野杏(Prunus armeniaca)是世界上广泛栽培杏的起源物种,也是我国新疆特色果树的重要种质资源,具有极高的科研价值、生态价值及应用价值[1]。近年来,穿孔病的发生已成为威胁野杏健康生长的重要影响因子,其中以嗜果刀孢菌(Wilsonomyces carpophilus)引起的穿孔病最为严重。该病原菌具有潜伏侵染的特性,主要危害核果类植物的叶片和果实,也可危害嫩枝、休眠芽及花萼,严重影响植株生长和果品质量[2-4]。目前,国内外关于嗜果刀孢菌的研究多集中在病原菌的种类和防治方面,少数学者研究了该菌的流行病学、真菌寄主范围、遗传多样性及次生代谢产物等[3-11]。关于嗜果刀孢菌引起的穿孔病致病机制的研究很少,尚未有嗜果刀孢菌致病过程中产生的酶的种类及活性的相关研究。
病原菌与寄主的相互作用是一个十分复杂的过程,病原菌产生对寄主正常生理活动有影响的代谢产物。酶、毒素和生长调节物质是国内外科研工作者研究最多的病原物主要的三大致病因子[12]。研究发现植物在受到病原物侵染后,膜系统受到伤害,细胞膜透性增强,过氧化物酶(POD)、超氧化物歧化酶(SOD)、过氧化氢酶(CAT)等抗氧化酶会参与植物的防御,这些指标被作为植物抗病性鉴定的重要生理指标[13]。此外,宿主细胞壁是阻止病原真菌侵入的一道屏障。病原真菌入侵时通过分泌多种细胞壁降解酶,降解组成宿主细胞壁的各种多糖物质,从而破坏细胞壁和胞间层,或导致细胞分离,组织溃散[14]。Adaskaveg[15]证实了扁桃穿孔病病原菌(W.carpophilus)在侵入寄主的过程中先破坏细胞壁,在一定程度上加速了病原菌侵入。Chiu等[16]的研究表明,果胶酶在褐腐菌(Monilinia fructicola)致病过程中发挥了重要的作用。王鹏程等[17]也证实了细胞壁降解酶在枣黑斑病菌(Alternaria sp.)侵入枣果过程中的作用。然而不同的病原菌所分泌的细胞壁降解酶种类是不同的,并且不同的降解酶在致病过程中发挥的作用也不同。目前关于嗜果刀孢菌(W.carpophilus)在危害野杏穿孔病的过程中产生的细胞壁降解酶的种类及其活性变化等研究未见报道,以及嗜果刀孢菌侵染过程中寄主体内抗氧化酶的生理指标是如何变化的尚不清楚。
笔者在本研究中通过嗜果刀孢菌活体外诱导培养和接种野杏叶片后,采用3,5-二硝基水杨酸(DNS)法和考马斯亮蓝(Bradford)法测定嗜果刀孢菌在野杏叶片内外产生的细胞壁降解酶活性,分析比较其变化趋势。研究结果为深入了解其致病机制提供理论依据及对开展穿孔病的预防和控制等工作具有重要意义。
1 材料和方法
1.1 材料
供试菌株:嗜果刀孢菌菌株XJAU-Y046-7m2源自天山野果林野杏叶片,种类鉴定由前期研究完成[18]。
供试寄主:栽植于新疆农业大学试验地的2~3 a(年)的野杏。
1.2 方法
1.2.1 嗜果刀孢菌体外细胞壁降解酶的提取 以Czaper 液体培养基(KNO3 2.0 g,KCl 0.5 g,FeSO4 0.01 g,K2HPO4 1.0 g,MgSO4·7 H2O 0.5 g)为基础培养基,分别添加10 g不同诱导物至锥形瓶中,主要有蔗糖、果胶、微晶纤维素、羧甲基纤维素(CMC)、滤纸粉、脱脂棉屑、野杏叶片,最后用蒸馏水定容至1 L。通过磷酸将培养基的pH 调至5.0,于121 ℃高温灭菌20 min。
将PDA平板上纯化培养10 d的嗜果刀孢菌,用直径0.5 cm 打孔器选取菌落生长一致的菌饼,选取5 个菌饼置于盛有100 mL 上述培养基的250 mL 的三角瓶内,25 ℃下振荡培养7 d 后过滤去除菌丝,4 ℃10 000 r·min-1 离心30 min,弃去沉淀,留上清液(粗酶液)备用[17,19]。
1.2.2 嗜果刀孢菌产生的细胞壁降解酶的活性测定 参考Douaiher等[20]和Siah等[21]的研究方法测定羧甲基纤维素酶(Cx)、β-葡萄糖苷酶、木聚糖酶、聚甲基半乳糖醛酸酶(PMG)、多聚半乳糖醛酸酶(PG)的活性。利用DNS比色法,在分光光度计540 nm处测定反应混合物吸光值,根据酶反应所释放的还原糖含量计算上述酶活性。酶活性单位为50 ℃下每分钟每毫升酶液(每毫克蛋白)催化底物产生1 μmol还原糖所需酶量,根据D-半乳糖醛酸标准曲线计算生成的还原糖。
果胶甲基反式消除酶(PMTE)的活性测定:在232 nm处测定反应混合物吸光值,30 ℃下每分钟每毫升酶液催化底物释放1 μmol 不饱和醛酸为一个酶活单位(U·mL-1)。酶蛋白浓度测定按照Bradford的方法,用考马斯亮蓝G-250 显色,在595 nm 比色测定,用牛血清做标准曲线。
