山楂叶螨(Tetranychus viennensis Zacher)又名山楂红蜘蛛,属于蛛形纲蜱螨目叶螨科,是中国北方地区苹果等落叶果树上的重要害虫[1]。山楂叶螨主要危害果树叶片、嫩梢和花萼,轻微危害时树体内膛叶片主脉两侧出现苍白色小点,影响光合作用,削弱树势,降低果实品质,严重危害时叶片干枯脱落,造成减产乃至绝收[2-3]。
果园中防治山楂叶螨主要依赖化学防治,化学农药浓度伴随时间推移不断降低,叶螨处于亚致死浓度环境中[4]。亚致死浓度对叶螨的生长发育、繁殖以及种群的扩散会产生明显的影响。国内与山楂叶螨亚致死效应研究相关的杀螨剂有螨酯、四螨嗪、阿维菌素、三唑锡、甲氰菊酯和螺虫乙酯等[5-7]。丙烯腈类杀螨剂乙唑螨腈和腈吡螨酯的杀螨机制是通过抑制线粒体呼吸链复合体Ⅱ起作用,与常见杀螨剂的作用机制不一致[8]。研究发现这2 种杀螨剂对柑橘全爪螨、二斑叶螨等叶螨的各种螨态均有良好的防治效果[9]。但是,乙唑螨腈和腈吡螨酯对山楂叶螨的亚致死效应研究尚未有报道。为全面了解乙唑螨腈和腈吡螨酯对果园害螨种群动态的影响,笔者在本研究中采用生命表法研究2种新型杀螨剂对山楂叶螨的亚致死效应,统计相应种群参数,并测定谷胱甘肽S-转移酶(glutathione S-transferase,GST)和羧酸酯酶(carboxylesterase,CarE)活性,以明确这2种杀螨剂对山楂叶螨亚致死效应,为科学应用新型杀螨剂提供理论依据。
1 材料和方法
1.1 供试材料
供试山楂叶螨采自中国农业科学院郑州果树研究所试验苹果园,采集后在室内使用苹果叶连续饲养10代以上。
1.2 主要仪器与试剂
30%腈吡螨酯悬浮液购自日产化学株式会社,30%乙唑螨腈悬浮剂购自沈阳科创化学品有限公司。紫外可见光光度计(上海菁华科技仪器有限公司,JH752)、匀浆研磨器、高速冷冻离心机(eppendorf,Centrifuge 5427R)、恒温水浴箱(上海精宏实验设备有限公司,HK-8D)、GST 酶活性检测试剂盒和CarE 酶活性检测试剂盒均购自北京盒子生工科技有限公司。
1.3 生物测定
采用叶碟喷雾法测定乙唑螨腈和腈吡螨酯室内毒力。用蒸馏水将制剂稀释5~7个浓度梯度。
1.3.1 卵毒力测定 海绵垫修剪为平整方形,充分吸水后放置于培养皿内,海绵上放置滤纸,将苹果叶片修剪为直径为25 mm圆形叶碟,苹果叶面向下放置在滤纸上,制成叶碟。培养皿内加蒸馏水,水面低于滤纸。在叶碟上接入状态一致的雌成螨10头,使其自由产卵24 h,检查统计叶片上卵粒数,每个处理3枚叶片,平均卵粒数在30粒左右。使用potter喷雾塔(Burkard,P=100 kPa,沉降1 min。下同)将药液雾化并均匀喷施到叶螨卵及叶片表面,晾干后放置到人工气候箱(温度为28 ℃±1 ℃,相对湿度为70%~80%,光周期为16 L∶8 D,下同)中观察孵化情况。
1.3.2 雌成螨毒力测定 同卵毒力方法制作相同叶碟。培养皿内加蒸馏水,水面低于滤纸。选择生长状态一致的雌成螨,接入到叶碟上,使用potter喷雾塔,将药液雾化并均匀喷施到叶螨及叶片表面,放置到人工气候箱中观察,24、48 h 后记录叶螨死亡情况。
1.4 亚致死效应
1.4.1 对卵的亚致死效应 在叶碟上接入交配后的雌成螨30头,自由产卵6 h,移除雌成螨。使用药剂亚致死剂量处理叶碟上卵,待卵孵化后,立即将幼螨移入新的叶碟,单头饲养。每个处理观察初孵幼螨50头,各处理剩余叶螨分别接入叶碟中相同条件下饲养备用,发育至成螨后接入雄螨(来自备用虫源)。每24 h观察并记录产卵量,收集当日所产卵继续饲养,明确卵的孵化率及雌雄比,直至所有成虫全部死亡。
1.4.2 对雌成螨的亚致死效应 在干净的叶碟中挑入生长一致、刚羽化1 d 之内的雌成螨50 头,静置1 h,待雌成螨稳定后使用potter 喷雾塔喷施亚致死剂量的药剂,晾干后放入人工气候箱。24 h 后挑选活泼的雌成螨接入新的叶碟中单头饲养,并为每个雌螨接入雄成螨。逐日记载成虫的产卵量,直至雌成虫全部自然死亡为止。将其所产的卵保留观察并记录孵化率及雌雄比。
1.5 解毒酶活性测定
1.5.1 成螨亚致死剂量酶活性测定 将处于产卵前期的山楂叶螨使用亚致死浓度药剂处理,后置于人工气候箱中,24 h 后收集活泼雌成螨120 头到2 mL 离心管中,使用液氮速冻,放入-80 ℃冰箱冷冻备用。
