糖和有机酸是影响果实品质的重要代谢物质,与挥发性物质一起决定果实的风味品质[1]。酿酒葡萄适宜的糖和酸含量是决定葡萄酒风味品质的重要因素,葡萄果实中最主要的可溶性糖是葡萄糖和果糖,有机酸主要为酒石酸和苹果酸,此外还含有少量的琥珀酸、草酸和柠檬酸,糖和有机酸的合成与积累主要受蔗糖代谢相关酶和三羧酸循环相关酶的调控[2]。目前生产中主要通过温度、光照、水分、土壤等环境因素的调控和栽培措施来提高葡萄果实糖分和有机酸的积累[3]。
γ-氨基丁酸(GABA)是一种广泛存在于植物体内的四碳非蛋白氨基酸,GABA 支路途径是GABA通过谷氨酸脱羧酶(GAD)生物合成,并绕过TCA循环中2 种关键酶α-酮戊二酸脱氢酶(α-KGDH)和琥珀酰辅酶A 连接酶(SCS)的反应,最后以琥珀酸的形式返回三羧酸循环[4]。研究表明,外源GABA 处理可以提高采后苹果果实中草酸、苹果酸、乌头酸、琥珀酸的水平[5]。Faraj 等[6]的研究结果表明GABA处理提高了柑橘叶片中琥珀酸水平,并且提升了GABA 转氨酶(GABA-T)、琥珀酸半醛脱氢酶(SSADH)、苹果酸脱氢酶和琥珀酸脱氢酶等的活性;GABA在碳代谢与氮代谢之间也发挥重要作用,谷氨酸脱羧酶(GAD)作为GABA合成的关键酶,在GAD基因敲除的拟南芥中发现,拟南芥种子中可溶性糖和有机酸含量明显下降[7-8]。但外源GABA 在葡萄果实发育过程中对果实糖酸积累及代谢的影响鲜有报道。因此,笔者在本研究中以10年生酿酒葡萄蛇龙珠为试材,对葡萄叶片进行不同浓度GABA处理,通过测定葡萄果实糖酸组分、糖酸代谢及GABA 合成相关酶活性,探讨GABA 对葡萄果实发育过程中糖酸代谢机制和GABA代谢的影响,以期为酿酒葡萄优质生产提供理论依据。
1 材料和方法
1.1 试验材料
试验在甘肃农业大学食品科学与工程学院露地葡萄园进行,供试品种为10年生酿酒葡萄品种蛇龙珠,株行距为0.75 m×1.5 m,单干双臂Y 形整形,南北走向。
1.2 试验设计
试验共设4 个GABA 浓度处理:5(T1)、10(T2)、15(T3)、20 mmol·L-1(T4),以蒸馏水处理为对照(CK),于盛花期、坐果期、膨大期、转色期对长势一致、无病虫害的植株进行叶面喷施,以叶片开始滴液为准,每个处理设3 个重复,每个重复5 株。果实采样时间为处理后第3天上午8:00,成熟期再取样1次,每株随机选取1 穗葡萄,冰盒贮运至实验室,液氮冷冻后放入超低温冰箱备用。
1.3 果实可溶性总糖、糖组分含量及蔗糖代谢相关酶活性测定
可溶性总糖含量采用蒽酮硫酸法测定[9],使用高效液相色谱仪(美国Waters Acquity Arc)参照贺雅娟等[10]的方法进行果实蔗糖、葡萄糖和果糖含量的测定。葡萄果肉加液氮研磨后称取0.5 g,加入5 mL 80%乙醇,35 ℃超声提取20 min,12 000 r·min-1离心15 min,重复提取2 次,每次加80%乙醇2 mL,合并上清液,定容至10 mL,真空离心浓缩仪旋转蒸发至全干(60 ℃),用1 mL 超纯水1 mL 乙腈复溶,用0.22 μm 有机相微孔滤膜过滤后加入样品瓶中待测。色谱条件:XBridge BEH Amide色谱柱(4.6 mm×150 mm,2.5 μm),柱温40 ℃,流动相为75%乙腈、0.2%乙胺以及24.8%超纯水,流速0.8 mL·min-1,进样量10 μL,检测波长为254 nm。蔗糖、葡萄糖和果糖的标准曲线分别为y=110 269 x-9 598.4,R2=0.999 3、y=110 132 x-9 849.6,R2=0.999 4、y=104 844 x-10 395,R2=0.999 2。
SuSy-s(蔗糖合酶合成方向)、SuSy-c(蔗糖合酶分解方向)、SPS(蔗糖磷酸合成酶)、AI(酸性转化酶)、NI(中性转化酶)酶液提取参考文献[11]的方法,酶活性测定参考文献[12]的方法。以鲜质量计。
1.4 果实GAD、GABA、有机酸组分含量及有机酸代谢相关酶活性测定
GAD 酶液提取及酶活性测定采用文献[13]的方法,GABA 含量测定采用文献[14]的方法。果实草酸、酒石酸、莽草酸、富马酸、柠檬酸、α-酮戊二酸、苹果酸含量测定参考文献[15]的方法,葡萄果肉加液氮研磨后称取1.5 g,加入7.5 mL 超纯水,4 ℃、10 000 r·min-1离心10 min,用0.22 μm 水相微孔滤膜过滤,将滤液加入样品瓶中待测,使用美国Waters Acquity Arc高效液相色谱仪,亚特兰蒂斯T3柱(4.6 mm×150 mm,3 μm),流动相为20 mmol·L-1 NaH2PO4 溶液(用H3PO4 将pH 调至2.7),流速为0.50 mL·min-1,柱温为30 ℃,检测波长为210 nm,进样量为20 μL。草酸、酒石酸、莽草酸、富马酸、柠檬酸、α-酮戊二酸、苹果酸的标准曲线分别为y=28 071 704.260 7 x-2 742.293 2,R2=0.999 4、y=4 250 792.452 8 x-3 184.606 5,R2=0.999 1、y=102 070 x-30 412,R2=0.997 9、y=174 769.779 9 x+13 427.704 9,R2=0.999 8、y=1 877 841.095 9 x-2 096.5616,R2=0.9986、y=18989x-43769,R2=0.9995、y=1 721 625.744 2 x+5 796.673 9,R2=0.999 9。