1.2.3 嗜果刀孢菌产生细胞壁降解酶对野杏叶片的降解作用 采用离体针刺法,将1.2.2 中得到的经7 种诱导物诱导的酶液各2 mL,分别滴加在无菌的棉球上,使棉球完全润湿。取野杏叶片,用灭菌后的3 号昆虫针在叶片的左右两侧对称针刺大小一致的伤口,右边接种以酶液润湿的棉花,左边以煮沸灭活的酶液为对照处理,每个处理3 次重复。25 ℃左右光照条件下培养,7 d 后观察叶片变化情况。
1.2.4 嗜果刀孢菌在野杏叶片内产生细胞壁降解酶的提取 将PDA 平板上纯化培养10 d 的嗜果刀孢菌,用直径0.5 cm打孔器选取菌落生长一致的菌饼,接种至健康的野杏叶片上,以接种空白培养基作为对照,于接种后2、4、6、8、10、12、14、16 d 分别取样。称取叶片1 g,放入研钵中,冰浴研磨,加入5 mL 1 mol·L-1 NaCl 提取液(20 mmol·L-1 Tris-HCl 缓冲液,pH 值7.4),4 ℃、5000 r·min-1下离心15 min,取上清液(粗酶液)备用,4 ℃保存。
1.2.5 野杏叶片中抗氧化酶的提取及活性测定 叶片的接种方法同1.2.4,于接种后1、3、5、10、15 d分别取样。称取1 g野杏叶片,剪碎放入研钵中,加适量磷酸缓冲液,冰浴研磨成匀浆,4 ℃,12 000 r·min-1,冷冻离心30 min,取上清液为提取粗酶液。POD 活性测定采用愈创木酚法[22]、SOD 活性测定采用四唑氮蓝还原法[23]、CAT活性测定参照高俊凤[24]的方法,酶活性根据公式计算,每个处理3次重复。
式中:0.01为酶活性单位;V是提取酶液的体积;Vt 为测定时酶液用量;w 表示用于提取酶的叶片质量;t 为▲OD 变化所用时间;酶活性单位为U/(鲜质量·时间)。
1.3 数据处理
将测定的全部数据采用Excel 2010进行处理和分析,计算3次重复的平均值,利用IBM SPSS Statistics 22.0 统计分析软件进行方差分析,采用Duncan检验法进行多重比较及差异显著性分析,使用Origin 2018 64Bit软件进行图表制作。
2 结果与分析
2.1 嗜果刀孢菌在不同外源诱导物下产生细胞壁降解酶的种类
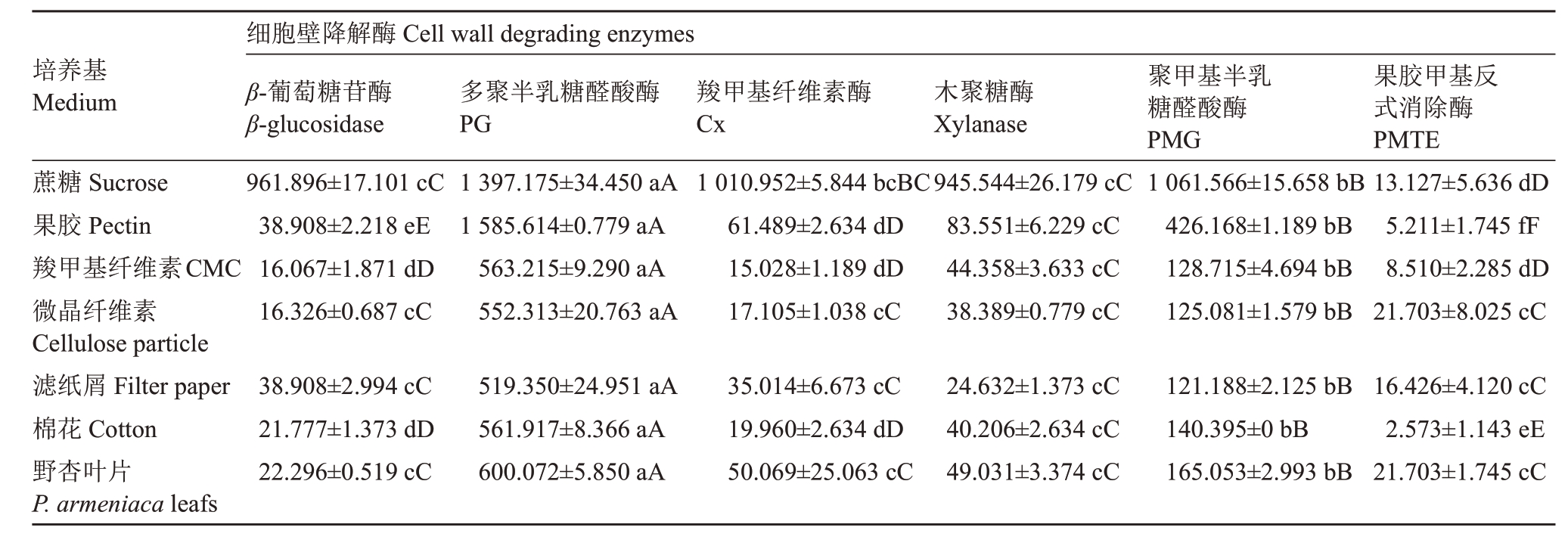
嗜果刀孢菌在7种不同外源诱导物培养基上均能产生羧甲基纤维素酶(Cx)、β-葡萄糖苷酶、木聚糖酶、聚甲基半乳糖醛酸酶(PMG)、多聚半乳糖醛酸酶(PG)、果胶甲基反式消除酶(PMTE),且酶活性呈现显著性差异(表1)。6 种酶中PG 酶活性最高,其次为PMG、木聚糖酶、Cx、β-葡萄糖苷酶,而PMTE酶活性最低,其中PG、PMG、PMTE 属于果胶酶,木聚糖酶、Cx、β-葡萄糖苷酶为纤维素酶。嗜果刀孢菌在分别以蔗糖、果胶、羧甲基纤维、微晶纤维素、滤纸粉、脱脂棉屑、野杏叶片为诱导物的培养基中,产生的PG酶活性依次为:(1 397.175±34.450)、(1 585.614±0.779)、(563.215±9.290)、(552.313±20.763)、(519.350±24.951)、(561.917±8.366)、(600.072±5.850)U·mL-1;产生的PMG酶活性依次为(1 061.566±15.658)、(426.168±1.189)、(128.715±4.694)、(125.081±1.579)、(121.188±2.125)、(140.395±0)、(165.053±2.993)U·mL-1;产生的PMTE 酶活性依次为(13.127±5.636)、(5.211±1.745)、(8.510±2.285)、(21.703±8.025)、(16.426±4.120)、(2.573±1.143)、(21.703±1.745)U·mL-1。PG、PMG、分别在果胶、蔗糖诱导培养基中酶活性最高,果胶、蔗糖为PG、PMG 的最佳诱导物。
表1 嗜果刀孢菌在不同外源诱导物下产生细胞壁降解酶的种类及活性
Table 1 Comparison of the activities of cell wall degrading enzymes produced in different inducer media (U·mL-1)
注:不同小写字母代表5%水平差异显著性;不同大写字母代表1%水平差异显著性。下同。
Note: Different small letters represent significant difference at p<0.05; different capital letters represent significant difference at p<0.01. The same below.
