番木瓜(Carica papaya L.)植株性别分为雄株、雌株和两性株3 种类型,其中两性株果肉厚、品质优,与雌果相比,具有更高的商品价值和经济价值[1-4]。番木瓜的性别由一对性染色体控制,即:雌株基因型为XX,雄株基因型为XY,两性株基因型为XYh[5-6]。与遗传性较稳定的X 染色体相比,雄性特异的Y 染色体(MSY)和两性特异的Yh 染色体(HSY)被高度甲基化或异染色质化,核酸多态性较高[7-8]。HSY 和MSY 染色体大约8.1 Mb,是番木瓜最大的1 号染色体,98.9%~99.6%的序列具有相似性,在非同源区段有1887 个插入缺失和21 088 个SNP,包含27 个差异基因,但大部分都不是性别决定基因[9-12]。其中,SVP-like被认为是雌性抑制基因,在MSY染色体上含MADS-box和K-box,而在HSY染色体上只含K-box,但至今尚无足够证据表明SVP-like参与番木瓜性别分化[3,13-14]。
植物性别决定存在两种机制,第一是遗传型性别决定,第二是环境型性别决定[15]。遗传因子主导番木瓜株性,外界环境影响花性转变,而花性转变主要决定于温度[16-17]。番木瓜的花性分化是在性别决定遗传因子的基础上进行雄性、雌性或两性性状的分化和发育,与外界因素有关,当内外因素有利于某一性别的发育时,就有可能产生跟性别决定遗传物质不一致的结果,在高温、干旱和缺氮等外界压力下,会导致番木瓜雌性不育[18]。番木瓜最适生长温度为26~32 ℃,当外界环境温度高于最适生长温度5 ℃以上时,就会对植物生长形成高温胁迫[1]。随外界环境由低温到高温,番木瓜两性株的花型从雌型两性花(雄蕊心皮化)转变为长圆形两性花(完全花),再转变为雄型两性花(雌蕊发育不全)和雄花(雌蕊完全退化),当环境温度过低时,番木瓜雄株也会形成两性花[2-3,19]。这可能与番木瓜花芽分化过程中植物激素浓度变化及稳态有关[20-21]。
中国番木瓜主产区属热带、南亚热带地区,高温季节时间长。番木瓜两性株在高温条件下性别转变导致间断结果和产量下降是制约番木瓜高效生产的主要因素之一。目前,有关高温导致番木瓜两性株性别转变的分子机制尚未完全阐明。笔者在本研究中基于番木瓜转录组数据,筛选番木瓜两性株雄花和两性花的差异表达基因,并分析两者的内源激素含量差异,为探索番木瓜两性株在高温条件下花性转变的分子调控机制提供新视角,为培育耐高温番木瓜新品种奠定理论依据。
1 材料和方法
1.1 材料及取样
以广东省农业科学院果树研究所自主选育的番木瓜品种GZY3-6两性株为研究对象。选取25株长势相近的组培苗,2019年3月15日定植于广东省农业科学院果树研究所番木瓜资源圃,常规栽培管理,生长状态良好。2019 年7 月16 日11:00—13:00(气温39~40 ℃)采集长度<5 mm 的主花芽,利用体视镜区分雌蕊退化的雄花和有功能性雌蕊的两性花(图1),用液氮速冻后贮存于-80 ℃超低温冰箱备用。样品委托杭州联川生物技术股份有限公司进行转录组测序与分析。
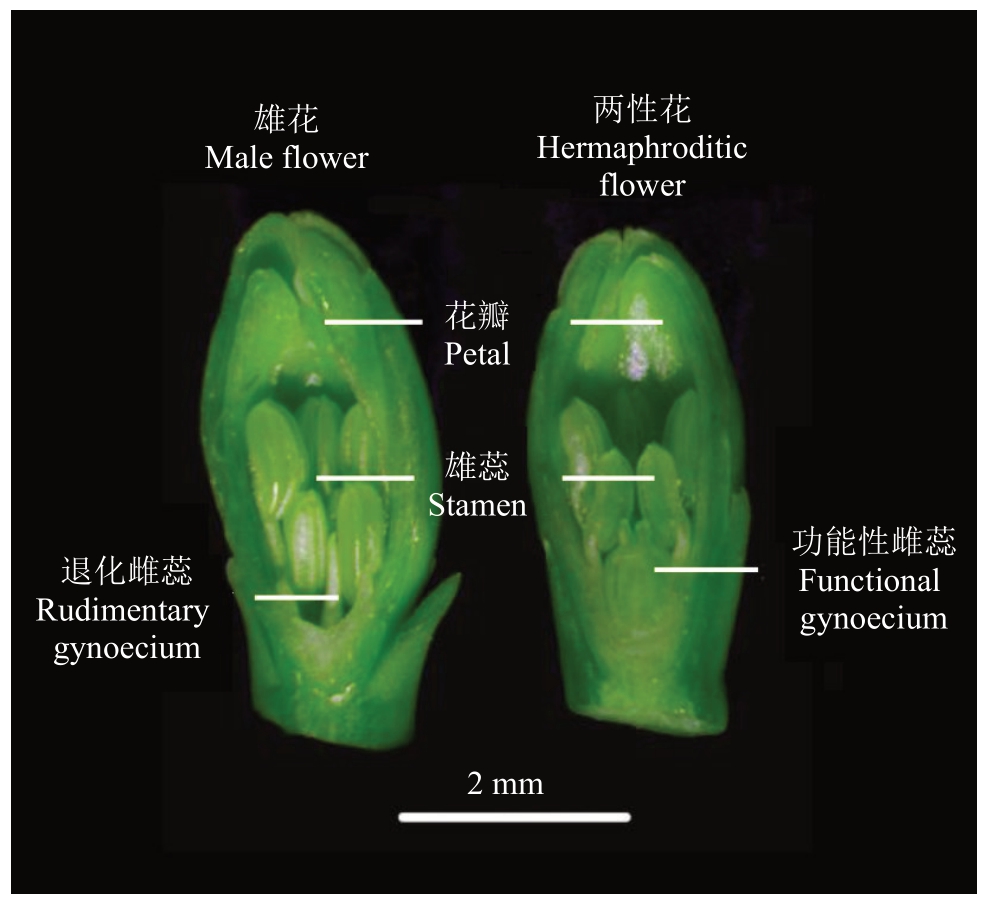
图1 番木瓜雄花和两性花
Fig.1 Male and hermaphroditic flowers in papaya
1.2 内源激素含量测定
取25 mg 样品,加入1 mL 在-40 ℃下预冷的50%乙腈水溶液,匀浆4 min,冰水浴超声5 min,匀浆超声步骤重复3 次,4 ℃下12 000 r·min-1 离心15 min,取上清液,氮气吹干,加入80 μL 10%乙腈水溶液复溶,移至带滤膜的EP管中,4 ℃下12 000 r·min-1离心15 min,取上清液,利用EXIONLC System超高效液相色谱仪和SCIEX 6500 QTRAP+三重四极杆质谱仪测定内源激素含量。设4 个生物学重复,每个生物学重复5~10个花芽。
色谱柱:Waters ACQUITY UPLC CSH C18(150 mm×2.1 mm,1.7 μm)。流动相A:0.01%甲酸水溶液;流动相B:0.01%甲酸乙腈溶液。柱温40 ℃,样品盘4 ℃,进样量5 μL。离子源参数:温度475 ℃,离子喷雾电压4 500 V(正离子模式)/-4 500 V(负离子模式),离子源气体1、气体2 和帘气分别设置为30、30和40 psi。通过SCIEX Analyst Work Station Software 1.6.3 和Sciex MultiQuant 3.0.3 软件 采集质谱数据和定量分析。
1.3 转录组测序与分析
用TRIzol 提取番木瓜花芽总RNA,NanoDROP ND-1000 对总RNA 的浓度与纯度进行质控。使用Oligo(dT)磁珠富集带多聚腺苷酸mRNA,NEB Fragmentation Module对mRNA随机打断,将片段化的mRNA 合成cDNA 第一条链,随后加入RNaseH和DNA polymerase Ⅰ合成cDNA 第二条链。加入dUTP Solution,将双链cDNA末端补齐为平末端,加A尾、连接测序接头。cDNA经消化后,在95 ℃预变性3 min,98 ℃变性15 s,共8个循环,60 ℃退火15 s,72 ℃延伸30 s,最后72 ℃延伸5 min,形成300 bp左右的cDNA 文库。使用Illumina Novaseq 6000 进行测序,测序模式为PE150。设3个生物学重复,每个生物学重复5~10个花芽。
测序后获得原始序列(raw reads),通过cutadapt软件去除接头、重复序列、低质量序列,获得过滤序列(clean reads)。使用HISAT2 v2.0.4将得到的过滤序列比对到番木瓜参考基因组(https://phytozome.jgi.doe.gov/pz/portal.html#!bulk?org=Org_Cpapaya)上,用String Tie 软件对基因或转录本进行初组装,用gffcompare 软件对转录本进行检测和组装注释。利用FPKM(指每百万碱基对测序的转录本序列片段的每千碱基片段的预期数量)计算基因表达水平。通过p 值<0.05 且|log2(fold change)|≥1 为阈值筛选差异表达基因(DEG)。
1.4 差异表达基因的功能注释与富集分析
利用R 包GOseq 软件进行GO(Gene Ontology)富集分析,获得生物学过程、细胞组成和分子功能的GO 注释结果。利用KOBAS 软件对差异表达基因进行KEGG(Kyoto Encyclopedia of Genes and Genomes)通路富集分析。以p 值<0.05 定义为GO 项或KEGG通路显著富集。
1.5 花发育相关基因启动子顺式作用元件分析
