葡萄在我国具有悠久的栽培历史,因其具有丰富的营养物质和较高的经济价值,深受广大民众的喜爱。近年来,随着葡萄栽培面积和产量的持续增长,病害造成的损失也逐年增加,其中葡萄白腐病是危害最严重的病害之一。该病是由白腐垫壳孢[Coniella diplodiella(Speg.)Petrk&Sydow.]引起的真菌性病害[1],19世纪在意大利首次报道,我国最早发现于1899年,目前主要发生在我国东北、华北、西北和华东北部地区,植株感病后,叶片边缘出现水渍状病斑,慢慢侵染到叶片中部,呈现出不太明显的同心轮纹状的褐色病斑;葡萄果粒逐渐变褐变软,发病后期整个果穗腐烂,造成大面积减产[2-3]。目前在农业生产上主要是化学药剂防治葡萄白腐病,化学防治法虽然效果好、见效快,但是长期用药,不仅增加成本、降低果实品质,而且还会存在农药残留,严重危害环境及食品安全。
不同葡萄种质对白腐病的抗性存在差异。欧亚种对白腐病的抗性较差,东亚种群的葡萄抗病性较强[4]。中国是葡萄属植物重要的起源地之一,基本包括了所有的东亚种群,野生葡萄种质资源丰富[5],利用野生种质资源导入异质抗性基因是解决栽培品种抗性基因贫乏的有效途径。我国的刺葡萄对包括白腐病在内的多种真菌性病害都具有很强的抗性[6-7],是葡萄白腐病抗性研究的良好材料。因此,解析葡萄抗病机制、挖掘抗性基因、培育具有较强抗病性的葡萄品种尤为重要。
转录组测序已被广泛用于解析拟南芥、番茄、葡萄和其他植物的抗病机制;研究结果能够提供植物在病原菌侵染下应答的信号通路和相关基因表达的信息。陈哲等[8]利用转录组测序分析,发现草莓受到炭疽病病原菌侵染后能够影响类黄酮生物合成和植物激素信号传导等通路中关键基因的表达水平,并鉴定出了19 个感病相关的差异表达基因。暹罗芽孢杆菌提高了贮藏杧果果实的抗病性,Jiang 等[9]利用暹罗芽孢杆菌的诱导机制,建立了杧果果实样品在贮藏期间的比较转录组分析,部分基因(JAZ、BAK1 和PR1)被暹罗芽孢杆菌处理上调,引发应激反应,并诱导酚类抗性物质的合成,从而提高杧果果实的抗病性。其次,一些基因(WRKY22、HSP90、CNGCs、SOD、PAL、4CL、CHS 和HCT)如过氧化物酶体、苯丙素、黄酮类和姜酚生物合成,在杧果果实中上调,从而增强系统的抗病能力,刺激免疫反应。姜长岳[10]以毛葡萄丹凤-2叶片为材料接种白粉菌进行转录组测序,从MYB家族中筛选出抗病相关转录因子VqMYB154,发现转录因子VqMYB154具有正向调控3个芪合酶靶基因表达以增强植物抗病性的功能。Jiao 等[11]通过转录组分析中国野生葡萄对白粉病的抗病机制,发现感病品种湖南一号下调基因比例明显高于山-24,而上调基因主要与防御反应和抗病代谢物的生物合成相关,同时发现12号和13号染色体上的NLR基因对白粉菌最敏感,其中许多基因在易感种质的所有感染阶段始终下调。Liu 等[12]利用转录组测序分析,从河岸葡萄中筛选了与抗病性相关的转录因子ERF、MYB、WRKY 和bHLH,确定了197 个候选基因,可能参与霜霉病菌引起的防御反应,其中有5 个基因富含亮氨酸重复序列(LRR)。沈才琦等[13]通过对感炭疽病欧亚种里扎马特和抗炭疽病刺葡萄黑珍珠的杂交后代中感炭疽病7-2-6株系和抗炭疽病7-1-8株系进行转录组测序分析,发现葡萄响应胶孢炭疽菌侵染的差异表达基因,显著富集在植物与病原体相互作用、类黄酮生物合成和MAPK 信号途径等3 个通路,并筛选出8 个抗病候选基因,包括MLO蛋白及WRKY、ERF转录因子等。转录组分析已广泛用于发掘植物响应病病原菌胁迫的调控因子,这为研究刺葡萄抗病机制提供了理论依据。笔者在本研究中对白腐病病原菌侵染前后的感病欧亚种美人指和抗病中国野生刺葡萄的果实进行转录组测序分析,通过转录组分析已经鉴定了几个可能在抗葡萄白腐病中起关键作用的基因。
1 材料和方法
1.1 试验材料
以抗白腐病刺葡萄0941 和感白腐病欧亚种美人指转色期的果实为试验材料,材料采自于国家葡萄种质资源圃(郑州)。
在葡萄果实的果柄处接种直径1 cm 的白腐病原菌菌块,每组接种18粒葡萄,保湿并置于28 ℃恒温箱中培养,同时在果实果柄处接种无菌水作为对照。分别在接菌0 h、24 h、48 h收集去除感染部分后的果实,每组均设置3 个生物学重复。采用多糖多酚植物总RNA提取试剂盒(华越洋,货号0416-50),参照说明书提取样品总RNA,置于-80 ℃超低温冰箱保存备用。
1.2 文库构建及测序
总RNA质量检测合格后,通过深圳华大基因科技服务有限公司进行转录组测序。对得到的原始数据进行质控,去除低质量的reads获得的高质量的质控数据,并储存于格式为fastq的数据文件中。以葡萄基因组12×(https://urgi.versailles.inra.fr/Species/Vitis)为参考基因组进行比对。利用HISAT2软件将得到的clean reads比对参考基因组进行组装。使用RSEM 软件计算基因表达水平,然后转换成FPKM(每千碱基转录序列的预期片段数/百万碱基对测序)值。使用对比工具Bowtie2 将clean reads 与参考基因组进行比对以统计基因比对率,比对率都在90%以上。利用组装的转录本与GO 数据库、KEGG 数据库、NR 数据库、Swiss-Prot 数据库、Pfam数据库、COG 数据库以及KOG 数据库进行基因功能注释。
1.3 差异表达基因筛选和富集分析
比对品种间基因的表达情况,筛选差异表达的部分进一步富集分析。使用DESeq2 软件进行差异基因的筛选,将|log2FoldChange|≥2、p≤0.05 的基因作为显著的差异表达基因,对筛选出的差异基因进行GO 功能富集和KEGG 通路分析,以获得差异表达基因的主要生物学功能及主要生化代谢途径。
1.4 WGCNA分析
