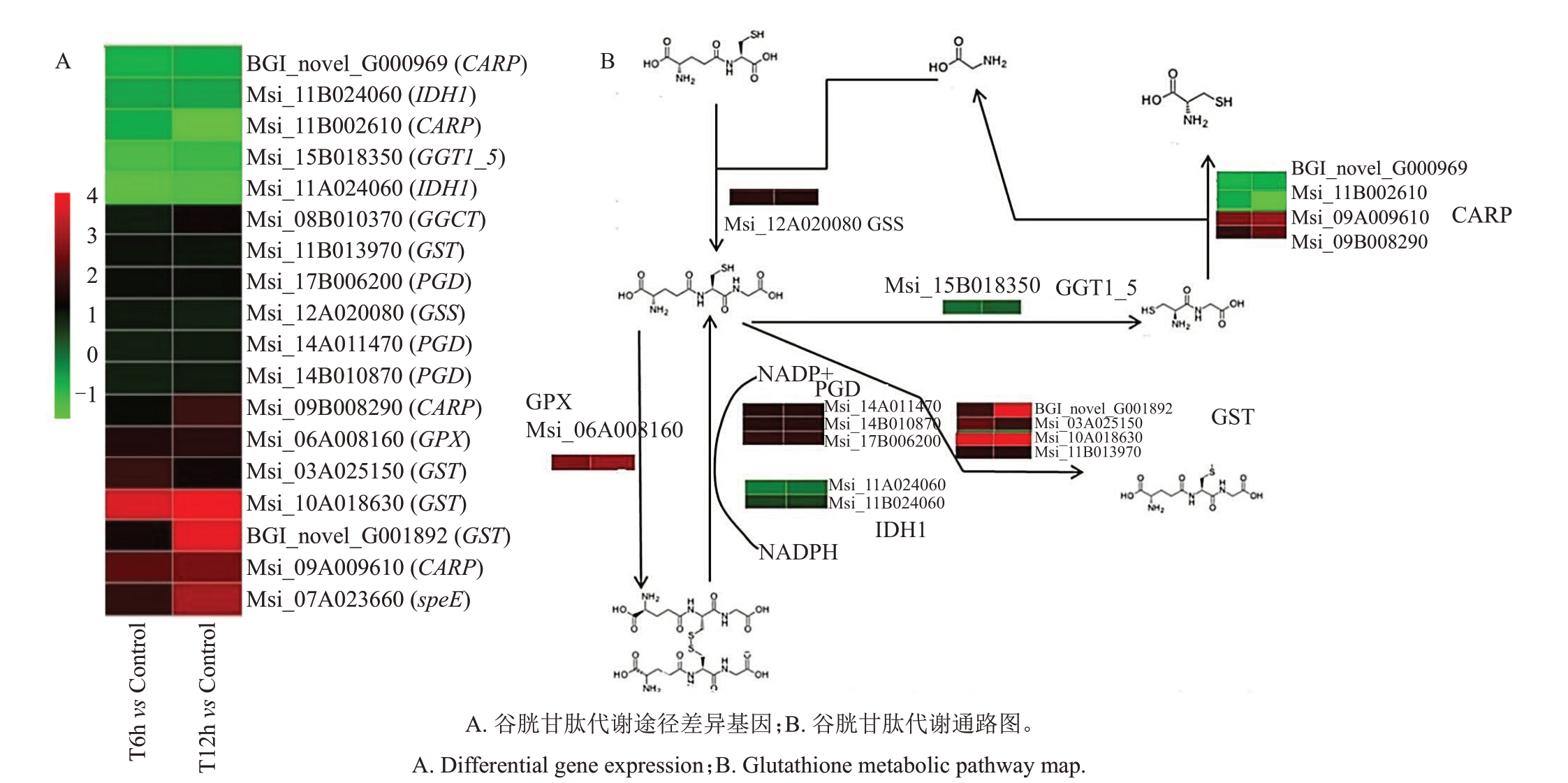
图4 冻害胁迫下新疆野苹果组培苗谷胱甘肽代谢差异基因转录分析
Fig.4 Analysis of transcription of glutathione metabolic differential genes of M.sieversii seedlings in vitro under freezing stress
如图4-B 所示,Msi_06A008160(DPX)、Msi_14A011470(PDG)、Msi_14B010870(PDG)、Msi_17B006200(PDG)、Msi_11A024060(IDH1)、Msi_11B024060(IDH1)参与GSH 与GSSG 的动态平衡,Msi_03A025150(GST)、Msi_10A018630(GST)、Msi_11B013970(GST)、BGI_novel_G001892(GST)参与到GSH 氨基转移反应中;Msi_15B018350(GGT1_5)、BGI_novel_G000969(CARP)、Msi_11B002610(CARP)、Msi_09A009610(CARP)、Msi_09B008290(CARP)、Msi_12A020080(GSS)参与到GSH降解和合成过程中。
2.5 新疆野苹果响应冻害谷胱甘肽代谢相关基因GO功能分析
对18 个参与谷胱甘肽代谢的差异表达基因进行GO 富集分析,由图5 可知,同一基因具有多个不同功能。在生物过程中发现Msi_06A008160、Msi_11B013970、Msi_12A020080 参与刺激反应;Msi_07A023660、Msi_08B010370、Msi_11B013970、Msi_14A011470、Msi_14B010870、Msi_15B018350、Msi_17B006200 参与细胞过程;Msi_07A023660、Msi_08B010370、Msi_14A011470、Msi_14B010870、Msi_15B018350、Msi_17B006200 参与代谢过程;Msi_09A009610、Msi_09B008290、Msi_11B013970 参与生物调节。在细胞组成中发现Msi_11B013970参与信号转导;Msi_06A008160、Msi_09A009610、Msi_09B008290、Msi_11B013970、Msi_12A020080、Msi_15B018350 参与细胞结构体构建;Msi_11B013970、Msi_12A020080参与内细胞构建。在分子功能中发现,Msi_03A025150、Msi_06A008160、Msi_07A023660、Msi_08B010370、Msi_10A018630、Msi_11A024060、Msi_11B013970、Msi_11B024060、Msi_12A020080、Msi_14A011470 参与催化活性激发;Msi_06A008160 参与抗氧化活性激发;Msi_09A009610、Msi_09B008290、Msi_11A024060、Msi_11B013970、Msi_11B024060、Msi_12A020080、Msi_14A011470、Msi_14B010870、Msi_17B006200 参与结合功能发挥。