1.5.2 卵亚致死剂量酶活性测定 将产有山楂叶螨卵的叶碟喷施亚致死剂量药剂,将叶碟移入人工气候箱中,待卵孵化后接入新的叶碟,置于人工气候箱中,继续生长至产卵前期,收集活泼雌成螨120头到2 mL离心管中,使用液氮速冻,放入-80 ℃冰箱冷冻备用。研磨前加入相应试剂盒的提取液,冰浴匀浆,匀浆液在4 ℃、12 000 r·min-1离心10 min,取20µL上清液用蒸馏水将其稀释5倍。后续步骤按GST和CarE检测试剂盒说明书进行。
1.6 数据分析
采用DPS12.0s软件计算毒力回归方程,确定亚致死剂量。生命表的组建参照耿书宝等[10]和涂洪涛等[11]的方法进行,按照下列公式计算种群生命表参数:周限增长率(λ):λ=erm;内禀增长率(rm):rm=lnR0/T;净增殖率(R0):R0=∑lxmx;种群加倍时间(DT):DT=ln(2)/rm,平均世代时间(T):T=∑xlxmx/R0。上述公式中,x表示雌成虫的日龄,lx表示在x日龄内雌成虫的存活率,mx 表示雌成虫在x 日龄内平均产雌量。使用SPSS 19.0 软件对山楂叶螨各处理的种群生命表参数进行显著性检验。
2 结果与分析
2.1 亚致死浓度的测定
毒力测定结果见表1,乙唑螨腈和腈吡螨酯对山楂叶螨的雌成螨和卵均有较强毒力。乙唑螨腈对山楂叶螨雌成螨的LC50 为4.991 0 mg·L- 1,LC15 为1.499 7 mg·L-1,LC30 为2.714 4 mg·L-1;乙唑螨腈对山楂叶螨卵的LC50 为3.765 9 mg·L-1,LC15 为1.033 7 mg·L-1,LC30为1.957 9 mg·L-1;乙唑螨腈对卵的毒力高于成螨。腈吡螨酯对山楂叶螨雌成螨的LC50为1.702 6 mg·L-1,LC15为0.782 7 mg·L-1,LC30为1.149 1 mg·L-1;腈吡螨酯对山楂叶螨卵的LC50为9.485 0 mg·L- 1,LC15 为4.077 5 mg·L- 1,LC30 为6.188 7 mg·L-1;腈吡螨酯对成螨的毒力强于卵。乙螨唑腈对雌成螨的毒力弱于腈吡螨酯,但乙螨唑腈对卵的毒力强于腈吡螨酯。
表1 乙唑螨腈和腈吡螨酯对山楂叶螨雌成螨和卵的室内毒力
Table 1 Toxicities of cyetpyrafen and cyenopyrafen to adult and egg of Tetranychus viennensis
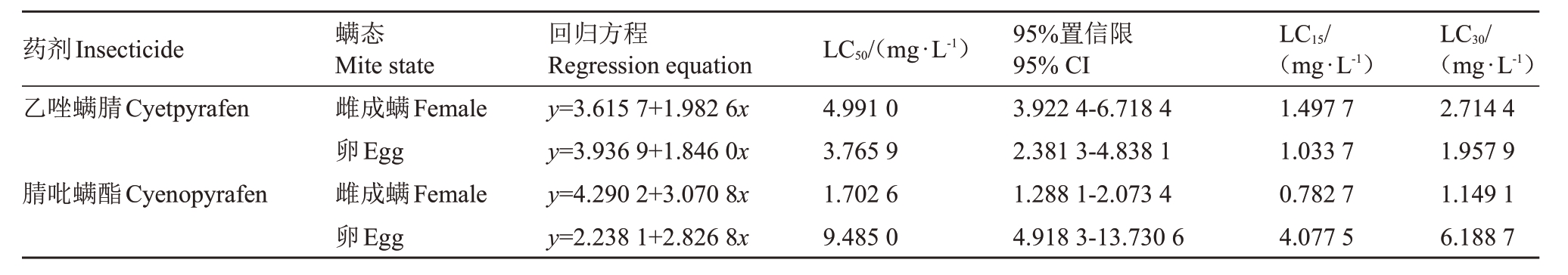
药剂Insecticide乙唑螨腈Cyetpyrafen腈吡螨酯Cyenopyrafen螨态Mite state雌成螨Female卵Egg雌成螨Female卵Egg回归方程Regression equation y=3.615 7+1.982 6x y=3.936 9+1.846 0x y=4.290 2+3.070 8x y=2.238 1+2.826 8x LC50/(mg·L-1)4.991 0 3.765 9 1.702 6 9.485 0 95%置信限95%CI 3.922 4-6.718 4 2.381 3-4.838 1 1.288 1-2.073 4 4.918 3-13.730 6 LC15/(mg·L-1)1.497 7 1.033 7 0.782 7 4.077 5 LC30/(mg·L-1)2.714 4 1.957 9 1.149 1 6.188 7
2.2 亚致死浓度处理卵对F0代成螨的影响
乙唑螨腈和腈吡螨酯亚致死剂量处理山楂叶螨卵后F0代雌成螨的产卵量、雌成螨寿命、子代卵孵化率和雌雄比结果见表2。在卵期喷施2 种杀螨剂的亚致死浓度,雌成螨寿命与对照相比无显著差异;乙唑螨腈LC15处理卵显著提高了单头叶螨平均总产卵量,相比对照组增加到41.26粒;乙唑螨腈LC30处理也提高了总产卵量,增加到37.44粒,但同对照差异不显著。腈吡螨酯2种亚致死浓度处理卵对雌成螨的产卵量无显著影响,在卵期喷施亚致死剂量后对成螨所产卵的孵化率和子代雌雄比相较于对照组无显著差异。
表2 乙唑螨腈和腈吡螨酯亚致死剂量处理卵对山楂叶螨雌成螨生物学特性的影响
Table 2 Effects of sublethal doses of cyetpyrafen and cyenopyrafen on biological characteristics of Tetranychus viennensis after egg treated

注:不同小写字母表示处理间差异显著(p<0.05)。下同。
Note:Different same letters indicate significant difference p<0.05.The same below.