从番木瓜参考基因组数据库中下载花发育相关基因的启动子序列(ATG 上游2000 bp 序列),用PlantCARE(http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)网站预测启动子序列的顺式作用元件,利用TBtools软件对预测结果进行可视化绘图[22]。
1.6 实时荧光定量PCR(qRT-PCR)分析
选取17 个花发育和植物激素相关差异表达基因,用Primer Premier 5 软件设计特异引物(表1)。参照周陈平等[23]的方法,使用天根RNAprep Pure 多糖多酚植物总RNA提取试剂盒提取总RNA,用Evo M-MLV RT Mix Kit with gDNA Clean for qPCR 试剂盒合成cDNA 作为PCR 模板,使用Bio-Rad CFX96仪进行扩增,反应体系和程序参照SYBR Green Pro Taq HS实时荧光定量PCR试剂盒方法进行,以番木瓜肌动蛋白基因(CpActin)为内参基因。用2-ΔΔCt方法计算基因相对表达量。设3 个生物学重复,每个生物学重复5~10个花芽。
表1 实时荧光定量PCR 引物
Table 1 Primers used for qRT-PCR

基因序列号Gene ID evm.TU.supercontig_211.22 evm.TU.supercontig_160.33 evm.TU.supercontig_6.397 evm.TU.supercontig_32.14 evm.TU.supercontig_367.3 evm.TU.supercontig_26.218 evm.TU.supercontig_458.2 evm.TU.supercontig_28.58 evm.TU.contig_30480.2 evm.TU.supercontig_6.164 evm.TU.supercontig_20.147 evm.TU.supercontig_20.141 evm.TU.supercontig_25.146 evm.TU.supercontig_17.12 evm.TU.supercontig_207.10 evm.TU.supercontig_2702.1 evm.TU.supercontig_7.12注释Annotated SAP转录调控因子Transcriptional regulator STERILE APETALA,SAP AP2类乙烯响应转录因子AP2-like ethylene-responsive transcription factor,ANT转录因子HHO5 transcription factor,HHO5碱性亮氨酸拉链蛋白Basic leucine zipper protein,ZIP21生长素-氨基酸水解酶IAA-amino acid hydrolase,ILL3细胞分裂素核苷5’-单磷酸盐磷酸核糖水解酶Cytokinin riboside 5’-monophosphate phosphoribohydrolase,LOG5脂氧合酶2 Linoleate lipoxygenase 2,LOX2 12-氧代植二烯酸还原酶3 12-oxophytodienoate reductase 3,OPR3 1-氨基环丙烷-1-羧酸氧化酶1-aminocyclopropane-1-carboxylate oxidase 1,ACO1水杨酸羧甲基转移酶Salicylate carboxymethyltransferase,SAMT1生长素响应蛋白Auxin-responsive protein,SAUR66生长素响应蛋白Auxin-responsive protein,SAUR67茉莉酮酸酯ZIM结构域蛋白6 Jasmonate-zim-domain protein 6,JAZ6茉莉酮酸酯ZIM结构域蛋白8 Jasmonate-zim-domain protein 8,JAZ8茉莉酮酸酯ZIM结构域蛋白10 Jasmonate-zim-domain protein 10,JAZ10碱性螺旋环螺旋转录因子13 Transcription factor bHLH13,bHLH13 WUSCHEL同源结构域转录因子9 WUSCHEL-related homeobox 9,WOX9引物序列(5’-3’)Primer sequence F:TCCCAACACCCTCATCG R:CACCAACGCCCACTACC F:GGAATGGTGGCGTGTT R:TTGGCTTCAGGTTGGTC F:CCCAGTTCAACCCAGCAA R:TGACCAGCACCGCCTCT F:AACAATCAAACGCCTCC R:CACCCAACCAAGAACAGA F:TGGGTCTGGGTCTCCTC R:CATTCGTTGTTGGTGGG F:CTGGTGACGAGGAGTT R:TCAGAGTCTTTGGGATG F:TGTTCTTGGAGGCACG R:TCGGCACATACGGAGT F:GTGACTCAGCCTCGTT R:CCACCGCTAGACATAA F:CAGCAGCCATACAGAT R:TGAAGAAACAAGCCAC F:TCCCACAATACACTCCATC R:CTCAGCCACAGACCTCAT F:AGATGGCGAAGAAGTG R:AGCGGATTGTTGAGAT F:AAGTGGCAGAGGGTAG R:TCAGCGGTGTAAACAA F:CGAAAGGACAGGGTTGC R:CGCCTCGTGATGATTGAC F:GTCTTCTTCCTCCCTCTG R:GTTTCTCCTCCATTGTTTC F:GGATTCCTCTGCCAAGT R:CCATTATCACGGTTCTGC F:GTGGTCTGGCGTGGAAT R:GTCTTGGAGCGGGAAAT F:TACGCCGTTACTGTGCC R:GAGTGGGTGGAGGGTGA
2 结果与分析
2.1 雄花和两性花中ACC和内源激素含量分析
在番木瓜两性花中,乙烯合成前体1-氨基环丙烷羧酸(ACC)、吲哚-3-乙酸(IAA)、反式玉米素(tZ)、水杨酸(SA)、茉莉酸(JA)含量分别是雄花的2.08、1.40、1.81、4.07、1.93 倍,两者的脱落酸(ABA)和赤霉素A3(GA3)含量无显著差异(图2)。
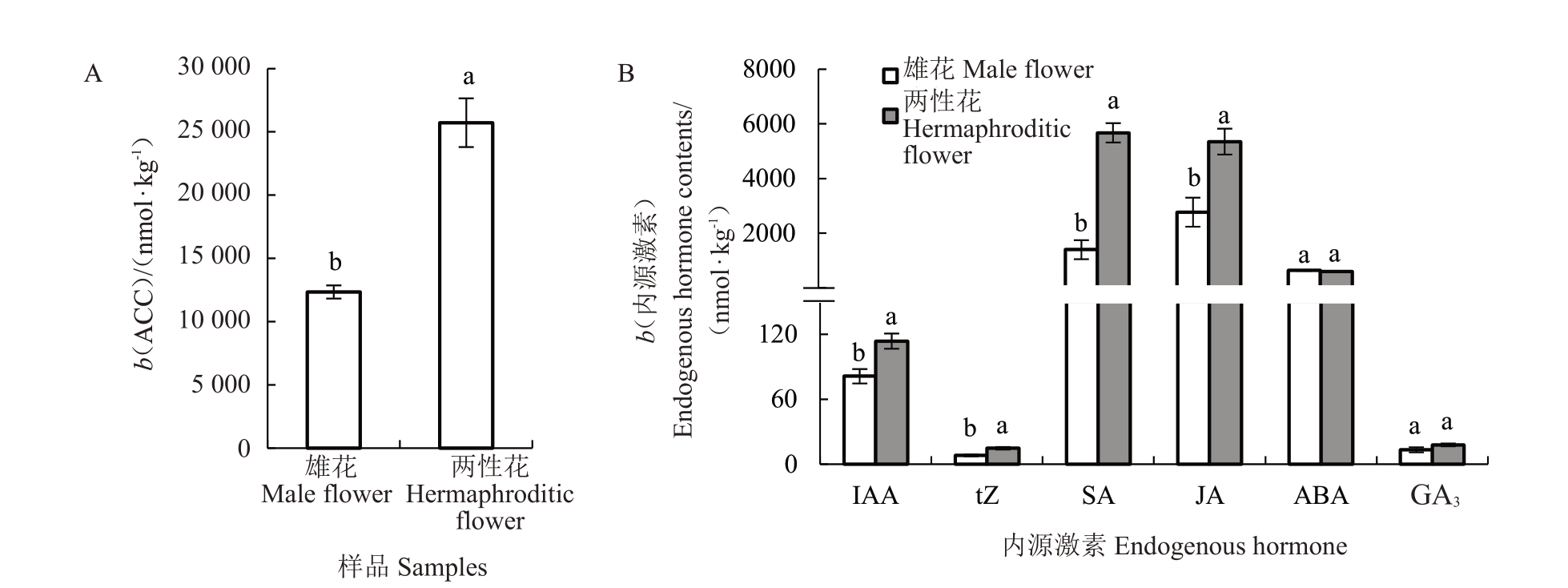
图2 番木瓜雄花和两性花中ACC(A)及内源激素(B)含量
Fig.2 The contents of ACC(A)and endogenous hormone(B)between the male and the hermaphroditic flowers in papaya
不同小写字母表示在p<0.05 水平差异显著。
Different small letters indicate significant difference at p<0.05.