培养基Medium蔗糖Sucrose果胶Pectin羧甲基纤维素CMC微晶纤维素Cellulose particle滤纸屑Filter paper棉花Cotton野杏叶片P.armeniaca leafs细胞壁降解酶Cell wall degrading enzymes β-葡萄糖苷酶β-glucosidase 961.896±17.101 cC 38.908±2.218 eE 16.067±1.871 dD 16.326±0.687 cC多聚半乳糖醛酸酶PG 1 397.175±34.450 aA 1 585.614±0.779 aA 563.215±9.290 aA 552.313±20.763 aA羧甲基纤维素酶Cx 1 010.952±5.844 bcBC 61.489±2.634 dD 15.028±1.189 dD 17.105±1.038 cC木聚糖酶Xylanase 945.544±26.179 cC 83.551±6.229 cC 44.358±3.633 cC 38.389±0.779 cC聚甲基半乳糖醛酸酶PMG 1 061.566±15.658 bB 426.168±1.189 bB 128.715±4.694 bB 125.081±1.579 bB果胶甲基反式消除酶PMTE 13.127±5.636 dD 5.211±1.745 fF 8.510±2.285 dD 21.703±8.025 cC 38.908±2.994 cC 21.777±1.373 dD 22.296±0.519 cC 519.350±24.951 aA 561.917±8.366 aA 600.072±5.850 aA 35.014±6.673 cC 19.960±2.634 dD 50.069±25.063 cC 24.632±1.373 cC 40.206±2.634 cC 49.031±3.374 cC 121.188±2.125 bB 140.395±0 bB 165.053±2.993 bB 16.426±4.120 cC 2.573±1.143 eE 21.703±1.745 cC
2.2 嗜果刀孢菌体外诱导产生细胞壁降解酶的降解作用
将添加不同诱导物蔗糖、果胶、微晶纤维素、羧甲基纤维素(CMC)、滤纸粉、脱脂棉屑、野杏叶片诱导产生的细胞壁降解酶液分别接种于野杏叶片后呈现不同程度的降解,对照叶片无症状。不同诱导物诱导产生的酶液接种至叶片2 d后接种点出现黄褐色斑点,7 d后出现黑褐色病斑(图1)。由病斑直径显著性分析可知(表2),以野杏叶片诱导产生的酶液降解野杏叶片的能力最强,发病病斑最大,其次是以蔗糖、果胶诱导产生的酶液,而以微晶纤维素、羧甲基纤维素(CMC)、滤纸粉、脱脂棉诱导产生的酶液作用于叶片后呈现的病斑较小,降解能力弱。

图1 嗜果刀孢菌体外诱导产生细胞壁降解酶的降解作用
Fig.1 Degradation of P.armeniaca leaves by cell wall degrading enzymes produce by W.carpophilus
叶片的左边为对照,右边为处理;A.蔗糖培养基诱导产生的酶液;B.野杏叶片培养基诱导产生的酶液;C.滤纸培养基诱导产生的酶液;D.微晶纤维素培养基诱导产生的酶液;E.羧甲基纤维素培养基诱导产生的酶液;F.脱脂棉屑培养基诱导产生的酶液;G.果胶培养基诱导产生的酶液。
