葡萄是一种深受大众喜爱的水果,果肉质地柔软、多汁,成熟后易落粒和腐烂,严重地影响货架期和商品质量[1]。根据葡萄果肉质地差异和耐压力强弱,葡萄果肉硬度分为硬、中和软三种类型,其中软肉型的果实极不耐贮藏[2]。解析软/硬肉葡萄果实硬度变化的生理基础和果实细胞结构变化对葡萄采后贮藏保鲜的影响具有重要意义。
果肉质地的软硬主要与果肉细胞壁组分变化、果肉细胞排列、大小等有关[3-4]。果实细胞壁组分主要由果胶、纤维素、半纤维素及少量结构蛋白等构成。果实的软化过程伴随着这些细胞壁组分降解和细胞壁结构的损坏[4-5]。研究发现细胞壁组分与降解酶活性的变化紧密相关,欧洲梨、安溪柿、杧果、番茄、香蕉、大樱桃、草莓等果实软化或采后贮藏过程中,果胶甲酯酶(PME)、多聚半乳糖醛酸酶(PG)、果胶酸裂解酶(PL)等酶活性升高加速了原果胶的降解,导致果实快速软化[4,6-10]。此外,果实质地差异与细胞壁结构有关,黑莓[11]、蓝莓[12]、山楂[13]等不同质地的果实,细胞壁的完整性和钙含量差异是造成果实软化和果肉质地差异的主要原因之一。
葡萄果实成熟软化主要与细胞壁组分的降解与重构[1,14,15]、果胶物质的解聚[15-16]、钙桥的形成[1,14]等有关。软/硬肉葡萄果实质地差异的生理和细胞学基础尚不清楚,笔者在本研究中以成熟期相近的软/硬肉葡萄为材料,系统分析了果实成熟软化过程中细胞壁组分和相关酶活性的变化,以期为揭示葡萄果实软化和质地差异的生理基础提供理论依据。
1 材料和方法
1.1 植物材料
于2020 年5 月至2020 年8 月,以硬肉品种黎明无核和软肉品种灰比诺为试材[2],供试葡萄果实取自于中国农业科学院郑州果树研究所国家葡萄种质资源圃。分别在葡萄花后21、35、49、63、77、91 d,选取大小一致、无病虫害的果实0.5~2.0 kg。每次采样后将果实随机分为两组,一组用于硬度等生理指标测定,另一组用液氮速冻,置于-80 ℃超低温冰箱中保存备用。
1.2 方法
1.2.1 果实硬度测定 每组样品分别选取20~30个葡萄果粒,使用数显果实硬度计(艾德堡,GY4)进行硬度测定,竖直向下匀速缓慢按压至果实发生形变,以峰值均值作为果实硬度,结果以N表示。
1.2.2 果实细胞壁物质含量的测定 细胞壁纤维素和半纤维素含量均使用试剂盒测定(CLL-1-Y,BXW-1-G,科铭生物,江苏苏州)。首先进行细胞壁物质提取,称取约0.3 g 果实,先后进行匀浆、水浴、离心、洗涤,获得粗细胞壁;然后加入1 mL的DMSO溶液浸泡15 h去除淀粉,4000 g离心10 min,沉淀干燥后获得细胞壁物质(CWM)。纤维素和半纤维素含量的测定方法均参考试剂盒说明书。原果胶和可溶性果胶含量测定参照曹建康等[17]的方法进行。
