苹果(Malus pumila Mill.)是属于蔷薇科苹果属的落叶乔木,是我国第一大水果[1]。在苹果栽培中,常通过施用大量的化肥以提高其生产力,这不仅无法保证苹果的产量和质量,还会造成严重的环境影响[2]。生物肥料是微生物的活细胞,能为植物提供营养、控制病害,同时还能改良土壤,是化肥的最佳替代品[3]。利用植物微生物开发生物菌肥可实现减肥增效的直观效果,从而促进苹果产业的可持续发展。
植物及植物微生物共同构成了一个协调互作的单元,称为植物全功能体[4]。在长期的互作过程中,与植物紧密结合的微生物与微生物之间很可能也会进化出群落水平的互作策略,使它们能够在植物全功能体内持续存在,并在植物生长过程中发挥至关重要的作用[5]。植物内生真菌是指其生活史中一定阶段或全部阶段生活于健康植物组织内部对植物无害的微生物。相比根际微生物(菌根真菌、共生菌或根围真菌),内生真菌在时间和空间上都存在优先权,它生活在植物组织内部可免受温度、渗透势和紫外线辐射等不利外部环境因素的影响[6-7]。其中,内生真菌为丝状生长,接触面积大,生物量大,可分解糖类、单宁等多种有机物,参与腐殖质的形成和分解、氨化作用和硝化作用等,因而对植物促生长、环境修复方面的效果优于内生细菌和内生放线菌[8-10]。由内生真菌制成的生物肥具有生物环境安全性和使土壤质量提高、保持生态平衡等特点,更适用于农业传统栽培系统[11]。
内生真菌对植物生长和发育的有益作用已有很好的证明,如内生真菌能产生生物控制剂、植物激素(吲哚乙酸、赤霉素、细胞分裂素等)、能产生次生代谢产物铁载体、水解酶等,在植物生长发育过程中发挥着重要的作用[12-13]。此外,内生真菌还能够改善土壤,并促进植物对营养元素的运输[7]。侯姣姣等[14]从古国槐叶片内分离筛选解磷内生真菌,回接后可侵染宿主无菌苗根部组织,建立共生关系,并影响宿主的叶绿素含量、可溶性蛋白质含量、相关防御酶活性等。Turbat 等[15]从苦参中分离出15 种内生真菌,筛选菌株的间接或直接促进植物生长能力,并进行体外评估。目前针对苹果内生真菌,大多学者以分离鉴定为基础,筛选苹果重要病害如苹果腐烂病[16-18]、炭疽病[19]、轮纹病[20]等的内生生防真菌。Liu等[21]也对苹果内生真菌的群落多样性进行研究,表明受组织类型、品种和地点的影响,内生真菌的种类不同。但对苹果内生真菌的促生功能菌株研究方面还属空白。笔者在本研究中从红富士苹果不同组织部位分离内生真菌,并研究其产IAA、铁载体、溶磷、解钾、固氮等促生功能,为苹果的可持续栽培提供试验基础。
1 材料和方法
1.1 材料
分离材料采自于西南林业大学树木园红富士苹果树(砧木为八棱海棠Malus robusta Rehder)的根、枝、叶,苹果树龄为15年。
供试培养基:PDA培养基、PDB培养基、孟加拉红培养基[22]、LB培养基、King’s B培养基、溶解无机磷(NPA)培养基、蒙金娜有机磷培养基、解钾培养基、MAS-CAS 琼脂培养基、阿须贝氏(Ashby)培养基[23-25]。
1.2 内生真菌的分离及鉴定
取红富士苹果树的根、枝、叶,用自来水冲洗干净,自然风干后剥离枝和根韧皮部,将根、枝、叶置于75%酒精中表面消毒30 s,5%的次氯酸钠溶液中浸泡(根4 min、枝3 min、叶2 min),最后无菌水中漂洗4 次,每次至少1 min。之后用灭菌滤纸吸干水分并剪成1 cm×1 cm 的小块,分别置于PDA、LB 和孟加拉红培养基,每皿放3 块,每处理3 个重复。取最后一次漂洗用的无菌水涂抹于PDA平板,对组织表面消毒效果进行验证。置于26 ℃培养箱中黑暗培养两周左右长出菌丝,挑取菌落边缘菌丝进行纯化,4 ℃低温斜面试管保存菌种。
内生真菌的鉴定:①形态学鉴定:内生真菌鉴定参考《真菌鉴定手册》[26]来进行初步的鉴定分析;②分子生物学鉴定:使用生工的Ezup柱式真菌基因组DNA抽提试剂盒,按照试剂盒步骤提取内生真菌菌株DNA,用 引 物ITS4(5’- TCCTCCGCTTATTGATATG-3’)和ITS5(5’-GGAAGTAAAAGTCGTAACAAGG-3’)PCR 扩 增 菌 株DNA 的ITS 序 列。PCR 反应体系(50 μL):DNA 2 μL、ITS4 和ITS5 各1.5 μL、Mixs 25 μL、ddH2O 20 μL。PCR 反应条件:95 ℃预变性3 min;95 ℃变性30 s;56 ℃退火40 s;72 ℃延伸1 min;共35 个循环;72 ℃终延伸10 min;4 ℃保温。PCR 产物电泳检测及测序:把提取的DNA 进行PCR 扩增,将扩增产物进行1%琼脂糖凝胶电泳检测,后将PCR产物送至擎科生物技术公司进行测序,将ITS 数据序列通过NCBI 数据库进行BLAST比对,确定菌株分类地位。
1.3 内生真菌促生功能筛选
1.3.1 分泌吲哚乙酸(IAA)能力测定 ①定性分析:采用Salkowski 比色法[27],将菌株分别接种到含诱导物色氨酸和不含色氨酸的King’s B 培养液中,于28 ℃摇床培养7 d进行液体发酵,设3次重复,取2 mL 发酵液,12 000 r·min-1离心15 min,取1 mL 上清液置于离心管中,加入等量氯化铁比色液,于暗处静置30 min,观察其颜色是否出现变化以及颜色的变化情况。
②定量分析:以标品IAA 配制0.5、1.0、5.0、10.0、15.0、20.0 mg·L-1的吲哚乙酸标准液,按上述显色方法,使用紫外分光光度计在530 nm处测定吸光度,绘制标准曲线,计算出标准曲线方程,将①中的显色菌株置于紫外分光光度计波长530 nm处测OD值,代入方程中计算出菌株产吲哚乙酸的量。
