温度是植物生长发育过程中重要的环境因子之一。近年来,人类活动排放的CO2等气体增加了温室气体浓度,全球温室效应致使气温逐年上升。据联合国政府间气候变化专门委员会(Intergovernmental Panel on Climate Change,IPCC)报告显示,全球平均气温每10 a(年)将上升0.3 ℃[1]。2021 年IPCC第一工作组最新评估报告指出,通过对未来20年的平均温度变化预计,全球温度升高将达到或超过1.5 ℃。高温已逐渐成为严重危害植物生长发育的非生物胁迫因子之一,抑制了植物的生理代谢[2]。植物热胁迫应对机制一直是研究关注的热点,也是后期耐热品种筛选的基础。在生理水平上,植物活性氧(reactive oxygen species,ROS)清除系统相关酶活性、光合特性及渗透调节物质的变化等被广泛研究;在分子水平上,与热应激反应相关的热休克转录因子(heat shock transcription factors,HSFs)和热休克蛋白(heat shock proteins,HSPs)、Ca+介导的信号通路亦受到高度关注[3-4]。高温处理使葡萄叶片的过氧化物酶(peroxidase,POD)、过氧化氢酶(catalase,CAT)、超氧化物歧化酶(superoxide dismutase,SOD)等抗氧化酶活性升高[5],净光合速率Pn显著降低[6]。连续高温显著降低光系统(photosystem Ⅱ,PSⅡ)反应中心活性,PSⅡ正常功能受阻[5,7],同时,超显微结构观察表明叶绿体结构发生改变[8]。单粒质量及可滴定酸、花色苷含量均显著降低,导致果实品质下降[7]。在草莓[9]、香梨[10]、越橘[11]等果树树种上均有类似报道。自噬相关基因MdATG18a 可通过保护叶绿体、维持高水平光合作用、清除ROS 等方面增强苹果的耐热性[12],MYB、ERF 等基因家族在物质合成与代谢、激素信号转导相关方面的基础耐热性研究中起着关键作用[13-14]。
甜樱桃(Pruns avium L.)在我国集中栽培于渤海湾地区,具有较高的经济价值,同时南方春季升温早,因此逐渐受以采摘游为主的南方果园青睐。浙江省夏季温度较高,7—9月期间40 ℃以上极端高温天气年均4.1 d[15]。本课题组结合杭州市气象统计数据,连续多年对杭州市夏天(6—8月)高温天数进行统计后发现,2016—2020 年连续5 a 夏天30 ℃以上高温天气在55 d以上,35 ℃以上高温天气在25 d以上,其中2016—2018 年连续3 a 夏天35 ℃以上高温天气均在35 d以上。对于适应于北方冷凉气候的甜樱桃而言,杭州地区夏季温度过高,而高温与双雌蕊的发生密切相关[16],会影响翌年坐果率[17]。由此可见,高温是制约甜樱桃在南方地区发展的因子之一,筛选耐高温的品种是南方地区甜樱桃引种适栽的研究目标之一,也是后期耐高温品种创制的基础。目前,关于高温胁迫对甜樱桃生理生化、光合作用影响的研究较少。孟祥丽[18]以早大果为接穗,对5种不同砧木组成的砧穗组合进行高温处理,指出45 ℃处理对PSⅡ中心造成破坏,并通过生理和形态学指标分析对5种砧穗组合的耐热性进行排序。孟聪睿[19]通过模拟高温测定了樱桃组培苗叶片渗透物质及抗氧化酶含量。吴晔[20]为探寻试管苗热处理脱毒法的温度条件,对10个樱桃砧木组培试管苗进行耐热性差异观测。
吉塞拉(Gisela)系列是目前南方地区甜樱桃栽培中最常用的砧木,为矮化/半矮化砧,具有亲和性较好、适应性强、抗病、提前结果等优点,但关于品种在吉塞拉砧木上的耐热性表现尚未见报道。本课题组通过多年引种进行适应性栽培,筛选出适合于杭州地区的甜樱桃品种萨米脱(Summit)。该品种在本地多年适栽后,具有相对稳定的产量,但在夏季温度较高的年份有一定比例的双雌蕊率[17]。笔者在本研究中以此为切入点,以甜樱桃萨米脱为接穗、吉塞拉作为砧木的嫁接苗为试材,通过模拟高温或自然环境,探究其植株在不同的高温处理下叶片生理、叶绿素荧光指标的变化,以期从生理水平分析甜樱桃对高温的响应机制,为下一步甜樱桃耐热性评价体系建立提供参考,也为今后南方地区甜樱桃耐高温品种(系)的筛选及育种、耐高温栽培技术研究奠定理论基础。
1 材料和方法
1.1 试验材料
试验于2019 年在浙江农林大学官塘基地避雨大棚内进行。试材为1 年生萨米脱盆栽嫁接苗,砧木为吉塞拉6号。
1.2 采样处理方法
试验中高温处理分别采用人工气候箱、田间自然高温2种方式,设为A、B、C共3组。其中,A、B组均使用人工气候箱模拟高温,C组为田间自然高温。A、B组试验于2019年8月25日至2019年8月26日进行。A组:试材为植株盆栽苗。随机挑取生长一致且健壮的盆栽苗6盆,分别置于25、45 ℃的人工气候箱内进行处理12 h,湿度70%,光照度12 000 lx,处理后随机采集无病害成熟叶片,10枚叶片为一组,3次重复;B组:试材为植株盆栽苗离体叶片。摘取无病害成熟叶片用湿棉球包裹叶柄,基部覆以保鲜膜以保持叶柄水分,分别置于25、45 ℃的人工气候箱内进行处理12 h,湿度70%,光照度12 000 lx,10枚叶片为一组,3次重复;C组:于2019年8月25日至2019年8月30日期间利用大棚内自然高温进行处理。试材为植株盆栽苗。将盆栽苗移入塑料大棚内,使用WS-TH23PRO温湿度记录仪记录大棚内的温湿度(图1)。分别于移入0、3(高温后)、5 d(恢复期)时随机采集无病害成熟叶片,10片为一组,3次重复。以上样品采集后洗净表面浮土灰尘等,液氮冷冻后置于-80℃冰箱中保存,用于生理指标测定。将3 组处理(7 种状态)分别标记为:A-25 ℃(T1)、A-45 ℃(T2)、B-25 ℃(T3)、B-45 ℃(T4)、C-0 d(T5)、C-3 d(T6)、C-5 d(T7)。

图1 田间(大棚自然高温)处理期间温湿度变化情况
Fig.1 temperature and humidity records infield treatment
1.3 测定指标及方法
生理指标测定方法:可溶性糖含量采用蒽酮法测定,可溶性蛋白含量采用考马斯亮蓝G-250法测定,脯氨酸含量采用茚三酮法测定,丙二醛含量采用硫代巴比妥酸法测定,SOD活性采用愈创木酚比色法测定,POD 活性采用氮蓝唑(NBT)法测定,CAT活性采用紫外吸收法测定,抗坏血酸过氧化物酶(ascorbate peroxidase,APX)活性根据刘冬峰[10]的方法测定,谷胱甘肽还原酶(glutathione reductase,GR)活性参考张腾国等[21]的方法测定,O2·-生成速率参照孟祥丽[18]的方法测定。采用德国Walz 公司PAM-2500 便携式叶绿素荧光仪测定初始荧光(Fo)、最大光化学效率(Fm)、PSⅡ最大光化学效率(Fv/Fm)、非光化学淬灭系数(non-photochemical quenching,NPQ)、表观电子传递速率(electron transport rate,ETR)。每个参数设3次生物学重复。
透射电镜观察方法:取A组25、45 ℃植株中部的功能叶片,于其主脉两侧部分用刀片切成1 mm×1 mm小方块放入2 mL离心管中,使用Alfa Aesar电镜固定液固定,并用真空泵抽气直至沉底,室温放置2 h后转入4 ℃储存。使用PBS缓冲液漂洗3次后用1%锇酸室温固定5 h。再次使用PBS缓冲液漂洗3次后用乙醇梯度脱水。使用丙酮和包埋剂渗透随后包埋。Leica UC60超薄切片机切片,采用铀铅双染色,在日立HT7650型透射电镜下观察拍照。
采用Excel 2019 和DPS 18.10 软件对数据进行统计分析,采用单因素方差分析和Duncan 法比较差异显著水平(p<0.05)。
2 结果与分析
2.1 高温对甜樱桃叶片抗氧化系统酶活性的影响
由图2 可知,高温后A 组叶片内5 种酶活性均升高。其中,SOD、POD活性升幅较大,其值较高温前分别升高了46.14%、131.63%。B组叶片内SOD、POD、GR活性均升高,但CAT、APX活性下降,其值分别较高温前下降了43.00%、27.83%。

图2 高温对甜樱桃叶片抗氧化系统酶活性的影响
Fig.2 Effects of high temperature on several enzyme activities in sweet cherry leaves
不同小写字母表示在p<0.05 差异显著。下同。
Different small letters indicate significant difference at p<0.05. The same below.