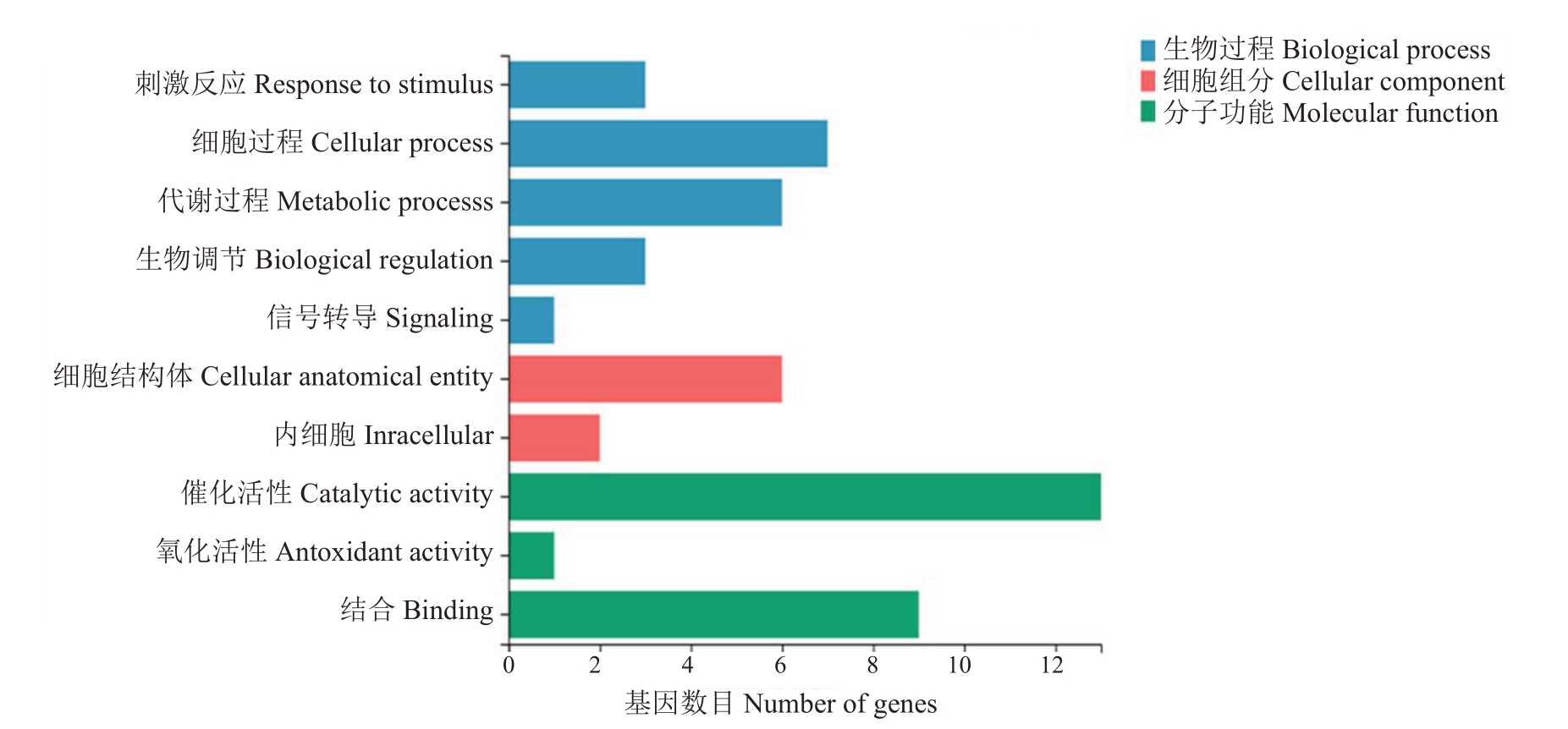
图5 冻害胁迫新疆野苹果组培苗谷胱甘肽代谢相关差异表达基因的GO 富集图
Fig.5 GO enrichment map of glutathione metabolic related differentially expressed genes of M.sieversii seedlings in vitro under freezing stress
2.6 qPCR验证转录组数据
如图6 所示,在谷胱甘肽代谢途径差异基因中选取6 个差异显著基因进行qPCR 检测,qPCR 实验结果显示,与CK相比,在T6h Msi_11B013970、Msi_06A008160、Msi_09B008290、BGI_novel_G001892、Msi_17B006200 上调表达,Msi_11A024060 下调表达。在T12h Msi_11B013970、Msi_06A008160、Msi_09B008290、BGI_novel_G001892、Msi_17B006200上调表达,Msi_11A024060下调表达。qPCR实验结果与转录组测序结果表达趋势一致。
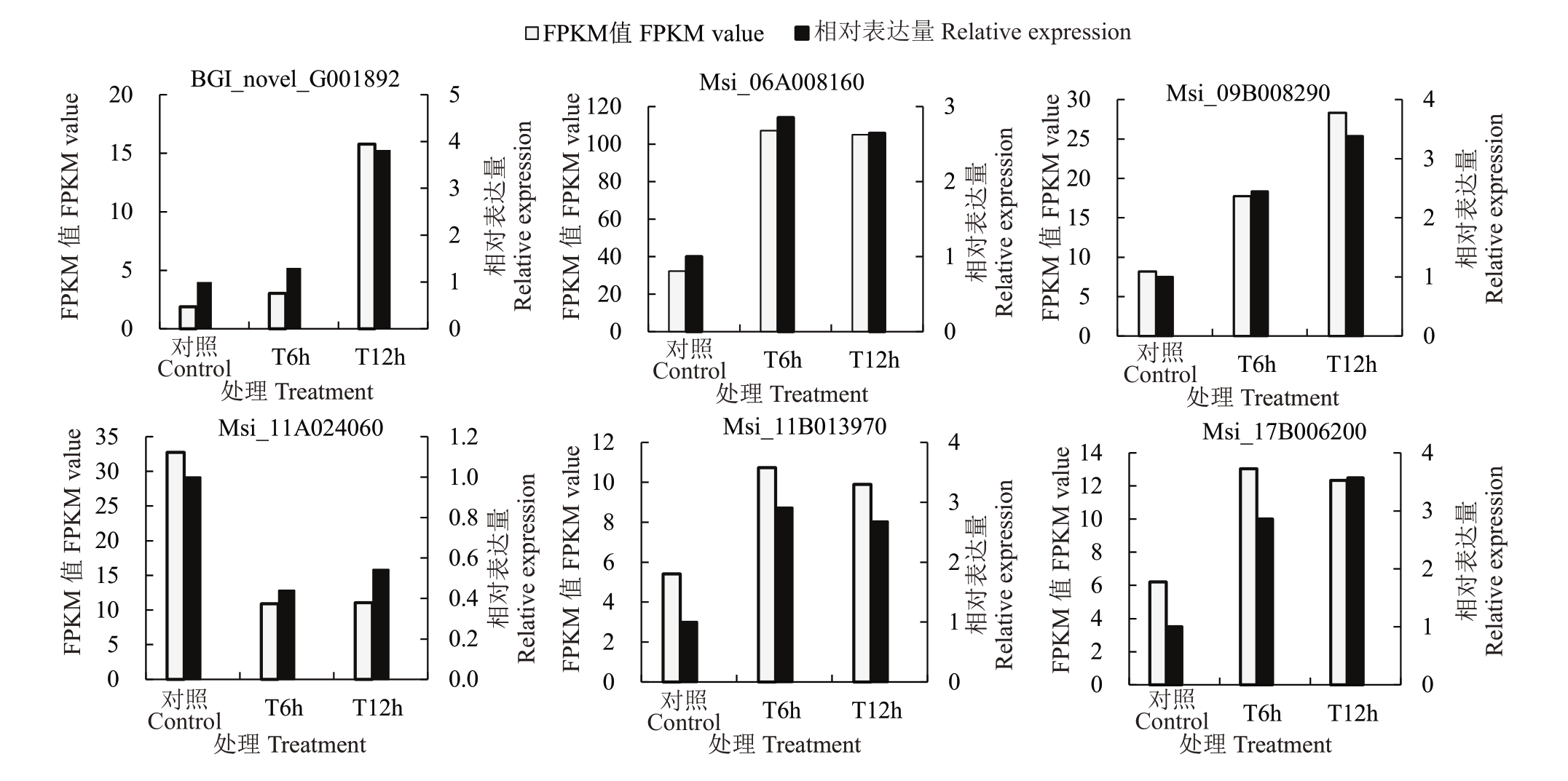
图6 差异基因的转录组表达量与qRT-PCR 验证
Fig.6 Transcriptome expression and qRT-PCR validation of differential genes
2.7 GPX和GST在新疆野苹果冻害处理与冻害恢复中的表达
如图7 所示,利用qPCR 对在T36h 和HF24h,Msi_06A008160(GPX)、BGI_novel_G001892(GST)、Msi_03A025150(GST)、Msi_10A018630(GST)、Msi_11B013970(GST)的表达量水平进行分析,与CK 相比,在T36h 处理下,Msi_06A008160(GPX)、Msi_11B013970(GST)上调表达,BGI_novel_G001892(GST)、Msi_03A025150(GST)、Msi_10A018630(GST)下调表达;在HF24h 处理下,Msi_06A008160(GPX)、Msi_11B013970(GST)、Msi_03A025150(GST)、Msi_10A018630(GST)上调表达,BGI_novel_G001892(GST)下调表达。HF24h 相比于T36h,Msi_06A008160(GPX)、Msi_03A025150(GST)、Msi_10A018630(GST)、Msi_11B013970(GST)都是上调表达,BGI_novel_G001892(GST)没有显著变化。