1.2.3 细胞壁降解酶活性测定 PME 活性和PL 活性均使用试剂盒进行测定(PME-2-G,PL-1-G,科铭生物,江苏苏州)。PG 活性测定参照曹建康[17]的方法进行。纤维素酶(Cx)活性测定参照王鸿飞等[18]的方法进行。
1.2.4 果实细胞解剖结构观察 将新鲜采集的葡萄果实用超薄刀片横切成5 mm厚的薄片,迅速投入FAA溶液(无水乙醇、水、37%甲醛、冰醋酸体积比10∶7∶2∶1)进行真空固定,并低速震荡24 h(4 ℃,50 r·min-1)。依次用50%、70%、85%、95%和100%的乙醇进行逐级脱水,分别用25%和100%二甲苯透明1次和3次,最后用25%的石蜡和75%的二甲苯进行渗蜡和石蜡包埋。
使用石蜡切片机(RM2016,徕卡仪器,上海)连续切制厚度为4 μm的切片,挑选完整的切片放置在载玻片上进行烤片(60 ℃),石蜡烤化后取出常温保存备用。将石蜡切片依次放入二甲苯和无水乙醇浸泡2次,使用75%酒精和自来水漂洗干净;放入甲苯胺蓝染液中染色2~5 min,用自来水漂洗2 次后烤干;放入二甲苯透明10 min后,使用中性树胶封片。最后进行显微镜镜检(Eclipse E100,尼康,日本)和图像采集分析(DS-U3,尼康,日本)。
1.2.5 数据分析 每组试验至少3 次重复,试验数据分别采用Excel 2016和SPSS 22.0进行统计和相关性分析,并使用Duncan新复极差法检验差异显著性。
2 结果与分析
2.1 软/硬肉葡萄果实硬度变化分析
在葡萄果实的发育过程中,两个品种的果实硬度均表现为先上升后下降的趋势(图1)。黎明无核和灰比诺果实硬度分别在花后35 d 和49 d 出现峰值,随后快速软化,花后63 d 灰比诺果实开始转色(图1)。在果实发育的各个时期,除花后第49天外,黎明无核硬度均显著高于灰比诺;在果实完熟时(花后91 d),黎明无核的果实硬度比灰比诺的果实硬度高2.8倍。
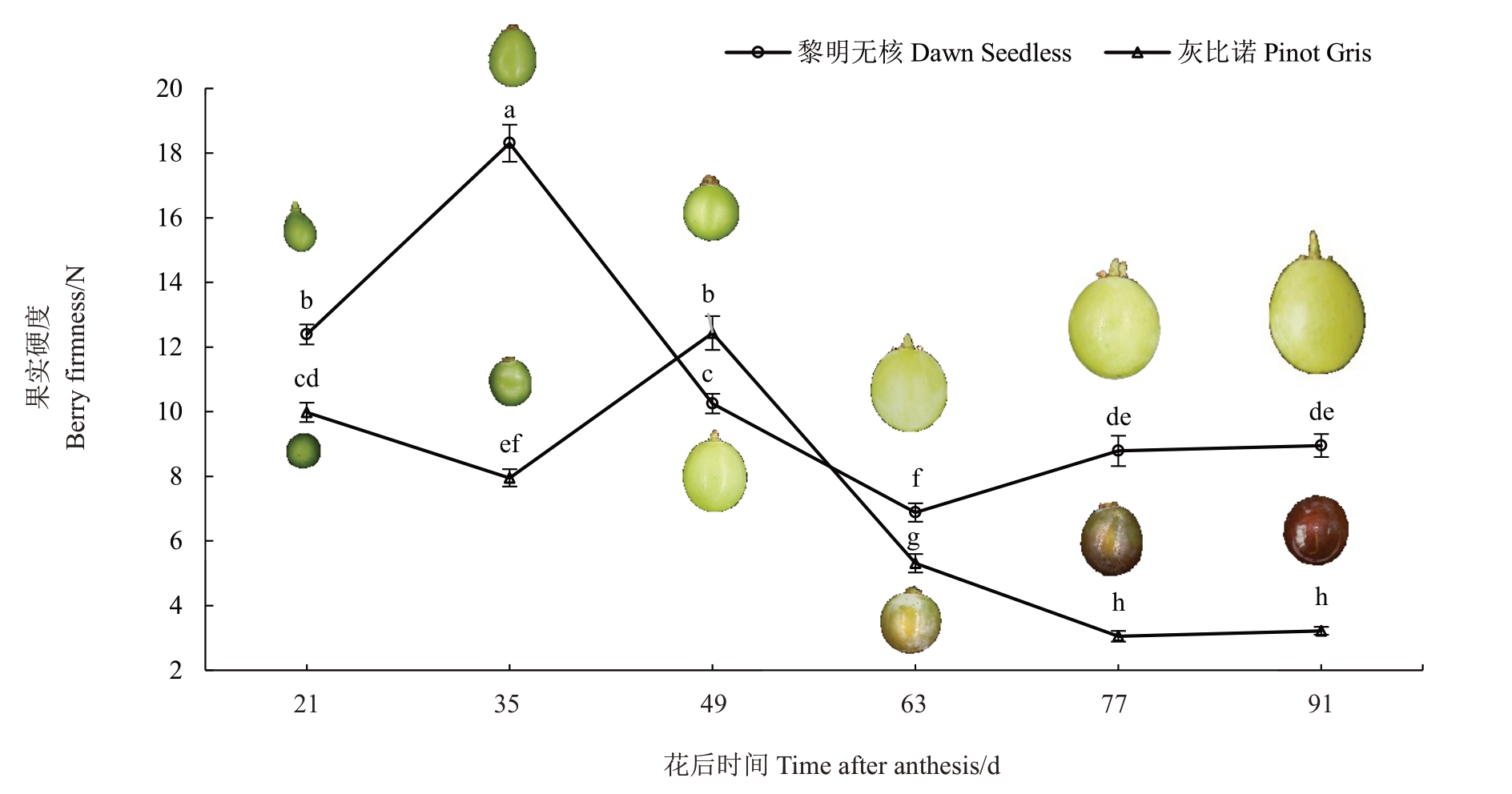
图1 葡萄果实硬度变化
Fig.1 Changes in firmness of grape fruits
不同小写字母表示处理之间在p<0.05 水平上存在显著差异;误差线代表标准误。下同。
The different lowercase letters indicate a significant difference among the treatments at the p<0.05 level. Error bars represent standard error.The same below.