1.3.2 产铁载体能力测定 采用MAS-CAS 检测法[28],将待测菌株的菌丝接种于CAS 琼脂培养基上,在黑暗条件下28 ℃培养7 d。通过观察CAS 琼脂上真菌菌落周围的颜色变化来确定是否具有产载体能力,菌落在平板有橙黄色带包围的认为是产铁载体的菌株。以未接种的CAS 为对照,每株菌测3个重复。
1.3.3 溶磷解钾能力测定(1)溶磷。①定性检测:将待测菌株用打孔器(d=5 mm)截取菌饼接种至无机磷培养基(NPA)和蒙金娜有机磷培养基上,每个菌株设置3次重复,置于26 ℃恒温培养7 d。根据溶磷圈直径与菌落直径的比值(D/d)大小定性判断该菌株是否具有溶磷能力。
②定量测定:采用钼锑抗比色法[29]测定培养液中有效磷含量。用打孔器(d=5 mm)截取菌饼接种至蒙金娜液体培养基中,每个处理组设置3次重复,置于28 ℃恒温摇床、150 r·min-1振荡培养7 d,将培养液于10 000 r·min-1离心15 min,用紫外分光光度计在880 nm 处比色并测定OD 值,以此判断菌株对有机磷的溶解能力,绘制标准曲线,以标准曲线计算菌株有效磷含量。有效磷含量计算公式如下:
式中:P 为标准曲线中查的磷浓度;V 为显色时定容体积;Ts分取倍数;V0为测定发酵液的体积;AP为有效磷含量。
(2)解钾。将待测菌株接种至解钾培养基上,3次重复。置于恒温培养箱26 ℃培养7 d,观察菌落周边是否会形成透明圈,将透明圈作为参考指标筛选出具有解钾活性的菌株。
1.3.4 固氮能力测定 将活化好的待测菌株接种于阿须贝氏(Ashby)培养基上,置于恒温培养箱28 ℃培养7 d,连续继代培养3次,若菌株在Ashby培养基上正常生长,则表明该菌株具有固氮活性。
1.4 内生真菌对组培苗的促生作用
将苹果组培苗(红富士)培养7 d观察无污染后,用打孔器打取5 mm×5 mm 的菌饼接种于组培苗根部,每一株苗3块菌饼,设5个重复,以空白组培苗为对照。在组培瓶中培养50 d后取苹果组培苗根、茎、叶,用自来水冲洗干净,置于20% KOH 水溶液中90 ℃水浴10~30 min,蒸馏水冲洗数次后用5%醋酸进行酸化,再滴加入墨水染色5~10 min,用清水脱色后即可镜检。同时对苹果组培苗进行生长指标测定,用直尺测量组培苗的株高及根长;采用叶绿素测定仪测定组培苗叶绿素和叶总氮含量。
1.5 数据分析
采用Photoshop、Excel 软件对数据进行图表处理,采用SPSS 26.0软件对不同处理间的差异进行显著性分析。
2 结果与分析
2.1 内生真菌分离鉴定
对15 年生红富士苹果树的根、枝、叶,采用PDA、LB、孟加拉红3 种培养基进行内生真菌的分离,共分离获得87株内生真菌。通过形态学初步鉴定后选取51 株真菌进行分子生物学鉴定。将51 株真菌归类为1 门4 纲8 目16 科21 属29 种,比对相似度均在97%以上。其中菌株LJ03、LJ08与已知菌种的相似度低于90%,疑为新种(表1)。
表1 苹果内生真菌分子生物学鉴定
Table 1 Molecular biological identification of apple endophytic fungi
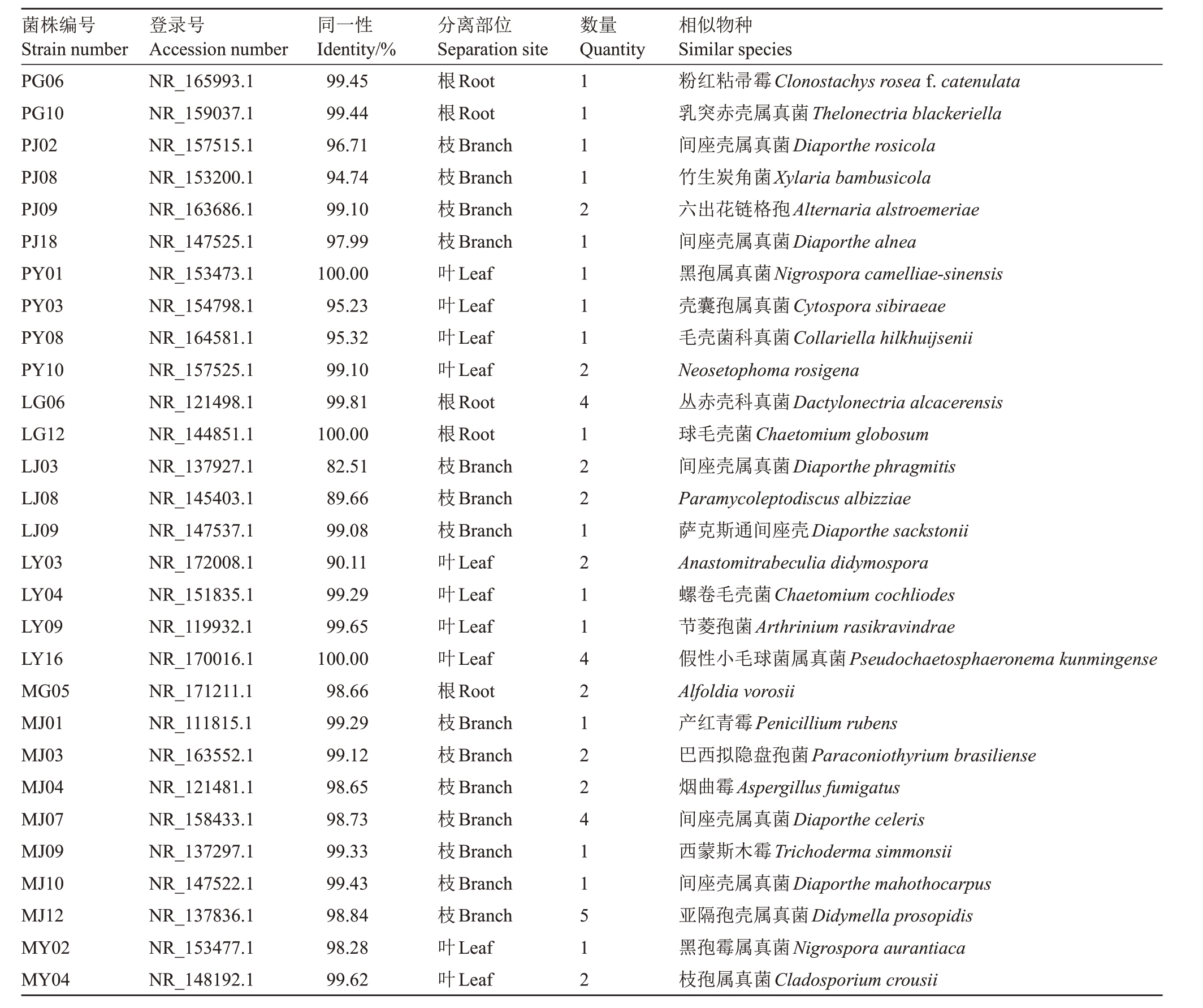
注:菌株编号首字母为培养基类型,P.PDA 培养基;L.LB 培养基;M.孟加拉红培养基。
Note:The initial letter of the strain number is the medium type,P.PDA medium;L.LB medium;M.Bengal red medium.
菌株编号Strain number PG06 PG10 PJ02 PJ08 PJ09 PJ18 PY01 PY03 PY08 PY10 LG06 LG12 LJ03 LJ08 LJ09 LY03 LY04 LY09 LY16 MG05 MJ01 MJ03 MJ04 MJ07 MJ09 MJ10 MJ12 MY02 MY04相似物种Similar species粉红粘帚霉Clonostachys rosea f.catenulata乳突赤壳属真菌Thelonectria blackeriella间座壳属真菌Diaporthe rosicola竹生炭角菌Xylaria bambusicola六出花链格孢Alternaria alstroemeriae间座壳属真菌Diaporthe alnea黑孢属真菌Nigrospora camelliae-sinensis壳囊孢属真菌Cytospora sibiraeae毛壳菌科真菌Collariella hilkhuijsenii Neosetophoma rosigena丛赤壳科真菌Dactylonectria alcacerensis球毛壳菌Chaetomium globosum间座壳属真菌Diaporthe phragmitis Paramycoleptodiscus albizziae萨克斯通间座壳Diaporthe sackstonii Anastomitrabeculia didymospora螺卷毛壳菌Chaetomium cochliodes节菱孢菌Arthrinium rasikravindrae假性小毛球菌属真菌Pseudochaetosphaeronema kunmingense Alfoldia vorosii产红青霉Penicillium rubens巴西拟隐盘孢菌Paraconiothyrium