钙离子(Ca2+)作为第二信使,参与植物体内许多信号转导途径[1]。各种生物和非生物胁迫在内的外部刺激均会导致胞质内的游离Ca2+浓度发生改变,而被钙结合蛋白或钙信号传感器识别,从而导致下游表达事件的发生[2-3]。钙依赖性蛋白激酶(calcium-dependent protein kinases,CDPKs/CPKs)作为植物中主要的Ca2+感受器之一[4],可不依赖于钙调素而经EF-hand 直接与Ca2+结合,感受Ca2+浓度变化后,蛋白激酶活性恢复,将Ca2+信号转导为磷酸化级联反应,这使得CDPK 具有Ca2+传感器和应答器的双重功能而被广泛研究[5-7]。
CDPKs/CPKs是植物和一些原生生物特有的一类丝氨酸/苏氨酸(Ser/Thr)型蛋白激酶,以其独特的结构而闻名[8],具有N 端可变域、Ser/Thr 蛋白激酶域、自抑制连接域和C 端具有EF-hand 结构的类钙调素调控域这4 个典型的结构域[9]。目前已在多种植物中鉴定出CDPK 基因,且不同物种中CDPK 基因数量差异较大,如模式植物拟南芥[Arabidopsis thaliana(L.)Heynh.]中有34 个[10],水稻(Oryza sativa L.)中有31 个[11-12],对萼猕猴桃(Actinidia valvata Dunn.)[13]、玉米(Zea mays L.)[14]、番茄(Solanum lycopersicum L.)[15]、黄瓜(Cucumis sativus L.)[16]、天蓝苜蓿(Medicago lupulina L.)[17]中分别有63、40、29、17、10 个。CDPK 广泛分布于植物体中,在植物生长发育和形态建成、生物及非生物胁迫应对中发挥着重要作用,且此过程也离不开Ca2+的调控[9]。如黄玉婷[18]发现外源Ca2+处理能使CDPK 基因大幅上调表达而提高茶树低温耐受力;CDPK 与Ca2+结合后活性被激发,从而调控玉米热激蛋白的表达来提高耐性[19];GsCPK16 可通过响应温度和光刺激而被提高的细胞溶质Ca2+水平激活,从而调节龙胆花的重新开放[20]。
刺梨(Rosa roxburghii Tratt.)属蔷薇科植物,是西南地区的特色树种之一,在土壤交换性钙含量(w)463~4526 mg·kg-1 的贵州喀斯特地区生长良好[21]。目前,刺梨全基因组测序已完成,但关于刺梨CDPK基因家族的分析及其对供钙水平的响应尚未见报道。本研究基于全基因组水平通过生物信息学方法,鉴定和分析刺梨CDPK 基因家族特征和系统进化关系、启动子顺式作用元件,分析该家族的时空表达特性,并研究刺梨CDPK 基因家族成员对不同供钙水平的响应,为后续探明CDPK 家族在刺梨中对外界钙环境的响应机制提供相关信息。
1 材料和方法
1.1 材料
本试验于2021年4月在贵州大学农学院刺梨资源圃中选取长势基本一致的刺梨贵农5 号(R. roxburghii Tratt.‘Guinong 5’)扦插苗,先用自来水冲洗除去根部泥土,再用蒸馏水培养,而后使用1/8 改良的Hoagland and Aron完全营养液进行预培养,每3 d更换1 次营养液,将水培刺梨苗置于人工气候室内培养。用去离子水将预培养2个月刺梨扦插苗的根和叶冲洗干净后饥饿24 h,转入以下3 种不同供钙水平的营养液中。经文献查找及一系列预试验筛选,最终试验设置3个Ca2+浓度梯度:0、0.5、2 mmol·L-1,分别配制成无钙、低浓度钙及高浓度钙的营养液,并在培养0、1、7 d后取其水培根和叶,经液氮处理后保存于-80 ℃备用。各处理均3 次生物学重复。在保持其他营养元素浓度不变的条件下,去掉营养液中所有含钙成分,由醋酸钙供钙,并用40 mg·L-1 NH4NO3 补齐氮素差异。试验中所用营养液均用0.1 mol·L-1 NaOH 和H2SO4调整pH 值为6.5,培养温度20 ℃。
1.2 方法
1.2.1 刺梨CDPK基因家族成员的鉴定以及染色体定位、蛋白质理化性质和亚细胞定位分析 从NCBI数据库(https://blast.ncbi.nlm.nih.gov/)下载刺梨基因组文件,在Tair数据库(https://www.arabidopsis.org/)下载拟南芥CDPK 家族的蛋白序列,运用TBtools v1.098696 进行本地Blast 比对(E-value <1×10-5),取bit score>100 的结果,随后将得到的序列在NCBI 中进行Protein Blast,结合SMART(http://smart.embl-heidelberg.de/)及Pfam 数 据 库(https://pfam.xfam.org/),以CDPK 蛋白典型结构域Ser/Thr 蛋白激酶区(Pfam 数据库ID:PF00069)以及EF-手型结构区(PF13499)进行筛选,删除重复值及不含上述结构域的序列,进而得到刺梨CDPK基因家族的成员。
在刺梨基因组注释信息GFF 文件中提取出RrCDPK基因染色体位置信息,并利用TBtools软件[22]绘图。根据RrCDPK在染色体上的排列顺序,依次对其命名。利用在线工具ExPASy(https://web.expasy.org/protparam/)对RrCDPK蛋白的分子质量、等电点等进行分析。利用WoLF PSORT 网站(https://wolfpsort.hgc.jp)预测RrCDPK蛋白的亚细胞定位信息。
1.2.2 系统进化树的构建及基因结构、保守基序和保守结构域分析 从Swiss-Prot(https://www.uniprot.org/)及NCBI 数据库下载水稻CDPK 家族蛋白文件[11-12],从Tair数据库下载拟南芥CDPK家族蛋白文件[10];从GDR数据库(https://www.rosaceae.org)下载苹果和草莓蛋白序列,依据上述方法得到苹果及草莓的CDPK家族蛋白文件。使用MEGA7软件对水稻、拟南芥、苹果、草莓及刺梨的CDPK 家族进行Muscle 多序列比对,采用邻接法(neighbor-joining,NJ)构建系统进化树,bootstrap 值设置为1000。使用iTOL在线工具(https://itol.embl.de)进行美化。
