梨树作为我国第三大水果树种,产量与品质已成为影响梨产区农户收入的重要因素[1-2]。而长期不合理施肥导致梨园土壤板结、酸化及盐渍化等问题加剧,进而降低肥料利用率[2-4],引发树体养分失调、产量与品质的下降[5]。土壤健康与果树植株健康是果业可持续发展的基础[6],其中土壤健康参与维持水果生产与提供生态系统等服务功能,影响果树养分吸收与果实品质的提升[7]。因此,在守护土壤健康的前提下,改善梨园土壤环境与微生物群落结构是提升梨树养分利用率与果实品质的有效途径。
低分子有机酸(Low molecular weight organic acid, LMWOAs)作为根际常见分泌物[8],通过直接影响根系微环境以驱动根际微生物群落的改变,促进某些难溶性矿质养分的溶解,进而提高其在根际养分有效态含量[9-10]。LMWOAs 具有较强的螯合力,易与多种金属离子形成复合物进而促进植物对金属离子的吸收[10]。另外,LMWOAs作为微生物碳源,能够吸引中性和有益微生物趋向根际,并促进益生菌在根际的定殖与增殖,以避免病原菌的入侵[11]:有益微生物参与固氮、溶解及螯合矿质元素,分解与转化有机物等以改善根际养分环境[12-14];此外,有益微生物产生抗生素、水解酶及挥发性物质,直接抑制病原体生长与毒力[15],并通过营养与空间竞争间接抑制病原体[16];有益菌能够在根部诱导植物系统抗性,从而增强植物对干旱、温度、盐及重金属等的耐受力[17]。因此,LMWOAs具有提升土壤养分环境与改善土壤微生物群落的潜能,目前其在果树上应用较少[18],而对梨园土壤质量的改良等方面的研究暂未有报道。
本研究在红宝石梨树栽培条件下,研究不同质量分数苹果酸、柠檬酸及草酸对梨树土壤微生物群落及养分的影响,探讨LMWOAs 对梨树土壤微生物群落及养分的作用效果及其与梨果实品质之间的关系,从土壤方向解析LMWOAs 对梨果实品质的作用机制,为新型果树专用水溶肥的研发以及果业绿色高效发展提供理论依据与技术支撑。
1 材料和方法
1.1 试验地基本情况
试验在中国农业科学院郑州果树研究所国家园艺种质资源库梨分库(34°42′47″N,113°41′49″E)进行。该地区属于黄河流域,年平均降水量约为542.15 mm。
1.2 供试材料
供试土壤:表层土壤pH 为7.16,有机质含量(w,后同)0.93%,有效磷含量131.80 mg·kg-1,有效钾含量241.02 mg·kg-1,铵态氮含量10.25 mg·kg-1,硝态氮含量14.85 mg·kg-1。
供试低分子有机酸:苹果酸、柠檬酸及草酸的纯度≥98.0%,购于阿法埃莎(Alfa Aesar)化学有限公司。
供试肥料:施用氮磷钾肥由尿素、硝酸钾及磷酸二氢钾组成,依据梨树产量设定全年施肥量(kg·hm-2)为N(367.5)-P2O5(247.5)-K2O(315)。
供试作物:5年生红宝石[Bayuehong(hybrid cultivar)×Suli(Pyrus bretschneideri)]梨树。
1.3 试验处理
2019年选取长势一致供试梨树(4 m×1 m)为试验材料。试验设置7 个处理(如表1),包括对照(氮磷钾肥)、LM(氮磷钾肥+苹果酸5%)、HM(氮磷钾肥+苹果酸10%)、LC(氮磷钾肥+柠檬酸5%)、HC(氮磷钾肥+柠檬酸10%)、LO(氮磷钾肥+草酸5%)和HO(氮磷钾肥+草酸10%)。其中,5%与10%是指LMWOAs质量占LMWOAs与全年施肥量总质量的比值(LMWOAs与氮磷钾肥直接混合称样),用量分别为84.72、178.86 kg·hm-2。每个处理设置4个重复小区,每个小区4株树,小区之间间隔2株梨树,各小区完全随机排列。按照梨树需肥规律,分别在萌芽期(4月11日)、第1次膨大期(5月5日)、第2次膨大期(6 月14 日)及采摘前20 d(8 月2 日)4 个时期施肥,以施肥枪施入树冠2/3 深度20 cm 处,其他栽培与病虫害等相关田间管理均保持一致。2019年9月4日每个重复小区随机选择3株梨树,避开施肥区域采用五点采样法采集每株树冠投影2/3 处0~20 cm土层与>20~40 cm 土层土壤,各层土壤样品混合。每个小区土壤混成1 个样,因此每个处理相同土层有4 个重复土样。所采集土壤冰上保存送至实验室,一部分土壤样品自然风干用于测定土壤养分指标,另一部分存于-80 ℃用于微生物功能多样性的测定。
表1 不同LMWOAs 处理下梨树土壤微生物群落功能多样性指数
Table 1 Functional diversity indices of pear tree soil microbial community with different LMWOAs

土层Soil layer/cm 0~20>20~40处理Treatment对照Control LM HM LC HC LO HO对照Control LM HM LC HC LO HO均匀度指数Pielou substrate evenness,E 0.98±0.01 b 1.02±0.01 ab 0.99±0.01 ab 1.00±0.01 ab 1.02±0.02 a 1.00±0.00 ab 1.01±0.02 ab 1.03±0.01 abc 1.02±0.01 bc 0.99±0.02 c 1.11±0.05 a 1.05±0.01 abc 1.08±0.01 ab 1.03±0.01 bc优势度指数Simpson index,D 0.95±0.00 bc 0.95±0.00 c 0.96±0.00 a 0.96±0.00 a 0.95±0.00 ab 0.96±0.00 a 0.96±0.00 a 0.95±0.00 a 0.94±0.00 a 0.94±0.01 a 0.94±0.00 a 0.93±0.00 a 0.90±0.01 b 0.93±0.01 a多样性指数Shannon’s diversity index,H’3.11±0.04 c 3.12±0.01 bc 3.27±0.02 a 3.20±0.01 b 3.19±0.02 b 