处理Treatment乙唑螨腈LC15Cyetpyrafen LC15乙唑螨腈LC30Cyetpyrafen LC30腈吡螨酯LC15Cyenopyrafen LC15腈吡螨酯LC30 Cyenopyrafen LC30对照CK子代雌雄比Sex ratio 4.511 4±0.586 7 ab 3.437 9±0.536 9 a 3.118 7±0.894 1 a 4.762 5±1.296 8 ab 5.948 7±1.300 9 b雌成螨寿命Adult longevity/d 10.36±1.19 a 10.76±1.19 a 10.43±2.58 a 10.63±0.60 a 9.99±1.79 a每雌产卵量Eggs per female 41.26±5.75 a 37.44±9.68 ab 28.00±9.13 b 25.53±4.03 b 26.51±3.11 b子代卵孵化率F1 hatchability/%77.92±4.41 a 81.18±8.48 a 74.15±7.74 a 76.94±2.02 a 86.47±5.27 a
从表3可知,乙唑螨腈和腈吡螨酯亚致死剂量LC15和LC30处理山楂叶螨卵后,与对照相比,乙唑螨腈LC15处理种群的净增殖率显著提高,乙唑螨腈LC30处理种群净增殖率也提高,但差异不显著,世代平均历期显著延长,这2 种处理在内禀增长率、周限增长率和种群加倍时间上并无差异;腈吡螨酯LC15和LC30处理后种群内禀增长率、周限增长率和种群加倍时间与对照组和乙唑螨腈处理组相比均无显著差异,净增殖率与对照组无显著差异。腈吡螨酯LC30处理世代平均历期与对照相比显著缩短。
表3 乙唑螨腈和腈吡螨酯亚致死剂量处理卵对山楂叶螨种群生命表参数的影响
Table 3 Effects of sublethal doses of cyetpyrafen and cyenopyrafen on life table parameters of Tetranychus viennensis after egg treated

处理Treatment乙唑螨腈LC15 Cyetpyrafen LC15乙唑螨腈LC30 Cyetpyrafen LC30腈吡螨酯LC15 Cyenopyrafen LC15腈吡螨酯LC30 Cyenopyrafen LC30对照CK净增殖率Net reproductiverate,R0 33.647 8±4.687 1 a世代平均历期Mean generation time,T 15.28±0.62 ab内禀增长率Intrinsic rate of increase,rm 0.229 7±0.007 8 a周限增长率Finite rate of increase,λ 1.258 3±0.009 8 a种群加倍时间Population doubling time,t 3.019 2±0.102 4 a 29.091 8±7.519 3 ab 15.69±0.55 a 0.213 5±0.021 5 a 1.238 2±0.026 5 a 3.269 3±0.350 7 a 21.079 0±6.844 3 b 13.02±0.47 c 0.231 7±0.035 4 a 1.261 2±0.044 3 a 3.042 4±0.501 1 a 21.065 2±3.322 5 b 15.44±0.51 ab 0.196 8±0.012 3 a 1.217 6±0.015 0 a 3.529 5±0.218 7 a 22.529 4±2.641 5 b 14.39±0.87 b 0.216 3±0.004 9 a 1.241 5±0.006 1 a 3.204 5±0.0738 5 a
2.3 亚致死剂量处理成螨对F0代雌成螨的影响
乙唑螨腈和腈吡螨酯亚致死剂量LC15和LC30处理山楂叶螨成螨后F0代雌成螨的产卵量、雌成螨寿命、子代卵孵化率和雌雄比结果见表4。相比于对照组,对雌成螨喷施2 种农药的不同亚致死浓度都缩短雌成螨的寿命,且喷施剂量越高雌成螨的寿命缩短的越多。乙唑螨腈LC30和腈吡螨酯LC30处理同对照相比均显著缩短,腈吡螨酯LC30处理的雌成螨寿命只有5.43 d。乙唑螨腈LC30和腈吡螨酯LC30处理后平均总产卵量分别降低为13.58 粒和12.95 粒,都显著低于对照组;2 种药LC15处理也降低平均总产卵量但相对于对照差异不显著。乙唑螨腈LC30和腈吡螨酯LC30处理后子代卵的孵化率也显著降低。乙唑螨腈LC30降低了子代雌雄比。