使用MEME 在线工具(https://meme-suite.org/meme/index.html)预测其蛋白序列的保守基序;通过NCBI-CDD进行RrCDPK家族保守结构域信息分析;根据RrCDPK家族GFF文件分析内含子-外显子结构;最终使用TBtools进行可视化。
1.2.3 启动子顺式作用元件分析 利用TBtools工具从刺梨基因组中提取出RrCDPK 编码序列的启动子上游2000 bp序列,使用在线数据库plantCARE(http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)预测其顺式作用元件。
1.2.4 刺梨不同组织及不同果实发育时期的转录组分析 从刺梨转录组数据[23-24]中提取出RrCDPK 基因家族在刺梨不同组织及果实发育时期的表达数据,采用TBtools软件绘制热图并进行聚类分析。
1.2.5 qRT-PCR 分析 使用Primer blast(https://www.ncbi.nlm.nih.gov/tools/primer blast)设 计qRTPCR 引物,由生工生物工程股份有限公司(上海)合成(表1)。取不同供钙水平处理的刺梨苗叶和根,用TaKaRa MiniBEST Plant RNA Extraction Kit 总RNA 提取试剂盒(大连宝生物工程有限公司)提取总RNA,纯化 的RNA 用TaKaRa RNA PCR Kit Ver.2.1 试剂盒(大连宝生物工程有限公司)合成cDNA。使用ABI ViiA7荧光定量PCR仪(杭州博日科技有限公司)进行PCR反应,反应体系为20 μL:2 μL cDNA、各0.8 μL 上下游引物、6 μL 灭菌水、10 μL TB Green Premix Ex Taq Ⅱ(Tli RNaseH Plus)(2×)(大连宝生物工程有限公司)和0.4 μL ROX Reference Dye Ⅱ,以UBQ为内参基因校正表达量[24]。采用2−ΔΔCT法[25]分析数据。所测基因表达量为其相对表达水平,以RrCDPK 测定值/UBQ 测定值表示,每个数据3次重复,并取其平均值。
表1 qRT-PCR 引物
Table 1 Primers used for quantitative real-time PCR
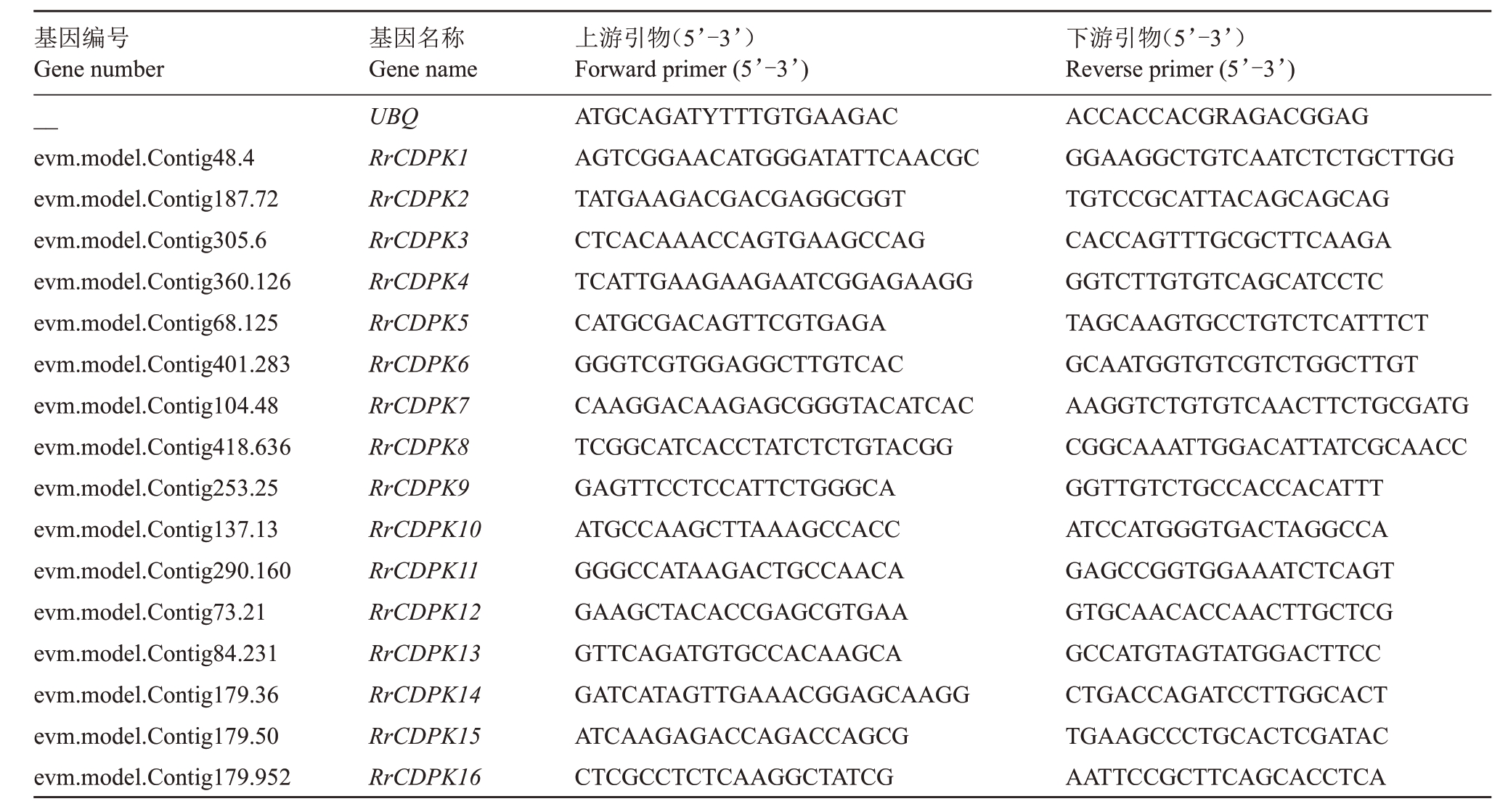
基因编号Gene number__evm.model.Contig48.4 evm.model.Contig187.72 evm.model.Contig305.6 evm.model.Contig360.126 evm.model.Contig68.125 evm.model.Contig401.283 evm.model.Contig104.48 evm.model.Contig418.636 evm.model.Contig253.25 evm.model.Contig137.13 evm.model.Contig290.160 evm.model.Contig73.21 evm.model.Contig84.231 evm.model.Contig179.36 evm.model.Contig179.50 evm.model.Contig179.952基因名称Gene name UBQ RrCDPK1 RrCDPK2 RrCDPK3 RrCDPK4 RrCDPK5 RrCDPK6 RrCDPK7 RrCDPK8 RrCDPK9 RrCDPK10 RrCDPK11 RrCDPK12 RrCDPK13 RrCDPK14 RrCDPK15 RrCDPK16上游引物(5’-3’)Forward primer(5’-3’)ATGCAGATYTTTGTGAAGAC AGTCGGAACATGGGATATTCAACGC TATGAAGACGACGAGGCGGT CTCACAAACCAGTGAAGCCAG TCATTGAAGAAGAATCGGAGAAGG CATGCGACAGTTCGTGAGA GGGTCGTGGAGGCTTGTCAC CAAGGACAAGAGCGGGTACATCAC TCGGCATCACCTATCTCTGTACGG GAGTTCCTCCATTCTGGGCA ATGCCAAGCTTAAAGCCACC GGGCCATAAGACTGCCAACA GAAGCTACACCGAGCGTGAA GTTCAGATGTGCCACAAGCA GATCATAGTTGAAACGGAGCAAGG ATCAAGAGACCAGACCAGCG CTCGCCTCTCAAGGCTATCG下游引物(5’-3’)Reverse primer(5’-3’)ACCACCACGRAGACGGAG GGAAGGCTGTCAATCTCTGCTTGG TGTCCGCATTACAGCAGCAG CACCAGTTTGCGCTTCAAGA GGTCTTGTGTCAGCATCCTC TAGCAAGTGCCTGTCTCATTTCT GCAATGGTGTCGTCTGGCTTGT AAGGTCTGTGTCAACTTCTGCGATG CGGCAAATTGGACATTATCGCAACC GGTTGTCTGCCACCACATTT ATCCATGGGTGACTAGGCCA GAGCCGGTGGAAATCTCAGT GTGCAACACCAACTTGCTCG GCCATGTAGTATGGACTTCC CTGACCAGATCCTTGGCACT TGAAGCCCTGCACTCGATAC AATTCCGCTTCAGCACCTCA