3.15±0.02 bc 3.20±0.03 b 3.07±0.03 a 3.01±0.03 ab 3.02±0.08 ab 3.05±0.04 ab 2.85±0.06 ab 2.50±0.15 c 2.80±0.09 b均一性指数McIntosh idex,U 4.01±0.16 ab 3.76±0.23 b 4.24±0.14 ab 3.96±0.06 ab 4.33±0.05 a 4.41±0.08 a 3.90±0.23 ab 3.32±0.24 ab 3.17±0.23 bc 3.90±0.26 a 2.38±0.056 d 2.60±0.25 cd 2.28±0.25 d 2.69±0.08 bcd丰富度指数Substrate richness,S 23.67±0.67 b 21.67±0.33 b 27.00±1.00 a 24.67±0.33 ab 22.67±0.88 b 23.33±0.33 b 23.67±1.76 b 19.67±0.33 ab 19.33±0.67 ab 21.33±2.67 a 16.33±2.85 abc 15.00±0.58 bc 10.67±2.03 c 15.67±1.76 abc
1.4 测定项目与方法
1.4.1 土壤养分、pH 及EC 指标的测定 用Clever Chem 380(德国)间断化学分析仪进行土壤硝态氮与铵态氮的测定[19];以中性NH4OAc浸提土壤溶液后上原子吸收测定土壤速效钾含量[19];以0.5 mol·L-1 NaHCO3(pH 8.5)浸提土壤溶液,并用分光光度法测定有效磷含量[19];采用水土比例为2.5∶1 的水浸提-电位法测定土壤pH[20];土壤与超纯水1∶5混合后,用电导率仪测定土壤EC 值[20]。用碳氮分析仪(Primacs100,Skalar,Breda,Netherlands)测定土壤中的总碳(TC)与无机碳(IC)含量。土壤有机质(SOM)=1.724×(TC-IC)。
1.4.2 微生物功能多样性指数的测定与计算 将相当于1 g 风干土的新鲜土样以土水质量比1∶100用0.85%灭菌NaCl 溶液稀释,28 ℃200 r·min-1振荡20 min,4 ℃静置30 min,每孔取上清液150 μL加入Bio-Eco 微孔板中。25 ℃培养168 h,每24 h用Biolog 自动读取仪1 次,并计算微生物功能多样性指数[21-23]。平均吸光度AWCD 值、McIntosh 指数(U)、Shannon-Wiener 指数(H’)、Simpson 指数(D)、Pielou 指数(E)及丰富度指数S 分别表示微生物群落的利用碳源整体能力、均一性、多样性、优势度、均匀度以及利用的碳源的总数。选取72 h进行数据分析微生物功能多样性指数与碳源利用强度。根据31 种碳源化学性质的不同,分为聚合物、碳水化合物、酚类、羧酸、氨基酸及胺类6 类碳源,分别计算每一类碳源的平均吸光度,用于表示微生物对于六大碳源的利用强度,以评估不同处理特异性代谢碳源活性[24-25]。
1.4.3 果实品质与养分含量的测定 可溶性固形物含量采用手持式数字折光仪(PR-101;ATAGO)测定;可溶性糖含量使用蒽酮法测定[26];维生素C(Vc)含量采用2,6-二氯酚靛酚法测定[27];可滴定酸(Titratable Acid,TA)含量采用NaOH滴定法测定[20]。L、a、b、C与h°值均采用便捷式色差仪(CR-400,Konica Minolta,日本)测定。叶片与果实氮磷钾含量采用H2SO4-H2O2消煮[20]。叶片与果实氮含量采用全自动间断化学分析仪(Clever Chem 380,德国)测定;叶片与果实中的磷含量采用钼蓝比色法测定;叶片与果实钾含量采用原子吸收(AAS ZEEnit 700P,Jena,德国)测定。
1.5 数据分析
采用Microsoft Excel 2007 进行数据处理;SPSS 22.0进行单因素方差分析,以p<0.05作为显著性的标准;主成分分析法(principal component analysis,PCA)采用Canoco 4.5分析与作图。
2 结果与分析
2.1 不同LMWOAs对梨土壤pH、EC的影响
不同LMWOAs处理下梨树土壤pH、EC及有机质含量列于图1。LMWOAs处理降低了梨树0~20 cm土壤pH,其中HM、柠檬酸(LC与HC)与草酸(LO与HO)处理较对照显著降低,而LM 则与对照无显著差异。而在>20~40 cm土壤中:柠檬酸与草酸较对照显著降低,而苹果酸处理(LM与HM)与对照之间无显著差异,其中HM较对照升高。LMWOAs处理降低了土壤EC 值,其中HM、柠檬酸与草酸较对照显著降低,而LM 与对照之间无显著差异。比较不同LMWOAs低浓度与高浓度处理对土壤EC的影响可知,柠檬酸与草酸的高浓度处理土壤EC值较低浓度有所提升,而LM较HM升高了土壤EC值。与对照相比,LMWOAs 处理均降低了0~20 cm土层土壤有机质含量,苹果酸、柠檬酸以及LO较对照显著降低,其中苹果酸处理(LM与HM)较对照下降幅度最大,分别降低49.44%与36.52%。在>20~40 cm 土层:柠檬酸与LO 处理土壤有机质含量较对照显著下降,而苹果酸与HO 处理较对照升高,其中HM 与HO较对照显著升高35.90%与34.62%。
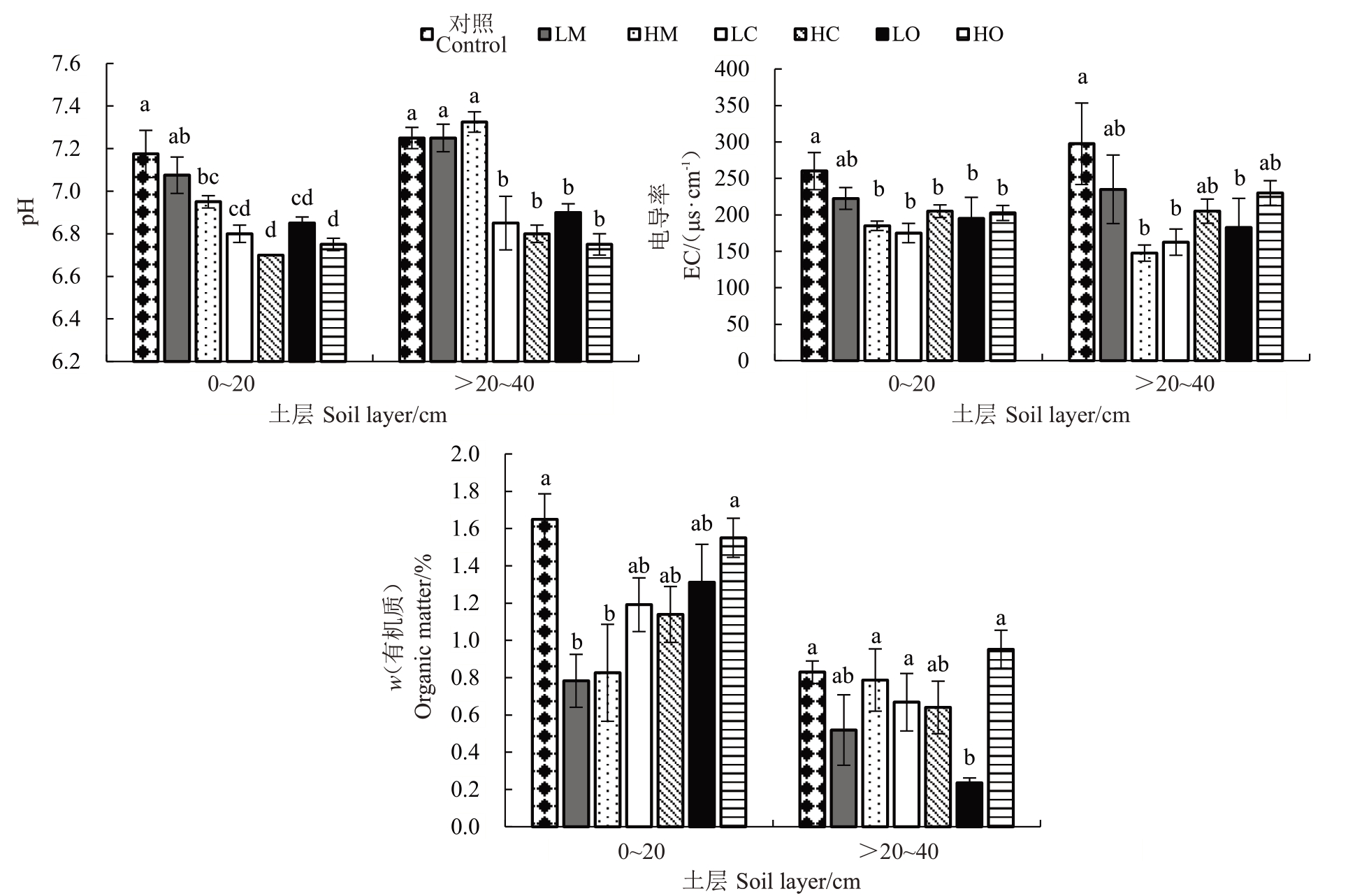
图1 不同LMWOAs 处理下梨树土壤pH、EC 及有机质含量
Fig.1 Soil pH,EC and organic matter content of pear trees with different LMWOAs
同一土层不同小写字母表示不同处理之间差异显著(p<0.05)。下同。
Different small letters in the same soil layer indicate significant differences among different treatments(p<0.05).The same below.
