葡萄(Vitis L.)是全球重要的经济类果树作物之一,具有重要的经济价值[1]。近年来,由于全球气候变化加剧,“倒春寒”等极端天气频发,造成葡萄萌动新梢全部或部分受冻,易出现冻芽、冻花、甚至冻果的现象,造成树势受损,产量下降,对我国的葡萄生产造成了严重影响。目前,葡萄栽培生产上一般通过葡萄园喷灌水、熏烟、风机吹风以及喷施药剂等措施来预防“倒春寒”[2]。因此,如何高效缓解葡萄“倒春寒”危害已成为葡萄栽培生产中亟待解决的问题。
海藻糖(Trehalose)是一种非还原性二糖,广泛存在于细菌、酵母和无脊椎动物体内。植物中海藻糖于19 世纪由Firdos 等[3]从黑麦麦角菌中首次发现,其合成途径为海藻糖-6-磷酸合酶(Trehalose-6-phosphatesynthase,TPS)-海藻糖-6-磷酸磷酸酶(Trehalose-6-pho-sphate phosphatase,TPP)途径[4]。海藻糖作为植物生长调控因子、渗透保护剂或稳定分子,在植物生长发育和逆境响应中发挥着重要作用[5]。例如,海藻糖可通过调节代谢调控信号来抵御植株干旱、低温和盐害等非生物胁迫[6-9]。逆境条件下,海藻糖可以保护细胞内的活性物质,促进蛋白质合成,增强质膜流动性以及功能酶的稳定性[10]。海藻糖可作为植物适应逆境胁迫的应激代谢产物,协助植物细胞内其他组分抵御外界不良环境,广泛存在于耐低温作物中[11]。此外,外源海藻糖通过显著提高小花内源亚精胺水平,增加小花内谷胱甘肽和抗坏血酸含量,促进谷胱甘肽-抗坏血酸循环,降低小花内活性氧含量,提高顶端小穗的小花育性,从而显著缓解了低温引起的每穗粒数下降[12]。目前,外源海藻糖在提高小麦幼苗、藜麦、冬小麦和玉米抗寒性方面的研究也已有报道[13-16]。然而,外源海藻糖是否能减轻低温胁迫对葡萄幼苗生长发育的不利影响,生理机制尚不明晰。
笔者在本研究中以萌芽期赤霞珠葡萄自根苗为试验材料,人工模拟“倒春寒”胁迫,采用叶面喷施不同浓度海藻糖,以喷施清水为对照组,初步探讨海藻糖对低温胁迫下赤霞珠幼苗的表型、生理生化指标及内源激素的影响,以期为海藻糖在酿酒葡萄抵御低温胁迫中的应用提供理论基础和实践依据。
1 材料和方法
1.1 试验材料
供试葡萄为1 年生赤霞珠自根苗,种植于含V 土∶V 蛭石∶V 珍珠岩为3∶1∶1 的营养钵中(10 cm×10 cm),在25 ℃温室下培育8周。
1.2 试验方法
选取健康、株型一致的幼苗,连续进行3 d 叶面喷施10 mmol·L-1、15 mmol·L-1和30 mmol·L-1的海藻糖溶液(索莱宝生物科技有限公司)(含有0.1%Tween-20),对照组喷施蒸馏水,置于低温培养箱(RUMED P350,Rubarth Apparate GmbH;Germany)进行人工模拟“倒春寒”处理,设定程序为1 ℃·h-1进行程序性降温,起始温度为25 ℃,最低温度为(-3±0.5)℃并保持4 h,设置16、4、0、-3 ℃0 h和-3 ℃4 h 5个采样点分别进行表型观察,采集中上部(从下向上第3~5枚)大小一致的叶片,液氮速冻并于-80 ℃冰箱保存备用。
1.3 存活率、MDA含量和叶绿素含量的测定
幼苗经模拟“倒春寒”处理后对冻芽进行修剪,在25 ℃下缓苗1 个月,后统计成活率(存活率=存活棵数/处理总棵数×100%);采用硫代巴比妥酸法测定丙二醛(Malondialdehyde,MDA)含量[17];采用无水乙醇提取法测定叶绿素含量[17];每个处理3 次重复。
1.4 O2·-与H2O2含量的测定
采用羟胺氧化法测定超氧阴离子(Superoxide anion,O2·-)[18];采用分光光度法测定过氧化氢(Hydrogen peroxide,H2O2)[19];每个处理3次重复。
1.5 抗氧化酶活性的测定
采用愈创木酚显色法测定过氧化物酶(Peroxidase,POD)活性[20];采用紫外吸收比色法测定过氧化氢酶(Catalase,CAT)活性[21];采用氮蓝四唑光还原法测定超氧化物歧化酶(Superoxide dismutase,SOD)活性[22];每个处理3次重复。
1.6 内源激素含量的测定
采用超高效液相色谱法[23]。收集并称取0.7 g叶片鲜样,加液氮充分研磨,加入80%甲醇(含0.05%柠檬酸)混匀置于4 ℃提取4 h;后经低温离心15 min,分离上清液,提取2次,合并上清液,氮吹至5 mL,用1.5×石油醚(7.5 mL)萃取3 次,弃醚相;用1×甲酸甲酯(5 mL)萃取3 次,收集酯相,氮吹至干,用2 mL 甲醇溶解后用0.45 μm 滤膜过滤,每个处理3次重复。
1.7 糖含量的测定
采用超高效液相色谱法[24]。称取叶片0.2 g,加入ddH2O,定容至2 mL,60 ℃超声提取30 min,90 ℃恒温水浴60 min,冷却至室温后离心15 min,吸出上清液并进行过滤,放入检测器进行液相分析检测。将标准葡萄糖、果糖、蔗糖均配制成2.5 mg·mL-1的混标母液,并分别稀释为0.05~1.00 mg·mL-1等一系列标准溶液浓度,经0.45 μm 滤膜过滤后进行液相测定,每个处理3次重复。
1.8 数据处理与统计分析