C组变化趋势与A 组相同,高温后甜樱桃叶片内5种酶活性均升高。其中,CAT、APX、GR活性在高温后显著升高,分别为高温前活性的1.91、2.36、1.41倍。当温度降低植株进入恢复期,除POD活性仍升高外,其余4 种酶活性显著降低。此时,CAT、APX活性仍显著高于处理前的水平,SOD、GR活性则低于处理前的水平。
2.2 高温对甜樱桃叶片渗透调节物质含量和O2·-产生速率的影响
图3 表明,高温后A 组甜樱桃叶片内MDA、脯氨酸含量及O2·-产生速率均升高。其中,脯氨酸含量、O2·-产生速率升高幅度较大,A、B组脯氨酸含量分别为高温前的3.07、4.03倍,O2·-产生速率分别为高温前的2.37、2.48 倍。可溶性蛋白和可溶性糖含量变化不同,A组甜樱桃叶片的可溶性蛋白含量增加,而B组的含量下降,为处理前的54.18%;高温后A组甜樱桃叶片的可溶性糖含量降低,而B组的增加了1.32%。
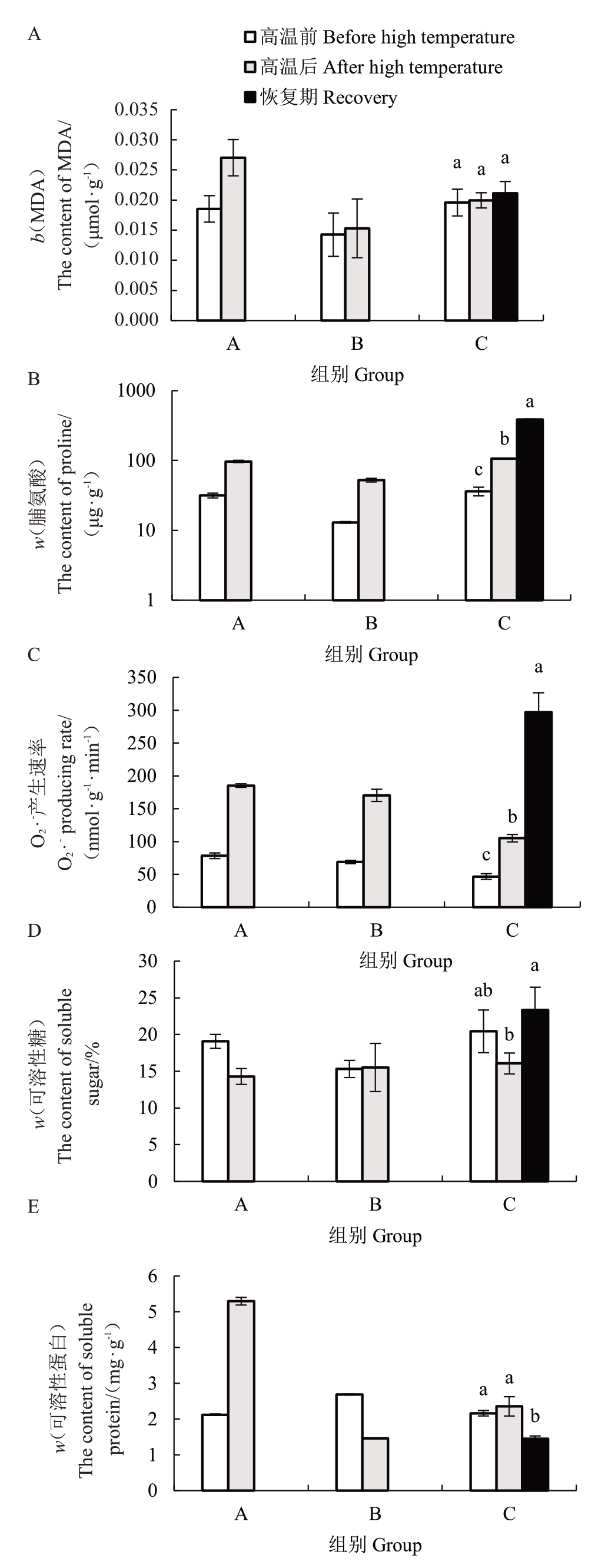
图3 高温对甜樱桃叶片渗透调节物质和O2·-产生速率的影响
Fig.3 Effects of high temperature on osmotic substances and O2·-producting rate in sweet cherry leaves
C组各个指标的变化趋势与A组相同。高温后甜樱桃叶片内MDA、可溶性蛋白含量少量增加,脯氨酸含量、O2·-产生速率则显著升高,脯氨酸含量为高温前的2.94倍,O2·-产生速率为高温前的2.25倍,而可溶性糖含量降低了21.39%。当温度降低植株进入恢复期,甜樱桃叶片内MDA、脯氨酸含量及O2·-产生速率仍呈升高趋势,此时MDA、脯氨酸含量、O2·-产生速率为高温前的1.08、10.63、6.36倍。恢复期叶片内可溶性蛋白含量回降,显著低于高温前的水平,同时可溶性糖含量回升,高于高温前的水平。
2.3 高温对甜樱桃叶片叶绿素荧光参数的影响
由图4 可知,3 组甜樱桃在高温处理后,Fv/Fm、Fv/Fo、ETR、NPQ 4 个参数值均呈现不同程度的下降。A组Fv/Fo下降幅度较大,其值比高温前降低了37.00%;B组Fv/Fo、NPQ下降幅度较大,其值分别比高温前降低了22.34%、32.04%;C 组Fv/Fo、ETR、NPQ 3 个参数值显著降低,分别比高温前降低了7.62%、40.90%、17.42%。恢复期内,甜樱桃叶片NPQ仍呈下降趋势,而Fv/Fm、Fv/Fo、ETR 3个参数值有所回升但仍低于处理前的水平。

图4 高温对甜樱桃叶片叶绿素荧光参数的影响
Fig.4 Effects of high temperature on ChlorophyⅡfluorescence in sweet cherry leaves
2.4 高温对甜樱桃叶片叶肉细胞局部超显微结构的影响
由图5 可见,高温前细胞内胞间隙较小,叶绿体呈长椭圆形,紧贴于细胞壁,基粒排列整齐有序,可见到有少量嗜锇颗粒分布。线粒体近圆形,多分布于2个叶绿体之间,含有丰富的嵴。高温后,叶绿体肿胀,部分形态扭曲呈不规则状,逐渐向细胞中央靠拢,整个细胞中散布有淀粉粒和大量细胞碎片组成的团块(图5-b)。叶绿体内有体积较大的淀粉粒,受其挤压类囊体片层出现扭曲,基质片层明显松散,结构趋于紊乱。嗜锇颗粒增大、积累并相互聚集(图5-d)。线粒体嵴开始变得模糊,可见到明显结晶(图5-f)。

图5 高温对甜樱桃叶片叶肉细胞局部超显微结构的影响
Fig.5 Effects of high temperature on ultrastructural changes of mesophyll cells in sweet cherry leaves
C.叶绿体;M.线粒体;S.淀粉粒;P.嗜锇颗粒;G.基粒片层。
C.Chloroplast;M.Mitochondrion;S.Starch grain;P.Plastoglobule;G.Granalamella.