2.2 果实细胞壁物质含量变化
在葡萄果实成熟软化过程中,两个品种的原果胶含量总体呈下降趋势,且灰比诺的原果胶含量均显著高于黎明无核原果胶含量(图2-A)。在黎明无核葡萄成熟过程中,果实的原果胶含量在花后35 d最高,然后迅速下降,但花后49~91 d 含量变化差异不显著;而WSP 含量与原果胶的变化趋势相反,WSP含量在花后35~77 d无显著差异,而在花后91 d显著高于其他时期(图2-A,B)。灰比诺的原果胶含量在果实成熟过程中一直呈下降趋势,而WSP含量在各个时期均无显著差异(图2-B)。
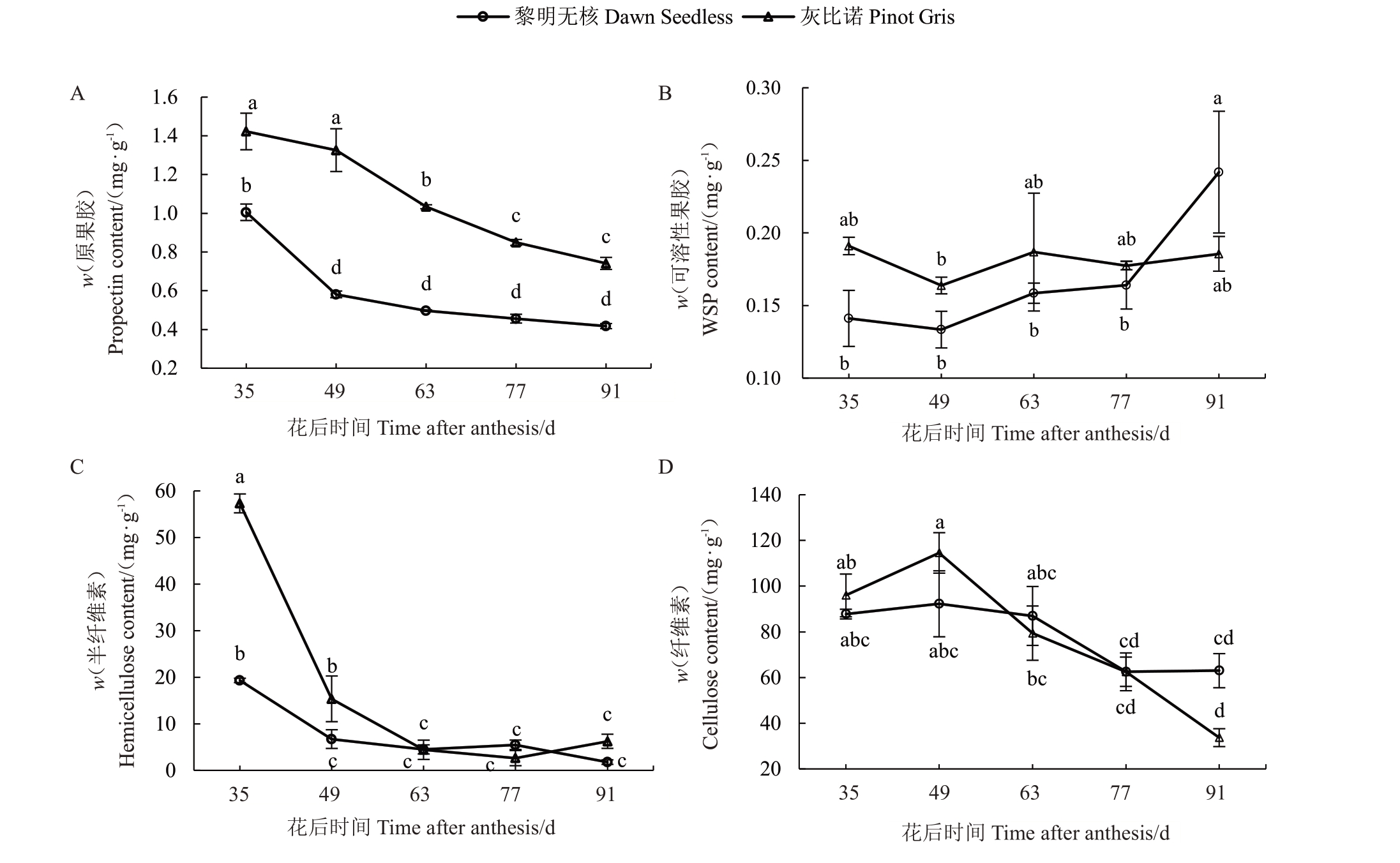
图2 葡萄果实细胞壁物质含量变化
Fig.2 Changes in cell wall components in grape fruits
对葡萄果实发育中半纤维素和纤维素含量分析结果显示,半纤维素含量变化较为显著(图2-C)。黎明无核和灰比诺的半纤维素含量均在花后35~49 d快速下降,如灰比诺的半纤维素含量从花后35 d 的57.32 mg·g-1下降到花后49 d的15.37 mg·g-1,降低了73%。同时,在花后35~49 d灰比诺半纤维素含量显著高于黎明无核的含量。