利用Microsoft Office Excel 2016 进行数据统计处理,分别使用Origin 2021b 中插件Principal Component Analysis 进行主成分分析(PCA),Correlation Plot 进行相关性分析以及Paired Comparison Plot 进行柱状图作图和显著性差异分析。
2 结果与分析
2.1 缓解低温伤害最适海藻糖浓度的筛选
对赤霞珠幼苗进行连续3 d 叶面喷施不同浓度的海藻糖,以喷施清水为对照组,后放入低温培养箱中进行模拟“倒春寒”处理(图1-a~t)。在模拟“倒春寒”胁迫条件下,随着低温胁迫加剧,在-3 ℃时喷施清水对照组的赤霞珠幼苗局部叶柄出现发紫现象,在该温度保持4 h,叶片与茎段出现严重萎蔫的现象(图1-d、1-e)。喷施不同浓度海藻糖的叶片受到程序性降温的影响不同。其中,10 mmol·L-1海藻糖预处理的叶片与嫩梢比30 mmol·L-1海藻糖预处理受到低温胁迫的萎蔫程度弱,而15 mmol·L-1海藻糖处理组则几乎未出现萎蔫,无明显冻害。

图1 不同浓度海藻糖预处理在人工模拟“倒春寒”低温胁迫下的表型
Fig.1 Phenotypes of different concentrations of trehalose pre-treatment under artificially simulated“cold spell in the later spring”
红色箭头指示为萎蔫或发紫,横向为同一处理下不同采样点,纵向为同一采样点下不同处理。
Red arrows indicate wilting or purple,horizontal for different sampling points under the same treatment,vertical for different treatments under the same sampling point.
2.2 海藻糖处理对低温胁迫下赤霞珠存活率、MDA含量和叶绿素含量的影响
在模拟“倒春寒”胁迫条件下,对不同浓度海藻糖处理组植株的存活率、MDA和叶绿素含量进行统计分析。结果表明,不同浓度海藻糖处理与清水对照组相比,赤霞珠幼苗的存活率不同。其中,CK组的存活率为10.53%,10 mmol·L-1、15 mmol·L-1海藻糖处理组存活率分别为25.00%、71.05%,为对照组的2.37倍和6.75倍,而30 mmol·L-1海藻糖处理组的存活率为8.33%,低于CK 组存活率。MDA 含量测定结果表明,各处理组MDA含量均呈上升趋势,与清水对照相比,16 ℃时各处理组(10、15、30 mmol·L-1)叶片MDA 含量分别降低了32.49%、33.72%、16.98%;4 ℃、0 ℃、-3 ℃0 h 3 个采样点,外源海藻糖处理组(10、15、30 mmol·L-1)叶片MDA 含量分别 降 低 了26.02% 、27.05% 、9.93% 和20.35% 、12.23%、7.74%和24.89%、23.29%、7.71%;-3 ℃4 h时,10 mmol·L-1、15 mmol·L-1海藻糖处理组MDA含量分别降低了28.63%、21.16%,而30 mmol·L-1海藻糖处理组MDA 含量增加了2.71%(图2-A)。此外,叶绿素含量测定结果表明,各处理组叶片叶绿素含量均有所下降,其中15 mmol·L-1海藻糖处理组5个采样点叶绿素含量较CK分别提高了3.55%、14.71%、10.49%、3.55%和31.17%,其余处理组(10 mmol·L-1、30 mmol·L-1)效果不显著(图2-B)。由此可见,15 mmol·L-1海藻糖处理组的赤霞珠幼苗叶片细胞膜受损程度最轻,表明15 mmol·L-1海藻糖处理可以有效缓解低温对赤霞珠幼苗的伤害。

图2 人工模拟“倒春寒”条件下不同浓度海藻糖处理组植株丙二醛(A)和叶绿素含量(B)
Fig.2 Malondialdehyde(A)and chlorophyll content(B)of plants treated with trehalose at different concentrations under artificially simulated“cold spell in the later spring”
不同小写字母表示同一采样点不同处理差异达到显著性水平(p<0.05),横坐标为不同采样点,-3 ℃0 h 和-3 ℃4 h 分别代表在-3 ℃维持0 h 和4 h,下同。
Different lowercase letters indicate that the difference between different treatments at the same sampling point reaches the significance level(p<0.05),the abscissa is different sampling points,and-3 ℃0 h and-3 ℃4 h represent 0 and 4 h maintenance at-3 ℃respectively,the same below.