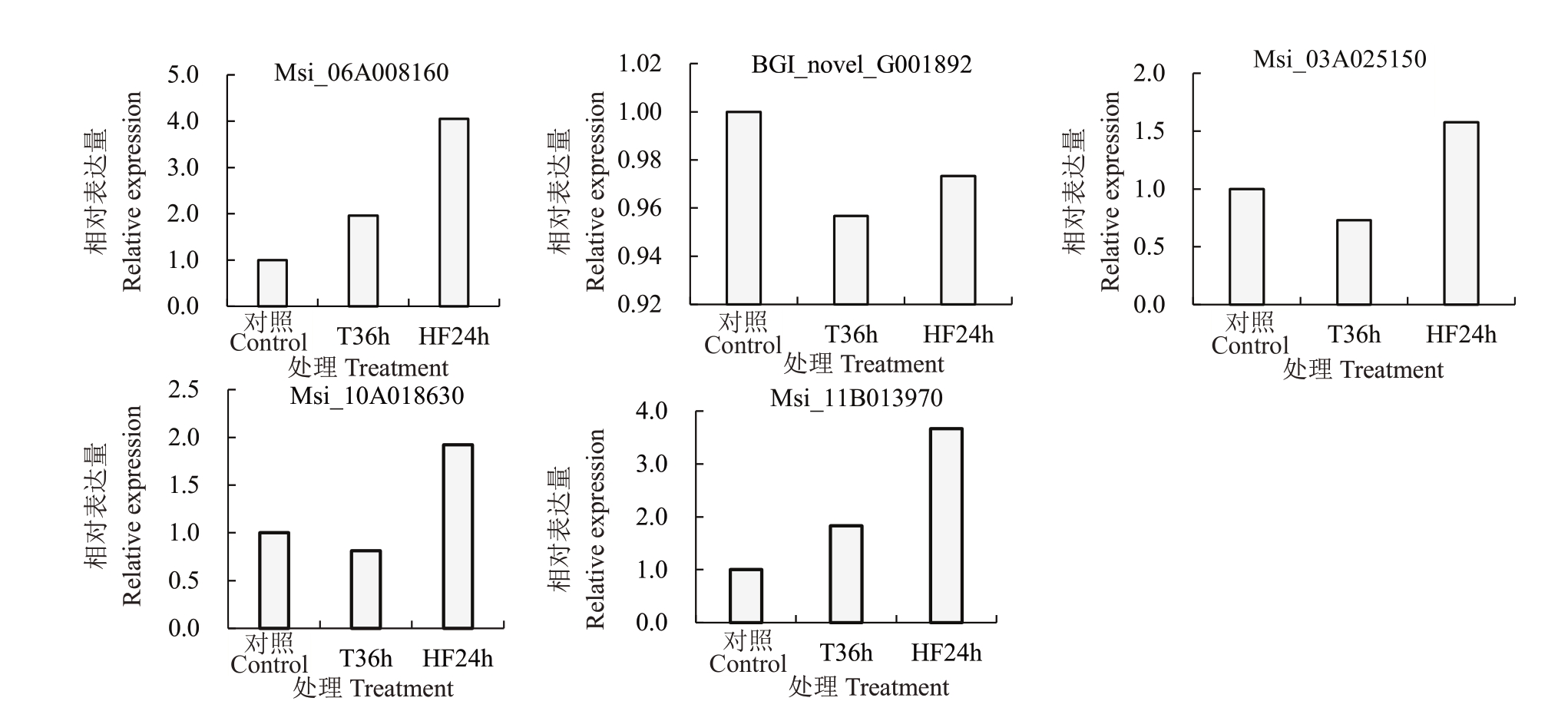
图7 新疆野苹果GPX 和GST 相关基因在T36h 和HF24h 表达分析
Fig.7 Expression analysis of GPX and GST related genes at T36h and HF24h in Malus sieversii
3 讨 论
植物活性氧的积累激活了植物防御基因的表达[30],GSH 库的大小以及氧化和还原状态与植物对胁迫环境的抵抗力高度相关[19]。GSH可以消除过氧化物来保护植物细胞免受氧化胁迫[31]。雷阳等[32]在探究辣椒对铅胁迫抗性中发现,GSH可有效缓解铅胁迫对辣椒造成的生理损害,进而提高辣椒抵抗铅胁迫的能力。韩敏等[33]在研究番茄嫁接苗抗寒性中发现,低温处理后的番茄叶片还原性谷胱甘肽含量高于对照,草莓在0 ℃低温驯化72 h,草莓叶片中GSH 含量达到最大值后下降,但明显高于对照[34]。各种逆境胁迫均可诱导植物细胞内活性氧浓度的增加而导致氧化胁迫[35],而植物在胁迫应答过程中主要调动抗氧化酶类和抗氧化物质来清除活性氧,从而减缓逆境对植物自身的伤害[36]。本研究结果显示,在T6h、T12h、T36h 还原性谷胱甘肽含量都显著高于对照,新疆野苹果组培苗恢复处理24 h,相较T36h GSH 含量没有显著差异,这表明GSH 在新疆野苹果抗冻害中起到重要作用。
对差异基因所参与的通路分析表明,相比于对照,在T6h 和T12h,Msi_12A020080(GSS)上调表达,Msi_15B018350(GGT1_5)下调表达,Msi_15B018350(GGT1_5)、BGI_novel_G000969(CARP)、Msi_11B002610(CARP)、Msi_A009610(CARP)、Msi_09B008290(CARP)、Msi_12A020080(GSS)参与到GSH 降解和合成过程中。这就解释了新疆野苹果组培苗在冻害胁迫下GSH 的积累可能的途径之一为抑制GSH 降解和促进GSH 合成。相比于对照,在T6h 和T12h,Msi_06A008160(GPX)、Msi_14A011470(PDG)、Msi_14B010870(PDG)、Msi_17B006200(PDG)上调表达,Msi_06A008160(DPX)、Msi_14A011470(PDG)、Msi_14B010870(PDG)、Msi_17B006200(PDG)参与GSH 与GSSG的动态平衡通路;谷胱甘肽还原酶(GR)、谷胱甘肽过氧化物酶(GPX)是GSH 与GSSG 动态平衡中的关键酶,谷胱甘肽还原酶(GR)是真核和原核生物中都存在的一类黄素蛋白氧化还原酶,主要分布于叶绿体、线粒体和细胞质中,是AsA-GSH 循环过程中重要的酶类,在氧化胁迫过程中能够有效清除ROS[37-40]。