2.5 甜樱桃生理指标、叶绿素荧光参数相关性分析
对C 组中14 个生理指标相关性分析可知,在p<0.01差异水平下,多个指标之间存在极显著相关关系(图6)。APX活性与CAT活性表现出极显著正相关,与荧光参数ETR(r=-0.807)、Fv/Fo(r=-0.922)呈极显著负相关,且与CAT 活性相关性极强(r=0.951);CAT 活性与荧光参数ETR(r=-0.862)、Fv/Fo(r=-0.906)呈极显著负相关;GR 活性与可溶性蛋白含量(r=0.909)呈极显著正相关,与可溶性含量(r=-0.822)呈极显著负相关;O2·-产生速率与脯氨酸含量(r=0.988)呈极显著正相关且相关性极强,与可溶性蛋白含量(r=-0.844)呈极显著负相关;脯氨酸含量与可溶性蛋白(r=-0.874)含量呈极显著负相关;ETR 与NPQ(r=0.832)、Fv/Fo(r=0.924)呈极显著正相关。SOD 活性与GR 活性呈显著正相关(r=0.716,p<0.05),GR活性与O2·-产生速率(r=-0.758,p<0.05)、脯氨酸含量呈显著负相关(r=-0.783,p<0.05),O2·-产生速率与NPQ(r=-0.763,p<0.05)呈显著负相关,脯氨酸含量与NPQ(r=-0.777,p<0.05)呈显著负相关,可溶性蛋白含量与可溶性糖含量(r=-0.700,p<0.05)呈显著负相关。综合可知,在田间试验中,甜樱桃叶片中部分酶活性(CAT、APX)与部分荧光参数(ETR、Fv/Fo)之间、GR 活性与多种渗透调节物质(脯氨酸、可溶性蛋白、可溶性糖)含量存在相关关系,而POD 活性、MDA 含量、Fv/Fm与其他指标间并无显著相关关系。
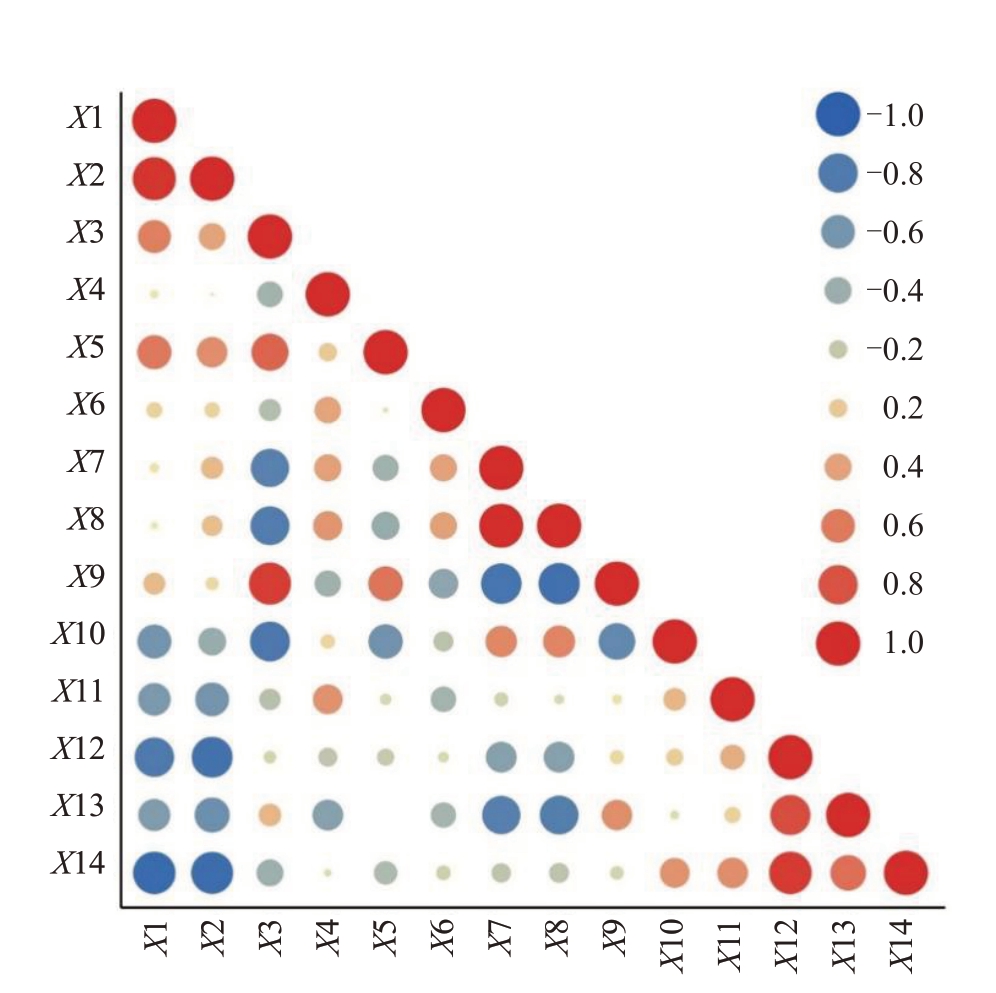
图6 甜樱桃各个生理指标、叶绿素荧光参数的相关性分析
Fig.6 Correlation analysis of physiological indexes and fluorescence parameters of sweet cherry
X1. APX 活性;X2. CAT 活性;X3. GR 活性;X4. POD 活性;X5. SOD 活性;X6.MDA 含量;X7.O2·-产生速率;X8.脯氨酸含量;X9. 可溶性蛋白含量;X10. 可溶性糖含量;X11. Fv/Fm;X12. ETR;X13.NPQ;X14.Fv/Fo。图8 相同。
X1.The activities of APX; X2.The activities of CAT; X3.The activities of GR; X4. The activities of POD; X5. The activities of SOD;X6. The content of MDA; X7. O2·- producing rate; X8. The content of proline;X9.The content of soluble protein;X10.The content of soluble sugar;X11.Fv/Fm;X12.ETR;X13.NPQ;X14.Fv/Fo.Fig.8 is the same.