表4 乙唑螨腈和腈吡螨酯亚致死剂量处理成螨对山楂叶螨雌成螨生物学特性的影响
Table 4 Effects of sublethal doses of cyetpyrafen and cyenopyrafen on biological characteristics of Tetranychus viennensis after female adult mite treated
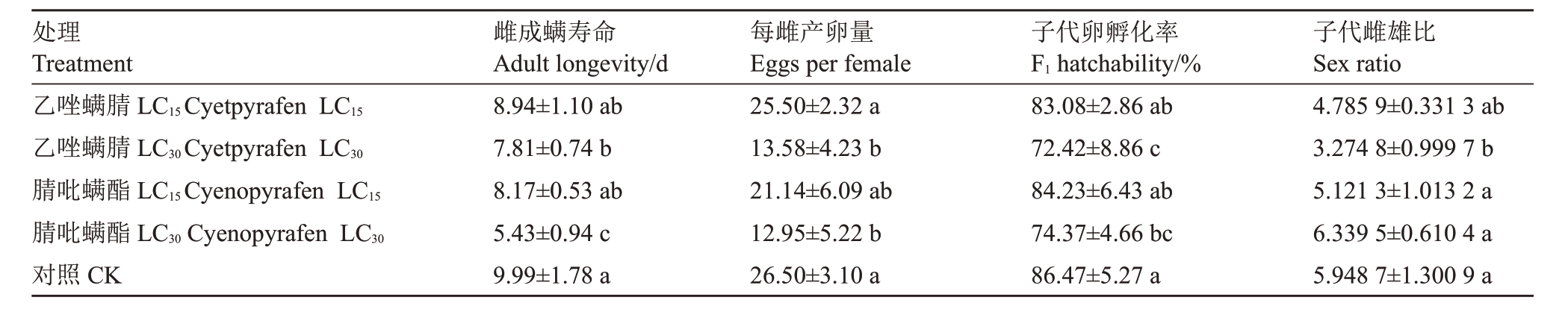
处理Treatment乙唑螨腈LC15Cyetpyrafen LC15乙唑螨腈LC30Cyetpyrafen LC30腈吡螨酯LC15Cyenopyrafen LC15腈吡螨酯LC30 Cyenopyrafen LC30对照CK雌成螨寿命Adult longevity/d 8.94±1.10 ab 7.81±0.74 b 8.17±0.53 ab 5.43±0.94 c 9.99±1.78 a每雌产卵量Eggs per female 25.50±2.32 a 13.58±4.23 b 21.14±6.09 ab 12.95±5.22 b 26.50±3.10 a子代卵孵化率F1 hatchability/%83.08±2.86 ab 72.42±8.86 c 84.23±6.43 ab 74.37±4.66 bc 86.47±5.27 a子代雌雄比Sex ratio 4.785 9±0.331 3 ab 3.274 8±0.999 7 b 5.121 3±1.013 2 a 6.339 5±0.610 4 a 5.948 7±1.300 9 a
从表5可知,与对照相比,乙唑螨腈LC15处理后世代历期显著减少,净增殖率、内禀增长率、周限增长率和种群加倍时间差异不显著。乙唑螨腈LC30处理后,净增殖率显著降低,种群加倍时间增加,世代平均历期、内禀增长率和周期增长率均降低,但与对照比差异不显著。腈吡螨酯LC15 和LC30 处理后,LC30的净增殖率和世代平均历期显著降低,其他种群生命表参数变化不显著。
表5 乙唑螨腈和腈吡螨酯亚致死剂量处理雌成螨对山楂叶螨种群生命表参数的影响
Table 5 Effects of sublethal doses of cyetpyrafen and cyenopyrafen on life table parameters of Tetranychus viennensis after female adult mite treated
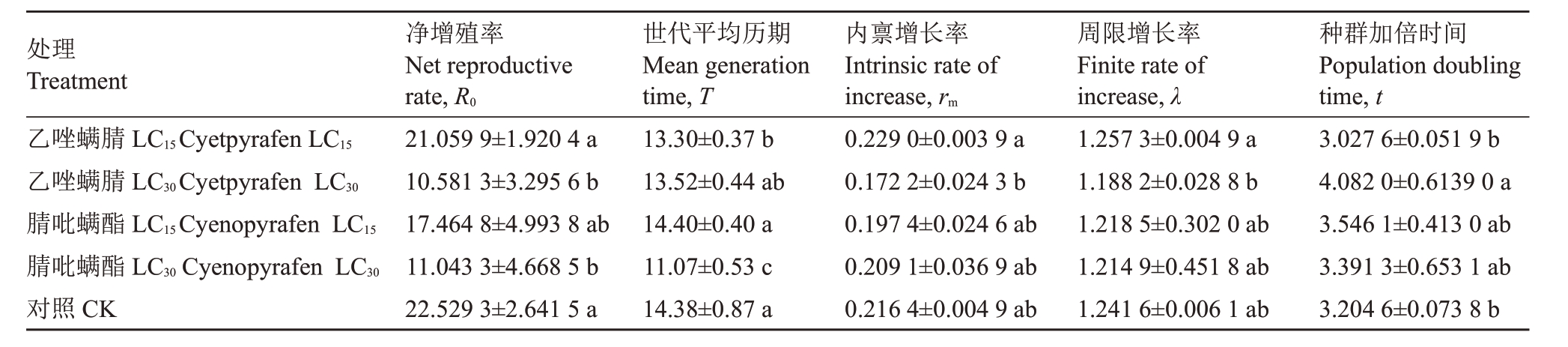
处理Treatment乙唑螨腈LC15Cyetpyrafen LC15乙唑螨腈LC30Cyetpyrafen LC30腈吡螨酯LC15Cyenopyrafen LC15腈吡螨酯LC30 Cyenopyrafen LC30对照CK净增殖率Net reproductive rate,R0 21.059 9±1.920 4 a 10.581 3±3.295 6 b 17.464 8±4.993 8 ab 11.043 3±4.668 5 b 22.529 3±2.641 5 a世代平均历期Mean generation time,T 13.30±0.37 b 13.52±0.44 ab 14.40±0.40 a 11.07±0.53 c 14.38±0.87 a内禀增长率Intrinsic rate of increase,rm 0.229 0±0.003 9 a 0.172 2±0.024 3 b 0.197 4±0.024 6 ab 0.209 1±0.036 9 ab 0.216 4±0.004 9 ab周限增长率Finite rate of increase,λ 1.257 3±0.004 9 a 1.188 2±0.028 8 b 1.218 5±0.302 0 ab 1.214 9±0.451 8 ab 1.241 6±0.006 1 ab种群加倍时间Population doubling time,t 3.027 6±0.051 9 b 4.082 0±0.6139 0 a 3.546 1±0.413 0 ab 3.391 3±0.653 1 ab 3.204 6±0.073 8 b
由图1和图2可知,在卵期和产卵前期喷施亚致死浓度杀螨剂对雌成螨期产卵高峰的出现时间影响并不明显,但是卵期喷亚致死剂量的药,其日均最高产卵量有所增加,且产卵高峰期维持的时间更长。