CS(柠檬酸合酶)、Cyt-ACO(细胞质乌头酸酶)、Mit-ACO(线粒体乌头酸酶)、NAD-IDH(NAD-异柠檬酸脱氢酶)、PEPC(磷酸烯醇式丙酮酸羧化酶)、NAD-MDH(NAD-苹果酸脱氢酶)、NADP-ME(NADP-苹果酸酶)酶液提取及活性测定参考文献[16-17]的方法,以鲜质量计。
1.5 数据统计分析
采用SPSS 23.0 进行数据处理,方差分析采用Duncan’s多重比较。采用Excel进行图表绘制。
2 结果与分析
2.1 GABA对葡萄果实可溶性总糖及糖组分含量的影响
如图1-A所示,在果实发育过程中,膨大期和转色期可溶性总糖含量迅速增加,成熟期增加缓慢;膨大期GABA处理葡萄可溶性总糖含量较CK提高效果最明显,其中T3处理较CK提高了0.81倍;成熟期GABA 处理均显著提高了可溶性总糖含量(p<0.05),其中T2 处理较CK 提高了0.09 倍,说明外源GABA处理有利于果实可溶性总糖的积累。
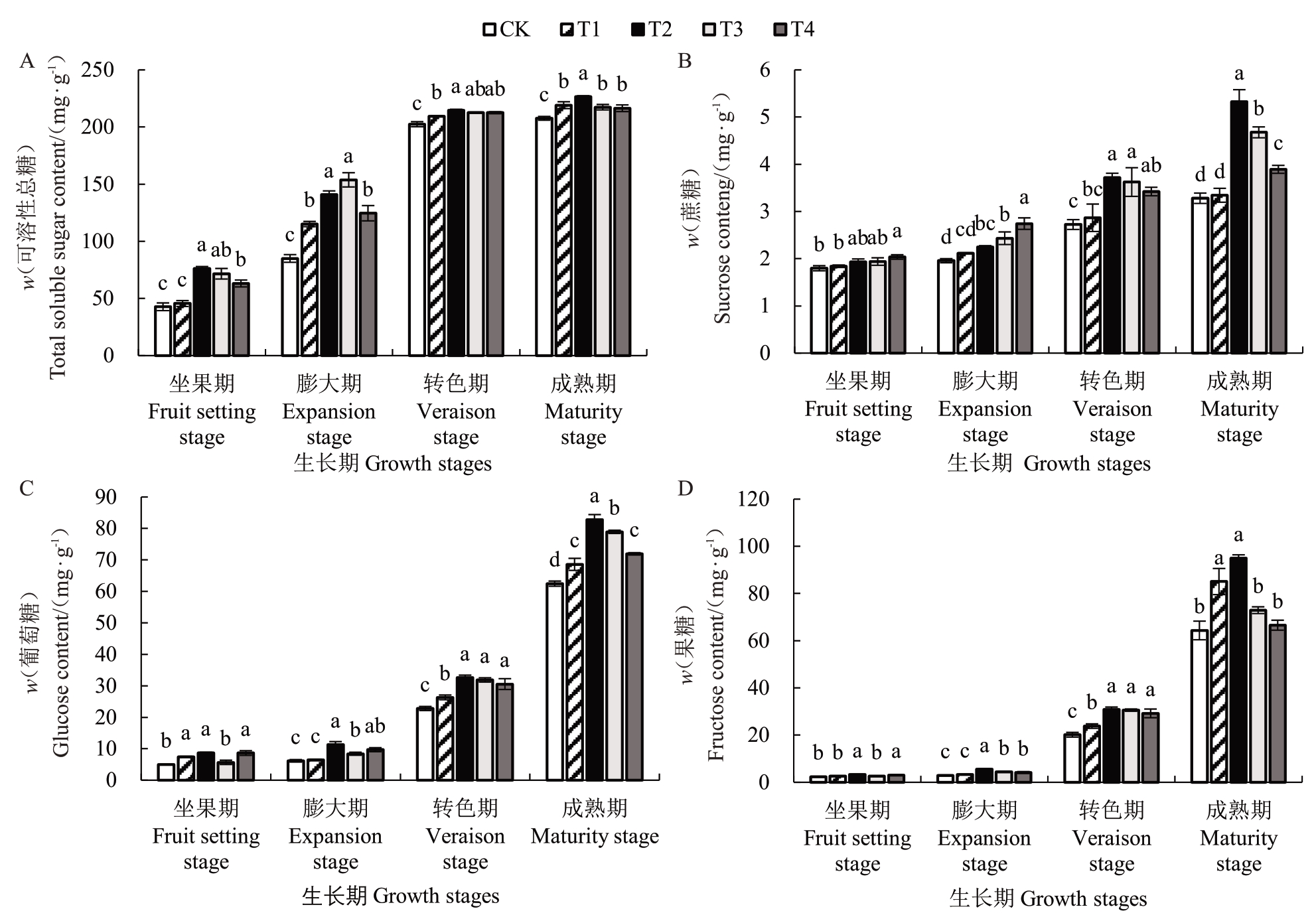
图1 GABA 对葡萄果实可溶性总糖及糖组分含量的影响
Fig.1 Effect of GABA on total soluble sugars and sugar fractions in grape fruit
坐果期和膨大期蔗糖(图1-B)、葡萄糖(图1-C)、果糖(图1-D)含量缓慢增加,随着葡萄果实成熟,葡萄糖、蔗糖、果糖含量迅速增加。成熟期GABA 处理均显著提高了葡萄糖含量(p<0.05),其中T2处理效果最好,较CK提高了0.33倍;成熟期T2、T3和T4处理蔗糖含量较CK均显著提高(p<0.05),其中T2 处理提高效果最为显著,较CK 提高了0.63倍;成熟期T1 和T2 处理果糖含量提高显著(p<0.05),分别较CK提高了0.32倍、0.48倍。
2.2 GABA对葡萄果实蔗糖代谢酶活性的影响
在果实发育过程中CK处理SuSy-s活性呈先升高后降低的变化趋势,葡萄转色期达到最高(图2-A)。经过GABA处理后,各处理SuSy-s活性随着时间的推移,均显著高于CK,其中T2处理效果最为显著(p<0.05),较CK 坐果期、膨大期、转色期和成熟期分别提高了1.79、0.59、0.45、1.11倍。
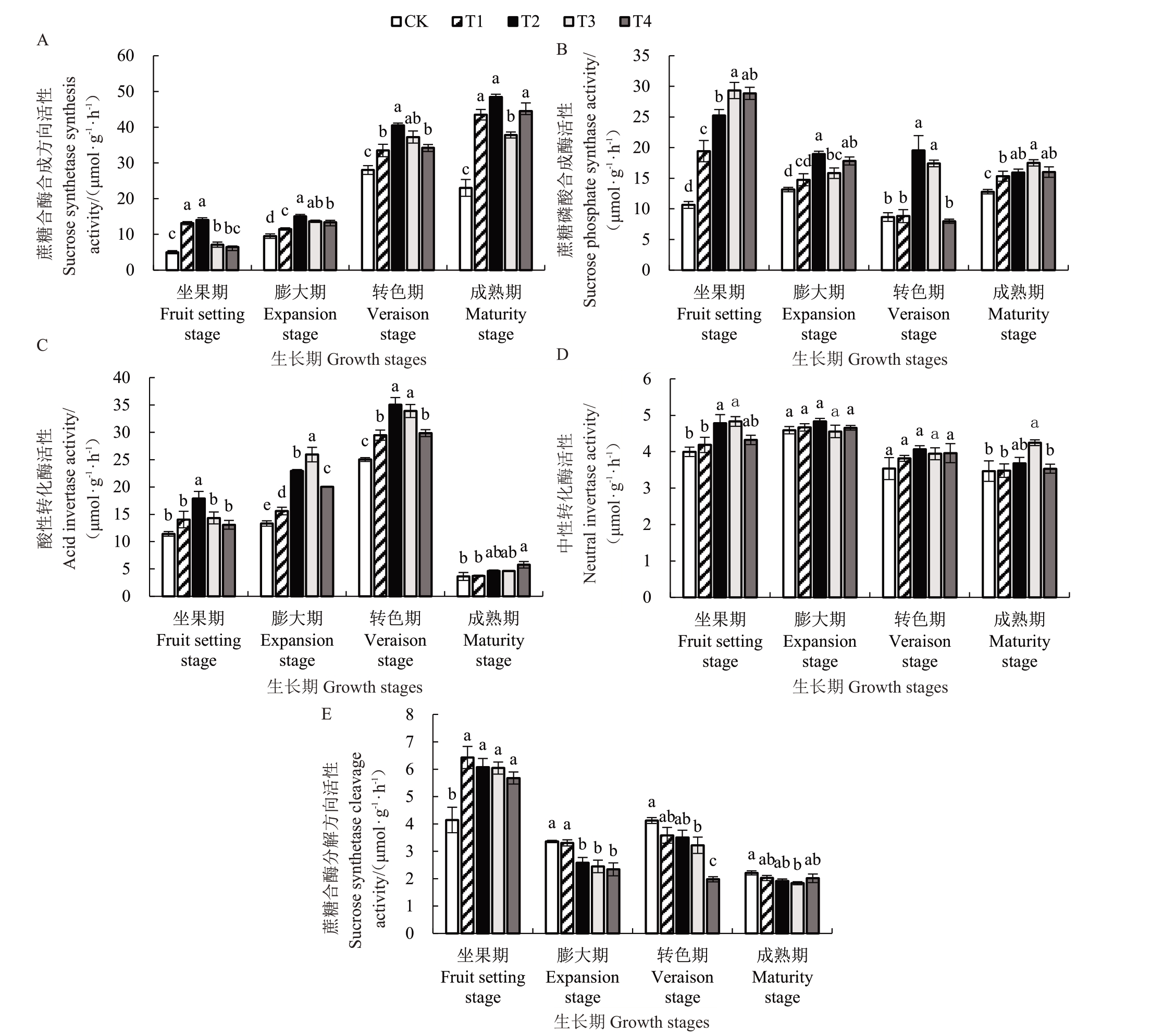
图2 GABA 处理对葡萄果实蔗糖代谢酶活性的影响
Fig.2 Effect of GABA treatment on the enzymatic activity of sucrose metabolism in grape fruit
如图2-B所示,CK处理SPS活性在果实发育过程中呈波动型变化趋势,在膨大期缓慢上升,在转色期到达最低点,随后又开始上升。坐果期GABA处理均显著提高了SPS 活性(p<0.05),分别是CK 的0.83、1.37、1.76、1.71倍;从膨大期至成熟期,T2处理和T3 处理SPS 活性较CK 提高显著且波动较小,转色期效果最为显著(p<0.05),分别是CK 的1.25、1.01 倍。成熟期GABA 处理SPS 活性均显著高于CK(p<0.05),但不同浓度GABA处理差异不明显。
SuSy-c活性(图2-E)在坐果期最高,随着果实的发育呈迅速下降-缓慢升高-迅速下降趋势,坐果期GABA处理均显著增强了SuSy-c的活性(p<0.05),其中,T1 处理效果最好,较CK 提升了0.55 倍;但从膨大期开始至成熟期,GABA 处理表现出明显的抑制作用,膨大期和转色期T4处理的抑制作用表现最强,成熟期T3处理抑制作用最显著(p<0.05),较CK降低了17%。说明T2 处理能够提高SuSy-s、SPS 活性,降低SuSy-c活性,有利于葡萄果实蔗糖大量合成和积累,而T3处理抑制蔗糖分解作用最强。
AI(图2-C)和NI(图2-D)活性随着果实发育均逐渐升高,成熟期AI活性迅速降低,而NI活性降低缓慢。膨大期、转色期GABA处理AI活性均显著提升,其中膨大期T3 处理效果最显著(p<0.05),较CK 提高了0.95 倍,转色期T2 处理最显著(p<0.05),较CK提高了0.40倍,成熟期各处理间差异均不显著。膨大期和转色期各处理NI 活性均没有表现出显著差异,成熟期T3处理效果显著(p<0.05),较CK 提高了0.22 倍。AI 和NI 活性的提高提升了蔗糖转化为葡萄糖和果糖的速率,从而促进了葡萄糖和果糖的积累。
2.3 GABA 对葡萄果实GABA 含量和有机酸组分含量的影响
如图3-A所示,CK处理葡萄GABA含量在果实发育过程中呈先升高后降低的趋势,经过GABA处理后,各处理GABA 含量随着时间的推移,均显著高于对照(p<0.05),其中坐果期T3 处理对GABA含量提升的效果最显著,是CK的3.91倍,说明外源GABA处理能提高葡萄果实GABA含量。
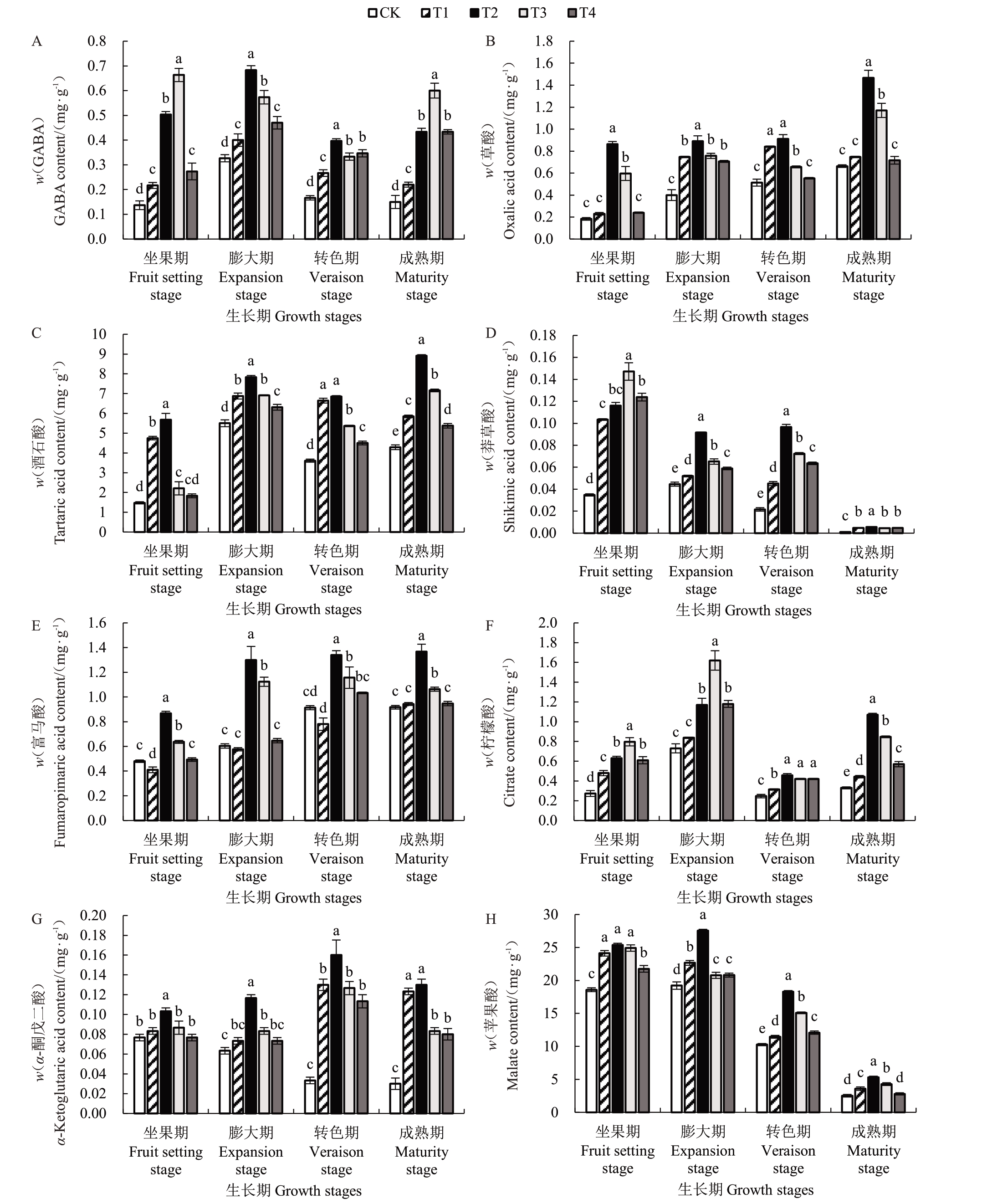
