梨是蔷薇科(Rosaceae)梨属(Pyrus)植物,是中国三大果树之一,在农村区域经济发展、农民脱贫致富、乡村振兴等方面起了重要作用[1]。
梨自花结实率低,生长周期长、影响因素复杂多样,基因组高度杂合[2]。这些特性给梨遗传转化和品种改良带来严重障碍。花药培养是由花粉小孢子产生单倍体植株的一种重要途径,可以有效地缩短育种年限,加快新品种选育进程,其在遗传育种中的意义重大。因此,非常有必要开展梨花药培养的研究。花药培养是利用植物组织培养技术,将发育到特定阶段的花药取出转移到培养基中生长,从而改变花药内部的生理状态,使其在离体条件下分化成胚状体或形成无分化的愈伤组织。目前,国内外关于梨树花药离体培养的研究比较少。1975 年,Jordan[3]在西洋梨花药培养上获得小原胚;1978 年,山东农学院梨花药培养诱导出愈伤组织[4];1996年,薛光荣等[5]在锦丰梨的花药培养上诱导出胚状体,但诱导率不稳定。
花药的细胞全能性较高,由其诱导出来的愈伤组织具有较强的分裂和分化能力;以花药愈伤组织为材料进行植物悬浮培养的细胞,具有材料均一、重复性好、条件易于控制、培养周期短等优点[6]。植物悬浮细胞是遗传转化的理想受体之一,用悬浮细胞作为转化受体可提高转化率,因此,稳定的悬浮细胞系对遗传转化起到了巨大的促进作用。悬浮细胞培养是指在离体的条件下,将愈伤组织或者其他易于分散的组织置于液体培养基中,通过振荡的方式得到分散的细胞或小细胞团,使其保持良好的生长状态且可以不断增殖的技术。1958年,美国的Steward等[7]建立了胡萝卜的悬浮体系,随后悬浮细胞培养技术被广泛应用于草本植物。对木本植物细胞悬浮培养的研究起步较晚,1975年,Wilson等[8]建立了橡胶悬浮体系。1991 年,王影等[9]建立了小叶杨的悬浮细胞体系。此后,经过20 多年的发展,中国在木本植物的研究中也取得了一系列的进展,如荔枝、葡萄、花椒树等也建立了细胞悬浮培养体系,但关于梨细胞悬浮培养方面未见报道[10-13]。
笔者在本研究中以新梨7 号花药为试材,对花药愈伤组织的获得、增殖及悬浮培养等条件进行了研究,以期获得高质量的梨细胞悬浮系,悬浮培养获得的小细胞团和单细胞可以直接用于原生质体的分离,也可以作为遗传转化的直接受体,为梨大规模细胞培养以及遗传转化奠定基础。
1 材料和方法
1.1 试验材料
以河北农业大学标本园栽植的新梨7号花药为试材。
1.2 试验方法
1.2.1 愈伤组织的诱导及继代 选取处于“紧凑期”(花蕾横径为2.7~3.0 mm,纵径为8.0~8.5 mm)的花蕾,将花蕾表面用清水冲洗3~5次洗去灰尘,然后在无菌工作台上按照先75%乙醇浸泡25 s,再用0.1%HgCl2消毒8 min,最后用无菌水冲洗3~4 次。剥开花蕾取出花药,将获得的无菌花药接种于1/2 MS+1.0 mg·L-1 2,4-D+0.4 mg·L-1 NAA+30 g·L-1蔗糖+7 g·L-1琼脂(pH 值5.8~6.0)培养基中,(24±1)℃暗培养30 d。
选取黄白色、较松散且活性较高的愈伤组织,接种于6-BA(0.5、1.0、1.5 mg·L-1)与2,4-D(1.0、1.5、2.0 mg·L-1)的激素配比组合(基础培养基为MS +30 g·L-1蔗糖+7 g·L-1琼脂)中进行继代增殖培养,完全随机组合,共9个处理。每个处理3次重复。培养条件与诱导培养条件相同。
1.2.2 悬浮细胞系的初代培养 细胞悬浮无菌培养基以MS+30 g·L-1蔗糖为基本培养基,添加不同质量浓度的生长调节剂2,4-D、6-BA(表1),以不添加生长调节剂的为对照,pH值5.8~6.0。
表1 梨愈伤组织液体培养基激素配比
Table 1 Hormone ratio of pear callus liquid medium
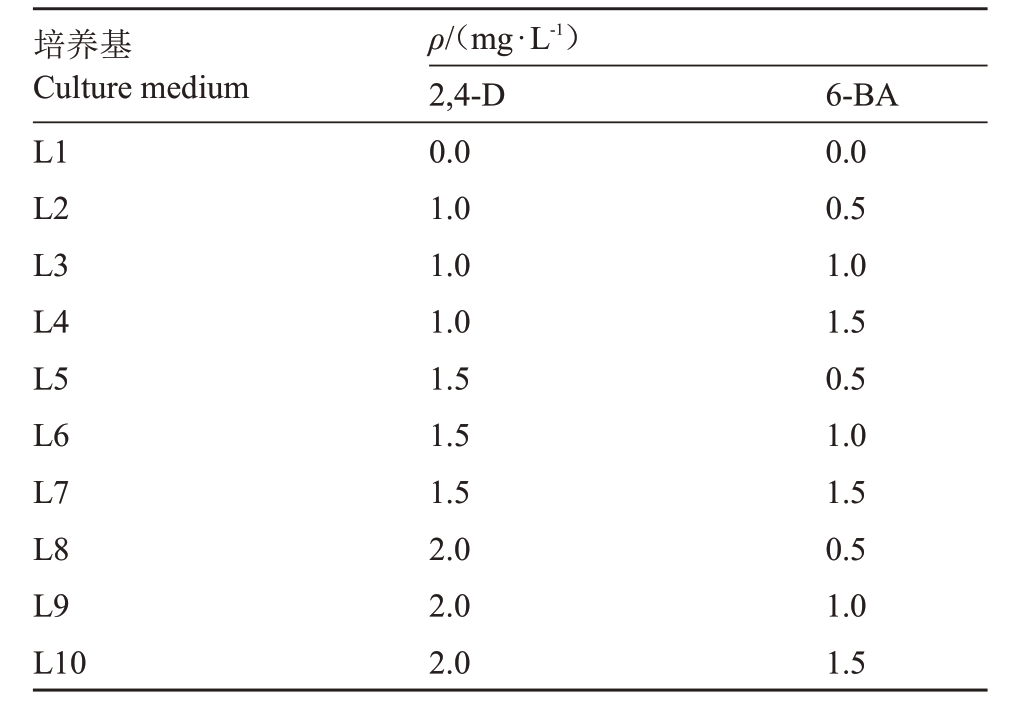
培养基Culture medium L1 L2 L3 L4 L5 L6 L7 L8 L9 L10 ρ/(mg·L-1)2,4-D 0.0 1.0 1.0 1.0 1.5 1.5 1.5 2.0 2.0 2.0 6-BA 0.0 0.5 1.0 1.5 0.5 1.0 1.5 0.5 1.0 1.5
将分散性好、疏松的愈伤组织,用镊子夹碎将其转移到装有40 mL液体培养基的三角瓶中进行悬浮振荡培养。28 ℃,暗培养,培养周期为8 d,接种量为1.5 g,摇床转速为200 r·min-1,进行启动培养,每个处理3次重复。