2.2 不同LMWOAs对梨土壤养分含量的影响
不同LMWOAs 处理梨树土壤养分变化列于图2。柠檬酸处理较对照降低了0~20 cm 土壤硝态氮含量,其中LC较对照显著降低34.28%,而苹果酸与草酸低浓度处理(LM 与LO)高于高浓度处理(HM与HO)。HM 与LC 显著降低了>20~40 cm 土壤硝态氮含量,较对照降低54.46%与44.22%,HC显著低于LM,而HC 与HO 显著高于LC 及LO。与对照相比,LMWOAs 处理均提高了土壤铵态氮含量,其中0~20 cm 草酸处理(LO 与HO)较对照显著提高49.37%与54.54%,而>20~40 cm 土层LO 较对照显著升高51.84%。与对照相比,LMWOAs显著降低了0~20 cm土层土壤有效P含量,其中柠檬酸处理(LC与HC)较对照减低44.26%与41.93%。LM与HO较对照显著提高>20~40 cm 土层土壤有效P,分别升高31.31%与25.18%,而柠檬酸较对照降低,其中HC较对照显著降低31.67%。与对照相比,LMWOAs显著降低了0~20 cm 土层土壤速效K 含量,其中HM、柠檬酸及草酸较对照显著降低。苹果酸较对照提高了>20~40 cm 土壤速效K 含量,其中LM 较对照显著升高37.23%。而柠檬酸与草酸较对照降低了>20~40 cm 土壤速效K 含量,其中HC、LO 及HO 较对照显著降低35.60%、38.28%及43.61%。与低浓度LMWOAs相比,高浓度LMWOAs降低了0~40 cm土壤速效K含量。
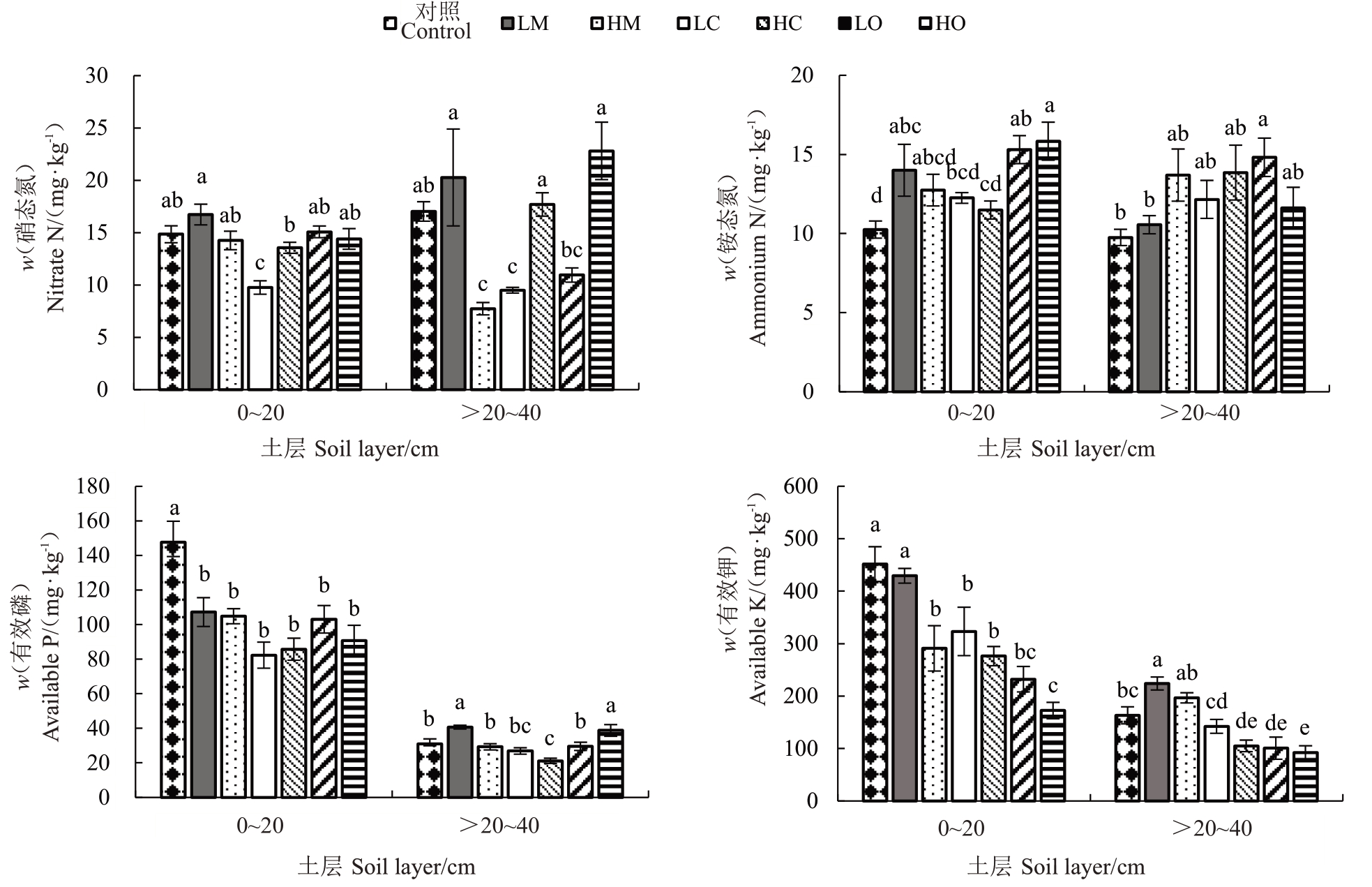
图2 不同LMWOAs 处理下梨树土壤养分含量
Fig.2 Soil nutrient content of pear trees with different LMWOAs
2.3 不同LMWOAs对梨土壤微生物群落的影响
各LMWOAs 处理梨树土壤微生物功能多样性指数列于表1。柠檬酸处理(LC 与HC)与LO 较对照提高了土壤均匀度指数E,其中在0~20 cm 土层HC较对照显著提高。HM、柠檬酸以及草酸较对照提高了0~20 cm 土层土壤微生物优势度指数D 值,其中HM、LC、草酸较对照显著升高。在>20~40 cm土层:LO 较对照显著降低了D 值,而其他处理与对照之间无显著差异。与对照相比,LMWOAs升高了0~20 cm 土壤微生物多样性指数H’值,其中HM、柠檬酸及HO较对照显著升高,而LMWOAs降低了>20~40 cm 土壤微生物多样性指数H’值,其中草酸处理(LO 与HO)较对照显著下降。在0~20 cm,LMWOAs 处理与对照的土壤微生物群落均一指数U 无显著差异,其中HC 与LO 较LM 显著升高。在>20~40 cm,柠檬酸与草酸较对照降低,其中LC、HC 及LO 较对照显著升高,而苹果酸与对照之间无显著差异。HM 较对照显著升高了0~20 cm 土壤微生物丰富度指数S,而其他处理与对照无显著差异。LO 较对照显著降低了>20~40 cm 土壤丰富度指数S值,而其他处理与对照无显著差异。
不同LMWOAs 处理下梨树土壤微生物群落碳源利用呈现多样性,选取第一主成分与第二主成分进行作图(图3),PC1与PC2分别为57.8%与8.9%。相同土层土样成簇,土层差异主要表现在PC1上,其中0~20 cm土层土样分布在第2、3象限,而>20~40 cm土层土样分布在第1、3及4象限。LM与对照处理微生物群落碳源代谢相似,因此,在0~20 cm 与>20~40 cm土层中2个处理均为相近。LMWOAs添加量的差异主要表现在PC2 上,其中柠檬酸与草酸低浓度处理在PC2上的得分高于高浓度。
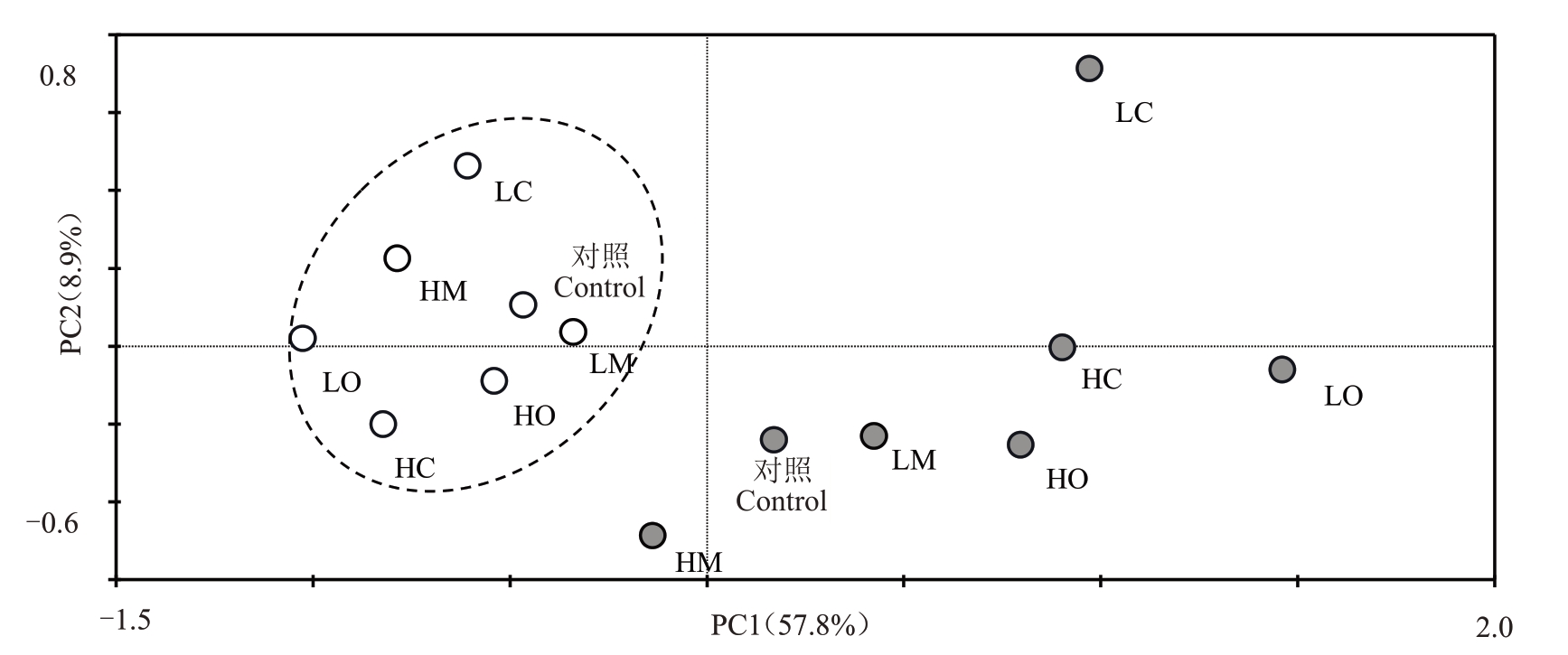
图3 不同LMWOAs 处理下梨树土壤微生物群落功能多样性主成分分析
Fig.3 Principal component analysis of functional diversity of pear soil microbial community with different LMWOAs
白色与灰色圆形分别表示0~20 cm 与>20~40 cm 土层土样。
Soil samples of 0-20 cm and >20-40 cm soil layers are represented by white and gray circles,respectively.
