苹果是世界上生产和消费最多的水果之一。中国苹果产业协会数据表明,我国2020年苹果产量超过4400 万t,居世界第一位。为保持苹果新鲜品质并做到全时空供应,1/5~1/4 的苹果会进入冷藏环节[1]。然而,苹果冷藏过程中会发生一些生理病害,导致果实品质下降。其中,果肉褐变是苹果贮藏期间常见病害之一。
苹果冷藏期间的果肉褐变通常发生在果肉部分,有时也会波及果心或果皮,是单一或多种因素综合作用的结果[1-2]。发生果肉褐变的苹果不但风味和营养价值降低,而且会因为与健康果实在外观上无明显区别导致病害发生情况难以监控,造成巨大的经济损失[2]。因此,对苹果贮藏期间果肉褐变的成因、机制和控制技术进行研究具有重要的理论价值和实践意义。
笔者在本文中综述了近年来在苹果冷藏期间常见的果肉褐变的分型、成因、发生机制和控制技术等方面的研究进展,以期为苹果冷藏期间果肉褐变的理论和防控研究提供参考。
1 果肉褐变的分型
苹果果肉褐变在冷藏期间或脱离原冷藏环境后都有发生,褐变部位的特征各有不同。质地或硬或软,或湿润或干燥,味道或苦或甜,有时还会形成空腔[3-5]。特征不同,成因也不同。区分不同的褐变特征,可以快速找到褐变的成因。常见苹果冷藏期间的果肉褐变可以分为辐射型、弥漫型、空洞型和崩溃型4类(图1)。
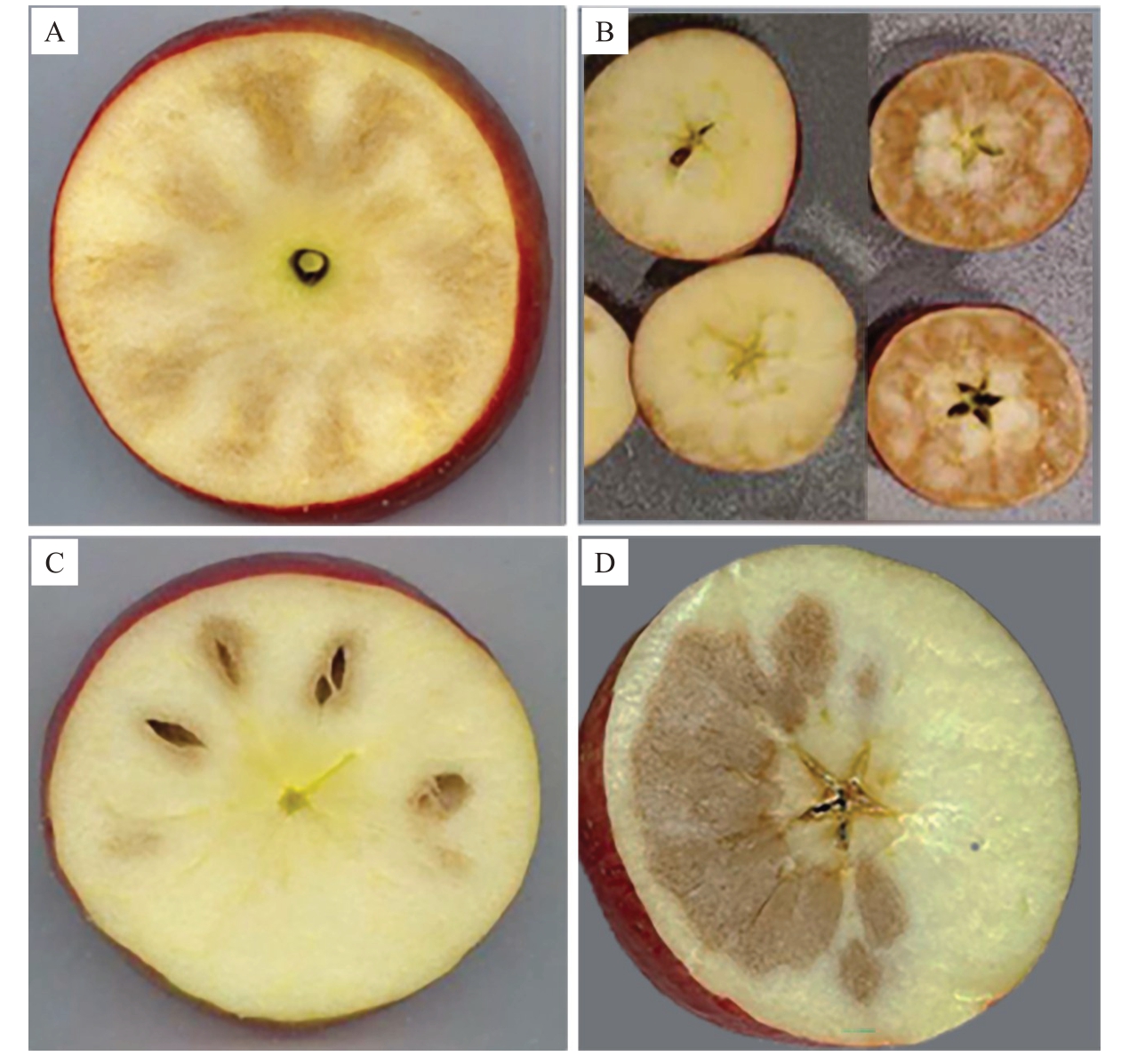
图1 苹果冷藏期间果肉褐变的4 种类型
Fig.1 Four types of flesh browning in apples during cold storage
A.辐射型[6];B.弥漫型[7];C.空洞型[6];D.果肉崩溃型。
A.Radial flesh browning[6];B.Diffusion flesh browning[7];C.Flesh cavities[6];D.Internal breakdown.