2.2 雄花和两性花转录组分析
对番木瓜两性株雄花和两性花样品进行转录组测序,共获得45.17 Gb 原始数据,过滤后,样品的平均序列数量为7.41 Gb,Q20>99.9%,Q30>98.7%,与参考基因组比对率超过88.9%,其中约78.2%比对到基因组唯一序列(表2)。说明转录组测序结果可靠性和精准性较好,可进一步分析。
表2 番木瓜雄花和两性花转录组测序数据统计情况
Table 2 Summary of RNA-seq data between male and hermaphroditic flowers in papaya
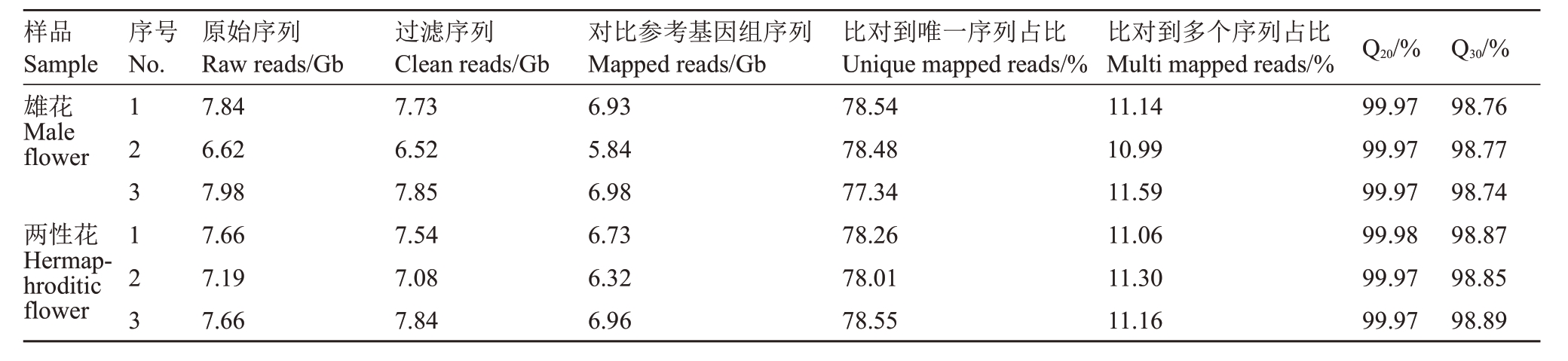
样品Sample雄花Male flowerr序号No.两性花Hermaphroditic flower 123123原始序列Raw reads/Gb 7.84 6.62 7.98 7.66 7.19 7.66过滤序列Clean reads/Gb 7.73 6.52 7.85 7.54 7.08 7.84对比参考基因组序列Mapped reads/Gb 6.93 5.84 6.98 6.73 6.32 6.96比对到唯一序列占比Unique mapped reads/%78.54 78.48 77.34 78.26 78.01 78.55比对到多个序列占比Multi mapped reads/%11.14 10.99 11.59 11.06 11.30 11.16 Q20/%99.97 99.97 99.97 99.98 99.97 99.97 Q30/%98.76 98.77 98.74 98.87 98.85 98.89
为分析雄花和两性花不同表达水平基因数量的分布情况,将基因表达水平划分为4 个等级,2 组样品在4 个等级中的基因数量分布情况相近(图3-A)。在2 组样品转录组数据中,共组装拼接得到27 793 个基因,不表达(FPKM<1)的基因数量约占33.9%,在100>FPKM ≥10 区间的基因数量最多,占比为39.2%,其次是10>FPKM ≥1 区间,占比为20.3%,FPKM ≥100 占比最少。在p 值<0.05 且|log2(fold change)| ≥1 的阈值条件下,在雄花和两性花中共鉴定得到517个差异表达基因,其中214个基因上调表达,303个基因下调表达(图3-B)。
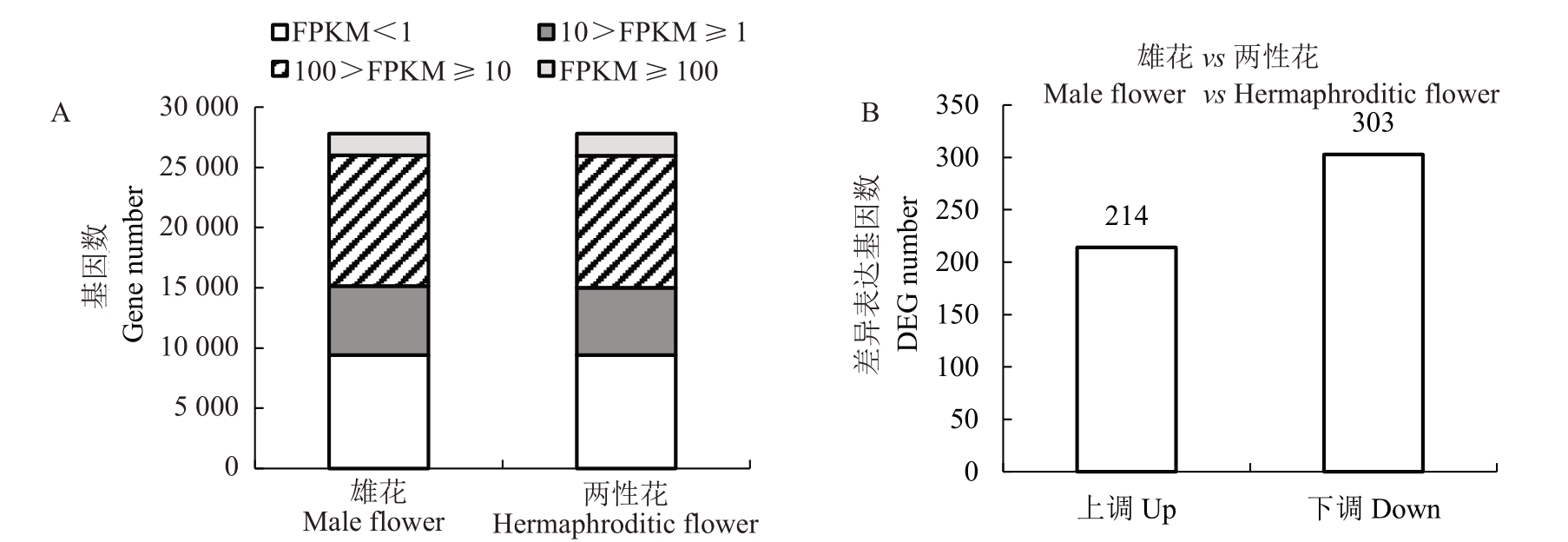
图3 番木瓜雄花和两性花不同表达水平基因数量分布(A)及差异表达基因数量(B)
Fig.3 Distribution of gene numbers at different expression levels(A)and the number of differently expressed genes(B)between the male and the hermaphroditic flowers in papaya
FPKM ≥100:高表达;100>FPKM ≥10:中表达;10>FPKM ≥1:低表达;FPKM<1:不表达。
FPKM ≥100.High expression;100>FPKM ≥10.Middle expression;10>FPKM ≥1.Low expression;FPKM<1.No expression.
2.3 雄花和两性花中差异表达基因GO 注释与KEGG功能富集分析
对番木瓜两性株雄花和两性花中517 个差异表达基因进行GO功能富集分析,主要涉及生物学过程、细胞组成、分子功能。显著富集(p 值<0.05)在生物学过程的有85 项、细胞组成有11 项、分子功能有49 项。选择富集最显著的前50 项条目进行展示(表3)。在生物学过程中,大多数基因主要富集到转录与转录调控、植物激素响应与信号调控、细胞分化与器官生长调控、胁迫响应与调控等相关的GO 条目中。在细胞组分中,以质膜、胞外区为主。在分子功能中,以转录调控区域DNA 结合转录调控、DNA 结合转录因子活性、DNA特异序列结合为主。
表3 番木瓜雄花和两性花差异表达基因显著富集的前50 个GO 条目
Table 3 The top 50 GO terms of the differently expressed genes between the male and the hermaphroditic flowers in papaya

GO 分类GO classification生物学过程Biological process基因数Gene number 5 14 5 14 56 674 13 50 62336338247 12 321 1422细胞组成Cellular component分子功能Molecular function GO序列号GO accession GO:1903507 GO:0009611 GO:2000022 GO:0009733 GO:0006355 GO:0031347 GO:0006855 GO:0046620 GO:0043161 GO:0006351 GO:0042744 GO:0046202 GO:0051762 GO:0009691 GO:0046323 GO:0009638 GO:0030162 GO:0009751 GO:0080037 GO:0031408 GO:0009753 GO:0030154 GO:1905392 GO:0071323 GO:0006979 GO:0019760 GO:0010500 GO:0071470 GO:0005887 GO:0005576 GO:0090406 GO:0005886 GO:0020037 GO:0044212 GO:0003700 GO:0003714 GO:0016799 GO:0043565 GO:0016614 GO:0005355 GO:0004601 GO:0004103 GO:0046593 GO:0008061 GO:0050660 GO:0016832 GO:0010334 GO:0005351 GO:0019825 GO:0018685注释功能GO Term转录负调控Negative regulation of transcription创伤响应Response to wounding茉莉酸介导的信号途径调控Regulation of jasmonic acid mediated signaling pathway生长素响应Response to auxin转录调控Regulation of transcription防御响应调控Regulation of defense response药物跨膜转运Drug transmembrane transport器官生长调控Regulation of organ growth泛素依赖性蛋白质分解代谢Ubiquitin-dependent protein catabolic process转录Transcription过氧化氢分解代谢过程Hydrogen peroxide catabolic process氰化物生物合成过程Cyanide biosynthetic process倍半萜生物合成过程Sesquiterpene biosynthetic process细胞分裂素生物合成过程Cytokinin biosynthetic process葡萄糖输入Glucose import向光性Phototropism蛋白水解调控Regulation of proteolysis水杨酸响应Response to salicylic acid细胞分裂素信号转导负调控Negative regulation of cytokinin-activated signaling pathway羟脂生物合成过程Oxylipin biosynthetic process茉莉酸响应Response to jasmonic acid细胞分化Cell differentiation植物器官形态发生Plant organ morphogenesis几丁质细胞响应Cellular response to chitin氧化胁迫响应Response to oxidative stress硫代葡萄糖苷代谢过程Glucosinolate metabolic process传递组织发育Transmitting tissue development渗透压胁迫细胞响应Cellular response to osmotic stress质膜组分Integral component of plasma membrane胞外区Extracellular region花粉管Pollen tube质膜Plasma membrane血红素结合Heme binding转录调控区域DNA结合Transcription regulatory region DNA binding DNA结合转录因子活性DNA binding transcription factor activity转录阻遏物活性Transcription corepressor activity水解酶活性Hydrolase activity DNA特异序列结合Sequence-specific DNA binding氧化还原酶活性Oxidoreductase activity葡萄糖跨膜转运蛋白活性Glucose transmembrane transporter activity过氧化物酶活性Peroxidase activity胆碱激酶活性Choline kinase activity扁桃腈裂解酶活性Mandelonitrile lyase activity几丁质结合Chitin binding黄素腺嘌呤二核苷酸结合Flavin adenine dinucleotide binding醛裂解酶活性Aldehyde-lyase activity倍半萜合酶活性Sesquiterpene synthase activity质子转运体活性Proton symporter activity氧结合Oxygen binding烷烃1-单加氧酶活性Alkane 1-monooxygenase activity 15 38 5 74 12 21 57 53 37 568224623642