通过WGCNA分析进一步探究差异表达基因之间的调控网络,旨在鉴定相关基因模块及模块内的hub 基因。对每个基因模块进行评估,检验每个模块中基因的相关性,利用Cytoscape 3.8.0 计算每个基因的相关性,鉴定每个模块内的hub 基因。通过计算每个模块的特征基因值,检验各个基因白腐病之间的相关性。此外,为了进一步探究模块中基因的功能,利用R包(ClusterProfiler)进行GO和KEGG富集分析。
1.5 实时荧光定量PCR(qRT-PCR)分析
为验证RNA-seq 测序检测到DEGs,本研究基于分析挑取9 个差异表达基因进行qRT-PCR 分析。使用Primer3plus 在线网站(https://www.primer3plus.com/index.html)设计引物,并由上海生工生物公司进行合成,引物具体信息见表1。然后用Fasting-King RT Kit(With gDNA)反转录试剂盒(天根)将RNA 反转录为cDNA。每个样本3 个重复,使用FastStart Essential DNA Green Master 试剂盒按照其说明在ABI-7300 系统进行qRT-PCR 分析,反应程序:94 ℃30 s;94 ℃5 s,60 ℃30 s,40 个循环。以VvEF1-α 为内参基因[14],用2-ΔΔCT 公式计算相对表达量。
表1 RT-qPCR 所用引物
Table 1 Primers for RT-qPCR
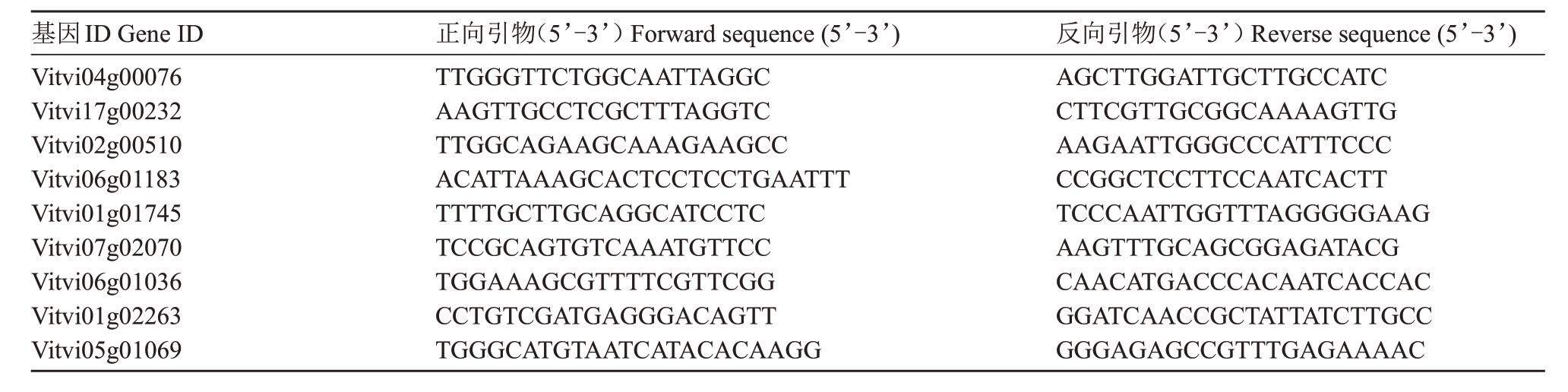
基因ID Gene ID Vitvi04g00076 Vitvi17g00232 Vitvi02g00510 Vitvi06g01183 Vitvi01g01745 Vitvi07g02070 Vitvi06g01036 Vitvi01g02263 Vitvi05g01069正向引物(5’-3’)Forward sequence(5’-3’)TTGGGTTCTGGCAATTAGGC AAGTTGCCTCGCTTTAGGTC TTGGCAGAAGCAAAGAAGCC ACATTAAAGCACTCCTCCTGAATTT TTTTGCTTGCAGGCATCCTC TCCGCAGTGTCAAATGTTCC TGGAAAGCGTTTTCGTTCGG CCTGTCGATGAGGGACAGTT TGGGCATGTAATCATACACAAGG反向引物(5’-3’)Reverse sequence(5’-3’)AGCTTGGATTGCTTGCCATC CTTCGTTGCGGCAAAAGTTG AAGAATTGGGCCCATTTCCC CCGGCTCCTTCCAATCACTT TCCCAATTGGTTTAGGGGGAAG AAGTTTGCAGCGGAGATACG CAACATGACCCACAATCACCAC GGATCAACCGCTATTATCTTGCC GGGAGAGCCGTTTGAGAAAAC
2 结果与分析
2.1 转录组测序质量分析
转录组测序后对原始数据进行过滤,一共得到798.77百万条高质量的过滤序列,共产生119.82 Gb过滤碱基,每个样本的Q30均大于92%,清洁读数率都在96%以上。转录组测序结果与葡萄参考基因组的比对率高,测序质量好,可用于后续分析(表2)。对各组重复之间进行相关性分析,每组样品之间的相关系数均高于0.8(图1),以确保试验结果的可靠性。
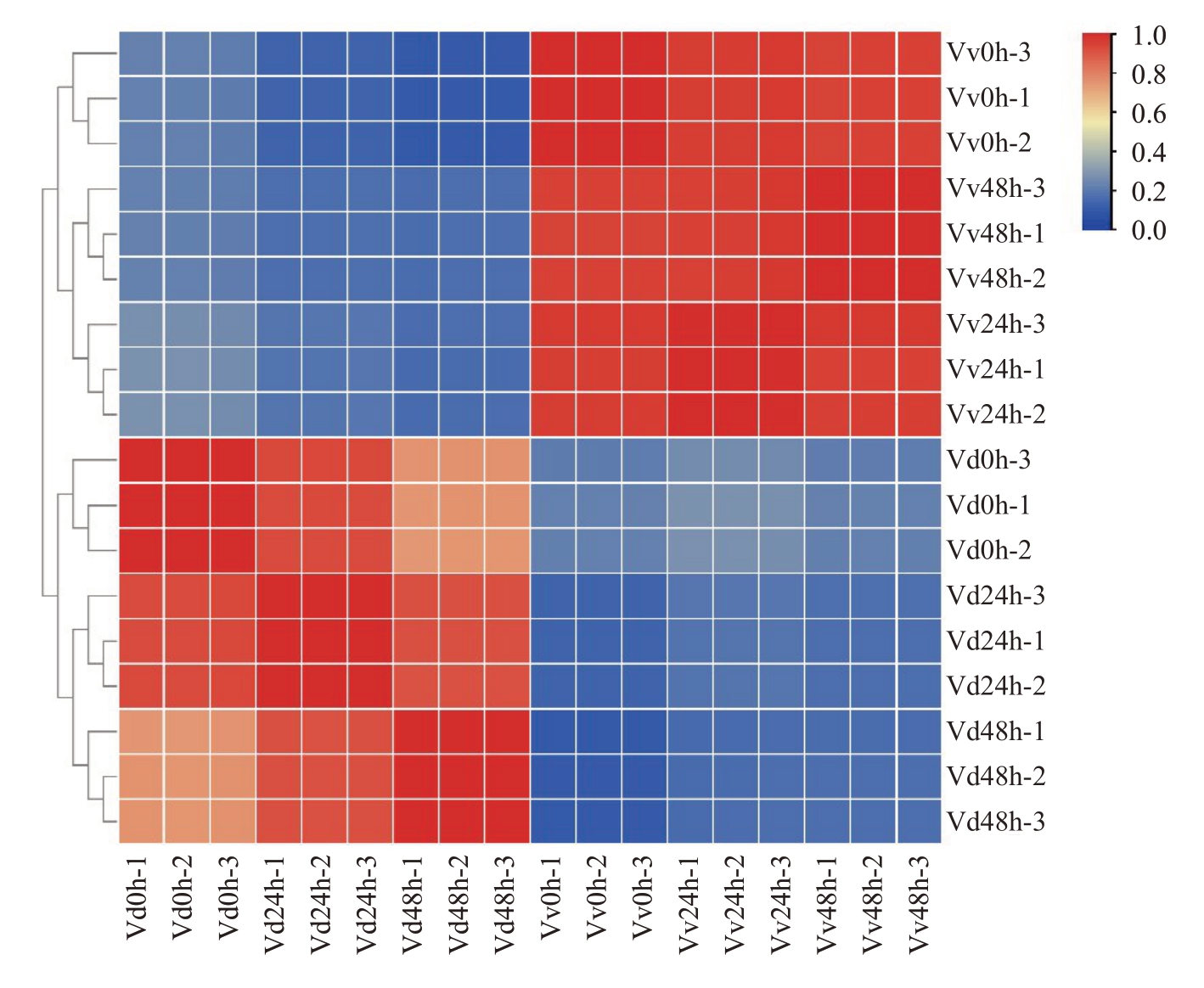
图1 各组重复之间的相关性分析
Fig.1 Correlation analysis among groups of repetitions
Vd 0 h、Vd 24 h、Vd 48 h 分别为刺葡萄接种白腐菌0 h、24 h、48 h 的样本。Vv 代表美人指,Vd 代表刺葡萄。
Vd 0 h、Vd 24 h and Vd48h are samples of Vitis davidii inoculated with white rot fungus for 0 h,24 h and 48 h,respectively.Vv stands for Vitis vinifera Manicure Finger and Vd stands for Vitis davidii.
表2 参试样品的转录组数据的质量
Table 2 Quality of the transcriptome data of each sample
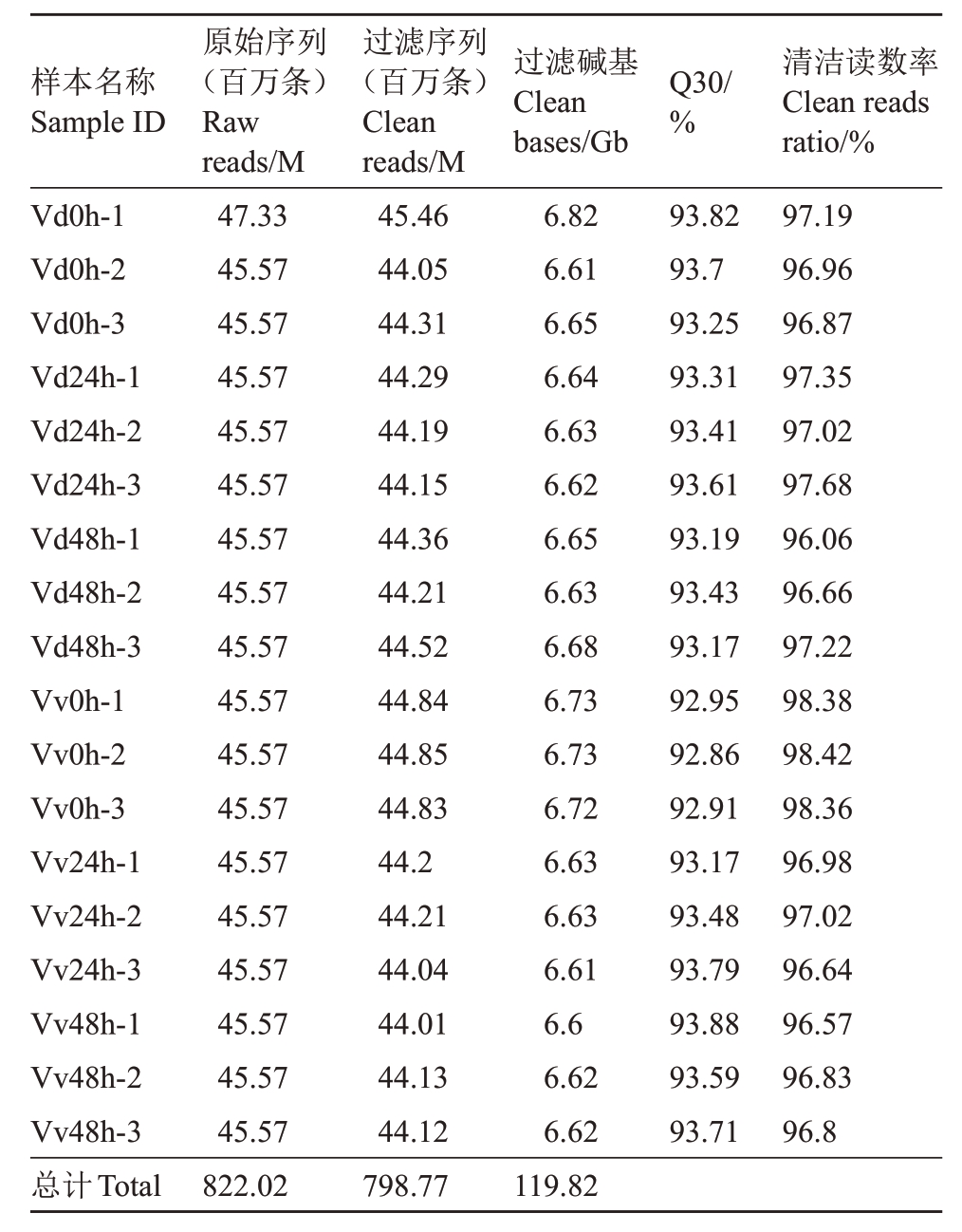
样本名称Sample ID Q30/%Vd0h-1 Vd0h-2 Vd0h-3 Vd24h-1 Vd24h-2 Vd24h-3 Vd48h-1 Vd48h-2 Vd48h-3 Vv0h-1 Vv0h-2 Vv0h-3 Vv24h-1 Vv24h-2 Vv24h-3 Vv48h-1 Vv48h-2 Vv48h-3总计Total原始序列(百万条)Raw reads/M 47.33 45.57 45.57 45.57 45.57 45.57 45.57 45.57 45.57 45.57 45.57 45.57 45.57 45.57 45.57 45.57 45.57 45.57 822.02过滤序列(百万条)Clean reads/M 45.46 44.05 44.31 44.29 44.19 44.15 44.36 44.21 44.52 44.84 44.85 44.83 44.2 44.21 44.04 44.01 44.13 44.12 798.77过滤碱基Clean bases/Gb 6.82 6.61 6.65 6.64 6.63 6.62 6.65 6.63 6.68 6.73 6.73 6.72 6.63 6.63 6.61 6.6 6.62 6.62 119.82 93.82 93.7 93.25 93.31 93.41 93.61 93.19 93.43 93.17 92.95 92.86 92.91 93.17 93.48 93.79 93.88 93.59 93.71清洁读数率Clean reads ratio/%97.19 96.96 96.87 97.35 97.02 97.68 96.06 96.66 97.22 98.38 98.42 98.36 96.98 97.02 96.64 96.57 96.83 96.8