2.3 海藻糖处理对低温胁迫下赤霞珠叶片活性氧系统的影响
赤霞珠在遭受低温胁迫时,活性氧系统失衡,大量的活性氧在细胞中积累,会破坏植物的细胞结构[25]。对叶片H2O2和O2·-含量测定的结果表明,随着模拟“倒春寒”胁迫加剧,对照组和处理组H2O2和O2·-含量均呈上升趋势,且在-3 ℃4 h时达到峰值。与清水对照相比,15 mmol·L-1海藻糖处理组植株叶片5 个采样点H2O2含量分别降低了11.68%、8.72%、19.91%、22.48%和23.82%(图3-A);在-3 ℃0 h、-3 ℃4 h 2个采样点,15 mmol·L-1海藻糖处理组O2·-含量分别降低了10.02%、6.21%(图3-B)。综上,15 mmol·L-1海藻糖处理可降低低温胁迫下赤霞珠幼苗叶片中活性氧的积累。
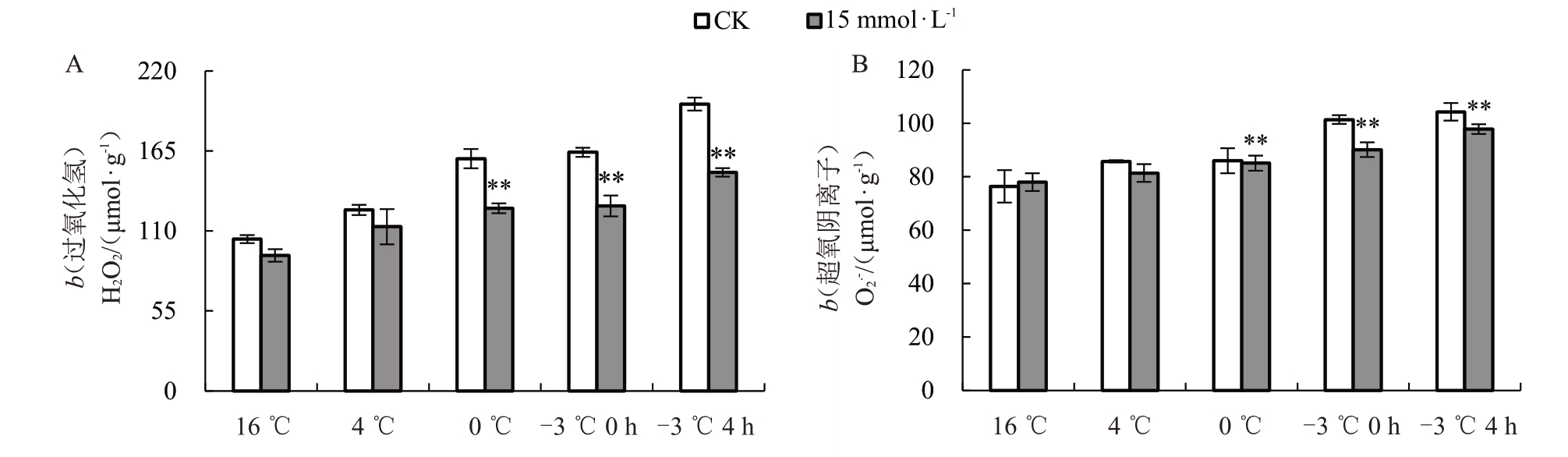
图3 人工模拟“倒春寒”条件下15 mmol· L-1海藻糖处理的叶片H2O2含量(A)和O2· -含量(B)变化
Fig.3 Changes of H2O2 content(A)and O2· -content(B)in leaves of 15 mmol· L-1trehalose treated groups under artificially simulated“cold spell in the later spring”
*p≤0.05,**p≤0.01。下同。
*p≤0.05,**p≤0.01.The same below.
2.4 海藻糖处理对低温胁迫下赤霞珠叶片抗氧化酶活性的影响
植物细胞膜的稳定性及酶保护系统与植物抗寒能力紧密关联,抗氧化酶可有效清除植物体内活性氧等物质的积累,从而减轻逆境胁迫下植物体受到的危害[26]。随着低温胁迫时间的延长,-3 ℃4 h 较16 ℃,CK 组CAT 活性增加了5.91 U·g-1,而15 mmol·L-1海藻糖处理组增加了25.92 U·g-1,相比对照组提高了221.73%(图4-A);15 mmol·L-1海藻糖处理对低温胁迫下SOD 活性仅在4 ℃下有显著差异,其他处理时间点与CK 无明显差异(图4-B)。模拟“倒春寒”处理后,与CK相比,15 mmol·L-1海藻糖处理显著提高了葡萄幼苗的POD 活性,在5 个采样点的POD 活性分别比CK 高出3.77%、44.08%、39.69%、46.85%和54.53%(图4-C)。说明15 mmol·L-1海藻糖处理可以提高低温胁迫下赤霞珠幼苗叶片的抗氧化酶活性,从而提高活性氧的清除能力。

图4 人工模拟“倒春寒”条件下15 mmol· L-1海藻糖处理组叶片CAT 活性(A)、SOD 活性(B)和POD 活性(C)变化
Fig.4 Changes of CAT activity(A),SOD activity(B)and POD activity(C)in leaves treated with CK and 15 mmol· L-1 trehalose under artificially simulated“cold spell in the later spring”
2.5 海藻糖处理对低温胁迫下赤霞珠叶片4种内源激素含量的影响
当植物遭遇低温、干旱、盐碱等非生物胁迫时,可以通过调控体内相关激素的表达来应对环境胁迫[27]。本研究中对15 mmol·L-1海藻糖预处理赤霞珠幼苗在低温胁迫下的4 种内源激素(tZR、GA3、IAA 和ABA)含量进行了测定分析。结果表明,随着处理时间的延长,CK 组赤霞珠幼苗叶片tZR 与GA3含量呈现先上升后下降的趋势(0 ℃出现拐点),而处理组大体呈现上升趋势(图5-A);其中,在-3 ℃4 h 时,15 mmol·L-1海藻糖处理组tZR、GA3含量较CK 分别提高了80.03%、127.66%。CK 组与处理组中IAA含量均呈下降趋势,-3 ℃4 h较16 ℃,CK中IAA 含量比处理组分别降低了13.54 ng·g-1 和12.66 ng·g-1,在-3 ℃维持4 h 后对照组IAA 含量降为0 ng·g-1,15 mmol·L-1海藻糖处理组IAA含量降为0.94 ng·g-1(图5-C)。CK组和处理组中ABA含量随处理时间的延长均呈先升高后降低趋势(4 ℃时对照组略有下降),但出现峰值的时间不同。在0 ℃、-3 ℃0 h、-3 ℃4 h 3 个采样点,处理组ABA含量较CK 组分别高出39.14%、5.47%、701.21%(图5-D)。综上,15 mmol·L-1海藻糖处理对低温胁迫下葡萄幼苗的激素合成系统具有一定的保护作用。