brasiliense烟曲霉Aspergillus fumigatus间座壳属真菌Diaporthe celeris西蒙斯木霉Trichoderma simmonsii间座壳属真菌Diaporthe mahothocarpus亚隔孢壳属真菌Didymella prosopidis黑孢霉属真菌Nigrospora aurantiaca枝孢属真菌Cladosporium crousii登录号Accession number NR_165993.1 NR_159037.1 NR_157515.1 NR_153200.1 NR_163686.1 NR_147525.1 NR_153473.1 NR_154798.1 NR_164581.1 NR_157525.1 NR_121498.1 NR_144851.1 NR_137927.1 NR_145403.1 NR_147537.1 NR_172008.1 NR_151835.1 NR_119932.1 NR_170016.1 NR_171211.1 NR_111815.1 NR_163552.1 NR_121481.1 NR_158433.1 NR_137297.1 NR_147522.1 NR_137836.1 NR_153477.1 NR_148192.1同一性Identity/%99.45 99.44 96.71 94.74 99.10 97.99 100.00 95.23 95.32 99.10 99.81 100.00 82.51 89.66 99.08 90.11 99.29 99.65 100.00 98.66 99.29 99.12 98.65 98.73 99.33 99.43 98.84 98.28 99.62分离部位Separation site根Root根Root枝Branch枝Branch枝Branch枝Branch叶Leaf叶Leaf叶Leaf叶Leaf根Root根Root枝Branch枝Branch枝Branch叶Leaf叶Leaf叶Leaf叶Leaf根Root枝Branch枝Branch枝Branch枝Branch枝Branch枝Branch枝Branch叶Leaf叶Leaf数量Quantity 11112111124122121142122411512
其中,PDA 培养基上共分离出13 株,鉴定为9属10 种,占分离鉴定的26%;LB 上共分离出16 株,鉴定为7 属9 种,占分离鉴定的31%;孟加拉红上共分离出22 株,鉴定为9 属10 种,占分离鉴定的43%。根部共分离出9 株,鉴定为5 属5 种,占分离鉴定的18%,其中Dactylonectria alcacerensis分离获得4 株,为优势种;枝部共分离出26 株,鉴定为9 属14 种,占分离鉴定的51%,其中Didymella prosopidis 分离获得5 株,为优势种;叶部共分离出16 株,鉴定为9 属10 种,占分离鉴定的31%,其中Pseudochaetosphaeronema kunmingense分离获得4株,为优势种。
分析鉴定结果表明,优势纲为粪壳菌纲(Sordariomycetes)占分离获得菌株种类数的62%,优势科为间座壳科(Diaporthaceae),占分离获得菌株种类数的21%,优势属为间座壳属(Diaporthe),占分离获得菌株种类数的21%,优势种为Didymella prosopi-dis,占分离获得菌株数的10%。其次为Dactylonectria alcacerensis、Pseudochaetosphaeronema kunmingense、Diaporthe celeris,占分离获得菌株总数的8%。
2.2 促生能力筛选
2.2.1 分泌IAA 菌株筛选及其能力测定 29 种内生真菌均表现出不同程度的产IAA 能力。复筛后根据IAA 标准曲线(y=0.015 5 x+0.025)计算产量,加色氨酸的菌株产IAA 分泌量高于不加色氨酸的菌株。含色氨酸的各菌株产IAA 的量在14.62~90.85 μg·mL-1范围内;不含色氨酸的各菌株产IAA的量(ρ,后同)在6.55~13.17 μg·mL-1范围内。
加色氨酸的菌株PG06分泌IAA能力最强,分泌量高达(90.85±2.77)μg·mL-1,与其他菌株存在显著性差异(p<0.05),其次是菌株PJ09,分泌量(26.55±3.26)μg·mL-1,菌株MJ10 分泌IAA 能力最弱,分泌量(14.62±1.69)μg·mL-1。不加色氨酸的菌株MJ09产IAA 分泌量最高,为(13.17±1.56)μg·mL-1,其次是菌株PJ09,分泌量(12.47±2.31)μg·mL-1。最低是菌株PY03,分泌量只有(6.55±0.04)μg·mL-1。其中,含色氨酸分泌量最高的菌株来源于根部,不含色氨酸分泌量最高的菌株来源于枝部。枝部菌株PJ09不论是在含色氨酸或不含色氨酸中都呈现了较高的分泌量(图1)。
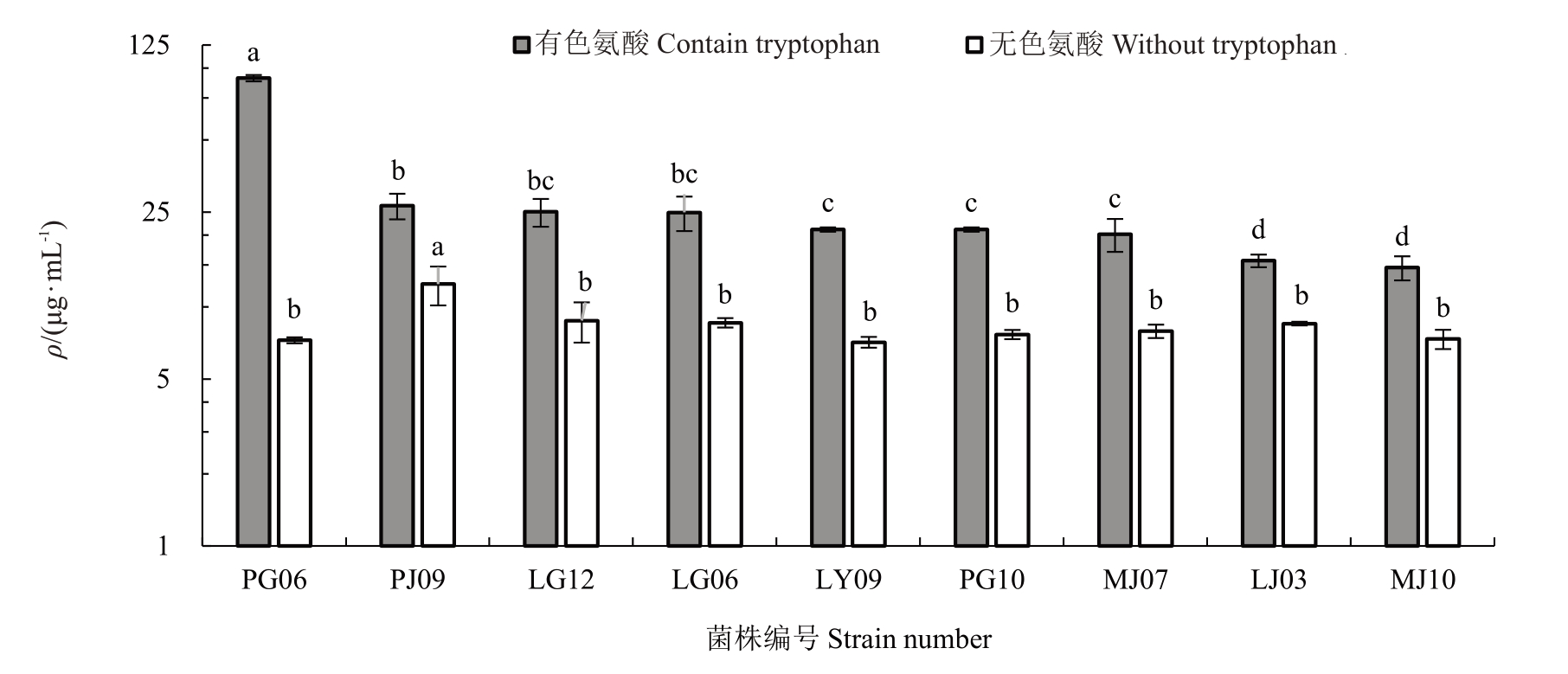
图1 部分内生真菌添加和不添加色氨酸诱导物的IAA 产量
Fig.1 IAA production of endophytic fungi with and without tryptophan inducers
不同小写字母表示在p<0.05 差异显著。下同。
Different small letters indicate significant difference at p<0.05.The same below.