2.2 差异表达基因筛选
筛选|log2FoldChange|≥2、p≤0.05的基因作为显著差异表达基因,在0 h、24 h、48 h抗病品种刺葡萄(Vd)和感病品种美人指(Vv)之间的差异表达基因分别有5266、4725、4653个(图2-A)。其中上调基因数分别为1737、1788、1727 个,下调基因数分别为3529、2937、2926 个。在抗病品种和感病品种之间的差异表达基因均是下调基因数目远大于上调基因数目,且24 h 和48 h 较0 h 下调基因数目大幅减少,3 个时期对比组共有差异表达基因有2684 个(图2-B)。
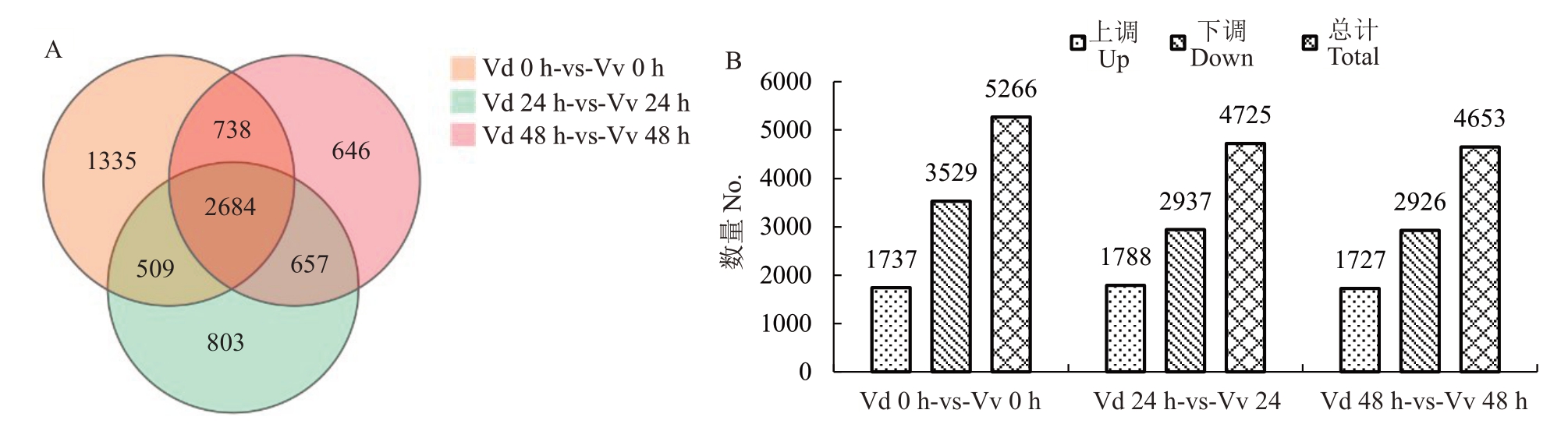
图2 抗病品种和感病品种间DEGs 数目统计及韦恩图
Fig.2 Statistics of DEGs between resistant and susceptible varieties
2.3 GO、KEGG功能注释及富集分析
将筛选出来的所有时期的差异表达基因进行GO富集分析,结果分为3类,分别为生物学过程、细胞组分和分子功能。结果仅展示出富集程度最为显著的前20条的代谢途径(图3)。在膜、细胞、细胞部分等组分中具有较高程度的富集,在生物学过程中防御反应、跨膜运输、代谢过程、细胞过程等具有较高程度的富集,在分子功能中转录调节活性和催化活性等具有较高程度的富集,结果表明这些功能在刺葡萄、美人指与白腐病的防御反应中发挥着重要作用。
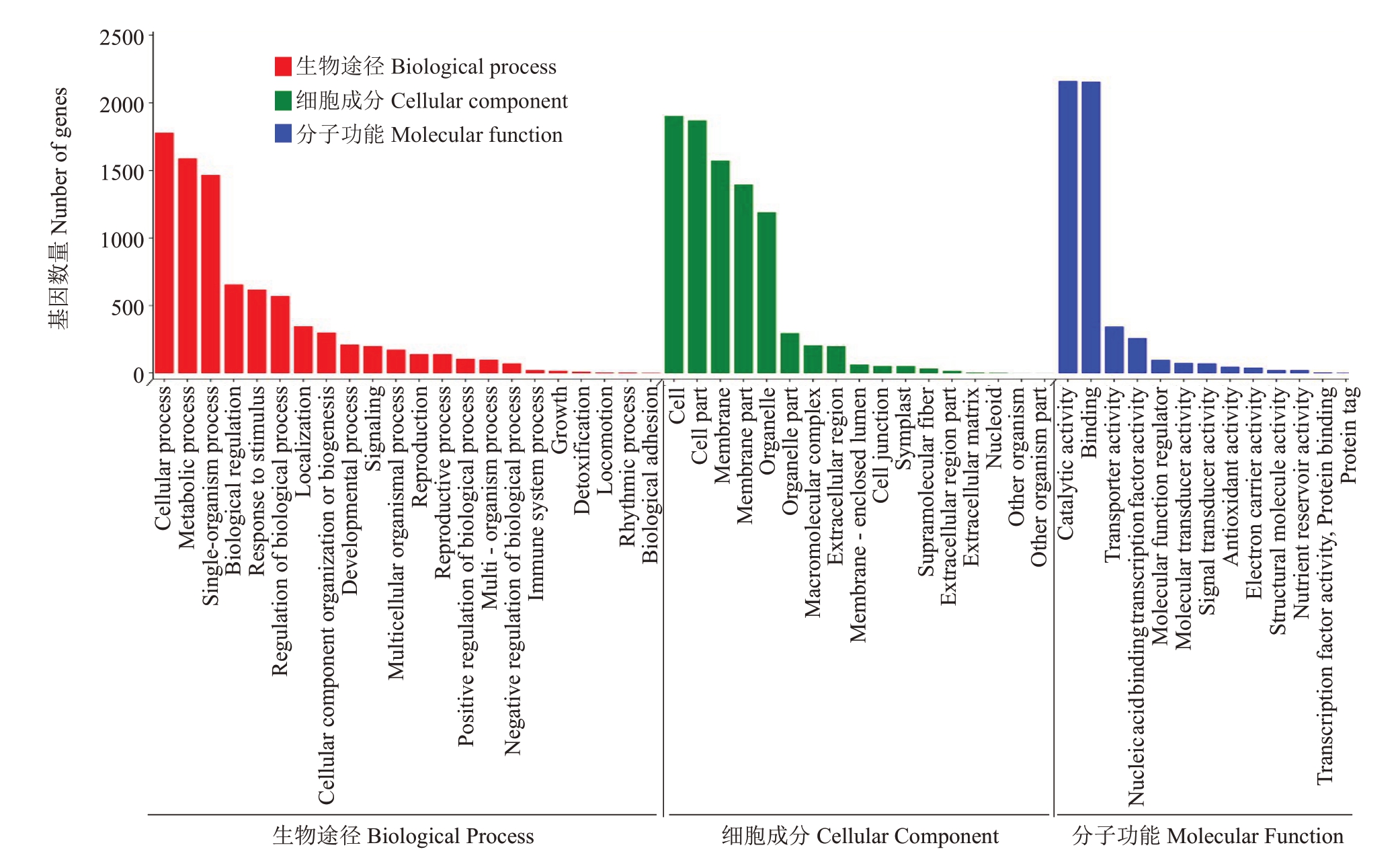
图3 差异表达基因GO 富集分析
Fig.3 GO enrichment analysis of DEGs
对刺葡萄和美人指之间的差异表达基因进行KEGG 代谢通路富集分析,取富集最为显著的前20个通路进行分析(图4)。通过KEGG 分析,表明上调差异基因主要富集在类黄酮生物合成、黄酮和黄酮醇生物合成、苯丙烷生物合成、芪类和二芳基庚烷类及姜酚生物合成、α-亚麻酸代谢、亚油酸代谢等代谢途径中,而下调差异基因主要富集在植物与病原菌的相互作用、类黄酮生物合成、苯丙烷生物合成、植物激素信号和丝裂原活化蛋白激酶(MAPK)信号转导途径、ABC运输、苯丙氨酸代谢、氨基糖和核苷酸糖代谢、二萜生物合成、DNA复制等通路中。
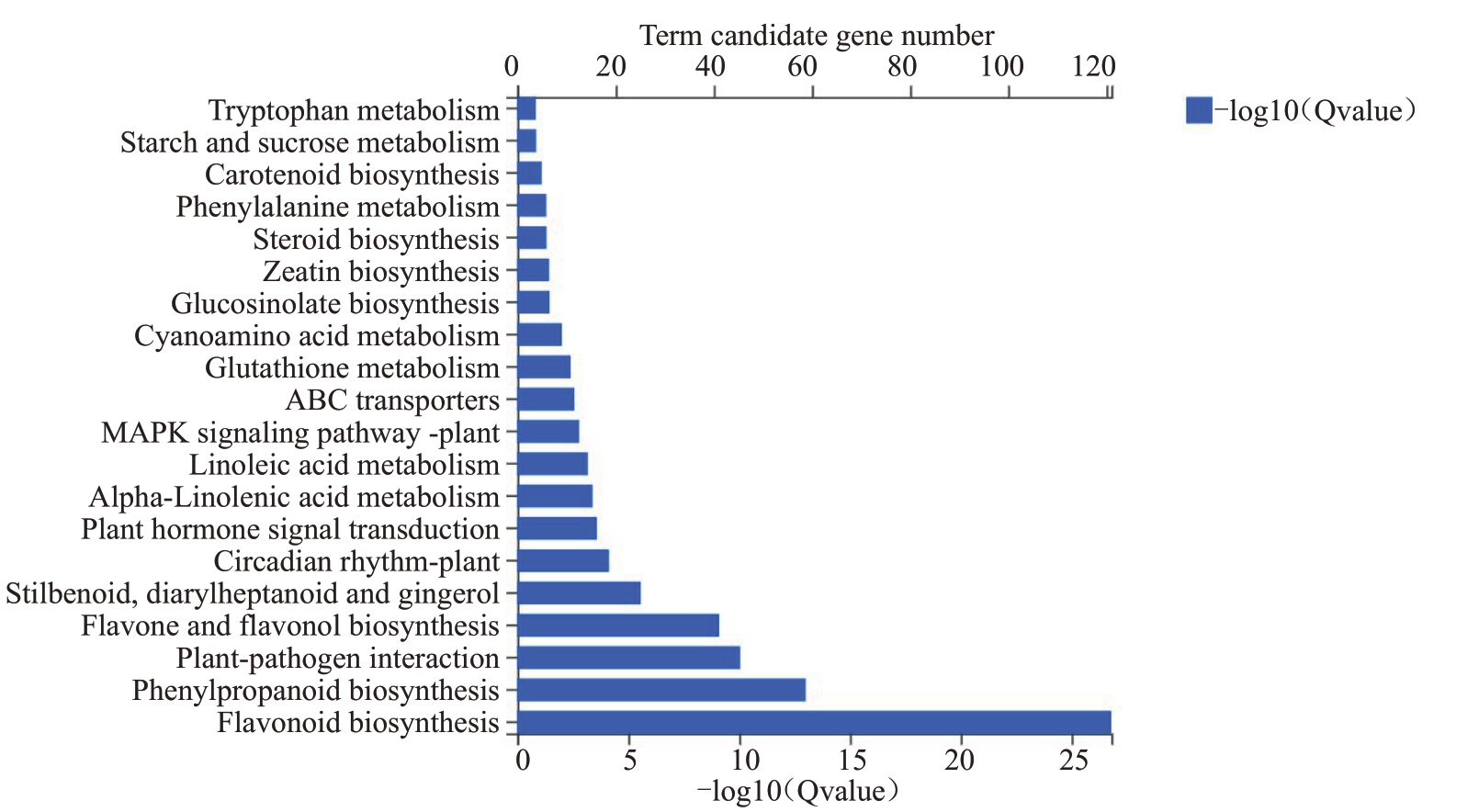
图4 差异表达基因KEGG 富集分析
Fig.4 KEGG enrichment analysis of DEGs
2.4 刺葡萄抗白腐病的相关差异表达基因
刺葡萄和美人指接种白腐病病原菌0 h、24 h、48 h 后进行转录组分析,从KEGG 富集的通路中选择白腐病病原菌诱导后表达差异较大的基因,作为重要的差异表达基因。差异表达基因主要来自次生代谢和植物激素信号转导两个通路。
葡萄果实接种白腐病病原菌后,与苯丙烷、类黄酮、黄酮和黄酮醇生物合成等次生代谢途径的相关基因在24 h和48 h均受到诱导而产生表达差异。图5展示了0 h、24 h和48 h中苯丙烷、类黄酮、黄酮和黄酮醇生物合成途径相关的差异表达基因(differentially expressed genes,DEGs)及其转录水平。其中苯丙氨酸解氨酶PAL(Vitvi00g01431、Vitvi16g01503)、四香豆酸酯CoA 连接酶4CL(Vitvi16g00139)、查耳酮合成酶CHS(Vitvi16g00990、Vitvi16g01480)、莽草酸/奎宁酸羟基肉桂酰转移酶HCT(Vitvi11g00735)、黄酮醇合酶FLS(Vitvi08g01575)这7个基因在受到白腐病原菌诱导48 h后,在抗病品种刺葡萄中的表达量远高于感病品种美人指。芪合酶STS(Vitvi16g01465、Vitvi16g01466)的表达量在受到病原菌诱导后,在刺葡萄和美人指中均呈现上升趋势,且美人指中的表达量高于刺葡萄。

图5 苯丙烷、类黄酮、黄酮和黄酮醇生物合成通路及其相关DEGs 的表达分析
Fig.5 Phenylpropane,flavonoids,Flavone and flavonol biosynthetic pathways and the expression levels of related DEGs
A.热图表示所有与通路相关DEGs 的表达水平,由各样本的FPKM 值进行log2 转换生成并按行进行标准化;B.通路图中的红色字体表示各通路上注释到的DEGs。下同。
A.The heat map indicates the expression levels of all pathway-related DEGs,generated by log2 transformation of the FPKM values for each sample and standardize by rows;B.The red font in the pathway map indicates the DEGs annotated on each pathway.The same below.