1.2.6 数据处理 各处理设置3次生物学重复、3次技术重复。使用SPSS 26 软件进行方差分析,用Duncan 法进行差异显著性分析。使用WPS 进行数据处理并作图。
2 结果与分析
2.1 刺梨CDPK 基因家族成员的鉴定及基本特征分析
通过与34 个AtCDPK 基因的蛋白序列进行比对及保守结构域筛选,共鉴定出16个RrCDPK基因家族成员,并基于其染色体定位(图1),依次命名为RrCDPK1~16。16个RrCDPK基因不均等地分布在刺梨的6条染色体上,4号染色体没有成员定位,而1号和7 号染色体上数量最多,为4 个。RrCDPKs 为亲水性蛋白,基因长度在1496(RrCDPK6)~5524(RrCDPK14)bp 之间,编码蛋白质长度在393(RrCDPK6)~561(RrCDPK16)aa,蛋白分子质量介于44.02(RrCDPK6)~62.98(RrCDPK16)ku 之间,各成员的蛋白长度及分子质量差异较小;除RrCDPK5/8 为碱性蛋白外,其余成员的蛋白等电点都小于7,为酸性蛋白;RrCDPK1/4/11/12/14/15属于不稳定蛋白,其他RrCDPK 成员均为稳定蛋白(表2)。亚细胞定位预测结果(表3)显示,RrCDPKs 在细胞核、细胞骨架、细胞膜和多种细胞器中均有定位,主要定位在细胞质中。
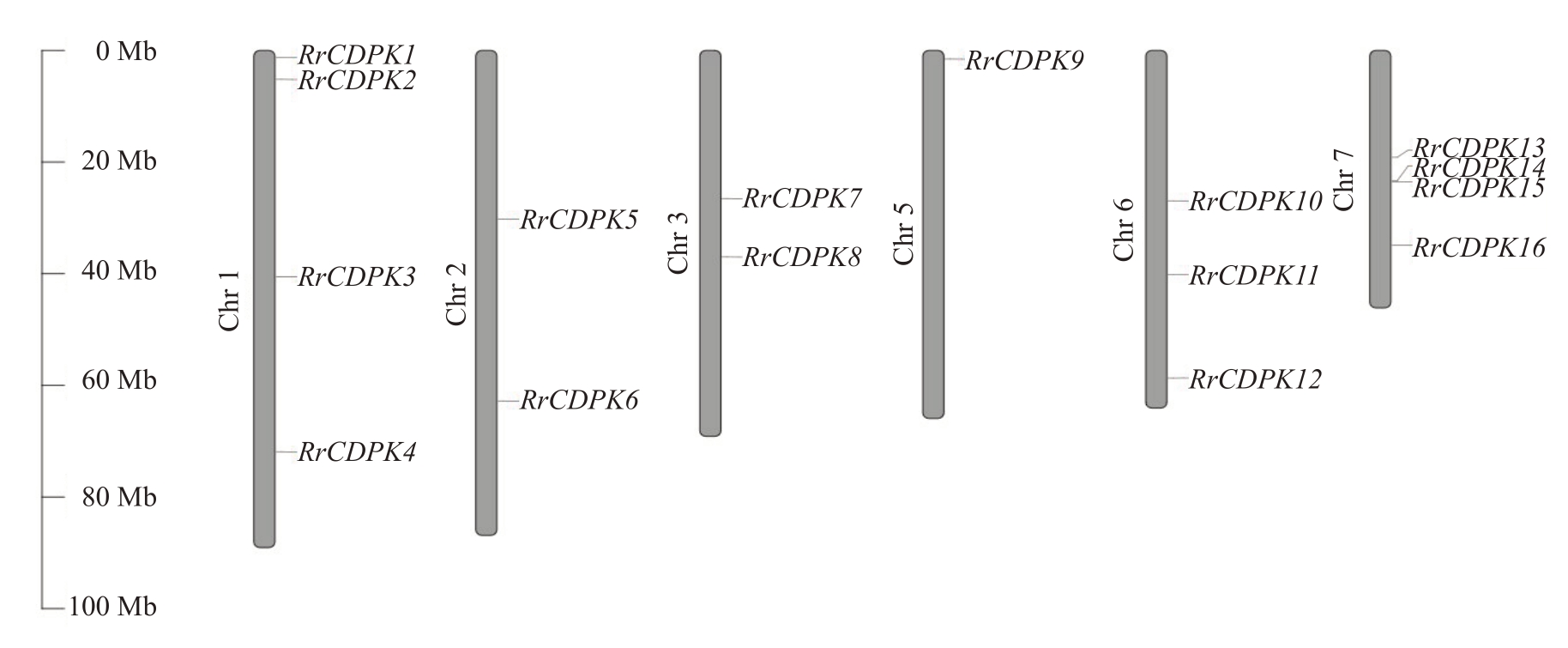
图1 刺梨CDPK 基因家族染色体定位图
Fig.1 Chromosome mapping of CDPK gene family in R.roxburghii
表2 刺梨CDPK 基因家族的蛋白质理化性质分析
Table 2 Analysis of protein physicochemical properties of CDPK gene family in R.roxburghii
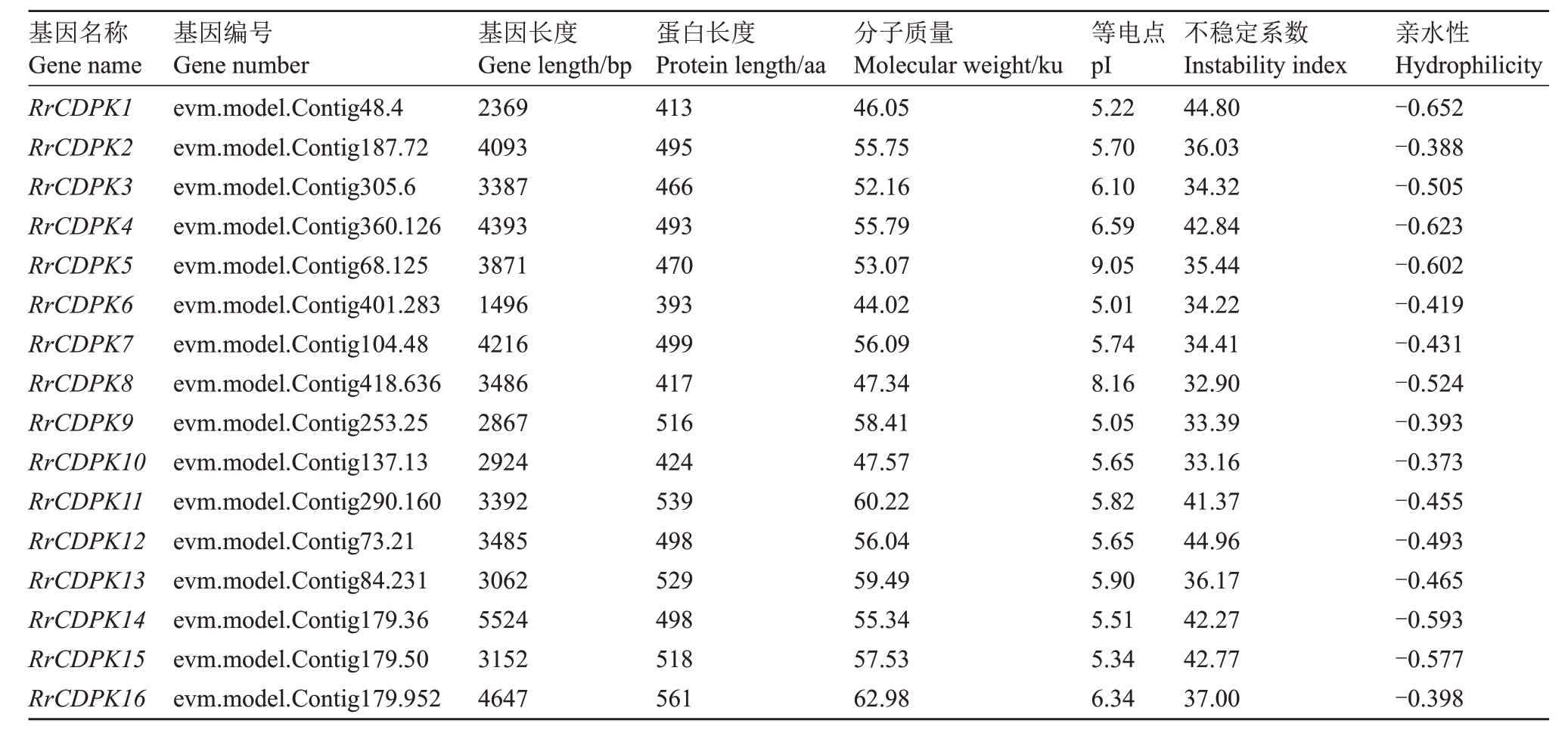
基因名称Gene name RrCDPK1 RrCDPK2 RrCDPK3 RrCDPK4 RrCDPK5 RrCDPK6 RrCDPK7 RrCDPK8 RrCDPK9 RrCDPK10 RrCDPK11 RrCDPK12 RrCDPK13 RrCDPK14 RrCDPK15 RrCDPK16基因编号Gene number evm.model.Contig48.4 evm.model.Contig187.72 evm.model.Contig305.6 evm.model.Contig360.126 evm.model.Contig68.125 evm.model.Contig401.283 evm.model.Contig104.48 evm.model.Contig418.636 evm.model.Contig253.25 evm.model.Contig137.13 evm.model.Contig290.160 evm.model.Contig73.21 evm.model.Contig84.231 evm.model.Contig179.36 evm.model.Contig179.50 evm.model.Contig179.952基因长度Gene length/bp 2369 4093 3387 4393 3871 1496 4216 3486 2867 2924 3392 3485 3062 5524 3152 4647蛋白长度Protein length/aa 413 495 466 493 470 393 499 417 516 424 539 498 529 498 518 561分子质量Molecular weight/ku 46.05 55.75 52.16 55.79 53.07 44.02 56.09 47.34 58.41 47.57 60.22 56.04 59.49 55.34 57.53 62.98等电点pI 5.22 5.70 6.10 6.59 9.05 5.01 5.74 8.16 5.05 5.65 5.82 5.65 5.90 5.51 5.34 6.34不稳定系数Instability index 44.80 36.03 34.32 42.84 35.44 34.22 34.41 32.90 33.39 33.16 41.37 44.96 36.17 42.27 42.77 37.00亲水性Hydrophilicity-0.652-0.388-0.505-0.623-0.602-0.419-0.431-0.524-0.393-0.373-0.455-0.493-0.465-0.593-0.577-0.398
表3 刺梨CDPK 基因家族的亚细胞定位分析
Table 3 Subcellular localization analysis of CDPK gene family in R.roxburghii
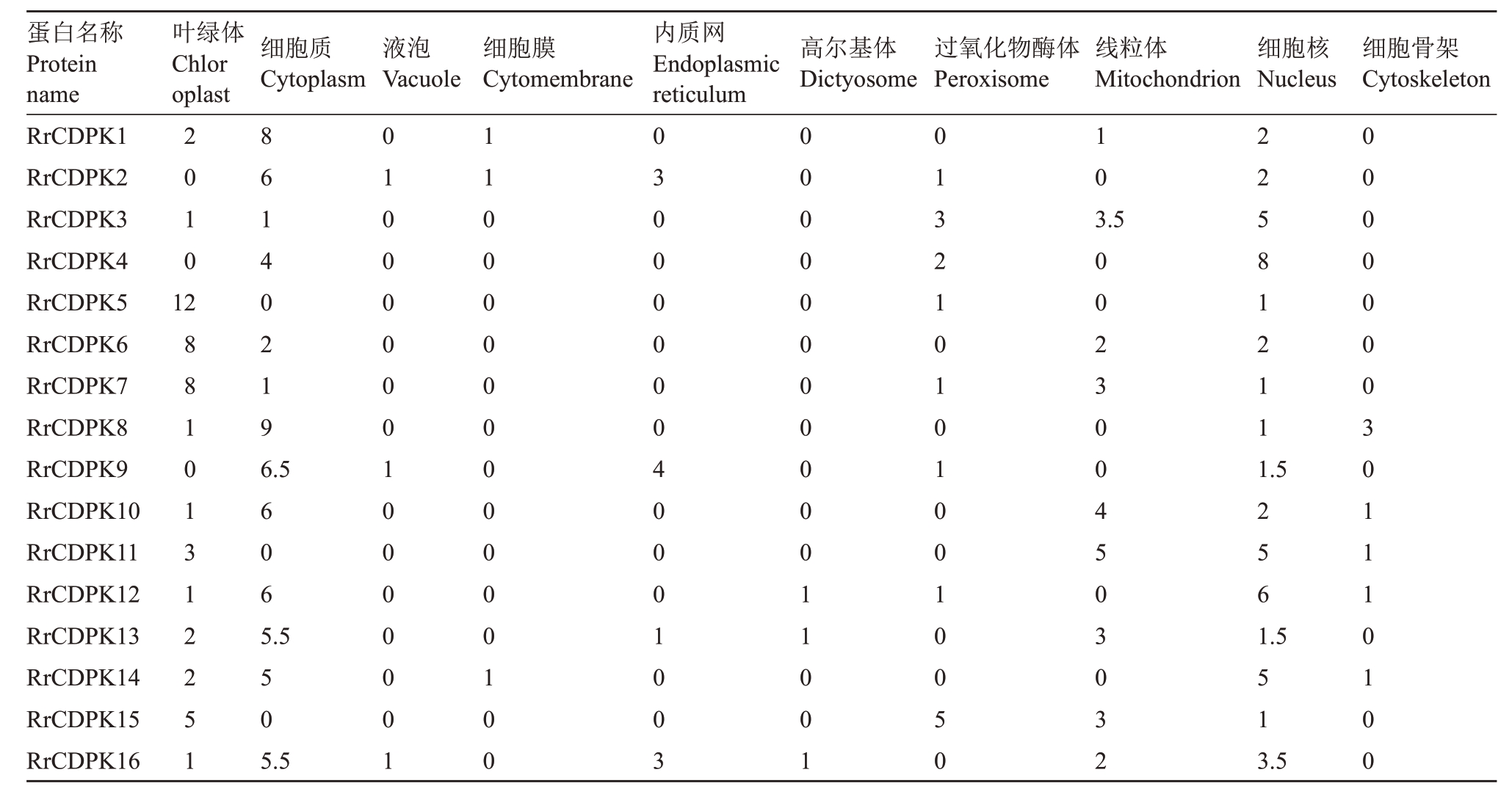
注:表中数字表示WoLF PSORT 网站中与目标蛋白序列最相似且亚细胞定位已经确定的蛋白数目。数目越大表示目标蛋白可能参与的亚细胞结构间的物质交流越多。
Note:The numbers in the table indicated the number of proteins in the WoLF PSORT website that were the most similar to the target protein sequence and whose subcellular localization had been determined. The larger the number, the more material exchange between the subcellular structures that the target protein might be involved in.