将番木瓜两性株雄花和两性花中差异表达基因比对到KEGG 数据库中,共富集到66 个KEGG 通路。以p 值<0.05 为标准,筛选差异表达基因显著富集的通路,包括植物激素信号转导、α-亚麻酸代谢、单萜类生物合成、亚麻酸代谢、ABC 转运蛋白、植物-病原体相互作用、谷胱甘肽代谢和促丝裂原活化蛋白激酶信号通路等8 个KEGG 通路(表4)。显著富集基因最多的是植物激素信号转导,其次是植物-病原体相互作用、促丝裂原活化蛋白激酶信号通路等。
表4 番木瓜雄花和两性花差异表达基因显著富集的KEGG 通路
Table 4 The significantly enriched KEGG pathways of differently expressed genes between the male and the hermaphroditic flowers in papaya

通路编号Pathway ID ko04075 ko00592 ko00902 ko00591 ko02010 ko04626 ko00480 ko04016通路名称Pathway name植物激素信号转导Plant hormone signal transduction α-亚麻酸代谢α-Linolenic acid metabolism单萜类生物合成Monoterpenoid biosynthesis亚麻酸代谢Linoleic acid metabolism ABC转运蛋白ABC transporters植物-病原体相互作用Plant-pathogen interaction谷胱甘肽代谢Glutathione metabolism促丝裂原活化蛋白激酶信号通路MAPK signaling pathway基因数Gene number 26 7348 13 6 10 p值p-value 3.42E-07 0.000 075 0.001 422 0.001 568 0.001 619 0.005 467 0.022 351 0.034 762
2.4 雄花和两性花中植物激素相关差异表达基因分析
植物激素在花器官发育过程中发挥重要作用。为分析番木瓜雄花和两性花中植物激素相关基因的表达情况,通过差异表达基因的功能注释,共鉴定到70个植物激素相关差异表达基因(表5)。在这些基因中,有13个基因参与脱落酸信号转导和响应生物学过程,其中10 个基因在雄花中下调表达,包括脱落酸受体基因PYL4;3个基因上调表达,包括在雄花中特异表达基因RGGA。
表5 番木瓜雄花和两性花中植物激素相关差异表达基因
Table 5 Differently expressed hormone-related genes between the male and the hermaphroditic flowers in papaya

p值p-value 0.002 345 1.95E-06 0.000 931 0.010 374 0.000 915 7.72E-08 0.003 539 6.05E-11 0.007789 0.000 164 0.000 126 0.014 717 0.000 143 0.002 607 0.003 342 4.99E-06 1.79E-17 9.86E-10 0.000 60 0.025 30 2.40E-08 0.026 61 0.002 954 1.03E-05 0.000 358 0.002 547 0.012 565 8.57E-07 0.011 811 1.97E-09 0.007 734 0.003 922 8.04E-05 8.52E-09 0.000 15 0.008 929 0.010 433数对的数倍异Log2(fold change)Hermaphroditic flower差1.12-1.14-1.03 1.07-1.10-1.41 16.47-1.29-1.34-1.36-1.19-1.27-1.53-1.61-1.18-2.37-2.53 1.94-1.15-1.06-3.22-1.12-1.75-1.42-1.29-1.48-1.23-1.28-1.20-2.59-1.44 1.41-1.90 1.40 1.25-1.46-1.23 170.52花FPKM 性0.00 3.15 2.44 2.35 124.89 60.29 1.38 56.01 1.48 11.38 194.32 6.59 13.92 3.84 13.26 84.79 9.88 5.72 2.88 153.06 3.16 6.12 33.90 45.97 4.22 4.71 37.92 26.93 41.03 10.01 3.63 19.18 48.25 16.77 2.96 3.42平水雄Male flowerr两6.02达花370.20 1.47 60.97 0.92 9.10 0.96 23.80 2.91 21.83 4.98 0.61 2.16 67.17 6.15 0.74 2.57 14.63 1.39 37.95 1.45 16.38 1.82 12.70 1.51 18.75 2.01 15.63 6.81 11.72 3.68 9.67 5.15 127.21 1.07 39.91 1.45 BTB/POZ and TAZ domain protein 4,BT4表Homeobox-leucine zipper protein,HB20 4 CDPK-related kinase 4,CRK4白酸23 WRKY transcription factor 23,WRKY23酶拟Homologous gene name in Arabidopsis Serine/threonine-protein kinase,WAG1 2C5 Phosphatase 2C5,PP2C5 Indole-3-acetic acid-amido synthetase,GH3.1 Indole-3-acetic acid-amido synthetase,GH3.9相32 Calcium-dependent protein kinase 32,CDPK32白Auxin response factor,ARF2蛋3 Zinc finger protein 3,ZFP3蛋F-box/kelch-repeat protein,KMD1 F Respiratory burst oxidase F,RBOHF A RNA binding protein A,RGGA F-box/kelch-repeat protein,KMD2 1 Annexin D1,ANN1激Alcohol dehydrogenase,ADH1 AAA-ATPase,ASD IAA-amino acid hydrolase,ILL3 2 Transcription factor,MYB2 27 Cold-regulated gene 27,COR27酶酶Abscisic acid receptor,PYL4域链Auxin-responsive protein,SAUR67 Auxin-responsive protein,SAUR66.1白白Auxin-responsive protein,SAUR66.2称Auxin-responsive protein,SAUR66酶3 Protein BIG GRAIN 3,BG3关激Dof zinc finger protein,DOF3.4酶成成构Auxin-responsive protein,IAA32拉Two-component response regulator,ARR4 Auxin-induced protein,IAA19 Two-component response regulator,ARR9白名解合合Auxin-induced protein,SAUR51白Auxin-responsive protein,SAUR6 Auxin-responsive protein,SAUR48器器酶酶因酶蛋白白白白蛋子水化化TAZ结白白氨子白白白节节基激激化合酶子蛋蛋蛋蛋酸因酸基基白蛋蛋亮因蛋蛋蛋调调源白白氧结酶苷因因体应应应应氨应酶蛋蛋白发酸白氢腺录Protein FD基氨氨基 受 响 响 响 响/苏 响 白-氨 酸 酸蛋MYB107 Transcription factor,MYB107应应同指响诱型响响芥素素异素素素因酯赖赖蛋爆核蛋脱酸分分南磷钙钙锌呼核膜乙三MYB转白控酸素素素素酸素蛋素乙乙酸依依指吸糖联醇磷FD蛋调落长长长长氨长粒长哚哚冷脱生生生生丝生大生吲吲Dof锌BTB/POZ和应导域录导应应生生同WRKY转诱响响子长长源长长长录组组生生生转F-box/kelch-repeat蛋F-box/kelch-repeat蛋双双号列基Gene ID序因evm.TU.supercontig_2.433 evm.TU.supercontig_3.143 evm.TU.supercontig_92.31 evm.TU.supercontig_127.8 evm.TU.supercontig_201.3 evm.TU.contig_46241 evm.TU.supercontig_36.126 evm.TU.supercontig_49.68 evm.TU.supercontig_14.57 evm.TU.supercontig_145.21 evm.TU.supercontig_26.287 evm.TU.supercontig_62.6 evm.TU.supercontig_5.226 evm.TU.supercontig_20.141 evm.TU.supercontig_20.144 evm.TU.supercontig_20.145 evm.TU.supercontig_20.147 evm.TU.supercontig_229.7 evm.TU.supercontig_9.161 evm.TU.supercontig_28.87 