图3 GABA 对葡萄果实GABA 含量和有机酸组分含量的影响
Fig.3 Effect of GABA on the GABA content and organic acid fraction content of grape fruit
草酸含量随着果实成熟逐渐升高(图3-B),而酒石酸含量在果实发育过程中呈现出先升高后略微降低再升高的趋势(图3-C)。经过对GABA 处理后,成熟期草酸及酒石酸含量均显著提高(p<0.05),其中T2 处理的效果最为显著,分别是CK 的1.21、1.08倍,说明不同浓度GABA处理均能提高草酸及酒石酸含量。
莽草酸(图3-D)含量呈先升高后降低的趋势,坐果期各处理莽草酸含量达到峰值,其中T3处理莽草酸含量最高,是CK 的3.23 倍,但随着果实的成熟,各处理莽草酸含量均较低且差异较小;而富马酸含量(图3-E)呈缓慢升高的趋势,转色期和成熟期变化较小,成熟期GABA处理显著提高了富马酸含量(p<0.05),以T2处理效果最显著,富马酸含量较CK 提升了0.49 倍;说明适宜浓度GABA 处理有利于莽草酸及富马酸的积累。
柠檬酸含量(图3-F)随着果实的发育呈先迅速升高、略微降低后再升高的趋势,在膨大期达到峰值,成熟期GABA 处理均显著提高了柠檬酸含量(p<0.05),其中T2处理效果最好,是CK的2.26倍,GABA处理能促进果实柠檬酸的积累。
在果实发育过程中,CK 处理α-酮戊二酸含量(图3-G)呈逐渐降低的趋势,但GABA 处理呈先上升后下降的趋势,转色期达到峰值,其中T2 处理效果最为显著(p<0.05),转色期T2 处理是CK 的3.74倍,成熟期T2处理是CK的3.41倍。
如图3-H所示,CK处理苹果酸含量随着果实发育呈逐渐降低的趋势,但T2处理呈先上升后下降的趋势,与CK相比,T2处理均显著提高了苹果酸含量(p<0.05),其中膨大期T2处理苹果酸含量最高,随着果实成熟迅速降低,成熟期T2 处理是CK 的1.11倍。
2.4 GABA对葡萄果实GAD及有机酸代谢相关酶活性的影响
GAD可以通过谷氨酸合成GABA。如图4-A所示,在果实发育过程中,CK处理葡萄GAD活性缓慢降低,经过GABA 处理后,各处理GAD 活性随着时间的推移,均显著高于CK(p<0.05),且降低幅度较小,其中,成熟期T4 处理提升效果最为显著,较CK提高了0.50倍。
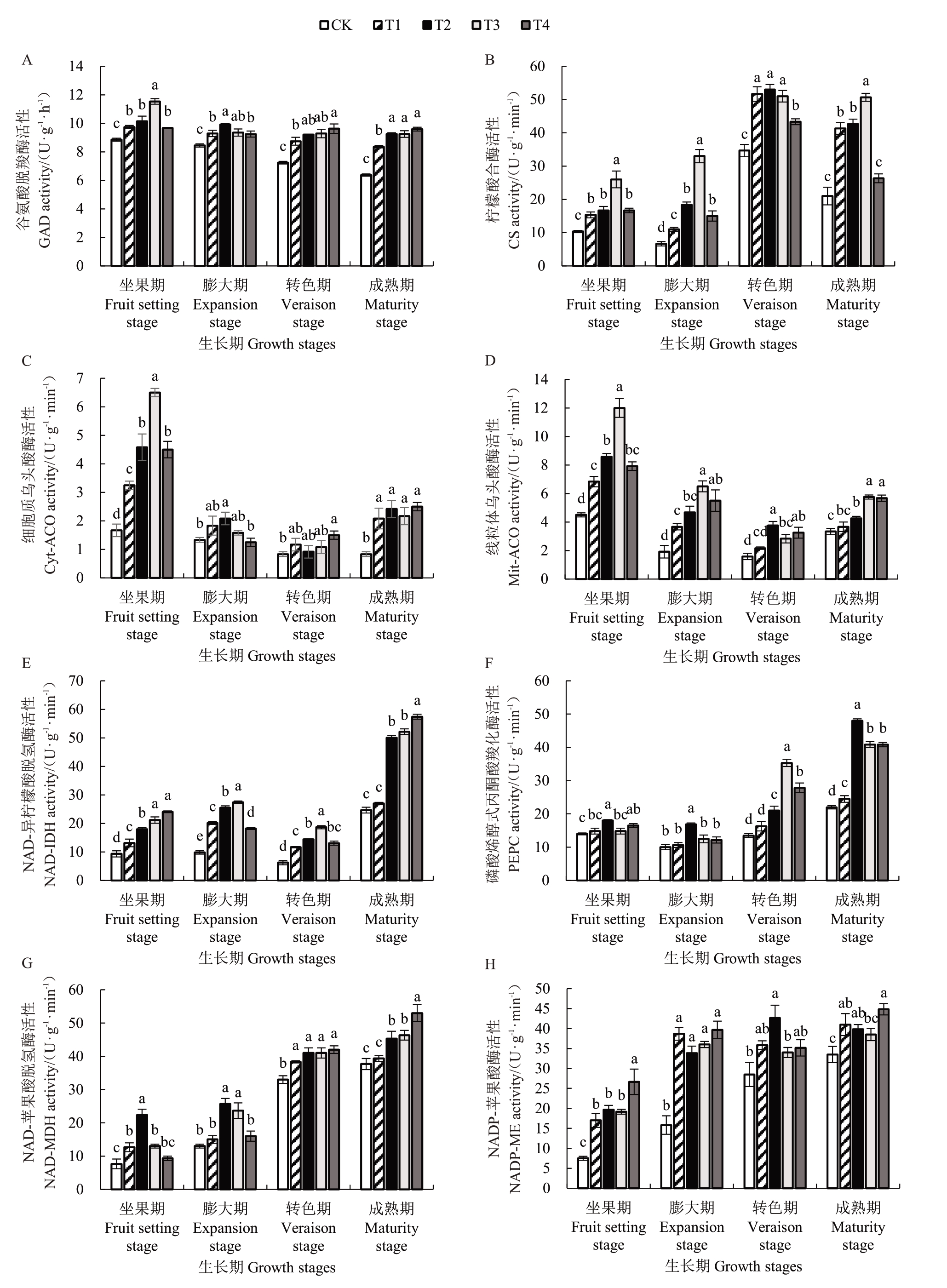
图4 GABA 对葡萄果实GAD 及有机酸代谢相关酶活性的影响
Fig.4 Effect of GABA on the activities of enzymes related to GAD and organic acid metabolism in grape fruit
如图4-B所示,CK处理CS活性呈缓慢降低-迅速升高-迅速降低的趋势,转色期活性最高,成熟期迅速降低。坐果期、膨大期和转色期GABA处理CS活性均显著高于CK(p<0.05),其中膨大期T3 处理的提升效果最显著,CS活性是CK的3.95倍。
ACO 催化柠檬酸产生水和顺乌头酸,Cyt-ACO和Mit-ACO是植物中的2种同工酶。如图4-C、D所示,Cyt-ACO活性在坐果期最高,随着果实发育逐渐降低,各处理Mit-ACO活性在果实发育过程中均呈迅速降低-缓慢上升的变化趋势,转色期Mit-ACO活性最低。坐果期GABA 处理均显著提高了Cyt-ACO 和Mit-ACO 活性(p<0.05),其中T3 处理效果最为显著,分别是CK 的2.90 和1.67 倍。ACO 活性的提高可加快柠檬酸的利用,但ACO 活性较CS 活性低,有利于柠檬酸积累,NAD-IDH 也是柠檬酸降解的控制因子,NAD-IDH 活性(图4-E)在果实发育过程中呈先缓慢降低后迅速升高的变化趋势,成熟期NAD-IDH 活性最高。NAD-IDH 活性成熟期T4处理提升效果最为显著(p<0.05),是CK的1.58倍。
如图4-F 所示,PEPC 活性随果实成熟逐渐上升,成熟期酶活性最高且各处理均差异显著(p<0.05),其中,T2处理提升效果最为显著,较CK提高了0.86倍。NAD-MDH活性在果实发育过程中先迅速升高后缓慢升高(图4-G);成熟期T2、T3 和T4 处理NAD-MDH 活性均与CK 差异显著(p<0.05),其中T4 处理提升效果最显著,较CK 提高了0.41 倍。CK 处理NADP-ME 活性在果实发育过程中逐渐升高(图4-H)。GABA 处理与CK 相比均显著提升了NADP-ME 活性(p<0.05),以膨大期提升效果最显著,分别是CK 的1.44、1.14、1.27、1.51 倍,但不同浓度GABA处理的效果不显著。
2.5 葡萄果实发育过程中GABA 代谢与糖组分含量及糖代谢相关酶活性的相关性
利用SPSS软件对葡萄果实GABA含量、GAD、糖组分含量及糖代谢相关酶活性进行相关性分析,获得相关系数矩阵(表1)。结果表明,GABA 与GAD、NI 呈极显著正相关(p<0.01),与SPS 呈显著正相关(p<0.05)。蔗糖与可溶性糖、葡萄糖、果糖、SuSy-s 呈极显著正相关(p<0.01),与SuSy-c、NI 呈极显著负相关(p<0.01)。葡萄糖和果糖与可溶性糖、蔗糖、SuSy-s 呈极显著正相关(p<0.01),与SuSy-c、NI 呈极显著负相关(p<0.01),与AI 呈显著负相关(p<0.05)。