蛋白名称Protein name RrCDPK1 RrCDPK2 RrCDPK3 RrCDPK4 RrCDPK5 RrCDPK6 RrCDPK7 RrCDPK8 RrCDPK9 RrCDPK10 RrCDPK11 RrCDPK12 RrCDPK13 RrCDPK14 RrCDPK15 RrCDPK16叶绿体Chlor oplast细胞质Cytoplasm液泡Vacuole细胞膜Cytomembrane内质网Endoplasmic reticulum高尔基体Dictyosome过氧化物酶体Peroxisome线粒体Mitochondrion细胞核Nucleus细胞骨架Cytoskeleton 2010 10 3.5 12 88101312251 86140219 22581211 6.5 1.5 606 256 5.5 1.5 50 51 5.5 0100000010000001 1100000000000100 0300000040001003 0000000000011001 0132101010010050 0023004503032 3.5 0000000301110100
2.2 系统进化及结构特征分析
为研究刺梨CDPK 蛋白家族的进化关系,以拟南芥、水稻、草莓和苹果的CDPK蛋白序列为参考进行系统进化树的构建,其中草莓及苹果的CDPK 家族是通过与RrCDPK 家族同样的鉴定方式,分别得到36和34个成员。聚类结果显示,RrCDPK家族可分为4 个亚家族(图2)。亚家族Ⅰ、Ⅱ、Ⅲ中均有5个RrCDPK 成员,分别为RrCDPK6/10/11/14/15、RrCDPK1/3/4/7/12、RrCDPK2/8/9/13/16,而亚家族Ⅳ中仅包括1个RrCDPK5成员。进一步观察可知,与刺梨CDPK 家族亲缘关系最近的是草莓,其次是苹果,较远的是拟南芥和水稻。
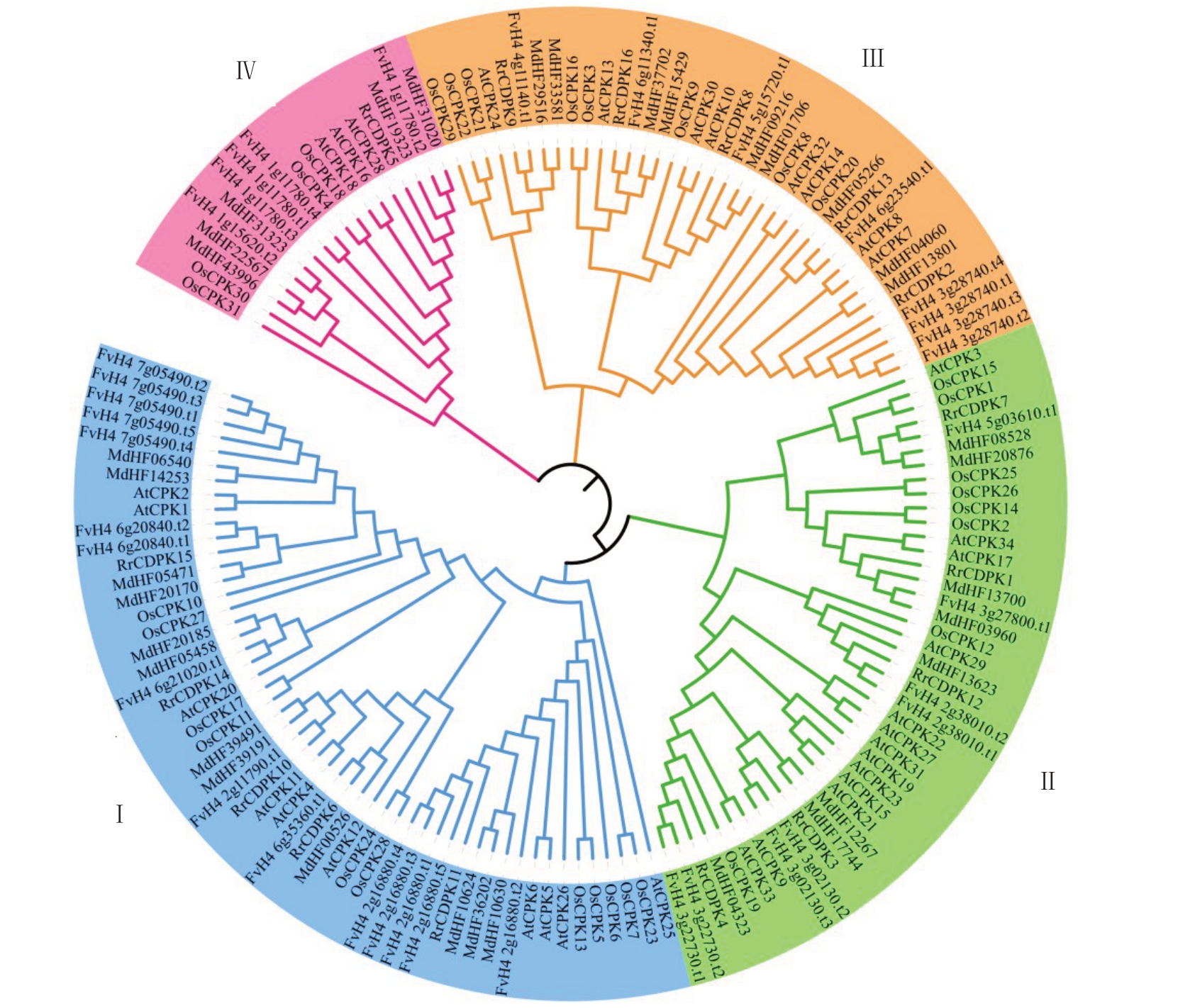
图2 5 个物种CDPK 家族成员的系统进化分析
Fig.2 Phylogenetic analysis of CDPK family members in five plant species
At.拟南芥;Os.水稻;Fv.草莓;Md.苹果;Rr.刺梨。
At.Arabidopsis thaliana;Os.Oryza sativa;Fv.Fragaria×ananassa;Md.Malus pumila;Rr.Rosa roxburghii.
为了更直观地了解刺梨CDPK基因家族成员的结构特征,分析了刺梨该家族的基因结构、保守基序和保守结构域(图3),结果表明,同一亚家族的成员基因结构较为相似,外显子数量为2~10 个,其中RrCDPK6最少(2个),RrCDPK5最多(10个)。刺梨CDPK 家族成员都含有2 个高度保守的结构域[Pkinase(即Ser/Thr 蛋白激酶)和EF-hand 结构域],以及6个保守基序(motif),且亲缘关系越近的成员,结构域及基序的数量和排列也越相似。
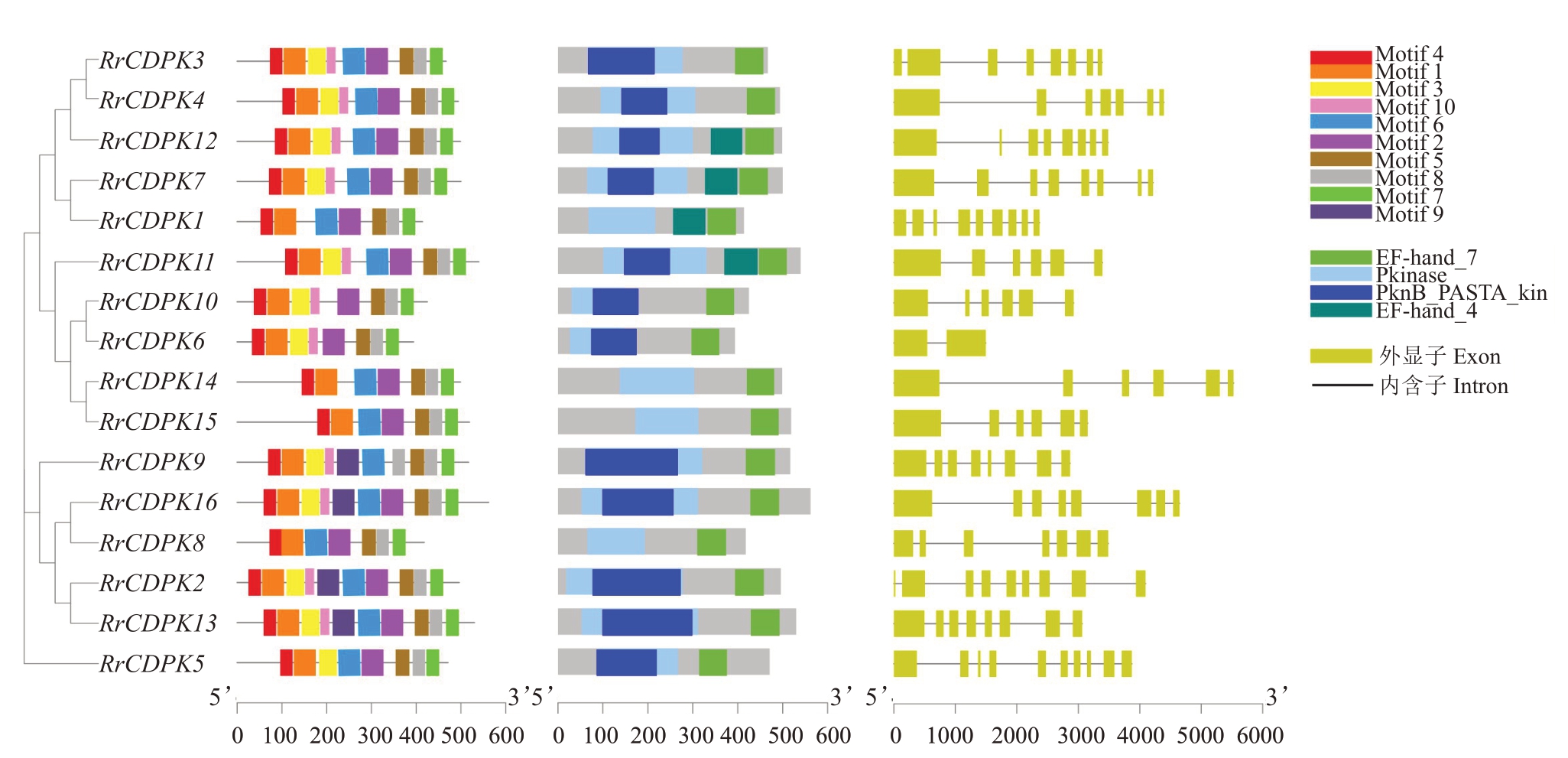
图3 刺梨CDPK 基因家族成员的结构特征
Fig.3 An analytical view of CDPK gene family in R.roxburghii
从左至右依次为保守基序、保守结构域及基因结构。
The figures from left to right were conserved motifs,conserved domains and gene structures,respectively.