图5 人工模拟“倒春寒”条件下15 mmol· L-1海藻糖处理组叶片内源tZR 含量(A)、GA3 含量(B)、IAA 含量(C)和ABA 含量(D)变化
Fig.5 Changes of endogenous tZR content(A),GA3 content(B),IAA content(C)and ABA content(D)in leaves of CK and 15 mmol· L-1 trehalose groups under artificially simulated“cold spell in the later spring”
2.6 海藻糖处理对低温胁迫下赤霞珠叶片内源糖组分的影响
糖作为供能物质,为植株的生长发育供能,在遭受逆境胁迫时,抗逆调节和生长发育存在一定的竞争关系,植物为了抵御低温胁迫会以生长发育受损为代价,造成糖含量积累[13]。因此,本研究对低温胁迫下内源糖组分进行测定,结果表明,与CK 组相比,15 mmol·L-1海藻糖处理组5 个采样点果糖含量分别提高了10.80%、31.26%、18.99%、19.88%和12.56%(图6-A)。此外,葡萄糖含量分别提高11.39%、23.02%、23.47%、15.31%和11.20%(图6-B)。蔗糖含量在16 ℃与4 ℃时CK 组几乎检测不到,而对照组分别为487 mg·L-1 与483 mg·L-1,在0 ℃时,CK 组蔗糖快速累积,与处理组累积的蔗糖无显著差异;然而,后续处理时间点,两者差异显著。其中,在-3 ℃4 h时,15 mmol·L-1海藻糖处理组中蔗糖含量较CK组提高了68.53%(图6-C)。综上,15 mmol·L-1海藻糖处理有利于低温胁迫下赤霞珠幼苗叶片中糖分的积累。

图6 人工模拟“倒春寒”条件下15 mmol· L-1海藻糖处理组叶片果糖(A)、葡萄糖(B)、蔗糖(C)含量的变化
Fig.6 Changes of fructose(A),glucose(B),sucrose(C)contents in leaves of CK and 15 mmol· L-1 trehalose treated groups under artificially simulated“cold spell in the later spring”
2.7 各项生理指标间的主成分分析
为了验证15 mmol·L-1海藻糖处理对人工模拟“倒春寒”的缓解效果,对5 个采样点的生理生化指标进行主成分分析(principal analysis,PCA),每个处理相对于PC1和PC2轴的距离显示了处理间的差异大小(图7-A)。PC1 45.9%和PC2 29.9%显示占总方差的75.8%,较好地解释了整体数据的特征。为了分析不同处理与各项生理生化指标间的相关关系进行冗余分析(redundancy analysis,RDA)(图7-B),RDA 图中各指标与处理间夹角的余弦值表示相关性,夹角越小,则相关性越大,如果箭头同向,表示正相关;箭头反向,表示负相关。RDA 图中的第一组变量与PC1 呈正相关,涉及到蔗糖、葡萄糖、果糖、SOD、POD、CAT、ABA、GA3、tZR、H2O2、O2.-、MDA等变量,相反,PC1变量与IAA和叶绿素含量呈显著负相关。
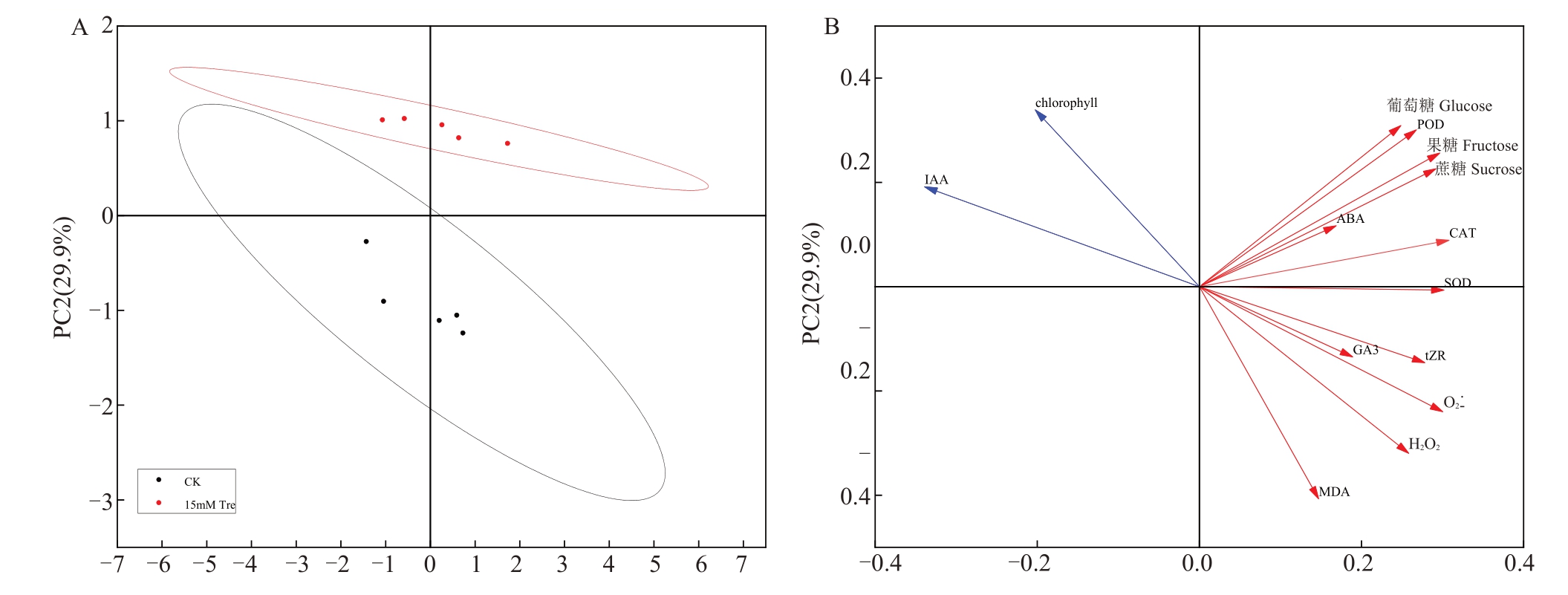
图7 各项生理指标间主成分分析
Fig.7 Principal component analysis between each physiological index
3 讨 论