evm.TU.supercontig_367.3 evm.TU.supercontig_6.74 evm.TU.supercontig_9.205 evm.TU.supercontig_15.49 evm.TU.supercontig_6.28 evm.TU.supercontig_65.69 evm.TU.supercontig_946.4 evm.TU.supercontig_14.18 evm.TU.supercontig_18.82 evm.TU.supercontig_26.29 evm.TU.supercontig_37.223 evm.TU.supercontig_54.76 evm.TU.supercontig_34.62 evm.TU.supercontig_10.159 evm.TU.supercontig_10.160 evm.TU.supercontig_64.98 evm.TU.supercontig_64.99生Biological process激Hormone signal transduction导激Hormone signal transduction应Hormone response Hormone receptor体输Hormone transportation导程转转成Hormone biosynthesis合导Hormone signal transduction激Hormone response转过号号物应号学信响受运信生响信物素素素素素素素素激激激激激激Hormone脱Abscisic acid裂酸分素落素Auxin长胞生素Cytokinin细
表5 (续) Table 5 (Continued)

p值p-value 1.19E-51 0.033 571 0.011 265 4.25E-12 0.005 059 0.000 754 0.002 162 6.89E-09 1.72E-37 0.000 308 2.22E-05 0.018 706 0.028 433 1.18E-09 0.008 266 0.003 414 7.12E-05 0.001 066 0.000 300 2.95E-09 6.12E-09 4.56E-08 2.95E-09 0.008 329 1.49E-06 1.80E-07 1.74E-09 5.95E-05 0.001 373 0.000 522 1.37E-07 0.000 691 0.000 146 9.83E-10数对的数倍异Log2(fold change)-6.63 1.22-1.04-5.09-1.26 2.51-1.58-1.77-7.14 1.25 1.27-1.56-1.14-1.81 1.03 1.22-1.15-1.43-1.35-5.37-2.65-2.67-5.37-1.24-3.91-2.06-2.47 1.21 1.47 1.04-2.04-2.00-1.52 2.53 FPKM Hermaphroditic flower差花性16.49 2.08 7.16 1.87 4.50 1.07 1.92 26.74 4.69 199.52 141.33 1.72 10.06 53.57 19.08 1.29 45.01 82.94 358.05 148.46 61.58 961.01 148.46 16.69 10.21 11.68 95.89 53.37 0.63 32.06 113.53 76.92 21.61 3.31平水达花0.17 4.87 3.47 0.05 1.88 6.08 0.64 7.84 0.03 0.58 4.56 3.00 3.60 9.82 3.60 7.06 0.68 2.80 1.74 7.52表雄Male flowerr两475.09 340.01 15.29 39.02 20.28 30.72 140.75 150.96 17.32 123.43 66.11 27.54 19.23 19.04酶酶Microtubule-binding protein,Mss4酶解解解水水糖核糖核核酸9子酸酸磷因磷盐磷9 Jasmonate-zim-domain protein 9,JAZ9糖1 Jasmonate-zim-domain protein 1,JAZ1盐8 Jasmonate-zim-domain protein 8,JAZ8 10 Jasmonate-zim-domain protein 10,JAZ10 6 Jasmonate-zim-domain protein 6,JAZ6 8 Jasmonate-zim-domain protein 8,JAZ8录盐9 AP2-like ethylene-responsive transcription factor,ERF9白白白白白白拟Homologous gene name in Arabidopsis酸3 12-oxophytodienoate reductase 3,OPR3 13 Transcription factor,bHLH13酸磷转化003 Ethylene-responsive transcription factor,ERF003因子磷蛋蛋蛋蛋蛋蛋4 Ethylene-responsive transcription factor 4,ERF4 Salicylate carboxymethyltransferase,SAMT1子磷5’-单Pathogenesis-related protein,PR-4域烯Metalloendoproteinase,MMP Zinc metalloprotease,EGY1 5’-单因酶水5 Ethylene-responsive transcription factor 5,ERF5 Ethylene-responsive transcription factor,ESR2酸构14 Gibberellin-regulated protein 14,GASA14酸1 Gibberellin-regulated protein 1,GASA1构构构构构构5’-单原E,E-geranyllinalool synthase,GES录移结子子子1 Gibberellin 20 oxidase 1,GA20OX1氧名子Scarecrow-like protein 3,SCL3 50 WRKY transcription factor 50,WRKY50 Cytosolic sulfotransferase,ST2A还醇C9 Glutaredoxin-C9,GRXC9转40 WRKY transcription factor 40,WRKY40转苷因域域域域域域称AP2类乙烯响应转录因苷因因因苷白白酶白子酶酸合旋子-1-羧基基白酶录核白核录录录蛋蛋化蛋ZIM结ZIM结核ZIM结源ZIM结烷ZIM结ZIM结因移应酯酯酯酯酯酯录转螺因甲源蛋素素丙WUSCHEL同2 Linoleate lipoxygenase 2,LOX2素二酶樟白环录WUSCHEL-related homeobox 9,WOX9蛋白转TGA8 Transcription factor,TGA8酶基裂转转转裂关蛋应应应应调调20-氧响酸酸酸酸酸酸羧细Cytokinin riboside 5’-monophosphate phosphoribohydrolase,LOG3芥结分酶节节分同合裂分响响响Cytokinin riboside 5’-monophosphate phosphoribohydrolase,LOG5相属响酶环素素素素酮酮酮酮酮酮磺合代蛋基还螺因酸南管胞胞胞病金烯烯烯烯基霉霉霉霉莉莉莉莉莉莉胞 脂12-氧 属 叶 氧 性细Cytokinin riboside 5’-monophosphate phosphoribohydrolase,LOG5.1录乙乙乙1-氨杨赤茉茉茉茉茉茉WRKY转基酶植白芳蛋旋致锌乙微细1-aminocyclopropane-1-carboxylate oxidase 1,ACO1质氧赤赤赤金香谷碱WRKY转子转水号列基Gene ID序因evm.TU.supercontig_7.12 evm.TU.supercontig_327.3 evm.TU.supercontig_26.218 evm.TU.supercontig_320.1 evm.TU.supercontig_92.106 evm.TU.supercontig_1476.2 evm.TU.supercontig_161.27 evm.TU.supercontig_127.4 evm.TU.supercontig_25.116 evm.TU.supercontig_83.80 evm.TU.supercontig_89.42 evm.TU.supercontig_33.29 evm.TU.contig_30480.2 evm.TU.supercontig_14.90 evm.TU.supercontig_26.40 evm.TU.supercontig_4.188 evm.TU.supercontig_84.12 evm.TU.supercontig_116.63 evm.TU.supercontig_1667.1 evm.TU.supercontig_17.12 evm.TU.supercontig_207.10 evm.TU.supercontig_25.146 evm.TU.supercontig_17.12 evm.TU.supercontig_919.2 evm.TU.supercontig_266.1 evm.TU.supercontig_458.2 evm.TU.supercontig_28.58 evm.TU.supercontig_5.334 evm.TU.supercontig_596.2 evm.TU.supercontig_1080.2 evm.TU.supercontig_2702.1 evm.TU.supercontig_10.75 evm.TU.supercontig_1.202 evm.TU.supercontig_6.164生Biological process激Hormone biosynthesis Hormone response成程合导Hormone signal transduction应Hormone response转合导Hormone signal transduction成Hormone biosynthesis转合Hormone response导Hormone signal transduction成Hormone biosynthesis转Hormone metabolic成Hormone biosynthesis合Hormone response转Hormone metabolic过应物号物号物应号谢物激Hormone response应应号谢学响生响信生信生响信代生响响信代物素素素素素素素素素素素素素素素激激激激激激激激激激激激导Hormone signal transduction激激Hormone酸素烯Ethylene素Gibberellic acid霉酸Jasmonic acid莉杨乙赤茉水Salicylic acid