白腐病病原菌侵染葡萄果实时,植物激素信号转导途径中生长素、细胞分裂素、赤霉素、脱落酸、油菜素甾醇、茉莉酸和水杨酸等重要防御相关的信号通路均发生富集,82个差异表达基因显著富集于植物激素信号转导途径(图6),39 个DEGs(TCH4,4个;AUXIAA,1 个;CH3,2 个;TF,1 个;JAZ,2 个;SAUR,16 个;PR1,2 个;MYC2,1 个;ETR;2 个;TIR,1 个)在刺葡萄中的表达水平明显高于美人指。其中,水杨酸信号途径下游基因PR1(Vitvi03g00860)和茉莉酸信号途径下游基因MYC2(Vitvi02g00231)在刺葡萄接菌处理后表达上调,且PR1(Vitvi03g00860)在接菌48 h 表达水平很高,最高上调 1000 倍(0.27<FPKM<1100),MYC2(Vitvi02g00231)在接菌0 h 和24 h 表达水平无明显变化,但在48 h 上调10 倍(0<FPKM<13);茉莉酸信号途径下游基因JAZ(Vitvi01g02293)在刺葡萄接菌24 h 下调表达3 倍,而后上调100 倍;乙烯信号途径上游基因ETR(Vitvi05g00684)在刺葡萄和美人指接菌后都上调。
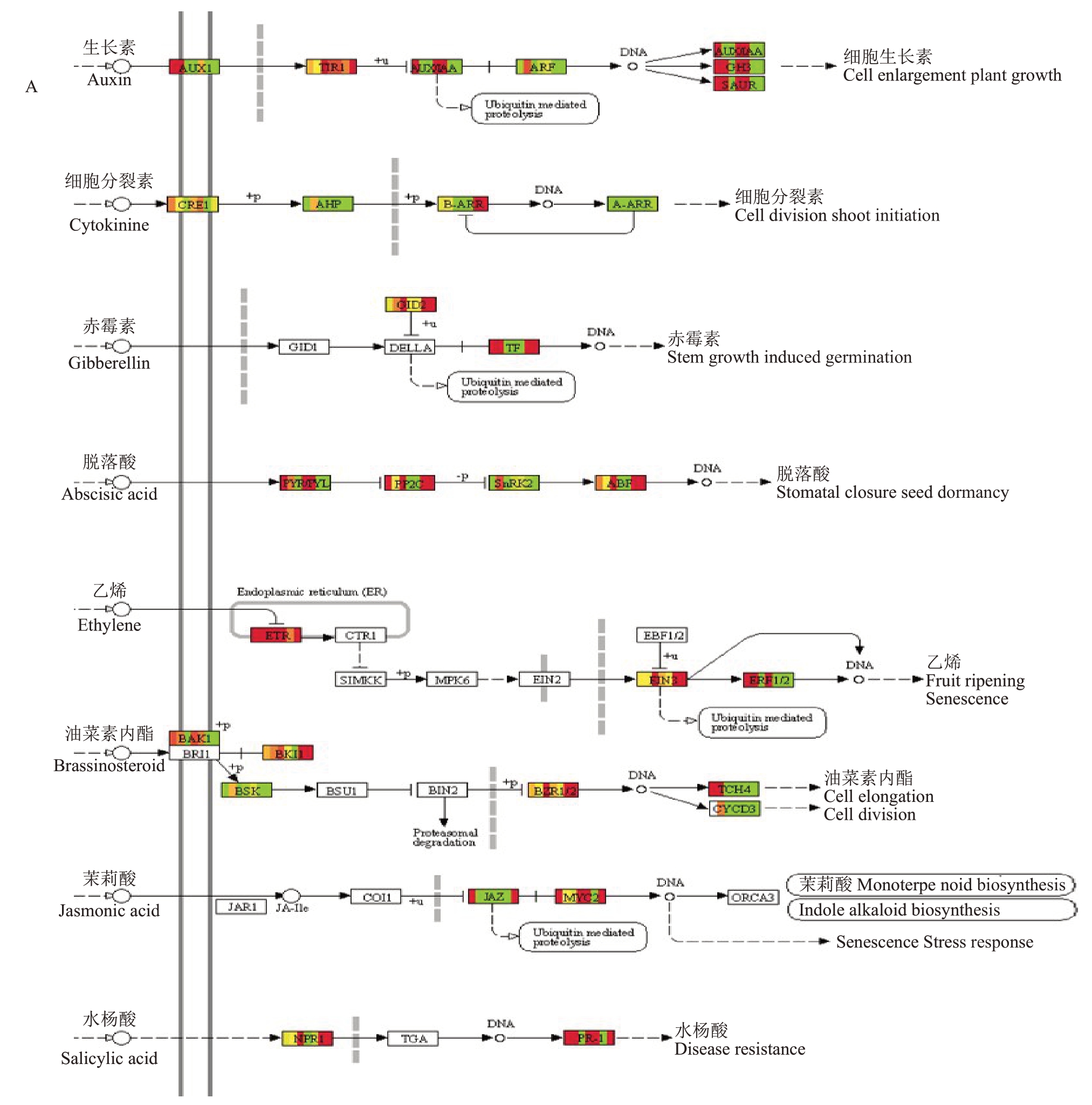
图6 植物激素信号转导通路及其相关DEGs 的表达分析
Fig.6 Phytohormone signal transduction pathway and the expression analysis of related DEGs
A.DEGs 富集到的植物激素信号通路图,红色模块表示上调的基因,绿色模块表示下调的基因,黄色表示没有变化的基因;B. 热图表示与植物激素信号转导相关DEGs 的表达水平。
A.Map of plant hormone signaling pathways enriched by DEGs,red modules indicate up-regulated genes,green modules indicate down-regulated genes and yellow indicate genes with no change;B.Heat map indicating expression levels of DEGs associated with plant hormone signaling.
