钙离子(Ca2+)作为第二信使参与植物细胞的信号转导,在植物生长发育以及抗逆境胁迫中均发挥着重要作用[1]。在植物中,钙离子结合蛋白(Ca2+-binding proteins)分为2 类,分别是Ca2+传感蛋白和Ca2+受体蛋白[2]。其中,钙依赖性蛋白激酶(Calcium dependent protein kinases,CDPKs)是植物细胞中最常见的一类Ca2+受体蛋白,在植物的生长发育、种子萌发、生物及非生物胁迫应答、离子通道运输等信号转导过程中起着重要作用[3-5]。
CDPK是一种广泛存在于植物中具有特殊结构特征的蛋白[6],结构主要由4 部分组成,包括N 端可变结构域(variable N-terminal domain)、Ser/Thr激酶结构域(Ser/Thr kinase domain)、自抑制连接结构域(auto-inhibitory junction domain)和类钙调素结构域(Calmodulin-like domain)[7-8]。其中,N 端可变区对其结合的底物有识别作用,结构域长度变化差异大,对CDPKs 的亚细胞定位有重要作用[9]。Ser/Thr 激酶区域具有高度保守的催化序列,在不同物种间具有较高的同源性[10]。自抑制域高度保守一般由20~30个氨基酸组成,通过假底物机制调节CDPKs的激酶活性[11]。类钙调素结构域包含1~4 个EF-hands,用于与Ca2+结合[12]。Ca2+与EF-hands 结合可诱导CDPK 构象变化,从而导致激酶结构域活性位点的折叠和暴露,激活CDPK使一系列底物磷酸化[13]。
CDPK 蛋白于1982 年首次在豌豆中被报道[14],然而直到1991年才在大豆中被克隆和鉴定[15]。CDPK 在植物生长发育以及生物和非生物胁迫方面均发挥着关键作用。在拟南芥中AtCPK2、AtCPK11、AtCPK17、AtCPK20、AtCPK24、AtCPK34被报道广泛参与花粉管的萌发[16-17];AtCPK1、AtCPK3、AtCPK4、AtCPK5、AtCPK6、AtCPK8、AtCPK10、AtCPK11、AtCPK12 和AtCPK21 参与环境对植物非生物胁迫[18-21];AtCPK28 调控植物的生长发育[22]。在水稻中,OsCPK7、OsCPK13、OsCPK17 和OsCPK24 被报道参与低温胁迫[23- 25];而OsCPK4、OsCPK9、Os-CPK12、OsCPK13和OsCPK21被研究发现参与干旱和盐胁迫[26-28]。除生长发育和非生物胁迫外,CDPK在生物胁迫中也发挥着广泛作用。前人研究表明拟南芥CPK28 与质膜相关的BIK1 相互作用并磷酸化,从而负调控植物PTI 免疫反应[29];拟南芥AtCPK5、AtCPK6 和AtCPK11 通过调控乙烯生物合成酶ACS 基因的表达控制乙烯的产生,来参与灰霉病感染免疫应答[30]。马铃薯StCDPK4 及StCDPK5通过磷酸化NADPH 氧化酶促进ROS 产生,同时调控病原胁迫下相关基因的表达,从而激活防御机制[31]。在小麦中,TaCDPK2 迅速响应小麦叶锈病的胁迫,在小麦抗叶锈病中发挥重要作用[32]。在番茄Cf-9抗性蛋白转基因烟草中发现,当Cf-9与枝孢霉Avr9 互作后激活了CDPK 的表达,并作为抗原诱导反应中重要的钙传感器发挥功能。将CDPK基因沉默后发现植物丧失Cf-Avr诱导的特异性超敏反应,表明CDPK 对于介导Cf-9/Avr9 诱导的植物防御体系不可或缺[33]。另外,玉米ZmCPK11被报道参与机械损伤胁迫[34]。在烟草中,NaCDPK4 和NaCDPK5被报道参与机械损伤和食草动物啃食胁迫[35]。