在胁迫条件下油菜抗性品种具有比敏感型品种更高的GR 活性[21],在Cd 胁迫下S 元素过剩的根中发现谷胱甘肽合成酶(MsGS)和植物螯合素合成酶(MsPCS1)基因的上调以及谷胱甘肽和植物螯合素浓度的增加,谷胱甘肽含量升高使植物螯合素能够与过量的Cd 结合[41]。高温胁迫下耐热性强的芦苇GR 活性显著高于耐热性弱的芦苇(Phragmites australis)[42]。在本研究中发现,-3 ℃条件下,在T6h和T12h基因GR表达量与对照相比没有显著差异,这可能是在新疆野苹果中存在能够代替GR功能的其他酶。谷胱甘肽过氧化物酶(GPX)是含有疏基的过氧化物酶类,它们利用GSH来减少过氧化物从而保护植物细胞免受氧化胁迫[31],木薯细菌性枯萎病侵染后MeGPX1、MeGPX5 和MeGPX6 的表达量呈显著上调趋势[43]。冻害胁迫下新疆野苹果组培苗基因Msi_06A008160(GPX)上调表达,这表明冻害胁迫下新疆野苹果组培苗可能通过GSHGSSG 动态平衡消除对其有害的H2O2和超氧阴离子等物质。相比于对照,在T6h 和T12h,Msi_03A025150(GST)、Msi_10A018630(GST)、Msi_11B013970(GST)、BGI_novel_G001892(GST)上调表达,携带棉花GST的转基因烟草也增强了对氧化胁迫的抗性[44]。GST转基因水稻幼苗对低温胁迫的抗性显著提高,利用农杆菌介导法将盐地碱蓬的GST基因转入低温敏感水稻品种中花11号中,并对T4代转基因水稻幼苗的抗低温特性进行了分析,结果显示,低温处理后转基因植株的GST活性比未转入这2 种基因的对照高[45]。在探究细长聚球藻对Cd2+胁迫的耐受性时发现,GST 转基因细长聚球藻对Cd2+胁迫的抗性显著提高[46]。谷胱甘肽转硫酶(GST)最早被发现的功能是它能催化GSH 与细胞废物形成中间体,这个过程是细胞废物代谢解毒的关键步骤[47]。植物细胞废物来源于细胞膜碎片或植物次生代谢物,例如,高粱[48]和小麦[49]细胞膜氧化损害释放的4 羟基壬烯酸(4-hydroxynonenal)以及植物在受到食草动物伤害或病原物侵染后产生的有毒次生代谢物[50],如美迪紫檀素(medicarpin)或异类黄酮类,它们被谷胱甘肽转硫酶(GST)催化GSH化后通过液泡排出体外。在拟南芥中发现,GST 具有GSH依赖的过氧化物酶功能(GSH-dependent peroxidase activity,GPOX),GPOX 以GSH 作为电子供体,还原脂肪酸和核酸的过氧化产物。也有研究发现杂草的GST具有相同的抗氧化功能[51-52]。在毛根复合大豆中GST 的过表达可以增强叶片的抗氧化能力,以及提高叶绿素含量和叶绿素荧光的量子效率[53],这表明冻害胁迫下新疆野苹果组培苗可能通过GSH消除对其有害的物质。