1.2.3 悬浮细胞增殖培养 取出装有悬浮物质的三角瓶,静置15~20 min,倒掉2/3 的液体培养基,然后加入与倒掉液体相同体积的新鲜培养基并用无菌玻璃棒将里面的大块愈伤组织压碎。以后每隔7 d继代1次,21 d后用150 μm的细胞筛过滤,获得的细小均匀的悬浮物质继续悬浮培养,得到颗粒分散、稳定性好的悬浮细胞系。全过程无菌操作,培养条件1.2.2。
1.2.4 悬浮细胞生长量的测定 自稳定悬浮系被继代的当天起,每隔1 d取悬浮细胞进行细胞生长量测定。每隔1 d取2 mL悬浮物质,离心称质量,即为鲜质量。
60 ℃下烘干12 h后称质量,即为干质量。
取悬浮液2 mL, 放入2 mL 刻度的离心管中,4500 r·min-1下离心5 min,得到的细胞体积即为细胞密实体积(PCV)。每个处理3次重复。
1.2.5 悬浮细胞死亡率的测定 选取生长稳定的悬浮细胞,每隔1 d 取1 mL 进行悬浮细胞死亡率测定。用0.4%的台盼蓝染色3 min,蒸馏水洗涤3 次,于20倍显微镜下观察其死亡个数,统计死亡率。
1.3 悬浮细胞的转化
将经悬浮培养后获得的活力较强的愈伤组织材料进行转化,浸染流程参照王林苹果愈伤浸染方法并对浸染液浓度、浸染时间等稍作修改[14]。具体流程为将活化好含有pROK2-GFP 质粒的GV3101 农杆菌液离心重悬,重悬后的浸染液(OD600为0.5)与愈伤组织置于28 ℃恒温摇床(100 r·min-1)在黑暗条件培养浸染15 min;将浸染后的愈伤吸干水分后置于共生培养基(MS+1.5 mg·L-1 2,4-D+1.0 mg·L-1 6-BA),黑暗条件下共培养3 d,然后将其接种到筛选培养基(MS+1.5 mg·L-1 2,4-D+1.0 mg·L-1 6-BA+20 mg·L-1 Basta + 300 mg·L-1 Cef + 200 mg·L-1 Timentin),黑暗条件下培养20 d。用手持荧光蛋白观测灯(LUYOR-3415 RG Hand-Held Lamp,路阳,美国)拍照,计算转化率。
1.4 原生质体的分离及产量、活力测定
生长稳定悬浮细胞系,取其第4 天时悬浮细胞为材料,6000 r·min-1离心5 min。酶解液成分为:纤维素酶(1.0%)、离析酶(0.5%)、1 mL MES pH 5.7(20 mmol·L-1)、5 mL 甘露醇(0.5 mol·L-1)、2.5 mL KCl(20 mmol·L-1)、无菌水定容到10 mL。55 ℃水浴10 min,冷却至室温25 ℃,加10 mmol·L-1 CaCl2,0.1%牛血清蛋白(BSA)用0.45 μm的滤头过滤。25 ℃黑暗条件下30 r·min-1恒温振荡12 h。将酶解后的材料进行纯化,加入W5 solution[其成分为2 mmol·L-1 MES(pH 5.7)+ 154 mmol·L-1 NaCl+125 mmol·L-1 CaCl2+5 mmol·L-1 KCl],120 r·min-1离心4 min,离心3次。
用血球计数板统计原生质体的产量。检测活力采用FDA染色法:FDA溶于丙酮,用蔗糖稀释,4 ℃保存。使用时取1 滴母液与1 滴原生质体悬浮液混合到一起,室温下静置5~10 min后荧光下观察。
1.5 数据处理与分析
采用Excel 2013和SPSS 24进行数据分析。
2 结果与分析
2.1 愈伤组织的获得及增殖培养
接种10 d 后,开始从花药中部慢慢长出愈伤组织,没有长出愈伤组织的花药慢慢褐化死亡。花药接种30 d 后形成的愈伤组织块,一部分愈伤组织微褐化,呈柔软水渍状为湿软型愈伤;一部分愈伤为黄白色,结构紧密,含水量少,此类愈伤经过4 个月的继代培养后即可获得表面有颗粒状突起结构疏松的愈伤组织,可用来作为悬浮培养的材料。
新梨7 号花蕾(图1-A)在“紧凑期”愈伤诱导率达45.80%,此时期的花药(图1-B)颜色为粉白色,花药难以剥离。在6-BA 质量浓度一定时,随着2,4-D质量浓度的增加,结构由紧凑型(图1-C)向疏松型(图1-D)转变,愈伤组织状态相对较好,增殖较多;反之,在2,4-D质量浓度一定时,随着6-BA质量浓度的增加愈伤组织状态相对较差,质地坚硬增殖较少或基本不增殖(表2)。最终在MS+1.5 mg·L-1 2,4-D+1.0 mg·L-1 6-BA的激素质量浓度配比下经过4~5次的继代即可得到生长旺盛且活性强的疏松愈伤,可用来进行悬浮培养。

图1 梨愈伤组织的诱导和增殖
Fig.1 Callus induction and proliferation of pear
A.花蕾;B.接种当天;C.接种30 d 形成紧凑型愈伤组织;D.接种5 个月形成疏松型愈伤。