不同LMWOAs 处理对微生物群落碳源利用强度的情况列于图4。0~20 cm 土层中:HM 处理下微生物群落对聚合物的利用强度最高,显著高于LM与HO,其中LM 较对照显著降低。HC 处理下微生物群落对碳水化合物的利用强度最高,且显著高于对照、LM及LC。LMWOAs处理土壤微生物群落对酚类的利用与对照之间无显著差异,而LO 显著高于其他LMWOAs 处理。各LMWOAs 处理(除LM外)升高了氨基酸类利用率,其中HM、LO较对照显著升高。HC 较对照显著升高了胺类的利用率,而LM显著降低了对胺类的利用率。>20~40 cm土层中:各处理之间在聚合物的利用强度上无显著性差异,其中草酸处理较对照降低。HM 较对照显著升高了碳水化合物的利用强度,而LC较对照显著降低了碳水化合物的利用强度。LMWOAs 处理与对照在酚类的利用强度上无显著性差异,而HM较LO显著升高。与对照相比,LMWOAs处理显著降低了土壤微生物群落对羧酸类物质的利用率,其中LO 最低。与对照相比,草酸降低了土壤微生物对氨基酸的利用强度,其中LO 较对照显著降低。各处理对胺类的利用率无显著差异。

图4 LMWOAs 处理对于梨树土壤微生物群落对6 类碳源的利用强度
Fig.4 Utilization intensity of pear soil microbial community to six types of carbon sources with LMWOAs
PM.聚合物;CH.碳水化合物;PH.酚类;CA.羧酸类;AA.氨基酸;AN.胺类。
PM.Polymer;CH.Carbohydrate;PH.Phenol;CA.Carboxylic acid;AA.Amino acid;AN.Amine.
2.4 LMWOAs 处理下微生物群落与土壤养分、微生物群落之间的关系
梨树土壤微生物群落与土壤养分之间的相关性分析列于表2。0~20 cm 土层的微生物群落与土壤养分含量均呈显著负相关,而>20~40 cm土层微生物群落(除E 外)与有机质、速效K 含量呈显著正相关,与铵态氮含量呈显著负相关。
表2 LMWOAs 处理下梨土壤微生物群落与养分之间的相关性分析
Table 2 Correlation analysis between pear soil microbial community and nutrients with LMWOAs
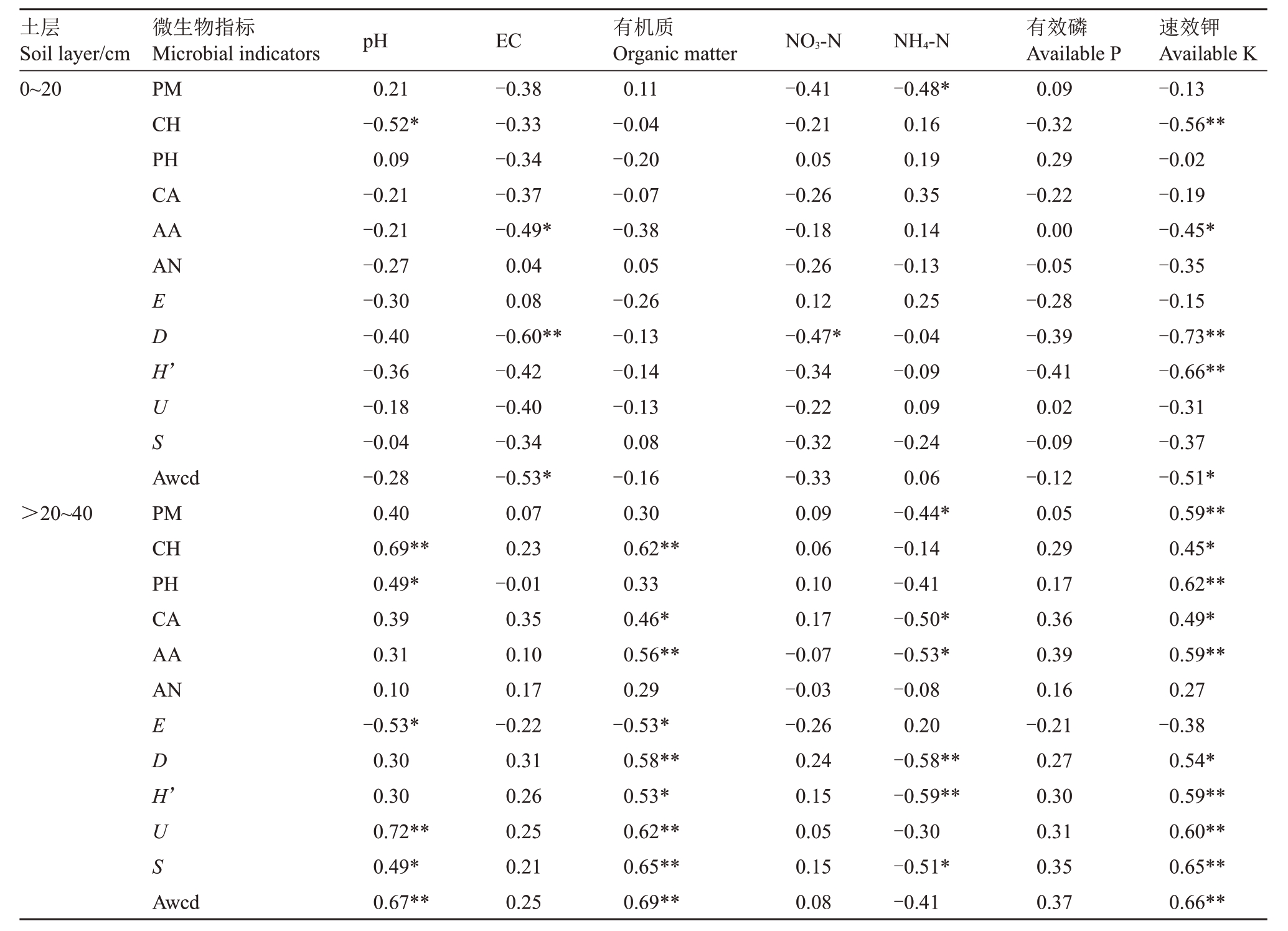
注:**表示p ≤0.01,*表示p ≤0.05;PM、CH、PH、CA、AA 及AN 分别表示聚合物、碳水化合物、酚类、羧酸类、氨基酸及胺类;E、D、H’、U及S 分别表示均匀度、优势度、多样性、均一性及丰富度指数。Awcd.平均颜色变化率。下同。
Note: **Correlation is significant at the 0.01 level. *Correlation is significant at the 0.05 level; PM, Polymer; CA, Carbohydrates; PH, Phenols;CA, Carboxylic acids;AA,Amino acid;AN,Amine; E, D, H’, U and S represent Pielou substrate evenness, Simpson index, Shannon's diversity index,McIntosh idex and substrate richness,respectively.Awcd.Average well color development.The same below.