有20个差异表达基因参与生长素运输、信号转导、代谢、响应等生物学过程,除生长素转运基因WAG1 和生长素响应基因SUR48 外,其余18 个基因在雄花中均下调表达。在两性花中,生长素合成基因ILL3、GH3.1和GH3.9表达量分别是雄花的9.32、2.17、3.36倍,4个参与生长素转运的SAUR家族基因表达量是雄花的2.27~5.78倍。
细胞分裂素是细胞分化的重要植物激素。有9个差异表达基因参与细胞分裂素信号转导、代谢、响应和生物合成等生物学过程。在两性花中,细胞分裂素生物合成基因LOG3、LOG5、LOG5.1 表达量分别是雄花的2.39、2.06、34.06倍。
另外,参与乙烯、赤霉素、茉莉酸、水杨酸相关差异表达基因分别有8、4、13、3个。在雄花中,赤霉素生物合成基因GA20OX1 和水杨酸代谢基因SAMT1的表达量分别是两性花的2.33和5.78倍。而在两性花中,茉莉酸生物合成基因LOX2、OPR3 和乙烯生物合成基因ACO1 表达量分别是雄花的4.17、5.54、2.20倍。
2.5 雄花和两性花中花发育相关差异表达基因分析
在番木瓜雄花和两性花中,共鉴定到6 个参与花发育相关基因。在两性花中,ANT、ANT2、CIB1、HHO5、ZIP21 基因表达量分别是雄花的2.27、2.08、2.17、2.22、2.08倍,而参与花和胚珠发育的SAP基因在两性花中特异表达(表6)。
表6 番木瓜雄花和两性花中花发育相关差异表达基因
Table 6 Differently expressed genes related to flower development between the male and the hermaphroditic flowers in papaya

基因序列号Gene ID拟南芥同源基因名称Homologous gene name in Arabidopsis表达水平FPKM雄花Male flowerr p值p-value基因功能Gene function evm.TU.supercontig_32.14 evm.TU.supercontig_6.397 evm.TU.supercontig_211.22 2.60两性花Hermaphroditic flower 5.47碱性亮氨酸拉链蛋白Basic leucine zipper protein,ZIP21转录因子HHO5 Transcription factor,HHO5 SAP转录调控因子Transcriptional regulator STERILE APETALA,SAP AP2类乙烯响应转录因子AP2-like ethylene-responsive transcription factor,ANT AP2类乙烯响应转录因子AP2-like ethylene-responsive transcription factor,ANT2隐花色素互作碱性螺旋环螺旋转录因子Cryptochrome-interacting basic-helix-loophelix,CIB1差异倍数的对数Log2(fold change)-1.07 0.015 315 31.56 69.57-1.14 0.000 037 0.00 5.68-15.79 5.75E-35花药发育Anther development花器官形成Floral organ formation花和胚珠发育Flower and ovule development evm.TU.supercontig_160.33 15.07 34.01-1.17 7.98E-06 evm.TU.supercontig_129.70 14.04 29.48-1.07 0.000 081 evm.TU.supercontig_62.160 3.77 8.27-1.13 0.001 310胚珠和花器官原基发育Ovule and floral organ primordia development胚珠和花器官原基发育Ovule and floral organ primordia development花发育Flower development
2.6 qRT-PCR验证差异表达基因
为验证番木瓜雄花和两性花转录组测序数据的准确性和有效性,选取17个花发育和植物激素相关差异表达基因进行qRT-PCR 分析,其中4 个花发育相关基因(SAP、ANT、HHO5、ZIP21)、5 个植物激素生物合成基因(ILL3、LOG5、LOX2、OPPR3、ACO1)、2 个生长素运输基因(SAUR66、SAUR67)、5 个植物激素信号转导与响应基因(JAZ6、JAZ8、JAZ10、bHLH13、WOX9)在雄花中下调表达,1 个水杨酸代谢基因(SAMT1)上调表达(图4)。将所得的基因相对表达量值转化为log2(fold change),与转录组数据的log2(fold change)进行比较,两者上、下调表达趋势一致,表明转录组数据真实、可靠。
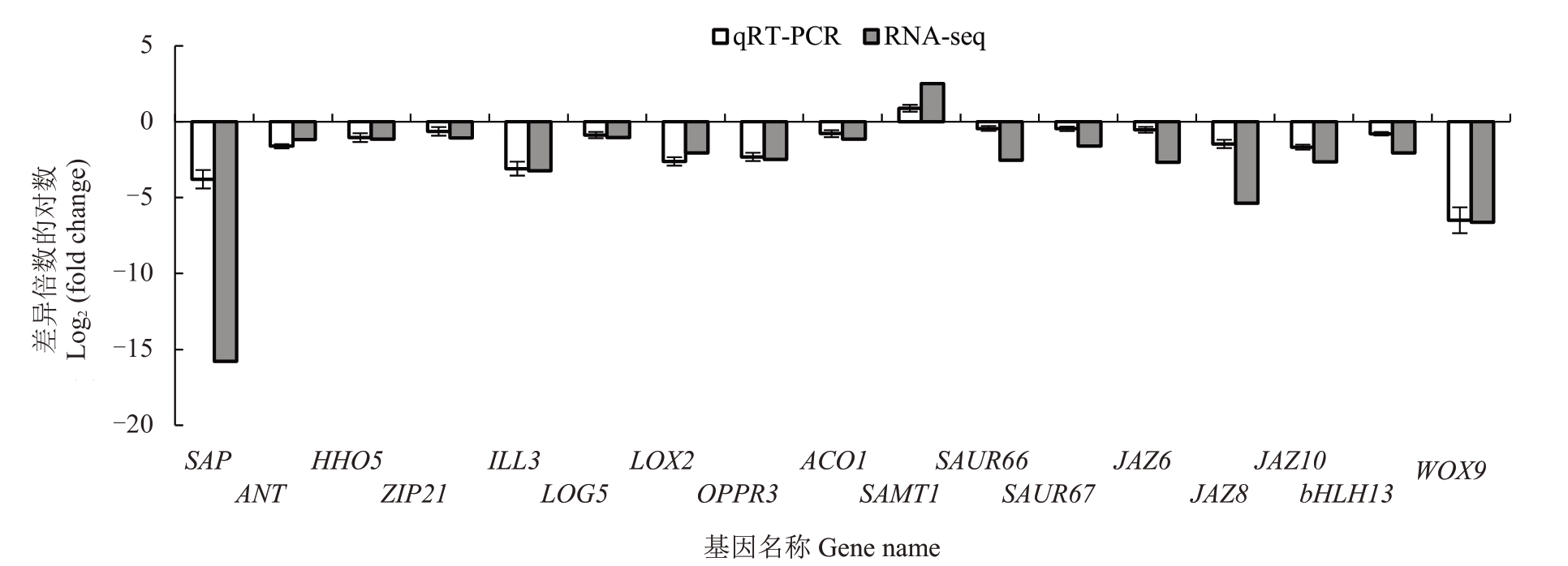
图4 差异表达基因qRT-PCR 验证
Fig.4 Verification of differently expressed genes by qRT-PCR
2.7 雄花和两性花中花发育相关基因启动子顺式作用元件预测
为了分析花发育相关基因ANT、ANT2、CIB1、HHO5、SAP、ZIP21(表6)的启动子元件的组成及可能的调控作用。除ANT2 基因未查找到相应启动子序列外,从番木瓜参考基因组数据库中下载其余5个基因启动子序列,采用PlantCARE 预测基因启动子顺式作用元件(图5)。这5 个基因启动子均含脱落酸响应和胁迫响应元件,此外,ANT 和CIB1 启动子含生长素响应、无氧诱导、茉莉酸甲酯响应、水杨酸诱导及响应元件,HHO5启动子含茉莉酸甲酯响应、水杨酸响应与诱导元件,SAP启动子含无氧诱导、低温响应和赤霉素响应元件,ZIP21启动子含无氧诱导、生长素响应、茉莉酸甲酯响应和赤霉素响应元件。
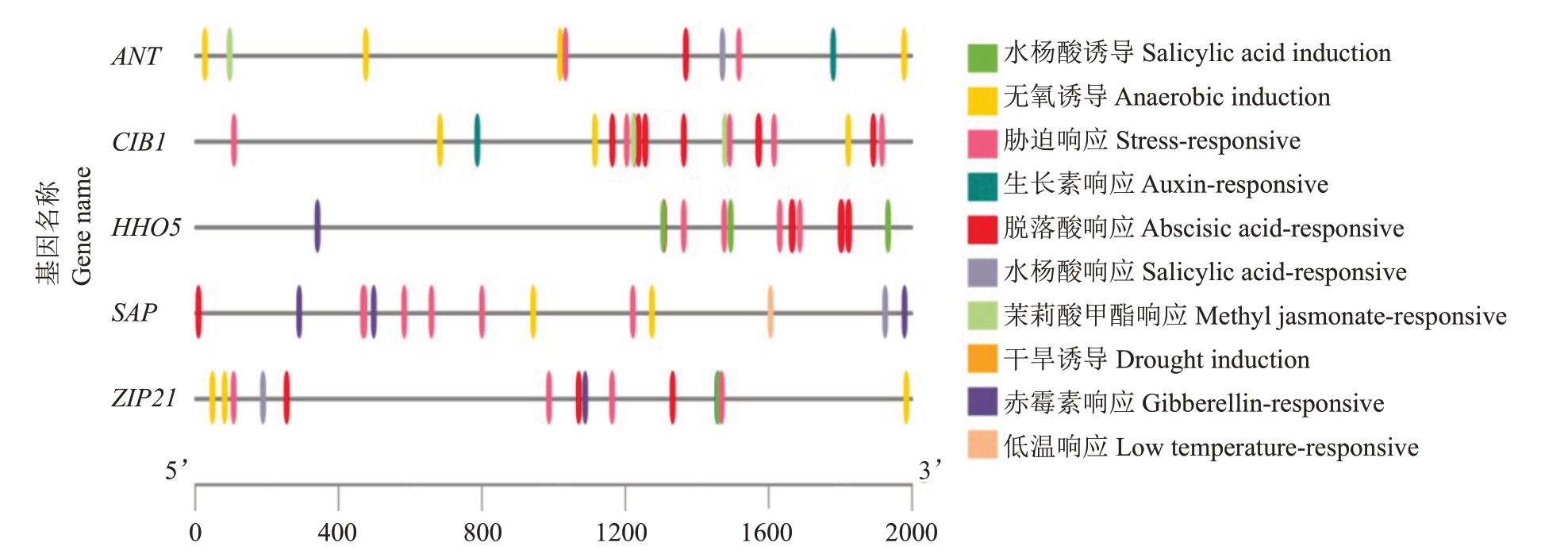
图5 ANT、CIB1、HHO5、SAP 和ZIP21 启动子顺式作用元件分析
Fig.5 Cis-acting elements on the promoters of ANT,CIB1,HHO5,SAP and ZIP21
启动子顺式作用元件分布
Distribution of cis-acting elements on the promoters
3 讨 论
性别分化除受遗传物质的调控外,环境条件也可以影响性别转变。高温是导致番木瓜两性株的花性向雄花转变的重要环境因素之一,但其分子调控机制尚不清楚。笔者在本研究中对在高温环境下采集的番木瓜两性株雄花和两性花进行转录组测序,转录组数据组装拼接得到27 793个基因,共鉴定到517 个差异表达基因。KEGG 富集分析表明,植物激素响应及信号转导通路富集最显著,说明植物激素相关差异表达基因可能在番木瓜两性株高温趋雄过程中起到关键作用。
植物激素通过相互作用和串扰,直接或间接参与性别分化和组织形态发生,生长素、乙烯、细胞分裂素在植物性别分化中起到促进雌性化的作用,而赤霉素起到促雄作用[24-27]。孙思琼等[28]研究表明,外施赤霉素会增加甜瓜雄花两性株的雄花数量。然而,Han 等[29]对番木瓜两性株和雌株的花芽外施GA3,并未观察到花性转雄,反而增加花梗长度和花序分枝数量。在本研究中,番木瓜雄花和两性花GA3含量无显著差异,与前人的研究结果一致。说明赤霉素对不同物种间花性转变的作用存在差异。而外施生长素(萘乙酸)[21]或乙烯利[30]可促进番木瓜雄株雌蕊的形成。在本研究中,生长素-氨基酸水解酶基因(ILL3)、吲哚乙酸氨基化合成酶基因(GH3.1、GH3.9)和1-氨基环丙烷-1-羧酸氧化酶基因(ACO1)在雄花中下调表达,导致雄花中生长素和乙烯的合成量减少。在拟南芥中,ANT 是调控胚珠生长发育的关键基因,受生长素调控,同时介导生长素信号通路相关基因参与花器官形成[31-32];CIB1 基因编码蛋白则直接调控FT 基因表达,调节植物成花[33]。对ANT 和CIB1 基因启动子的顺式元件进行分析,结果表明,这两个基因的启动子含生长素响应元件,番木瓜两性株雄花中生长素积累量的减少,可能降低ANT、CIB1 基因表达量,导致雄花雌蕊发育不全。也有研究报道,外施细胞分裂素(氯吡苯脲)可促进猕猴桃雄株雌蕊的形成[34]。本研究中,雄花细胞分裂素核苷5’-单磷酸盐磷酸核糖水解酶基因(LOG3、LOG5、LOG5.1)的下调表达,降低了雄花细胞分裂素的积累量,减缓雌蕊的分化。说明在高温环境下番木瓜两性株雌性不育的加剧与花芽生长素、乙烯和细胞分裂素合成量下降有关。