在果实发育的63~91 d,两个品种的半纤维素含量均维持较低水平且无显著差异(图2-C)。两个品种的纤维素含量分析的结果表明,在黎明无核果实成熟过程中,纤维素含量无显著变化,而灰比诺纤维素含量总体呈下降趋势(图2-D)。
2.3 细胞壁降解酶活性变化
多聚半乳糖醛酸酶(PG)能将果胶主链水解为单个半乳糖醛酸分子以降解果胶,实现果实的软化。在果实生长发育过程中,黎明无核果实PG 酶活性在花后35~77 d 无显著变化,在果实完熟时迅速上升,达到最高。灰比诺果实PG 酶活性呈先升高后降低的趋势,峰值出现在花后77 d(图3-A)。
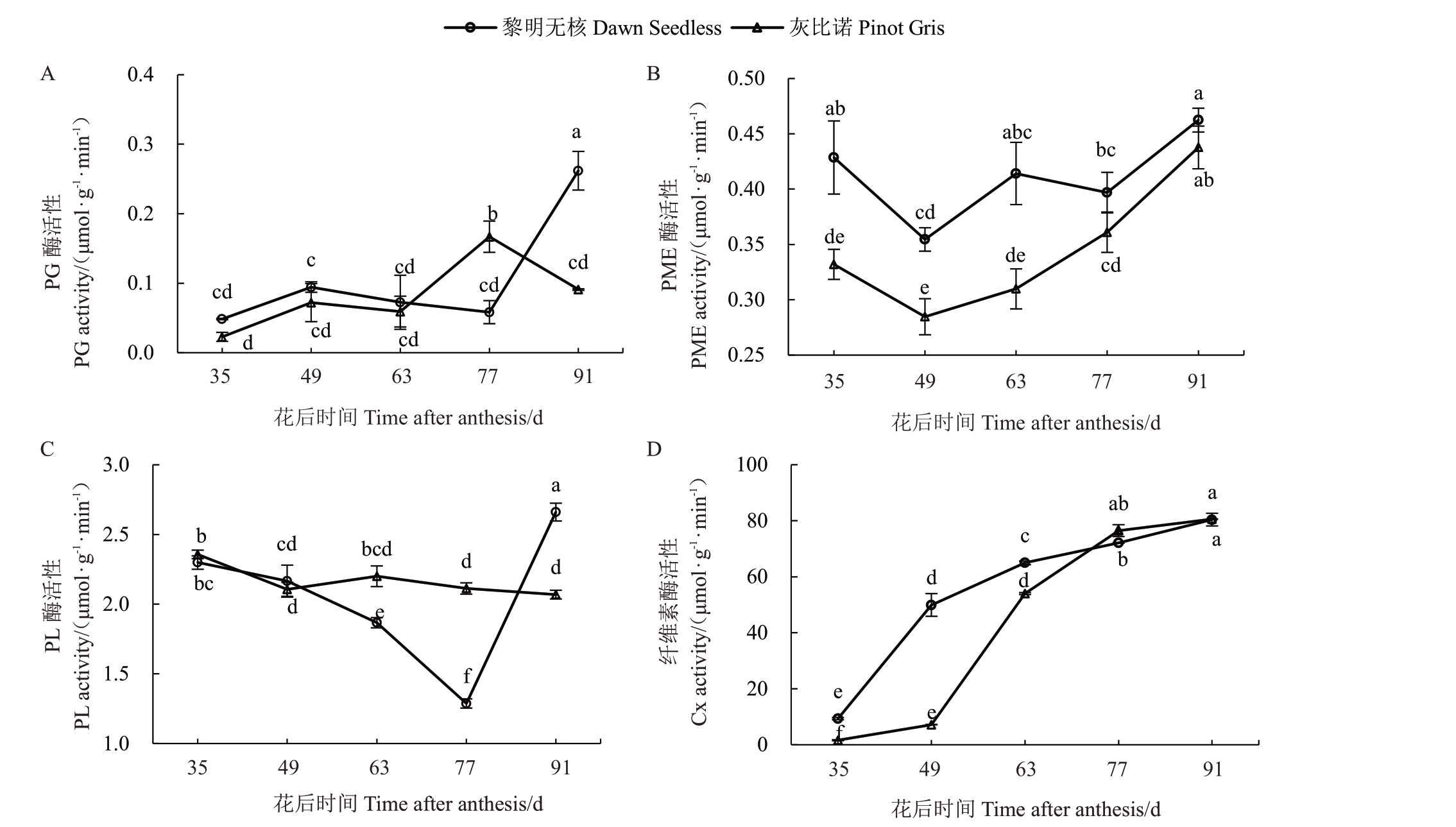
图3 葡萄果实细胞壁降解酶活性变化
Fig.3 Changes in the activities of the cell wall modifying enzymes in grape fruits
A.PG 酶活性;B.PME 酶活性;C.PL 酶活性;D.纤维素酶活性。
A.PG activity;B.PME activity;C.PL activity;D.Cx activity.