2.3 启动子顺式作用元件分析
基因启动子区域的顺式作用元件影响基因的表达模式。为了解RrCDPK基因家族成员的启动子序列功能,对启动子上游2000 bp 序列进行预测,结果如图4所示。RrCDPKs上游均具有大量的基本起始元件CAAT-box 和TATA-box,功能分别为启动子和增强子元件、核心启动子元件,符合真核生物特点。除RrCDPK16 启动子不含光响应元件外,其余成员启动子都含有或多或少的光响应元件,表明在光响应中发挥着重要作用。生长素(auxin,IAA)、赤霉素(gibberellin,GA)、水杨酸(salicylic acid,SA)、脱落酸(abscisic acid,ABA)以及茉莉酸甲酯(methyljasmonate,MeJA)5 种激素响应元件在RrCDPKs 启动子区普遍存在但又有差异,仅RrCDPK1/2/3/8/9/11/12 启动子区有生长素响应元件,而RrCDPK2/3/4/6/7/8/14、6/11/16、10/13 启动子区分别不存在水杨酸、脱落酸及赤霉素响应元件。RrCDPKs 还含有4种胁迫应答元件:防御和压力胁迫、低温胁迫、厌氧诱导及缺氧特异性诱导的应答元件。尤为突出的是,仅RrCDPK6/13 启动子无厌氧诱导应答元件,RrCDPK1 启动子具有缺氧特异性诱导有关的类增强子元件。RrCDPKs还含有转录因子结合位点:干旱诱导有关(不含RrCDPK1/6/8/10/16)、参与黄酮类生物合成基因调控(RrCDPK1/11)、光响应(RrCDPK1/4/9/15/16)的MYB 结合位点。此外,RrCDPKs含有多种与植物生长发育相关的应答元件,其中光敏色素下调表达、种子特异性调节、参与胚乳表达的应答元件分别为RrCDPK7、RrCDPK3、RrCDPK3/4启动子所特有。汇总可知,RrCDPK15 启动子含有的顺式作用元件种类及数目均相对较多,暗示较其他成员可能参与更多的生长发育过程。
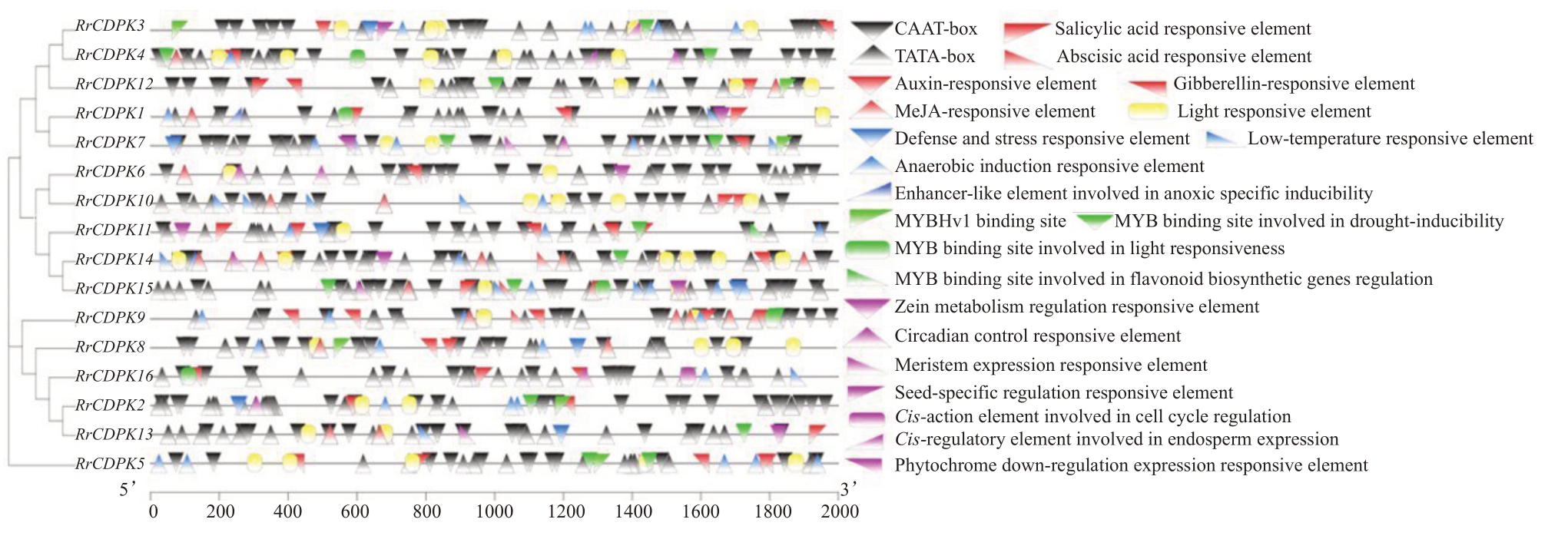
图4 刺梨CDPK 基因家族成员的顺式作用元件
Fig.4 Cis-acting elements of CDPK gene family in R.roxburghii
同一颜色表示一个大类顺式作用元件。其中黑色表示基本元件,红色表示激素响应元件,黄色表示光响应元件,蓝色表示胁迫响应元件,绿色表示转录因子结合位点,紫色表示与植物生长发育相关的应答元件。
The same color represented a broad class of cis-acting elements. The black. Basic elements; the red. Hormone-responsive elements; the yellow.Light-responsive elements; the blue. Stress-responsive elements; the green.Transcription factor binding sites; the purple. Response elements related to plant growth and development.
2.4 RrCDPKs的时空表达特点
为了解刺梨CDPK基因家族成员的时空表达特点,绘制了RrCDPKs在刺梨不同组织(茎、叶、花、30 d的果实)和果实不同发育时期(花后30、60、90 d)的表达模式热图。由图5-A 可知,RrCDPKs 在刺梨不同组织中有明显的表达差异:RrCDPK2/6/16在花中高表达,RrCDPK3/5/10/13 在茎中高表达,RrCDPK11/12 在果中高表达,RrCDPK4/8/9/15 在叶中高表达,RrCDPK1/7/14 在所测组织中的表达量均不高。由图5-B 可知,RrCDPK1/10/12/14 在果实发育前期表达量最高,中后期表达下调;仅RrCDPK7 在果实发育中期表达量最高;RrCDPK2/8/11/15的表达随果实的发育而不断上调,以至在后期表达最高;RrCDPK3/4/5/13/16在果实发育前期和果实膨大期的表达量都较高;仅RrCDPK6/9除在果实发育中期表达下调,其余两个时期表达量都稍高。
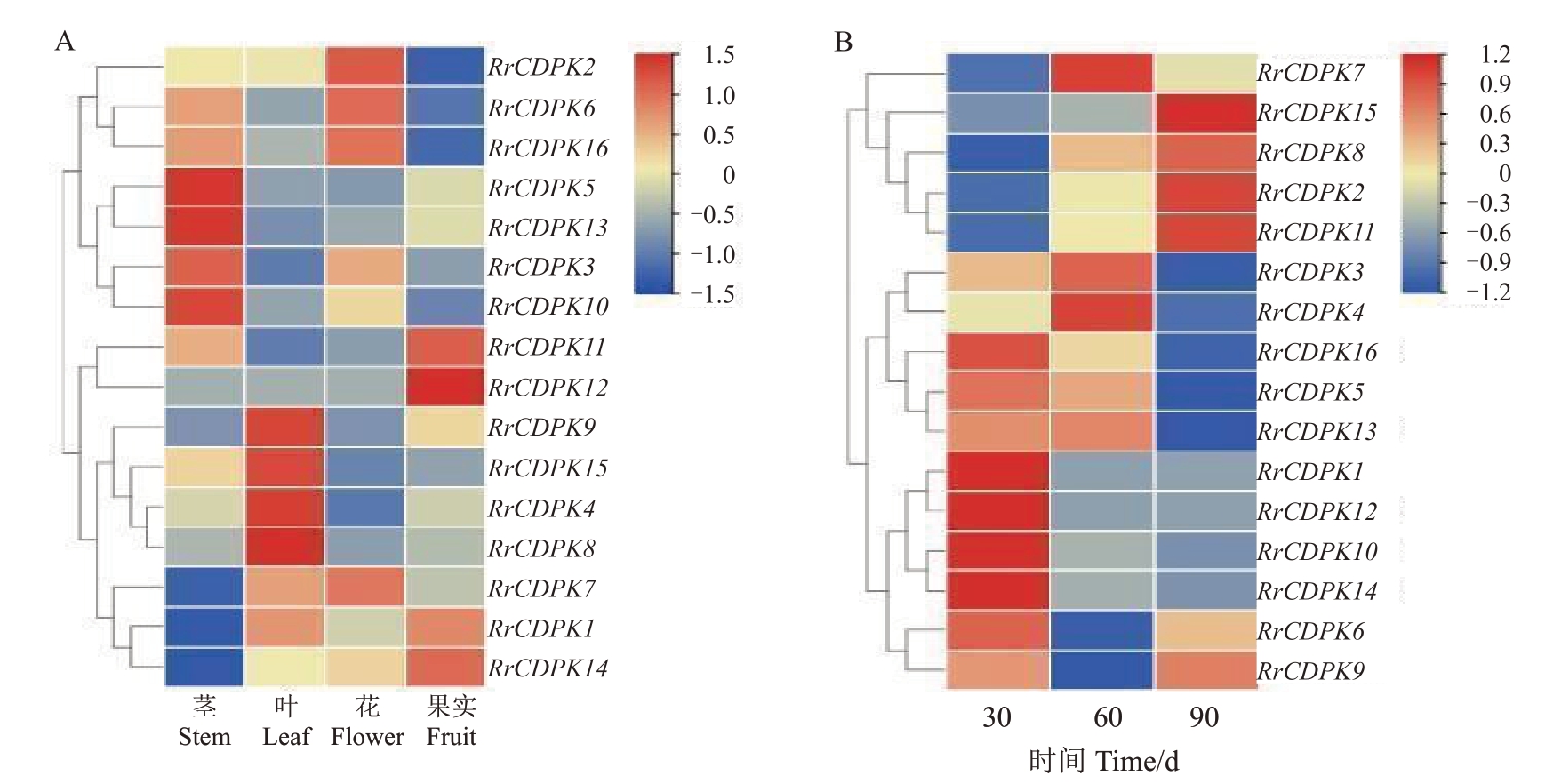
图5 刺梨CDPK 基因的时空表达分析
Fig.5 Spatio-temporal expression analysis of CDPK genes in R.roxburghii
A.RrCDPK 基因在刺梨茎、叶片、花瓣和30 d 果实中的表达热图;B.RrCDPK 基因在刺梨不同发育时期的果实中的表达情况。颜色从蓝到红表示基因的表达量从低到高。
A.The expression hot map of RrCDPK genes in stems,leaves,petals and 30-day fruits;B.The expression in R.roxburghii fruits at different development stages.Colors from blue to red indicated gene expression levels from low to high.