低温胁迫会引发植物产生一系列的生理生化反应[28-29],导致植物体内参与抗逆相关的调节物质大量积累,激发植物渗透调节能力,从而增强植物对逆境胁迫的响应[30]。MDA 是植物细胞内一种重要的渗透调节物质,可作为细胞膜遭遇逆境损伤程度的重要评价指标[31]。随着温度的降低,所有处理的赤霞珠叶片MDA含量均逐渐升高,与对照组相比,15 mmol·L-1海藻糖处理叶片5个采样点MDA含量分别降低了32.49%、26.02%、20.35%、24.89%和21.16%,效果最显著,这与Raza 等[32]的研究结果一致。叶绿素作为光合作用中最有效的色素,其含量在一定程度上反映了植物同化物质的能力[33],低温胁迫造成植物的光合能力下降[34]。本研究中,赤霞珠幼苗在模拟“倒春寒”过程中,叶绿素含量均有不同程度的下降,但15 mmol·L-1海藻糖处理组植株叶片5 个采样点的叶绿素含量较CK 分别提高了3.55%、14.71%、10.49%、3.55%和31.17%,很大程度上缓解了叶绿素含量的下降。因此,海藻糖在调节植物的抗寒性方面可能存在剂量效应。抗氧化酶通过清除活性氧,将植物体内氧自由基维持在较低水平,从而缓解植物质膜损伤,减轻氧自由基对细胞膜系统的伤害[35]。海藻糖则通过提高抗氧化酶活性和降低质膜损伤途径来提高植物对非生物胁迫的抗性[3]。随着处理温度的降低,赤霞珠叶片中CAT、SOD 和POD 活性均有不同程度的升高,但15 mmol·L-1 海藻糖处理组中3 种酶活性均高于对照组,其中CAT 和POD 的活性显著高于对照组,这与孙嘉华等[36]和杨娅倩[37]的研究成果不一致,推测3种抗氧化酶活性对不同植株响应低温胁迫的能力不同。当赤霞珠幼苗受到低温胁迫时,CAT、POD对活性氧的清除能力高于SOD,且15 mmol·L-1海藻糖处理下这种现象更为明显。综上,15 mmol·L-1海藻糖处理可有效缓解低温胁迫带来的损伤。
植物激素是一种广泛参与植物逆境胁迫的信号物质,在植物逆境胁迫响应过程中发挥重要作用,可引起植物体内适应性调节反应[38-40]。ABA作为最重要的植物激素之一,在植物抵抗低温、干旱等应激反应中具有重要作用。本研究中,经过-3~16 ℃低温胁迫后,15 mmol·L-1海藻糖处理组叶片ABA含量持续上升,而对照组ABA含量在-3 ℃维持4 h后显著降低,且IAA为0,表明对照组在-3 ℃维持4 h后丧失生长能力,其余激素合成也受到严重抑制,15 mmol·L-1海藻糖处理有效提高了赤霞珠幼苗对低温胁迫的耐受性,这与张盛楠等[41]的研究结果不一致,推测不同内源激素对低温的响应程度不同。低温胁迫下ABA 含量显著上升,加快细胞新陈代谢,从而提高植株的抗寒性,由半致死温度的测定可知,在-3 ℃维持4 h 后,赤霞珠幼苗细胞破裂甚至死亡,内源激素合成受损,造成细胞内激素含量急剧变化。
糖作为影响细胞膜和蛋白质的保护剂,可溶性糖的积累可以保护植物免受低温胁迫造成的伤害[42]。在遭遇“倒春寒”时,非还原性二糖蔗糖分解为单糖葡萄糖,参与机体供能[33]。在遭受低温胁迫时,植物的抗逆调节和生长发育之间存在竞争关系,植物通过调节渗透物质来抵御低温胁迫的侵害,而赤霞珠幼苗在遭遇“倒春寒”时,正处于一个这样的复杂关系。本研究中,随着温度的降低,果糖、葡萄糖和蔗糖均有不同程度的积累,在-3 ℃4 h时,15 mmol·L-1海藻糖处理组中蔗糖含量分别为对照组的1.69倍,差异显著,此前大量研究显示,蔗糖的积累有利于增强植物的抗逆性,这与Nemati等[43]和Zhou等[44]的研究成果一致。本研究结果表明,叶面喷施15 mmol·L-1海藻糖有效提高了内源糖组分的含量。本研究通过分析不同浓度海藻糖处理下赤霞珠幼苗抗寒性,结合生理生化指标综合评价筛选出缓解葡萄植株“倒春寒”冻害的最佳海藻糖浓度,对葡萄等园艺作物抗寒栽培提供了理论参考,但在栽培生产实践中,针对不同品种和树龄葡萄植株的缓解作用还有待于进一步验证。
4 结 论
研究筛选出了缓解“倒春寒”危害的最佳海藻糖浓度。在人工模拟的“倒春寒”胁迫下,叶面喷洒海藻糖促进了赤霞珠幼苗的生长,提高了叶绿素含量,诱导增强抗氧化酶的活性,维持了细胞渗透调节的水平,提高了内源激素的水平,调节了可溶性糖的水平,从而能够显著降低低温胁迫对赤霞珠幼苗的损伤效应,因此在生产上,可以通过叶面喷施15 mmol·L-1海藻糖溶液来应对“倒春寒”,减缓“倒春寒”对葡萄植株的危害。
[1] 程珊珊.葡萄VvMYB6 基因功能的研究[D].新乡:河南科技学院,2021.CHENG Shanshan. Study on the function of VvMYB6 gene in grapes[D]. Xinxiang:Henan Institute of Science and Technology,2021.