速效钾Available K-0.13-0.56**-0.02-0.19-0.45*-0.35-0.15-0.73**-0.66**-0.31-0.37-0.51*0.59**0.45*0.62**0.49*0.59**0.27-0.38 0.54*0.59**0.60**0.65**0.66**土层Soil layer/cm 0~20微生物指标Microbial indicators PM CH PH CA AA AN ED H ’US>20~40 Awcd PM CH PH CA AA AN ED H ’US Awcd pH 0.21-0.52*0.09-0.21-0.21-0.27-0.30-0.40-0.36-0.18-0.04-0.28 0.40 0.69**0.49*0.39 0.31 0.10-0.53*0.30 0.30 0.72**0.49*0.67**EC-0.38-0.33-0.34-0.37-0.49*0.04 0.08-0.60**-0.42-0.40-0.34-0.53*0.07 0.23-0.01 0.35 0.10 0.17-0.22 0.31 0.26 0.25 0.21 0.25有机质Organic matter 0.11-0.04-0.20-0.07-0.38 0.05-0.26-0.13-0.14-0.13 0.08-0.16 0.30 0.62**0.33 0.46*0.56**0.29-0.53*0.58**0.53*0.62**0.65**0.69**NO3-N-0.41-0.21 0.05-0.26-0.18-0.26 0.12-0.47*-0.34-0.22-0.32-0.33 0.09 0.06 0.10 0.17-0.07-0.03-0.26 0.24 0.15 0.05 0.15 0.08 NH4-N-0.48*0.16 0.19 0.35 0.14-0.13 0.25-0.04-0.09 0.09-0.24 0.06-0.44*-0.14-0.41-0.50*-0.53*-0.08 0.20-0.58**-0.59**-0.30-0.51*-0.41有效磷Available P 0.09-0.32 0.29-0.22 0.00-0.05-0.28-0.39-0.41 0.02-0.09-0.12 0.05 0.29 0.17 0.36 0.39 0.16-0.21 0.27 0.30 0.31 0.35 0.37
0~20 cm:pH 与碳水化合物的利用强度呈显著负相关,系数为-0.52。EC 与氨基酸的利用强度、awcd 呈显著负相关,而与指数D 呈极显著负相关(r =-0.60),硝态氮、铵态氮含量分别与指数D 及聚合物利用强度呈显著负相关,系数分别为-0.47与-0.48。速效K 含量与碳水化合物及氨基酸类利用强度呈显著负相关,与指数D及H’呈极显著负相关(r=-0.73与-0.66)。
>20~40 cm:pH 与碳水化合物、酚类利用强度呈显著正相关(r=0.69 与0.49),与微生物指数U、S及awcd呈显著正相关,与E呈显著负相关。有机质含量与碳水化合物、羧酸类及氨基酸类的利用强度呈显著正相关,与微生物指数(除E 外)呈显著正相关。铵态氮含量与聚合物、羧酸类及氨基酸类利用强度呈显著负相关,与指数D、H’及S呈显著负相关(系数分别为-0.58、-0.59 及-0.51)。速效K 含量与六大碳源(除胺类外)均呈显著正相关,其中与聚合物、酚类及氨基酸类呈极显著正相关,系数分别为0.59、0.62 及0.59。速效K 含量与微生物群落指数(除E外)呈显著正相关。
比较不同土层土壤养分含量与微生物群落之间的关系发现,>20~40 cm土层微生物群落与养分的关系更为紧密,而速效钾含量与0~20 cm 土层微生物群落指数的关系均为显著负相关,与>20~40 cm土层微生物群落的关系呈显著正相关。
梨树0~20 cm 土层微生物群落与果实品质及养分含量之间的关系如表3 所示。聚合物利用强度与Vc 含量呈极显著负相关(r =-0.61),与果皮着色L与b呈显著正相关。碳水化合物利用强度与可溶性糖含量、果实N 含量、果实K 含量呈显著负相关,与可滴定酸含量呈极显著正关。酚类利用强度与果实N 含量呈显著负相关。氨基酸利用强度与Vc 含量及果实氮含量呈显著负相关,系数分别为-0.46 与-0.58。胺类利用强度与可溶性固形物、Vc、果实K、果实P 含量以及公顷产量呈显著负相关,与可滴定酸含量呈显著正相关。微生物指数E与果品着色a、叶片P 含量呈显著正相关,与果皮着色H°呈显著负相关。指数U 与可溶性糖、Vc、果实N、果实K 含量呈显著负相关,与可滴定酸含量呈显著正相关。指数S 与叶片P 含量呈显著负相关。awcd 与可溶性糖、Vc、果实N、果实K 含量呈显著负相关。
表3 LMWOAs 处理下0~20 cm 土壤微生物群落与果实品质及养分之间的相关性分析
Table 3 Correlation analysis between pear soil microbial community and fruit quality and nutrients with LMWOAs at 0-20 cm soil layer
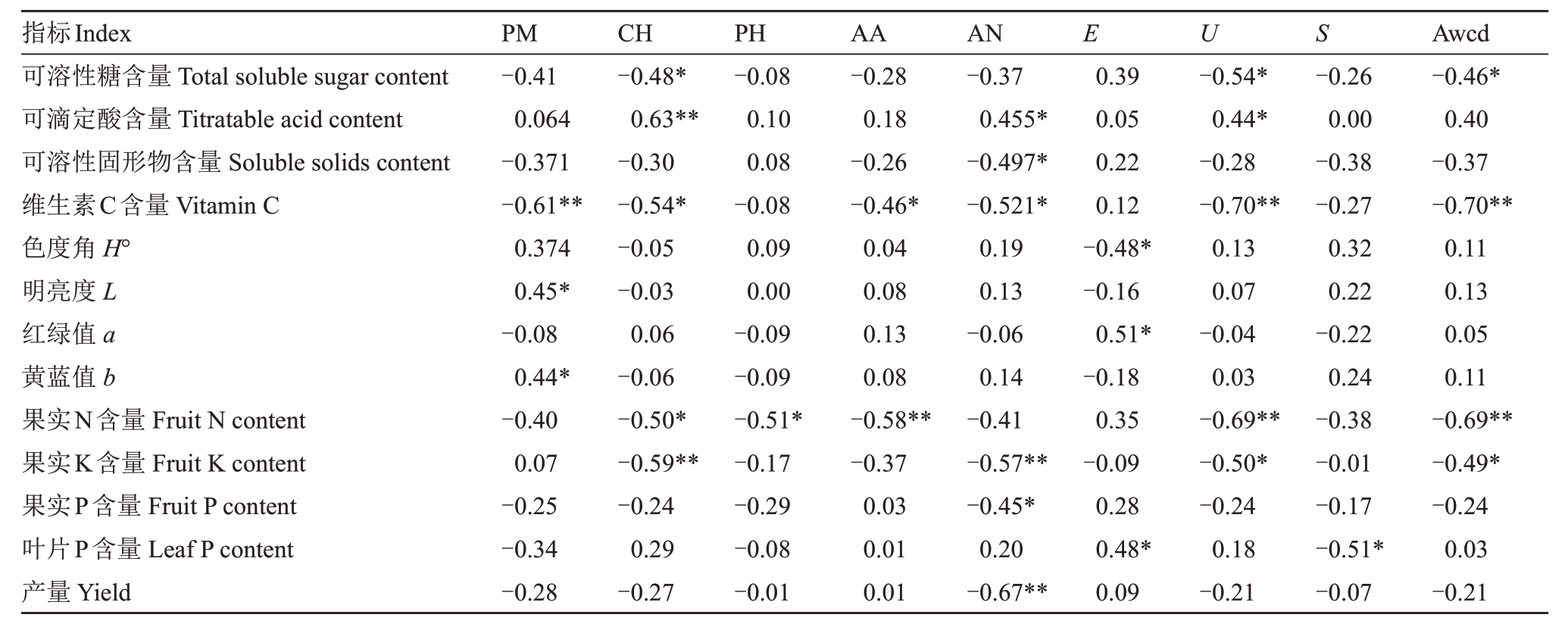
指标Index可溶性糖含量Total soluble sugar content可滴定酸含量Titratable acid content可溶性固形物含量Soluble solids content维生素C含量Vitamin C色度角H°明亮度L红绿值a黄蓝值b果实N含量Fruit N content果实K含量Fruit K content果实P含量Fruit P content叶片P含量Leaf P content产量Yield PM-0.41 0.064-0.371-0.61**0.374 0.45*-0.08 0.44*-0.40 0.07-0.25-0.34-0.28 CH-0.48*0.63**-0.30-0.54*-0.05-0.03 0.06-0.06-0.50*-0.59**-0.24 0.29-0.27 PH-0.08 0.10 0.08-0.08 0.09 0.00-0.09-0.09-0.51*-0.17-0.29-0.08-0.01 AA-0.28 0.18-0.26-0.46*0.04 0.08 0.13 0.08-0.58**-0.37 0.03 0.01 0.01 AN-0.37 0.455*-0.497*-0.521*0.19 0.13-0.06 0.14-0.41-0.57**-0.45*0.20-0.67**E U S 0.39 0.05 0.22 0.12-0.48*-0.16 0.51*-0.18 0.35-0.09 0.28 0.48*0.09-0.54*0.44*-0.28-0.70**0.13 0.07-0.04 0.03-0.69**-0.50*-0.24 0.18-0.21-0.26 0.00-0.38-0.27 0.32 0.22-0.22 0.24-0.38-0.01-0.17-0.51*-0.07 Awcd-0.46*0.40-0.37-0.70**0.11 0.13 0.05 0.11-0.69**-0.49*-0.24 0.03-0.21