对甜樱桃高温处理检测的11 个具有相关性的生理指标采用主成分分析法进行因子分析。提取特征值>1的因子,结果表明,前2个因子为主因子,其包含的信息量累计贡献率达89.881%(表1)。因子1的方差贡献率为45.929%,选择特征向量值较大的变量作为代表性变量为APX、SOD、GR 酶活性,定义为酶活参数因子。因子2 的方差贡献率为43.952%,选择特征向量值较大的变量作为代表性变量为ETR、NPQ,定义为荧光参数因子。
表1 甜樱桃高温条件下生理指标因子分析结果
Table 1 Factor analysis results of physiological indexes under high temperature in sweet cherry
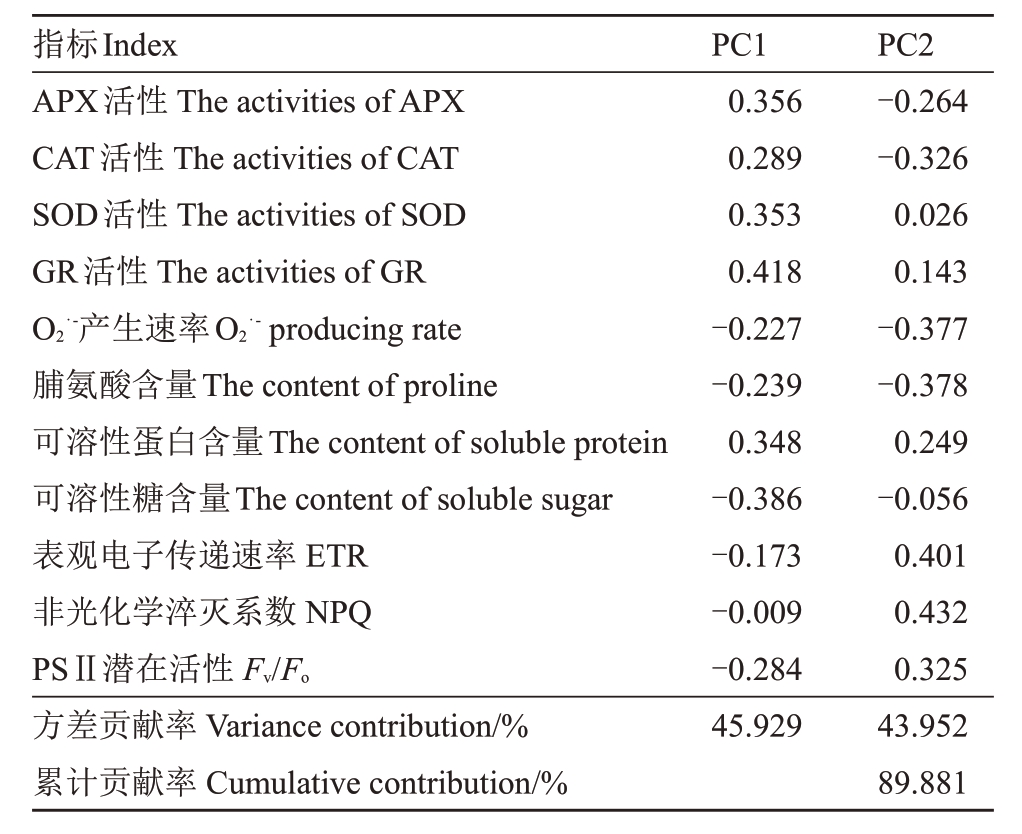
指标Index APX活性The activities of APX CAT活性The activities of CAT SOD活性The activities of SOD GR活性The activities of GR O2·-产生速率O2·-producing rate脯氨酸含量The content of proline可溶性蛋白含量The content of soluble protein可溶性糖含量The content of soluble sugar表观电子传递速率ETR非光化学淬灭系数NPQ PSⅡ潜在活性Fv/Fo方差贡献率Variance contribution/%累计贡献率Cumulative contribution/%PC1 0.356 0.289 0.353 0.418-0.227-0.239 0.348-0.386-0.173-0.009-0.284 45.929 PC2-0.264-0.326 0.026 0.143-0.377-0.378 0.249-0.056 0.401 0.432 0.325 43.952 89.881
11个具有相关性的生理指标经标准化转换后,采用可变类平均法进行聚类分析(图7)。将11个生理指标聚为3 类:第1 类包括APX、CAT 活性,为酶活指标;第2类为GR活性,为酶活指标;第3类包括SOD 活性、O2·-产生速率、脯氨酸含量、可溶性蛋白含量、可溶性糖含量、ETR、NPQ、Fv/Fo,为混合指标。结合相关性分析,APX活性与CAT活性呈极显著正相关,与ETR、NPQ、Fv/Fo呈极显著负相关,由此可见APX活性、GR活性、ETR可作为本试验中高温对萨米脱生理影响较为代表性的指标,可为今后甜樱桃对高温响应的生理指标筛选提供一定的参考。
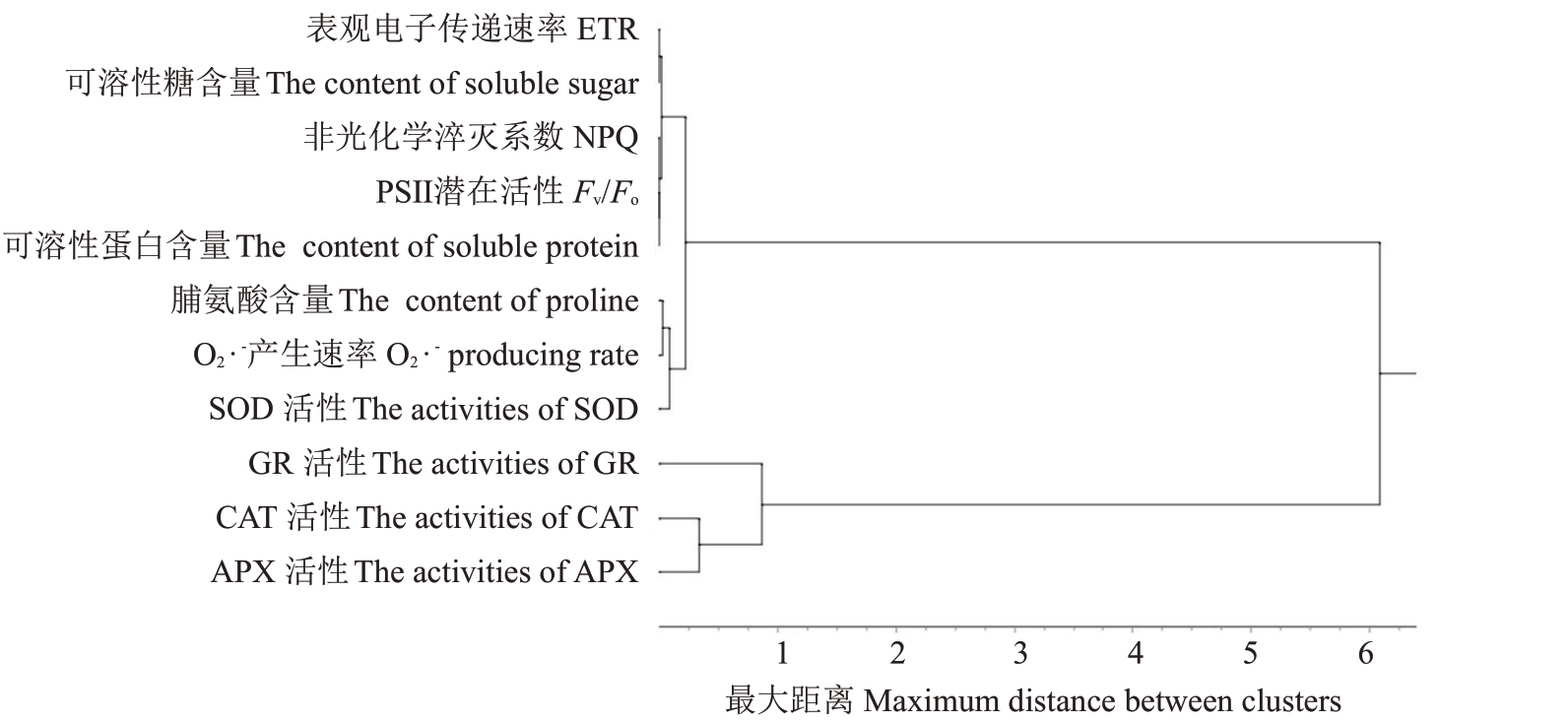
图7 11 项生理指标的系统聚类谱系
Fig.7 Hierarchical clustering pedigree of 11 physiological indexes
2.6 高温对甜樱桃生理影响代表性指标选择
将聚类分析后筛选出的3 个指标作为高温对甜樱桃生理影响较为代表性的指标,采用K-均值法迭代计算对试验中进行的3 组处理(7 种不同状态)的甜樱桃进行动态聚类。表2 结果表明,7 种不同状态的甜樱桃被分为3 类:第1 类包含处理T4,其对高温响应特征为高水平GR 活性、低水平APX活性;第2 类包含处理T1、T2、T3、T5,对高温响应特征为高ETR指数、低水平GR活性;第3类包含处理T6、T7,对高温响应特征为高水平APX 活性、低ETR 指数。结合甜樱桃生理指标热图(图8)可知,T4 组各生理指标值与其他处理有明显差异,单独为一类;T1、T3、T5 均代表各处理在高温胁迫前的生理状态,T2 各指标与这三组数据相近,同属于一类;T6、T7 为田间处理在高温胁迫后的生理状态,归属为一类。由此可知,聚类结果与7 种不同状态下甜樱桃生理指标热图(图8)分析结果相对应,上述筛选的APX 活性、GR 活性、ETR 3 个指标可作为本试验中高温对萨米脱生理影响较为代表性的指标,可为今后甜樱桃对高温响应的生理指标筛选提供一定的参考。