果胶甲酯酶(PME)主要催化甲基化的果胶甲酯转化为果胶酸,去甲基后由多聚半乳糖醛酸酶(PG)降解果胶主链。在生长发育过程中,两个品种PME活性变化均呈现先下降后上升的趋势,在花后49 d时PME 活性最低。黎明无核的PME 活性在花后35~63 d均显著高于灰比诺,在果实发育的77~91 d,两个品种PME酶活性无显著差异(图3-B)。
PL 活性在两个品种中也表现出不同的趋势。在黎明无核果实中,PL活性呈现先降低再升高的趋势,在花后77 d 达到最低。在灰比诺果实中,PL 活性无显著变化(图3-C)。
纤维素酶(Cx)降解细胞壁中的纤维素,使细胞壁结构松弛,导致果实软化。在葡萄生长发育过程中,两个葡萄品种Cx 酶活性均呈逐渐上升的趋势(图3-D)。在花后35~63 d,黎明无核Cx活性均显著高于灰比诺,而在花后77~91 d,两品种Cx活性无显著差异(图3-D)。
2.4 生理指标的相关性分析
在黎明无核果实发育过程中,果实硬度的变化与原果胶和半纤维素含量呈显著正相关,与Cx活性呈显著负相关;同时WSP 含量与PG 活性呈显著正相关,原果胶和半纤维素含量与Cx活性均呈极显著负相关(表1)。灰比诺果实在发育过程中,硬度与纤维素含量呈显著正相关,与Cx 活性呈负相关;此外纤维素含量与Cx活性和PME活性均呈显著负相关,原果胶含量与Cx活性呈负相关(表1)。
表1 葡萄果实细胞壁物质含量、细胞壁降解酶活性与硬度的相关性分析
Table 1 Correlation of the contents of cell wall components and activities of their degradaing enzymes activities with the firmness of grape fruits
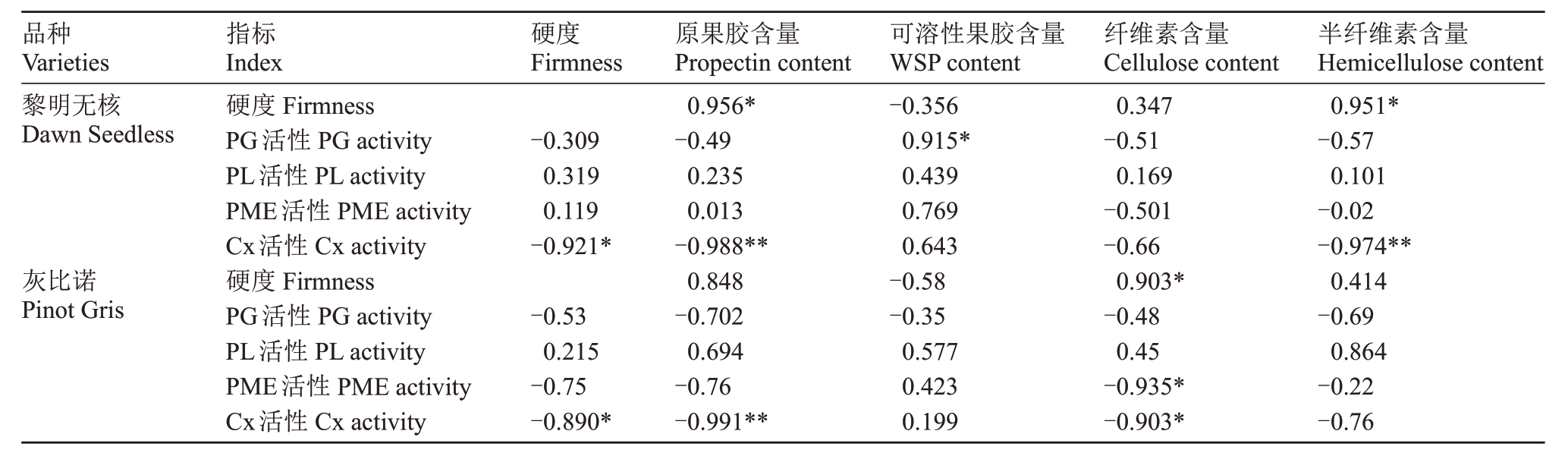
注:*显著线性相关,**极显著线性相关。
Note:*Significant linear correlation,**extremely significant linear correlation.
品种Varieties黎明无核Dawn Seedless硬度Firmness-0.309 0.319 0.119-0.921*灰比诺Pinot Gris半纤维素含量Hemicellulose content 0.951*-0.57 0.101-0.02-0.974**0.414-0.69 0.864-0.22-0.76指标Index硬度Firmness PG活性PG activity PL活性PL activity PME活性PME activity Cx活性Cx activity硬度Firmness PG活性PG activity PL活性PL activity PME活性PME activity Cx活性Cx activity-0.53 0.215-0.75-0.890*原果胶含量Propectin content 0.956*-0.49 0.235 0.013-0.988**0.848-0.702 0.694-0.76-0.991**可溶性果胶含量WSP content-0.356 0.915*0.439 0.769 0.643-0.58-0.35 0.577 0.423 0.199纤维素含量Cellulose content 0.347-0.51 0.169-0.501-0.66 0.903*-0.48 0.45-0.935*-0.903*
2.5 果实细胞形态变化
对葡萄果实硬度发生显著变化的四个时期的果肉细胞进行了显微结构观察,结果显示在果实发育过程中,两个品种的果肉细胞的大小和排列均发生了巨大的变化(图4)。在幼果期(21 d),黎明无核和灰比诺果肉细胞大小均匀、排列紧密整齐,二者在细胞结构和排列上无明显变化(图4-A1,B1)。随着果实的发育和膨大(35~49 d),两个品种的果肉细胞明显变大,但细胞大小不均匀、排列较松散(图4-A2,A3,B2,B3);其中灰比诺在花后49 d呈现果皮和果肉细胞结合不紧密(图4-B3)。在果实开始软化和着色时(63 d),两个品种的细胞排列和大小均杂乱无章,尤其灰比诺果肉细胞的细胞壁边界模糊,开始出现大量降解现象;相比较而言,黎明无核的细胞壁基本保持完整,未出现细胞壁降解现象(图4-A4)。

图4 葡萄果实细胞的显微结构观察
Fig.4 Changes in cell microstructure of grape fruits
A1~A4.黎明无核;B1~B4.灰比诺;A1,B1.21 d;A2,B2.35 d;A3,B3.49 d;A4,B4.63 d。
A1-A4.Dawn Seedless;B1-B4.Pinot Gris;A1,B1.21 d;A2,B2.35 d;A3,B3.49 d;A4,B4.63 d.