梨树>20~40 cm 土层微生物群落与果实品质及养分含量之间的关系如表4所示。聚合物利用强度与单果质量呈显著正相关,系数分别为0.49 与0.45。碳水化合物与叶片N含量呈显著正相关。羧酸类利用强度与果实着色指数H°、L 及b 呈显著正相关,系数分别为0.54、0.51 及0.56。果实K 含量与微生物群落呈显著正相关,其中与聚合物、氨基酸类利用强度的相关系数分别为0.45 与0.65,与微生物群落指数D、H’、U、S及awcd呈显著正相关,系数分别为0.65、0.46、0.51、0.49、0.48及0.53。
表4 LMWOAs 处理下>20~40 cm 土壤微生物群落与果实品质及养分之间的相关性分析
Table 4 Correlation analysis between pear soil microbial community and fruit quality and nutrients with LMWOAs at >20-40 cm soil layer

指标Index单果质量Mean Fruit mass/g色度角H°明亮度L黄蓝值b果实N含量Fruit N content果实K含量Fruit K content果实P含量Fruit P content叶片N含量Leaf N content PM 0.49*0.09-0.15-0.12 0.08 0.45*0.23-0.05 CH 0.22 0.27 0.16 0.15 0.19 0.33 0.01 0.48*CA-0.04 0.54*0.51*0.56**0.24 0.42-0.09-0.14 AA 0.30 0.03 0.07 0.14 0.42 0.65**0.22-0.21 D U S 0.28 0.20 0.00 0.07 0.41 0.46*0.21-0.15 H’0.27 0.20 0.08 0.15 0.40 0.51*0.21-0.25 0.28 0.32 0.26 0.28 0.32 0.49*0.06 0.31 0.30 0.19 0.02 0.06 0.39 0.48*0.12 0.03 Awcd 0.31 0.34 0.24 0.27 0.33 0.53*0.08 0.19
3 讨 论
3.1 LMWOAs改变梨园土壤养分吸收
LMWOAs作为植物根际常见的分泌物,与植物养分的吸收密切相关,原因是改变根系周围pH,提升某些难溶性养分的溶解度[9-10],通过其较强的螯合力,与多种金属离子形成复合物进而促进植物对金属离子的吸收[10]。
pH 作为影响土壤矿质元素有效态的因素之一,作用于植物养分吸收与利用[28]。笔者在本研究中发现,草酸与柠檬酸显著降低了梨树土壤pH,而苹果酸对土壤pH 的影响与土层深度及添加量有关。有机物添加后,土壤氮循环涉及的硝化作用会导致土壤pH 值的下降,这与本研究结果保持一致,即HO 显著降低了土壤pH,而HO 在>20~40 cm 土壤中硝态氮含量高于对照[29-30]。另外,苹果酸与氮磷钾复配降低了葡萄根际土壤pH[31]。而诸多文献表明,有机酸的添加提高了土壤pH,可能是由于有机酸添加后土壤微生物群落在降解羧酸的同时,消耗了H+并释放出OH-与CO2[32-34]。土壤电导率EC为评价土壤盐渍化的重要指标[35],而LMWOAs 则降低了梨园土壤EC 值,其中5%柠檬酸与草酸处理EC 值显著降低。而苹果酸则升高了葡萄根际土壤EC 值,其中5%苹果酸处理显著高于对照与10%苹果酸处理[31]。
LMWOAs作为碳氮代谢偶联的枢纽,与植物氮素代谢相关。苹果酸作为固氮菌呼吸的重要能量来源,为氮固定提供了大量的碳骨架[36-38]。氮矿化受土壤中有机质降解控制[39],而根际有机酸为微生物群落提供了大量能量,以生产催化有机物分解的胞外酶[40]。另外,有机酸的累积有助于土壤有机质从矿化物中的释放,进而进一步加速了氮素矿化[41]。因此苹果酸、柠檬酸及草酸提高了土壤铵态氮的含量,同时5%苹果酸提高了土壤硝态氮的含量。
速效磷含量影响着植物对磷的摄取[42],Wang等[43]发现低分子质量有机酸能够同时增强无机磷(Pi)与有机磷(Po)释放到土壤中。根际产生苹果酸、柠檬酸以及草酸等,用于溶解微溶性磷酸岩中的磷元素,而比较各有机酸的溶磷效率发现,草酸>柠檬酸>苹果酸[44]。因此,10%草酸处理显著提高了>20~40 cm 土层速效磷含量。植物根际分泌的苹果酸与土壤中磷含量密切相关,土壤磷缺乏时构树、桑树、诸葛菜以及欧洲油菜根际苹果酸分泌量较低磷与磷含量丰富状态下显著升高[45]。因此,5%苹果酸处理下梨树土壤有效磷含量较对照显著升高。
5%苹果酸促进了梨树对钾元素的吸收,而柠檬酸与草酸阻碍了梨树对钾的吸收。在葡萄上的研究也发现了苹果酸与氮磷钾复配有助于葡萄对于钾的吸收[31]。而高浓度草酸易与土壤中金属离子发生化学反应,形成草酸钙等沉淀[46],降低了金属离子的移动性,进而造成土壤板结等现象,最终抑制植物对于钾元素的吸收。
3.2 LMWOAs改变梨园土壤微生物群落
LMWOAs 作为植物根际常见的一类根际分泌物,对根际微生物群落有筛选作用[11,17,47-49],而LMWOAs与氮磷钾复配显著改变了梨树土壤微生物群落特征。而5%草酸与氮磷钾复配降低了梨树土壤微生物群落的多样性指数与丰富度。Li等[50]在研究草酸对富多环芳烃土壤微生物群落的影响时发现,草酸处理降低了土壤细菌群落的多样性指数。Ma等[51]发现10 mmol·kg-1草酸与苹果酸均能显著升高富铬土壤微生物群落多样性指数。
3.3 LMWOAs 处理下微生物群落与土壤养分、微生物群落之间的关系
笔者在本研究中发现梨树>20~40 cm 土层微生物群落与土壤养分及果实品质密切相关。这可能与梨树根的分布相关,在研究梨树施肥与其根系发育时,发现梨树根系在土壤中扎根较深,更易于吸收深层土壤的养分[52]。李宏等[53]在研究库尔勒香梨时发现,与0~20 cm土层相比,>20~40 cm土层吸收根与疏导根的根长密度明显增加。伍从成等[54]研究发现,梨树根尖数量随着土壤的垂直深度的增加呈先增加后减少的趋势,其中30~60 cm土层深度的根尖分布最多。因此,在本研究中>20~40 cm土层土壤与梨果实品质之间关系更为密切。
钾肥与根际微生物群落的多样性密切相关,Wei 等[55]发现葡萄根际土壤的速效钾含量作用于细菌群落结构,与Chloroflexi、Rokubacteria及Nitrospirae 等呈显著负相关。笔者在研究苹果酸与葡萄果实品质关系时发现[31],苹果酸处理下根际微生物群落的差异拥有提升葡萄果实品质的潜质,即葡萄根际Woeseiaceae、Bacillaceae 含量与果实钾及可溶性糖含量呈显著正相关。
4 结 论
LMWOAs 与氮磷钾复配显著影响红宝石梨园土壤有效态养分含量与微生物群落,其中5%苹果酸与氮磷钾肥复配明显改善了梨树土壤养分环境。另外,LMWOAs与氮磷钾肥配施下梨园土壤微生物群落与梨养分含量及果实品质之间密切相关。与0~20 cm 土层相比,>20~40 cm 土层梨园土壤微生物群落在梨树养分吸收与果实品质提升方面发挥更为重要的作用。
[1] 周江涛,赵德英,陈艳辉,康国栋,程存刚.我国梨生产布局变动分析[J].中国果树,2021(4):92-97.ZHOU Jiangtao,ZHAO Deying,CHEN Yanhui,KANG Guodong,CHENG Cungang. Empirical analysis of pear spatial distribution variation in China[J].China Fruits,2021(4):92-97.