通过qPCR检测,表明与CK相比在T36h时,基因BGI_novel_G001892(GST)、Msi_03A025150(GST)、Msi_10A018630(GST)下调表达,基因Msi_06A008160(GPX)、Msi_11B013970(GST)上调表达;在HF24h的表达量分析发现,除了BGI_nov-el_G001892(GST)下调表达,Msi_06A008160(GPX)、Msi_03A025150(GST)、Msi_10A018630(GST)、Msi_11B013970(GST)上调表达,这表明GSH 不仅通过自身与自由基以及有毒物质反应增强新疆野苹果组培苗抗寒性,同时GSH 代谢在修复冻害损伤的过程中也起着重要作用,然而GSH 和GSSG 分别可以作为还原形式和氧化形式的电子载体来调节细胞内氧化还原平衡状态,而细胞内氧化还原平衡状态是否可以作为一种信号响应冻害胁迫还需要进一步探究。
4 结 论
在-3 ℃模拟冻害条件下,新疆野苹果组培苗形态和生理指标显示受到的伤害随着处理时间的延长而不断加深,新疆野苹果组培苗谷胱甘肽代谢途径中关键基因GPX和GST上调表达能够响应冻害胁迫。
[1] 霍学喜,刘天军,刘军弟,魏延安,姚心省,马晓燕,卢斐.2020年度中国苹果产业发展报告(精简版)[J].中国果菜,2022,42(2):1-6.HUO Xuexi,LIU Tianjun,LIU Jundi,WEI Yan’an,YAO Xinsheng,MA Xiaoyan,LU Fei. China apple industry development report in 2020[J].China Fruit&Vegetable,2022,42(2):1-6.
[2] 王金政,陈汝,薛晓敏,王志刚.中国苹果产业防灾减灾的战略对策[J].落叶果树,2019,51(3):19-21.WANG Jinzheng,CHEN Ru,XUE Xiaomin,WANG Zhigang.Strategic countermeasures for disaster prevention and mitigation of apple industry in China[J].Deciduous Fruits,2019,51(3):19-21.
[3] 陈学森,韩明玉,苏桂林,刘凤之,过国南,姜远茂,毛志泉,彭福田,束怀瑞.当今世界苹果产业发展趋势及我国苹果产业优质高效发展意见[J].果树学报,2010,27(4):598-604.CHEN Xuesen,HAN Mingyu,SU Guilin,LIU Fengzhi,GUO Guonan,JIANG Yuanmao,MAO Zhiquan,PENG Futian,SHU Huairui. Discussion on today’s world apple industry trends and the suggestions on sustainable and efficient development of apple industry in China[J]. Journal of Fruit Science,2010,27(4):598-604.