2.2.2 产铁载体能力测定 将菌株接种于CAS 培养基上,培养后观察菌落在平板上是否有橙黄色带包围,有则认为菌株具产铁载体能力(图2)。29 株内生菌中10 株具有产铁载体的能力。其中,菌株PJ09、MJ10、MJ01、MJ03、MJ07、LJ03 来源于枝部,菌株LG06、LG12 来源于根部,菌株PY10、LY03 来源于叶部。
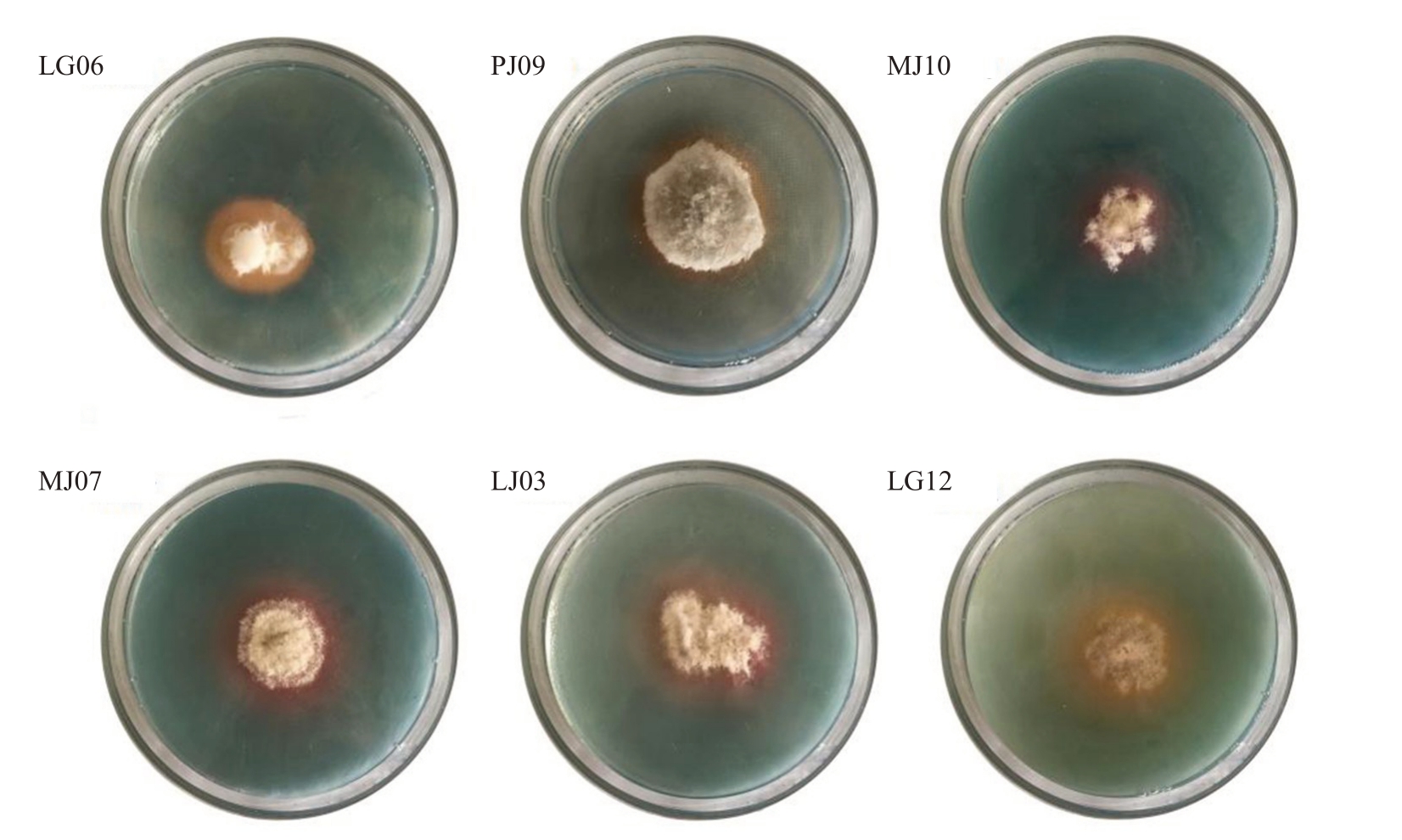
图2 部分内生真菌在CAS 平板上产铁载体能力检测
Fig.2 Producing siderophores capacity of some endophytic fungi on CAS plate
2.2.3 溶磷解钾能力测定 对内生真菌进行有机磷和无机磷的定性测定,没有检测到溶无机磷功能的菌株,其中有3 株内生真菌能在有机磷培养基上形成透明溶磷圈(图3),D/d 比值范围在1.21~1.44。由于D/d 值的大小与菌株自身代谢产物酶种类、酸种类及其释放快慢等相关,因此为了避免误差,透明圈法只能作为溶磷菌的初步筛选指标,菌株需进一步进行液体发酵复筛,才能准确掌握溶磷能力的大小。
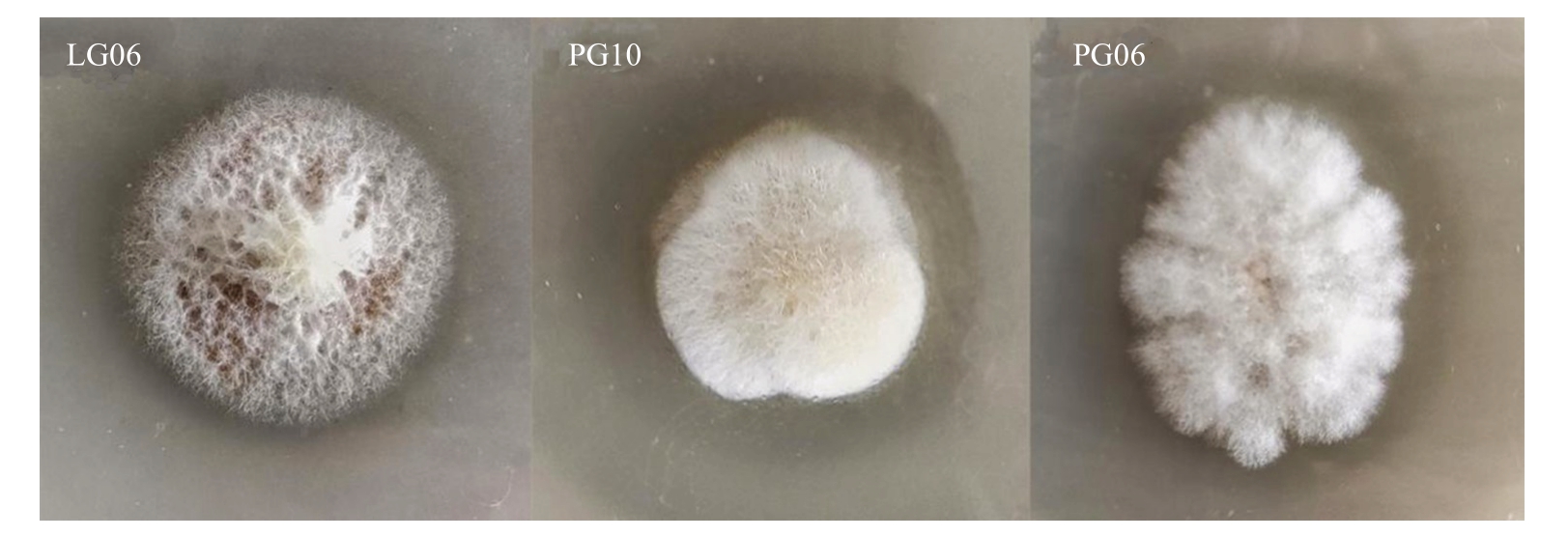
图3 内生真菌在孟金娜有机磷平板上溶磷能力检测
Fig.3 Phosphorus-solubilizing ability of endophytic fungi on Monkina organophosphorus plate
进一步用钼锑比色法判定菌株的解磷能力。根据公式计算得出菌株LG06 有效磷含量最高,达66.27 mg·mL-1,其次为PG10,磷含量20.01 mg·mL-1,菌株PG06含量最低只有3.30 mg·mL-1(表2)。依据有效磷含量可判断菌株LG06溶磷能力最强。
表2 内生真菌在蒙金娜平板上的菌落与溶磷圈直径比及溶磷含量测定
Table 2 The diameter ratio of endophytic fungi colony to the dissolved P circle and the dissolved P content on Monkinar plate
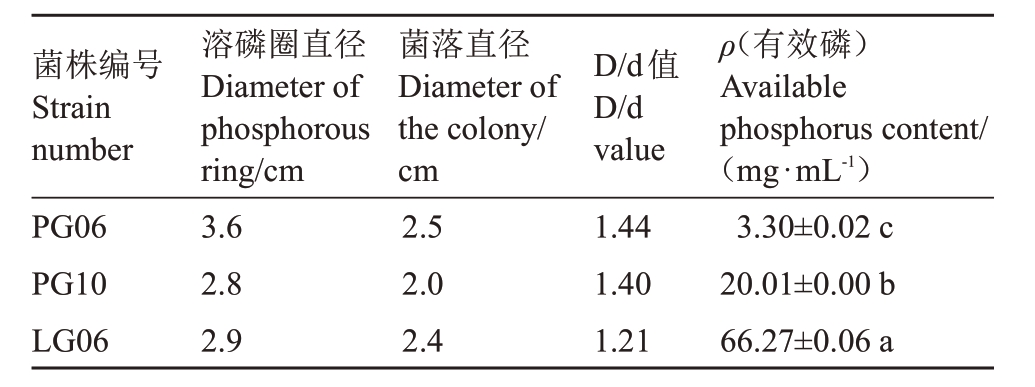
ρ(有效磷)Available phosphorus content/(mg·mL-1)3.30±0.02 c 20.01±0.00 b 66.27±0.06 a菌株编号Strain number PG06 PG10 LG06溶磷圈直径Diameter of phosphorous ring/cm 3.6 2.8 2.9菌落直径Diameter of the colony/cm 2.5 2.0 2.4 D/d值D/d value 1.44 1.40 1.21