A.Flower bud;B.The day of inoculation;C.Compact callus formed at 30 d of inoculation;D.The formation of loose callus after 5 months of inoculation.
表2 不同激素质量浓度培养对梨愈伤组织形态的影响
Table 2 Effects of different hormone concentrations on subculture of pear callus

注:“+”表示产生愈伤数量较少;“++”表示产生愈伤数量较多;“+++”表示产生愈伤数量多。
Note:“+”indicated that the number of callus was small;“++”indicates a large number of callus;“+++”indicates the number of callus generated.
培养基Culture medium L1 L2 L3 L4 L5 L6 L7 L8 L9 ρ/(mg·L-1)6-BA 0.5 0.5 0.5 1.0 1.0 1.0 1.5 1.5 1.5愈伤组织状态Callus status黄白色,颗粒(+)Yellow white,grainy(+)黄白色,松散(++)Yellow white,loose(++)黄白色,松散(++)Yellow white,loose(++)黄白色,湿软(+)Yellow white,wet and soft(+)黄白色,松散(+++)Yellow white,loose(+++)黄白色,松散(++)Yellow white,loose(++)灰白色,紧致(+)Grayish white,firm(+)白色,较硬(++)White,hard(++)灰白色,偏硬(++)Grayish white,hard(++)2,4-D 1.0 1.5 2.0 1.0 1.5 2.0 1.0 1.5 2.0
2.2 激素对梨愈伤悬浮细胞生长的影响
不同质量浓度的2,4-D 与6-BA 的组合对梨悬浮细胞状态存在较大影响(表3)。在2,4-D 质量浓度为1.0~1.5 mg·L-1时,随着6-BA 质量浓度的提高活细胞率及近圆细胞率数值均有所升高,悬浮液状态也达到好的状态;而在2,4-D质量浓度为2.0 mg·L-1时,随着6-BA质量浓度的提高其圆细胞及活细胞率数值均有所下降,但两者均显著高于对照(图2-B~C)。在2,4-D 质量浓度为1.5 mg·L-1,6-BA 为1.0 mg·L-1时,细胞活细胞(图2-F)率达到81.98%,近圆细胞(图2-E)率达到83.66%,此时悬浮液状态也达到最好(图2-D)。综上所述,L6培养基为最适合梨悬浮细胞培养的培养基。

图2 梨细胞悬浮体系观察
Fig.2 Observation of pear cell suspension system
A.褐化悬浮系外观(对照);B.褐化悬浮系细胞(对照);C.染色后的褐化悬浮系细胞(对照);D.稳定悬浮系外观(L6);E.稳定悬浮系细胞(L6);F.染色后的稳定悬浮系细胞(L6)。
A. The appearance of Browning suspension system (control); B. The Browning suspension line cell (control); C. The Browning suspension line cells after staining (control); D.The appearance of stable suspension system (L6); E. Stable suspension line cell (L6); F. Stable suspension line cells after staining(L6).
表3 不同激素质量浓度对梨细胞悬浮培养的影响
Table 3 Effects of different hormone concentrations on suspension culture of pear cells

注:采用Ducan’s multiple range test 方法分析,不同小写字母不同表示在p<0.05 水平差异显著。下同。
Note:The different small letters mean significant difference at p<0.05 by Duncan’s multiple range test.The same below.