以上结果表明,刺梨RrCDPK 基因的表达具有时空表达特异性,在不同组织器官和刺梨果实不同发育时期发挥着重要的作用。
2.5 RrCDPKs对不同供钙水平的响应
笔者在本试验中探索了所有刺梨RrCDPK基因家族对供钙水平的响应,结果如图6所示,以0 mmol·L-1钙处理为对照,发现供钙后,少数RrCDPKs 表现出明显的上调效应:0.5 mmol·L-1钙水平下,培养1 d后,叶中2 个基因(RrCDPK4/8)表达量显著提高(p<0.05),培养7 d 后,根中2 个基因(RrCDPK1/2)表达量显著提高;2 mmol·L-1钙水平下,培养1 d后,叶中3 个基因(RrCDPK4/10/12)、根中RrCDPK9 表达量显著提高,培养7 d 后,叶中RrCDPK9、根中RrCDPK2 表达量显著提高。表明这些基因在应对不同供钙水平时发挥了重要作用。此外,RrCDPKs对钙水平的响应随着培养时间延长而逐渐发生变化,如随着培养时间延长,RrCDPK3 在叶中的上调表达效应增强,而RrCDPK5/7在叶中的上调表达效应减弱,RrCDPK13/15/16在根中的上调表达效应增强,而RrCDPK6/10/11/12/14 在根中的上调效应减弱,说明在不同阶段响应钙水平的RrCDPKs基因不同;且比较各基因在2 种组织中的相对表达量可知,RrCDPKs 在叶和根中对钙水平的敏感性不同,特别是RrCDPK6/16 在叶中低表达,与图5-A 的表达模式吻合。
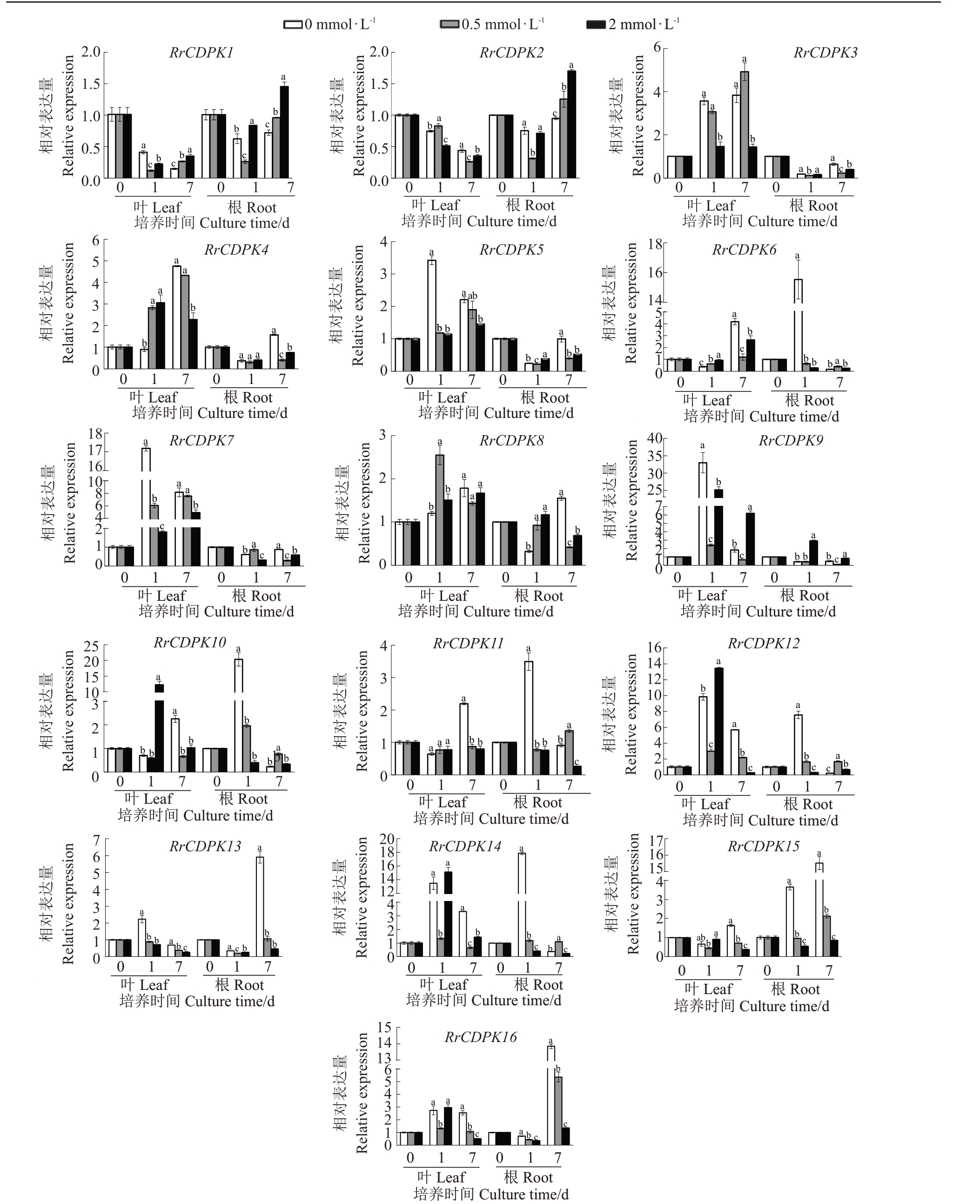
图6 刺梨CDPK 基因对不同供钙水平的响应
Fig.6 Response of CDPK genes in R.roxburghii to different calcium levels
以UBQ 基因为管家基因;以各基因分别在叶或根中0 d 时0 mmol·L-1 Ca2+处理下的表达量为对照。图中数值表示(平均值±标准差),n=3;同一基因在相同组织中同一时间的相对表达量用不同小写字母标识表示差异显著性(p<0.05)。
The UBQ was used as the housekeeping gene;the expression levels of each gene in leaves or roots under 0 mmol·L-1 Ca2+treatment at 0 days were used as the control. The numerical values in the figure represented (the mean±standard deviation), n=3; the relative expression levels of the same gene in the same tissue at the same time were marked with different lowercase letters to indicate significant differences(p<0.05).
3 讨 论
钙依赖性蛋白激酶(CDPKs)作为植物中广泛存在的一类丝氨酸/苏氨酸型蛋白激酶,具有Ca2+传感器和应答器的双重功能,可调节植物生长发育并诱导对环境胁迫的保护性反应[5-6]。其由多基因家族编码,首次被Hetherington 等[26]发现于豌豆(Pisum sativum)中,迄今已在多种植物中被鉴定。笔者在本研究中通过生物信息学方法,在刺梨基因组中鉴定出16 个RrCDPKs 成员。本研究中亚细胞定位预测,发现RrCDPKs 表现出多样化亚细胞分布,包括细胞质、细胞核、细胞骨架、细胞膜和多种细胞器,与Harper 等[27]的研究一致。此外,有证据表明一些CDPK 基因可以改变位置以响应压力,如在冰植物(Mesembryanthemum crystallinum)叶中表达的Mc-CPK1的异构体显示出从质膜到细胞核定位的明显转变,以应对盐胁迫或脱水胁迫[28]。本研究显示RrCDPKs的内含子数目较多,除RrCDPK6仅1个内含子外,其他成员均含5个内含子以上。赵娟等[29]发现,更多内含子的存在可能会通过可变剪接和外显子重排而增加CDPK基因的功能多样性。因此推测亚细胞分布的多样性及较多的内含子数目会使RrCDPKs功能增多。CDPK有4个典型的结构域,保守的激酶结构域是Ser/Thr蛋白激酶的典型特征[30]。钙传感器主要依赖于Ca2+与EF-hand基序的结合,该基序高度保守,且蛋白质中EF-hand基序对的存在能够增强对钙的稳定性和亲和力[31]。本研究表明RrCDPKs都含有2个高度保守的结构域:Pkinase(即Ser/Thr蛋白激酶)和EF-hand 结构域,以及6 个保守基序,且RrCDPKs的结构域和基序在组成及排列顺序上基本一致,这表明RrCDPKs在进化上相对保守。
本研究的系统进化分析显示RrCDPKs 与众多植物[10-17]一样可归为4个亚家族,表明物种之间仍存在一定共性。其中亚家族Ⅳ仅包括1个成员RrCDPK5,与其他植物Ⅳ亚族中成员数量少的特征一致,如28个薄壳山核桃和25个山核桃CDPKs中Ⅳ亚族均仅2个[29]。靳燕等[16]发现,CDPK最早存在于衣藻中,随着基因组的扩张,逐步出现于裸子植物、被子植物中,衣藻的2个CDPK基因都位于Ⅳ亚族,Ⅳ亚族的CDPKs内含子个数明显多于其他族,是最早扩张出的。因此,可通过内含子与外显子的分布情况来分析CDPK基因进化的先后顺序。本研究中Ⅳ亚族的外显子数最多,与前人研究结果[16]一致,推测为进化最早的RrCDPK 基因。茶树CsCDPK17 与AtCDPK17 的同源关系最近,能够不同程度地响应低温、干旱、渗透胁迫[32],而本试验中RrCDPK1 和AtCDPK17也同属于一个分支,且RrCDPK1启动子含有低温响应元件和缺氧特异性诱导有关的类增强子元件,说明RrCDPK1 可能参与低温响应。CDPK家族是植物有效响应和增强对非生物和生物胁迫的抵抗力的基本组成部分之一[33]。据报道,二穗短柄草中CDPK14 基因参与了植物的抗旱反应[34]。Li等[35]研究发现,ShCDPK家族成员在冷、干旱胁迫下的表达变化显著,用VIGS技术(病毒诱导的基因沉默)使ShCDPK6和ShCDPK26基因沉默的植物对寒冷和干旱胁迫的抗逆性降低,笔者推测可能是因为其启动子中存在大量与防御和干旱胁迫相关的顺式作用元件。刺梨CDPK家族成员是否参与调控植物对抗生物和非生物胁迫尚未可知。因此,笔者在本研究中分析了RrCDPKs启动子的顺式作用元件,发现RrCDPKs 含有防御和压力胁迫、低温胁迫、厌氧诱导及缺氧特异性诱导的应答元件,这表明刺梨CDPK 可能响应逆境胁迫。此外,还发现激素响应元件在RrCDPKs中普遍存在,特别是脱落酸及赤霉素。有研究表明,AtCPK10通过ABA和Ca2+信号通路参与气孔运动的调节,可能在植物响应干旱胁迫中发挥重要作用[36]。Li 等[37]研究表明,CDPKs 参与了多种激素的串扰,并且同源基因参与了甘薯及其两个二倍体近缘种的不同激素通路。因此,RrCDPKs也可能通过激素信号途径和Ca2+信号通路以响应环境胁迫。
CDPKs 广泛存在于各种植物组织中。本研究中利用转录组数据分析了RrCDPKs 在刺梨不同组织(茎、叶、花、30 d的果实)和果实不同发育时期(花后30、60、90 d)的表达模式,发现在刺梨的茎、叶、花、不同发育时期的果实中都可检测到RrCDPKs的表达,且各有不同,表明其成员不同程度地参与刺梨营养生长及生殖生长。Frattini 等[38]研究发现水稻OsCDPK2 在叶片对光的响应中起作用,OsCDPK2蛋白在暴露于光的绿叶中几乎检测不到,但转移到黑暗时水平急剧上升。本试验中RrCDPK1/4 基因的启动子都包含光响应元件和光响应的MYB 结合位点,且都与OsCDPK2位于Ⅱ亚族,RrCDPK1在叶中表达量不高,RrCDPK4 在叶中高表达,因此,RrCDPK1/4 可能协同参与应对外界的光变化。Martins 等[39]近几年研究发现钙对类黄酮的产生有显著影响,能促进槲皮素类黄酮醇的含量增加;外源钙处理的花生中类黄酮含量增加[40]。RrCDPK11 启动子包含参与黄酮类生物合成基因调控的MYB 结合位点,且在果实发育后期高表达,与类黄酮化合物的积累趋势[41]一致。因此,笔者推测RrCDPK11 参与了刺梨调控类黄酮化合物的合成,且其机制可能与Ca2+信号有关。
大量研究表明,CDPK 通过EF-hand 结构感知Ca2+浓度的变化,解除自我抑制,激活激酶结构域,进而传递信息调节植物的生理变化,广泛参与植物的生长发育和形态建成[7,30]。由于EF-hand基序数量和氨基酸序列的变化,CDPK对Ca2+的亲和力可能不同[42]。而本研究经结构域分析,发现RrCDPKs 所含EF-hand 结构域数目相差无几。因此,笔者进一步研究了刺梨CDPKs对钙离子的响应,与杨婳若等[43]处理相似,但经过前期试验摸索发现实生苗比扦插苗耐受更高浓度的hoagland 营养液和钙水平,因此本试验所选供钙水平适宜于后续试验及分析。同样,取样时间的选择也至关重要,本试验参考前人研究[18,44-45]发现响应金属离子的文献中取样时间有长有短,短期的多数为1 d,长期的多数为7 d。本试验结果发现RrCDPKs 对钙的响应随着培养时间延长而逐渐发生变化,表明RrCDPKs对长期和短期的钙处理的响应机制不同,这与张习敏[17]的研究讨论一致,也进一步验证了取样时间的合理性。本研究以0 mmol·L-1钙处理为对照,发现供钙后,少数RrCDPKs表现出明显的上调效应,分析可知,叶中RrCDPK4/8、根中RrCDPK1/2,叶中RrCDPK4/9/10/12、根中RrCDPK2/9分别在0.5、2 mmol·L-1钙水平下表现出显著的上调表达响应,表明这些基因在应对不同供钙水平时发挥了重要作用。周卫等[46]研究发现植物主要通过根系吸收钙,通过叶片的蒸腾拉力将钙运输至地上部提供营养。本研究结果表明,刺梨RrCDPKs家族可响应外界缺钙胁迫和钙浓度变化,可能通过诱导植株对钙吸收及运输以维持植株正常的生理功能,但其具体机制还有待研究。进一步分析可知,RrCDPKs在叶和根中对钙水平的敏感性不同,如RrCDPK6/16在叶中低表达,与其组织表达模式相吻合,这一定程度上说明RrCDPKs基因表达属于器官依赖型,与其他物种的研究结论一致[17]。也有研究总结,尽管目前所有被发现的CDPK 都含有与Ca2+结合的EF-hand结构域,但并不是每个CDPK的激酶活性都完全受Ca2+调控。如,拟南芥中至少有6 个成员(CPK7/8/10/13/25/30)的激酶活性基本上不受Ca2+调控,研究发现在没有Ca2+存在时,它们就已有较高的激酶活性;CPK32 的活性部分受Ca2+调控[47]。由本研究可知刺梨RrCDPK1/2 基因对缺钙的敏感性不如其他成员,尤其RrCDPK1与拟南芥CPK7/8同属于一个进化分支,推测其激酶活性基本不受Ca2+调控。因此,这些鉴定的刺梨钙依赖性蛋白激酶在响应不同供钙水平时发挥的作用不尽相同,为后续探明刺梨对外界钙环境的响应机制提供了相关信息。