在对29株内生真菌解钾功能检测中,并未检测到其解钾能力。
2.2.4 固氮能力测定 将29 株内生真菌接种于无氮Ashby 固体培养基上。有26 株内生真菌能正常生长且生长状况良好,表明这26株内生真菌具有固氮能力,其中PJ08 和PY03 在菌株边缘形成了透明圈,这表示菌株还可分解Ashby 培养基内的难溶化合物CaCO3(图4)。
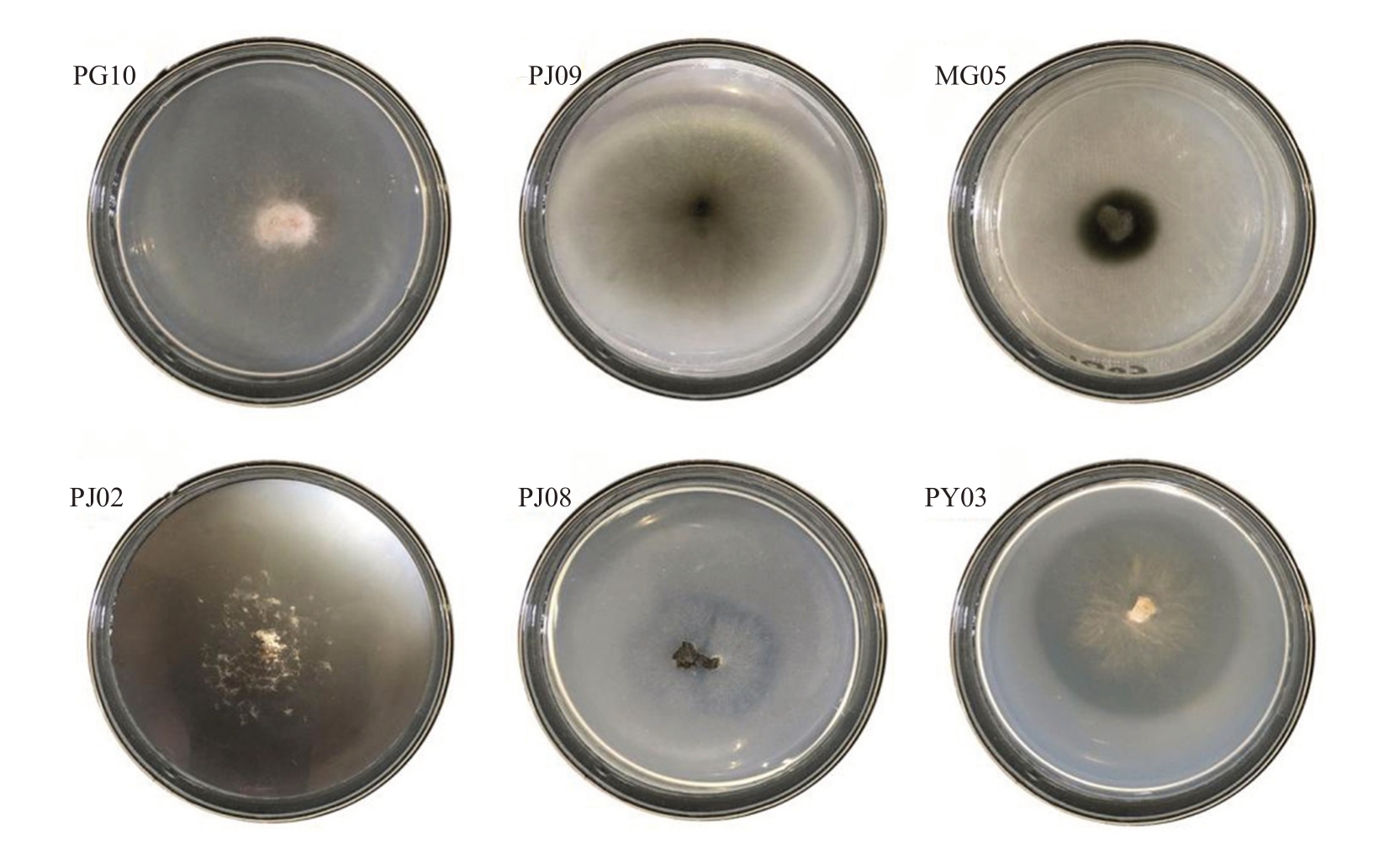
图4 部分内生真菌固氮菌株筛选
Fig.4 Screening of some endophytic fungi nitrogen-fixing strains
2.2.5 内生真菌对苹果组培苗的促生验证 依据IAA分泌量选取LG06、LG12、PG06、PJ09、LY09共5株内生真菌进行组培苗回接实验。在组培瓶里培养50 d,回接菌株PJ09的组培苗全部死亡,其余均可正常生长。其中,回接PG06 和LG12 菌株的组培苗成活率达100%,LG06 成活率为60%,LY09 成活率为40%。在定殖检测中,显微镜下可在苹果根的韧皮部观察到内生真菌,菌丝进入根表皮层,并沿纵轴延伸,具有可见的细胞间菌丝体形态,这表明菌株均在组培苗中定殖成功(图5)。
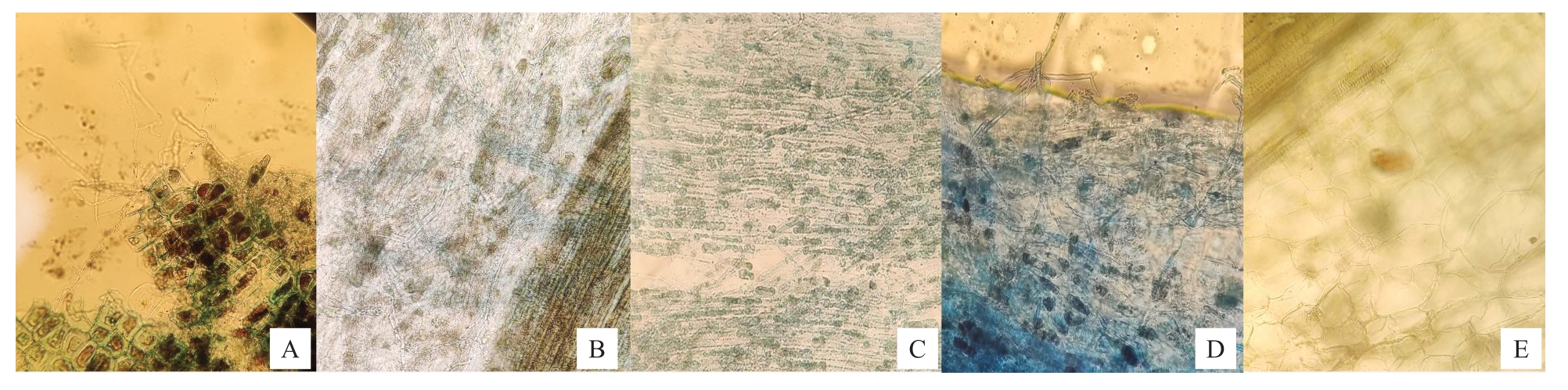
图5 内生真菌定殖情况显微观察
Fig.5 Microscopy of endophytic fungal colonization status
A 为PG06,在根部可见大量菌丝在组织断裂处延伸;B 为LY09,菌丝延伸于根部组织细胞中;C 为LG06,菌丝平行穿梭在根组织的皮层细胞中;D 为LG12,在枝部组织细胞中可见大量菌丝;E 为对照,在组织细胞中未见菌丝。
A is PG06,a large number of hyphae extending at the root at the tissue fracture;B is LY09,hyphae extending in the root tissue cells;C is LG06,hyphae shuttling parallel in the cortical cells of the root tissue;D is LG12,a large number of hyphae visible in the branch tissue cells;E is a control,and no hyphae in the tissue cells.