1.1 辐射型褐变
辐射型褐变主要表现为果实的维管束发生明显褐变,而果皮相对不受影响(图1-A)[6,8-9]。一般果柄端果肉褐变的严重程度略重于花萼端,褐变组织质地较软,细胞壁受损严重[8]。此类褐变被认为是由衰老导致的维管束损伤引起,会随着贮藏时间的增加而加剧,也会因为晚采、贮藏环境中高CO2和/或低O2浓度持续时间长以及贮藏温度过低而增强其易感性[6,8-9]。
1.2 弥漫型褐变
弥漫型褐变的果实维管组织往往不受影响或褐变较轻,而果皮组织褐变明显(图1-B)[8]。弥漫型褐变被认为是一种冷害,常见于低温生长的地区[7,9]。当果实发育受到低温胁迫时,果实的正常生理代谢活动会被破坏,导致果实发育滞后,以至于采后果实无法适应正常的低温贮藏,进而导致弥漫性褐变的发生[7]。此类褐变一般在果实两端较严重,中间部分较轻[6]。病害初期,仅在果皮组织附近形成小块褐变,褐变区域有明显界限(图1-B左);随着病害的加重,褐变向内发展,褐变面积变大,整个果皮组织附近全部褐变(图1-B右)。
1.3 空洞型褐变
空洞型褐变的特征是果肉褐变组织成点或片状分布,在果肉组织之间形成褐变圈,并随着症状的加重,褐变组织发展出透镜状的凹坑和空洞(图1-C)[2,9]。空洞型褐变发生初期,果皮色泽变暗,有些品种会出现浅褐色斑点,果实内部产生块状褐变,果肩和果心线组织发病率较高,褐变组织的质地坚实、湿润[2,10-11];随后病症迅速发展,褐变组织失水,质地变得松散、干燥,果实风味变淡,食之初甜后苦;最终,褐变组织产生凹坑和空洞,果皮出现褐斑和褶皱[3,5]。
1.4 崩溃型褐变
崩溃型褐变表现为果肉组织松散状褐变(图1-D)[12-13],可以细分为低温崩溃、衰老崩溃和水心崩溃等[12]。低温崩溃由果肉内层开始逐步向外侧发展,初期褐变组织质地坚硬、湿润,褐变颜色较浅,维管束无明显褐变,后期发展为以果核为核心的圆球形大面积褐变,多发于元帅等品种[13],近几年在富士、维纳斯黄金等品种上也常见到。衰老崩溃多发于苹果贮藏后期[14],常见有果肉粉棉病和橡皮病。果肉粉棉病发病初期,果肉发绵变软,随后变干形成易碎的粉质状,后期果肉发生褐变,严重时果皮变暗、变褐或出现果皮破裂、果肉绽裂等症状[2];橡皮病常发于维管束和靠近果皮的组织,质地绵软,病害前期果肉轻微褐变,随后果皮变暗明显,病害部位有轻微轮廓,手指按压时容易陷下去,多发于红玉苹果,因此也常称为红玉橡皮病[13-14]。水心崩溃常见于采收时过熟的苹果,表现为果肉的一部分成水浸状,一般在果心处或围绕维管束开始发展,具体发病部位及程度因品种而异,也会受到低温、采收时间等因素的影响[12,15]。
尽管果肉褐变的类型不尽一致,但在实践生产中往往发现2 种以上的褐变类型叠加发生,比如辐射型褐变后期往往也会发现空洞型褐变,而水心崩溃后期也往往伴随着衰老崩溃。这种不同的导致果肉褐变的因素叠加,有时会造成区分困难,同时也增加了苹果冷藏期间果肉褐变的风险和防控的难度。
2 影响因素
品种、采前气候条件、栽培技术以及采后贮藏条件等,都会影响苹果冷藏期间果肉褐变的发生。
2.1 品种
不同苹果品种对果肉褐变的敏感性不同。一些品种由于果肉细胞排列紧密,果肉中的气体交换较为困难,导致组织内部CO2浓度较高,易发生CO2伤害,进而造成果肉褐变,如富士、布瑞本(Breaburn)等[15]。Serra 等[16]对14 种苹果采后褐变情况研究发现,青苹和红粉佳人品种不易发生果肉褐变,而嘉年华(Fiesta)和米切嘎拉(Mondial Gala)等品种易发生严重的果肉褐变。国内的主栽培品种中,富士、长富2 号和王林等品种对水心崩溃有较高的易感性,而金冠、国光和元帅等品种则不敏感[17-18]。
2.2 采前因素
气候条件、土肥水管理、结果部位、作物负荷和采收期等栽培技术措施都可能影响到果实贮藏期间果肉褐变的发生。
2.2.1 气候条件(1)温度:苹果生长期温度过低或过高都会造成果肉褐变的产生。有研究发现,在寒冷地区生长的布瑞本苹果比在温暖地区生长的更容易发生褐变,并推测其可能原因为寒冷的生长条件改变了细胞代谢过程,通过降低表皮和组织的扩散性/增强对高CO2和低O2的敏感性,增加了果肉褐变的风险[19]。McCormick 等[20]还发现,采前4 周环境温度>10 ℃可以显著降低果肉褐变的发病率,但具体机制不清楚。
(2)干旱:干旱对营养生长和果实发育具有重要影响。干旱环境下,根部对钙等微量元素的吸收、利用和运转受限[21],增强了对果肉褐变的易感性[22-23]。此外,干旱导致的果实坐果率下降,也有可能通过降低果园负荷引起一系列包括果肉褐变易感性在内的果实品质差异[20]。
2.2.2 采收期 采收成熟度与果肉褐变联系十分密切,过早或过晚采收均会增加果肉褐变的风险。其中,早采的苹果易发生果心褐变[24],晚采的苹果的CO2 伤害(空洞型褐变)、水心崩溃的发病率更高[24-25]。
2.2.3 矿质元素 土壤中营养元素和施肥的种类、数量、方式也直接影响果实采后贮藏质量。
(1)Ca:Ca2+能通过结合细胞膜上的磷脂和蛋白质来维持细胞膜的功能和稳定性[26],而果肉褐变与细胞膜系统的稳定性显著相关。Ca2+含量过低会增大膜的通透性,甚至使细胞膜区室化被破坏,引起果肉褐变[23,27];但也有报道[28]指出,Ca2+含量过高会破坏细胞中Ca2+稳态机制,过多的Ca2+从细胞器或细胞膜外释放到细胞质中,对细胞产生毒害。因此,Ca2+含量过低或过高都有可能引起果肉褐变的发生。
(2)N:N 含量过高间接影响果肉褐变的发生。采前大量施氮能促进果实的生长发育,氮钙比增大,引起果实缺钙[14,21]。此外,采前氮肥施入量过多,会使果实呼吸强度增大,加快果实衰老,引起衰老崩溃。