The wound on the left-hand side of each leaf was mock-inoculated (control);A. Enzymes form sucrose medium; B. Enzymes form P. armeniaca leafs medium; C. Enzymes form filter paper medium; D. Enzymes form cellulose particle medium; E. Enzymes form CMC medium; F. Enzymes form cotton medium;G.Enzymes form pectin medium.
表2 嗜果刀孢菌在不同外源诱导物下产生的酶液对野杏叶片降解力测定结果
Table 2 The results of the degradation of the P.armeniaca leafs by enzyme solution produced by W.carpophilus under different exogenous inducers

诱导物Inducer平均病斑直径Average diameter of lesion/mm蔗糖Sucrose 2.620±0.110 bB野杏叶片P.armeniaca leafs 4.777±0.092 aA滤纸屑Filter paper 1.380±0.173 dD微晶纤维素Cellulose particle 1.340±0.032 deD羧甲基纤维素CMC 0.613±0.015 fE棉花Cotton 1.097±0.035 eD果胶Pectin 1.883±0.041 cC
2.3 嗜果刀孢菌侵染野杏叶片后细胞壁降解酶的活性变化
2.3.1 纤维素酶活性变化 野杏叶片接种嗜果刀孢菌2 d 后出现黄褐色小圆斑,随后病斑不断扩展。野杏叶片接种病菌后Cx 酶活性在2~4 d 呈急剧上升、4~8 d 波动上升、8~16 d 急剧下降的趋势,酶活性在接种后的第4 天和第8 天出现峰值(49.031、51.626 U·mL-1),依次为对照组的1.313 倍和2.096 倍,酶活性在接种第14 天时急剧下降至40.465 U·mL-1。对照组Cx 酶活性呈现先上升再逐渐下降最后缓慢上升,且处理组Cx酶活性始终高于对照组酶活性(图2-A)。
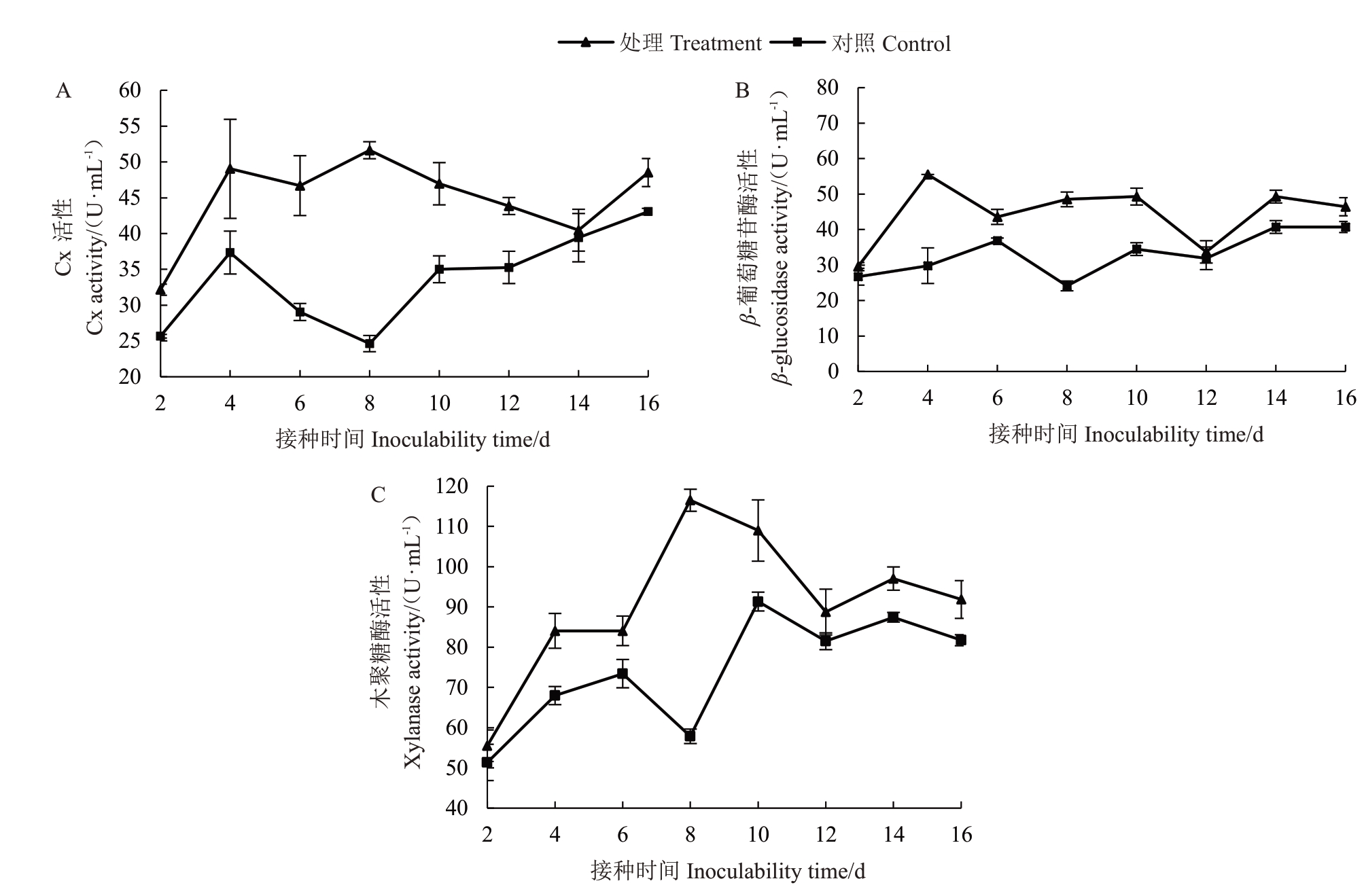
图2 嗜果刀孢菌侵染野杏叶片纤维素酶活性的变化
Fig.2 Changes of cellulase activity in the leaves of P.armeniaca infected by W.carpophilus
受嗜果刀孢菌侵染后野杏叶片内的β-葡萄糖苷酶活性在2~4 d 呈急剧升高、4~12 d 波动下降、12~16 d 缓慢上升的趋势,接种第4 天酶活性达55.519 U·mL-1为最高峰,为对照组的1.862倍,随后在接种第12 天酶活性急剧下降至33.717 U·mL-1。对照组β-葡萄糖苷酶活性随接种时间的增加呈缓慢波动上升的趋势(图2-B)。
木聚糖酶活性变化为2~8 d呈急剧升高、8~12 d后急剧下降、14~16 d波动下降的趋势,接种第8天达到高峰,酶活性为116.516 U·mL-1,为对照组的2.014倍,在接种第12天时酶活性急剧下降至88.743 U·mL-1,12 d后变化较平缓。对照组木聚糖酶活性呈先上升随后缓慢下降的趋势,且变化趋势没有处理组明显(图2-C)。
2.3.2 果胶酶活性变化 PG 酶活性在接种2~6 d短时间内急剧上升,随后在6~8 d 急剧下降,8~16 d 变化逐渐稳定,接种6 d 时达到高峰,酶活性为1 784.435 U·mL-1,为对照组的2.309 倍,随后在接种第8 天时酶活性急剧下降至632.776 U·mL-1,随后酶活性变化逐渐稳定。对照组PG 酶活性在接种后变化趋势平缓,PG 酶活性明显低于处理组(图3-A)。