培养基Culture medium L1 L2 L3 L4 L5 L6 L7 L8 L9 L10悬浮液状态Suspension state褐化、稳定性差Browning,poor stability乳白色、稳定性较差Milky white,poor stability乳白色、稳定性较差Milky white,poor stability乳白色、稳定性较好Milky white,better stability乳白色、稳定性好Milky white,good stability乳白色、稳定性好Milky white,good stability乳白色、稳定性较好Milky white,better stability乳白色、稳定性较好Milky white,better stability乳白色、稳定性较好Milky white,better stability乳白色、稳定性较好Milky white,better stability活细胞率Living cell rate/%16.56±0.79 f 54.79±4.46 e 65.38±4.36 d 66.41±1.96 d 79.23±0.63 ab 81.98±0.74 a 73.38±0.50 c 75.34±2.63 bc 72.09±4.28 c 71.84±3.08 c近圆细胞率Suborbicular cell rate/%25.66±1.64 f 69.33±0.75 e 70.84±4.01 de 73.68±2.20 cde 80.00±0.57 ab 83.66±1.67 a 73.25±2.78 cde 76.62±3.51 bc 73.78±0.63 cd 71.92±2.49 de
2.3 悬浮细胞系的建立及细胞学观察
将上述经继代培养得到的疏松愈伤组织,转接到液体培养基中进行悬浮培养。愈伤组织在悬浮培养1周后出现长形细胞(图3-D),细胞多为空的且活力不高,在以后的继代过程中逐步淘汰。2~3 周散出来的既有长形细胞又有圆形小细胞团(图3-E),但长细胞占比相对较大。4 周后长形细胞消失,多为圆形小细胞团,单细胞少量存在。最终建立起来的细胞悬浮系(图3-F)基本没有长形细胞,都是由单细胞和小细胞团组成,也会时常出现多细胞聚集现象,大多数细胞呈圆球形且含有丰富的内含物质(图3-C)。故在前三次继代时,应待悬浮系沉淀后倒去上层2/3的培养液,去除死细胞和空细胞。

图3 梨悬浮细胞系的建立
Fig.3 Establishment of pear suspension cell line
A.稳定增殖的愈伤组织;B.稳定悬浮系外观;C.稳定悬浮系细胞;D.培养1 周后的悬浮细胞;E.培养2~3 周后的悬浮细胞;F.含有丰富内含物质的悬浮细胞;G.培养1 周后的悬浮细胞(对照);H.培养2~3 周后的悬浮细胞(对照);I.稳定悬浮系细胞(对照)。
A.The callus with stable proliferation;B.The appearance of stable suspension system;C.Stable suspension line cells;D.Suspension cells cultured for 1 week;E.The suspension cells after 2-3 weeks of culture;F.Suspension cell with rich content;G.Suspension cells cultured for 1 week(control);H.The suspension cells after 2-3 weeks of culture(control);I.Stable suspension line cell(control).