(3)Mg 和K:Ca2+和Mg2+之间具有离子相似性,而K+又是Ca2+和Mg2+的拮抗剂,这导致Mg2+和K+与Ca2+之间的互相竞争引发细胞膜功能的紊乱[29]。Corrêa 等[30]发现,发生果肉褐变的果实具有更高的Mg/Ca、K/Ca和(K+Mg)/Ca。
2.2.4 结果部位 结果部位对果肉褐变易感性的影响主要通过光照、温度等因素实现。内膛果由于光照不足,在贮藏期间衰老更快,更易发生果肉粉绵病;而外层果常因光照过强,易发生水心崩溃[31]。此外,结果部位对果肉褐变的影响,还与其对钙含量分布的影响有关,如分枝根部的苹果往往比顶端的苹果Ca2+含量更高,长穗苹果的Ca2+含量比短穗苹果的Ca2+含量高[21],而Ca2+含量较低的果实通常具有更高的果肉褐变风险[32]。
2.2.5 树体负荷 相比于温度、矿质元素等采前因素,作物负荷对果实质量具有更大的影响。比如枝条生长旺盛、果实过度稀疏等低负荷的果树,发生苹果水心病和果肉褐变的风险就大[33]。这可能与低负荷增大了果实单果质量,从而降低了果实内包括Ca2+在内的微量元素浓度有关[20,34]。此外,低负荷导致的果个增大减缓了果实内气体交换,增加了果肉内部CO2的积累,增大了空洞型褐变的风险[31]。而高负荷量,则表现出与果心褐变的正相关性[20]。
2.3 采后因素
苹果采后贮藏环境的温度、气体成分和外源物质处理等都会影响到果肉褐变的发生。
2.3.1 温度 贮藏温度不适宜会导致果肉褐变的发生。温度过低容易引起低温诱导的、由外而内的果肉弥散型褐变,或者由内到外的低温崩溃。如Saba等[5]发现,与3 ℃贮藏的苹果相比,0.5 ℃贮藏诱导了恩派(Empire)苹果果肉褐变的发生;王志华等[4]的研究表明,-2 ℃贮藏的苹果褐变指数高于0 ℃贮藏的苹果褐变指数。
2.3.2 气体成分 空洞型果肉褐变是典型的由高CO2伤害引起的褐变症状。气调贮藏的苹果在CO2浓度过高,以及普通冷藏的苹果在因包装不合适及库内通风不良造成环境中CO2浓度过高等情况下,都可能引起空洞型果肉褐变的发生。低O2环境会加剧此类褐变的形成和发展[2,8],主要表现为果实硬度下降和果皮下陷的加速以及褐变组织扩大和空洞形成的加剧,且常伴随轻微发酵味和腐烂现象的发生[14]。此外,贮藏温度过高也会使呼吸强度提高从而引起CO2积累,导致空洞型果肉褐变的出现[3],或者因加快果实成熟引起贮藏后期的衰老崩溃。
Castroa 等[35]研究发现,红粉佳人在5 kPa CO2+1.5 kPa O2条件下冷藏2个月后,果肉褐变发病率显著高于1 或3 kPa CO2+1.5 kPa O2中冷藏的苹果,而在0.5 kPa CO2+1.5 kPa O2环境中没有观察到果肉褐变;同时发现,在1.5 kPa CO2条件下,将O2由1 kPa提升到19 kPa 时,果肉褐变发病率可由5%降低至1%。Argenta等[24,36]研究发现,在0.5 kPa CO2+1.5 kPa O2贮藏条件下,富士表现出弥漫型褐变的特征;在2.5 kPa CO2+1.5 kPa O2贮藏条件下,3种不同品系的富士苹果均会发生果肉褐变,且能同时观察到空洞型褐变和弥漫型褐变2种果肉褐变类型。
值得注意的是,贮藏环境中的高CO2含量一般不被认为是导致苹果弥漫型和辐射型果肉褐变的主要因素,而是褐变发生中的一个叠加因素[7]。
2.3.3 外源物质处理 研究表明,1-甲基环丙烯(1-methylcyclopropene,1-MCP)、钙和二苯胺(diphenylamine,DPA)处理都可能影响到苹果贮藏期间的果肉褐变。
(1)1-MCP:多篇文献均报道了1-MCP 可以显著延缓苹果等多种水果采后衰老进程,降低了果肉褐变、水心褐变等的发生[37-39],但也有1-MCP使用不当增加果肉褐变的报道。如Koushesh 等[5]研究发现,1-MCP处理后0.5 ℃贮藏的恩派苹果,果肉褐变的发病率和褐变严重程度均显著高于3 ℃贮藏的,并提出这与低温冷害关系密切;DeEll 等[40]也发现,1-MCP 处理的恩派苹果在2.5 kPa O2+2 kPa CO2或1.5 kPa O2+1.2 kPa CO2条件下,果肉褐变的发病率显著增加,并认为这可能与1-MCP处理增强了果实对CO2的敏感性有关。此外,1-MCP处理也被发现诱导了晚采的恩派货架期间果肉的褐变[41]。由此推测,1-MCP处理后对果肉褐变影响效果的差异,可能与苹果品种、冷藏温度和气体成分的控制等的差异有关。
(2)钙:许多报道表明,采后一定浓度的钙处理有利于苹果保持良好品质。一方面,钙可以作为直接或间接的自由基清除剂,维持细胞膜结构的正常功能;另一方面,钙也可以提高果实的抗坏血酸(ascorbic acid,AsA)含量[42]。AsA 作为一种重要的抗氧化剂,在抑制果肉褐变方面发挥关键作用。如Zhao 等[43]研究发现,首红经5%的CaCl2浸泡10 min处理后,显著维持了AsA等抗氧化物质的含量。
(3)DPA:DPA对低温崩溃和CO2伤害导致的果肉褐变有明显的抑制作用。有报道表明,DPA 可以降低苹果对CO2的易感性;在配合低温处理使用时,DPA也能明显降低果心褐变率[44]。但由于DPA使用后的废液对环境和人体存在潜在的危害,英国、德国等部分国家已禁止DPA的使用。
3 苹果果肉褐变的机制