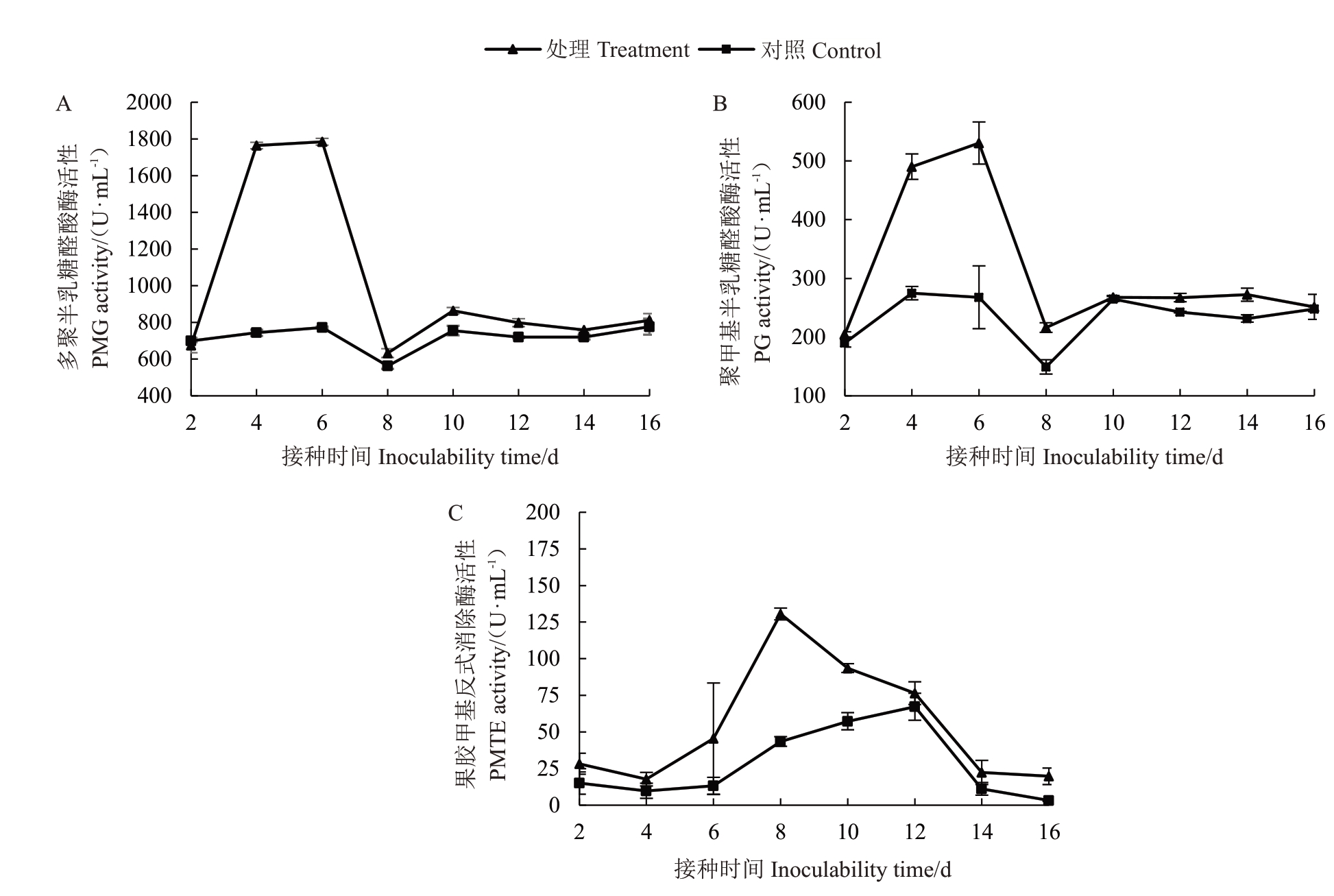
图3 嗜果刀孢菌侵染野杏叶片果胶酶活性的变化
Fig.3 Changes of pectinase activity in the leaves of P.armeniaca infected by W.carpophilus
PMG 酶活性在接种病菌后2~6 d 急剧上升,6~8 d急剧下降,8~16 d逐渐平稳,接种第6天时达到高峰,酶活性为530.511 U·mL-1,为对照组的1.981倍,在接种第8天时酶活性急剧下降至216.186 U·mL-1,随后酶活性变化稳定。对照组PMG 酶活性在接种后有缓慢上升的趋势,但其变化明显小于处理组(图3-B)。
PMTE酶活性在接种病菌后2~8 d急剧升高,8~16 d急剧下降。接种第8天时酶活性达130.549 U·mL-1为最高峰,为对照组的3.003 倍,随后酶活性急剧下降,16 d下降至19.724 U·mL-1。对照组PMTE酶活性在接种后呈缓慢上升的趋势,随后逐渐下降,其PMTE酶活性明显低于处理组(图3-C)。
2.4 嗜果刀孢菌侵染野杏后叶片内抗氧化酶的活性变化
野杏叶片受嗜果刀孢菌侵染后,POD活性随着侵染时间的增加呈上升趋势,在接种第5天时达到高峰,酶活性为323.889 U·mg-1·min-1,为对照组的5.949倍。对照组的POD活性随着时间的增加呈下降趋势(图4-A)。CAT活性动态变化呈1~5 d急剧上升、5~15 d波动下降的趋势,接种第5天后CAT活性达到高峰,为81.111 U·mg-1·min-1,为对照组的1.497倍,接种15 d后酶活性为72.778 U·mg-1·min-1。对照组的CAT 活性随着时间的增加呈缓慢上升趋势,但其酶活性变化明显小于处理组(图4-B)。SOD活性动态变化为1~3 d 呈缓慢下降、5~10 d 急剧上升、10~15 d 缓慢下降的趋势,接种第10 天时酶活性达最高峰,为228.675 U·mg-1·min-1,为对照组的1.85 倍。对照组的SOD 活性随病菌危害时间的增加呈下降趋势(图4-C)。
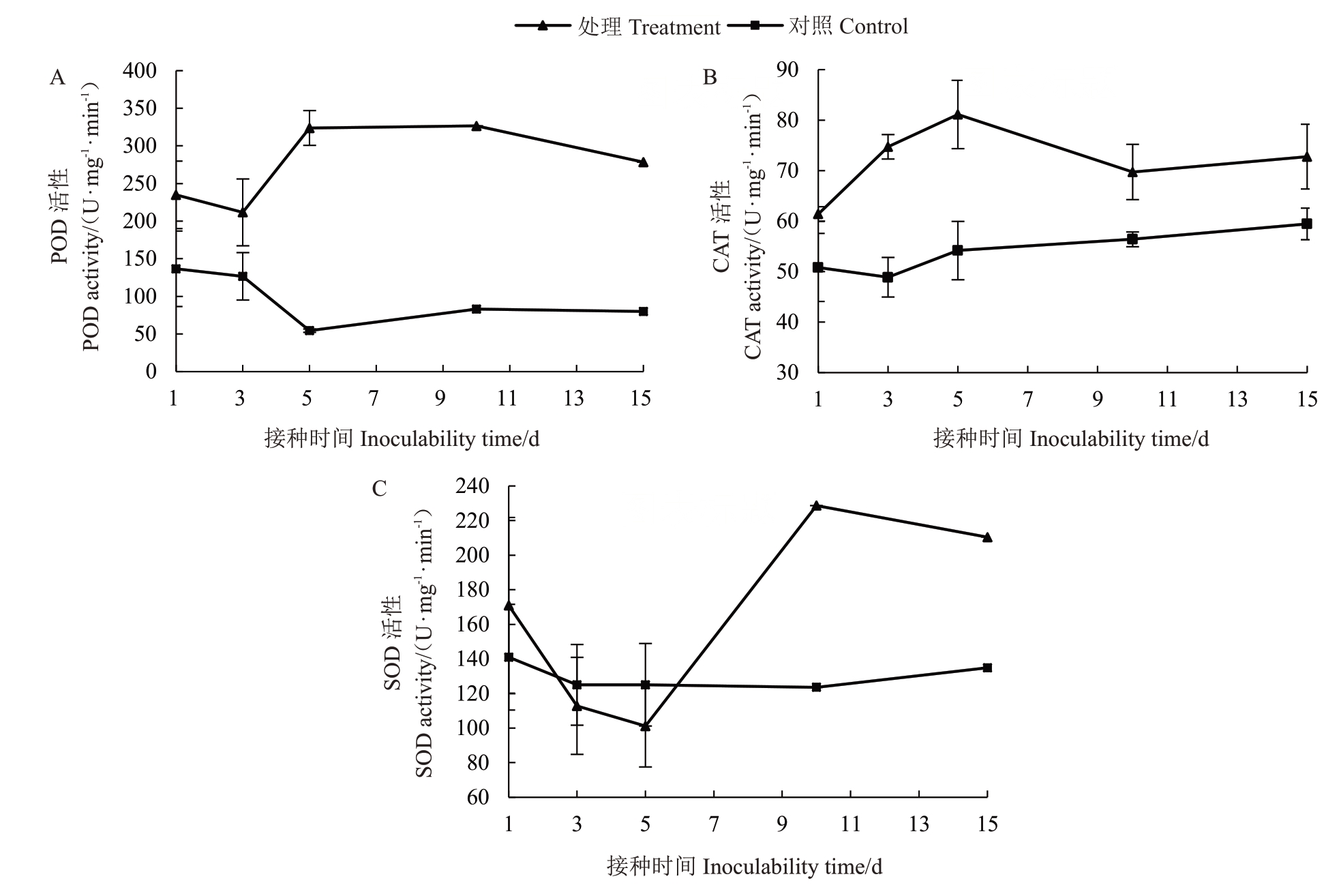
图4 嗜果刀孢菌侵染野杏后叶片内抗氧化酶的活性变化
Fig.4 Changes of antioxidant enzyme activities in leaves of P.armeniaca infected by W.carpophilus
3 讨 论
国内外学者研究发现细胞壁降解酶是病原菌致病过程中产生的关键酶。目前尚无嗜果刀孢菌致病过程中产生的酶的种类和活性的研究,笔者在本研究中初步明确嗜果刀孢菌产生的纤维素酶和果胶酶是引起穿孔病发生的重要致病因子。李宝聚等[19]发现黄瓜黑星病菌(Cladosporium cucumerinum)在不同底物改良的Czaper 液体培养基中和罹病黄瓜组织中均能产生羧甲基纤维素酶(Cx)及果胶甲基半乳糖醛酸酶(PMG)。陈晓林等[25]的研究得出苹果树腐烂病菌(Valsa ceratosperma)在培养基诱导和病组织侵染中均能产生β-葡萄糖苷酶、Cx、PG、PMG 和木聚糖酶,并且木聚糖酶活性高于其他种类。李沅泽等[26]测得苹果轮纹病菌(Botryosphaeria doyhide)在不同诱导培养基上均可产生PG、PMG、内切-4-1,4-葡聚糖酶、木葡聚糖酶、β-葡萄糖苷酶、木聚糖酶和滤纸酶(FPA),但是在发病苹果枝条中未检测到上述细胞壁降解酶。