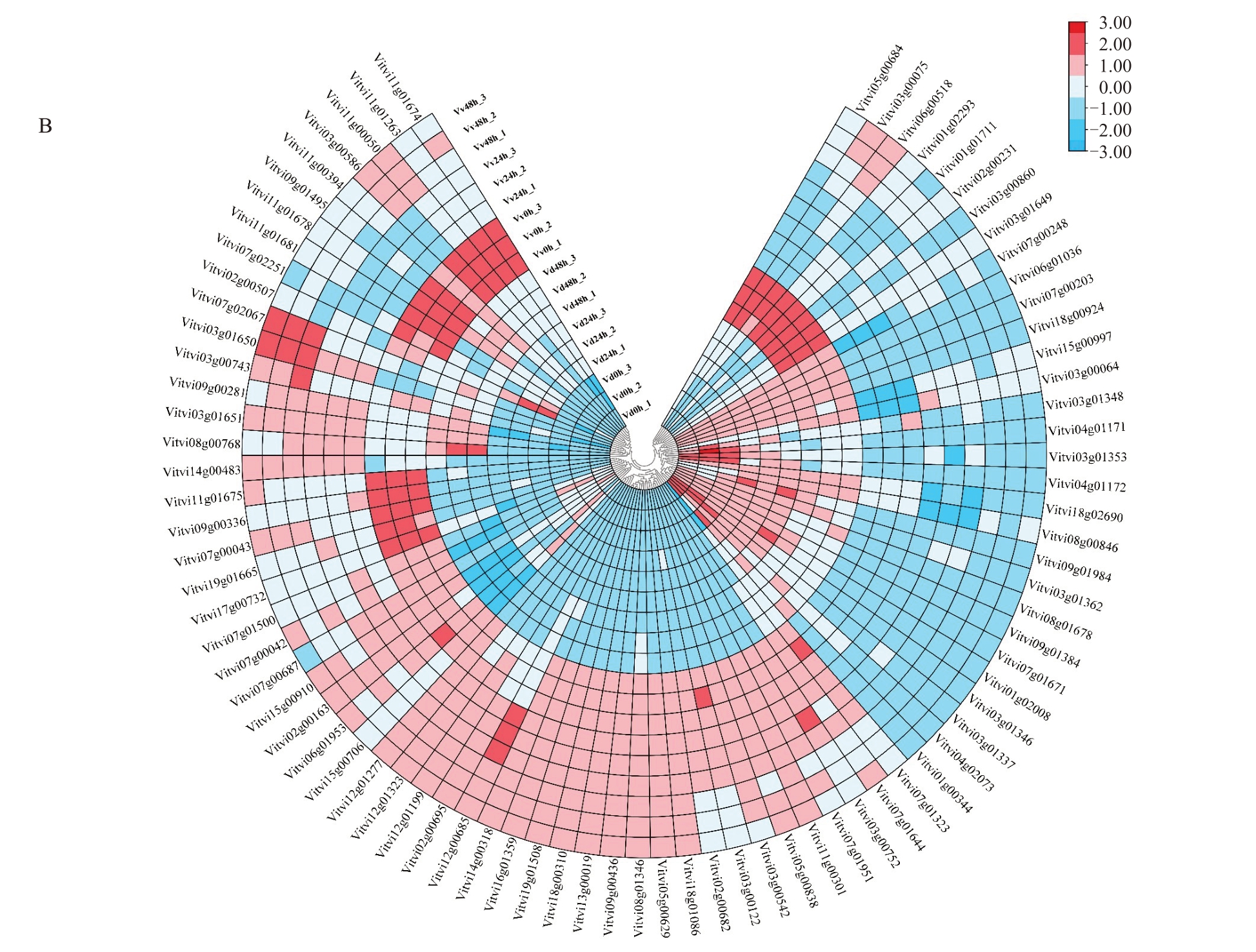
图6 (续) Fig.6 (Continued)
2.5 接菌处理后转录因子的表达模式
DEGs 中转录因子有344 个,属于39 个转录因子家族,主要包含40 个AP2/ERF、66 个MYB、22 个WRKY、35 个bHLH、24 个NAC、16 个LBD、16 个C2H2、11个HD-ZIP等(图7)。在接菌处理后,其中10 个转录因子在美人指中的表达量明显高于刺葡萄,例如AP2/ERF(Vitvi05g01073)在接菌24 h 美人指中的表达量是刺葡萄的60倍,在接菌48 h美人指的表达量是刺葡萄的35 倍。16 个转录因子在刺葡萄中的表达量显著高于美人指,例如参与植物苯丙烷类次生代谢途径的MYB(Vitvi02g01019)在接菌24 h 刺葡萄中的表达量是美人指的100 倍,在接菌48 h刺葡萄的表达量是美人指的40倍。8个转录因子在受到白腐病原菌的诱导后的刺葡萄和美人指中表达模式相反,例如响应激素信号转导的AP2/ERF(Vitvi16g01438)在接菌24 h 刺葡萄中表达上调12倍,而在美人指中下调表达10 倍;在接菌48 h 刺葡萄中表达下调表达10倍,而美人指上调3倍。
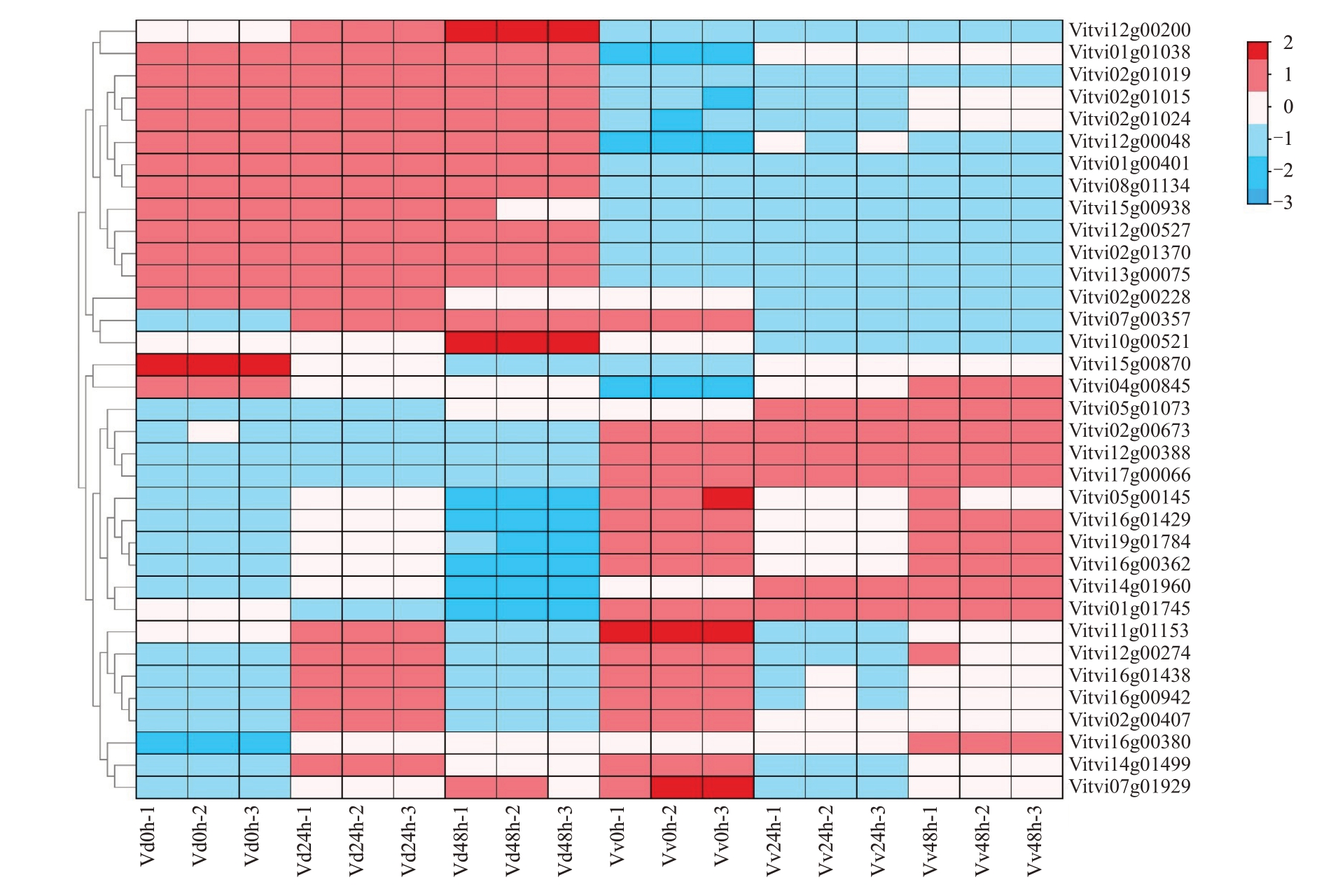
图7 转录因子家族在各个样本的表达谱
Fig.7 Relative expression profiles of transcription factor family in each sample
2.6 WGCNA分析鉴定刺葡萄抗病的关键基因
经过滤FPKM<1 的基因,4085 个差异基因用于构建加权基因共表达网络。通过计算18 个样本表达水平的相关系数聚类分析,样本间聚类良好,未出现离群样本。根据差异基因的FPKM 值进行相关度分析并聚类,相关度较高的基因被分配到同一个模块中,图中聚类树的不同分支代表不同的基因共表达模块,不同颜色代表不同的模块,共划分为7个模块,并绘制基因共表达网络热图(图8)。根据基因表达模块的趋势与抗病性状的相关性分析,以皮尔逊相关系数(r>0.5)和显著性p 值的大小(p<0.05)为筛选条件MEyellow、MEblack、MEgreen模块与抗病性状呈显著正相关,其中MEblack模块在Vd0h、Vd24h、Vd48h与抗病性状呈显著正相关,MEyellow模块在Vd48h与抗病性状呈显著正相关。对这两个抗病相关模块中的差异表达基因进行KEGG富集分析,其富集的通路主要集中在类黄酮生物合成、MAPK信号途径、苯丙烷生物合成、植物相互作用(图9)。
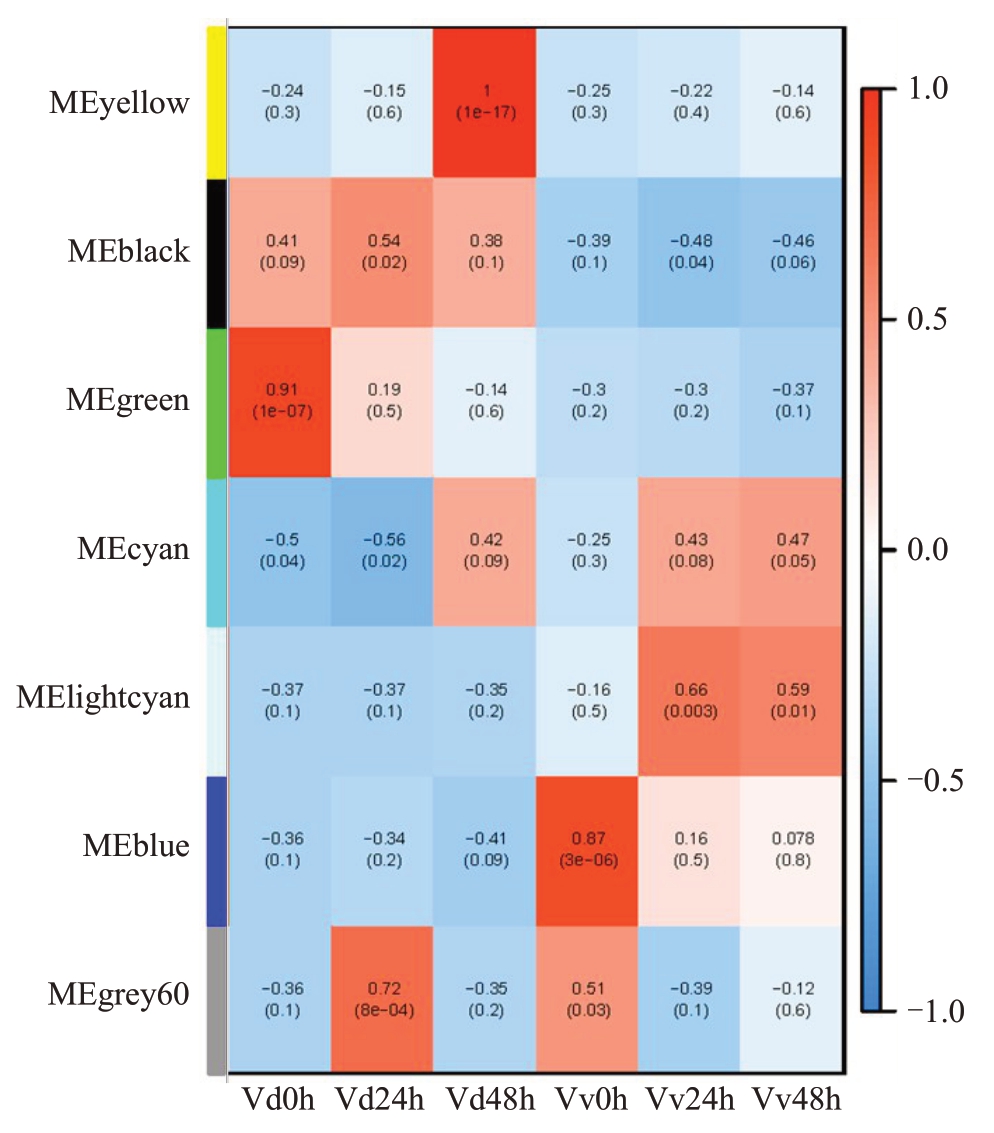
图8 模块与表型的相关性分析
Fig.8 Correlation analysis between modules and phenotypes
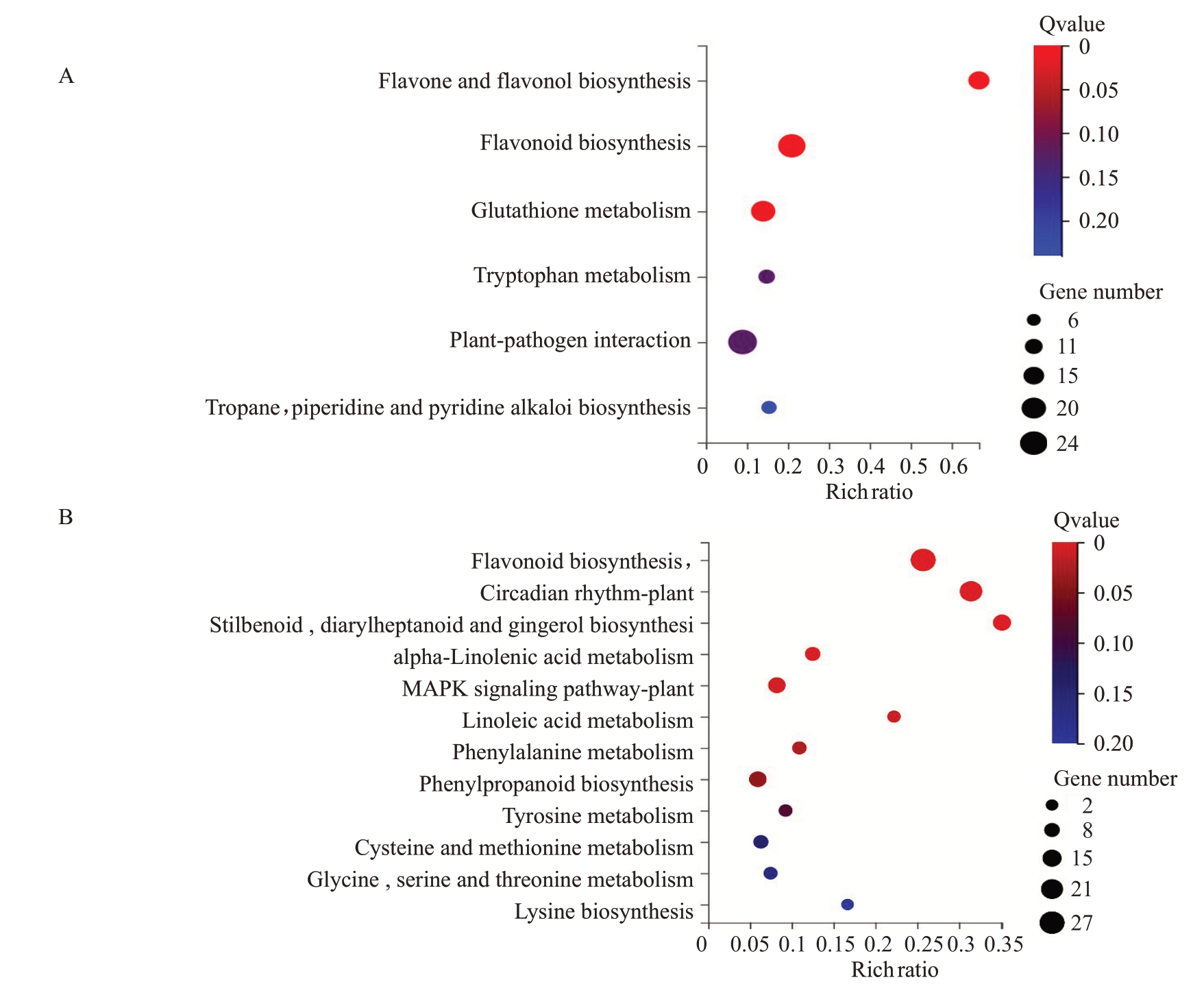
图9 Black(A)和Yellow(B)模块KEGG 富集气泡图
Fig.9 KEGG enriched bubble diagram of Black(A)and Yellow(B)module
选取Meyellow、MEblack 模块内连通度最高的前150 个基因作为模块的hub 基因,其中候选转录因子共有13 个,并通过WGCNA 预测与它们可能存在互作关系的DEGs,利用Cytoscape 软件进行可视化分析(图10)。在MEblcak 模块中有4 个转录因子(MYB,2 个;EIN 和TCP,各1 个)与植物防御反应有关,其中与MYB4-Like(Vitvi17g00232)相关DEGs 的数量最多,共有104 个;这些基因主要与植物的代谢反应有关,如苯丙氨酸生物合成、色氨酸代谢、糖酵解、泛素介导的蛋白水解等。在MEyellow 模块中有9 个转录因子(MYB,3 个;ERF,3 个;bHLH,2 个;bZIP,1 个)与植物防御反应有关,其中MYB1R1(Vitvi04g00076)有相关性的DEGs 数量为134 个,这些基因的功能注释发现与多种植物激素有关,如生长素、乙烯、水杨酸和茉莉酸,还和氧化还原反应、类黄酮生物合成等有关。