目前,主流理论认为,细胞膜区室化功能丧失造成的酶促褐变是导致苹果冷藏期间果肉褐变的主要机制。正常情况下,多酚氧化酶(polyphenol oxidase,PPO)位于细胞质中,酚类物质位于液泡中,由细胞的膜结构将两者分隔开;但当细胞膜结构被破坏或通透性发生改变时,苹果中的酚类物质泄露,并在O2的参与下被PPO催化形成醌类物质,而后醌类物质聚合生成棕色色素,导致果肉褐变的发生[45-46]。下文总结了可能导致苹果冷藏期间果肉细胞膜区室化功能损失的机制。
3.1 Ca2+浓度改变
Ca2+可以通过与细胞膜表面的脂质和蛋白质结合来维持细胞膜结构的功能和稳定性。据报道,维持膜结构的功能需要超过0.1 mmol·L-1的游离Ca2+,而原生质体自身Ca2+维持在一个极低的水平(0.1~0.2 μmol·L-1),无法发挥类似的作用[23]。因此,当原生质体外Ca2+减少或Ca2+与质膜的结合被抑制时,会导致细胞膜结构被破坏,使细胞膜区室化的功能丧失,随后酚类物质泄露并与多酚氧化酶反应生成醌类物质,最终导致果肉褐变。
一般认为,Ca2+减少导致的区室化功能丧失的可能机制有2种。一种机制是K+、Mg2+等与Ca2+竞争结合质膜上Ca2+结合位点,但结合后的K+、Mg2+等却不能替代Ca2+发挥维持细胞膜功能的作用,促使细胞膜通透性增加,最终区室化被破坏(图2)[23]。Freitas等[23]、Corrêa 等[30]和Shoffe 等[47]的研究均证实高K+/Ca2+比或Mg2+/Ca2+比增加了果肉褐变的发病风险。另一种机制是细胞壁Ca2+结合位点的增加,使细胞膜外侧有效Ca2+浓度减少,不足以维持细胞膜的功能,导致区室化被破坏。如Freitas等[23]研究发现,在苹果贮藏过程中,果胶甲酯酶(pectin methylesterases,PMEs)基因表达上调和PME活性增加并在细胞壁上创造了更多的Ca2+结合位点,降低了细胞膜外侧游离Ca2+的水平,膜透性增大,导致果肉褐变发病率升高。
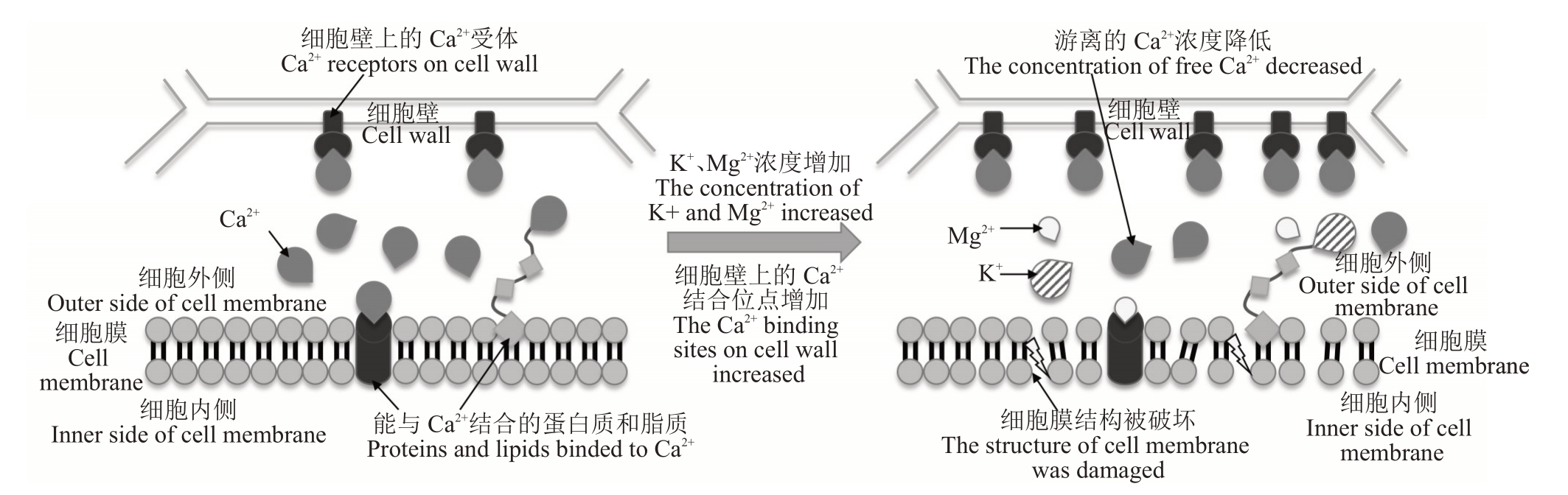
图2 Ca2+减少导致细胞膜区室化丧失的可能机制
Fig.2 Possible mechanism of cell membrane compartmentalization failure caused by reduction of Ca2+
此外,钙含量低会减弱果实耐CO2能力,加速了由高CO2引起的果肉褐变的发生[22],也使果实更易发生水心病、苦痘病等病害以及贮藏期间的果肉崩溃[48]。刘野等[49]提出,贮藏环境中的CO2进入细胞后形成的CO32-,可以与细胞质膜上水溶性的Ca2+结合,使质体外有效Ca2+减少,导致细胞透性增大,并指出这是CO2伤害引起果肉褐变的一个可能原因。
但也有报道提出,Ca2+浓度过高也可能造成苹果冷藏期间的果肉褐变,原因可能在于Ca2+浓度过高导致的钙稳态机制受损,使过多的Ca2+从细胞器或细胞膜外释放到细胞质中,从而对细胞产生了毒害作用[50-51]。
3.2 乙醇和乙醛毒害
在低温、低O2和高CO2等逆境条件下,果蔬呼吸强度降低,有氧代谢会部分转为无氧代谢。在此过程中,乙醇、乙醛等发酵代谢产物大量积累并对细胞膜结构产生毒害作用,导致细胞膜区室化功能丧失,果肉褐变风险增加(图3)。分子水平上的研究表明,高CO2与低O2条件可通过下调果实PcMDHAR和PcAPX 表达,降低氨基酸-谷胱甘肽循环功能,减少AsA 含量,积累大量乙醇与乙醛,增加细胞膜透性,最终导致果肉褐变加重[52];Shoffe 等[47]研究也发现,冷害的严重程度与乙醇和乙醛的积累有关;Liu等[53]研究表明,发生水心褐变的果实中的乙醇和乙醛的浓度分别增加了710.26%和100.54%。

图3 乙醇、乙醛毒害及氧化应激诱导果肉褐变的可能机制
Fig.3 Possible mechanism of ethanol,acetaldehyde toxicity and oxidative stress by which induces flesh browning
斜线和点填充表示代谢通路被促进和抑制,长划线-点和虚线箭头分别表示代谢过程被促进和抑制,上下箭头分别表示物质含量增加和减少。PDC.丙酮酸脱羧酶;ADH.乙醇脱氢酶;SOD.超氧化物歧化酶;CAT.过氧化氢酶;POX.过氧化物酶;PPOs.多酚氧化酶;DHAR.脱氢抗坏血酸还原酶;GR.谷胱甘肽还原酶;DHA.脱氢抗坏血酸;GSSH.氧化型谷胱甘肽。
Oblique lines and dot fillings indicate that the metabolic pathway are promoted and inhibited,long dash dot and dashed arrow indicate that the metabolic process is promoted and inhibited,up and down arrows indicate that the substance content is increased and decreased,respectively.PDC.Pyruvate decarboxylase; ADH. Ethanol dehydrogenase; SOD. Superoxide dismutase; CAT. Catalase; POX. Peroxidase; PPOs. Polyphenol oxidase;DHAR.Dehydroascorbic acid reductase;GR.Glutathione reductase;DHA.Dehydroascorbic acid;GSSH.Oxidized glutathione.