对回接处理的苹果组培苗进行生长指标的测定。结果表明与CK 相比,处理组叶片数量、叶绿素、叶片总氮含量存在显著差异(p<0.05),而株高和根长无显著性差异。处理菌株LY09 株高值最高,较对照提高了46.31%,LG06 根长值最大,较对照提高了67.77%。菌株PG06 叶片数量、叶绿素含量、叶片总氮含量较对照差异最为显著,依次增加了61.17%、44.08%、42.56%;其次为菌株LG12,叶绿素含量、叶片总氮含量较对照增加了42.92%、40.67%。这表明IAA 分泌量高的苹果促生内生真菌可以增加苹果的叶绿素含量,而叶绿素含量的增加会增强苹果的光合作用,进而促进组培苗的生长(图6、表3)。

图6 内生真菌对苹果组培苗的促生效果
Fig.6 Growth promoting results of endophytic fungi on apple tissue culture seedlings
表3 内生真菌对苹果组培苗的促生作用
Table 3 Growth promoting effect of endophytic fungi on apple tissue culture seedlings
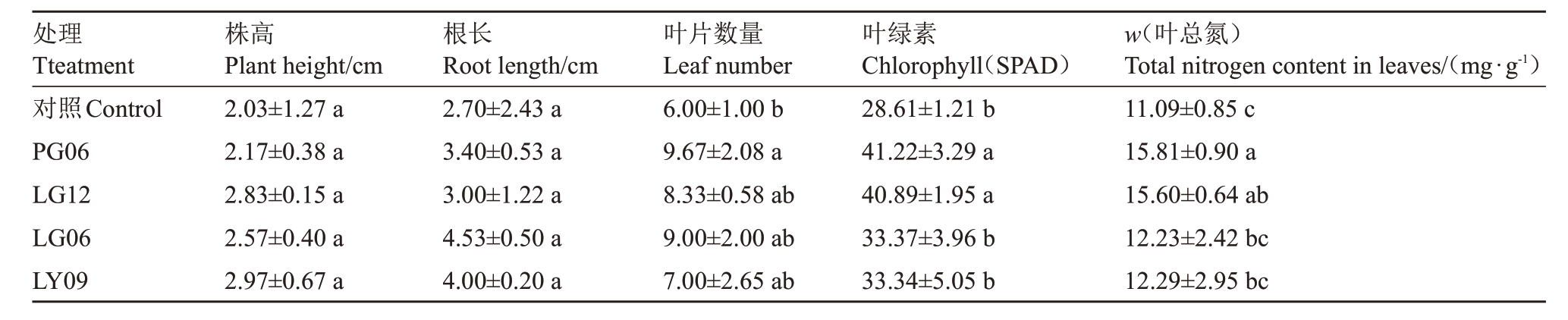
处理Tteatment对照Control PG06 LG12 LG06 LY09株高Plant height/cm 2.03±1.27 a 2.17±0.38 a 2.83±0.15 a 2.57±0.40 a 2.97±0.67 a根长Root length/cm 2.70±2.43 a 3.40±0.53 a 3.00±1.22 a 4.53±0.50 a 4.00±0.20 a叶片数量Leaf number 6.00±1.00 b 9.67±2.08 a 8.33±0.58 ab 9.00±2.00 ab 7.00±2.65 ab叶绿素Chlorophyll(SPAD)28.61±1.21 b 41.22±3.29 a 40.89±1.95 a 33.37±3.96 b 33.34±5.05 b w(叶总氮)Total nitrogen content in leaves/(mg·g-1)11.09±0.85 c 15.81±0.90 a 15.60±0.64 ab 12.23±2.42 bc 12.29±2.95 bc
3 讨 论
笔者在本研究中对苹果植株内生真菌进行促生功能研究,共分离获得内生真菌51株鉴定为29种。研究结果表明,孟加拉红培养基分离获得的苹果内生真菌种类多于PDA培养基,这与徐涛[30]的结果一致;不同组织部位分离的结果显示,苹果枝部获得内生真菌种类最多,王万周[31]也曾证明苹果枝部可培养内生真菌种类比根和叶部更丰富。本研究根部获得的优势属为丛赤壳科的Dactylonectria属,而邓振山等[19]从红富士根部分离得到的优势属为链格孢属(Alternaria),这可能与分离的季节、树龄或栽培区域有关。但徐涛[30]、李芳等[32]、王亚红等[33]从苹果枝条获得的枝孢属(Cladosporium)、间座壳属(Diaporthe)、黑孢属(Nigrospora)、毛壳菌属(Chaetomium)、节菱孢属(Arthrinium)等都与本文菌属一致,这表明相同的植物宿主中内生真菌的种类存在相似性。
IAA 是刺激细胞分裂、促进植物根系形成和增加生长量的植物激素。本次实验中筛选的内生真菌,含色氨酸时各菌株产IAA 的量在14.62~90.85 μg·mL-1;不含色氨酸时各菌株产IAA的量在6.55~13.17 μg·mL-1。在植物中,吲哚乙酸可由色氨酸前体(TRP)通过各种途径产生,多数研究者证明色氨酸介导下更适合IAA 的产生与合成[34-36]。Shah等[37]对铁皮石斛内生真菌研究表明所有菌株都能合成IAA,在添加色氨酸后菌株R10 产IAA 量接近60 μg·mL-1。在乔木中,闽楠内生真菌加色氨酸后IAA 含量最高达41.39 μg·mL-1 [27],杜仲内生真菌IAA 含量最高达38 mg·mL-1 [38],本研究苹果内生真菌加色氨酸后IAA 分泌量最高达90.85 μg·mL-1,处于相对较高的水平。
铁载体是可以螯合土壤中游离铁的低分子量化合物,是微生物代谢的重要分子,可以作为各种酶的辅助因子,抑制病原微生物的生长,促进植物生长。本研究中筛选出具有产生铁载体能力的内生真菌10 株,其中间座壳属(Diaporthe)有3 株都具有产铁能力,菊芋内生真菌的间座壳属菌株也表明具有产铁载体能力[12]。菌株PJ09为链格孢属(Alternaria),Turbat等[15]从苦参内生真菌中也筛选到链格孢属菌株具有产铁能力。菌株MJ01 为青霉属(Penicillium),冯美茹[23]在百花曼陀内生真菌中筛选到青霉属具有产铁能力。另5 株分别为LG06 Dactylonectria alcacerensis、LG12 球毛壳菌(Chaetomium globosum)、PY10 Neosetophoma rosigena、LY03 Anastomitrabeculia didymospora、MJ03 巴西拟隐盘孢菌(Paraconiothyrium brasiliense),尚没有关于其产铁载体能力的报道。
本次实验中并未筛选到溶解无机磷能力的菌株,但从根部筛选到3 株具有溶解有机磷能力的菌株。其中LG06 Dactylonectria alcacerensis 解有机磷能力达66.27 mg·mL-1,其次为PG10 Thelonectria blackeriella达20.01 mg·mL-1。马韦韦[39]对野生延胡索内生真菌进行解有机磷能力筛选,发现其内生真菌简青霉(Penicillium asturianum)和米曲霉(Aspergillus oryzae)有解有机磷能力。徐润冰[40]发现植物内生真菌H88-2c(Argyranthemum apiacea)具有解有机磷能力,且高达134.40 mg·L-1,本次筛选出的菌株与其相比解有机磷能力较低。
植物内生固氮菌是指定殖在植物内部与宿主联合固氮的菌,是植物内生菌中最受关注的类群之一[41]。对于内生固氮菌种类的研究目前主要集中于细菌,内生真菌的固氮能力还有待探索。冯美茹[23]对百花曼陀罗内生真菌的固氮研究发现,其分离的全部菌株都可在阿须贝氏培养基上正常生长。在本研究中共有29株内生真菌,有26株可在无氮培养基上正常生长。可见植物内生真菌也是开发固氮内生菌不容忽视的重要菌源。
为了进一步探究苹果内生真菌与苗木间的互利共生关系,选取IAA 含量高、兼具促生功能多的菌株回接至苹果组培苗,观察其对组培苗的生长影响。结果表明除PJ09 菌株外其余4 株内生真菌对苹果组培苗的促生效果明显。其中,菌株LG06 分离率达21%,是较有优势的苹果内生真菌,同时兼具有产IAA、铁载体、固氮和溶解有机磷能力,显示出了良好的促生潜力,回接苹果组培苗后也表现出明显的生长优势。菌株PG06 为粉红粘帚霉(Clonostachys rosea f. catenulata),分泌IAA 能力最强,分泌量达(90.85±2.77)μg·mL-1,同时兼具2 种促生特性。粉红粘帚霉作为一种重要的植物内生真菌,因其具有强大的破坏力,成为很多植物病原真菌的克星,如葡萄孢菌、草莓灰霉菌等,是目前极具潜力的生防菌之一[42]。菌株LG12 为球毛壳菌(Chaetomium globosum),可产IAA、铁载体和固氮。该属广泛分布于空气、土壤等多种自然环境中,也是植物最常见的内生真菌之一。球毛壳菌能产生种类繁多的次级代谢产物,且其次级代谢产物具有抗真菌和杀线虫等多种生物活性,对植物病原真菌和根结线虫(Meloidogyne spp.)有较大的生物防治潜力[43]。
以上菌株丰富了苹果内生促生菌资源,为开发苹果内生真菌生物菌肥提供了理论基础与科学依据。以上菌株的内生真菌的次级代谢产物、多样性及生物防治功能筛选等,将是未来进一步研究的重点。
4 结 论
从苹果植株根、枝、叶分离获得内生真菌51 株鉴定为29种。对促生潜力筛选发现,29株菌均有不同程度的产IAA能力,10株具有产铁载体功能,3株具有解有机磷能力,26 株具有固氮能力,29 株均不具有解钾能力。选取IAA含量高、兼具促生功能多的5 株菌回接苹果组培苗,结果表明,内生真菌PG06、LG12、LG06、LY09对组培苗均具有显著促生作用。
致谢:感谢中国农业科学院果树研究所董雅凤老师为本试验提供苹果组培苗。
[1] 崔挺.中国苹果产业国际竞争力研究[D].杨凌:西北农林科技大学,2008.