茉莉酸和水杨酸在植物生长发育及对高温、干旱、过量光、机械损伤等非生物胁迫响应中发挥重要作用[35-37]。在番茄中,JAI-1基因功能缺失,导致雌性不育,防御反应能力下降[38]。在小麦和拟南芥中,opr3 突变体幼苗受高温胁迫后,成活率显著低于野生型[39]。外施水杨酸可明显提高番茄、马铃薯、小麦、水稻、葡萄等作物的耐高温能力[40-41]。在本研究中,参与茉莉酸合成的脂氧合酶2 基因(LOX2)、12-氧代植二烯酸还原酶3 基因(OPR3)在雄花中下调表达,而参与水杨酸代谢的水杨酸羧基甲基转移酶基因(SAMT1)在雄花中上调表达,导致雄花中茉莉酸和水杨酸积累的减少,从而降低雄花对高温耐受能力。番木瓜HHO5 基因启动子含茉莉酸甲酯响应、水杨酸响应元件,雄花中茉莉酸和水杨酸含量下降,可能减弱HHO5基因的表达,影响雌蕊的发育。
SAP 转录因子属于F-box 蛋白,是E3 泛素连接酶复合物SKP1/Cullin/F-box 的组成部分,通过降解PPD蛋白来调节胚珠、花和花序发育[42]。SAP功能缺失会导致拟南芥胚珠、花和花序严重畸变[43]。干旱、高盐、低温等逆境胁迫会促使菠萝SAP 基因高效表达,影响花器官的大小[44]。龙眼SAP可能参与生长素和赤霉素等激素应答及非生物胁迫响应[45]。在本研究中,番木瓜SAP 基因在雄花中不表达(FPKM <1)。启动子顺式元件分析结果表明,SAP基因启动子含低温响应元件,说明SAP基因的表达受低温诱导,而在高温胁迫下,SAP基因转录活性可能被抑制,可能导致番木瓜雌蕊败育,促进雄花形成。
4 结 论
对番木瓜两性株雄花和两性花进行转录组测序和分析,筛选到70个植物激素相关差异表达基因和6个花发育相关差异表达基因。番木瓜两性株在高温条件下的花性转变可能与花芽中植物激素生物合成、代谢和转运相关基因表达量的改变,导致ACC、IAA、tZ、SA、JA 积累的减少,从而降低花发育相关基因ANT、CIB1、HHO5 的表达量,以及高温抑制SAP基因的表达有关。
[1] 熊月明,郭林榕,黄雄峰,张丽梅,林旗华.不同栽培技术措施对番木瓜两性株高温变性的抑制效应[J]. 福建农业学报,2011,26(6):981-984.XIONG Yueming,GUO Linrong,HUANG Xiongfeng,ZHANG Limei,LIN Qihua. Effect of cultivation conditions on high temperature gender alteration of hermaphroditic papaya (Carica papaya L.)[J]. Fujian Journal of Agricultural Sciences,2011,26(6):981-984.
[2] 李亚丽,沈文涛,言谱,周鹏.番木瓜性别决定的研究进展[J].广西农业科学,2009,40(2):198-202.LI Yali,SHEN Wentao,YAN Pu,ZHOU Peng.Advance of sex determination in papaya[J]. Guangxi Agricultural Sciences,2009,40(2):198-202.
[3] ARYAL R,JAGADEESWARAN G,ZHENG Y,YU Q,SUNKAR R,MING R. Sex specific expression and distribution of small RNAs in papaya[J].BMC Genomics,2014,15(1):20.
[4] ARYAL R,MING R. Sex determination in flowering plants:papaya as a model system[J].Plant Science,2014,217/218:56-62.
[5] LIU Z Y,MOOR P H,MA H,ACKERMAN C M,RAGIBA M,YU Q Y,PEARL H M,KIM M S,CHARLTON J W,STILES J L,ZEE F T,PATERSON A H,MING R.A primitive Y chromosome in papaya marks incipient sex chromosome evolution[J].Nature,2004,427(6972):348-352.
[6] MING R,YU Q Y,MOORE P H.Sex determination in papaya[J].Seminars in Cell & Developmental Biology,2007,18(3):401-408.
[7] ZHANG W L,WANG X,YU Q Y,MING R,JIANG J M.DNA methylation and heterochromatinization in the male-specific region of the primitive Y chromosome of papaya[J]. Genome Research,2008,18(12):1938-1943.
[8] BERGERO R,CHARLESWORTH D. Preservation of the Y transcriptome in a 10-million-year-old plant sex chromosome system[J].Current Biology,2011,21(17):1470-1474.
[9] VANBUREN R,WAI C M,ZHANG J S,HAN J,ARRO J,LIN Z C,LIAO Z Y,YU Q Y,WANG M L,ZEE F,MOORE R C,CHARLESWORTH D,MING R. Extremely low nucleotide diversity in the X-linked region of papaya caused by a strong selective sweep[J].Genome Biology,2016,17(1):230.
[10] VANBUREN R,ZENG F C,CHEN C X,ZHANG J S,WAI C M,HAN J,ARYAL R,GSCHWEND A R,WANG J P,NA J K,HUANG L X,ZHANG L M,MIAO W J,GOU J Q,ARRO J,GUYOT R,MOORE R C,WANG M L,ZEE F,CHARLESWORTH D,MOORE P H,YU Q Y,MING R. Origin and domestication of papaya Yh chromosome[J]. Genome Research,2015,25(4):524-533.
[11] WANG J P,NA J K,YU Q Y,GSCHWEND A R,HAN J,ZENG F C,ARYAL R,VANBUREN R,MURRAY J E,ZHANG W L,NAVAJAS-PÉREZ R,FELTUS F A,LEMKE C,TONG E J,CHEN C X,WAI C M,SINGH R,WANG M L,MIN X J,ALAM M,CHARLESWORTH D,MOORE P H,JIANG J M,PATERSON A H,MING R. Sequencing papaya X and Yh chromosomes reveals molecular basis of incipient sex chromosome evolution[J]. Proceedings of the National Academy of Siences of the United States of America,2012,109(34):13710-13715.
[12] YU Q Y,NAVAJAS-PÉREZ R,TONG E,ROBERTSON J,MOORE P H,PATERSON A H,MING R.Recent origin of dioecious and gynodioecious Y chromosomes in papaya[J]. Tropical Plant Biology,2008,1(1):49-57.
[13] LIAO Z Y,YU Q Y,MING R. Development of male-specific markers and identification of sex reversal mutants in papaya[J].Euphytica,2017,213(2):53.
[14] UENO H,URASAKI N,NATSUME S,YOSHIDA K,TARORA K,SHUDO A,TERAUCHI R,MATSUMURA H. Genome sequence comparison reveals a candidate gene involved in male-hermaphrodite differentiation in papaya (Carica papaya)trees[J]. Molecular Genetics and Genomics,2015,290(2):661-670.
[15] PIFERRER F.Epigenetics of sex determination and gonadogenesis[J].Developmental Dynamics,2013,242(4):360-370.
[16] BARNABÁS B,JÄGER K,FEHÉR A. The effect of drought and heat stress on reproductive processes in cereals[J]. Plant,Cell and Environment,2008,31(1):11-38.
[17] REZAEI E E,WEBBER H,GAISER T,NAAB J,EWERT F.Heat stress in cereals:Mechanisms and modelling[J]. European Journal of Agronomy,2015,64:98-113.