张大智等[27]测得杧果细菌性角斑病菌(Xanthomonas campestris pv. mangiferaeindicae)在羧甲基纤维素钠和柑橘果胶诱导培养基和感病杧果叶片中均可分泌Cx、β-glucosidase、PG、PMG、PMTE和PGTE 等细胞壁降解酶,且Cx 和PMG 的活性高于PMTE 和PGTE。金勤等[28]研究得出胶孢炭疽菌(Colletotrichum gloeosorioide)在 体 外产生 的PMG 活性远高于Cx 和漆酶。研究测得嗜果刀孢菌在寄主体内外均可产生Cx、木聚糖酶、PG、PMG、β-葡萄糖苷酶和PMTE 等6 种细胞壁降解酶,在寄主体内外酶活性最高的均为果胶酶中的PMG 和PG,纤维素酶活性最低,表明果胶酶在嗜果刀孢菌致病过程中起到了较为关键的作用。前人的研究表明不同种类的病菌分泌的细胞壁降解酶是不同的,在致病过程中起关键作用的降解酶也不同。通过将嗜果刀孢菌接种到野杏叶片上发现,在接种第4 天时PG 和PMG 的活性达到高峰,显著高于其他5 种细胞壁降解酶活性,木聚糖酶和PMTE 的活性在接种后8 d 达到高峰。表明果胶酶中的PMG 和PG 在嗜果刀孢菌致病过程中分泌最早且果胶酶比纤维素酶对野杏叶片致病作用明显。笔者研究发现野杏叶片接种病菌后细胞壁降解酶均表现出起伏变化,是否为野杏叶片的免疫对抗作用有待进一步研究。
在植物与病原物长期协同进化过程中,植物形成了一系列复杂的防御酶体系抵御病原物的入侵[29]。植物体内的POD、CAT、SOD等是防御酶系统中的关键保护酶,可清除植物细胞内产生的O2-.和H2O2 等活性氧物质,以此抵御病原菌入侵的危害[30]。金勤等[28]的研究得出油茶受到炭疽病菌的侵染后,植株体内的POD、PPO 等防御酶的活性显著升高。韩彦卿等[31]的研究证实白发病菌(Sclerospora graminicola)侵染抗病品种后CAT、POD、SOD 酶活性升高,且远远高于感病品种,相反在感病品种中这几种酶活性显著下降,表明POD等防御酶在抵御病原菌和保护植物细胞免受伤害过程中发挥重要抗病作用。笔者研究发现野杏叶片接种嗜果刀孢菌后,POD、CAT、SOD 活性均有所升高,野杏叶片的POD等抗氧化酶活性升高,表明野杏叶片在受到嗜果刀孢菌侵染后,会通过调节自身的抗氧化酶系统减轻植物体内活性氧产生的危害。
嗜果刀孢菌可危害杏(P. armeniaca)、扁桃(Amygdalus communis)、稠李(Padus avium)、西洋梨(Pyrus communis)、苹果(Malus domestica)和冬青栎(Quercus ilex)等多种寄主植物[32],其在不同寄主上产生的酶类及活性变化是否存在差异,以及嗜果刀孢菌在致病过程中是否会产生其他种细胞壁降解酶如漆酶和PGTE 等,还需进一步研究。植物病原菌侵入寄主是一个复杂的过程,除了能向外分泌细胞壁降解酶外,往往还可以产生毒素和激素等致病因子。由于病原菌致病过程中的机制是非常复杂的,关键致病因子与不同致病因子间如何协调作用等,有待深入研究。
4 结 论
笔者在本研究中首次发现嗜果刀孢菌在野杏叶片内外均可产生多聚半乳糖醛酸酶、羧甲基纤维素酶、β-葡萄糖苷酶、木聚糖酶、聚甲基半乳糖醛酸酶和果胶甲基反式消除酶等6 种细胞壁降解酶,在野杏叶片内外酶活性最高的均为果胶酶中的PMG 和PG,纤维素酶活性最低。嗜果刀孢菌细胞壁降解酶在野杏叶片致病过程中的动态变化表明果胶酶中的PMG 和PG 在嗜果刀孢菌致病过程中分泌最早,且果胶酶比纤维素酶对野杏叶片致病作用明显。野杏叶片受嗜果刀孢菌危害后,POD、CAT、SOD 等抗氧化酶活性升高。
[1] 刘黎明.新疆野杏生殖生态学初步研究[D].乌鲁木齐:新疆农业大学,2010.