[2] 于会丽,司鹏,邵微,徐国益,乔宪生,王玉红,杨晓静.海藻酸水溶肥对梨树生长与果实产量及品质的影响[J].果树学报,2019,36(5):603-611.YU Huili,SI Peng,SHAO Wei,XU Guoyi,QIAO Xiansheng,WANG Yuhong,YANG Xiaojing. Effect of water soluble alginic acid fertilizer on the growth,yield and quality of pear[J].Journal of Fruit Science,2019,36(5):603-611.
[3] 卢树昌,陈清,张福锁,贾文竹.河北果园主分布区土壤磷素投入特点及磷负荷风险分析[J].中国农业科学,2008,41(10):3149-3157.LU Shuchang,CHEN Qing,ZHANG Fusuo,JIA Wenzhu.Characteristics of soil phosphorus input and phosphorus load risk in majororchards region of Hebei[J]. Scientia Agricultura Sinica,2008,41(10):3149-3157.
[4] 董彩霞,姜海波,赵静文,徐阳春.我国主要梨园施肥现状分析[J].土壤,2012,44(5):754-761.DONG Caixia,JIANG Haibo,ZHAO Jingwen,XU Yangchun.Current fertilization in pear orchards in China[J].Soils,2012,44(5):754-761.
[5] 丁邦新,刘雪艳,何雪菲,陈波浪,柴仲平.‘库尔勒香梨’园测土配方推荐施肥研究[J].果树学报,2019,36(8):1020-1028.DING Bangxin,LIU Xueyan,HE Xuefei,CHEN Bolang,CHAI Zhongping.Recommendation of fertilization for‘Kuerlexiangli’pear orchards based on soil testing[J]. Journal of Fruit Science,2019,36(8):1020-1028.
[6] 王厚臣,史作安,梁美霞,武成伟,孙岳东.果园土壤健康状态与苹果健康栽培[J].落叶果树,2019,51(2):63-64.WANG Houchen,SHI Zuoan,LIANG Meixia,WU Chengwei,SUN Yuedong. Soil health status of orchard and healthy apple cultivation[J].Deciduous Fruits,2019,51(2):63-64.
[7] 张江周,李宝深.提升集约化香蕉园土壤健康水平的根层调控策略与途径[J].土壤通报,2021,52(2):398-407.ZHANG Jiangzhou,LI Baoshen. Root-zone management strategies to improve soil health in an intensive banana orchard[J].Chinese Journal of Soil Science,2021,52(2):398-407.
[8] UBEYNARAYANA N,JEYAKUMAR P,BISHOP P,PEREIRA RC,ANDERSON C W. Effect of soil cadmium on root organic acid secretion by forage crops[J]. Environmental Pollution,2021,268:115839.
[9] 韩振海,沈隽,王倩.园艺植物根际营养学的研究:文献述评[J].园艺学报,1993,20(2):116-122.HAN Zhenhai,SHEN Jun,WANG Qian. Studies on rhizosphere nutrition of horticultural crops:literature review[J]. Acta Horticulturae Sinica,1993,20(2):116-122.
[10] 许衡,杨和生,徐英,毛志泉,束怀瑞.果树根际微域环境的研究进展[J]. 山东农业大学学报(自然科学版),2004,35(3):476-480.XU Heng,YANG Hesheng,XU Ying,MAO Zhiquan,SU Huairui. Research progress on rhizosphere environment of fruit trees[J]. Journal of Shandong Agricultural University (Natural Science Edition),2004,35(3):476-480.
[11] YUAN J,RAZA W,SHEN Q R. Root exudates dominate the colonization of pathogen and plant growth-promoting rhizobacteria[J].Root Biology,2018,52:167-180.
[12] MEENA V S,BAHADUR I,MAURYA B R,KUMAR A,MEENA R K,MEENA S K,VERMA J P.Potassium-solubilizing microorganism in evergreen agriculture:An overview[M]. New Delhi:Springer,2016.
[13] SÁNCHEZ Ó J,OSPINA D A,MONTOYA S. Compost supplementation with nutrients and microorganisms in composting process[J].Waste Management,2017,69:136-153.
[14] FERY S D. Mycorrhizal fungi as mediators of soil organic matter dynamics[J].Annual Review of Ecology,Evolution,and Systematics,2019,50(1):237-259.
[15] RAZA W,LING N,LIU D Y,WEI Z,HUANG Q W,SHEN Q R.Volatile organic compounds produced by Pseudomonas fluorescens WR-1 restrict the growth and virulence traits of Ralstonia solanacearum[J].Microbiological Research,2016,192:103-113.
[16] DANIEL V D L,SAFIYH T,SÉBASTIEN M,JORG S,LISA M,RICHARD F I,ALISTAIR R,XIAO W,WEI Z,NELE W,JACO V,LEE N. Poplar and its bacterial endophytes:Coexistence and harmony[J].Critical Reviews in Plant Sciences,2009,28(5):346-358.
[17] LAKSHMANAN V,KITTO S L,CAPLAN J L,HSUEH Y H,KEARNS D B,WU Y S,BAIS H P.Microbe-associated molecular patterns-triggered root responses mediate beneficial rhizobacterial recruitment in Arabidopsis[J]. Plant Physiology,2012,160(3):1642-1661.
[18] 邵微,徐国益,于会丽,高登涛,刘远,司鹏.低分子有机酸水溶肥提升梨叶片光合、养分吸收及果实品质[J].果树学报,2022,39(6):992-1003.SHAO Wei,XU Guoyi,YU Huili,GAO Dengtao,LIU Yuan,SI Peng. Low molecular weight organic acid water-soluble fertilizer improves leaf photosynthesis and fruit quality of pear[J].Journal of Fruit Science,2022,39(6):992-1003.
[19] 鲍士旦.土壤农化分析[M].北京:中国农业出版社,2000.BAO Shidan.Soil agrochemical analysis[M].Beijing:China Agricultural Press,2000.
[20] 鲁如坤.土壤农业化学分析方法[M].北京:中国农业科技出版社,2000.LU Rukun. Method of soil agrochemical analysis[M]. Beijing:Chinese Agricultural Science and Technology Press,2000.
[21] 司鹏,邵微,于会丽,乔宪生,高登涛,王志强,杨健.樱桃大苗培育过程中土壤微生物功能多样性与酶活性的变化[J].生态与农村环境学报,2016,32(4):609-614.SI Peng,SHAO Wei,YU Huili,QIAO Xiansheng,GAO Dengtao,WANG Zhiqiang,YANG Jian. Changes in microbial functional diversity andenzyme activity in soil during cherry sapling cultivation[J].Journal of Ecology and Rural Environment,2016,32(4):609-614.
[22] 司鹏,邵微,于会丽,乔宪生,杨晓静,高登涛,王志强.小分子有机物对土壤酶活性及微生物多样性的影响[J].中国土壤与肥料,2019(2):75-82.SI Peng,SHAO Wei,YU Huili,QIAO Xiansheng,YANG Xiaojing,GAO Dengtao,WANG Zhiqiang. Response of microbial communities and enzyme activities to soil amendment with lowmolecular-weight organic substances [J]. Soil and Fertilizer Sciences in China,2019(2):75-82.
[23] 邵微,于会丽,张培基,徐国益,乔宪生,高登涛,王志强,田鹏,司鹏.不同落叶果树根际微生物群落代谢与组成的差异性研究[J].果树学报,2020,37(9):1371-1383.SHAO Wei,YU Huili,ZHANG Peiji,XU Guoyi,QIAO Xiansheng,GAO Dengtao,WANG Zhiqiang,TIAN Peng,SI Peng.Differences in metabolism and composition of microbial communities in rhizosphere soil with different deciduous fruit trees[J].Journal of Fruit Science,2020,37(9):1371-1383.
[24] JIANG Z,LI P,WANG Y H,LI B,WANG Y X.Effects of roxarsone on the functional diversity of soil microbial community[J].International Biodeterioration&Biodegradation,2013,76:32-35.
[25] WU L K,LI Z,LI J F,KHAN M A,HUANG W M,ZHANG Z Y,LIN W X. Assessment of shifts in microbial community structure and catabolic diversity in response to Rehmannia glutinosa monoculture[J].Applied Soil Ecology,2013,67:1-9.
[26] 王学奎,黄见良.植物生理生化实验原理与技术[M].北京:高等教育出版社,2015.WANG Xuekui,HUANG Jianliang. Principles and techniques of plant physiological biochemical experiment[M]. Beijing:Higher Education Press,2015.