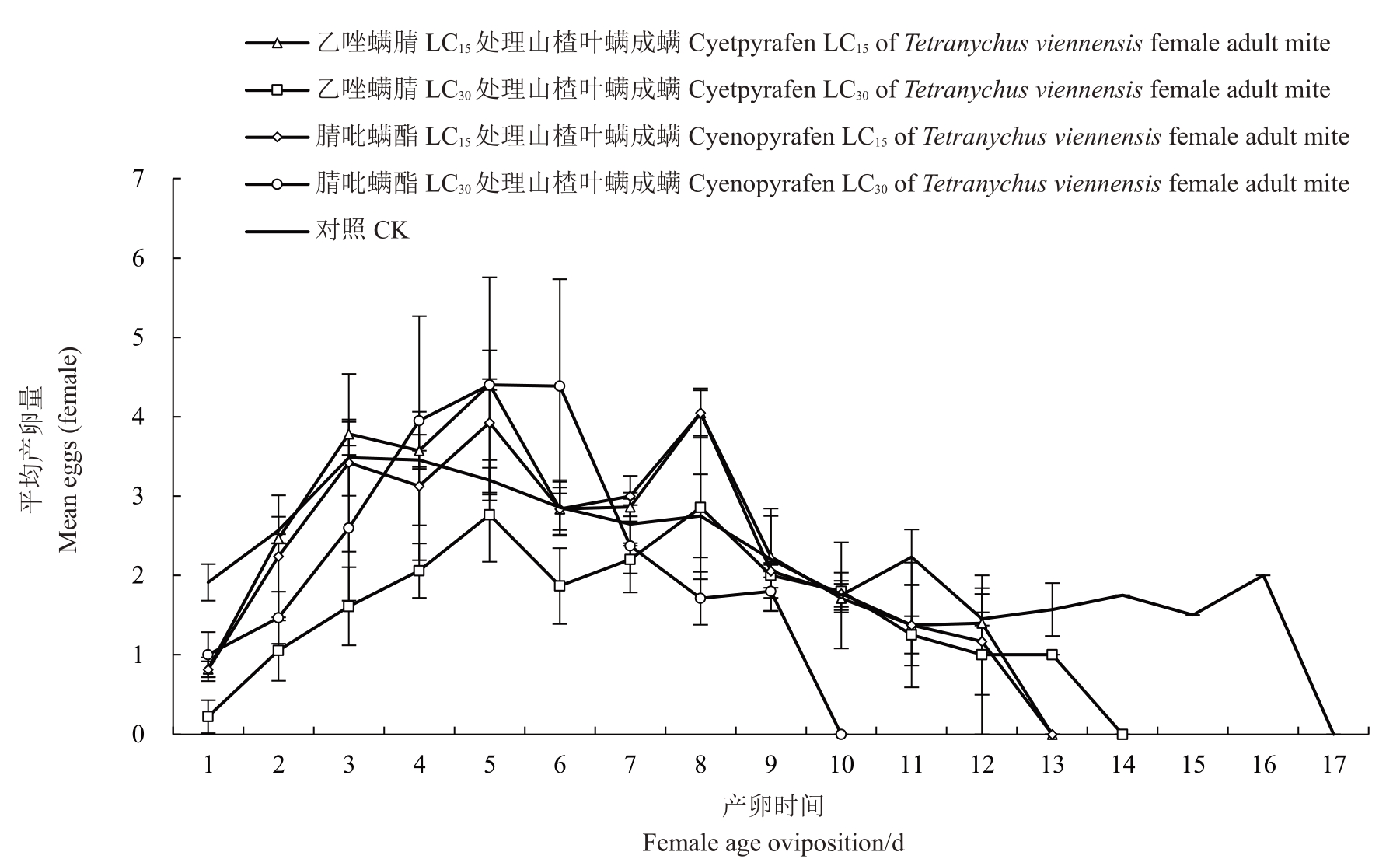
图1 乙唑螨腈和腈吡螨酯亚致死浓度处理对山楂叶螨雌成螨日均产卵量的影响
Fig.1 Effects of sublethal doses of cyetpyrafen and cyenopyrafen on survival rate(lx)and age-specific fecundity(mx)of Tetranychus viennensis after female adult mite treated
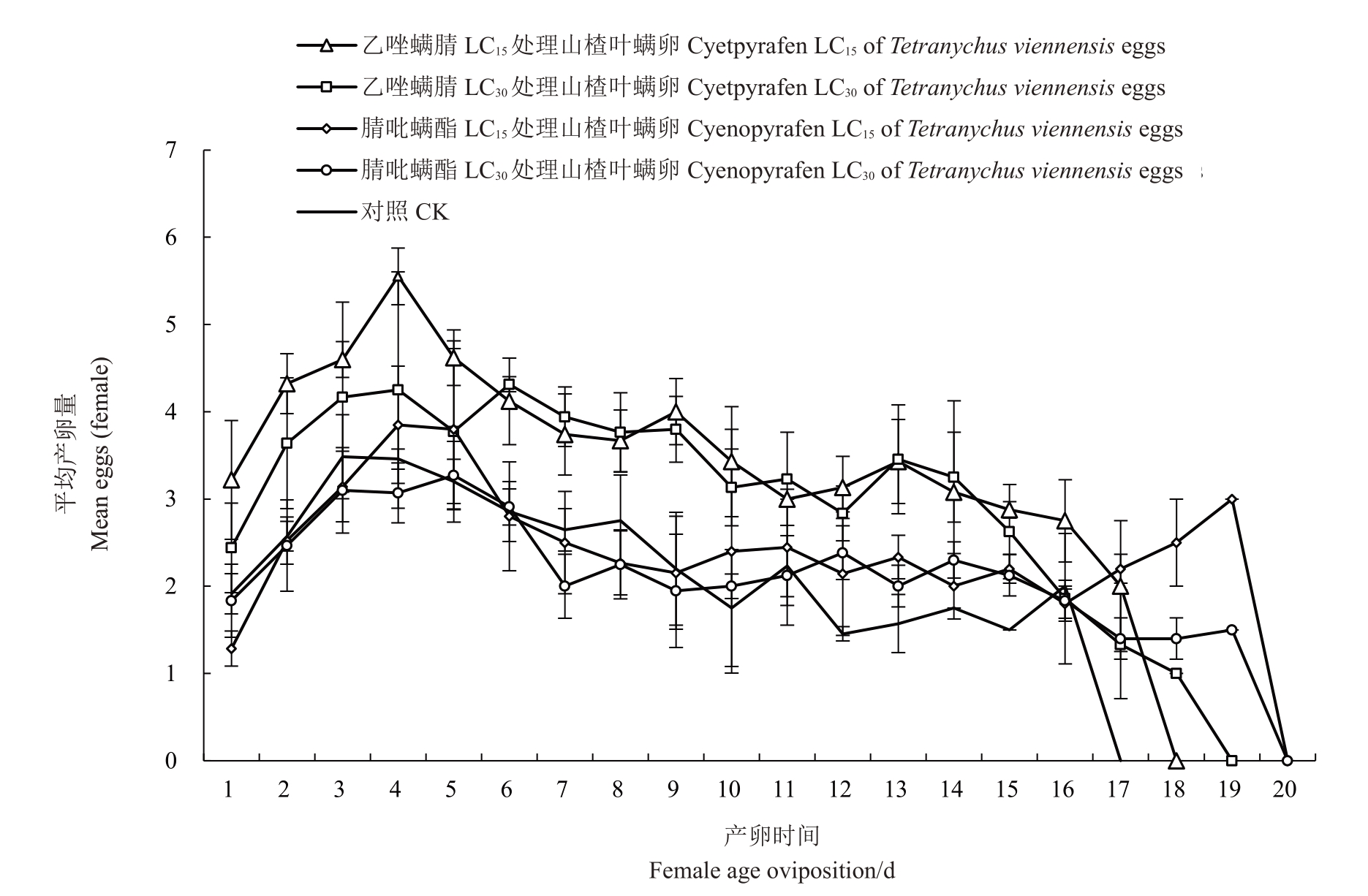
图2 乙唑螨腈和腈吡螨酯亚致死浓度处理卵对山楂叶螨雌成螨期日均产卵量的影响
Fig.2 Effects of sublethal doses of cyetpyrafen and cyenopyrafen on survival rate(lx)and age-specific fecundity(mx)of Tetranychus viennensis after eggs treated
2.4 解毒酶测定
如图3和图4所示,乙唑螨腈和腈吡螨酯的2种亚致死剂量LC15和LC30处理成螨和卵后,叶螨体内的GST酶活性都下降,除乙唑螨腈LC15处理成螨组未达到显著差异水平外,其余各处理相对于对照组均有显著差异,腈吡螨酯亚致死剂量LC15和LC30处理成螨后酶活性最低,均只有3.87 U·g-1。乙唑螨腈和腈吡螨酯亚致死剂量LC15和LC30各处理CarE 酶活性相对于对照组都升高。2种药剂亚致死剂量处理成螨后,乙唑螨腈LC30处理达到显著差异水平,其余组差异不显著;处理卵后,各处理的组酶活性显著提高,卵处理相比于成螨期处理酶活性提高更多,乙唑螨腈LC30处理组酶活性最高(247.20 U·g-1)。
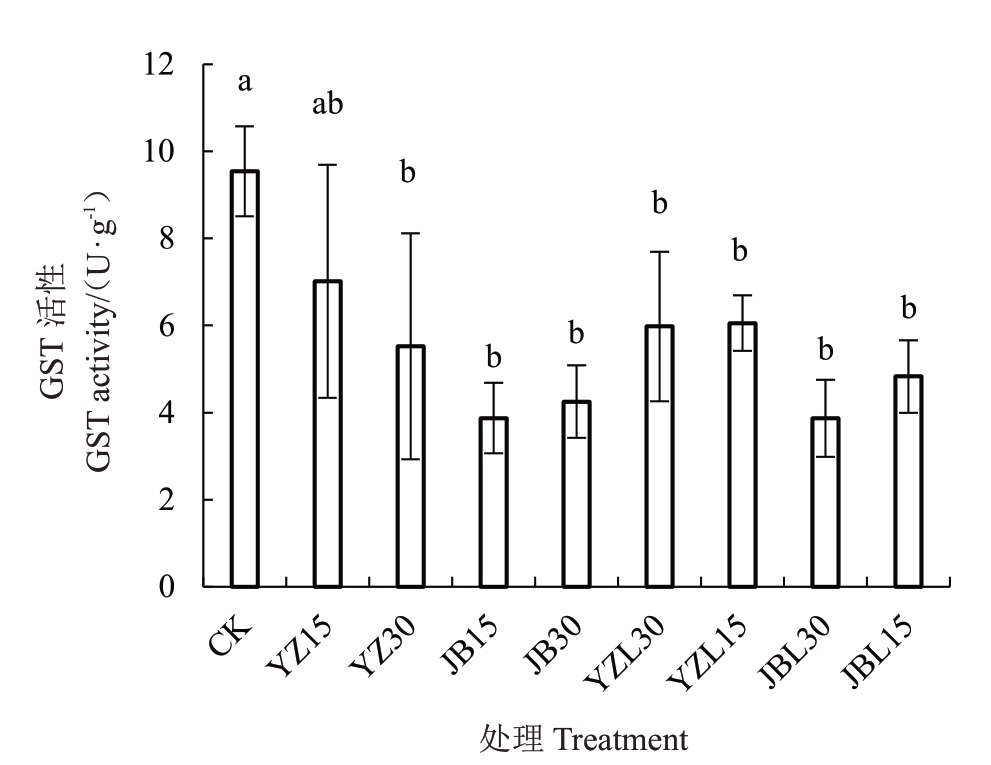
图3 乙唑螨腈和腈吡螨酯致死浓度处理成螨和卵对山楂叶螨雌成螨期谷胱甘肽S-转移酶活性的影响
Fig.3 Effects of sublethal doses of cyetpyrafen and cyenopyrafen on GST of Tetranychus viennensis after eggs and female adult mite treated
YZ 表示乙唑螨腈处理山楂叶螨成螨,YZL 表示乙唑螨腈处理山楂叶螨卵;JB 表示腈吡螨酯处理山楂叶螨成螨,JBL 表示腈吡螨酯处理山楂叶螨卵。15 和30 表示相应的亚致死浓度。下同。
YZ represents the female adult mite treated with Cyetpyrafen, and YZL represents the egg treated with Cyetpyrafen;JB represents the female adult mite treated with Cyenopyrafen, JBL represents the egg treated with Cyenopyrafen. 15 and 30 represent the corresponding sublethal concentration.The same below.

图4 乙唑螨腈和腈吡螨酯致死浓度处理成螨和卵对山楂叶螨雌成螨期羧酸酯酶活性的影响
Fig.4 Effects of sublethal doses of cyetpyrafen and cyenopyrafen on CarE of Tetranychus viennensis after eggs and female adult mite treated
3 讨 论