表2 聚类中心及7 种不同状态甜樱桃的聚类结果
Table 2 The final cluster center and cluster results of seven different states of sweet cherry
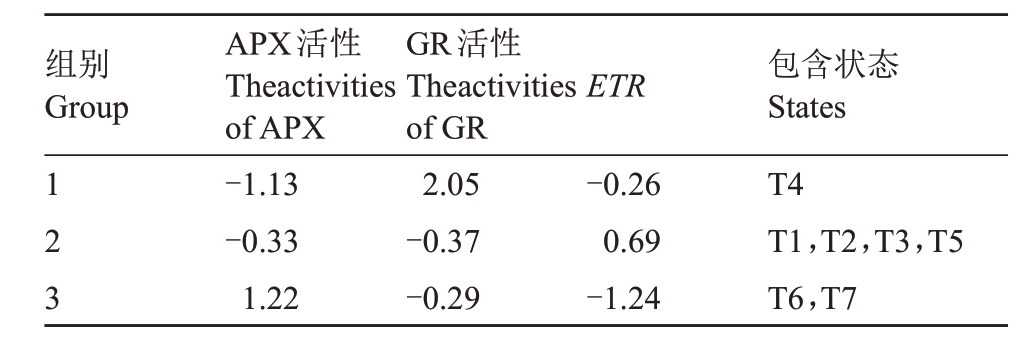
组别Group ETR 123 APX活性Theactivities of APX-1.13-0.33 1.22 GR活性Theactivities of GR 2.05-0.37-0.29-0.26 0.69-1.24包含状态States T4 T1,T2,T3,T5 T6,T7
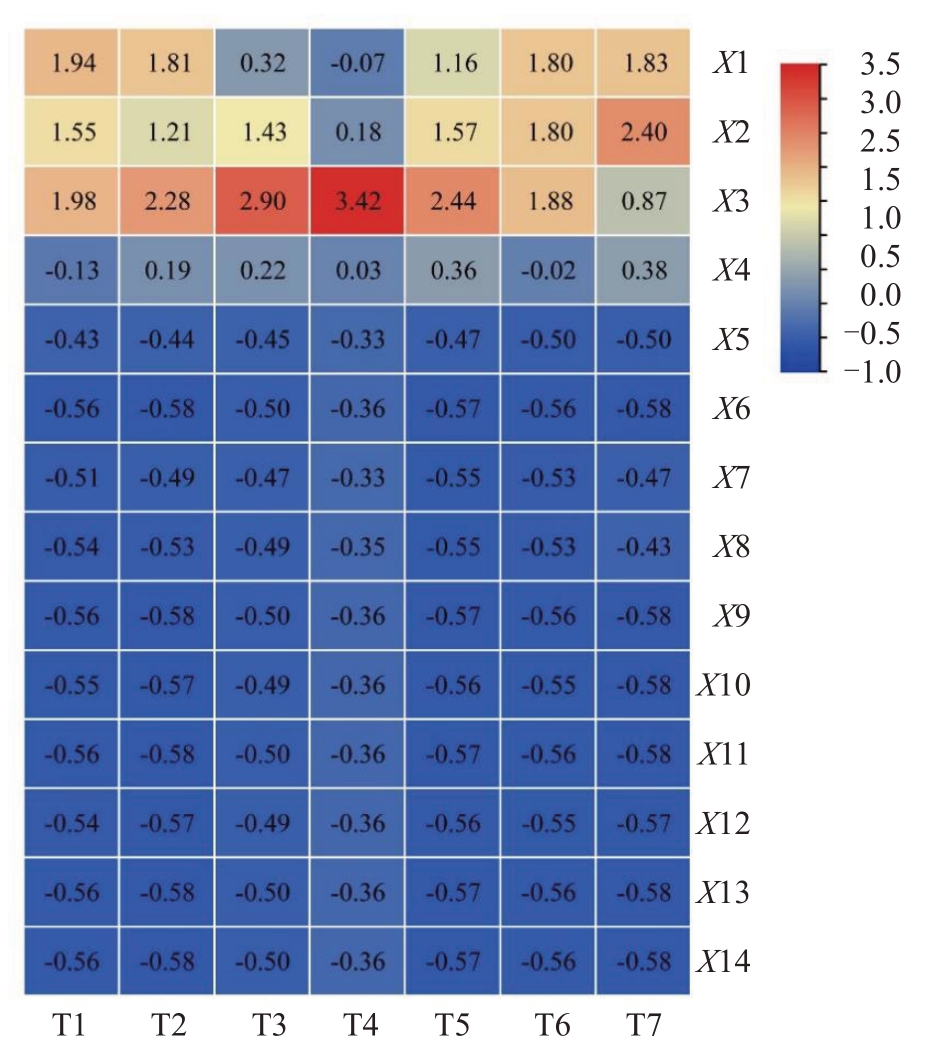
图8 不同状态甜樱桃生理指标热图
Fig.8 Heat map of physiological indexes of sweet cherry in different states
3 讨 论
极端高温天气频发给植物生长带来严峻挑战,植物对高温的生理响应及其分子机制的研究是耐高温品种选育的基础。正常情况下,植物体内ROS保持在平衡状态,高温胁迫导致ROS 大量积累,对膜质、蛋白质、核酸高分子造成损伤[2]。在长期进化过程中,植物已具备对各种非生物胁迫的适应机制,包括ROS清除机制、渗透物质调节、离子转运蛋白、信号级联和转录因子调节[22]。ROS清除机制分为酶促和非酶促防御系统。SOD、POD、CAT、APX、GR 均是酶促防御系统中的重要成员。SOD是植物体内唯一能够清除O2·-的酶,可有效减轻胁迫对膜系统的伤害,是抵御氧化胁迫的第一道防线。SOD催化光系统Ⅰ(photosystemⅠ,PSⅠ)中产生的O2·-转化为H2O2,其后在CAT 或其他还原剂参与下被APX、POD等转化为H2O。夏黑葡萄在35、45 ℃高温处理后POD、CAT 活性快速升高[5]。油桃在高温环境下SOD 活性缓慢升高,CAT 活性先升高后下降[23]。高温干旱处理下,香梨叶片中SOD、CAT 活性升高,POD 活性先升高随后下降[24]。但是,高温对北高丛越橘SOD 活性影响并不大,只对POD、CAT 活性有改变[11]。本试验中,经历田间高温后C 组甜樱桃叶片中SOD、POD、CAT活性均升高,人工高温处理后A组叶片中3种酶活性亦升高,而B组叶片中CAT活性显著降低,这很可能与试验材料的选择有关。B组试验材料为离体叶片,相对A、C组材料其代谢强度降低。通常认为,CAT活性与植物抗逆性及植物代谢强度密切相关[25]。APX 活性也有同样的变化趋势。APX和GR是抗环血酸-谷胱甘肽(ASA-GSH)循环中的限速酶,该循环在清除H2O2、调控细胞H2O2水平上有重要作用。在叶绿体中,GR 平衡ROS 浓度,防止膜脂过氧化以保护类囊体膜系统维持有效的光合作用[26]。因此,GR活性通常被认为是机体内抗氧化状态的重要标志[21]。高温处理下,杜鹃叶片的APX、GR活性随胁迫程度加深先升高后降低[27],野扇花APX活性也呈现同样变化[28]。本试验中,高温使A、C 2组中甜樱桃叶片的APX、GR酶活性均升高,但B组叶片中APX活性降低。结合相关性分析可知,APX活性与CAT活性之间存在极显著正相关,故B组中CAT和APX活性的变化呈现相同趋势。