3 讨 论
3.1 原果胶、纤维素及半纤维素的降解导致葡萄果实软化
纤维素是果实细胞壁的骨架,半纤维素与纤维素形成交联结构,共同维持细胞骨架的稳定。在苹果和梨果实成熟过程中,纤维素含量与秦冠的硬度变化呈显著相关,而半纤维素含量与富士苹果和库尔勒香梨硬度呈极显著相关[22-23]。在樱桃果实中,纤维素降解速率不同是导致质地差异的主要因素之一[10]。巨峰葡萄果实软化过程中,果胶和半纤维素的降解、半纤维素和纤维素含量的降低是前期软化的主要原因之一[16]。硬肉红地球和软肉玫瑰香的成熟软化与原果胶和可溶性果胶含量密切相关[15]。不溶性的原果胶降解为可溶性的果胶和果胶酸,引起胞间层溶解和细胞壁结构破坏,导致果实软化[19-20]。在大樱桃的果实成熟软过程中,硬度高、耐贮藏品种可溶性果胶含量低,软化速度慢,而硬度低,不耐贮藏的品种可溶性果胶含量高,软化速度快[10]。在桃果实成熟过程中共价紧密结合型果胶转变为离子可溶性果胶,软溶质品种下降速率明显高于硬溶质品种,细胞壁多糖的降解速度亦存在明显差异[21]。本研究结果显示,在细胞壁的结构物质中,原果胶、半纤维素和纤维素含量均与软/硬肉葡萄果实软化有关,尤其是原果胶和半纤维素含量在两个品种中差异较显著,这进一步表明了葡萄果实的软化与原果胶和半纤维素降解均有关系,是细胞壁组分解聚综合作用的结果。
3.2 葡萄细胞壁降解酶活性分析
与果实细胞壁组分降解中最为关键的水解酶是PME、PG和PL等,它们协同作用于果胶的降解,Cx和木葡聚糖内糖基转移酶(XTH)分别参与纤维素和半纤维素的水解。在苹果早熟品种(系)中,PME酶活性呈先上升后下降又上升的趋势[24]。在甜樱桃生长发育过程中,PME与美早和红灯硬度呈显著负相关,而软肉品种佳红PME 活性几乎没有变化[10]。在葡萄果实发育中,硬肉NN107的PME活性比软肉的Thompson Seedless高,且在果实完熟前活性无显著变化[1]。本研究的结果同样显示在果实转色前,硬肉品种黎明无核的PME 活性均比软肉品种灰比诺高,预示PME 活性变化可能不是造成软/硬肉葡萄果实质地差别的主要原因。
PG 将去酯化的果胶分子降解为半乳糖醛酸,PL 通过β-消除方式随机断裂多聚半乳糖醛酸来降解细胞壁。PG 活性在芒果和柿子的成熟软化过程中呈上升趋势,在猕猴桃和苹果呈先上升后下降趋势[25-27],在樱桃中,PG 可能是硬度变化的次要原因[10]。在香蕉成熟过程中,PL酶活性的高峰与呼吸跃变高峰相一致,跃变之后,PL 酶活性逐渐降低[6]。沉默表达番茄SlPL 基因显著降低果实可溶性果胶含量、提高硬度,由此认为PL 是番茄果实软化的关键因子之一[9]。本研究结果显示,在葡萄果实的成熟软化过程中,两个品种的PG 和PL 活性变化差异不显著,且与果实硬度的相关性不显著,这表明葡萄果实中果胶降解可能是PME、PG、PL等降解酶综合作用的结果。
木葡聚糖转移酶和纤维素酶分别是半纤维素与纤维素降解的关键酶,在果实的软化过程中起着重要的作用。在柿果实成熟和软化过程中,木葡聚糖转移酶关键基因DkXTH8 的表达量最高,过表达DkXTH8 基因导致了番茄细胞致密性变差、果实软化加速[28]。在大樱桃软化过程,Cx活性并没有影响到果实硬度[10]。但在本实验中发现,软硬肉葡萄品种的Cx活性均与果实的硬度呈显著相关,这表明纤维素酶活性变化可能是引起葡萄果实软化的重要因素之一。
3.3 果肉细胞壁结构的完整性影响了果实硬度的变化
随着果实成熟软化,原果胶降解,侧链中性糖损失,细胞壁发生不可逆转的变化,最终导致细胞的破裂[4,29-31]。在番茄果实成熟过程中,抑制细胞壁多糖解聚,保持细胞壁结构完整,可抑制成熟软化进程[32]。果胶结构完整的樱桃具有较高果实硬度[33]。耐压力强的红地球、秋红、秋黑果肉细胞大且分布均匀、紧密,而易软化的巨峰果肉细胞大小不均匀、松散[3];同时,硬肉品种NN107 的细胞壁解聚率较低,也是不易软化的重要原因[1]。笔者在本研究中发现果实开始软化和着色时(63 d),硬肉品种黎明无核的细胞壁结构保持完整,而软肉品种灰比诺已经部分降解;同时发现灰比诺转色前半纤维素含量急剧下降,这表明果肉细胞壁组分降解速度和维持细胞壁完整性是影响葡萄果肉质地的重要因素。
4 结 论
在葡萄果实成熟软化过程中,原果胶和半纤维素快速降解,导致了果肉细胞壁的破裂是葡萄果实软化的关键因素;果实细胞壁组分的降解是由多种酶共同作用的结果,其中以纤维素酶的作用较为显著。
[1] BALIC I,EjSMENTEWICZ T,SANHUEZA D,SILVA C,PEREDO T,OLMEDO P,BARROS M,VERDONK J C,PAREDES R,MENESES C,PRIETO H,ORELLANA A,DEFILIPPI B G,CAMPOS-VARGAS R. Biochemical and physiological study of the firmness of table grape berries[J]. Postharvest Biology and Technology,2014,93:15-23.