农药对害虫的致死剂量并不能准确预测其对害虫未来生长发育繁殖的影响。田间施用农药后,短期内昆虫处于致死剂量的状况,随着时间推移必定会处于一种亚致死剂量的状态[12]。亚致死剂量下生命表试验是评估农药毒性更有效的方法,生命表可以显示杀虫剂对幸存个体的影响,提供对种群增长率影响的衡量标准[13-14]。乙唑螨腈和腈吡螨酯以往主要在柑橘全爪螨上使用,近些年也增加登记在二斑叶螨和山楂叶螨上使用。笔者在本研究中发现,在山楂叶螨卵期喷施亚致死剂量LC15和LC30的乙唑螨腈,F0代产卵总量增加和净增殖率提高,同对照组相比达到显著差异水平,与程明明等[15]使用乙唑螨腈亚致死处理柑橘全爪螨后每雌产卵量增加,净增殖率升高但未达到显著差异水平的结论有相似性,而与田亚静等[16]研究发现乙唑螨腈亚致死浓度处理朱砂叶螨卵,总产卵量和净增殖率都降低的结论相反,这可能是由于2 种不同种螨之间的差异。腈吡螨酯的亚致死剂量LC15和LC30处理卵,世代平均历期相比于对照显著缩短,与程明明等[15]腈吡螨酯处理柑橘全爪螨的结论一致。笔者在本研究中,在雌成螨产卵前期喷施亚致死剂量的农药,F0代的产卵量和雌成螨寿命都降低,2 种杀螨剂的LC30亚致死剂量处理组同对照组相比均达到显著差异水平,这与徐淑等[17]在荔枝叶螨上的结论一致。李定旭等[5]发现,山楂叶螨中成螨接触亚致死剂量的甲氰菊酯后寿命缩短、产卵量减少;而若螨接触LC10剂量的甲氰菊酯后生殖力提高,这和乙唑螨腈处理山楂叶螨雌成螨和卵的结论有一定相似性。徐学农等[6]使用唑螨酯LC50处理山楂叶螨成螨同样刺激了雌成螨的产卵。这些现象是亚致死剂量刺激效应的表现[18]。
亚致死剂量的农药使用后可以诱导害虫对农药产生过度补偿反应称为亚致死剂量刺激效应[19],叶螨表现出亚致死剂量刺激效应或与其体内的酶活性的变化有关[20]。节肢动物主要解毒酶系有GST、CarE、多功能氧化酶和细胞色素P450 单加氧酶等,这些酶活性增强使节肢动物代谢抗性增强[21-22]。GST是昆虫体内常见的解毒酶,通过催化谷胱甘肽(glutathione,GSH)与环氧化合物结合,转化除草剂和杀虫剂[23]。CarE 通过过量表达增强对外源性化合物的水解作用实现解毒功能[24]。测定这2种在解毒和代谢途径中起到重要作用的酶,了解亚致死剂量的杀螨剂对山楂叶螨的生理影响意义重大。在各处理中,GST酶活性相较于对照都显著降低。昆虫接受到农药处理后,体内酶活性的变化是一个动态的过程,会随着时间的延长升高或降低。如辣椒碱类物质亚致死剂量处理朱砂叶螨后GST 酶活性低于对照组,在氯虫苯甲酰胺和甲维盐处理草地贪夜蛾幼虫后,GST酶活性随时间表现出诱导-抑制-诱导的现象[25-26]。笔者在本研究中选择药剂处理后24 h 这一个时间节点的酶,在某一个时间点可能存在着抑制或激活的现象,会随着时间变化而表现不同的结果。而CarE酶的活性相较于对照升高,乙唑螨腈卵期亚致死浓度处理后酶活性最高,这可能是引起亚致死剂量刺激效应的原因。
笔者在本试验中只做了2 个亚致死剂量,以及成螨和卵2 种螨态,事实上田间叶螨发生时是不同螨态共存,农药施用后是从高浓度逐渐向低浓度发展,在下一步研究中应当结合田间试验进行种群动态的调查,进一步深入研究2 种药剂对山楂叶螨的影响。当前新型杀螨剂乙唑螨腈和腈吡螨酯正在田间进行推广应用,笔者为2 种杀螨剂的合理轮换使用和叶螨的抗药性治理提供了理论依据。
4 结 论
乙唑螨腈和腈吡螨酯亚致死剂量处理山楂叶螨雌成螨抑制种群的发展。乙唑螨腈亚致死剂量处理山楂叶螨卵促进种群的繁殖,腈吡螨酯亚致死剂量处理山楂叶螨卵对种群则无影响。
[1] 蒋立奔,曹荣祥,童晓利,陈月红,韩金龙,郭成宝.不同杀螨剂对二斑叶螨的室内毒力及田间防效[J]. 江苏农业科学,2019,47(23):116-118.JIANG Liben,CAO Rongxiang,TONG Xiaoli,CHEN Yuehong,HAN Jinlong,GUO Chengbao.Indoor toxicity test and field efficacy of different acaricides against Tetranychus urticae Koch[J].Jiangsu Agricultural Sciences,2019,47(23):116-118.
[2] 封云涛,魏明峰,郭晓君,张润祥,庾琴,范仁俊.三种杀螨剂对山楂叶螨的毒力评价[J].植物保护学报,2018,45(3):640-646.FENGYuntao,WEI Mingfeng,GUO Xiaojun,ZHANG Runxiang,YU Qin,FAN Renjun. Toxicity evaluation of three acaricides against hawthorn spider mite Amphitetranychus viennensis Zacher[J].Journal of Plant Protection,2018,45(3):640-646.