MDA含量和O2·-产生速率是衡量膜脂过氧化、植物氧化胁迫程度的重要指标。高温胁迫下,油桃MDA含量则呈微增-减少-增加趋势[23]。本试验中,高温使得3 组甜樱桃叶片MDA 含量升高,O2·-产生速率较处理前显著升高,这与前人在樱桃中的研究结果相同[18-19]。在C 组中,即使后期温度下降,叶片内MDA含量和O2·-产生速率仍呈升高趋势,表明高温对甜樱桃造成损伤,膜脂过氧化程度较高。在正常状态下,叶绿体中O2·-产生速率约为240 μmol·s-1,但在逆境条件下速率为240~720 μmol·s-1[10]。本试验数据表明,甜樱桃正常状态下叶片中O2·-产生速率<100 μmol·s-1,但高温胁迫后速率升高至100~300 μmol·s-1。除抗氧化酶系统外,渗透调节物质(脯氨酸、可溶性蛋白、可溶性糖等)通过调节细胞渗透平衡来抵制外界胁迫对植物造成的伤害[2]。研究表明,耐热性强的品种脯氨酸含量更高,在高温干旱处理下,香梨叶片中可溶性蛋白含量下降,可溶性糖含量逐渐增加[24]。本试验C 组中,高温使甜樱桃叶片内可溶性糖含量降低,可溶性蛋白含量升高。A组叶片内同样指标亦呈相同变化,但B 组叶片中则呈相反趋势,这很可能与叶片离体后源库关系改变有关。
叶绿体是植物细胞中对热胁迫较敏感的细胞器。研究表明,高温胁迫会使叶绿体性状趋于圆形并向细胞中央转移,被膜出现不同程度的断裂,类囊体片层松散并随机分布着大量嗜锇颗粒。嗜锇颗粒的大小和数量通常与膜脂过氧化程度密切相关[8],颗粒体积变大并积累预示细胞老化和解体。光合作用的一部分同化产物以淀粉形式在植物体内积累。高温胁迫下植物韧皮部胼胝体增多导致维管束堵塞,从而影响同化产物在韧皮部的运输,淀粉粒逐渐积累[29]。本试验中,高温后甜樱桃叶绿体和线粒体受到不同程度损伤,表现为叶绿体外形膨胀变圆、类囊体片层扭曲、基质片层明显松散、结构趋于紊乱、淀粉粒及嗜锇颗粒增大并积累和线粒体嵴开始变得模糊等。葡萄、越橘在高温胁迫后叶绿体中出现大量巨型淀粉粒及大量嗜锇颗粒[8,11],与本试验中研究结果一致。
PSⅠ和PSⅡ反应中心是ROS产生的主要位点,胁迫条件下PSⅠ比PSⅡ稳定,PSⅡ对高温敏感[30]。叶绿素荧光可快速无损收集PSⅡ性能相关数据,是对类囊体膜功能变化情况的定量检测。Fv/Fm、Fv/Fo是鉴定植物是否遭受高温胁迫的重要荧光参数,其中最大光化学量子产率Fv/Fm,是植物PSⅡ系统初始光能转换效率的标志指标,通常认为该值与植物的耐热性具有良好一致性[31],是耐热性鉴定的重要指标。高温胁迫下快白菜Fv/Fm降低[32],同样的变化在草莓、葡萄、越橘[7,9,11,31]等多个树种上都有报道。叶绿体结构和光合性能密切相关。本试验中,高温处理使叶绿体结构受损,Fv/Fm、Fv/Fo、ETR、NPQ 均出现不同程度下降,表明高温抑制了甜樱桃光能转化效率,PSⅡ反应中心活性下降,同化力(NADPH、ATP)形成受阻,对碳固定同化造成了影响,热耗散的能量降低。PSⅡ的最大效率与积累的ROS 之间存在线性关系[33],这在本试验中相关性分析中也被证实。
主成分分析是考察多个变量间相关性的常用多元统计方法之一,在植物抗逆性鉴定与评价研究中广泛应用[31,34]。田间栽培是果树引种适应性栽培过程中各种性状考察的主要场所,在田间具有优良性状和较高抗性的品种才具备推广的价值。本研究对C 组各个指标,通过主成分分析将具有相关性关系的生理指标降维及系统聚类分析,筛选出3 个指标作为高温对甜樱桃生理影响的代表性指标。利用筛选出的3 个代表性指标将试验中3 组处理7 种状态进行动态聚类分析,分类结果与实际测得各状态间指标差异情况吻合。由此可知,上述筛选的APX活性、GR 活性、ETR 3 个指标可作为本试验中高温对萨米脱生理影响的代表性指标,为今后甜樱桃对高温响应的生理指标筛选提供一定的参考。
4 结 论
高温处理使甜樱桃受到氧化损伤,叶绿体、线粒体结构受损,抗氧化酶系统、渗透调节物质均对胁迫做出响应,以维持抗氧化酶系统的稳定,降低膜透性损害。APX 活性、GR 活性、ETR 3 个指标可作为高温对萨米脱生理影响的代表性指标,为今后甜樱桃对高温响应的生理指标筛选提供一定的参考。
[1] JONES P D,NEW M,PARKER D E,MARTIN S,RIGOR I G. Surface air temperature and its changes over the past 150 years[J]. Reviews of Geophysics,1999,37(2):173-199.