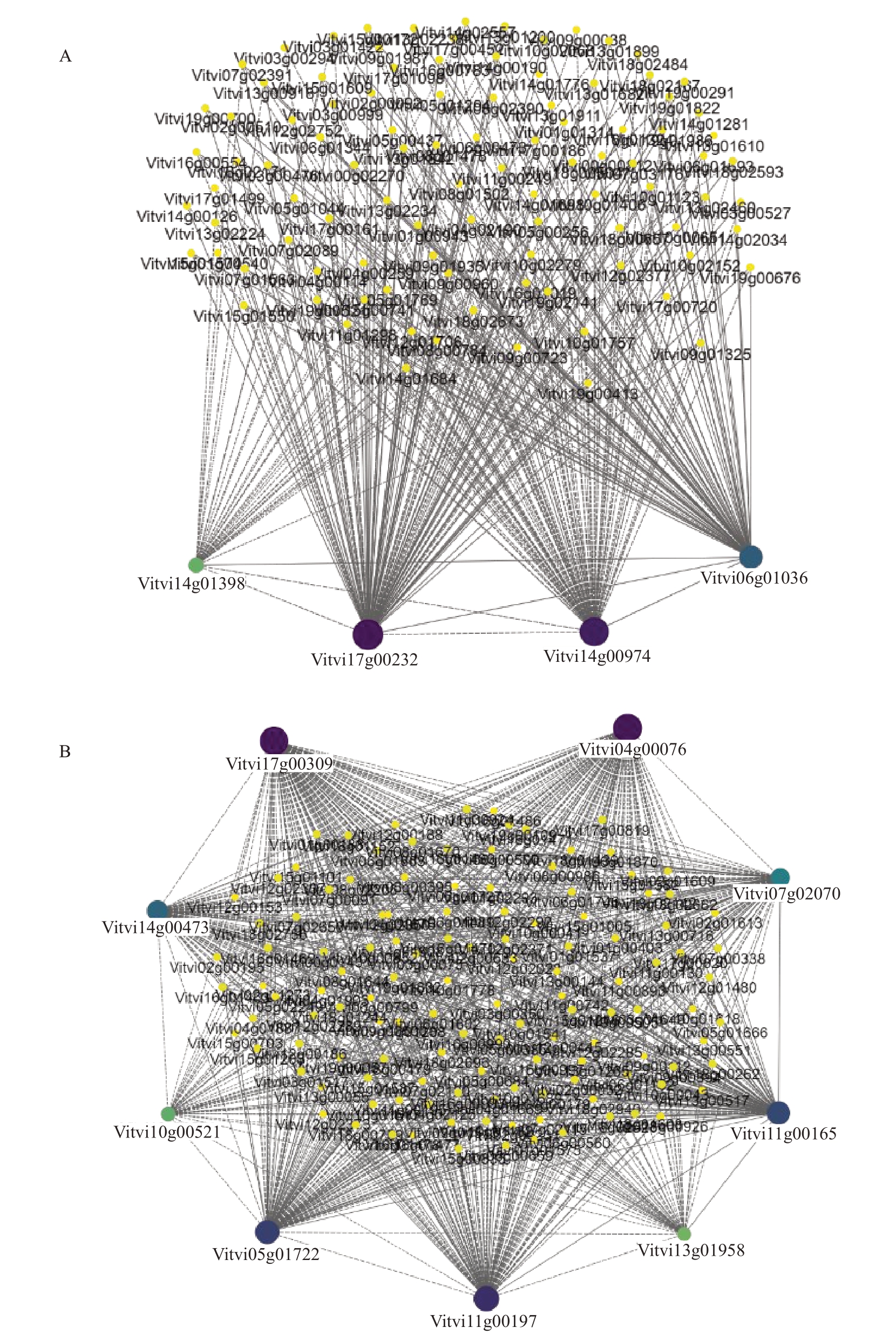
图10 Black(A)和Yellow(B)模块内抗病相关核心转录因子的基因共表达网络
Fig.10 Gene co-expression network of disease resistance related core transcription factors in Black(A)and Yellow(B)modules
通过KEGG 富集分析、转录因子家族分析以及WGCNA中与抗病性相关联的模块,结合基因注释,筛选出6 个与抗病相关的候选基因,分别是Vitvi01g02263(FAOMT)、Vitvi06g01183(F3’5’H)、Vitvi06g01036(EIN3)、Vitvi04g00076(MYB1R1)、Vit-vi17g00232(MYB4)和Vitvi07g02070(ERF098)。
2.7 DEGs的RT-qPCR
为了验证RNA-Seq 数据的可靠性,从DEGs 中选择9个基因,包括2个类黄酮代谢相关的DEGs、2个植物激素信号转导相关的DEGs、2个共表达模块筛选到的关键基因和3 个随机挑选的DEGs,通过RT-qPCR方法分析了这些基因在刺葡萄和美人指受白腐病病原菌侵染后的表达量变化,荧光定量显示以上基因在接种病菌前后的相对表达量与转录组数据一致,这说明转录组数据是可靠的(图11)。其中FAOMT、F3’5H、EIN3、MYB4的表达量在所有时期中刺葡萄均明显高于美人指;MYB1R1、ERF098 在接菌48 h 后,在刺葡萄中的表达量较其他时期和美人指中的均有显著提升。
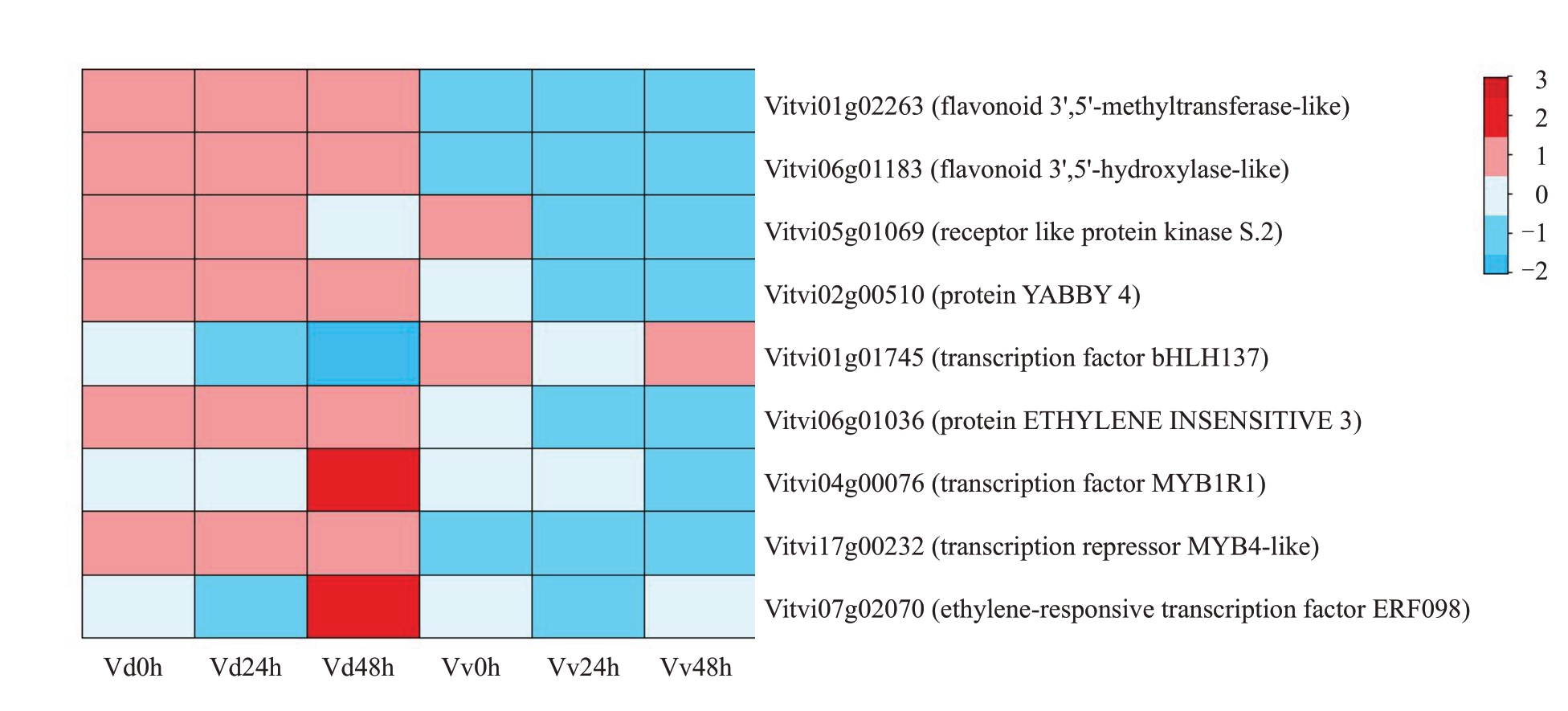
图11 差异表达基因qRT-PCR 鉴定
Fig.11 DEGs were identified by qRT-PCR
3 讨 论
本研究中,利用RNA-Seq技术对接种白腐病原菌后不同时间点的抗病和感病葡萄叶片进行转录组测序,在0 h、24 h 和48 h 三个时期两者之间分别筛得差异基因5266、4725和4653个。当白腐病病原菌侵染葡萄果实后,多个代谢通路的基因表达发生改变,其中次生代谢物可能积极参与葡萄抗病反应。
苯丙烷代谢物对植物的发育和生存至关重要,苯丙烷代谢物的生物合成和多样性可通过加氧酶、氧化还原酶、连接酶和转移酶等一系列酶来实现,这些酶通过酰基化、甲基化、糖基化和羟基化对代谢物的基本骨架进行化学修饰[15]。苯丙氨酸由莽草酸途径产生,苯丙类化合物是类黄酮、异黄酮和孜然芹素等多种酚类化合物的前体,研究发现苯丙氨酸在植物体内积累,能显著降低植物对病原菌的敏感性[16]。苯丙氨酸解氨酶(PAL)是苯丙烷代谢途径中的关键酶,能够将苯丙氨酸转为肉桂酸,是催化苯丙烷类次级代谢的第一步反应,起到枢纽的作用[17]。PAL参与花色苷、植物保护素、酚类代谢等物质的合成,该基因在大多数植物中是以多基因家族存在,PAL基因家族成员调控的代谢途径也各有差异。有研究表明,植物中PAL活性降低时,抵抗外界干扰的能力下降[18]。肉桂酸4-羟基化酶(C4H)为通用的苯丙氨酸途径限速酶,参与苯丙烷代谢途径第二步催化反应,起到承上启下的作用。该酶能提高自身的活性,来抵抗外界的环境胁迫。C4H将反式肉桂酸催化为β-香豆酸,通过4-香豆酰辅酶A 连接酶转化为香豆酞辅酶A,为下游类黄酮、木质素等次级代谢途径提供前体物质[19]。PAL催化苯丙烷代谢合成途径中的第一步反应产生的物质与C4H 催化生成4-香豆酸。PAL 与C4H 这两个酶通常情况下均以协同的方式进行表达,来控制黄酮物质的合成,但通常情况下,C4H 的活性比PAL 的活性低很多[20]。在本试验中PAL(Vitvi16g01503)、4CL(Vitvi16g00139)基因在受到病原菌诱导48 h后,其在抗病品种刺葡萄中的表达量远高于美人指。在植物体内PAL 作为苯丙烷类代谢定速酶,与植物的抗病性有重要关系。已报道在金针菇感染异型葡枝霉菌之后PAL活性明显增强[21],β-1,3-葡寡糖10倍诱抗剂处理后的葡萄叶片苯丙氨酸解氨酶(PAL)活性增强,说明处理之后提高了体内防御酶和抗病相关蛋白活性,进而诱导植株产生系统抗病性[22],以上结果表明PAL与植物的抗病性呈现正相关关系。