[18] 李惠华,何健,苏明华,赖瑞云.番木瓜性别分化研究进展(综述)[J].亚热带植物科学,2008,37(4):64-68.LI Huihua,HE Jian,SU Minghua,LAI Ruiyun. Review of studies on sex differentiation in Carica papaya[J]. Subtropical Plant Science,2008,37(4):64-68.
[19] LIN H,LIAO Z Y,ZHANG L M,YU Q Y.Transcriptome analysis of the male-to-hermaphrodite sex reversal induced by low temperature in papaya[J]. Tree Genetics & Genomes,2016,12(5):94.
[20] 廖芬,唐文忠,周主贵,黄茂康,崔素芬,何全光.番木瓜株性转变与叶片内源激素平衡关系[J].西南农业学报,2013,26(2):713-717.LIAO Fen,TANG Wenzhong,ZHOU Zhugui,HUANG Maokang,CUI Sufen,HE Quanguang. Endogenous hormonal balance in papaya leaf during sex conversion of hermaphrodite papaya(Carica papaya L.)[J].Southwest China Journal of Agricultural Scieneces,2013,26(2):713-717.
[21] ZHOU P,FATIMA M,MA X Y,LIU J,MING R.Auxin regulation involved in gynoecium morphogenesis of papaya flowers[J].Horticulture Research,2019,6(1):119.
[22] CHEN C J,CHEN H,ZHANG Y,THOMAS H R,FRANK M H,HE Y H,XIA R. TBtools:An integrative toolkit developed for interactive analyses of big biological data[J]. Molecular Plant,2020,13(8):1194-1202.
[23] 周陈平,杨敏,郭金菊,邝瑞彬,杨护,黄炳雄,魏岳荣.番木瓜成熟过程中全基因组DNA 甲基化和转录组变化分析[J].园艺学报,2022,49(3):519-532.ZHOU Chenping,YANG Min,GUO Jinju,KUANG Ruibin,YANG Hu,HUANG Bingxiong,WEI Yuerong.Dynamic changes in DNA methylome and transcriptome patterns during papaya fruit ripening[J].Acta Horticulturae Sinica,2022,49(3):519-532.
[24] 周賡,陈宸,刘晓虹,卢向阳,田云,陈惠明.黄瓜性别决定研究进展[J].植物生理学报,2019,55(7):902-914.ZHOU Geng,CHEN Chen,LIU Xiaohong,LU Xiangyang,TIAN Yun,CHEN Huiming. Research progress of sex determination in cucumber[J]. Plant Physiology Journal,2019,55(7):902-914.[25] HAMAD H,GEWAILY E,GHONEIM A,SHEHAB M,ELKHOLLY N. Improvement ability of male parent by gibberellic acid application to enhancing the outcrossing of cytoplasmic male sterility rice lines[J]. Acta agriculturae Slovenica,2021,117(3):1-11.
[26] LIU J,CHEN L Y,ZHOU P,LIAO Z Y,LIN H,YU Q Y,MING R.Sex biased expression of hormone related genes at early stage of sex differentiation in papaya flowers[J]. Horticulture Research,2021,8(1):147.
[27] SHIRLEY N J,AUBERT M K,WILKINSON L G,BIRD D C,LORA J,YANG X,TUCKER M R.Translating auxin responses into ovules,seeds and yield:Insight from Arabidopsis and the cereals[J]. Journal of Integrative Plant Biology,2019,61(3):310-336.
[28] 孙思琼,王惠林,王志文,徐宝林.赤霉素和乙烯利对甜瓜4种性型分化的影响[J].中国瓜菜,2021,34(11):68-73.SUN Siqiong,WANG Huilin,WANG Zhiwen,XU Baolin. Effects of gibberellin and ethephon on sex differentiation in four sex types of melon[J]. Chinese Cucurbits and Vegetables,2021,34(11):68-73.
[29] HAN J,MURRAY J E,YU Q Y,MOORE P H,MING R. The effects of gibberellic acid on sex expression and secondary sexual characteristics in papaya[J]. HortScience,2014,49(3):378-383.
[30] KUMAR A,JAISWAL V S. Sex reversal and fruit formation on male plants of Carica Papaya L. by ethrel and chlorflurenol[J].Proceedings Plant Sciences,1984,93(6):635-641.
[31] YAMAGUCHI N,JEONG C W,NOLE-WILSON S,KRIZEK B A,WAGNER D. AINTEGUMENTA and AINTEGUMENTALIKE6/PLETHORA3 induce LEAFY expression in response to auxin to promote the onset of flower formation in Arabidopsis[J].Plant Physiology,2016,170(1):283-293.
[32] KRIZEK B A,BLAKLEY I C,HO Y Y,FREESE N,LORAINE A. The Arabidopsis transcription factor AINTEGUMENTA orchestrates patterning genes and auxin signaling in the establishment of floral growth and form[J]. Plant Journal,2020,103(2):752-768.
[33] LIU Y W,LI X,MA D B,CHEN Z R,WANG J W,LIU H T.CIB1 and CO interact to mediate CRY2-dependent regulation of flowering[J].EMBO Reports,2018,19(10):e45762.
[34] AKAGI T,HENRY I M,OHTANI H,MORIMOTO T,BEPPU K,KATAOKA I,TAO R.A Y-encoded suppressor of feminization arose via lineage-specific duplication of a cytokinin response regulator in kiwifruit[J].Plant Cell,2018,30(4):780-795.
[35] 陈金焕,田玉如,李艾佳,夏新莉,尹伟伦.茉莉酸信号及其在木本植物中的研究进展[J]. 中国科学(生命科学),2020,50(2):215-226.CHEN Jinhuan,TIAN Yuru,LI Aijia,XIA Xinli,YIN Weilun.Jasmonic acid signaling and its research progress in woody plants[J].Scientia Sinnica Vitae,2020,50(2):215-226.
[36] BALFAGÓN D,SENGUPTA S,GÓMEZ-CADENAS A,FRITSCHI F B,AZAD R K,MITTLER R,ZANDALINAS S I. Jasmonic acid is required for plant acclimation to a combination of high light and heat stress[J]. Plant Physiology,2019,181 (4):1668-1682.
[37] KHAN F S,GAN Z M,LI E Q,REN M K,HU C G,ZHANG J Z. Transcriptomic and physiological analysis reveals interplay between salicylic acid and drought stress in citrus tree floral initiation[J].Planta,2021,255(1):24.
[38] LI L,LI C Y,HOWE G A. Genetic analysis of wound signaling in tomato. Evidence for a dual role of jasmonic acid in defense and female fertility[J]. Plant Physiology,2001,127(4):1414-1417.
[39] TIAN X J,WANG F,ZHAO Y,LAN T Y,YU K Y,ZHANG L Y,QIN Z,HU Z R,YAO Y Y,NI Z F,SUN Q X,ROSSI V,PENG H R,XIN M M.Heat shock transcription factor A1b regulates heat tolerance in wheat and Arabidopsis through OPR3 and jasmonate signalling pathway[J]. Plant Biotechnology Journal,2020,18(5):1109-1111.
[40] 孙军利,赵宝龙,郁松林.外源水杨酸对高温胁迫下葡萄几种抗氧化酶活性和抗氧化物含量的影响[J]. 植物生理学报,2014,50(7):1014-1018.SUN Junli,ZHAO Baolong,YU Songlin. Effect of exogenous salicylic acid on antioxidant enzymes activities and antioxidants contents in grape seedlings under high temperature stress[J].Plant Physiology Journal,2014,50(7):1014-1018.
[41] JHA U C,NAYYAR H,SIDDIQUE K H M. Role of phytohormones in regulating heat stress acclimation in agricultural crops[J]. Journal of Plant Growth Regulation,2022,41(3):1041-1064.
[42] WANG Z B,LI N,JIANG S,GONZALWZ N,HUANG X H,WANG Y C,INZÉ D,LI Y H.SCFSAP controls organ size by targeting PPD proteins for degradation in Arabidopsis thaliana[J].Nature Communications,2016,7(1):11192.
[43] BYZOVA M V,FRANKEN J,AARTS M G,DE ALMEIDAENGLER J,ENGLER G,MARIANI C,VAN LOOKEREN C M M,ANGENENT G C. Arabidopsis STERILE APETALA,a multifunctional gene regulating inflorescence,flower,and ovule development[J].Genes&Development,1999,13(8):1002-1014
[44] 夏杨,周佳炜,苏初连,叶子,蒲金基,陈华蕊,张贺.菠萝Ac-SAP 转录因子对非生物胁迫和生物胁迫的应答响应[J].分子植物育种,2019,17(3):739-745.XIA Yang,ZHOU Jiawei,SU Chulian,YE Zi,PU Jinji,CHEN Huarui,ZHANG He. Response of SAP transcription factor to abiotic and biotic stress in Ananas comosus[J]. Molecular Plant Breeding,2019,17(3):739-745.
[45] 韩婕,陈晓慧,申序,李晓斐,林玉玲,吴鹏飞,赖钟雄. 龙眼SAP-PPD-KIX-TPL 信号途径基因家族的全基因组鉴定与表达模式[J].应用与环境生物学报,2022,28(2):440-450.HAN Jie,CHEN Xiaohui,SHEN Xu,LI Xiaofei,LIN Yuling,WU Pengfei,LAI Zhongxiong. Genome-wide identification and expression pattern of gene family of SAP-PPD-KIX-TPL signal pathway in Dimocarpus longan Lour.[J].Chinese Journal of Applied and Environmental Biology,2022,28(2):440-450.