LIU Liming.Primary research on reproductive ecology of Armeniaca vulgaris Lam.[D]. Urumqi:Xinjiang Agricultural University,2010.
[2] 何苏琴,白滨,文朝慧,荆卓琼.甘肃省桃果实褐斑病病原鉴定[J].植物保护,2016,42(5):53-57.
HE Suqin,BAI Bin,WEN Chaohui,JING Zhuoqiong. Identification of the pathogen of the brown spot of peach fruit in Gansu,China[J].Plant Protection,2016,42(5):53-57.
[3] BUBICI G,D’AMICO M,CIRULLI M.Field reactions of plum cultivars to the shot hole disease in southern Italy[J]. Crop Protection,2010,29(12):1396-1400.
[4] NARMANI A,TEPONNO R B,ARZANLOU M,BABAIAHARI A,STADLER M,New secondary metabolites produced by the phytopathogenic fungus Wilsonomyces carpophilus[J].Phytochemistry Letters,2018,26:212-217.
[5] SCHERM H,SAVELLE A T,BOOZER R T,FOSHEE W G.Seasonal dynamics of conidial production potential of Fusicladium carpophilum on twig lesions in southeastern peach orchards[J].Plant Disease,2008,92(1):47-50.
[6] AHMADPOUR A,GHOSTA Y,JAVAN-NIKKHAH M,FATAHI R,GHAZANGARI K. Isolation and pathogenicity tests of Iranian cultures of the shot hole pathogen of Prunus species,Wilsonomyces carpophilus[J]. Australasian Plant Disease Notes,2009,4(1):133-134.
[7] LALANCETTR N,MCFARLAND K A,BURNETT A L. Modeling sporulation of Fusicladium carpophilum on nectarine twig lesions:Relative humidity and temperature effects[J]. Phytopathology,2012,102(4):421-428.
[8] AHMADPOUR A,GDOSTA Y,JAVAN-NIKKHAH M,GHAZANGARI K,FATAHI R. Study on morphology,pathogenicity and genetic diversity of Wilsonomyces carpophilus isolates,the causal agent of shot hole of stone fruit trees based on RAPDPCR in Iran[J] Archives Of Phytopathology And Plant Protection,2012,45(17):2076-2086.
[9] TOVAR-PEDRAZA J M,AYALA-ESCOBAR V,SEGURA-LEON O L. Thyrostroma carpophilum causing apricot shot-hole in Mexico[J].Australasian Plant Disease Notes,2013,8(1):31-33.
[10] THOMIDIS T,EXADAKTYLOU E. Effect of a plastic rain shield on fruit cracking and cherry diseases in Greek orchards[J].Crop Protection,2013,52:125-129.
[11] NABI A,SHAH M U D,PADDER B A,DAR M S,AHMAD M.Morphocultural,pathological and molecular variability in Thyrostroma carpophilum causing shot hole of stone fruits in India[J].European Journal of Plant Pathology,2017,151(3):613-627.
[12] 刘胜毅,许泽永,何礼远.植物与病原菌互作和抗病性的分子机制[J].中国农业科学,1999,32(S1):94-102.
LIU Shengyi,XU Zeyong,HE Liyuan.Plantparasite interactions and molecular mechanism of resistance[J]. Scientia Agricultura Sinica,1999,32(S1):94-102.
[13] KUMARESAN V,GNANAM A J,PASUPULETI M,ARASU M V,AL-DHABI N A,HARIKRISHNAN R,ACROCKIARAJ J.Comparative analysis of Cs Cu/ZnSOD defense role by molecular characterization:Gene expression-enzyme activity-protein level[J].Gene,2015,564(1):53-62.
[14] MAKELA M R,DONOFRIO N,VRIES R P.Plant biomass degradation by fungi[J].Fungal Genetics and Biology,2014,72:2-9.
[15] ADASKAVEG J E. Conidial morphology,host colonization and development of shot hole of almond caused by Wilsonomyces carpophilus[J]. Canadian Journal of Botany,1995,73(3):432-444.
[16] CHIU C M,YOU B J,CHOU C M,YU P L,YU F Y,PAN S M,BOSTOCK R M,CHUNG K R,LEE M H.Redox status-mediated regulation of gene expression and virulence in the brown rot pathogen Monilinia fructicola[J]. Plant Pathology,2013,62(4):809-819.
[17] 王鹏程,郝海婷,王兰,凌新慧.枣黑斑病菌细胞壁降解酶活性测定及致病性分析[J].果树学报,2019,36(7):903-910.
WANG Pengcheng,HAO Haitin,WANG Lan,LING Xinhui.Analysis of cell wall degrading enzymes from jujube black spot pathogen and its pathogenicity[J]. Journal of Fruit Science,2019,36(7):903-910.