[4] ZTTERSTRÖM R,EIJKMAN C,HOPKINSSIR F G.The dawn of vitamins and other essential nutritional growth factors[J].Acta Paediatrica,2006,95(11):1331-1333.
[5] 胡文琴,王恬,孟庆利.抗氧化活性肽的研究进展[J].中国油脂,2004,29(5):42-45.HU Wenqin,WANG Tian,MENG Qingli. Research advance of antioxidative bioactive peptides[J]. China Oils and Fats,2004,29(5):42-45.
[6] DIXON D P,SKIPSEY M,GRUNDY N M,EDWARDS R.Stress- induced protein S- glutathionylation in Arabidopsis[J].Plant Physiology,2005,138(4):2233-2244.
[7] MARTY L,BAUSEWEIN D,MÜLLER C,BANGASH S A K,MOSELER A,SCHWARZLÄNDER M,MÜLLER-SCHÜSSEL S J,ZECHMANN B,RIONDET C,BALK J,WIRTZ M,HELL R,REICHHELD J P,MEYER A J.Arabidopsis glutathione reductase 2 is indispensable in plastids,while mitochondrial glutathione is safeguarded by additional reduction and transport systems[J].New Phytologist,2019,224(4):1569-1584.
[8] HASANUZZAMAN M,BHUYAN M H M B,ZULFIQAR F,RAZA A,MOHSIN S M,MAHMUD J A,FUJITA M,FOTOPOULOS V,FOTOPOLOS V.Reactive oxygen species and antioxidant defense in plants under abiotic stress:Revisiting the crucial role of a universal defense regulator[J].Antioxidants,2020,9(8):681.
[9] HUNG S H,YU C W,LIN C H. Hydrogen peroxide functions as a stress signal in plants[J].Botanical Bulletin of Academia Sinica,2005,46(1):1-10.
[10] XING F S,SONG P,WANG F T,GAO Y Z.Response of glutathione-ascorbate cycle in rice leaves to photoinhibition[J]. Chinese Journal of Rice Science,1992,164(2):177-183.
[11] TAO D L,ÖQUIST G,WINGSLE G.Active oxygen scavengers during cold acclimation of Scots pine seedlings in relation to freezing tolerance[J].Cryobiology,1998,37(1):38-45.
[12] 刘攀,耿兴敏,赵晖.碱胁迫下杜鹃花抗氧化体系的响应及亚细胞分布[J].园艺学报,2020,47(5):916-926.LIU Pan,GENG Xingmin,ZHAO Hui. Subcellular distribution and responses of antioxidant systems in leaves of three Rhododendron cultivars under alkali stress[J].Acta Horticulturae Sinica,2020,47(5):916-926.
[13] FRATELLI M,GOODWIN L O,ØROM U A,LOMBARDI S,TONELLI R,MENGOZZI M,GHEZZI P. Gene expression profiling reveals a signaling role of glutathione in redox regulation[J]. Proceedings of the National Academy of Sciences of the United States of America,2005,102(39):13998-14003.
[14] MICHELET L,ZAFFAGNINI M,MARCHAND C,COLLIN V,DECOTTIGNIES P,TSAN P,LANCELIN J M,TROST P,MIGINIAC-MASLOW M,NOCTOR G,LEMAIRE S D.Glutathionylation of chloroplast thioredoxin f is a redox signaling mechanism in plants[J]. Proceedings of the National Academy of Sciences of the United States of America,2005,102(45):16478-16483.
[15] KOORMNEEF A,LEON-RREYESA A,RITSEMA T,VERHAGE A,DEN OTTER F C,VAN LOON L C,PIETERSE C M J. Kinetics of salicylate-mediated suppression of jasmonate signaling reveal a role for redox modulation[J]. Plant Physiology,2008,147(3):1358-1368.
[16] AKTER N,SOBAHAN M A,HOSSAIN M A,URAJI M,NAKAMURA Y,MORI I C,MURATA Y.The involvement of intracellular glutathione in methyl jasmonate signaling in Arabidopsis guard cells[J].Bioscience,Biotechnology,and Biochemistry,2010,74(12):2504-2506.
[17] KUMAR D,HAZRA S,DATTA R,CHATTOPADHYAY S.Transcriptome analysis of Arabidopsis mutants suggests a crosstalk between ABA,ethylene and GSH against combined cold and osmotic stress[J].Scientific Reports,2016,6:36867-36867.
[18] SULTANA A,BORO P,MANDAL K,CHATTOPADHYAY S.AAL-toxin induced stress in Arabidopsis thaliana is alleviated through GSH-mediated salicylic acid and ethylene pathways[J].Plant Cell,Tissue and Organ Culture,2020,141(2):299-314.
[19] 陈坤明,宫海军,王锁民.植物谷胱甘肽代谢与环境胁迫[J].西北植物学报,2004,24(6):1119-1130.CHEN Kunming,GONG Haijun,WANG Suomin. Glutathione metabolism and environmental stresses in plants[J].Acta Botanica Boreali-Occidentalia Sinica,2004,24(6):1119-1130.
[20] MELSTER A,ANDERSON M. Glutathnione[J]. Annual Review of Biochemistry,1983,52:711-760.
[21] 张腾国,聂亭亭,孙万仓,史中飞,王娟.逆境胁迫对油菜谷胱甘肽还原酶基因表达及其酶活性的影响[J].应用生态学报,2018,29(1):213-222.ZHANG Tengguo,NIE Tingting,SUN Wancang,SHI Zhongfei,WANG Juan.Effects of diverse stresses on gene expression and enzyme activity of glutathione reductase in Brassica campestris[J].Chinese Journal of Applied Ecology,2018,29(1):213-222.