[27] 曹建康,姜微波,赵玉梅.果蔬采后生理生化实验指导[M].北京:中国轻工业出版社,2007.CAO Jiankang,JIANG Weibo,ZHAO Yumei.Physiological and biochemical experiment guidance after fruit and vegetable harvest[M].Beijing:China Light Industry Press,2007.
[28] GONDAL A H,HUSSAIN I,IJAZ A B,ZAFAR A,CH B I,ZAFAR H,SOHAIL M D,KHAN A A,NIAZI H,YOUSAF H,USAMA M,TOUSEEF M,TARIQ M. Influence of soil pH and microbes on mineral solubility and plant nutrition:A review[J].International Journal of Agriculture and Biological Sciences,2021,5(1):71-81.
[29] MARSCHNER B,NOBLE A D. Chemical and biological processes leading to the neutralization of acidity in soil incubated with litter materials[J]. Soil Biology and Biochemistry,2000,32(6):805-813.
[30] YAN F,SCHUBERT S. Soil pH changes after application of plant shoot materials of faba bean and wheat[J]. Plant and Soil,2000,220(1/2):279-287.
[31] SI P,SHAO W,YU H L,XU G Y,DU G Q. Differences in microbial communities stimulated by Malic acid have the potential to improve nutrient absorption and fruit quality of grapes[J].Frontiers in Microbiology,2022,13:850807.
[32] GRAMSS G,VOIGT K D,BUBLITZ F,BERGMANN H. Increased solubility of (heavy) metals in soil during microbial transformations of sucrose and casein amendments[J].Journal of Basic Microbiology,2003,43(6):483-498.
[33] SHI S J,RICHARDSON A E,O’CALLAGHAN M,DEANGELIS K M,JONES E E,STEWART A,FIRESTONE M K,CONDRON L M.Effects of selected root exudate components on soil bacterial communities[J]. FEMS Microbiology Ecology,2011,77(3):600-610.
[34] LIU Y,EVANS S E,FRIESEN M L,TIEMANN L K.Root exudates shift how N mineralization and N fixation contribute to the plant-available N supply in low fertility soils[J]. Soil Biology and Biochemistry,2022,165:108541.
[35] 周林虎,王昊宇,张秉来,祁兆鑫,曹荣泰,范延彬,孙生海,刘宇平.硫酸盐渍土表观电导率与水分、盐分及粒径关系研究[J].干旱区研究,2021,38(4):1020-1030.ZHOU Linhu,WANG Haoyu,ZHANG Binglai,QI Zhaoxin,CAO Rongtai,FAN Yanbin,SUN Shenghai,LIU Yuping. The relationship between ECaof sulfate saline soil and moisture content,salt content,and particle size[J].Arid Zone Research,2021,38(4):1020-1030.
[36] ROSENDAHL L,VANCE C P,PEDERSEN W B. Products of dark CO2 fixation in pea root nodules support bacteroid metabolism[J].Plant Physiology,1990,93(1):12-19.
[37] VANCE C P,HEICHEL G H. Carbon in N2 fixation:Limitation or exquisite adaptation[J]. Annual Review of Plant Biology,1991,42(1):373-390.
[38] SCHULZE J,TESFAYE M,LITJENS R H M G,BUCCIARELLI B,TREPP G,MILLER S,SAMAC D,ALLAN D,VANCE C P.Malate plays a central role in plant nutrition[J].Plant and Soil,2002,247(1):133-139.
[39] HENNERON L,KARDOL P,WARDLE D A,CROS C,FONTAINE S. Rhizosphere control of soil nitrogen cycling:A key component of plant economic strategies[J]. New Phytologist,2020,228(4):1269-1282.
[40] CHENG W X,KUZYAKOV Y.Root effects on soil organic matter decomposition[M]//ZOBEL R W,WRIGHT S F. Roots and Soil Management:Interactions between Roots and the Soil.ASACSSA-SSA,2005:119-143.
[41] KEILUWEIT M,BOUGOURE J J,NICO P S,PETT-RIDGE J,WEBER P K,KLEBER M. Mineral protection of soil carbon counteracted by root exudates[J].Nature Climate Change,2015,5(6):588-595.
[42] 李军,袁亮,赵秉强,李燕婷,温延臣,李伟,林治安.磷肥中腐植酸添加比例对玉米产量、磷素吸收及土壤速效磷含量的影响[J].植物营养与肥料学报,2017,23(3):641-648.LI Jun,YUAN Liang,ZHAO Bingqiang,LI Yanting,WEN Yanchen,LI Wei,LIN Zhian. Effect of adding humic acid to phosphorous fertilizer on maize yield and phosphorus uptake and soil available phosphorus content[J]. Journal of Plant Nutrition and Fertilizer,2017,23(3):641-648.
[43] WANG Y Z,CHEN X,WHALEN J K,CAO Y H,QUAN Z,LU C Y,SHI Y. Kinetics of inorganic and organic phosphorus release influenced by low molecular weight organic acids in calcareous,neutral and acidic soils[J]. Journal of Plant Nutrition and Soil Science,2015,178(4):555-566.
[44] DE OLIVEIRA M G,MURTA H M,VALADARES R V,DA SILVEIRA W B,DA SILVA I R,COSTA M D. Oxalic acid is more efficient than sulfuric acid for rock phosphate solubilization[J].Minerals Engineering,2020,155:106458.
[45] WU Y Y,ZHAO K. Root-exuded malic acid versus chlorophyll fluorescence parameters in four plant species under different phosphorus levels[J]. Journal of Soil Science and Plant Nutrition,2013,13(3):604-610.
[46] 孙榕.高表面活性磷灰石材料应用于吸附草酸和硝基苯的研究[D].南京:南京农业大学,2009.SUN Rong.The study of the application of apatite materials with high surface activity to adsorb oxalic acid and nitrobenzene[D].Nanjing:Nanjing Agricultural University,2009.
[47] BERENDSEN R L,PIETERSE C M J,BAKKER P A H M.The rhizosphere microbiome and plant health[J].Trends in Plant Science,2012,17(8):478-486.
[48] CHEN Y,CAO S G,CHAI Y R,CLARDY J,KOLTER R,GUO J H,LOSICK R. A Bacillus subtilis sensor kinase involved in triggering biofilm formation on the roots of tomato plants[J].Molecular Microbiology,2012,85(3):418-430.
[49] BEAUREGARD P B,CHAI Y R,VLAMAKIS H,LOSICK R,KOLTER R.Bacillus subtilis biofilm induction by plant polysaccharides[J]. Proceedings of the National Academy of Sciences,2013,110(17):E1621-E1630.
[50] LI X N,SONG Y,WANG F,BIAN Y R,JIANG X. Combined effects of maize straw biochar and oxalic acid on the dissipation of polycyclic aromatic hydrocarbons and microbial community structures in soil:A mechanistic study[J]. Journal of Hazardous Materials,2019,364:325-331.
[51] MA H,LI X D,HOU S Y,PENG D H,WANG Y,XU F,XU H.The activation and extraction systems using organic acids and Lentinus edodes to remediate cadmium contaminated soil[J].Environmental Pollution,2019,255:113252.
[52] 邱玉玲.三年施肥对梨园土壤和叶果氮磷钾及产量品质的影响[D].重庆:西南大学,2021.QIU Yuling. Impacts of three-year fertilizations on nitrogen,phosphorus and potassium in soil,leaves and fruits,and fruit yield and quality in pear orchards[D]. Chongqing:Southwest University,2021.
[53] 李宏,程平,郑朝晖,郭光华,杨婵.库尔勒香梨根系空间分布特征[J].西北农业学报,2012,21(12):97-104.LI Hong,CHENG Ping,ZHENG Chaohui,GUO Guanghua,YANG Chan. Spatial distribution characteristics of root of Korla Fragrant pear tree[J].Acta Agriculturae Boreali-Occidentalis Sinica,2012,21(12):97-104.
[54] 伍从成,姜海波,赵静文,范学山,董彩霞,沈其荣,徐阳春.连续5 年施用生物有机肥对梨树根系形态及分布的影响[J].南京农业大学学报,2017,40(3):473-480.WU Congcheng,JIANG Haibo,ZHAO Jingwen,FAN Xueshan,DONG Caixia,SHEN Qirong,XU Yangchun. Effect of continuous application of bio-organic fertilizer for five years on morphology and distribution of pear root[J].Journal of Nanjing Agricultural University,2017,40(3):473-480.
[55] WEI M M,LIU X C,HE Y H,XU X L,WU Z S,YU K,ZHENG X. Biochar inoculated with Pseudomonas putida improves grape (Vitis vinifera L.) fruit quality and alters bacterial diversity[J].Rhizosphere,2020,16:100261.