2.4 悬浮细胞系生长曲线和死亡率的测定
由图4可知,梨悬浮细胞生长量呈“S”型生长曲线,0~2 d 悬浮物质生长量小为迟滞期,细胞死亡率维持在13.97%~22.50%;2~4 d 以后细胞体积、鲜质量、干质量明显增加即为进入对数期,是细胞增殖、生长发育最明显的时期,该时期的细胞死亡率最低,为13.97%~15.40%;4~6 d 细胞生长较平稳,生长速度逐渐降低,此时鲜质量为0.22 g,干质量为0.05 g,均达到最大值进入了静止期,这一时期的细胞死亡率为15.40%~19.14%;6 d 以后开始进入衰亡期,细胞鲜质量、密实体积逐渐趋于下降趋势,此时则认为是悬浮培养物质进入了衰亡期,细胞死亡率为19.14%~19.18%;同时观察到厚厚一层的衰败细胞堆集在瓶壁上,不利于悬浮细胞系的生长。在0~8 d的继代周期内,以花药为外植体获得的愈伤组织细胞悬浮系存活率均超过77.50%,细胞的死亡率均维持在22.5%以下(图4),说明悬浮细胞的活性较强,绝大数细胞的生长状态良好。
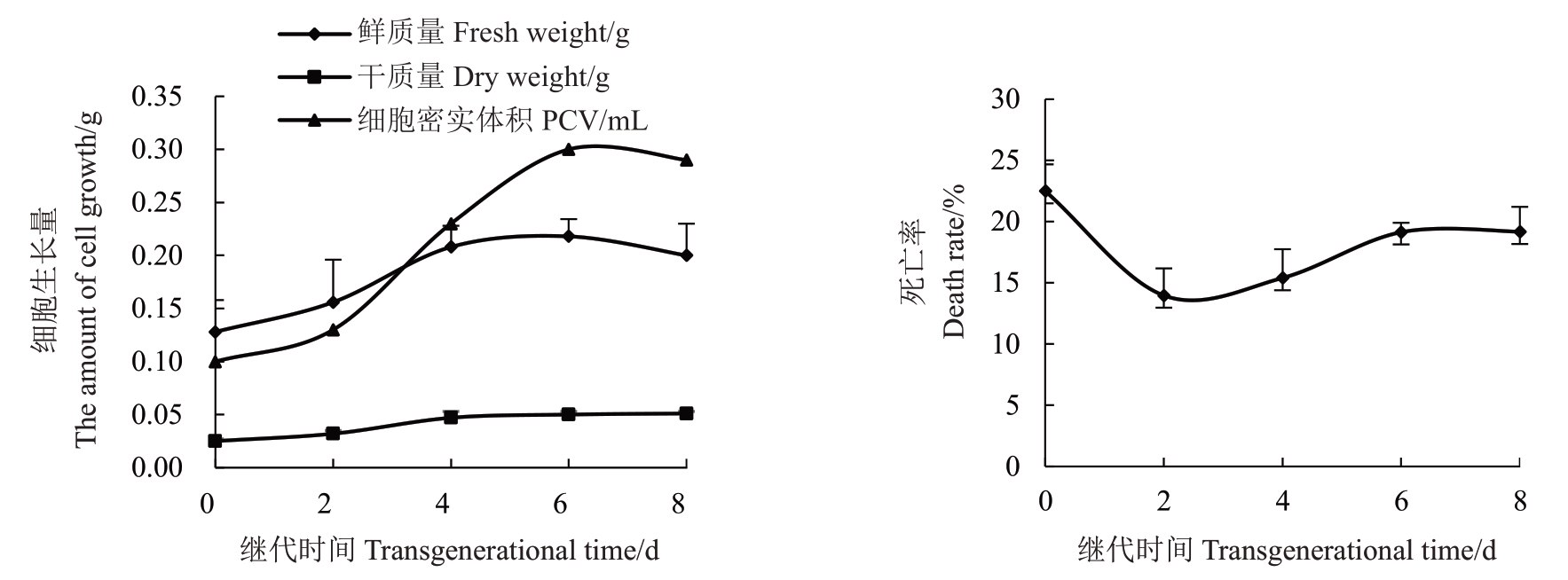
图4 梨悬浮细胞系生长曲线及死亡率测定
Fig.4 Growth curve and mortality of pear suspension cell line
综上,细胞悬浮系生长曲线、悬浮细胞死亡率之间存在着一定的对应关系,建立了梨悬浮细胞系,并且确定了2~4 d内的悬浮细胞生长状态最好,活力最高,这一时期的细胞分裂速度很快。因此,可以选择此时期的悬浮液来开展后续工作。
2.5 悬浮细胞系生长情况
将经悬浮培养后的愈伤组织接种于固体培养基中,得到了活性很强的愈伤组织,分裂速度快,状态良好,一般15~20 d即可达到试验所需生长量;而未经悬浮培养的愈伤组织15~20 d才进入快速生长期(图5)。光培养或暗培养1个月后,愈伤组织的状态基本相同,光培养下颜色稍微偏深乳黄色一点并且经悬浮培养的愈伤组织继代后分裂速度也比未经悬浮培养的愈伤组织分裂速度快,颜色差异也较明显(图6)。经悬浮培养得到的愈伤组织呈乳黄色(图6-C),活力以及分裂能力均较强;而未经悬浮培养的愈伤组织呈黄白色(图6-D),生长量和活性均不如经悬浮培养的愈伤组织(图5)。

图5 梨悬浮细胞培养对愈伤组织生长量的影响
Fig.5 Effect of pear suspension cell culture on callus growth
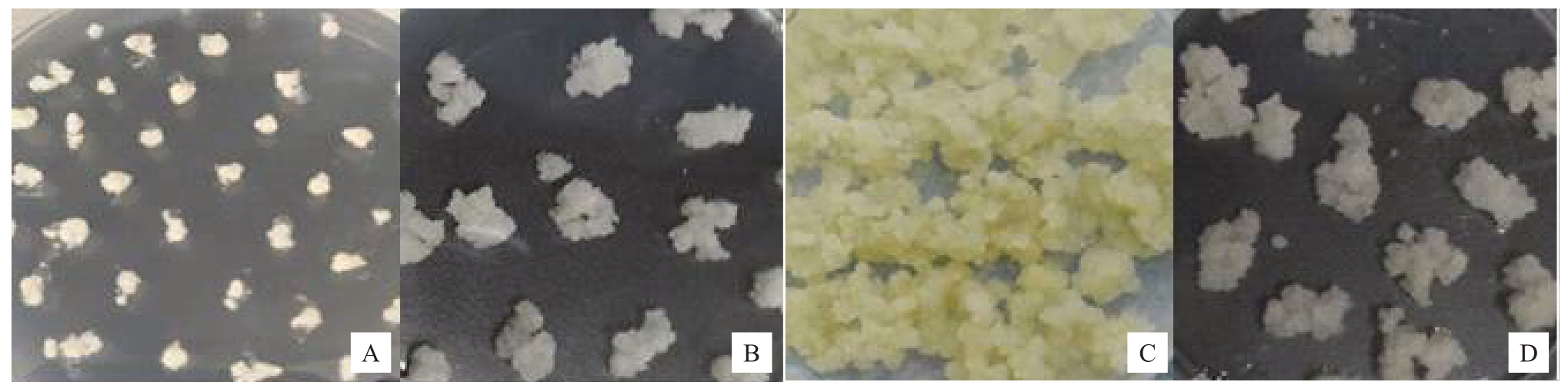
图6 梨愈伤组织生长情况(暗培养)
Fig.6 Growth of pear callus(dark culture)
A.刚接种的经悬浮后的愈伤组织;B.刚接种的未经悬浮培养的愈伤组织;C.经悬浮培养后接种20 d 的愈伤组织;D.未经悬浮培养接种20 d的愈伤组织。
A.The suspension callus after inoculation; B. Newly inoculated callus without suspension culture; C.The callus inoculated 20 days after suspension culture;D.The callus inoculated for 20 days without suspension culture.