3.3 自由基及其清除体系失衡
正常环境下,果蔬组织内活性氧(reactive oxygen species,ROS)等自由基的产生与清除处于动态平衡,少量的ROS 不会对组织产生伤害,且充当了一种重要的稳态信号传导实体[54]。但当果蔬处于逆境胁迫时,组织内ROS的异常积累超出了系统的清除能力,导致多种蛋白质和酶的结构与功能受到破坏,膜脂中的不饱和脂肪酸因ROS、脂氧合酶(lipoxygenase,LOX)等的攻击发生过氧化反应,膜通透性增大,最终导致果肉褐变(图3)[55]。
此外,许多非酶类物质,如AsA和谷胱甘肽(reduced glutathione,GSH)等可以参与ROS的清除,因而也在防止果肉褐变方面发挥着重要作用[45,56-57]。AsA 还是一种高效的酚酶抑制剂,具有很强的抗褐变能力;GSH 可以通过与醌类物质相互作用,产生无色物质,抑制褐变的发生。但是,这些抗氧化物的活性很可能会在逆境胁迫下遭到破坏(图3)。
3.4 能量亏缺
生物膜完整性的维持与足够的细胞能量状态密切相关,充足的三磷酸腺苷(adenosine triphosphate,ATP)可以稳定细胞膜的结构;较高的ATP含量也可以保持细胞内较高的ROS清除能力,减轻ROS对膜脂的过氧化作用,从而减少果肉褐变的发生[4,58]。当果蔬处于逆境胁迫下(如低温、高CO2等),有氧呼吸的三羧酸循环(tricarboxylic acid cycle,TCA)减弱后,电子传递链偶联的ATP减少,无法提供正常能量以维持细胞膜的结构和功能(图4)[4,15];同时,乙醇、乙醛和ROS含量增加破坏了细胞膜结构完整性(图3),进一步导致了ATP 合成受阻最终造成细胞膜区室化快速丧失,褐变加重[27]。王志华等[4]发现,低温减少了ATP 和二磷酸腺苷(adenosine diphosphate,ADP)含量,导致细胞供能不足,从而加重了果肉褐变,且温度越低,能量亏缺越严重,褐变指数越高。Hatoum 等[15]研究发现,当将CO2从0.7 kPa 升高到3.7 kPa 时,褐变发病率随之升高,并认为这是高浓度CO2将呼吸代谢从三羧酸循环转移到发酵过程,使ATP生成减少,导致维持细胞膜的能量不足,最终引起褐变。Wang 等[58]也发现,ATP 合成酶、NADH脱氢酶和无机焦磷酸化酶基因表达下调和磷脂酶D基因表达上调所导致的能量亏缺和细胞膜稳定性下降,是果肉褐变增加的一个重要原因。

图4 能量亏缺诱导果肉褐变的可能机制
Fig.4 Possible mechanism of energy deficiency by which induces flesh browning
斜线和点填充分别表示代谢通路被促进和抑制,黑白渐变填充表示物质含量减少,上下箭头分别表示物质含量增加和减少。NAD+.烟酰胺腺嘌呤二核苷酸;NADP+.烟酰胺腺嘌呤二核苷磷酸;NADH.还原型烟酰胺腺嘌呤二核苷酸;NADPH.还原型烟酰胺腺嘌呤二核苷酸磷酸;FAD.黄素腺嘌呤二核苷酸;FADH2.还原型黄素二核苷酸。
Oblique lines and dot fillings indicate that the metabolic pathways are promoted and inhibited, respectively, black and white gradient filling indicates that the content of substances is decreased,up and down arrows indicate that the content of substances is increased and decreased,respectively.NAD+.Nicotinamide adenine dinucleotide;NADP+.Nicotinamide adenine dinucleotide phosphate;NADH.Reduced nicotinamide adenine dinucleotide phosphate;NADPH.Reduced nicotinamide adenine dinucleotide phosphate;FAD.Flavin adenine dinucleotide;FADH2.Reduced flavin dinucleotide.