4 结 论
在刺梨全基因组中,共鉴定出16个刺梨RrCDPK 家族成员,所有成员均具有丝氨酸/苏氨酸蛋白激酶和EF-hand 结构域。通过构建系统进化树,获得4个亚家族,亚族内成员间具有高度保守性,而各亚族成员间的序列和结构特征存在一定差异,进一步发现与刺梨CDPK 家族亲缘关系最近的是草莓,其次是苹果,较远的为拟南芥和水稻。各基因成员启动子顺式作用元件预测它们大多含有光响应元件、多种激素响应元件及胁迫响应元件等。不同器官及果实发育时期的转录组数据显示RrCDPKs 具有时空表达特异性。RrCDPKs 对不同供钙水平的表达响应试验表明,以0 mmol·L-1无钙处理为对照,0.5 mmol·L-1钙水平下RrCDPK1/2/4/8 表达显著上调,2 mmol·L-1钙水平下RrCDPK2/4/9/10/12表达显著上调;RrCDPKs在叶和根中对供钙水平及处理时间的表达响应也不同。研究结果为后续探明CDPK家族在刺梨对外界钙环境的响应机制提供了相关信息。
[1] TUTEJA N,MAHAJAN S. Calcium signaling network in plants:An overview[J]. Plant Signaling and Behavior,2007,2(2):79-85.
[2] JING X,CAI C J,FAN S H,LUO H Y. Physiological response characteristics of Moso bamboo under drought stress based on calcium signal[J].Forests,2021,12(12):1699.
[3] ALLAN C,MORRIS R J,MEISRIMLER C N.Encoding,transmission,decoding,and specificity of calcium signals[J]. Journal of Experimental Botany,2022,73(11):3372-3385.
[4] OLIVER B,JÖRG K.Analysis of calcium signaling pathways in plants[J]. Biochimica et Biophysica Acta,2012,1820(8):1283-1293.
[5] 姜珊珊,张丹,孔祥培,周严,李德全.植物中的钙依赖蛋白激酶(CDPK)的结构特征和功能研究进展[J]. 生物技术通报,2013,29(6):12-19.
JIANG Shanshan,ZHANG Dan,KONG Xiangpei,ZHOU Yan,LI Dequan. Research progress of structural characteristics and functions of calcium-dependent protein kinases in plants[J].Biotechnology Bulletin,2013,29(6):12-19.
[6] SCHULZ P,HERDE M,ROMEIS T. Calcium-dependent protein kinases:Hubs in plant stress signaling and development[J].Plant Physiology,2013,163(2):523-530.
[7] 原增艳,宋小锋,朱畇昊.地黄RgCDPK 基因的克隆与表达分析[J].广西植物,2020,40(12):1816-1823.
YUAN Zengyan,SONG Xiaofeng,ZHU Yunhao. Cloning and expression analysis of calcium-dependent protein kinase genes in Rehmannia glutinosa[J]. Guangxi Plants,2020,40(12):1816-1823.
[8] LIU Y,XU C J,ZHU Y F,ZHANG L N,CHEN T Y,ZHOU F,CHEN H,LIN Y J. The calcium-dependent kinase OsCPK24 functions in cold stress responses in rice[J]. Journal of Integrative Plant Biology,2018,60(2):173-188.
[9] WU P,WANG W L,DUAN W K,LI Y,HOU X L.Comprehensive analysis of the CDPK-SnRK superfamily genes in Chinese cabbage and its evolutionary implications in plants[J]. Frontiers in Plant Science,2017,8:162.
[10] CHENG S H,WILLMANN M R,CHEN H C,SHEEN J.Calcium signaling through protein kinases. The Arabidopsis calciumdependent protein kinase gene family[J]. Plant Physiology,2002,129(2):469-485.
[11] ASANO T,TANAKA N,YANG G X,HAYASHI N,KOMATSU S. Genome-wide identification of the rice calcium-dependent protein kinase and its closely related kinase gene families:Comprehensive analysis of the CDPKs gene family in rice[J].Plant and Cell Physiology,2005,46(2):356-366.
[12] RAY S,AGARWAL P,ARORA R,KAPOOR S,TYAGI A K.Expression analysis of calcium-dependent protein kinase gene family during reproductive development and abiotic stress conditions in rice(Oryza sativa L.ssp.indica)[J].Molecular Genetics and Genomics,2007,278(5):493-505.
[13] 张永杰,白丹凤,MUHAMMAD Abid,李志,方金豹,钟云鹏.对萼猕猴桃CDPK 基因家族鉴定及非生物胁迫应答分析[J].果树学报,2021,38(10):1653-1667.
ZHANG Yongjie,BAI Danfeng,MUHAMMAD Abid,LI Zhi,FANG Jinbao,ZHONG Yunpeng.Identification of CDPK family genes and their response to abiotic stress in Actinidia valvata[J].Journal of Fruit Science,2021,38(10):1653-1667.
[14] ZHAO Y L,DU H W,WANG Y K,WANG H L,YANG S Y,LI C H,CHEN N,YANG H,ZHANG Y H,ZHU Y L,YANG L Y,HU X L. The calcium-dependent protein kinase ZmCDPK7 functions in heat-stress tolerance in maize[J].Journal of Integrative Plant Biology,2021,63(3):510-527.
[15] WANG J P,XU Y P,MUNYAMPUNDU J P,LIU T Y,CAI X Z. Calcium-dependent protein kinase (CDPK) and CDPK-related kinase(CRK)gene families in tomato:Genome-wide identification and functional analyses in disease resistance[J]. Molecular Genetics and Genomics,2016,291(2):661-676.