目前,CDPK 家族基因在多个物种中被克隆和鉴定。在拟南芥中被发现有34 个CDPK 基因[36];在水稻中被发现有31 个CDPK 基因[37];在玉米中被发现有40 个CDPK 基因[38];在小麦中被发现有20 个CDPK 基因[32];在大豆中被鉴定有39 个CDPK 基因[8]。CDPK 基因的研究在荔枝中还未见报道。栽培荔枝品种妃子笑全基因组序列已被释放[39],这使得荔枝基因家族鉴定成为可能。中国是全球最大的荔枝生产国,同时荔枝在食品、医药和化妆品等方面起着重要作用[40]。然而,荔枝在生长发育过程中,受到各种病害的影响。其中,荔枝霜疫病是危害荔枝的一种重要病害[41]。荔枝霜疫病是由荔枝霜疫霉菌(Peronophthora litchi)侵染荔枝所引起的,严重危害着荔枝的产量和品质[42]。笔者针对荔枝霜疫病这一问题,结合CDPK 在植物生长以及抗病中的重要作用,对荔枝CDPK 成员进行了全基因组家族鉴定,并对其基因结构、蛋白结构、染色体定位,以及组织表达和在荔枝霜疫病胁迫条件的表达模式进行分析,为进一步研究荔枝CDPK 功能提供参考。
1 材料和方法
1.1 植物材料及生长条件
试验所用276份荔枝种质材料由广东省农业科学院果树研究所提供。荔枝种质资源种植于国家果树种质广州荔枝资源圃。荔枝病原菌材料荔枝霜疫霉菌由华南农业大学提供。
1.2 LcCDPK基因家族的鉴定
所用的荔枝基因组序列均下载于荔枝基因组数据库[39]。为鉴定荔枝CDPK 基因家族成员,笔者在本研究中使用拟南芥数据库TAIR(https://www.arabidopsis.org/)下载AtCPK 氨基酸序列作为参考,比对荔枝基因组数据库筛选得到候选基因。搜索到的候选基因利用InterProScan program(http://www.ebi.ac.uk/interpro)进行确认蛋白激酶区域是否同时存在Protein kinase结构域和EF手性结构。最后,所有确认的蛋白序列利用Pfam(http://pfam.sanger.ac.uk/search)和SMART(http://smart.embl-heidelberg.de/)工具进行重新评估。
1.3 LcCDPK序列及进化分析
利用Clustal X2.1对荔枝、拟南芥和水稻的CDPK 氨基酸序列进行多重序列比对。使用MEME(http://meme-suite.org/tools/meme)工具分析LcCDPK 蛋白保守Motif;利用GSDS 2.0(http://gsds.cbi.pku.edu.cn)进行基因结构分析;从荔枝基因组数据库中分别提取CDPK基因上游2000 bp的序列,利用PlantCARE(http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)分析启动子顺式作用元件[43]。最后,使用TBtools 对保守Motif、基因结构、顺式作用元件和染色体定位进行可视化。利用PROSITE(https://web.expasy.org/myristoylator/)网站预测LcCDPK 的N-myristoylation 位 点;利 用wolfpsort(https://wolfpsort.hgc.jp)对荔枝CDPK 蛋白亚细胞定位进行预测。在MEGA5.0软件中使用Neighborjoining构建荔枝CDPK家族进化树,设置参数为:距离模型,Bootstrap法,重复1000次[44]。
1.4 荔枝霜疫病处理方法
荔枝霜疫霉菌的培养和孢子悬浮液配置参考Xing等[45]的方法。简要为荔枝霜疫霉菌培养于萝卜琼脂培养基(胡萝卜200 g,琼脂粉20 g,水1000 mL),培养温度为27 ℃。荔枝霜疫霉菌孢子释放于灭菌水中,用血球计数板计算并调整孢子悬浮液的浓度。