2.6 梨悬浮细胞系的转化
利用农杆菌介导法将35S::GFP 植物表达载体转化至经悬浮培养的梨愈伤组织。荧光观察结果表明,愈伤组织呈现绿色(图7),表明35S::GFP已转化至其中,转化率为20.00%。

图7 梨悬浮细胞遗传转化
Fig.7 Genetic transformation of pear suspension cells
2.7 原生质体产量及活力鉴定
用1%的纤维素酶+0.5%离析酶组合提取的原生质体(图8-A)产量达到3.67×106个·mL-1,用FDA染色(图8-B)进行活力鉴定,原生质体活力为92.00%。

图8 梨原生质体的活力鉴定
Fig.8 Identification of vigor of pear protoplasts
A.悬浮细胞系提取的原生质体;B.FDA 染色图。
A.Protoplast extracted from suspension cell line;B.FDA staining diagram.
3 讨 论
悬浮细胞悬浮液状态、活细胞率及近圆细胞率、悬浮细胞生长量以及后期生长情况是确定悬浮细胞生长是否正常和悬浮培养体系是否建立成功的重要指标[15]。笔者在本文中研究了不同质量浓度激素对梨愈伤组织继代培养的影响,并通过对梨悬浮培养条件的研究,建立了悬浮细胞培养体系,结果表明除要选择疏松状态的愈伤组织为材料进行培养外,还要考虑到培养基的渗透压、激素种类及质量浓度,以及合适的转速、继代周期及继代比例等,才能最终得到一个好的悬浮细胞系。
前人研究表明,适宜的培养基激素种类及配比是愈伤组织以及细胞悬浮系快速增殖的关键因素[16-17]。本研究结果表明,2,4-D 质量浓度对愈伤的诱导与增殖起着关键作用,增殖量随2,4-D 质量浓度升高而升高[18-20],这与前人研究结果一致。李艳敏等[21]将2,4-D 与NAA、KT 组合使用,作为芍药花药愈伤组织培养基。在适宜的增殖质量浓度下,经过4~5 次继代后即可得到表面有颗粒状突起结构的疏松愈伤,与前人研究结果一致。2,4-D 和6-BA 搭配为细胞悬浮培养的最佳组合,因此本试验采用MS + 1.5 mg·L-1 2,4-D+1.0 mg·L-1 6-BA 建立了悬浮细胞体系。还有其他文献研究表明在悬浮细胞的长期继代培养过程中添加KT(细胞分裂素),KT 以一个较低质量浓度,一般选择为0.1 mol·L-1为宜,较高质量浓度会造成褐化现象[22]。
在整个悬浮培养的过程中植物悬浮细胞开始培养时的接种量以及继代周期、新旧培养基比例等是重要的影响因子,对于不同发育时期生长周期的长短及细胞生长增殖周期有显著影响。张正雪等[23]认为起始接种量为3 g时生长速率可达到最高,本研究起始接种量为1.5 g,究其原因可能是因为不同植物所需要的最低临界生长密度不同。其新旧培养基比例为2∶1,梨悬浮细胞的生长曲线呈“S”型[24],与前人研究一致。0~2 d为迟滞期,2~4 d为对数期,4~6 d为静止期。在一个继代周期内,细胞的死亡率维持在23%以下,表明悬浮细胞状态良好,活性很强。王磊等[25]研究表明接种15 d是悬浮细胞继代培养的较佳时期。本文适宜继代周期为7 d,与前人研究不同,这可能是因为梨愈伤组织在适宜的悬浮培养条件下分裂能力较强,导致液体培养基中的营养成分被过早的消耗殆尽,从而缩短了继代周期。2~4 d内的细胞分裂最快,可以以此阶段的细胞来开展各项研究。
细胞壁的化学成分和结构因品种不同而不同,所以不同外植体的最佳酶液组合和浓度也不同。张宁等[26]研究表明如果酶液浓度过高,会导致原生质体中毒和破裂;反之,如果浓度过低,会导致原生质体成团。杜小云等[27]用1%纤维素酶+0.5%离析酶+0.01%半纤维素酶组合,得到草莓悬浮系原生质体;本文悬浮细胞酶液组合为1%纤维素酶+0.5%离析酶。
经悬浮培养后的愈伤组织增殖快,活力强是良好的遗传转化的受体,但受品种、染液浓度和时间的影响,愈伤组织转化率较低。李亚梅等[28]表明在浸染液浓度OD600为0.6~0.8 和浸染时间30 min 时,酸枣愈伤组织转化效率超过20%。本文中,以OD600为0.5,浸染时间15 min 为最好,浸染30 min 的愈伤组织会在后期培养过程中被菌吞没,造成褐化死亡。
4 结 论
本研究以无菌的新梨7 号花药为外植体诱导愈伤组织,将其接种到1/2 MS+1.0 mg·L-1 2,4-D+0.4 mg·L-1 NAA+30 g·L-1蔗糖+7 g·L-1琼脂的固体培养基上即可诱导出愈伤组织。将得到的疏松愈伤组织接种到MS+1.5 mg·L-1 2,4-D+1.0 mg·L-1 6-BA+30 g·L-1蔗糖的液体培养基中,在(26±1)℃,200 r·min-1的黑暗条件下悬浮振荡培养,每隔7 d继代1 次,新旧培养基比例2∶1,经30 d 后获得了良好的悬浮细胞系,悬浮液的颜色澄清透明,分散性及均一性好,生长分裂速度快,活细胞率可达81.98%,近圆细胞率达83.66%;用悬浮细胞系提取的原生质体产量达3.67×106个·mL-1,活力可达92.00%,愈伤组织转化率达20.00%。本研究结果为建立一套稳定优良的梨细胞悬浮培养体系提供了理论依据及数据支撑。
[1] 李致慧.梨花药离体培养及多倍体诱导技术研究[D].杨凌:西北农林科技大学,2012.LI Zhihui. The study on anther culture and polyploid induction of pear[D].Yangling:Northwest A&F University,2012.