表1 葡萄果实发育过程中GABA 代谢与糖组分含量及糖代谢相关酶活性的相关性
Table 1 Correlation of GABA metabolism with sugar content and enzymes activity related to sugar metabolism during grape fruit development
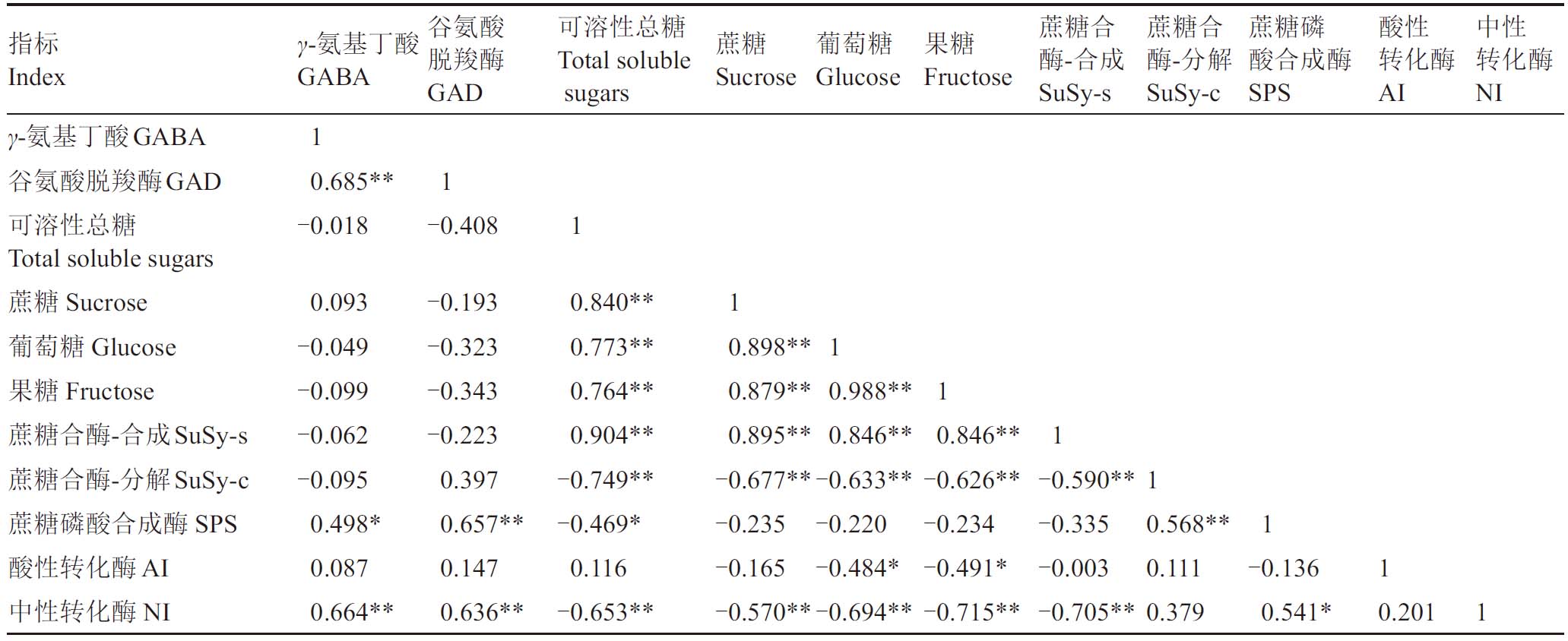
注:*表示在0.05 水平显著相关,**表示在0.01 水平极显著相关。下同。
Notes:*means significant difference(p<0.05),**means extremely significant difference(p<0.01).The same below.
指标Index γ-氨基丁酸GABA谷氨酸脱羧酶GAD可溶性总糖Total soluble sugars蔗糖Sucrose葡萄糖Glucose果糖Fructose蔗糖合酶-合成SuSy-s蔗糖合酶-分解SuSy-c蔗糖磷酸合成酶SPS酸性转化酶AI中性转化酶NI γ-氨基丁酸GABA 1 0.685**-0.018谷氨酸脱羧酶GAD可溶性总糖Total soluble sugars蔗糖Sucrose葡萄糖Glucose果糖Fructose蔗糖合酶-合成SuSy-s蔗糖合酶-分解SuSy-c蔗糖磷酸合成酶SPS酸性转化酶AI中性转化酶NI 1-0.408 1 0.093-0.049-0.099-0.062-0.095 0.498*0.087 0.664**-0.193-0.323-0.343-0.223 0.397 0.657**0.147 0.636**0.840**0.773**0.764**0.904**-0.749**-0.469*0.116-0.653**1 0.898**0.879**0.895**-0.677**-0.235-0.165-0.570**1 0.988**0.846**-0.633**-0.220-0.484*-0.694**1 0.846**-0.626**-0.234-0.491*-0.715**1-0.590**-0.335-0.003-0.705**1 0.568**0.111 0.379 1-0.136 0.541*1 0.201 1
2.6 不同浓度GABA 处理对葡萄果实发育过程中GABA代谢与有机酸组分含量及有机酸代谢相关酶活性的相关性
利用SPSS软件对葡萄果实GABA含量、GAD活性、有机酸组分含量及有机酸代谢相关酶活性进行相关性分析,获得相关系数矩阵(表2)。结果表明,GABA与GAD、柠檬酸呈极显著正相关(p<0.01),与 草 酸、Mit-ACO 呈显著正相关(p<0.01)。草酸与富马酸、酒石酸、IDH、MDH、ME、PEPC呈极显著正相关(p<0.01),与GABA、CS呈显著正相关(p<0.05)。富马酸与草酸、酒石酸、α-酮戊二酸、CS、MDH、ME、PEPC 呈极显著正相关(p<0.01)。酒石酸与草酸、富马酸、ME呈极显著正相关(p<0.01),与柠檬酸、MDH 呈显著正相关(p<0.05)。莽草酸与GAD、苹果酸、Cyt-ACO、Mit-ACO 呈极显著正相关(p<0.01),与MDH、PEPC 呈显著负相关(p<0.05)。柠檬酸与GABA呈极显著正相关(p<0.01),与酒石酸呈显著正相关(p<0.05)。苹果酸与GAD、莽草酸、MDH、PEPC 呈极显著正相关(p<0.01),与Mit-ACO 呈显著正相关(p<0.05),与CS、IDH、ME 呈显著负相关(p<0.05)。α-酮戊二酸与富马酸呈极显著正相关(p<0.01),与GAD、草酸、CS呈显著正相关(p<0.05)。
表2 葡萄果实发育过程中GABA代谢与有机酸组分含量及有机酸代谢相关酶活性的相关性
Table 2 Correlation of GABA metabolism with the content of organic acid components and enzymes activity related to organic acid metabolism during grape fruit development
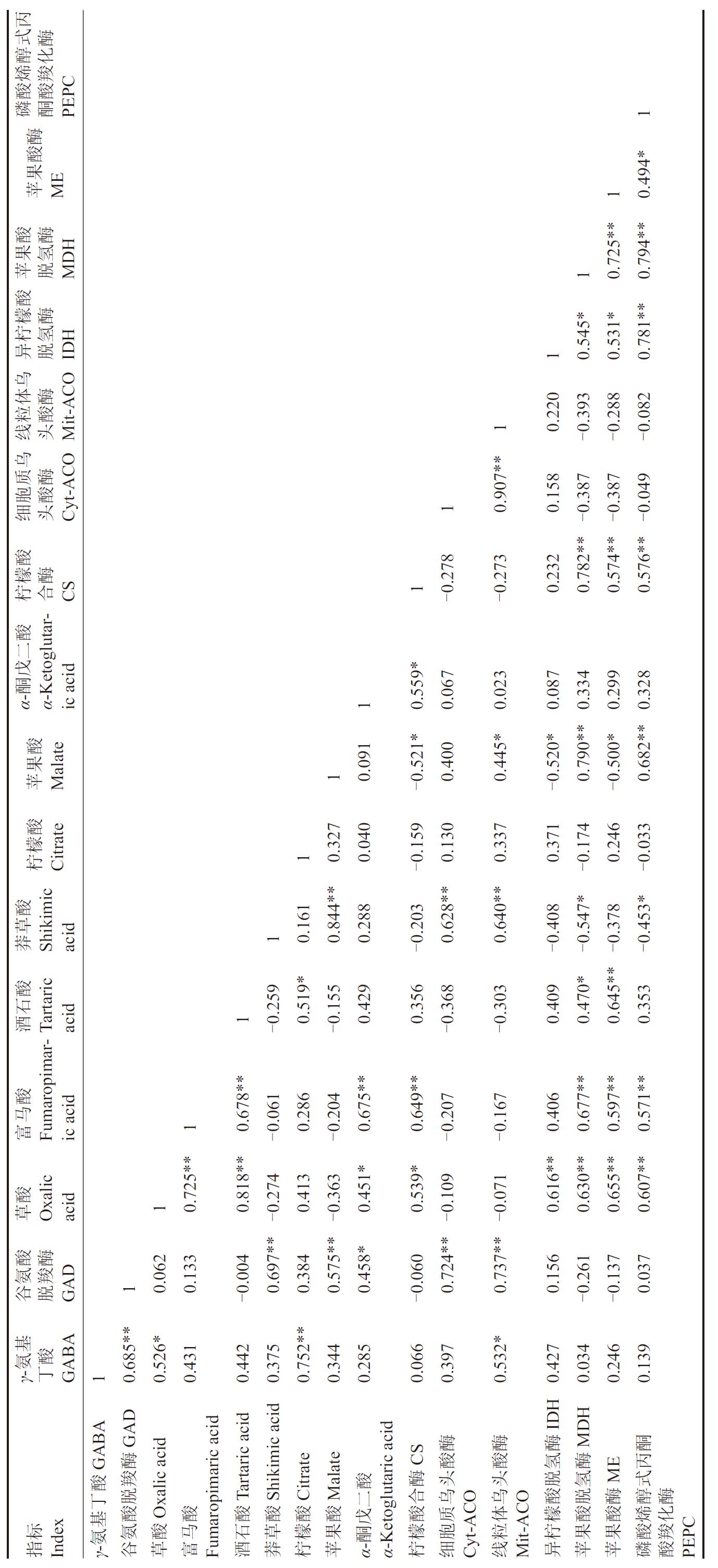