综上所述,能量亏缺和氧自由基伤害导致的膜通透性改变,可能是苹果贮藏期间果肉褐变的主要原因,Ca2+等因为参与了细胞膜的稳定性,因而也对果实褐变有一定的影响,而无氧呼吸可以促进褐变的进程。
4 采后防止果肉褐变的措施
苹果的品种,采前的气候条件、采收期、土肥水管理、疏花疏果以及修剪等栽培技术措施都对苹果贮藏期间的果肉褐变有着明显的影响(见2.2 所述)。因此,选择不易褐变的苹果品种、适期采收、防止过度干旱以及合理负荷等都在一定程度上降低了果实贮藏期间果肉褐变的风险。
4.1 预冷
研究发现,采后及时预冷可以降低多种果蔬在贮藏过程中果肉褐变的发病率[59]。笔者在实践生产中也发现,苹果采后及时预冷,能有效减缓果实冷藏期间的果肉褐变,推测其机制可能是预冷可以快速降低果实的呼吸作用。这不仅减缓了果实成熟,推迟或减轻冷害的发生,还减少了呼吸产生的CO2过度积累带来伤害的风险。
4.2 贮藏温度
对苹果应用低温贮藏或气调贮藏时,应综合考虑果实的成熟度、采收期、品种特性等多种因素,以防止冷害造成的果肉褐变。对于富士等晚熟品种而言,适宜贮藏的温度为(-1~1)℃(机械冷藏)或(-0.5~0.5)℃(气调贮藏)。
4.3 气体成分
用于气调贮藏的苹果需注意库内气体浓度,以防止O2浓度过低或CO2浓度过高导致空洞型褐变、辐射型褐变的发生。尤其是对于环境CO2敏感的富士和维纳斯黄金,长期贮藏的这2 种苹果理想气体指标为CO2不超过1%,O2在5%左右。
4.4 外源物质
1-MCP 是目前国内外在富士等苹果品种上常用的保鲜处理技术措施。1-MCP 的使用方法和效果取决于苹果的品种、贮藏时间及贮藏温度等因素[60]。对富士苹果而言,1-MCP 的常用体积分数为0.5~1.0 μL·L-1,一般在常温(20~25 ℃)或低温(0~2 ℃)条件下处理12~24 h,就可以有效减轻果肉褐变,同时提高果实其他贮藏品质[36,61]。
5 展 望
目前,果肉褐变机制的研究仍集中于细胞膜区室化功能的丧失。然而,细胞膜衰老是果蔬采后成熟衰老过程中不可避免的自然事件。因此,启动细胞膜衰老的关键因子和关键步骤是什么,贮藏环境中哪些因素可以导致细胞膜衰老加速或者有利于保持细胞膜结构的稳定性,值得研究。特别是对于不同类型的果肉褐变,它们之间细胞膜结构和功能变化有什么差异;为什么会出现辐射型、弥散型、空洞型和果肉崩溃型等明显不同的褐变形式;这些不同褐变类型的发生,分别对应着哪些诱导果肉褐变的因素;它们之间有无共性的问题,存不存在互相影响的情况等,需要深入研究。只有深入研究和了解细胞膜衰老的机制、区分出不同褐变类型的诱导因素,才能更好地、有针对性地采取果肉褐变的防治措施。
对于有特殊需求,比如为追求贮藏期或者风味品质而特意早采或者晚采的苹果,以及需要特定贮藏环境或特定采后处理需求的苹果等,研究这些特定的农业技术措施或采前采后处理措施对果肉褐变发生规律的影响,探讨其影响机制,寻找有针对性的防控技术措施,也具有很大的实际应用价值。
针对发生果肉褐变的苹果外观无明显差异、仅靠肉眼无法准确判断是否发病及发病程度的情况,开发更加灵敏的基于声波、光波等原理的无损检测技术及分检设备,将有助于在苹果贮藏和出库、上市期间发生褐变果实的筛选分级,减少病果流入市场带来的经济和信誉损失。
根据生理状态、采收期和贮藏环境等的差异和果肉褐变发生的机制,筛选鉴定果肉褐变相关的特定生理成分(标志物),研究建立基于标志物的苹果果肉褐变发生的数学模型和预测预警技术,实现在苹果入库初期即对苹果贮藏期间果肉褐变发生情况进行预测,或者根据生物标志物对地区或果园整体果实冷藏期间果肉褐变发生的概率提出预测预警,都有助于贮藏企业提前采取有针对性的预防或控制措施,减轻因果肉褐变带来的风险和经济损失。
[1] 李永新,王超,王丽岩,茹磊,陈敬鑫.苹果采后贮藏褐变研究进展[J].渤海大学学报(自然科学版),2020,41(3):202-210.LI Yongxin,WANG Chao,WANG Liyan,RU Lei,CHEN Jingxin. Research progress on postharvest storage browning of apple[J]. Journal of Bohai University (Natural Science Edition),2020,41(3):202-210.
[2] 石建新,闫晓芳.苹果贮藏期间常见果肉褐变类型及防治[J].落叶果树,1995,27(3):35-36.SHI Jianxin,YAN Xiaofang.Types and control of common flesh browning of apple during storage[J]. Deciduous Fruits,1995,27(3):35-36.
[3] 王杰,里程辉,王颖达. 苹果贮藏期二氧化碳伤害及贮藏建议[J].北方果树,2019(3):50-51.WANG Jie,LI Chenghui,WANG Yingda. Carbon dioxide damage during apple storage and storage suggestions[J]. Northern Fruits,2019(3):50-51.
[4] 王志华,贾朝爽,王文辉,佟伟,姜云斌.低温贮藏对‘金红’苹果能量代谢和品质的影响[J].园艺学报,2020,47(12):2277-2289.WANG Zhihua,JIA Chaoshuang,WANG Wenhui,TONG Wei,JIANG Yunbin. Effects of low temperature storage on energy metabolism,related physiology and quality in‘Jinhong’apple fruit[J].Acta Horticulturae Sinica,2020,47(12):2277-2289.
[5] SABAM K,WATKINS C B.Flesh browning development of‘Empire’apple during a shelf life period after 1-methylcyclopropene(1-MCP)treatment and controlled atmosphere storage[J/OL].Scientia Horticulturae,2020,261:108938. DOI:10.1016/j.scienta.2019.108938.
[6] JAMES H,JOBLING J. The flesh browning disorder of‘Pink Lady’apples[J].New York Fruit Quarterly,2008,16(2):23-28.
[7] TONG C B S,CHANG H Y,BOLDT J K,et al. Diffuse flesh browning in‘Honeycrisp’apple fruit is associated with low temperatures during fruit growth[J]. HortScience,2016,51(10):1256-1264.
[8] JAMES H J,JOBLING J J. Contrasting the structure and morphology of the radial and diffuse flesh browning disorders and CO2 injury of‘Cripps Pink’apples[J]. Postharvest Biology and Technology,2009,53(1/2):36-42.
[9] JAMES H,JOBLING J,TANNER D. Investigating structural and physiological differences between radial and diffuse types of flesh browning in cripps pink apples[J]. Acta Horticulturae,2008,768:77-84.
[10] 李喜宏,宋壮兴,田勇,冯小元,曹恩义.新红星苹果贮藏前期高CO2处理的极限值[J].中国果树,1993(1):6-9.LI Xihong,SONG Zhuangxing,TIAN Yong,FENG Xiaoyuan,CAO Enyi.Limit value of high CO2 treatment in early storage of Xinhongxing apple[J].China Fruits,1993(1):6-9.
[11] 曲怡宁.苹果果实贮藏期二氧化碳伤害特性观察与研究[D].杨凌:西北农林科技大学,2019.QU Yining. Observation and study on CO2 damage characteristics of apple fruit during storage[D].Yangling:Northwest A&F University,2019.
[12] 福田博之,章祖涵.苹果果肉褐变的发生机制及其分类法[J].烟台果树,1982(3):51-57.FUTIAN Bozhi,ZHANG Zuhan. Mechanism and classification of apple flesh browning[J].Yantai Fruits,1982(3):51-57.
[13] 董启凤,杨克钦,肖永年.苹果的果内崩溃[J].辽宁果树,1986(1):49-52.DONG Qifeng,YANG Keqin,XIAO Yongnian. Intra fruit collapse of apple[J].Liaoning Fruits,1986(1):49-52.