类黄酮代谢途径是苯丙烷代谢途径通过查尔酮合成酶(CHS)延伸的另一个重要分支,类黄酮代谢途径广泛存在于各种植物体内,类黄酮化合物是类黄酮代谢途径中产生的大量次生代谢物质,大自然中类黄酮化合物一般分为六大类:黄酮、黄酮醇、黄烷酮、黄烷醇、花色素、异黄酮[23]。在植物中,叶片和花等器官的类黄酮化合物含量比较高,类黄酮化合物作为次生代谢物质在植物中可以作为植物和微生物之间的关键信号物质发挥植物防御素的功能,在植物中有很强的抗病抑菌作用。CHS 可以通过催化形成不同的类黄酮化合物来提高植物的抗病能力,也可通过改变植保素、黄酮素、抗氧化酶SOD和POD 等的积累以及防御酶的活性来提高防御能力[24-27]。研究表明,在番茄和苹果中过表达CHS 能够增强对病原菌等环境胁迫的抗性[28-29]。抗枯萎病的棉花品种中CHS表达量明显上调后,类黄酮的含量提高从而增强其对枯萎病的抗性[30]。此外,郭泽西[31]已经证明抗性品种中查尔酮合成酶基因CHSI的表达量比感病品种高,过表达CHSl能够提高感病葡萄品种对灰霉病和霜霉病的抗性。在本研究中发现接种白腐病病原菌诱导48 h后,在抗病品种刺葡萄中的表达量远高于感病品种美人指,这与前人的研究一致。同时笔者通过加权共表达网络分析发现的两个候选基因:F3’5’H和FAOMT,均是类黄酮代谢途径中的关键基因,且FAOMT是F3’5’H的下游基因[32],二者在刺葡萄中的表达量均明显高于美人指。类黄酮代谢通路由结构基因和调节基因共同控制,调节基因包括MYB、bHLH 等转录因子[33],此前发现MYB4能够控制肉桂酸4-羟化酶(C4H)的负表达。笔者发现类黄酮代谢途径中的关键基因和转录因子在抗病品种刺葡萄和感病品种美人指中表现出明显的差异,因此,在刺葡萄响应白腐病病原菌侵染时,类黄酮物质可能起到重要作用。
此外,EIN3 和ERF098 是模块中筛选出刺葡萄响应白腐病侵染的关键基因,转录因子EIN3是乙烯信号转导途径中的调控因子,可激活乙烯反应因子(ERF)[34]。本研究中乙烯信号途径下游基因EIN3的表达水平在处理不同时间点中刺葡萄均高于美人指,而EIN3 下游基因ERF098 在48 h 抗病品种刺葡萄中的表达量明显高于其他时间和感病品种美人指,而在刺葡萄中48 h 的表达量明显上升这可能与反应时间有关。由此可知,乙烯可能在刺葡萄抗白腐病反应的激素信号转导中发挥了重要作用。
[1] AKKURT M,WELTER L,MAUL E,TOPFER R,ZYPRIAN E.Development of scar markers linked to powdery mildew (Uncinula necator) resistance in grapevine (Vitis vinifera L. and Vitis
sp.)[J].Molecular Breeding,2007,19(2):103-111.
[2] ALI K,MALTESE F,ZYPRIAN E,REX M,CHOI Y H,VERPOORTE R. NMR metabolic fingerprinting based identification of grapevine metabolites associated with downy mildew resistance[J]. Journal of Agricultural and Food Chemistry,2009,57(20):9599-9606.
[3] 王跃进,贺普超.中国葡萄属野生种抗白腐病机制研究[J].北方园艺,1988(1):5-8.
WANG Yuejin,HE Puchao. Study of white rot disease resistant mechanism in Chinese Vitis wild species[J]. Northern Horticulture,1988(1):5-8.