[2] 岳慧欣,何延波,姜建福,樊秀彩,张颖,刘崇怀,杨英军.葡萄主产区春霜冻风险分析[J]. 中国农业气象,2021,42(3):221-229.YUE Huixin,HE Yanbo,JIANG Jianfu,FAN Xiucai,ZHANG Ying,LIU Chonghuai,YANG Yingjun. Risk analysis of spring frost in main grape producing areas[J].Chinese Journal of Agrometeorology,2021,42(3):221-229.
[3] FIRDOS K,NUDRAT A A,MUHAMMAD S,FAHAD A Q,MUHAMMAD A. Trehalose:A key organic osmolyte effectively involved in plant abiotic stress tolerance[J]. Journal of Plant Growth Regulation,2018,38(2):606-618.
[4] TIAN L F,XIE Z J,LU C Q,HAO X H,WU S,HUANG Y,LI D P,CHEN L B.The trehalose-6-phosphate synthase TPS5 negatively regulates ABA signaling in Arabidopsis thaliana[J].Plant Cell Reports,2019,38(8):869-882.
[5] LI Z,LIANG F P,ZHANG T B,FU N,LONG Y.Enhanced tolerance to drought stress resulting from Caragana korshinskii Ck-WRKY33 in transgenic Arabidopsis thaliana[J]. BMC Genetics,2021,22(1):1-10.
[6] 李佳馨,谢寅峰,李霞,王净.海藻糖对转C4 型PEPC 水稻种子萌发耐旱性的影响[J].核农学报,2021,35(12):2879-2892.LI Jiaxin,XIE Yinfeng,LI Xia,WANG Jing.Effects of trehalose on drought tolerance of transgenic C4 PEPC rice seeds[J]. Journal of Nuclear Agricultural Sciences,2021,35(12):2879-2892.
[7] JANG I C,OH S J,SEO J S,CHOI W B ,SONG S I,KIM C H,KIM Y S,SEO H S,DO CHOI Y,NAHM B H,KIM J K. Expression of a bifunctional fusion of the Escherichia coli genes for trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in transgenic rice plants increases trehalose accumulation and abiotic stress tolerance without stunting growth[J].Plant Physiology,2003,131(2):516-524.
[8] NARANG P K,DEY J,MAHAPATRA S R,GHOSH M,MISRA N,SUAR M,KUMAR V,RAINA V. Functional annotation and sequence-structure characterization of a hypothetical protein putatively involved in carotenoid biosynthesis in microalgae[J].South African Journal of Botany,2021,141:219-226.
[9] YANG Y,YAO Y D,LI J,ZHANG J,ZHANG X D,HU L X,DING D X,BAKPA E P,XIE J M. Trehalose alleviated salt stress in tomato by regulating ROS metabolism,photosynthesis,osmolyte synthesis,and trehalose metabolic pathways[J]. Frontiers in Plant Science,2022,13:1-18.
[10] SHIMA S,MATSUI H,TAHARA S,IMAI R.Biochemical characterization of rice trehalose-6-phosphate phosphatases supports distinctive functions of these plant enzymes[J]. FEBS Journal,2007,274(5):1192-1201.
[11] 丁泽红,铁韦韦,付莉莉,颜彦,胡伟.木薯海藻糖合成酶基因MeTPS6 克隆及其在非生物胁迫下的表达分析[J].南方农业学报,2017,48(11):1923-1929.DING Zehong,TIE Weiwei,FU Lili,YAN Yan,HU Wei. Cloning of trehalose-6-phosphate synthase gene MeTPS6 from cassava and its expression under abiotic stress[J].Journal of Southern Agriculture,2017,48(11):1923-1929.
[12] LIANG Z,LUO J,WEI B,LIAO Y C,LIU Y.Trehalose can alleviate decreases in grain number per spike caused by low- temperature stress at the booting stage by promoting floret fertility in wheat[J]. Journal of Agronomy and Crop Science,2021,207(4):717-732.
[13] 谢冬微,王晓楠,付连双,孙健,李卓夫,郑伟.外源海藻糖对冬小麦低温下胚芽长及幼苗抗寒性的影响[J].麦类作物学报,2015,35(2):215-223.XIE Dongwei,WANG Xiaonan,FU Lianshuang,SUN Jian,LI Zhuofu,ZHENG Wei. Effect of exofenous trehalose on germ length and seedling freeze resistance of winter wheat under cold stress[J].Journal of Triticeae Crops,2015,35(2):215-223.
[14] ABDALLAH M S,ABDELGAWAD Z A,EL-BASSIOUNY H.Alleviation of the adverse effects of salinity stress using trehalose in two rice varieties[J]. South African Journal of Botany,2016,103:275-282.
[15] 郝晓华,王晓洁,刘可心.外源海藻糖对干旱胁迫下藜麦生理特性的影响[J]. 山东农业大学学报(自然科学版),2021,52(5):739-745.HAO Xiaohua,WANG Xiaojie,LIU Kexin. Effects of exogenous trehalose on the physiological characteristics of quinoa under drought stress[J].Journal of Shandong Agricultural University(Natural Science Edition),2021,52(5):739-745.
[16] LUO Y,GAO Y M,WANG W,ZOU C J.Application of trehalose ameliorates heat stress and promotes recovery of winter wheat seedlings[J].Biologia Plantarum,2014,58(2):395-398.