丙式酶醇化烯羧磷酮PEPC酸酸1 Table 2 Correlation of GABA metabolism with the content of organic acid components and enzymes activity related to organic acid metabolism during grape fruit development酶苹ME10.494*酸果 酸酶苹脱MDH果氢10.725**0.794**酸檬酶异脱IDH柠氢10.545*0.531*0.781**乌体酶粒酸线头Mit-ACO 10.220-0.393-0.288-0.082乌质酶胞酸细头Cyt-ACO 10.907**0.158-0.387-0.387-0.049酸檬酶柠合CS1-0.278-0.273 0.232 0.782**0.574**0.576**酸二戊α-酮α-Ketoglutaric acid 10.559*0.067 0.023 0.087 0.334 0.299 0.328苹Malate酸果10.091 0.400 0.445*0.790**0.682**-0.521*-0.520*-0.500*柠Citrate酸檬10.327 0.040 0.130 0.337 0.371 0.246-0.159-0.174-0.033莽Shikimic酸草acid10.161 0.844**0.288-0.203 0.628**0.640**-0.408-0.547*-0.378-0.453*酒Tartaric酸石0.519*0.429 0.356 0.409 0.470*0.645**0.353 acid1-0.259-0.155-0.368-0.303酸富Fumaropimar-马ic acid 10.678**-0.061 0.286-0.204 0.675**0.649**-0.207-0.167 0.406 0.677**0.597**0.571**草Oxalic酸acid10.725**0.818**-0.274 0.413-0.363 0.451*0.539*-0.109-0.071 0.616**0.630**0.655**0.607**酸酶氨羧谷脱GAD10.062 0.133-0.004 0.697**0.384 0.575**0.458*-0.060 0.724**0.737**0.156-0.261-0.137 0.037基γ-氨酸丁GABA 10.685**0.526*0.431 0.442 0.375 0.752**0.344 0.285 0.066 0.397 0.532*0.427 0.034 0.246 0.139 GABA GAD IDH Oxalic acid富Fumaropimaric acid Tartaric acid Shikimic acid Citrate Malate α-Ketoglutaric acid CS 酶 酶 酶MDH酮酶酸 酸 氢酶 丙酸羧 酶酸 头 头 脱氢ME式脱乌丁合 乌 酸脱酶醇酶酸基指Index 酸 酸 酸酸酸二 酸质 体 檬酸酸烯化标戊γ-氨氨酸马 石草檬果 檬胞 粒 柠果果酸羧谷草 酒莽柠苹α-酮 柠细Cyt-ACO线Mit-ACO异苹苹磷酸PEPC
3 讨 论
糖、有机酸是构成果实风味物质的重要组成部分,而在酿酒葡萄中,糖和有机酸含量是影响葡萄酒品质的重要因素。研究表明,酿酒葡萄中的糖类大部分转化为酒精,约10%转化为脂类和酚酸类物质,蔗糖、葡萄糖及果糖含量的提高可以增加葡萄酒的风味强度,而有机酸种类及含量的变化不仅对葡萄酒的口感、色泽及稳定性都有影响,还具有调节酸碱平衡的作用[18];张扬等[19]研究表明酒石酸等有机酸含量的提高可以增强葡萄酒的色泽和香气。在蛇龙珠葡萄发育过程中,各种糖类物质含量逐渐上升,有机酸含量逐渐降低,在果实成熟期,蛇龙珠葡萄中葡萄糖含量略高于果糖含量,蔗糖含量较低,有机酸中酒石酸和苹果酸含量最高,10 mmol·L-1GABA处理后,蔗糖、葡萄糖及果糖含量均显著高于对照,且果糖含量略高于葡萄糖含量,酒石酸、苹果酸、柠檬酸等有机酸含量也显著提高。因此,外源GABA处理提高了酿酒葡萄糖和有机酸含量,使酿酒葡萄果实达到适宜的糖酸含量,可能是提高葡萄酒品质的重要措施。
研究表明,外源喷施GABA 通过提高正常条件和低温条件下番茄叶片叶绿素含量,提高抗氧化酶活性和叶片净光合速率,从而提高可溶性糖、还原糖及非还原糖含量[20]。GAD 作为GABA 支路中GABA 合成的关键酶,拟南芥GAD 突变体种子表现出部分糖和有机酸含量降低[8]。在本研究中,通过喷施不同浓度的GABA,各处理均表现出可溶性糖、葡萄糖、果糖、蔗糖含量增加。
在植物中,蔗糖、葡萄糖及果糖的积累主要由SuSy、SPS、AI 及NI 共同调控,蔗糖的合成通过SuSy和SPS催化,而葡萄糖和果糖的积累则来自蔗糖的分解,由转化酶和SuSy催化[21]。在蛇龙珠葡萄果实发育过程中,在果实发育初期SPS活性较高,在果实发育后期活性下降,SuSy-s 活性则在果实发育的中后期迅速升高,而果实中的蔗糖含量随着果实的成熟逐渐升高,因此,SuSy 可能是决定果实蔗糖积累的重要因素。通过相关性分析发现,蔗糖含量与SuSy-s 活性呈极显著正相关,与SuSy-c 活性、NI活性呈极显著负相关,说明SuSy可能是调控果实蔗糖含量的关键酶,而NI可能是果实成熟过程中调控蔗糖分解的关键酶;这与寇单单等[22]的结果一致。通过分析发现,GABA 含量与SPS 活性呈显著正相关,GABA可能通过影响SPS活性影响蔗糖含量,但其调控机制仍需进一步研究。外源GABA 处理显著提高了葡萄糖和果糖的水平和转化酶活性,而GABA 处理在果实发育中后期,SuSy-c 活性在果实发育过程中呈缓慢降低的趋势,且酶活性均较低,AI 活性在果实发育过程中逐渐升高,且活性较高,AI 可能在调控葡萄糖和果糖积累过程中发挥关键作用,与文献[23-24]结论一致。通过相关分析发现,GABA含量与NI活性呈极显著正相关,说明GABA也可能通过提高NI活性,从而影响蔗糖代谢。研究表明,蔗糖可作为信号分子调控基因的表达,从而影响酶催化活性[25]。蔗糖水平的提高可能提高了转化酶活性,进而促进了葡萄糖和果糖的积累[26]。本研究结果显示外源GABA处理提高了蔗糖合成相关酶的活性,降低SuSy-c活性,促进了蔗糖的积累,通过提高转化酶活性提高了葡萄糖和果糖含量。但其调控机制仍需进一步研究。