[22] 王咏梅.自由基与谷胱甘肽过氧化物酶[J].解放军药学学报,2005,21(5):369-371.WANG Yongmei. Free radicals and glutathione peroxidase[J].Pharmaceutical Journal of Chinese People’s Liberation Army,2005,21(5):369-371.
[23] 单雪萌,杨克彬,李广柱,王新悦,李英,高志民.毛竹谷胱甘肽过氧化物酶基因分子特征及其表达模式分析[J].植物科学学报,2022,40(3):344-354.SHAN Xuemeng,YANG Kebin,LI Guangzhu,WANG Xinyue,LI Ying,GAO Zhimin. Molecular characteristics and expression pattern analysis of glutathione peroxidase genes in Phyllostachys edulis (Carriere) J. Houzeau[J]. Plant Science Journal,2022,40(3):344-354.
[24] 地丽热巴·地里夏提.盐穗木谷胱甘肽过氧化物酶(HcGPX)和谷胱甘肽转硫酶(HcGST)基因的克隆与功能研究[D].乌鲁木齐:新疆大学,2013.Dilraba · Dilxat. Molecular cloning and functional analysis of glutathione peroxides gene and glutathiones-transferase gene from Halostachys caspica[D]. Urumqi:Xinjiang University,2013.
[25] 王光勇.火把梨谷胱甘肽S-转移酶基因的克隆与功能分析[D].昆明:昆明理工大学,2012.WANG Guangyong. Isolation and functional analysis of a glutathione-S-transferase gene from Pyrus pyrifolia Nakai cv.Huobali[D]. Kunming:Kunming University of Science and Technology,2012.
[26] 何晨晨,刘俐君,鲁晓燕.基于转录组测序分析NaCl 胁迫下新疆野苹果叶和根糖酵解相关基因的表达[J]. 果树学报,2020,37(7):951-961.HE Chenchen,LIU Lijun,LU Xiaoyan.Analysis of genes related to glycolysis in the leaves and roots of Malus sieversii under NaCl stress based on transcriptome sequencing[J]. Journal of Fruit Science,2020,37(7):951-961.
[27] 范宗民,孙军利,赵宝龙,刘怀锋,于坤,章智钧,刘晶晶.不同砧木‘赤霞珠’葡萄枝条抗寒性比较[J]. 果树学报,2020,37(2):215-225.FAN Zongmin,SUN Junli,ZHAO Baolong,LIU Huaifeng,YU Kun,ZHANG Zhijun,LIU Jingjing. Evaluation of cold resistance of one-year shoots from‘Cabernet Sauvignon’grape vine grafted on different rootstocks[J]. Journal of Fruit Science,2020,37(2):215-225.
[28] 李合生.植物生理生化实验原理和技术[M].北京:高等教育出版社,2000:261-263.LIN HeSheng. Principles and techniques of plant physiological and biochemical experiments [M]. Beijing:Higher Education Press,2000:261-263.
[29] 马红喜,刘俐君,苏永峰,张德恩,袁引燕,鲁晓燕.基于转录组测序筛选新疆野苹果组培苗应答冻害光合特性相关基因[J].果树学报,2022,39(9):1529-1539.MA Hongxi,LIU Lijun,SU Yongfeng,ZHANG Deen,YUAN Yinyan,LU Xiaoyan. Screening of freezing stress-responsive genes related to photosynthesis in in vitro seedlings of Malus sieversii via RNA-seq[J]. Journal of Fruit Science,2022,39(9):1529-1539.
[30] FOYER C H,NOCTOR G. Redox homeostasis and antioxidant signaling:A metabolic interface between stress perception and physiological responses[J]. The Plant Cell,2005,17(7):1866-1875.
[31] NOCTOR G,GOMEZ L,VANACKER H,FOYER C H.Interactions between biosynthesis,compartmentation and transport in the control of glutathione homeostasis and signalling[J]. Journal of Experimental Botany,2002,53(372):1283-1304.
[32] 雷阳,乔宁,苗如意,杨玉花,吴越莉.谷胱甘肽对铅胁迫下辣椒幼苗生理特性的影响[J].中国瓜菜,2022,35(5):74-80.LEI Yang,QIAO Ning,MIAO Ruyi,YANG Yuhua,WU Yueli.Glutathione affects physiological characteristics of pepper seedlings under severe lead stress[J]. China Cucurbits and Vegetables,2022,35(5):74-80.
[33] 韩敏,曹逼力,刘树森,徐坤.低温胁迫下番茄幼苗根穗互作对其抗坏血酸—谷胱甘肽循环的影响[J].园艺学报,2019,46(1):65-73.HAN Min,CAO Bili,LIU Shusen,XU Kun. Effects of rootstock and scion interactions on ascorbate-glutathione cycle in tomato seedlings under low temperature stress[J]. Acta Horticulturae Sinica,2019,46(1):65-73.
[34] YONG Z,LUO Y,HOU Y X,JIANG H,CHEN Q,TANG H R.Chilling acclimation induced changes in the distribution of H2O2 and antioxidant system of strawberry leaves[J]. Agricultural Journal,2008,3(4):286-291.
[35] BALL L,ACCOTTO G P,BECHTOLD U,CREISSEN G,FUNCK D,JIMENEZ A,KULAR B,LEYLAND N,MEJIACARRANZA J,REYNOLDS H,KARPINSKI S,MULLINEAUX P M. Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis[J].The Plant Cell,2004,16(9):2448-2462.