[2] 姜建福.葡萄果肉质地性状的评价、QTL 定位及候选基因预测[D].杨凌:西北农林科技大学,2020.
JIANG Jianfu.Evaluation,QTL analysis and candidate gene prediction for berry texture in Vitis vinifera L.[D].Yangling:Northwest A&F University,2020.
[3] 周会玲,李嘉瑞.葡萄浆果耐压力、耐拉力与果实结构的关系[J].西北农林科技大学学报(自然科学版),2007,35(2):106-109.
ZHOU Huiling,LI Jiarui. The relationship between fruit structure with pressure and pulling force of berry of grapes[J]. Journal of Northwest A&F University (Natural Science Edition),2007,35(2):106-109.
[4] WANG D,YEATS T H,ULUISIK S,ROSE J K C,SEYMOUR G B. Fruit softening:Revisiting the role of pectin[J]. Trends in Plant Science,2018,23(4):302-310.
[5] AIRIANAH O B,VREEBURG R A M,FRY S C. Pectic polysaccharides are attacked by hydroxyl radicals in ripening fruit:evidence from a fluorescent fingerprinting method[J].Annals of Botany,2016,117(3):441-455.
[6] PAYASI A,SANWAL G G. Pectate lyase activity during ripening of banana fruit[J].Phytochemistry,2003,63(3):243-248.
[7] 庄军平,苏菁,陈维信.香蕉果实果胶裂解酶基因cDNA 克隆及序列分析[J].果树学报,2006,23(2):227-231.
ZHUANG Junping,SU Jing,CHEN Weixin. Cloning and sequence of cDNA fragments encoding pectate lyase genes from banana fruit[J].Journal of Fruit Science,2006,23(2):227-231.
[8] 周厚成.草莓果实成熟软化相关基因的研究[D].杨凌:西北农林科技大学,2014.
ZHOU Houcheng. Research on genes related to fruit ripening and softening of strawberry[D].Yangling:Northwest A&F University,2014.
[9] YANG L,HUANG W,XIONG F J,XIAN Z Q,SU D D,REN M Z,LI Z G. Silencing of SlPL,which encodes a pectate lyase in tomato,confers enhanced fruit firmness,prolonged shelf-life and reduced susceptibility to grey mould[J]. Plant Biotechnology Journal,2017,15(12):1544-1555.
[10] 沈颖,李芳东,王玉霞,张序,李延菊,赵慧,张福兴.甜樱桃果实发育过程中细胞壁组分及其降解酶活性的变化[J].果树学报,2020,37(5):677-686.
SHEN Ying,LI Fangdong,WANG Yuxia,ZHANG Xu,LI Yanju,ZHAO Hui,ZHANG Fuxing.A study on the variation of cell wall components and activities of their degradation enzymes in sweet cherry during fruit development[J]. Journal of Fruit Science,2020,37(5):677-686.
[11] 熊振豪,张春红,李维林,吴文龙.不同硬度黑莓果实发育成熟进程中的解剖构造[J]. 南京林业大学学报(自然科学版),2019,43(1):149-153.
XIONG Zhenhao,ZHANG Chunhong,LI Weilin,WU Wenlong.Anatomical structure characteristics of blackberry fruits with differential firmness during ripening[J]. Journal of Nanjing Forestry University(Natural Science Edition),2019,43(1):149-153.
[12] MONTECCHIARINI M L,SILVA-SANZANA C,VALDERRAMO L,ALEMANO S,GOLLAN A,RIVADENEIRA M F,BELLO F,VAZQUEZ D,BLANCO-HERRERA F,PODESTA F E,TRIPODI K E J.Biochemical differences in the skin of two blueberries (Vaccinium corymbosum) varieties with contrasting firmness:Implication of ions,metabolites and cell wall related proteins in two developmental stages[J]. Plant Physiology and Biochemistry,2021,162:483-495.
[13] XU J Y,ZHAO Y H,ZHANG X,ZHANG L J,HOU Y L,DONG W X.Transcriptome analysis and ultrastructure observation reveal that hawthorn fruit softening is due to cellulose/hemicellulose degradation[J]. Frontiers in Plant Science,2016,7:1524.
[14] ZEPEDA B,OLMEDO P,EjSMENTEWICZ T,SEPULVEDA P,BALIC I,BALLADARES C,DELGADO- RIOSECO J,FUENTEALBA C,MORENO A A,DEFILIPPI B G,MENESES C,PEDRESCHI R,CAMPOS-VARGAS R. Cell wall and metabolite composition of berries of Vitis vinifera (L.) cv.Thompson Seedless with different firmness[J]. Food Chemistry,2018,268:492-497.
[15] MA L,SUN L J,GUO Y S,LIN H,LIU Z D,LI K,GUO X W.Transcriptome analysis of table grapes (Vitis vinifera L.) identified a gene network module associated with berry firmness[J].PloS One,2020,15(8):e0237526.
[16] YAKUSHIJI H,SAKURAI N,MORINAGA K.Changes in cellwall polysaccharides from the mesocarp of grape berries during veraison[J].Physiologia Plantarum,2010,111(2):188-195.