谷氨酸脱羧酶(GAD)是谷氨酸合成GABA的限速酶,外源GABA 通过提高GAD、GABA-T 活性和CmGAD基因表达量,促进GABA的生物合成,进而增强了GABA 支路的效率[27-28]。这与本研究的结果一致,外源GABA条件下,GAD活性和GABA含量显著提高,相关性分析表明,GABA含量与GAD活性呈极显著正相关,说明GAD可能是GABA合成的关键酶。
GABA 通过GABA 支路连接三羧酸循环,而三羧酸循环是果实中碳水化合物、有机酸和氨基酸代谢的桥梁[29]。果实中各种有机酸的积累取决于有机酸的合成、降解和利用的平衡[30]。酒石酸、苹果酸、琥珀酸、草酸和柠檬酸是影响葡萄果实风味的主要有 机 酸,受PEPC、NADP-ME、NADP-IDH、Cyt-ACO、NAD-MDH 和CS 直接或间接调节。PEPC 将磷酸烯醇丙酮酸催化为草酰乙酸,随后在NADMDH 存在下降解为苹果酸或通过CS 降解为柠檬酸,而苹果酸可通过NADP-ME 进一步降解为丙酮酸,柠檬酸盐可通过ACO 和NAD-IDH 分解为α-酮戊二酸和二氧化碳[31]。喷施使用同位素标记的GABA 发现,GABA 能快速转化为琥珀酸并进入TCA循环[32]。有学者发现外源GABA 能提高TCA 循环的速率,增强酸代谢相关酶活性,从而促进了有机酸的积累[6,33]。Shoukun[5]认为GABA 处理的果实可加速苹果酸的生物合成和抑制其分解,通过上调MdGAD、MdGABA-T和MdSSADH,促进GABA分流的活性,这可能导致琥珀酸和GABA 的积累。笔者在本研究中发现外源GABA增加了果实中CS、Cyt-ACO、Mit- ACO、NAD- IDH、PEPC、NAD- MDH、NADP-ME的活性,果实中草酸、酒石酸、莽草酸、富马酸、柠檬酸、α-酮戊二酸和苹果酸含量也显著提高,通过相关性分析发现,GABA含量与柠檬酸含量呈极显著正相关,与草酸含量呈显著相关,说明GABA 处理能促进柠檬酸和草酸的积累。可能是外源喷施GABA 后,能使果实中GABA 含量迅速升高,GABA 通过GABA 支路转化为琥珀酸进入TCA 循环,琥珀酸含量的增加通过负反馈调节使α-酮戊二酸含量增加,从而降低柠檬酸的分解速率,促进了柠檬酸的积累;而琥珀酸含量的增加也可提高苹果酸合成的底物水平,从而促进苹果酸的积累;TCA 循环速率的提高为其他中间代谢产物提供了较高水平的底物,促进了草酸、酒石酸、莽草酸、富马酸的积累。有研究表明,GABA调节ALMT(铝活化阴离子蛋白)的活性来引发跨膜电位差的变化,从而影响三羧酸循环的中间代谢产物[34]。Alexis[35]的研究表明VvALMT9 能够介导向内整流苹果酸盐和酒石酸盐电流,促进这些二羧酸在葡萄浆果液泡中的积累。
4 结 论
研究结果表明,外源GABA通过提高SuSy-s和SPS活性,抑制SuSy-c活性来促进蔗糖的积累,通过提高转化酶活性来促进葡萄糖和果糖的积累。外源喷施GABA 通过提高GAD 活性来促进GABA 的生物合成,从而提高TCA循环速率,柠檬酸合酶、细胞质乌头酸酶和线粒体乌头酸酶、NAD-异柠檬酸脱氢酶、磷酸烯醇式丙酮酸羧化酶、NAD-苹果酸脱氢酶和NADP-苹果酸酶活性均显著提高,促进了草酸、酒石酸、莽草酸、富马酸、柠檬酸、α-酮戊二酸和苹果酸的积累,进而改善了葡萄风味品质,以叶面喷施10 mmol·L-1GABA效果最好。
[1] JULIA B,CLAUDIO O B,LUCÍA P,MARTIN A L,VERÓNICA A L,RICARDO M,CARLOS S A,MARÍA F D,MARÍA V L. Carbon metabolism of peach fruit after harvest:changes in enzymes involved in organic acid and sugar level modifications[J]. Journal of Experimental Botany,2009,60(6):1823-1837.
[2] CRYSTAL S,LAURENT G D,GRANT R C,CHRISTOPHER M F,KATHLEEN L S. Regulation of malate metabolism in grape berry and other developing fruits[J]. Phytochemistry,2009,70(11):1329-1344.
[3] 苏静,祝令成,刘茜,彭云静,马百全,马锋旺,李明军.果实糖代谢与含量调控的研究进展[J]. 果树学报,2022,39(2):266-279.