[17] 高俊凤. 植物生理学实验指导[M]. 北京:高等教育出版社,2006.GAO Junfeng. Plant physiology experiment instruction[M]. Beijing:Higher Education Press,2006.
[18] ELSTNER E F,HEUPEL A.Inhibition of nitrite formation from hydroxylammoniumchloride:a simple assay for superoxide dismutase[J].Analytical Biochemistry,1976,70(2):616-620.
[19] VELIKOVA V,YORDANOV I,EDREVA A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants-Protective role of exogenous polyamines[J]. Plant Science Limerick,2000,151(1):59-66.
[20] 陈耀兵,江念,孙紫薇,滕树锐,郑小江.藤茶不同繁殖方式有效成分测定及抗氧化酶活性分析[J]. 中成药,2018,40(2):399-403.CHEN Yaobing,JIANG Nian,SUN Ziwei,TENG Shurui,ZHENG Xiaojiang. Determination of effective components and analysis of antioxidant enzyme activities of ampelopsis grossedentata under different breeding modes[J]. Chinese Traditional Patent Medicine,2018,40(2):399-403.
[21] 李文欣,李程,史瑞基,张雪梅,郭素萍,顾玉红.不同品种红树莓果实发育过程中抗氧化性的研究[J].河北农业大学学报,2018,41(3):67-71.LI Wenxin,LI Cheng,SHI Ruiji,ZHANG Xuemei,GUO Suping,GU Yuhong. Study on the antioxidant activity of red raspberry fruit during the development process[J].Journal of Agricultural University of Hebei,2018,41(3):67-71.
[22] 王秀梅,安毅,秦莉,林大松,霍莉莉.镉胁迫下土壤理化因子与过氧化氢酶活性的通径分析[J]. 中国农学通报,2018,34(11):59-65.WANG Xiumei,AN Yi,QIN Li,LIN Dasong,HUO Lili. Path analysis of soil physicochemical factors and catalase activities under cadmium stress[J].Chinese Agricultural Science Bulletin,2018,34(11):59-65.
[23] 高江曼,孟莹,刘庆,王童孟,刘美迎,李汶冰,惠竹梅,张振文.赤霞珠葡萄生长发育过程中内源激素的变化及其与果实成熟的关系[J].食品科学,2017,38(7):167-175.GAO Jiangman,MENG Ying,LIU Qing,WANG Tongmeng,LIU Meiying,LI Wenbing,HUI Zhumei,ZHANG Zhenwen.Changes in endogenous hormones during the development of Vitis vinifera L.cv.Cabernet Sauvignon and their relationship with berry ripening[J].Food Science,2017,38(7):167-175.
[24] 李梦鸽.避雨栽培对葡萄果实糖分积累及蔗糖代谢酶活性的影响[D].雅安:四川农业大学,2015.LI Mengge.Effects of rain shelter cultivation on sugar accumulation and sucrose metabolism enzyme activities of grape fruit[D].Ya’an:Sichuan Agricultural University,2015.
[25] ZHAO Y Q,HAN Q H,DING C H,HUANG Y,LIAO J Q,CHEN T,FENG S L,ZHOU L J,ZHANG Z W,CHEN Y E,YUAN S,YUAN M. Effect of low temperature on chlorophyll biosynthesis and chloroplast biogenesis of rice seedlings during greening[J]. International Journal of Molecular Sciences,2020,21(4):1390-1411.
[26] 王文霞,陈丽明,王海霞,刘有清,吴自明,曾勇军,谭雪明,潘晓华,石庆华,曾研华.淹水缓解直播早籼稻苗期低温冷害的生理特性研究[J].中国水稻科学,2021,35(2):166-176.WANG Wenxia,CHEN Liming,WANG Haixia,LIU Youqing,WU Ziming,ZENG Yongjun,TAN Xueming,PAN Xiaohua,SHI Qinghua,ZENG Yanhua. Study on physiological characteristics behind mitigative effects of flooding on low temperature-caused chilling damage to direct seeded early indica rice at the seedling stage[J].Chinese Journal of Rice Science,2021,35(2):166-176.
[27] 李钠钾.低温弱光胁迫下5-氨基乙酰丙酸对烟草幼苗生长及生理特性的影响与转录组测序分析[D].重庆:西南大学,2019.LI Najia.Effects of 5-Aminolevulinic acid on growth and physiological characteristics of tobacco seedlings under low temperature and low light stress and analysis of transcriptome sequencing[D].Chongqing:Southwest University,2019.
[28] 李辰彦,李祖军,田雪飞,方加海,石庆华,曾勇军,李辉婕,吴自明.抽穗扬花期低温胁迫对双季晚稻生理特性的影响[J].核农学报,2021,35(11):2634-2644.LI Chenyan,LI Zujun,TIAN Xuefei,FANG Jiahai,SHI Qinghua,ZENG Yongjun,LI Huijie,WU Ziming.Effects of low temperature stress at heading and flowering stage on physiological characteristics of double cropping late rice[J]. Journal of Nuclear Agricultural Sciences,2021,35(11):2634-2644.
[29] IBRAHIM N,NASE F. Short hot water as safe treatment induces chilling tolerance and antioxidant enzymes,prevents decay and maintains quality of cold-stored cucumbers[J]. Postharvest Biology and Technology,2018,138:1-10.
[30] 陈璐,张小丽,高柱,卢玉鹏,王小玲.喷施硝酸镧对脐橙叶片渗透调节物质的影响[J]. 中国农学通报,2021,37(29):114-119.CHEN Lu,ZHANG Xiaoli,GAO Zhu,LU Yupeng,WANG Xiaoling.Effects of lanthanum nitrate spraying on osmotic regulatory substances in navel orange leaves[J].Chinese Agricultural Science Bulletin,2021,37(29):114-119.