[3] 蒋世铮,任德新,张文忠,赛买提江.香梨园山楂叶螨种群动态的研究[J].植物保护,2012,38(4):54-56.JIANG Shizheng,REN Dexin,ZHANG Wenzhong,Smtjiang.Population dynamics of Amphitetranychus viennensis in Korla pear orchards[J].Plant Protection,2012,38(4):54-56.
[4] 古德就,余明恩,侯任环,李哲怀.农药亚致死剂量对菜蚜茧蜂搜索行为影响的研究[J].生态学报,1991,11(4):324-330.GU Dejiu,YU Ming’en,HOU Renhuan,LI Zhehuai. The effects of sublethal doses of insecticides on the foraging behaviour of parasitoid,Diaeretiella rapae (Hym.:Braconidae)[J]. Acta Ecologica Sinica,1991,11(4):324-330.
[5] 李定旭,田娟,沈佐锐.不同药剂对山楂叶螨的亚致死效应[J].植物保护学报,2006,33(2):187-192.LI Dingxu,TIAN Juan,SHEN Zuorui. Sublethal effects of selected insecticides on the hawthorn spider mite,Tetranychus viennensis[J].Journal of Plant Protection,2006,33(2):187-192.
[6] 徐学农,王刚,高仕朋.杀螨王的亚致死浓度处理桃叶对山楂叶螨雌成螨生殖的影响[J].安徽农业大学学报,1998,25(4):352-355.XU Xuenong,WANG Gang,GAO Shipeng. Effects of sublethal concentration of fenpyroximate on reproduction of Tetranychus viennensis Zacher[J]. Journal of Anhui Agricultural University,1998,25(4):352-355.
[7] 张坤鹏,武海斌,宫庆涛,孙永川,孙瑞红.螺虫乙酯对山楂叶螨种群的影响[J].果树学报,2015,32(4):689-695.ZHANG Kunpeng,WU Haibin,GONG Qingtao,SUN Yongchuan,SUN Ruihong.Effects of spirotetramat on Tetranychus viennensis population[J]. Journal of Fruit Science,2015,32(4):689-695.
[8] FURUYA T,MACHIYA K,FUJIOKA S,NAKANO M,INAGAKI K. Development of a novel acaricide,pyflubumide[J].Journal of Pesticide Science,2017,42(3):132-136.
[9] 张俊龙,刘少武,冯聪,宋玉泉.30%乙唑螨腈·螺螨酯悬浮剂对不同害螨的药效试验[J].农药,2022,61(4):305-308.ZHANG Junlong,LIU Shaowu,FENG Cong,SONG Yuquan.Control effects of cyetpyrafen·spirodiclofens 30% SC against different mite targets in field[J]. Agrochemicals,2022,61(4):305-308.
[10] 耿书宝,侯贺丽,何帅洁,刘甲浩,王国君,尹健,乔利.温度对灰茶尺蛾实验种群生命表参数的影响[J]. 福建农业学报,2021,36(5):572-577.GENG Shubao,HOU Heli,HE Shuaijie,LIU Jiahao,WANG Guojun,YIN Jian,QIAO Li. Effect of temperature on life table parameters of Ectropis grisescens experimental populations[J].Fujian Journal of Agricultural Sciences,2021,36(5):572-577.