CUI Ting. Research on international competitiveness of chinese apple industry[D]. Yangling:Northwest Agriculture & Forestry University,2008.
[2] 杨建彬.苹果树施化肥过多有“五害”[J].果树实用技术与信息,2020(1):16-17.
YANG Jianbin. Apple trees Suffer from“Five Hazards”caused by excessive fertilizer[J].Fruit Tree Practical Technology and Information,2020(1):16-17.
[3] KOUR D,RANA K L,YADAV A N,YADAV N,KUMARC M,KUMAR V,VYAS P,DHALIWAL H S,SAXENAE A K. Microbial biofertilizers:Bioresources and ecofriendly technologies for agricultural and environmental sustainability[J]. Biocatalysis and Agricultural Biotechnology,2020,23:101487.
[4] LIU J,RIDGWAY H J,JONES E E.Apple endophyte community is shaped by tissue type,cultivarand site and has members with biocontrol potential against neonectria ditissima[J]. Journal of Applied Microbiology,2020,128(6):1735-1753.
[5] MIDDLETON H,YERGEAU É,MONARD C,COMBIER P J,EL AMRANI A. Rhizospheric plant-microbe interactions:miRNAs as a key mediator[J].Trends in Plant Science,2021,26(2):132-141.
[6] 钟睿. 醉马草及其根部真菌对甘肃内生真菌(Epichloë gansuensis)的响应[D].兰州:兰州大学,2021.
ZHONG Rui. Responses of achnatherum inebrians and root-associated fungi to Epichloë gansuensis endophyte[D]. Lanzhou:Lanzhou University,2021.
[7] RANA K L,KOUR D,KAUR T,DEVI R,YADAV A N,YADAV N,DHALIWAL H S,SAXENA A K. Endophytic microbes:biodiversity,plant growth-promoting mechanisms and potential applications for agricultural sustainability[J]. Antonie Van Leeuwenhoek,2020,113(8):1075-1107.
[8] MONDAL S,HALDER S K,YADAV A N,MONDAL K C.Microbial consortium with multifunctiona plant growth-promoting attributes:future perspective in agriculture[M]. Singapore:Springer Singapore,2020:219-258.
[9] 曹苗文.铜尾矿库白羊草内生真菌、根域及非根域土壤微生物多样性格局[D].太原:山西大学,2018.
CAO Miaowen. Diversity patterns of microbial communities of endophyte,root zone of bothriochloa ischaemum,and soils on dam of copper mine tailings[D]. Taiyuan:Shanxi University,2018.
[10] 庞师婵.伴生栽培对番茄根际土壤及根系内生微生物多样性的影响[D].南宁:广西大学,2021.
PANG Shichan. Effects of associated cultivation on soilmicrobial diversity in rhizosphere and endophytic microbial diversity in root of tomatoes[D].Nanning:Guangxi University,2021.
[11] BARON N C,RIGOBELO E C. Endophytic fungi:A tool for plant growth promotion and sustainable agriculture[J]. Mycology,2022,13(1):39-55.
[12] SUEBRASRI T,HARADA H,JOGLOY S,EKPRASERT J,BOONLUE S. Auxin- producing fungalendophytes promote growth of sunchoke[J].Rhizosphere,2020,16:100271.
[13] ZHENG R H,LI S J,ZHANG X,ZHAO C Q.Biological activities of some new secondary metabolites isolated from endophytic fungi:A review study[J]. International Journal of Molecular Sciences,2021,22(2):959.
[14] 侯姣姣,布芳芳,余仲东,康永祥.古国槐叶片溶磷内生真菌的筛选及其促生潜力初探[J].西北植物学报,2016,36(7):1456-1463.
HOU Jiaojiao,BU Fangfang,YU Zhongdong,KANG Yongxiang.Screening of phosphate-solubilizing endophytic fungi from ancient Sophora japonica leaves and their potential for plant growth-promoting[J]. Acta Botanica Boreali-Occidentalia Sinica,2016,36(7):1456-1463.
[15] TURBAT A,RAKK D,VIGNESHWARI A,KOCSUBEÉ S,THU H,SZEPESI Á,BAKACSY L,ŠKRBIĆ B D,JIGJIDDORJ E A,VÁGVӦLGYI C. Characterization of the plant growth-promoting activities of endophytic fungi isolated from Sophora flavescens[J].Microorganisms,2020,8(5):683.
[16] 薛应钰,李兴昱,李发康,苟攀宁,李龙,徐秉良.苹果树腐烂病生防真菌Z-12A 的鉴定及其生防效果[J]. 微生物学通报,2021,48(1):57-69.
XUE Yingyu,LI Xingyu,LI Fakang,GOU Panning,LI Long,XU Bingliang. Identification of Z-12A fungus for biocontrol of apple tree canker[J]. Chinese Journal of Microbiology,2021,48(1):57-69.
[17] 袁雪,王珊珊,赵晓萌,杨佳玥,胡海文,张国庆.苹果腐烂病病原菌的鉴定及拮抗菌筛选[J].中国农学通报,2017,33(1):33-37.
YUAN Xue,WANG Shanshan,ZHAO Xiaomeng,YANG Jiayue,HU Haiwen,ZHANG Guoqing. Identification and antagonistic studies on a pathogenic fungus causing apple Valsa canker[J].Chinese Agricultural Science Bulletin,2017,33(1):33-37.
[18] 赵倩.苹果树皮内生链格孢的种类鉴定及其对苹果树腐烂病的生防潜力[D].保定:河北农业大学,2014.
ZHAO Qian. Identification and antagonistic studies on a pathogenic fungus causing apple Valsa canker[D]. Baoding:Agricultural University of Hebei,2014.
[19] 邓振山,陈苗,张伟民,贺晓龙.苹果树内生真菌抗腐烂病和炭疽病活性菌株的筛选[J].微生物学杂志,2015,35(5):61-66.
DENG Zhenshan,CHEN Miao,ZHANG Weimin,HE Xiaolong.Screening of endophytic fungus from apple tree with anti-Valsa ceratosperma and Anti-Glomerella cingulata activities[J]. Journal of Microbiology,2015,35(5):61-66.
[20] 侯晓杰,梁魁景,丛日征,芦站根.贮藏期苹果轮纹病拮抗菌的筛选研究[J].北方园艺,2015(23):115-119.
HOU Xiaojie,LIANG Kuijing,CONG Rizheng,LU Zhangen.Screening of antagonistic bacteria for apple rutta during storage[J].Northern Horticulture,2015(23):115-119.
[21] LIU J,ABDELFATTAH A,NORELLI J,BURCHARD E,SCHENA L,DROBY S,WISNIEWSKI M. Apple endophytic microbiota of different rootstock/scion combinations suggests a genotype-specific influence[J].Microbiome,2018,6(18):1-11.
[22] 杨涛,赵疆,魏亚琴,方彦昊,王治业,李鑫,杨晖.华重楼内生真菌聚多曲霉的促生与拮抗作用[J].微生物学通报,2021,48(8):2665-2680.