SU Jing,ZHU Lingcheng,LIU Xi,PENG Yunjing,MA Baiquan,MA Fengwang,LI Mingjun. Research progress on sugar metabolism and concentration regulation in fruit[J]. Journal of Fruit Science,2022,39(2):266-279.
[4] ALAN W B,BARRY J S. Does the GABA shunt regulate cytosolic GABA?[J].Trends in Plant Science,2020,25(5):422-424.
[5] SHOUKUN H,YUYU N,WEI Q,YIHENG H,QIUYAN B,YANRONG L,JINGPING R. Exogenous γ-aminobutyric acid treatment that contributes to regulation of malate metabolism and ethylene synthesis in apple fruit during storage[J]. Journal of Agricultural and Food Chemistry,2018,66(51):13473-13482.
[6] FARAJ H,NABIL K. Exogenous GABA is quickly metabolized to succinic acid and fed into the plant tca cycle[J]. Plant Signaling&Behavior,2019,14(3):e1573096.
[7] LI L,DOU N,ZHANG H,WU C X. The versatile GABA in plants[J].Plant Signaling&Behavior,2021,16(3):1862565.
[8] AARON F,ADRIANO N N,RUTHIE A,MARTIN L,PHUONG A P,LUHUA S,RICHARD P H,JOHNATHAN A N,GAD G,ALISDAIR R F.Targeted enhancement of glutamate-toγ-aminobutyrate conversion in Arabidopsis seeds affects carbonnitrogen balance and storage reserves in a development-dependent manner[J].Plant Physiology,2011,157(3):1026-1042.
[9] 刘晓涵,陈永刚,林励,庄满贤,方晓娟.蒽酮硫酸法与苯酚硫酸法测定枸杞子中多糖含量的比较[J]. 食品科技,2009,34(9):270-272.
LIU Xiaohan,CHEN Yonggang,LIN Li,ZHUANG Manxian,FANG Xiaojuan. Comparison of methods in determination of polysaccharide in Lycium barbarum L. [J]. Food Science and Technology,2009,34(9):270-272.
[10] 贺雅娟,马宗桓,韦霞霞,李玉梅,李彦彪,马维峰,丁孙磊,毛娟,陈佰鸿.黄土高原旱塬区不同品种苹果果实糖及有机酸含量比较分析[J].食品工业科技,2021,42(10):248-254.
HE Yajuan,MA Zonghuan,WEI Xiaxia,LI Yumei,LI Yanbiao,MA Weifeng,DING Sunlei,MAO Juan,CHEN Baihong. Comparative analysis of sugar and organic acid contents of different apple cultivars in dryland of loess plateau[J]. Science and Technology of Food Industry,2021,42(10):248-254.
[11] 张弦.不同施钾水平对‘嘎拉’苹果果实糖、酸生理代谢的影响[D].杨凌:西北农林科技大学,2016.
ZHANG Xian. Effects of different potassium level on sugar and acid metabolism in Gala apple fruit[D]. Yangling:Northwest A&F University,2016.
[12] 潘俨.库尔勒香梨果实发育及采后糖代谢与呼吸代谢关系的研究[D].乌鲁木齐:新疆农业大学,2016.
PAN Yan.The relationship between sugar metabolism and respiratory metabolism throughout fruit development and postharvest of Korla Fragrant pear (Pyrus Sinkiangensis Yu)[D]. Urumqi:Xinjiang Agricultural University,2016.