[2] 张宏宇,朱心慰,侯鹏奇,胡锐峰,黄玺,侯义龙.果树单倍体和多倍体研究进展[J].分子植物育种,2019,17(2):606-611.ZHANG Hongyu,ZHU Xinwei,HOU Pengqi,HU Ruifeng,HUANG Xi,HOU Yilong. Research progress and prospect pf fruit tree haploid and polyploid[J]. Molecular Plant Breeding,2019,17(2):606-611.
[3] JORDAN M. In vitro culture of anther from Prunus,Pyrus and Ribes[J].Planta Medica,1975,28(1):59-65.
[4] 山东农学院组织培养:梨花药培养研究初探[J].山东农学院科研资料选编,1978,13(5):7-15.Tissue culture of Shandong Agricultural College:Preliminary study on the culture of pear flower[J].Selection of Scientific Research data of ShandongAgricultural University,1978,13(5):7-15.
[5] 薛光荣,杨振英,史永忠,方成泉,贾敬贤.锦丰梨花粉植株的诱导[J].园艺学报,1996,23(2):123-127.XUE Guangrong,YANG Zhenying,SHI Yongzhong,FANG Chengquan,JIA Jingxian.Induction of pollen plantlets by anther culture in vitro in‘Jinfeng’pear[J]. Acta Horticulturae Sinica,1996,23(2):123-127.
[6] 吕春茂,范海延,姜河,孟宪军.植物细胞培养技术合成次生代谢物质研究进展[J].云南农业大学学报,2007,22(1):1-7.LÜ Chunmao,FAN Haiyan,JIANG He,MENG Xianjun. Research advances on synthesis of secondary metabolities by plant cell culture[J].Journal of Yunnan Agricultural University,2007,22(1):1-7.
[7] STEWARD F C,MAPES M O,SMITH J. Growth and organized development of cultured cells Ⅰ. Growth and division of freely suspended cells[J].American Journal of Botany,1958,45(9):693-703.
[8] WILSON H M,STREET H E. The growth,anatomy and morphogenetic potential of callus and cell suspension cultures of Hevea brasiliensis[J].Annals of Botany,1975,39(62):140-150.
[9] 王影,黄敏仁,陈道明,许明.杨树细胞悬浮培养及体细胞胚胎发生的研究[J].南京林业大学学报(自然科学版),1991,9(3):31-37.WANG Ying,HUANG Minren,CHEN Daoming,XU Ming. A study on cell suspension culture and somatic embryogenesis of Populus simonii[J].Journal of Nanjing Forestry University(Natural Science Edition),1991,9(3):31-37.
[10] 赖钟雄,黄浅,林秀莲,林玉玲,陈义挺,赖呈纯,蔡英卿.荔枝胚性悬浮细胞系的快速建立及其体胚植株的再生[J].中国农学通报,2007,10(1):12-17.LAI Zhongxiong,HUANG Qian,LIN Xiulian,LIN Yuling,CHEN Yiting,LAI Chengchun,CAI Yingqing. Rapid establishment of embryogenic cell suspensions and plan regeneration via somatic embryogenesis in litchi[J]. Chinese Agricultural Science Bulletin,2007,10(1):12-17.
[11] 范琪.葡萄胚性悬浮系建立及原生质体分离研究[D].兰州:甘肃农业大学,2018.FANG Qi. Establishment of grapevine embryogenic suspension system and protoplast isolation[D]. Lanzhou:Gansu Agricultural University,2018.
[12] 周正君.花椒细胞悬浮培养及原生质体分离[D].杨凌:西北农林科技大学,2016.ZHOU Zhengjun. Studies on cell suspension culture and protoplast isolation of Zanthoxylum bungeanum[D].Yangling:Northwest A&F University,2016.
[13] 黄振.青杨悬浮细胞系建立与原生质体培养研究[D].北京:北京林业大学,2015.HUANG Zhen. Studies on suspension cell line establishment and protoplast culture of Populus spp.[D]. Beijing:Beijing Forestry University,2015.
[14] DONG Q L,LIU D D,AN X H,HU D G,YAO Y X,HAO Y J.MdVHP1 encodes an apple vacuolar H+-PPase and enhances stress tolerance in transgenic apple callus and tomato[J].Journal of Plant Physiology,2011,168(17):2124-2133.
[15] 欧巧明,厚毅清,包梅年,胡达,陈玉梁,岳春玲.中药半夏单细胞悬浮培养、胚胎发生及植株再生[J].中国生物工程杂志,2012,32(10):39-49.OU Qiaoming,HOU Yiqing,BAO Meinian,HU Da,CHEN Yuliang,YUE Chunling.The suspended single cell culture and embryogeny and plant regeneration of pinellia ternata[J]. China Biotechnology,2012,32(10):39-49.