[4] 刘崇怀.中国葡萄属(Vitis L.)植物分类与地理分布研究[D].郑州:河南农业大学,2012.
LIU Chonghuai.Studies on taxonomy and geographical distribution of Chinese wild grape species[D]. Zhengzhou:Henan Agricultural University,2012.
[5] 董阳辉,徐佩娟,王艺平,钱剑锐,何铁海,朱朝磊,陈宝忠,王洪兴,张建萍. 葡萄白腐病研究[J]. 江西农业学报,2011,23(2):107-110.
DONG Yanghui,XU Peijuan,WANG Yiping,QIAN Jianrui,HE Tiehai,ZHU Chaolei,CHEN Baozhong,WANG Hongxing,ZHANG Jianping. Study on white rot of grape[J].Acta Agriculturae Jiangxi,2011,23(2):107-110.
[6] 张颖,樊秀彩,孙海生,姜建福,刘崇怀.葡萄种质资源对叶片白腐病的抗性鉴定及评价[J]. 果树学报,2017,34(9):1095-1105.
ZHANG Ying,FAN Xiucai,SUN Haisheng,JIANG Jianfu,LIU Chonghuai. Identification and evaluation of resistance to white rot in grape resources[J]. Journal of Fruit Science,2017,34(9):1095-1105.
[7] 贾云飞.刺葡萄0943 VdWRKY70 转录因子抗病功能分析[D].洛阳:河南科技大学,2018.
JIA Yunfei. Function analysis of VdWRKY70 transcription factor disease resistance from Vitis davidii 0943[D]. Luoyang:Henan University of Science and Technology,2018.
[8] 陈哲,黄静,赵佳,梁宏.草莓应答炭疽菌侵染的转录组分析[J].植物保护,2020,46(3):138-146.
CHEN Zhe,HUANG Jing,ZHAO Jia,LIANG Hong.Transcriptomics analysis of strawberry response to Colletotrichum theobromicola infection[J].Plant Protection,2020,46(3),138-146.
[9] JIANG Z C,LI R,TANG Y,CHENG Z Y,QIAN M J,LI W,SHAO Y Z. Transcriptome analysis reveals the inducing effect of Bacillus siamensis on disease resistance in postharvest mango[J]. Foods,2022,11(1):107.
[10] 姜长岳.中国野生毛葡萄VqMYB154 转录因子调控抗白粉病机理研究[D].杨凌:西北农林科技大学,2021.
JIANG Changyue. Mechanism analysis of transcription factor VqMYB154 regulating the resistance to powdery mildew in Chinese wild Vitis quinquangularis[D]. Yangling:Northwest A&F University,2021.
[11] JIAO C,SUN X P,YAN X X,XU X Z,YAN Q,GAO M,FEI Z J,WANG X P. Comparative transcriptome profiling of Chinese wild grapes provides insights into powdery mildew resistance[J].Phytopathology,2021,111(11):2041-2051.
[12] LIU L,ZHANG B,WANG H,YU S Y,GUAN T S,HUANG Y F,LIU C Y. Candidate resistance genes selection and transcriptome analysis for the early responses to Plasmopara viticola infection in grape cultivars[J]. Journal of Plant Pathology,2020,102(3):857-869.
[13] 沈才琦,孙磊,张颖,姜建福,刘崇怀,樊秀彩.胶孢炭疽菌侵染不同抗性葡萄叶片的转录组学分析[J].果树学报,2022,39(5):730-742.
SHEN Caiqi,SUN Lei,ZHANG Ying,JIANG Jianfu,LIU Chonghuai,FAN Xiucai. Transcriptomic analysis of different resistant leaves infected by Colletotrichum gloeosporioides in grape[J].Journal of Fruit Science,2022,39(5):730-742.
[14] 查倩,奚晓军,蒋爱丽,田益华,王世平.葡萄实时定量PCR 中稳定内参基因的筛选[J].果树学报,2016,33(3):268-274.
ZHAQian,XI Xiaojun,JIANGAili,TIAN Yihua,WANG Shiping.Identification of the appropriate reference gene through using a real-time quantitative PCR in grapes[J]. Journal of Fruit Science,2016,33(3):268-274.
[15] WANG S C,ALSEEKH S,FERNIE A R,LUO J. The structure and function of major plant metabolite modifications[J].Molecular Plant,2019,12(7):899-919.
[16] KUMAR V,HATAN E,BAR E,DAVIDOVICH-RIKANATI R,DORON-FAIGENBOIM A,SPITZER-RIMON B,ELAD Y,ALKAN N,LEWINSOHN E,OREN-SHAMIR M. Phenylalanine increases chrysanthemum flower immunity against Botrytis cinerea attack[J].The Plant Journal,2020,104(1):226-240.
[17] 梁冬.大豆PAL2-3 基因的功能研究[D].哈尔滨:东北农业大学,2017.
LIANG Dong. Functional study of PAL2-3 gene in soybean[D].Harbin:Northeast Agricultural University,2017.
[18] 董艳珍.植物苯丙氨酸解氨酶基因的研究进展[J].生物技术通报,2006(S1):31-33.
DONG Yanzhen. Research advances on plant phenylalanine ammonia-lyase gene[J].Biotechnology Bulletin,2006(S1):31-33.
[19] 邹广平,李雅丽,冯梦薇,杨修,许智伟.甘草悬浮细胞肉桂酸-4-羟基化酶(C4H)基因的克隆及表达分析[J].生物技术通报,2017,33(11):96-100.
ZOU Guangping,LI Yali,FENG Mengwei,YANG Xiu,XU Zhiwei.Cloning and expression analysis of cinnamate 4-hydroxylase gene from Glycyrrhiza uralensis suspension cultured cells[J].Biotechnology Bulletin,2017,33(11):96-100.
[20] 陈蒙.山葡萄苯丙烷代谢相关基因的克隆表达与功能验证[D].延吉:延边大学,2019.
CHEN Meng. Cloning and expression and functional verification of phenypropane pathway gene in Vitis amurensis[D].Yanji:Yanbian University,2019.
[21] 黄静,李长田,贾传文,王晓敏,吕瑞娜.异型葡枝霉感染不同金针菇后防御酶系的活性变化[J].中国食用菌,2021,40(1),103-108.
HUANG Jing,LI Changtian,JIA Chuanwen,WANG Xiaomin,LÜ Ruina. Changes of defensive enzyme activities in different Flammulina filiformis varieties after inoculation with Cladobotryum varium[J].Edible Fungi of China,2021,40(1):103-108.
[22] 王晓琳,吴琴燕,彭燕琼,邬劼,黄洁雪,毛妮妮,吉沐祥.2 种生物诱抗剂对葡萄霜霉病的诱导抗病作用[J].中国农学通报,2021,37(32):127-131.
WANG Xiaolin,WU Qinyan,PENG Yanqiong,WU Jie,HUANG Jiexue,MAO Nini,JI Muxiang. Effects of two inducers on induced resistance to grapevine downy mildew[J]. Chinese Agricultural Science Bulletin,2021,37(32):127-131.
[23] TAYLOR L P,GROTEWOLD E. Flavonoids as developmental regulators[J].Current Opinion in Plant Biology,2005,8(3):317-323.
[24] QIAO Y,MENG X J,JIN X X,DING G H.Genes expression of key enzymes in phenylpropanes metabolism pathway in cucumber with RT-PCR[J].Advanced Materials Research,2013,746:53-57.
[25] CHEN L J,GUO H M,LIN Y,CHENG H M. Chalcone synthase EaCHS1 from Eupatorium adenophorum functions in salt stress tolerance in tobacco[J]. Plant Cell Reports,2015,34(5):885-894.
[26] 陈帅. 烟草类黄酮代谢途径中关键酶CHS 基因与R2R3 MYB 类转录抑制因子功能研究[D]. 雅安:四川农业大学,2017.
CHEN Shuai. Function analysis of CHS genes and R2R3 MYB repressors related to flavonoid biosynthesis pathway in Nicotiana tabacum[D].Ya’an:Sichuan Agricultural University,2017.
[27] RAHMAN F U,KHAN I A,ASLAM A,LIU R T,SUN L,WU Y D,ASLAM M M,KHAN A U,LI P,JIANG J F,FAN X C,LIU C H,ZHANG Y.Transcriptome analysis reveals pathogenesis-related gene 1 pathway against salicylic acid treatment in grapevine(Vitis vinifera L.)[J].Front Genet,2022,13,1033288.
[28] KROL P,IGIELSKI R,POLLMANN S,KEPCZYNSKA E.Priming of seeds with methyl jasmonate induced resistance to hemi-biotroph Fusarium oxysporum f. sp. lycopersici in tomato
via 12-oxo-phytodienoic acid,salicylic acid,and flavonol accumulation[J].Journal of Plant Physiology,2015,179:122-132.
[29] JIAO C,SUN X P,YAN X X,XU X Z,YAN Q,GAO M,FEI Z J,WANG X P.Grape transcriptome response to powdery mildew infection:comparative transcriptome profiling of Chinese wild grapes provides insights into powdery mildew resistance[J].Phytopathology,2022,111(11):2041-2051.
[30] 黄启秀.海岛棉类黄酮代谢途径中与枯萎病抗性相关基因的克隆及功能验证[D].乌鲁木齐:新疆农业大学,2017.
HUANG Qixiu. Cloning and functional verification of genes related to Fusarium wilt resistance in flavonoid mtabolism pathway of island cotton (Gossypium barbadense L.)[D]. Urumqi:Xinjiang Agricultural University,2017.
[31] 郭泽西,孙大运,曲俊杰,潘凤英,刘露露,尹玲.查尔酮合成酶基因在葡萄抗灰霉病和霜霉病中的作用[J].中国农业科学,2022,55(6):1139-1148.
GUO Zexi,SUN Dayun,QU Junjie,PAN Fengying,LIU Lulu,YIN Ling. The role of chalcone synthase gene in grape resistance to gray mold and downy mildew[J]. Scientia Agricultura Sinica,2022,55(6):1139-1148.
[32] LI P Q,RUAN Z,FEI Z X,YAN J J,TANG G H. Integrated transcriptome and metabolome analysis revealed that flavonoid biosynthesis may dominate the resistance of Zanthoxylum bungeanum against stem canker[J]. Journal of Agricultural and Food Chemistry,2021,69(22):6360-6378.
[33] SHVACHKO N A,LOSKUTOV I G,SEMILET T V,POPOV V S,KOVALEVA O N,KONAREV A V. Bioactive components in oat and barley grain as a promising breeding trend for functional food production[J].Molecules,2021,26(8):2260.
[34] GU S Y,WANG L C,CHEUH C M,LO W S. CHITINASE LIKE1 regulates root development of dark-grown seedlings by modulating ethylene biosynthesis in Arabidopsis thaliana[J].Frontiers in Plant Science,2019,10:600.