YANG Tao,ZHAO Jiang,WEI Yaqin,FANG Yanhao,WANG Zhiye,LI Xin,YANG Hui. Growth promotion and antagonistic effects of endophytic fungus Aspergillus sydowii from Paris polyphylla var. chinensin[J]. Chinese Journal of Microbiology,2021,48(8):2665-2680.
[23] 冯美茹. 白花曼陀罗内生真菌多样性及抗菌与促生特性研究[D].广州:广东药科大学,2021.
FENG Meiru. Diversity,antimicrobial activities and the growth promoting characteristics of endophytic fungi from Datura metel L.[D]. Guangzhou:Guangdong Pharmaceutical University,2021.
[24] 罗阳兰.蕙兰内生真菌多样性及其促生能力的研究[D].汉中:陕西理工大学,2019.
LUO Yanglan. Diversity and growth-promoting ability of endophytic fungi in cymbidium faberi[D]. Hanzhong:Shaanxi University of Technology,2019.
[25] 燕红,于彩莲,包鑫,乔晓飞.高效解磷兼解钾活性菌株分离筛选的初步研究[J]. 扬州大学学报(农业与生命科学版),2016,37(1):81-85.
YAN Hong,YU Cailian,BAO Xin,QIAO Xiaofei. Preliminary study on isolation and screening of highly-effective strain in decomposing phosphate and potassium[J]. Journal of Yangzhou University (Agriculture & Life Science Edition),2016,37(1):81-85.
[26] 魏景超.真菌鉴定手册[M].上海:上海科学技术出版社,1979.
WEI Jingchao. Fungal identification manual[M]. Shanghai:Shanghai Science and Technology Press,1979.
[27] 姚英.闽楠内生真菌多样性及其促生作用机制研究[D].贵阳:贵州大学,2020.
YAO Ying. Study on the diversity of endophytic fungi and its promoting mechanism in Phoebe bournei[D]. Guiyang:Guizhou University,2020.
[28] SARBADHIKARY S B,MANDAL N C. Elevation of plant growth parameters in two solanaceous crops with the application of endophytic fungus[J]. Indian Journal of Agricultural Research,2018,52(4):424-428.
[29] 刘娜.解磷解钾促生微生物肥料用菌株的分离[D].沈阳:沈阳农业大学,2020.
LIU Na. The separation of microbial fertilizer strains was promoted by the hydrolysis of phosphorus and potassium[D].Shenyang:Shenyang Agricultural University,2020.
[30] 徐涛.苹果树皮内生真菌的分离及其对腐烂病的生防作用[D].保定:河北农业大学,2012.
XU Tao. Isolation of endophytic fungi from apple bark and their biological control effect on Valsa canker[D]. Baoding:Agricultural University of Hebei,2012.
[31] 王万周.苹果树主要病害内生拮抗真菌的筛选及拮抗特性[J].农业与技术,2018,38(18):68.
WANG Wanzhou. Screening and antagonistic characteristics of endophytic fungi against apple tree diseases[J]. Agriculture &Technology,2018,38(18):68.
[32] 李芳,邹妍,刘彬,张云秀,龙鸿,阎国荣.新疆野苹果(Malus sieversii)内生菌的分离与鉴定[J].天津农学院学报,2015,22(4):1-5.
LI Fang,ZOU Yan,LIU Bin,ZHANG Yunxiu,LONG Hong,YAN Guorong. Isolation and identification of endophytic fungi in Malus sieversii[J]. Journal of Tianjin Agricultural College,2015,22(4):1-5.
[33] 王亚红,李永丽,常乐,余海如,周洲,曲良建.对三种苹果病原菌具抑制作用的新疆野苹果内生真菌的分离与鉴定[J].果树学报,2020,37(3):390-396.
WANG Yahong,LI Yongli,CHANG Le,YU Hairu,ZHOU Zhou,QU Liangjian. Isolation and identification of endophytic fungi resistant to three apple pathogens from the branches of Malus sieversii[J]. Journal of Fruit Science,2020,37(3):390-396.
[34] MEHMOOD A,HUSSAIN A,IRSHAD M,HAMAYUN M,IQBAL A,KHAN N. In vitro production of IAA by endophytic fungus Aspergillus awamori and its growth promoting activities in Zea mays[J].Symbiosis,2019,77(3):225-235.
[35] MOHAMED A,KHALIL A M A,HASSAN E D,ALSHARIF S M,EID A M,EWAIS E D,AZAB E,GOBOURI A A,ELKELISH A,FOUDA A.Isolation and characterization of fungal endophytes isolated from medicinal plant Ephedra pachyclada as plant growth-promoting[J].Biomolecules,2021,11(2):140.
[36] ZAKHAROVA E A,SHCHERBAKOV A A,BRUDNIK V V,SKRIPKO N G,BULKHIN N S,IGNATOV V V. Biosynthesis of indole-3-acetic acid in Azospirillum brasilense insights from quantum chemistry[J].European Journal of Biochemistry,1999,259(3):572.
[37] SHAH S,SHRESTHA R,MAHARJAN S,SELOSSE M A,PANT B. Isolation and characterizationof plant growth-promoting endophytic fungi from the roots of dendrobium moniliforme[J].Plants,2018,8(1):5.
[38] 何凤,吕庚鑫,孟益德,庆军,杜庆鑫,杜兰英,杜红岩,王璐,刘攀峰.干旱胁迫及复水对杜仲苗激素含量的影响[J].植物生理学报,2021,57(12):2279-2290.
HE Feng,LÜ Gengxin,MENG Yide,QING Jun,DU Qingxin,DU Lanying,DU Hongyan,WANG Lu,LIU Panfeng.Effects of drought stress and rehydration on hormone contents of Eucommia ulmoides seedling[J]. Plant Physiology Journal,2021,57(12):2279-2290.
[39] 马韦韦.延胡索内生真菌的分离鉴定及产功能酶、解磷菌株初探[D].汉中:陕西理工学院,2016.
MA Weiwei. Isolation and identification of endophytic fungi from medical plants Corydalis yanhusuo,preliminary exploration on endophyte fungi with functional enzymes activity and phosphate dissolving ability[D]. Hanzhong:Shaanxi University of Technology,2016.
[40] 徐润冰.深色有隔内生真菌嗜鱼外瓶霉(Exophiala pisciphila)促进玉米磷吸收的机制研究[D].昆明:云南大学,2017.
XU Runbing. The mechanisms of maiza phosphorus absorption enhanced by a dark septate endophyte (Exophiala pisciphila)colonization[D].Kunming:Yunnan University,2017.
[41] 金玲月,路晶晶,冯燕辉,张沁怡,吴寒,李容丹,黄薇,蓝灿华,田宝玉.患根结线虫病番茄根内生固氮菌的筛选、鉴定和系统发育分析[J].福建农业科技,2020(12):46-51.
JIN Lingyue,LU Jingjing,FENG Yanhui,ZHANG Qinyi,WU Han,LI Rongdan,HUANG Wei,LAN Canhua,TIAN Baoyu.Screening,identification and phylogenetic analysis of endophytic nitrogen-fixing bacteria in tomato roots infected with rootknot nematode[J]. Fujian Agricultural Science and Technology,2020(12):46-51.
[42] 舒雪纯,张影波,官玲亮,陈振夏,黄梅,陈晓鹭,袁媛,元超.艾纳香内生真菌粉红粘帚霉抗菌次生代谢产物[J].生物工程学报,2020,36(8):1650-1658.
SHU Xuechun,ZHANG Yingbo,GUAN Lingliang,CHEN Zhenxia,HUANG Mei,CHEN Xiaolu,YUAN Yuan,YUAN Chao. Antibacterial secondary metabolites of Clonostachys rosea,an endophytic fungus from Blumea balsamifera (L.) DC[J].Chinese Journal of Bioengineering,2020,36(8):1650-1658.
[43] 廖宏娟,张志斌,江玉梅,朱笃.球毛壳菌对植物病原真菌和根结线虫的生物防治潜力[J]. 天然产物研究与开发,2022,34(6):1-24.
LIAO Hongjuan,ZHANG Zhibin,JIANG Yumei,ZHU Du.Biocontrol potential of Chaetomium globosum against plant pathogenic fungi and root-knot nematodes:A review[J].Natural Products Research and Development,2022,34(6):1-24.