[17] 曹建康,姜微波,赵玉梅.果蔬采后生理生化实验指导[M].北京:中国轻工业版社,2007:79-88.
CAO Jiankang,JIANG Weibo,ZHAO Yumei. Experimental instruction of postharvest physiology and biochemistry of fruits and vegetables[M]. Beijing:China Light Industry Press,2007:79-88.
[18] 王鸿飞,邵兴锋.果品蔬菜贮藏与加工实验指导[M].北京:科学出版社,2012:56-59.
WANG Hongfei,SHAO Xingfeng. Experimental guidance for storage and processing of fruit and vegetables[M]. Beijing:Science Press,2012:56-59.
[19] COSGROVE D J. Growth of the plant cell wall[J]. Nature Reviews Molecular Cell Biology,2005,6(11):850-861.
[20] ROLLE L,SIRET R,SEGADE S R,MAURY C,GERBI V,JOURJON F. Instrumental texture analysis parameters as markers of table-grape and winegrape quality:A review[J].American Journal of Enology and Viticulture,2012,63(1):11-28.
[21] 阚娟.不同溶质型桃果实成熟软化机理研究[D].扬州:扬州大学,2011.
KAN Juan. Study on the mechanism of peach ripening and softening with different solutes[D]. Yangzhou:Yangzhou University,2011.
[22] 雷琴,任小林.秦冠和富士苹果果实成熟过程中的质地变化特性[J].西北农业学报,2007,16(1):213-216.
LEI Qin,REN Xiaolin. Characteristics of texture change with Qinguan and Fuji apples during ripening[J]. Acta Agriculturae Boreali-occidentalis Sinica,2007,16(1):213-216.
[23] 陈湘颖,丁想,牛莹莹,尤璐瑶,廖康.‘库尔勒香梨’及其芽变品种‘沙01’果实硬度差异的相关因素分析[J]. 西南农业学报,2020,33(5):952-957.
CHEN Xiangying,DING Xiang,NIU Yingying,YOU Luyao,LIAO kang. Analysis on related factors of difference of fruit firmness between‘Korla Fragrant Pear’and its bud mutation‘Sha 01’[J]. Southwest China Journal of Agricultural Sciences,2020,33(5):952-957.
[24] 李世军,尹宝颖,李佳,李中勇,张学英,徐继忠.早熟苹果果实软化过程中乙烯、相关酶及其基因表达变化[J].华北农学报,2019,34(6):104-109.
LI Shijun,YIN Baoying,LI Jia,LI Zhongyong,ZHANG Xueying,XU Jizhong. Changes of ethylene,related enzymes and their genes expression during softening of premature apple fruits[J].Acta Agriculturae Boreali-Sinica,2019,34(6):104-109.
[25] HUANG W J,CHEN M Y,ZHAO T T,HAN F,ZHANG Q,LIU X L,JIANG C Y,ZHONG C H. Genome-wide identification and expression analysis of polygalacturonase gene family in kiwifruit (Actinidia chinensis) during fruit softening[J]. Plants,2020,9(3):327.
[26] LI L,LI C B,SUN J,SHENG J F,ZHOU Z G,XIN M,YI P,HE X M,ZHENG F J,TANG Y Y,LI J M,TANG J.The effects of 1-Methylcyclopropene in the regulation of antioxidative system and softening of mango fruit during storage[J]. Journal of Food Quality,2020,2020:6090354.
[27] WANG H,CHEN Y H,LIN H T,LIN M S,CHEN Y H,LIN Y F. 1-Methylcyclopropene containing-papers suppress the disassembly of cell wall polysaccharides in Anxi persimmon fruit during storage[J]. International Journal of Biological Macromolecules,2020,151:723-729.
[28] HAN Y,BAN Q Y,LI H,HOU Y L,JIN M J,HAN S K,RAO J P. DkXTH8,a novel xyloglucan endotransglucosylase/hydrolase in persimmon,alters cell wall structure and promotes leaf senescence and fruit postharvest softening[J]. Scientific Reports,2016,6:39155.
[29] TOIVONEN P M A,BRUMMELL D A.Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables[J].Postharvest Biology and Technology,2008,48(1):1-14.
[30] CHRISTIAENS S,BUGGENHOUT S V,HOUBEN K,CHAULA D,LOEY A M V,HENDRICKX M E. Unravelling processinduced pectin changes in the tomato cell wall:An integrated approach[J].Food Chemistry,2012,132(3):1534-1543.
[31] POSÉ S,PANIAGUA C,MATAS A J,GUNNING A P,MORRIS V J,QUESADA M A,MERCADO J A. A nanostructural view of the cell wall disassembly process during fruit ripening and postharvest storage by atomic force microscopy[J]. Trends in Food Science and Technology,2019,87:47-58.
[32] XIE F,YUAN S Z,PAN H X,WANG R,CAO J K,JIANG W B. Effect of yeast mannan treatments on ripening progress and modification of cell wall polysaccharides in tomato fruit[J].Food Chemistry,2017,218:509-517.
[33] ZHANG L F,CHEN F S,AN H J,YANG H S,SUN X Y,GUO X F,LI L T. Physicochemical properties,firmness,and nanostructures of sodium carbonate-soluble pectin of 2 Chinese cherry cultivars at 2 ripening stages[J]. Journal of Food Science,2008,73(6):N17-N22.