[18] YE S H,JIA H Y,CAI G F,TIAN C M,MA R. Morphology,DNA phylogeny,and pathogenicity of Wilsonomyces carpophilus isolate causing shot-hole disease of Prunus divaricata and Prunus armeniaca in Wild-Fruit Forest of Western Tianshan Mountains,China[J].Forests,2020,11(3):319.
[19] 李宝聚,周长力,赵奎华,李凤云,陈红漫.黄瓜黑星病菌致病机理的研究.Ⅱ.细胞壁降解酶及其在致病中的作用[J].植物病理学报,2000,30(1):13-18.
LI Baoju,ZHOU Changli,ZHAO Kuihua,LI Fengyun,CHEN Hongman. Pathogenic mechanism of scab of cucumber caused by Cladosporium cucumerinum.Ⅱ.The cell wall-degrading enzymes and ITS Pathogenic action[J].Acta Phytopathologica Sinica,2000,30(1):13-18.
[20] DOUAIHER M N,NOWAK E,DURAND R,HALAMA P,REIGNAULT P. Correlative analysis of Mycosphaerella graminicola pathogenicity and cell wall-degrading enzymes produced in vitro:The importance of xylanases and polygalacturonases[J].Plant Pathology,2007,56(1):79-86.
[21] SIAH A,DEWEER C,DUYME F,SANSSENE J,DURAND R,HALAMA P,REIGNAULT P. Correlation of in planta endobeta-1,4-xylanase activity with the necrotrophic phase of the hemibiotrophic fungus Mycosphaerella graminicola[J].Plant Pathology,2010,59(4):661-670.
[22] BEAUCHAMP C,FRIDOVICH I. Superoxide dismutase:improved assays and an assay applicable to acrylamide gels[J].Analytical Biochemistry,1971,44(1):276-287.
[23] El-MOSHATY F I B,PIKE S M,NOVACKY A J,SEHGAL O P.Lipid peroxidation and superoxide production in cowpea(Vigna unguiculata) leaves infected with tobacco ringspot virus or southern bean mosaic virus[J]. Physiological and Molecular Plant Pathology,1993,43(2):109-119.
[24] 高俊凤.植物生理学实验技术[M].西安:世界图书出版公司,2000:214-215.
GAO Junfeng. Experimental techniques of plant physiology[M].Xi’an:World Publishing Corporation,2000:214-215.
[25] 陈晓林,牛程旺,李保华,李桂舫,王彩霞.苹果树腐烂病菌产生细胞壁降解酶的种类及其活性分析[J].华北农学报,2012,27(2):207-212.
CHEN Xiaolin,NIU Chengwang,LI Baohua,LI Guifang,WANG Caixia. The kinds and activities of cell wall-degrading enzymes produced by Valsa ceratosperma[J].Acta Agriculturae Boreali-Sinica,2012,27(2):207-212.
[26] 李沅泽,李保华,王彩霞.苹果轮纹病菌产生细胞壁降解酶种类及其活性分析[J].青岛农业大学学报(自然科学版),2021,38(2):85-90.
LI Yuanze,LI Baohua,WANG Caixia. The varieties and activities of cell wall-degrading enzymes produced by Botryosphaeria dothidea form apple fruit[J]. Journal of Qingdao Agricultural University(Natural Science Edition),2021,38(2):85-90.
[27] 张大智,詹儒林,柳凤,李国平,赵艳龙,常金梅.杧果细菌性角斑病菌细胞壁降解酶的致病作用[J].果树学报,2016,33(5):585-593.
ZHANG Dazhi,ZHAN Rulin,LIU Feng,LI Guoping,ZHAO Yanlong,CHANG Jinmei.Pathogenic effect of cell wall degrading enzymes produced by pathogencausing mango bacterial leaf spot[J].Journal of Fruit Science,2016,33(5):585-593.
[28] 金勤,周国英,刘君昂,何苑皞.细胞壁降解酶在油茶炭疽病菌致病过程中的作用研究[J].植物保护,2017,43(3):97-102.
JIN Qin,ZHOU Guoying,LIU Junang,HE Yuanhao.The role of cell-degrading enzymes in the pathogenic process of Camellia oleifera diease caused by Colletotrichum gloeosporioides[J].Plant Protection,2017,43(3):97-102.
[29] 王晨芳,黄丽丽,张宏昌,韩青梅,朱琳,冯浩,康振生.小麦-条锈菌互作过程中活性氧及保护酶系的变化研究[J].植物病理学报,2009,39(1):52-60.
WANG Chenfang,HUANG Lili,ZHANG Hongchang,HAN Qingmei,ZHU Lin,FENG Hao,KANG Zhensheng. Changes of reactive oxygen species and protective enzymes in the interaction of wheat and Puccinia striiformis f.sp.tritici[J].Acta Phytopathologica Sinica,2009,39(1):52-60.
[30] 孙嘉曼,傅俊范,严雪瑞,周如军.相关防御酶对人参锈腐病诱导抗性的响应[J].植物病理学报,2012,42(4):396-403.
SUN Jiaman,FU Junfan,YAN Xuerui,ZHOU Rujun. Response of the defense enzymes to induced resistance of ginseng rusty root rot[J]. Acta Phytopathologica Sinica,2012,42(4):396-403.
[31] 韩彦卿,王鹤,王慧娜,程富英,田娜娜,闫鑫,韩渊怀.谷子抵御白发病菌侵染的生理生化及基因表达分析[J].植物病理学报,2020,50(6):657-665.
HAN Yanqing,WANG He,WANG Huina,CHENG Fuying,TIAN Nana,YAN Xin,HAN Yuanhuai.Physiological,biochenical and gene expression analysis of foxtail millet aganist infection of Sclerospora graminicola[J].Acta Phytopathologica Sinica,2020,50(6):657-665.
[32] 叶双华.伊犁野果林中Juglanconis 和Wilsonomyces 属引起的病害病原菌种类研究[D].乌鲁木齐:新疆农业大学,2020.
YE Shuanghua. Identification of Juglanconis and Wilsonomyces pathogens species in Ili Wild-Fruit Forest[D]. Urumqi:Xinjiang Agricultural University,2020.