[14] 刘兴华,陈维信.果品蔬菜贮藏运销学[M].北京:中国农业出版社,2002.LIU Xinghua,CHEN Weixin.Fruit&vegetable storage and transportation science[M].Beijing:China Agriculture Press,2002.
[15] HATOUM D,BUTS K,HERTOG M,GEERAERD A,SCHENK A,VERCAMMEN J,NICOLAI B. Effects of preand postharvest factors on browning in braeburn[J]. Horticultural Science,2018,41:19-26.
[16] SERRA S,ANTHONY B,BOSCOLO S F,MASIA A,MUSACCHI S.Determination of post-harvest biochemical composition,enzymatic activities,and oxidative browning in 14 apple cultivars[J].Foods,2021,10(1):186.
[17] DU M J,LIU Z T,ZHANG X T,LI H D,LIU Z Y,LI X H,SONG J X,JIA X Y,WANG L Y.Effect of pulsed controlled atmosphere with CO2 on the quality of watercored apple during storage[J/OL]. Scientia Horticulturae,2021,278:109854. DOI:10.1016/j.scienta.2020.109854.
[18] 姜云斌,王志华,贾朝爽.苹果水心病的发病机理研究进展[J].中国果树,2022(1):8-14.JIANG Yunbin,WANG Zhihua,JIA Chaoshuang. Research progress on pathogenesis of watercore in apple[J].China Fruits,2022(1):8-14.
[19] LAU O L. Effect of growing season,harvest maturity,waxing,low O2 and elevated CO2 on flesh browning disorders in‘Braeburn’apples[J]. Postharvest Biology and Technology,1998,14(2):131-141.
[20] MCCORMICK R J,BIEGERT K,STREIF J. Occurrence of physiological browning disorders in stored‘Braeburn’apples as influenced by orchard and weather conditions[J/OL].Postharvest Biology and Technology,2021,177:111534.DOI:10.1016/j.postharvbio.2021.111534.
[21] SAURE M C. Calcium translocation to fleshy fruit:its mechanism and endogenous control[J]. Scientia Horticulturae,2005,105(1):65-89.
[22] 王志华,姜云斌,贾朝爽,王文辉,刘伟.河北承德寒富苹果贮藏期果肉褐变原因分析与建议[J].果树实用技术与信息,2020(6):43-45.WANG Zhihua,JIANG Yunbin,JIA Chaoshuang,WANG Wenhui,LIU Wei. Analysis and suggestions on flesh browning of‘Hanfu’apple in Chengde,Hebei province during storage[J].Practical Technology and Information of Fruit Trees,2020(6):43-45.
[23] DE FREITAS S T,AMARANTE C V T D,LABAVITCH J M,MITCHAMA,E J.Cellular approach to understand bitter pit development in apple fruit[J]. Postharvest Biology and Technology,2010,57(1):6-13.
[24] ARGENTA L C,DO AMARANTE C V T,BETINELLI K S,BRANCHER T L,NESI C N,VIEIRA M J. Comparison of fruit attributes of‘Fuji’apple strains at harvest and after storage[J/OL]. Scientia Horticulturae,2020,272:109585. DOI:10.1016/j.scienta.2020.109585.
[25] KWEON H J,KANG I K,KIM M J,LEE J,MOON Y S,CHOI C,CHOI D G,WATKINS C B. Fruit maturity,controlled atmosphere delays and storage temperature affect fruit quality and incidence of storage disorders of‘Fuji’apples[J]. Scientia Horticulturae,2013,157:60-64.
[26] HIRSCHI K D. The calcium conundrum. Both versatile nutrient and specific signal[J].Plant Physiology,2004,136(1):2438-2442.
[27] 王雷. 苹果果实不同O2/CO2 简易气调贮藏CO2 伤害特性研究[D].杨凌:西北农林科技大学,2020.WANG Lei. Study on the injury characteristics of CO2 in different O2/CO2 storage of apple fruits[D]. Yangling:Northwest A&F University,2020.
[28] WOOD R,PROSKE M,DE FREITAS S D,SCHEER C,VÖGELE T,NEUWALD D A. Seasonal variation in calcium and ascorbic acid content at harvest related to internal browning in‘Braeburn’apple during controlled atmosphere storage[J].Scientia Horticulturae,2022,297:110943.
[29] PRETI R,TAROLA A M. Study of polyphenols,antioxidant capacity and minerals for the valorisation of ancient apple cultivars from Northeast Italy[J].European Food Research and Technology,2021,247(1):273-283.
[30] CORRÊA T R,STEFFENS C A,AMARANTE C V T D,MIQUELOTO A,BRACKMANN A,ERNANI P R. Multivariate analysis of mineral content associated with flesh browning disorder in‘Fuji’apples produced in Southern Brazil[J]. Bragantia,2017,76(2):327-334.
[31] FERGUSON I,VOLZ R,WOOLF A. Preharvest factors affecting physiological disorders of fruit[J]. Postharvest Biology and Technology,1999,15(3):255-262.
[32] KALCSITS L,MATTHEIS J,GIORDANI L,REID M,MULLIN K. Fruit canopy positioning affects fruit calcium and potassium concentrations,disorder incidence,and fruit quality for‘Honeycrisp’apple[J]. Canadian Journal of Plant Science,2019,99(5):761-771.
[33] KANAYAMA Y,KOCHETOV A.Abiotic stress biology in horticultural plants[M].Tokyo:Springer,2015:127-145.
[34] MUSACCHI S,SERRA S.Apple fruit quality:Overview on preharvest factors[J].Scientia Horticulturae,2018,234:409-430.
[35] DE CASTRO E,BARRETT D M,JOBLING J,MITCHAM E J.Biochemical factors associated with a CO2-induced flesh browning disorder of Pink Lady apples[J]. Postharvest Biology and Technology,2008,48(2):182-191.
[36] ARGENTA L,FAN X T,MATTHEIS J. Delaying establishment of controlled atmosphere or CO2 exposure reduces‘Fuji’apple CO2 injury without excessive fruit quality loss[J].Postharvest Biology and Technology,2000,20(3):221-229.
[37] LEE J,KANG I K,NOCK J F,WATKINS C B. Effects of preharvest and postharvest applications of 1-methylcyclopropene on fruit quality and physiological disorders of‘Fuji’apples during storage at warm and cold temperatures[J]. HortScience,2019,54(8):1375-1383.
[38] MIN D D,DONG L L,SHU P,CUI X X,ZHANG X H,LI F J.The application of carbon dioxide and 1-methylcyclopropene to maintain fruit quality of‘Niuxin’persimmon during storage[J].Scientia Horticulturae,2018,229:201-206.