[11] 涂洪涛,张金勇,陈汉杰.毒死蜱亚致死剂量对二斑叶螨实验种群动态的影响[J].应用昆虫学报,2016,53(1):83-88.TU Hongtao,ZHANG Jinyong,CHEN Hanjie. Sublethal effect of chlorpyrifos on the population dynamics of an experimental Tetranychus urticae Koch population[J]. Chinese Journal of Applied Entomology,2016,53(1):83-88.
[12] 韩文素,王丽红,孙婳婳,高希武.杀虫剂对昆虫的亚致死效应的研究进展[J].中国植保导刊,2011,31(11):15-20.HAN Wensu,WANG Lihong,SUN Huahua,GAO Xiwu. Research progress on sublethal effects of insecticides on insect[J].China Plant Protection,2011,31(11):15-20.
[13] STARK J D,BANKS J E. Population-level effects of pesticides and other toxicants on arthropods[J]. Annual Review of Entomology,2003,48:505-519.
[14] CHITGAR M G,KHOSRAVI R,JALALISENDI J,GHADAMYARI M.Sublethal effects of Thymus vulgaris essential oil on life-table parameters of two-spotted spider mite,Tetranychus urticae Koch (Acari:Tetranychidae)[J].Archives of Phytopathology and Plant Protection,2013,46(7):781-788.
[15] 程明明,成禄艳,王莉,傅云梅,雷双,丁莉莉,方云洪,魏志堂,于士将,丛林,冉春.乙唑螨腈和腈吡螨酯对柑橘全爪螨的亚致死效应[J].果树学报,2021,38(5):782-791.CHENG Mingming,CHENG Luyan,WANG Li,FU Yunmei,LEI Shuang,DING Lili,FANG Yunhong,WEI Zhitang,YU Shijiang,CONG Lin,RAN Chun.Sublethal effects of cyetpyrafen and cyenopyrafen on Panonychus citri(Acari:Tereanychidae)[J].Journal of Fruit Science,2021,38(5):782-791.
[16] 田亚静.乙唑螨腈和丁氟螨酯对朱砂叶螨及加州新小绥螨的亚致死效应研究[D].雅安:四川农业大学,2017.TIAN Yajing. Sublethal effects of B-azolemiteacrylic and cyflumetofen on Tetranychus cinnabarinus Boisduval and Neoseiulus californicus(McGregor)[D].Yaan:Sichuan Agricultural University,2017.
[17] 徐淑,贾涛,陈炳旭,冯日碧.亚致死浓度阿维菌素对荔枝叶螨种群发育的影响[J].农药学学报,2017,19(3):388-392.XU Shu,JIA Tao,CHEN Binxu,FENG Ribi.Effects of sublethal concentrations of abamectin on development of Oligonychus litchii[J].Chinese Journal of Pesticide Science,2017,19(3):388-392.
[18] 黄柯程,曾鑫年,黎卓莹. 低剂量杀虫剂对昆虫的兴奋性效应[J].生态毒理学报,2010,5(1):26-31.HUANG Kecheng,ZENG Xinnian,LI Zhuoying. Insecticidesinduced hormesis on insects[J].Asian Journal of Ecotoxicology,2010,5(1):26-31.
[19] STEBBING A R D.Hormesis:The stimulation of growth by low levels of inhibitors[J]. Science of the Total Environment,1982,22(3):213-234.
[20] 赵明,沈国清,陆贻通,余月书.低剂量吡虫啉对异色瓢虫的Hormesis 效应及其抗氧化酶活性的影响[J].扬州大学学报(农业与生命科学版),2012,33(4):77-80.ZHAO Ming,SHEN Guoqing,LU Yitong,YU Yueshu. Hormetic effect of imidacloprid on Harmonia axyridis and impact on the antioxidant enzyme activity[J]. Journal of Yangzhou University(Agricultural and Life Science Edition),2012,33(4):77-80.
[21] CAMPOS F,KRUPA D A,DYBAS R A.Susceptibility of populations of twospotted spider mites (Acari:Tetranychidae) from Florida,Holland,and the canary Islands to abamectin and characterization of abamectin resistance[J].Journal of Economic Entomology,1996,89(3):594-601.
[22] 汝阳,陈耀年,尚素琴,张新虎.阿维菌素亚致死剂量对二斑叶螨解毒酶系的影响[J].甘肃农业大学学报,2017,52(1):87-91.RU Yang,CHEN Yaonian,SHANG Shuqin,ZHANG Xinhu.Effect of sublethal dose of avermectin on the activities of detoxifying enzymes in Tetranychus urticae[J].Journal of Gansu Agricultural University,2017,52(1):87-91.
[23] YAO J X,ZHU Y C,ADAMCZYK J,LUTTRELL R. Influences of acephate and mixtures with other commonly used pesticides on honey bee (Apis mellifera) survival and detoxification enzyme activities[J]. Comparative Biochemistry and Physiology Part C:Toxicology&Pharmacology,2018,209:9-17.
[24] KONTOGIANNATOS D,MICHAIL X,KOURTI A. Molecular characterization of an ecdysteroid inducible carboxylesterase with GQSCG motif in the corn borer,Sesamia nonagrioides[J].Journal of Insect Physiology,2011,57(7):1000-1009.
[25] 倪婧,谢道燕,杨振国,柴建萍,江秀均.天然辣椒碱类物质对朱砂叶螨各发育阶段的杀螨活性及对谷胱甘肽S-转移酶的影响[J/OL]. 西南农业学报,2022:1-10[2022-07-29]. http://kns.cnki.net/kcms/detail/51.1213.S.20220218.1415.021.html.NI Qian,XIE Daoyan,YANG Zhenguo,CHAI Jianping,JIANG Xiujun.Acaricidal activity and effects on glutathione S-transferase of natural capsaicin against different development stages of Tetranychus cinnabarinus[J/OL]. Southwest China Journal of Agricultural Sciences,2022:1-10[2022-07-29]. http://kns.cnki.net/kcms/detail/51.1213.S.20220218.1415.021.html.
[26] 蒋兴川,沈怿丹,孙劲超,李秀霞,黄勇,董永成,操海群.氯虫苯甲酰胺和甲维盐对草地贪夜蛾幼虫的毒力及解毒酶活性的影响[J].环境昆虫学报,2019,41(5):961-967.JIANG Xingchuan,SHEN Zedan,SUN Jinchao,LI Xiuxia,HUANG Yong,DONG Yongcheng,CHAO Haiqun. Effect of chlorantraniliprole and emamectin benzoate on toxicity and detoxification enzymes activity in Spodoptera frugiperda larva[J].Journal of Environmental Entomology,2019,41(5):961-967.