[16] 靳燕,王谢琴,卿华,马政,姚慧鹏.黄瓜CDPK 基因家族的鉴定与进化特征分析[J].四川农业大学学报,2021,39(1):19-26.
JIN Yan,WANG Xieqin,QING Hua,MA Zheng,YAO Huipeng. Identification and evolutionary characterization of cucumber CDPK gene family[J].Journal of Sichuan Agricultural University,2021,39(1):19-26.
[17] 张习敏.高钙诱导天蓝苜蓿叶片草酸钙积累及其在环境适应中的作用研究[D].厦门:厦门大学,2018.
ZHANG Ximin.High calcium promotes the accumulation of calcium oxalate in Medicago lupulina leaves and its role in environmental adaptation[D].Xiamen:Xiamen University,2018.
[18] 黄玉婷. 茶树CsCaMs、CsCDPKs 基因家族的克隆与表达分析[D].北京:中国农业科学院,2016.
HUANG Yuting. Cloning and expression analysis of CsCaMs and CsCDPKs family genes in tea plant (Camellia sinensis)[D].Beijing:Chinese Academy of Agricultural Sciences,2016.
[19] 李娜娜. Ca2+/CaM 及CDPK 在干旱高温复合胁迫诱导玉米sHSPs 表达增加中的作用[D].郑州:河南农业大学,2016.
LI Nana.The functions of Ca2+/CaM and CDPK in the enhancement of maize sHSPs-induced by combined drought and heat stress[D].Zhengzhou:Henan Agricultural University,2016.
[20] NEMOTO K,NIINAE T,GOTO F,SUGIYAMA N,WATANABE A,SHIMIZU M,SHIRATAKE K,NISHIHARA M. Calcium-dependent protein kinase 16 phosphorylates and activates the aquaporin PIP2;2 to regulate reversible flower opening in Gentiana scabra[J].The Plant Cell,2022,34(7):2652-2670.
[21] 李兆康.贵州省主要产区刺梨果实品质比较及高产单株叶片的养分特征[D].贵阳:贵州大学,2021.
LI Zhaokang. Comparison of fruit quality and leaves nutrient characteristics of per high-yielding plant of Rosa roxburghii in main producing areas of Guizhou province[D]. Guiyang:Guizhou University,2021.
[22] CHEN C J,CHEN H,ZHANG Y,THOMAS H R,FRANK M H,HE Y H,XIA R. TBtools:An integrative toolkit developed for interactive analyses of big biological data[J]. Molecular Plant,2020,13(8):1095-1228.
[23] HUANG X L,YAN H Q,ZHAI L S,YANG Z T,YI Y. Characterization of the Rosa roxburghii Tratt. transcriptome and analysis of MYB genes[J].PLoS One,2019,14(3):e0203014.
[24] LU M,MA W T,LIU Y Q,AN H M,RICHARD A L.Transcriptome analysis reveals candidate lignin-related genes and transcription factors in Rosa roxburghii during fruit ripening[J].Plant Molecular Biology Reporter,2020,38(2):331-342.
[25] LIVAK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method[J].Methods,2001,25(4):402-408.
[26] HETHERINGTON A,TREWAVAS A. Calcium-dependent protein kinase in pea shoot membranes[J]. Febs Letters,1982,145(1):67-71.
[27] HARPER J F,BRETON G,HARMON A.Decoding Ca2+signals through plant protein kinases[J].Annual Review of Plant Biology,2004,55:263-288.
[28] PATHARKAR O R,CUSHMAN J C. A novel coiled-coil protein co-localizes and interacts with a calcium-dependent protein kinase in the common ice plant during low-humidity stress[J].Planta,2006,225(1):57-73.
[29] 赵娟,朱凯凯,范平桦,谭鹏鹏,彭方仁,李永荣.薄壳山核桃和山核桃CDPK 基因家族的鉴定及表达分析[J].农业生物技术学报,2022,30(3):442-456.
ZHAO Juan,ZHU Kaikai,FAN Pinghua,TAN Pengpeng,PENG Fangren,LI Yongrong.Identification and expression analysis of CDPK gene family in pecan (Carya illinoinensis) and Chinese hickory (Carya cathayensis)[J]. Journal of Agricultural Biotechnology,2022,30(3):442-456.
[30] 武志刚,武舒佳,王迎春,郑琳琳. 植物中钙依赖蛋白激酶(CDPK)的研究进展[J].草业学报,2018,27(1):204-214.
WU Zhigang,WU Shujia,WANG Yingchun,ZHENG Linlin.Advances in studies of calcium-dependent protein kinase (CDPK)in plants[J].Acta Prataculturae Sinica,2018,27(1):204-214.
[31] REDDY V S,REDDY A S N. Proteomics of calcium-signaling components in plants[J]. Phytochemistry,2004,65(12):1745-1776.
[32] 雷蕾,王璐,姚丽娜,郝心愿,曾建明,丁长庆,王新超,杨亚军.茶树钙依赖性蛋白激酶基因CsCDPK17 的鉴定及表达分析[J].茶叶科学,2019,39(3):267-279.
LEI Lei,WANG Lu,YAO Lina,HAO Xinyuan,ZENG Jianming,DING Changqing,WANG Xinchao,YANG Yajun. Identification and expression analysis of the calcium-dependent protein kinase gene CsCDPK17 in tea plant (Camellia sinensis)[J].Journal of Tea Science,2019,39(3):267-279.
[33] AHMED B,ALAM M,HASAN F,EMDAD E M,ISLAM S,RAHMAN N. Jute CDPK genes and their role in stress tolerance and fiber development:A genome-wide bioinformatic investigation of Chorchorus capsularis and C. olitorius[J]. Plant Gene,2020,24(5):100252.
[34] 韦淑亚,赵旭东,杨广笑,何光源.二穗短柄草钙依赖型蛋白激酶基因BdCDPK14 克隆及表达分析[J].南方农业学报,2017,48(5):761-767.
WEI Shuya,ZHAO Xudong,YANG Guangxiao,HE Guangyuan.Clone and expression of calcium- dependent protein kinase BdCDPK14 gene in Brachypodium distachyon[J]. Southern Agricultural Journal,2017,48(5):761-767.
[35] LI Y Y,ZHANG H X,LIANG S B,CHEN X L,LIU J Y,ZHANG Y,WANG A X.Identification of CDPK gene family in Solanum habrochaites and its function analysis under stress[J].International Journal of Molecular Sciences,2022,23(8):4227.
[36] ZOU J J,WEI F J,WANG C,WU J J,RATNASEKERA D,LIU W X,WU W H.Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress[J]. Plant Physiology,2010,154(3):1232-1243.
[37] LI X,ZHAO L M,ZHANG H,LIU Q C,ZHAI H,ZHAO N,GAO S P,HE S Z. Genome-wide identification and characterization of CDPK family reveal their involvements in growth and development and abiotic stress in sweet potato and its two diploid relatives[J]. International Journal of Molecular Sciences,2022,23(6):3088.
[38] FRATTINI M,LAURA M,DIEGO B. Rice calcium-dependent protein kinase isoforms OsCDPK2 and OsCDPK11 show different responses to light and different expression patterns during seed development[J]. Plant Molecular Biology,1999,41(6):753-764.
[39] MARTINS V,UNLUBAYIR M,TEIXEIRA A,GERÓS H,LANOUE A. Calcium and methyl jasmonate cross-talk in the secondary metabolism of grape cells[J]. Plant Physiology and Biochemistry,2021,165(3):228-238.
[40] CUI L,GUO F,ZHANG J L,YANG S,MENG J J,GENG Y,LI X G,WAN S B. Synergy of arbuscular mycorrhizal symbiosis and exogenous Ca2+ benefits peanut (Arachis hypogaea L.)growth through the shared hormone and flavonoid pathway[J].Scientific Reports,2019,9(1):1-11.
[41] 樊卫国,潘学军,陈红,穆瑞,官纪元,周禹佳.春梢叶损失对刺梨生长和果实产量与品质的影响机理分析[J].西北植物学报,2021,41(11):1863-1875.
FAN Weiguo,PAN Xuejun,CHEN Hong,MU Rui,GUAN Jiyuan,ZHOU Yujia. Influence mechanism of spring shoot leaf loss on growth,fruit yield and quality of Rosa roxburghii[J].Acta Botanica Boreali-Occidentalia Sinica,2021,41(11):1863-1875.
[42] KLIMECKA M,MUSZYNSKA G. Structure and functions of plant calcium-dependent protein kinases[J].Acta Biochimica Polonica,2007,54(2):219-233.
[43] 杨婳若,樊卫国.不同供钙水平对刺梨苗生长、矿质元素吸收及相关生理生化特性的影响[J].果树学报,2022,39(10):1891-1902.
YANG Huaruo,FAN Weiguo. Effects of different calcium supply levels on growth,mineral element absorption and related physiological and biochemical characteristics of Rosa roxburghii seedlings[J]. Journal of Fruit Science,2022,39(10):1891-1902.
[44] FU X Z,ZHOU X,XING F,LING L L,CHUN C P,AARTS M G M,PENG L Z.Genome-wide identification,cloning and functional analysis of the zinc/iron-regulated transporter-like protein(ZIP) gene family in trifoliate orange (Poncirus trifoliata L.Raf.)[J].Frontiers in Plant Science,2017,8:588.
[45] 王照,鲁敏,南红,安华明.缫丝花OSCA 基因家族的鉴定与表达分析[J].植物生理学报,2022,58(2):350-362.
WANG Zhao,LU Min,NAN Hong,AN Huaming.Identification and expression analysis of OSCA gene family in Rosa roxburghii Tratt.[J].Plant Physiology Journal,2022,58(2):350-362.
[46] 周卫,汪洪. 植物钙吸收、转运及代谢的生理和分子机制[J].植物学报,2007,42(6):762-778.
ZHOU Wei,WANG Hong. The physiological and molecular mechanisms of calcium uptake,transport and metabolism in plants[J].Chinese Bulletin of Botany,2007,42(6):762-778.
[47] 刘舞贞.拟南芥CPKa 调控ABA 响应与ROS 累积的分子机制研究[D].杨凌:西北农林科技大学,2016.
LIU Wuzhen.Research on the molecular mechanism of CPKa regulation ABA response and ROS accumulation in Arabidopsis[D].Yangling:Northwest A&F University,2016.