[2] WAHID A,GELANI S,ASHRAF M,FOOLAD M R. Heat tolerance in plants:An overview[J]. Environmental and Experimental Botany,2007,61(3):199-223.
[3] ZHANG H M,ZHU J H,GONG Z Z,ZHU J K.Abiotic stress responses in plants[J]. Nature Reviews Genetics,2022,23(2):104-119.
[4] ZHU J K. Abiotic stress signaling and responses in plants[J].Cell,2016,167(2):313-324.
[5] 查倩,奚晓军,蒋爱丽,田益华,黄健.高温胁迫对葡萄幼树叶绿素荧光特性和抗氧化酶活性的影响[J]. 植物生理学报,2016,52(4):525-532.
ZHA Qian,XI Xiaojun,JIANG Aili,TIAN Yihua,HUANG Jian.Effects of heat stress on chlorophyll fluorescence characteristics and antioxidant activity in grapevines(Vitis vinifera L. cv. Xiahei)[J].Plant Physiology Journal,2016,52(4):525-532.
[6] 娄玉穗,尚泓泉,吕中伟,李政,王鹏,吴文莹.基于光合特性的8 个葡萄品种耐弱光、抗高温特点比较[J].果树学报,2021,38(9):1491-1502.
LOU Yusui,SHANG Hongquan,LÜ Zhongwei,LI Zheng,WANG Peng,WU Wenying. Comparison of low light tolerance and high temperature resistance among eight grape cultivars based on analysis of photosynthetic characteristics[J]. Journal of Fruit Science,2021,38(9):1491-1502.
[7] 张睿佳,李瑛,虞秀明,娄玉穗,许文平,张才喜,赵丽萍,王世平.高温胁迫与外源油菜素内酯对巨峰葡萄叶片光合生理和果实品质的影响[J].果树学报,2015,32(4):590-596.
ZHANG Ruijia,LI Ying,YU Xiuming,LOU Yusui,XU Wenping,ZHANG Caixi,ZHAO Liping,WANG Shiping. Effects of heat stress and exogenous brassinolide on photosynthesis of leaves and berry quality of Kyoho grapevine[J]. Journal of Fruit Science,2015,32(4):590-596.
[8] 刘敏,房玉林.高温胁迫对葡萄幼树生理指标和超显微结构的影响[J].中国农业科学,2020,53(7):1444-1458.
LIU Min,FANG Yulin.Effects of heat stress on physiological indexes and ultrastructure of grapevines[J]. Scientia Agricultura Sinica,2020,53(7):1444-1458.
[9] 徐超,王明田,杨再强,韩玮,郑盛华.高温对温室草莓光合生理特性的影响及胁迫等级构建[J]. 应用生态学报,2021,32(1):231-240.
XU Chao,WANG Mingtian,YANG Zaiqiang,HAN Wei,ZHENG Shenghua.Effects of high temperature on photosynthetic physiological characteristics of strawberry seedlings in greenhouse and construction of stress level[J].Chinese Journal of Applied Ecology,2021,32(1):231-240.
[10] 刘冬峰.砂梨对高温胁迫的响应及耐热机理研究[D].杭州:浙江大学,2014.
LIU Dongfeng. Studies on the pesponse of sand pear to hightemperature and heat-tolerance mechanism[D]. Hangzhou:Zhejiang University,2014.
[11] 黄磊,孙耀清,郝立华,党承华,朱玉,王贺新,程东娟,张运鑫,郑云普.高温对北高丛越橘叶片结构和生理代谢的影响[J].园艺学报,2016,43(6):1044-1056.
HUANG Lei,SUN Yaoqing,HAO Lihua,DANG Chenghua,ZHU Yu,WANG Hexin,CHENG Dongjuan,ZHANG Yunxin,ZHENG Yunpu. Effects of high temperatures on leaf structures and physiological metabolism of north highbush blueberry[J].Acta Horticulturae Sinica,2016,43(6):1044-1056.
[12] HUO L Q,SUN X,GUO Z J,JIA X,CHE R M,SUN Y M,ZHU Y F,WANG P,GONG X Q,MA F W.MdATG18a overexpression improves basal thermotolerance in transgenic apple by decreasing damage to chloroplasts[J]. Horticulture Research,2020,7:21.
[13] HAO S X,LU Y F,PENG Z,WANG E Y,CHAO L K,ZHONG S L,YAO Y C. McMYB4 improves temperature adaptation by regulating phenylpropanoid metabolism and hormone signaling in apple[J].Horticulture Research,2021,8:182.
[14] WANG Y C,JIANG H Y,MAO Z L,LIU W J,JIANG S H,XU H F,SU M Y,ZHANG J,WANG N,ZHANG Z X,CHEN X S.Ethylene increases the cold tolerance of apple via the MdERF1BMdCIbHLH1 regulatory module[J]. The Plant Journal:For Cell and Molecular Biology,2021,106(2):379-393.
[15] 孙科,娄伟平,刘昌杰.1961—2017 年浙江省夏季高温热浪时空变化特征[J].科技通报,2019,35(7):53-58.
SUN Ke,LOU Weiping,LIU Changjie.Temporal-spatial change characteristics of summer heatwaaves in Zhejiang Province during 1961-2017[J].Bulletin of Science and Technology,2019,35(7):53-58.
[16] BEPPU K,IKEDA T,KATAOKA I. Effect of high temperature exposure time during flower bud formation on the occurrence of double pistils in‘Satohnishiki’sweet cherry[J]. Scientia Horticulturae,2001,87(1/2):77-84.