[13] PHAIWAN P,PARITA T,TIPAWAN T,PANATDA J,FENG C,SUDARAT J. Glutamate decarboxylase (gad) extracted from germinated rice: enzymatic properties and its application in soymilk[J]. Journal of Nutritional Science and Vitaminology,2019,65:S166-S170.
[14] PHUONG H L,LIESA V,THIEN T L,YANNICK V,KATLEEN R.Implementation of hplc analysis for γ-aminobutyric acid (GABA) in fermented food matrices[J]. Food Analytical Methods,2020,13(5):1190-1201.
[15] 李彦彪,马维峰,贾进,牟德生,李生保,毛娟.河西走廊不同产地‘赤霞珠’酿酒葡萄果实品质评价[J].西北植物学报,2021,41(5):817-827.
LI Yanbiao,MA Weifeng,JIA Jin,MU Desheng,LI Shengbao,MAO Juan. Evaluation on fruit quality of Cabernet Sauvignon wine grapes from different producing areas in hexi corridor[J].Acta Botanica Boreali Occidentalia Sinica,2021,41(5):817-827.
[16] 罗安才. 柑橘果实有机酸代谢生理和奉节脐橙芽变株系的AFLP 分析研究[D].重庆:西南农业大学,2003.
LUO Ancai. Research on the organic acids metabolism in citrus fruits and the AFLP analysis of the fengjie navel orange mutants[D].Chongqing:Southwest Agricultural University,2003.
[17] 刘丽媛.山葡萄糖酸积累规律及代谢调控机理研究[D].杨凌:西北农林科技大学,2016.
LIU Liyuan.The physiological study on the sugar and acid accumulations and metabolic regulation mechanisms of Vitis amurensis Rupr. grape[D]. Yangling:Northwest A & F University,2016.
[18] 曹炜玉,路文鹏,舒楠,杨义明,范书田.葡萄酒风味物质及其影响因素研究进展[J].中国酿造,2022,41(5):1-7.
CAO Weiyu,LU Wenpeng,SHU Nan,YANG Yiming,FAN Shutian. Research progress on wine flavor substances and their influencing factors[J].China Brewing,2022,41(5):1-7.
[19] 张扬,彭晶晶,李坤一,杨洁,郭安鹊. 发酵前添加有机酸对‘西拉’红葡萄酒颜色和感官质量的影响[J/OL].食品与发酵工业:[2022-08-20]. https://kns.cnki.net/kcms/detail/11.1802.TS.20220712.1408.009.html.
ZHANG Yang,PENG Jingjing,LI Kunyi,YANG Jie,GUO Anque.Effects of organic acid added before fermentation on color and sensory quality of Syrah red wine[J/OL]. Food and Fermentation Industries:[2022-08-20]. https://kns.cnki.net/kcms/detail/11.1802.TS.20220712.1408.009.html.
[20] OLA H AE,AMR E,GNIEWKO N,REHAM F,TOMASZ W,SOUMYA M,AYMAN F A,HUSSIEN M E,AHMED A E,HANY G A E,EHAB A,ADIL A G,NIHAL E N,AHMED M E,AHMED B,MOHAMED F M I.Protective effect of γ-aminobutyric acid against chilling stress during reproductive stage in tomato plants through modulation of sugar metabolism, chloroplast integrity,and antioxidative defense systems[J].Frontiers in Plant Science,2021,12:663750.
[21] MUHAMMAD J U,LUQMAN B S,HAILESLASSIE G,SHENGJIE Z,PINGLI Y,HONGJU Z M O K,MUHAMMAD A,XUQIANG L,NAN H,CHENGSHENG G,WENGE L.Identification of key gene networks controlling organic acid and sugar metabolism during watermelon fruit development by integrating metabolic phenotypes and gene expression profiles[J].Horticulture Research,2020,7(1):193.
[22] 寇单单,张叶,王朋飞,李东东,张学英,陈海江.‘仓方早生’桃及其早熟芽变果实蔗糖和苹果酸积累与相关基因表达[J].园艺学报,2019,46(12):2286-2298.
KOU Dandan,ZHANG Ye,WANG Pengfei,LI Dongdong,ZHANG Xueying,CHEN Haijiang. Differences in sucrose and malic acid accumulation and the related gene expression in‘Kurakato Wase’peach and its early-ripening mutant[J]. Acta Horticulturae Sinica,2019,46(12):2286-2298.
[23] 龚荣高,张光伦,吕秀兰,曾秀丽,罗楠,胡强.脐橙在不同生境下果实蔗糖代谢相关酶的研究[J].园艺学报,2004,31(6):719-722.
GONG Ronggao,ZHANG Guanglun,LÜ Xiulan,ZENG Xiuli,LUO Nan,HU Qiang. Studies on the sucrose-metabolizing enzymes in navel orange fruit from different habitats[J].Acta Horticulturae Sinica,2004,31(6):719-722.
[24] 高彦婷,张芮,李红霞,魏鹏程.水分胁迫对葡萄糖分及其蔗糖代谢酶活性的影响[J].干旱区研究,2021,38(6):1713-1721.
GAO Yanting,ZHANG Rui,LI Hongxia,WEI Pengcheng. Effect of water stress on sugar accumulation and sucrose metabolism enzyme activities of greenhouse grape fruit[J]. Arid Zone Research,2021,38(6):1713-1721.
[25] JULIA W,SJEF S,JOHANNES H. Sucrose: metabolite and signaling molecule[J].Phytochemistry,2010,71(14):1610-1614.
[26] BERNARD F,PHILIPPE J,MARC C,COLETTE G,OLIVIER H,DOMINIQUE R,DIDIER M.Acid invertase as a serious candidate to control the balance sucrose versus(glucose+fructose)of banana fruit during ripening[J]. Scientia Horticulturae,2011,129(2):197-206.
[27] YUXING L,BOYANG L,YUXIAO P,CHENLU L,XIUZHI Z,ZHIJUN Z,WEI L,FENGWANG M,CUIYING L. Exogenous GABA alleviates alkaline stress in malus hupehensis by regulating the accumulation of organic acids[J]. Scientia Horticulturae,2020,261:108982.
[28] 梁静宜,郭凡,赵科,王鸿飞,许凤.外源γ-氨基丁酸对鲜切南瓜品质和γ-氨基丁酸代谢的影响[J].食品工业科技,2022,43(19):385-392.
LIANG Jingyi,GUO Fan,ZHAO Ke,WANG Hongfei,XU Feng. Effect of exogenous quality and γ-aminobutyric acid on GABA metabolism in fresh-cut pumpkins[J]. Science and Technology of Food Industry,2022,43(19):385-392.
[29] ETIENNE A,GÉNARD M,LOBIT P,MBEGUIÉ- AMBÉGUIÉ D,BUGAUD C. What controls fleshy fruit acidity?A review of malate and citrate accumulation in fruit cells[J].Journal of Experimental Botany,2013,64(6):1451-1469.
[30] YANG C,CHEN T,SHEN B R,SUN S X,SONG H Y,CHEN D,XI W P. Citric acid treatment reduces decay and maintains the postharvest quality of peach (Prunus persica L. )fruit[J].Food Science&Nutrition,2019,7(11):3635-3643.
[31] FAMIANI F,BONGHI C,CHEN Z H,DRINCOVICH M F,FARINELLI D,LARA M V,PROIETTI S,ROSATI A,VIZZOTTO G,WALKER R P. Stone fruits:Growth and nitrogen and organic acid metabolism in the fruits and seeds:A review[J].Frontiers in Plant Science,2020,572601.
[32] FARAJ H,NABIL K.The use of deuterium-labeled gamma-aminobutyric(d6-GABA)to study uptake,translocation,and metabolism of exogenous GABA in plants[J].Plant Methods,2020,16(1):24.
[33] ZHOU L,JINGJIN Y,YAN P,BINGRU H.Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera)[J]. Scientific Reports,2016,6(1):30338.
[34] MATTHEW G,STEPHEN D T. Linking metabolism to membrane signaling: the GABA- malate connection[J]. Trends in Plant Science,2016,21(4):295-301.
[35] ALEXIS D A,ULRIKE B,RITA F,JINGBO Z,MARIA M C,ANA R.The vacuolar channel vvalmt9 mediates malate and tartrate accumulation in berries of Vitis vinifera[J]. Planta,2013,238(2):283-291.