[36] 钱琼秋,宰文珊,朱祝军,喻景权.外源硅对盐胁迫下黄瓜幼苗叶绿体活性氧清除系统的影响[J].植物生理与分子生物学学报,2006,32(1):107-112.QIAN Qiongqiu,ZAI Wenshan,ZHU Zhujun,YU Jingquan.Effects of exogenous silicon on active oxygen scavenging systems in chloroplasts of cucumber (Cucumis sativus L.) seedlings under salt stress[J]. Journal of Plant Physiology and Molecular Biology,2006,32(1):107-112.
[37] DOULIS A G. Seasonal changes in antioxidants in red spruce(Picea rubens Sarg) at two sites in the eastern united states[J].New Phytologist,1993,123:365-374.
[38] FOYER C H,SOURIAU N,PERRET S,LELANDAIS M,KUNERT K J,PRUVOST C,JOUANIN L. Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees[J].Plant Physiology,1995,109(3):1047-1057.
[39] POTTERS G,HOREMANS N,BELLONE S,CAUBERGS R J,TROST P,GUISEZ Y,ASARD H. Dehydroascorbate influences the plant cell cycle through a glutathione-independent reduction mechanism[J].Plant Physiology,2004,134(4):1479-1487.
[40] CHEW O,WHELAN J,MILLAR A H. Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants[J]. The Journal of Biological Chemistry,2003,278(47):46869-46877.
[41] DAS U,RAHMAN M A,ELA E J,LEE K W,KABIR A H.Sulfur triggers glutathione and phytochelatin accumulation causing excess Cd bound to the cell wall of roots in alleviating Cd-toxicity in alfalfa[J].Chemosphere,2021,262:128361.
[42] DING W,SONG L,WANG X,BI Y. Effect of abscisic acid on heat stress tolerance in the calli from two ecotypes of Phragmites communis[J].Biologia Plantarum,2010,54(4):607-613.
[43] 曹敏,王海,冯亚亭,陈银华,骆凯.木薯谷胱甘肽过氧化物酶(MeGPX)基因全基因组鉴定及表达分析[J].分子植物育种,2022,20(3):722-732.CAO Min,WANG Hai,FENG Yating,CHEN Yinhua,LUO Kai. Genome identification and expression analysis of glutathione peroxidase (MeGPX) gene in cassava[J]. Molecular Plant Breeding,2022,20(3):722-732.
[44] YU T,LI Y S,CHEN X F,HU J,CHANG X,ZHU Y G.Transgenic tobacco plants overexpressing cotton glutathione S-transferase (GST) show enhanced resistance to methyl viologen[J].Journal of Plant Physiology,2003,160(11):1305-1311.
[45] 赵凤云,王晓云,赵彦修,张慧.转入盐地碱蓬谷胱甘肽转移酶和过氧化氢酶基因增强水稻幼苗对低温胁迫的抗性[J].植物生理与分子生物学学报,2006,32(2):231-238.ZHAO Fengyun,WANG Xiaoyun,ZHAO Yanxiu,ZHANG Hui.Transferring the Suaeda salsa glutathione S-transferase and catalase genes enhances low temperature stress resistance in transgenic rice seedlings[J].Journal of Plant Physiology and Molecular Biology,2006,32(2):231-238.
[46] 顾梓鹏,任玉东,程芬,张晓雯,徐东,叶乃好,梁成伟.巨藻中谷胱甘肽S 转移酶基因对细长聚球藻PCC7942 耐镉性的影响[J].渔业科学进展,2023,44(2):127-136.GU Zipeng,REN Yudong,CHENG Fen,ZHANG Xiaowen,XU Dong,YE Naihao,LIANG Chengwei. Effects of the glutathione S-transferase gene extracted from giant kelp (Macrocystis pyrifera) on the cadmium tolerance of Synechococcus elongatus PCC7942[J]. Progress in Fishery Sciences,2023,44(2):127-136.
[47] 李岗,吴声敢,俞瑞鲜,陈丽萍,苍涛,蔡磊明,王强,吴长兴.植物谷胱甘肽转硫酶及其与杂草抗药性的关系[J].杂草科学,2012,30(3):1-8.LI Gang,WU Shenggan,YU Ruixian,CHEN Liping,CANG Tao,CAI Leiming,WANG Qiang,WU Changxing. Plant glutathione S-transferases and its relation to herbicide-resistance of weed[J].Journal of Weed Science,2012,30(3):1-8.
[48] GRONWALD J W,PLAISANCE K L. Isolation and characterization of glutathione S-transferase isozymes from Sorghum[J].Plant Physiology,1998,117(3):877-892.
[49] CUMMINS I,COLE D J,EDWARDS R. Purification of multiple glutathione transferases involved in herbicide detoxification from wheat (Triticum aestivum L.) treated with the safener fenchlorazole- ethyl[J]. Pesticide Biochemistry and Physiology,1997,59(1):35-49.
[50] LI Z S,ALFENITO M,REA P A,WALBOT V,DIXON R A.Vacuolar uptake of the phytoalexin medicarpin by the glutathione conjugate pump[J].Phytochemistry,1997,45(4):689-693.
[51] ROXAS V P,SMITH R K,ALLEN E R,ALLEN R D. Overexpression of glutathione S-transferase/glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress[J]. Nature Biotechnology,1997,15(10):988-991.
[52] CUMMINS I,COLE D J,EDWARDS R.A role for glutathione transferases functioning as glutathione peroxidases in resistance to multiple herbicides in black-grass[J]. Plant Journal,1999,18(3):285-292.
[53] LI X J,WANG Y,LIU F,PI B Y,ZHAO T J,YU B J.Transcriptomic analysis of Glycine soja and G. max seedlings and functional characterization of GsGSTU24 and GsGSTU42 genes under submergence stress[J].Environmental and Experimental Botany,2020,171:103963.