[31] 曹建东,陈佰鸿,王利军,毛娟,赵鑫.葡萄抗寒性生理指标筛选及其评价[J].西北植物学报,2010,30(11):2232-2239.CAO Jiandong,CHEN Baihong,WANG Lijun,MAO Juan,ZHAO Xin. Cold resistance indexes identification and comprehensive evaluation of grape varieties[J]. Acta Botanica Boreali-Occidentalia Sinica,2010,30(11):2232-2239.
[32] RAZA A,SU W,JIA Z Q,LUO D,ZHANG Y,GAO A,HUSSAIN M A,MEHMOOD S S,CHEN Y,LV Y,ZOU X L.Mechanistic insights into trehalose-mediated cold stress tolerance in Rapeseed (Brassica napus L.) seedlings[J]. Frontiers in Plant Science,2022,13:1-20.
[33] AZIZI F,AMIRI H,ISMAILI A. Melatonin improves salinity stress tolerance of Phaseolus vulgaris L.cv.Pak by changing antioxidant enzymes and photosynthetic parameters[J].Acta Physiologiae Plantarum,2022,44(4):1-12.
[34] 项洪涛,郑殿峰,何宁,李琬,王曼力,王诗雅.植物对低温胁迫的生理响应及外源脱落酸缓解胁迫效应的研究进展[J].草业学报,2021,30(1):208-219.XIANG Hongtao,ZHENG Dianfeng,HE Ning,LI Wan,WANG Manli,WANG Shiya.Research progress on the physiological response of plants to low temperature and the amelioration effcectiveness of exogenous ABA[J].Acta Prataculturae Sinica,2021,30(1):208-219.
[35] KHEDIA J,DANGARIYA M,NAKUM A K,AGARWAL P,PANDAA,PARIDA A K,GANGAPUR D R,MEENA R,AGARWAL P K. Sargassum seaweed extract enhances Macrophomina phaseolina resistance in tomato by regulating phytohormones and antioxidative activity[J]. Journal of Applied Phycology,2020,32(6):1-12.
[36] 孙嘉华,李雨萌,凌翰,惠竹梅.2,4-表油菜素内酯对多菌灵处理下葡萄抗氧化代谢系统的影响[J]. 干旱地区农业研究,2021,39(5):71-75.SUN Jiahua,LI Yumeng,LING Han,HUI Zhumei.Effects of 2,4-epibrassinolide on antioxidant system of grape seedlings treated with carbendazim[J]. Agricultural Research in the Arid Areas,2021,39(5):71-75.
[37] 杨娅倩.外源亚精胺对高温胁迫下葡萄幼苗生理生化特性的影响[D].泰安:山东农业大学,2020.YANG Yaqian. Effects of exogenous spermidine on physiological and biochemical characteristics of grape seedlings under high temperature stress[D]. Tai’an:Shandong Agricultural University,2020.
[38] 王志恒,赵延蓉,黄思麒,王悦娟,起金娥,魏玉清.外源海藻糖影响甜高粱幼苗抗旱性的生理生化机制[J].植物生理学报,2022,58(4):654-666.WANG Zhiheng,ZHAO Yanrong,HUANG Siqi,WANG Yuejuan,QI Jin’e,WEI Yuqing. Physiological and biochemical mechanism of exogenous trehalose on drought resistance in sweet sorghum seedlings[J]. Plant Physiology Journal,2022,58(4):654-666.
[39] ZHANG Y,LAN H X,SHAO Q L,WANG R Q,CHEN H,TANG H J,ZHANG H S,JI H. An A20/AN1-type zinc finger protein modulates gibberellins and abscisic acid contents and increases sensitivity to abiotic stress in rice(Oryza sativa)[J].Journal of Experimental Botany,2016,27(1):315-326.
[40] 李馨园,杨晔,张丽芳,左师宇,李丽杰,焦健,李晶.外源ABA对低温胁迫下玉米幼苗内源激素含量及Asr1 基因表达的调节[J].作物学报,2017,43(1):141-148.LI Xinyuan,YANG Ye,ZHANG Lifang,ZUO Shiyu,LI Lijie,JIAO Jian,LI Jing. Regulation on contents of endogenous hormones and Asr1 gene expression of maize seedling by exogenous ABA under low-temperature stress[J].Acta Agronomica Sinica,2017,43(1):141-148.
[41] 张盛楠,贾琰,瞿炤珺,杨亮,李晓,张妍,王喆,赵宏伟.孕穗期冷水灌溉对寒地粳稻相关生理指标及叶绿体超微结构的影响[J].西北农业学报,2020,29(6):886-897.ZHANG Shengnan,JIAYan,QU Zhaojun,YANG Liang,LI Xiao,ZHANG Yan,WANG Zhe,ZHAO Hongwei.Effects of cold water irrigation at booting stage on physiological indexes and chloroplast ultrastructure of japonica rice in cold region[J].Acta Agricultural Boreali-Occidentalis Sinica,2020,29(6):886-897.
[42] LIU Y S,GENG J C,SHA X Y,ZHAO Y X,HU T M,YANG P Z. Effect of rhizobium symbiosis on low-temperature tolerance and antioxidant response in alfalfa (Medicago sativa L.) [J].Frontiers in Plant Science,2019,10:538. DOI:10.3389/fpls.2019.00538.
[43] NEMATI F,GHANATI F,GAVLIGHI H A,SHARIFI M. Comparison of sucrose metabolism in wheat seedlings during drought stress and subsequent recovery[J]. Biologia Plantarum,2018,62(3):595-599.
[44] ZHOU L M,ZHANG F,LIU C A.Improved yield by harvesting water with ridges and subgrooves using buried and surface plastic mulchs in a semiarid area of China[J]. Soil & Tillage Research,2015,150:21-29.