[17] 沈国正,刘辉,张琛,郗笃隽,裴嘉博,黄康康.杭州和泰安地区3 个甜樱桃品种花器官发育质量比较分析[J].浙江大学学报(农业与生命科学版),2020,46(2):168-176.
SHEN Guozheng,LIU Hui,ZHANG Chen,XI Dujun,PEI Jiabo,HUANG Kangkang. Comparison and analyzation on the floral organ development and quality of three sweet cherry cultivars in Hangzhou and Tai’an areas[J]. Journal of Zhejiang University(Agriculture and Life Sciences),2020,46(2):168-176.
[18] 孟祥丽.五种不同甜樱桃砧木旱涝和高温胁迫适应性评价[D].金华:浙江师范大学,2011.
MENG Xiangli. Comprehensive evaluation of the tolerance of sweet cherry drafted on five different rootstocks under drought,waterlogging and high-temperature stress by subordinate function values analysis[D].Jinhua:Zhejiang Normal University,2011.
[19] 孟聪睿.干旱高温胁迫对樱桃的生理影响[D].太谷:山西农业大学,2013.
MENG Congrui. Drought and heat stress on cherry physiological effects[D].Taigu:Shanxi Agricultural University,2013.
[20] 吴晔.10 个樱桃砧木组培试管苗耐热差异性观察[J].山西果树,2015(6):5-7.
WU Ye. Observation on the difference of heat resistance of 10 cherry rootstocks in vitro[J].Shanxi Fruits,2015(6):5-7.
[21] 张腾国,聂亭亭,孙万仓,史中飞,王娟.逆境胁迫对油菜谷胱甘肽还原酶基因表达及其酶活性的影响[J].应用生态学报,2018,29(1):213-222.
ZHANG Tengguo,NIE Tingting,SUN Wancang,SHI Zhongfei,WANG Juan. Effects of diverse stresses on gene expression and enzyme activity of glutathione reductase in Brassica campestris[J]. Chinese Journal of Applied Ecology,2018,29(1):213-222.
[22] OHAMA N,SATO H,SHINOZAKI K,YAMAGUCHI-SHINOZAKI K. Transcriptional regulatory network of plant heat stress response[J].Trends in Plant Science,2017,22(1):53-65.
[23] 尤超,沈虹,郭世荣,孙锦.高温逆境胁迫对油桃生理特征影响的研究[J].中国农学通报,2016,32(10):79-84.
YOU Chao,SHEN Hong,GUO Shirong,SUN Jin. Physiological characteristics of nectarine seedlings under high temperature stress[J]. Chinese Agricultural Science Bulletin,2016,32(10):79-84.
[24] 穆蓁蓁.高温干旱对库尔勒香梨光合特性的影响研究[D].乌鲁木齐:新疆农业大学,2015.
MU Zhenzhen.Research on the impacts of high temperature and drought on Korla fragrant pear photosynthetic characteristics[D].Urumqi:Xinjiang Agricultural University,2015.
[25] HAMEED A,GOHER M,IQBAL N. Heat stress-induced cell death,changes in antioxidants,lipid peroxidation,and protease activity in wheat leaves[J]. Journal of Plant Growth Regulation,2012,31(3):283-291.
[26] MÜLLER-SCHÜSSELE S J,WANG R,GÜTLE D D,JILL R,MARTA R F,MARTIN S,FELIX B,VOLKER M L,STANISLAV K,PETER D,MARKUS S,RALF R,MICHAEL H,ANDREAS J M. Chloroplasts require glutathione reductase to balance reactive oxygen species and maintain efficient photosynthesis[J].The Plant Journal,2020,103(3):1140-1154.
[27] 潘向东,王进文,胡玥,池艺,金松恒.高温胁迫下4 种杜鹃叶片的生理生化响应[J/OL].分子植物育种,2021:1-9.(2021-02-04).https://kns.cnki.net/kcms/detail/46.1068.S.20210203.1903.017.html.
PAN Xiangdong,WANG Jinwen,HU Yue,CHI Yi,JIN Songheng. Physiological and biochemical responses of leaves of four Rhododendron cultivars under heat stress[J/OL]. Molecular Plant Breeding,2021:1-9.(2021-02-04).https://kns.cnki.net/kcms/detail/46.1068.S.20210203.1903.017.html.
[28] 张俊波,李志怡,孙清,杜红梅.高温胁迫对野扇花植株生长及叶中生理生化指标和极性代谢产物的影响[J].植物资源与环境学报,2021,30(2):52-58.
ZHANG Junbo,LI Zhiyi,SUN Qing,DU Hongmei. Effect of high temperature stress on plant growth and physiological and biochemical indexes and polar metabolites in leaves of Sarcococca ruscifolia[J]. Journal of Plant Resources and Environment,2021,30(2):52-58.
[29] 张洁,李天来.日光温室亚高温对番茄光合作用及叶绿体超微结构的影响[J].园艺学报,2005,32(4):614-619.
ZHANG Jie,LI Tianlai.Effects of daytime sub-high temperature on photosynthesis and chloroplast ultrastructure of tomato leaves in greenhouse[J].Acta Horticulturae Sinica,2005,32(4):614-619.
[30] HASANUZZAMAN M,NAHAR K,ALAM M M,ROYCHOWDHURY R,FUJITA M. Physiological,biochemical,and molecular mechanisms of heat stress tolerance in plants[J].International Journal of Molecular Sciences,2013,14(5):9643-9684.
[31] 窦飞飞,张利鹏,王永康,于坤,刘怀锋.高温胁迫对不同葡萄品种光合作用和基因表达的影响[J].果树学报,2021,38(6):871-883.
DOU Feifei,ZHANG Lipeng,WANG Yongkang,YU Kun,LIU Huaifeng. Effects of high temperature stress on photosynthesis and gene expression of different grape cultivars[J]. Journal of Fruit Science,2021,38(6):871-883.
[32] 陈连珠,张雪彬,杨小锋.根际高温对快白菜根系结构、光合及叶绿素荧光参数的影响[J].中国瓜菜,2020,33(2):48-52.
CHEN Lianzhu,ZHANG Xuebin,YANG Xiaofeng. Effects of rhizosphere high temperature on root,photosynthesis and chlorophyll fluorescence parameters of Chinese cabbages[J].China Cucurbits and Vegetables,2020,33(2):48-52.
[33] KILLI D,RASCHI A,BUSSOTTI F. Lipid peroxidation and chlorophyⅡfluorescence of photosystem Ⅱperformance during drought and heat stress is associated with the antioxidant capacities of C3 sunflower and C4 maize varieties[J]. International Journal of Molecular Sciences,2020,21(14):4846.
[34] 王晚霞,高立杨,张瑞,赵婷,张仲兴,王双成,王延秀.2,4-表油菜素内酯对盐碱胁迫下垂丝海棠光合及生理特性的影响[J].果树学报,2021,38(9):1479-1490.
WANG Wanxia,GAO Liyang,ZHANG Rui,ZHAO Ting,ZHANG Zhongxing,WANG Shuangcheng,WANG Yanxiu. Effects of 2,4 epbrassinolide on photosynthetic and physiological characteristics of Malusa halliana under saline-alkali stress[J].Journal of Fruit Science,2021,38(9):1479-1490.