[39] DIAS C,RIBEIRO T,ROBDRIGUES A C,FERRANTE A,VASCONCELOS A W,PINTADO M. Improving the ripening process after 1-MCP application:Implications and strategies[J].Trends in Food Science&Technology,2021,113:382-396.
[40] DEELL J R,LUM G B. Effects of low oxygen and 1-methylcyclopropene on storage disorders of‘Empire’apples[J]. Hort-Science,2017,52(9):1265-1270.
[41] JUNG S K,CHOI H S. Browning of early and late-harvested‘Empire’apples affected by cold storage and 1-MCP[J].Agronomy,2020,10(7):1050.
[42] NXUMALO K A,MATSUANE C,MASARIRAMBI M T. Calcium-related post-harvest physiological disorders of fruits and vegetables in eswatini:A review[J]. Current Journal of Applied Science and Technology,2019,33(6):1-10.
[43] ZHAO Y,WANG C. Effect of calcium chloride in combination with salicylic acid on post-harvest freshness of apples[J]. Food Science and Biotechnology,2015,24(3):1139-1146.
[44] 刘丽丹,吴仲珍,李育生,张璐.二苯胺控制采后果蔬生理病害的机制[J].保鲜与加工,2014,14(1):53-56.LIU Lidan,WU Zhongzhen,LI Yusheng,ZHANG Lu. The mechanism of diphenylamine on controlling physiological diseases of fruits and vegetables during storage[J]. Storage & Process,2014,14(1):53-56.
[45] GLAGOLEVAAY,SHOEVA O Y,KHLESTKINA E K.Melanin pigment in plants:Current knowledge and future perspectives[J].Frontiers in Plant Science,2020,11:770.
[46] TANG T T,XIE X F,REN X,WANG W J,TANG X M,ZHANG J,WANG Z D.A difference of enzymatic browning unrelated to PPO from physiology,targeted metabolomics and gene expression analysis in Fuji apples[J/OL]. Postharvest Biology and Technology,2020,170:111323. DOI:10.1016/j.postharvbio.2020.111323.
[47] AL SHOFFE Y,NOCK J F,BAUGHER T A,MARINI R P.WATKINS C B. Bitter pit and soft scald development during storage of unconditioned and conditioned‘Honeycrisp’apples in relation to mineral contents and harvest indices[J/OL]. Postharvest Biology and Technology,2020,160:111044. DOI:10.1016/j.postharvbio.2019.111044.
[48] 王田利.钙对苹果生长的影响及预防缺钙措施[J].西北园艺(果树),2020(5):31-32.WANG Litian. Effect of calcium on apple growth and measures to prevent calcium deficiency[J]. Northwest Horticulture,2020(5):31-32.
[49] 刘野,胡小松,张飞.二氧化碳导致鸭梨褐变与细胞内钙的关系[J].食品科学,2011,32(13):62-65.LIU Ye,HU Xiaosong,ZHANG Fei. Relationship between carbon dioxide-induced browning and intracellular calcium in Yali pears (Pyrus bretschneideri Rehd.)[J]. Food Science,2011,32(13):62-65.
[50] DE FREITAS S T,PAREEK S. Postharvest physiological disorders in fruits and vegetables[M].Boca Raton:CRC Press,2019:127-162.
[51] SCHMIDT R R,WEITS D A,FEULNER C F J,VAN DONGEN J T. Oxygen sensing and integrative stress signaling in plants[J].Plant Physiology,2018,176(2):1131-1142.
[52] DEUCHANDE T,CARVALHO S M P,GINÉ-BORDONABA J,VASCONCELOS M W,LARRIGAUDIÈRE C.Transcriptional and biochemical regulation of internal browning disorder in‘Rocha’pear as affected by O2 and CO2 concentrations[J].Postharvest Biology and Technology,2017,132:15-22.
[53] LIU X,FAN H M,LIU D H,LIU J,SHEN Y,ZHANG J,WEI J,WANG C L.Transcriptome and metabolome analyses provide insights into the watercore disorder on Akibae pear fruit[J].International Journal of Molecular Sciences,2021,22(9):4911.
[54] OLSON K R,GAO Y,DELEON E R,ARIF M,ARIF F,ARORA N,STRAUB K D. Catalase as a sulfide-sulfur oxido-reductase:An ancient (and modern?) regulator of reactive sulfur species(RSS)[J].Redox Biology,2017,12:325-339.
[55] CHEN C,JIANG A L,LIU C H,WAGSTAFF C,ZHAO Q,ZHANG Y,HU W. Hydrogen sulfide inhibits the browning of fresh-cut apple by regulating the antioxidant,energy and lipid metabolism[J/OL]. Postharvest Biology and Technology,2021,175:111487.DOI:10.1016/j.postharvbio.2021.111487.
[56] LEE J,CHENG L L,RUDELL D R,FOCK J F,WATKINS C B.Antioxidant metabolism in stem and calyx end tissues in relation to flesh browning development during storage of 1-methylcyclopropene treated‘Empire’apples[J]. Postharvest Biology and Technology,2019,149:66-73.
[57] LI Z L,LI B R,LI M Q,FU X D,ZHAO X M,MIN D D,LI F J,LI X A,ZHANG X H. Hot air pretreatment alleviates browning of fresh-cut pitaya fruit by regulating phenylpropanoid pathway and ascorbate-glutathione cycle[J].Postharvest Biology and Technology,2022,190:111954.
[58] WANG J W,JIANG Y G,LI G D,LÜ M,ZHOU X,ZHOU Q,FU W W,ZHANG L,CHEN Y F,JI U J.Effect of low temperature storage on energy and lipid metabolisms accompanying peel browning of‘Nanguo’pears during shelf life[J]. Postharvest Biology and Technology,2018,139:75-81.
[59] DUAN Y,WANG G B,FAWOLE O A,VERBOVEN P,ZHANG X R,WU D,OPARA U L,NICOLAI B,CHEN K S.Postharvest precooling of fruit and vegetables:A review[J].Trends in Food Science&Technology,2020,100:278-291.
[60] ZHANG X H,LI F J,ZHAI H,SHU H R.Advances in the mechanism of 1-MCP inhibiting ethylene action and its application in apple storage[J]. International Agricultural Engineering Journal,2011,20(2):135-140.
[61] LEE J,JEONG M C,KU K H. Chemical,physical,and sensory properties of 1- MCP- treated Fuji apple (Malus domestica Borkh.) fruits after long-term cold storage[J]. Applied Biological Chemistry,2017,60(4):363-374.