[16] LIU L,GU Y H,MENG K.Establishment of suspension cell cultures and cell growth characteristics of Xanthoceras sorbifolia[J].Scientia Silvae Sinicae,2010,46(9):79-83.
[17] 荆瑞勇,王丽艳,郭永霞.丹参愈伤组织培养中激素浓度的优化研究[J].激光生物学报,2015,24(4):377-381.JING Ruiyong,WANG Liyan,GUO Yongxia. Study on optimization of hormone concentration in callus culture of Saliae miltiorrhiza Bge.[J]. Acta Laser Biology Sinica,2015,24(4):377-381.
[18] 芦笛,杨清,陆巍.影响禾谷类作物胚性细胞悬浮系建立的一些因素[J].植物生理学通讯,2007,43(3):399-406.LU Di,YANG Qing,LU Wei.Several influencing factors on establishment of embryogenic suspension cell lines of cereal crops[J].Plant Physiology Communications,2007,43(3):399-406.
[19] 谷立坤,张建云,张世敏,贾新成.不同质量浓度2,4-D 对硬粒小麦成熟胚愈伤组织诱导及植株再生的影响[J].河南农业大学学报,2004,38(4):452-455.GU Likun,ZHANG Jianyun,ZHANG Shimin,JIA Xincheng.Effect on inducing of calllus from mature embryos of five wheats and plant regeneration of different concentration of 2,4-D[J].Journal of Henan Agricultural University,2004,38(4):452-455.
[20] CARSONO N,JUWENDAH E,LIBER T.Optimize 2,4-D concentration and callus induction time enhance callus proliferation and plant regeneration of three rice genotypes[J]. Biodiversitas Journal of Biological Diversity,2021,22(7):2555-2560.
[21] 李艳敏,蒋慧,符真珠,张晶,袁欣,王慧娟,高杰,董晓宇,王利民,张和臣.芍药花药愈伤组织诱导及体细胞胚发生[J].植物学报,2021,56(4):443-450.LI Yanmin,JIANG Hui,FU Zhenzhu,ZHANG Jing,YUAN Xin,WANG Huijuan,GAO Jie,DONG Xiaoyu,WANG Limin,ZHANG Hechen. Callus induction and somatic embryogenesis in anther culture of Paeonia lactiflora[J]. Chinese Bulletin of Botany,2021,56(4):443-450.
[22] 郝艳芳,王良群,刘勇,张微,杨伟,白鸿雁,武擘.高粱幼叶细胞悬浮系的建立[J].作物杂志,2018(1):35-40.HAO Yanfang,WANG Liangqun,LIU Yong,ZHANG Wei,YANG Wei,BAI Hongyan,WU Bo. Establishment of sorghum cell suspensions with young leaves[J].Crops,2018(1):35-40.
[23] 张正雪,蓝增全,吴田.基于诺丽叶片愈伤组织的细胞悬浮系的建立[J].生物技术通报,2018,34(5):142-147.ZHANG Zhengxue,LAN Zengquan,WU Tian. Establishment of cell suspension culture system based on the leaf callus in Noni[J].Biotechnology Bulletin,2018,34(5):142-147.
[24] WANG G L,XU C Y,WANG G D. Study on vitrification cryopreservation condition of Anthurium andraeanum embryonic suspension cells[J]. Journal of Plant Resources and Environment,2010,19(1):26-31.
[25] 王磊,曹逼力,徐坤.生姜离体叶片愈伤组织悬浮细胞系建立与植株再生[J].激光生物学报,2017,26(2):176-182.WANG Lei,CAO Bili,XU Kun. Establishment of cell suspension culture from leaf-derived callus and plant regeneration of ginger[J].Acta Laser Biology Sinica,2017,26(2):176-182.
[26] 张宁,李威,顾钊宇,陈秋菊,段续伟,杨清,李天忠.‘富士’苹果花粉原生质体分离初探[J]. 园艺学报,2015,42(6):1167-1170.ZHANG Ning,LI Wei,GU Zhaoyu,CHEN Qiuju,DUAN Xuwei,YANG Qing,LI Tianzhong. Preliminary study on the isolation of mature pollen protoplasts in‘Fuji’apple[J].Acta Horticulturae Sinica,2015,42(6):1167-1170.
[27] 杜小云,王震,王翠华,曾弓箭,沈祥陵.‘红颜’草莓原生质体制备及瞬时转化体系的建立[J].生物资源,2019,41(6):532-538.DU Xiaoyun,WANG Zhen,WANG Cuihua,ZENG Gongjian,CHEN Xiangling. Preparation of protoplasts and establishment of tansient transformation system of‘Hongyan’strawberry[J].Biotic Resources,2019,41(6):532-538.
[28] 李亚梅,马福利,张山奇,黄锦秋,陈梦婷,周军永,孙其宝,孙俊.酸枣愈伤组织转化体系构建及在ZjBRC1 调控ZjYUCCA表达中的应用[J].园艺学报,2022,49(4):749-757.LI Yamei,MA Fuli,ZHANG Shanqi,HUANG Jinqiu,CHEN Mengting,ZHOU Junyong,SUN Qibao,SUN Jun.Optimization of jujube callus transformation system and application of Zj-BRC1 in regulating ZjYUCCA expression[J]. Acta Horticulturae Sinica,2022,49(4):749-757.