对于荔枝成熟果实的荔枝霜疫病的处理方法,笔者在参考Sun等[41]的方法基础上并稍作修改。简要方法为,在接种荔枝霜疫霉菌孢子前用无菌水清洗荔枝成熟果实3次。后将每个荔枝果实用移液器接种5 μL荔枝霜疫霉菌孢子悬浮液(孢子个数为1×104个·mL-1);接种后的果实转移至保鲜盒中并放置恒温培养箱培养,培养温度为27 ℃,昼夜各12 h。3次重复,每个重复10粒荔枝果实。对于荔枝叶片的荔枝霜疫病的处理方法,笔者在参考Xing等[45]的方法基础上并稍作修改。具体为,采集荔枝新鲜叶片,平铺于培养皿中,每个培养皿中放入4枚荔枝叶片,并加入10 mL 无菌水,用移液器接种5 μL 荔枝霜疫霉菌孢子悬浮液(孢子个数为1×104个·mL-1);盛有叶片的培养皿转移至恒温培养箱培养,培养温度为27 ℃,昼夜时间各12 h。3次重复,每个重复10枚荔枝叶片。
荔枝叶片和成熟果实病级参考Xing 等[45]的方法。病情指数(Disease index,DI)的计算参考Sun等[41]的方法。
RNA-Seq 以及RT-PCR 所用的荔枝材料为裕荣1 号(YR1)。所用荔枝霜疫病的处理方法同前文一致。简要为分别用5 μL 荔枝霜疫霉菌孢子悬浮液(孢子个数为1×104个·mL-1)和5 μL灭菌水(对照组,Mock)接种荔枝新鲜叶片。接种后的材料转移至恒温培养箱中培养,培养条件同前文一致。在处理后的24 h 收集并液氮保存目标材料,各材料3 个生物学重复用于后续分析。
1.5 转录组数据分析
植物总RNA的提取使用Plant RNA Kit(R6827,Omega)试剂盒完成。使用Ultra RNA样本制备试剂盒(Illumina)构建RNA-seq 文库。RNA 测序由北京奥维森基因科技有限公司完成,双端测序(Paired-End,Illumina HiSeq 4000)。计算RPKM作为基因的表达量,使用R语言heatmap函数进行数据可视化。
1.6 试验数据
本文中所用荔枝组织特异性表达原始数据来自SRA(NCBI Sequence Read Archive)数据库,登录号为PRJNA747875。共9 种组织:雌花子房(female flowers ovary)、根(root)、果皮(pericarp)、假种皮(aril)、胚(embryo)、外种皮(episperm)、雄花花药(male flowers anther)、叶(leaf)和种子(seed)。
本文中荔枝霜疫霉菌处理荔枝叶片转录组数据已经上传至GEO(NCBI Gene Expression Omnibus database)数据库,登录号为GSE201243。
1.7 实时荧光定量分析
使用qRT-PCR 进一步分析荔枝响应荔枝霜疫霉菌胁迫下的候选基因。植物叶片提取试剂盒为Plant RNA Kit(R6827,Omega)。RNA 反转录试剂盒为SYBR Green master mix(Vazyme,Cat# Q711-02)。使用LightCycler480 thermal cycler(Roche)进行qRT-PCR反应。每个样品3个生物学重复。使用引物见表1。以荔枝Actin基因为内参基因[41]。基因相对表达量用2-ΔΔCt计算[46]。
表1 文中所用引物序列
Table 1 Sequences of primers used in this study
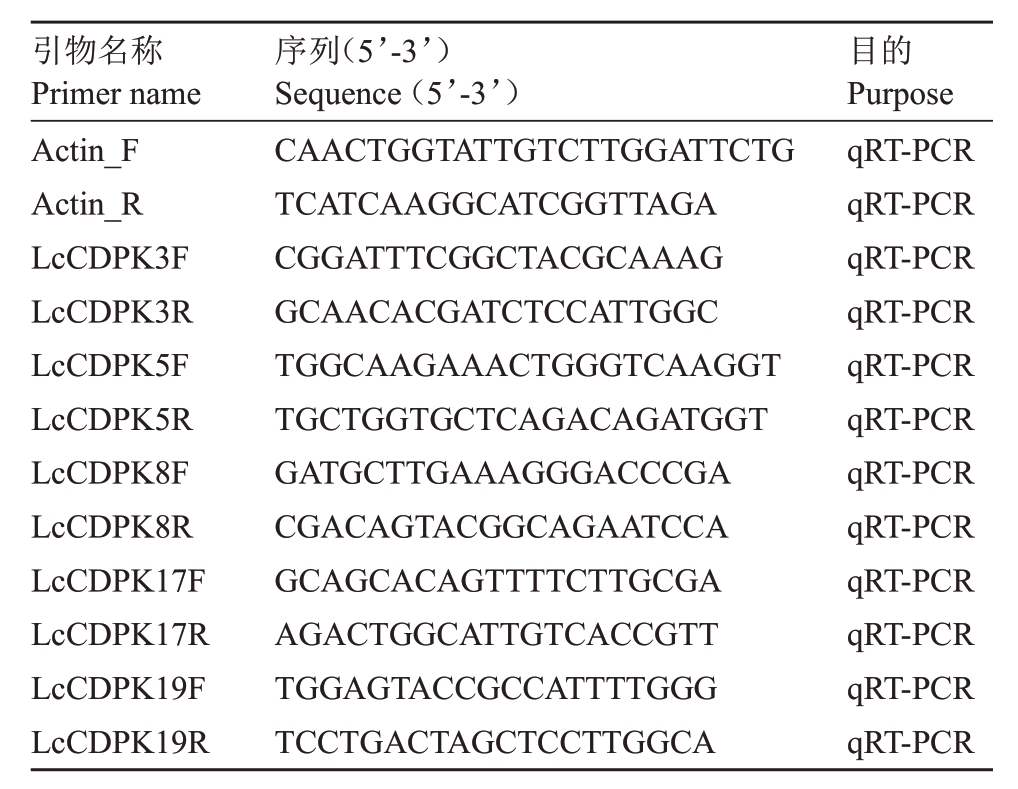
引物名称Primer name Actin_F Actin_R LcCDPK3F LcCDPK3R LcCDPK5F LcCDPK5R LcCDPK8F LcCDPK8R LcCDPK17F LcCDPK17R LcCDPK19F LcCDPK19R序列(5’-3’)Sequence(5’-3’)CAACTGGTATTGTCTTGGATTCTG TCATCAAGGCATCGGTTAGA CGGATTTCGGCTACGCAAAG GCAACACGATCTCCATTGGC TGGCAAGAAACTGGGTCAAGGT TGCTGGTGCTCAGACAGATGGT GATGCTTGAAAGGGACCCGA CGACAGTACGGCAGAATCCA GCAGCACAGTTTTCTTGCGA AGACTGGCATTGTCACCGTT TGGAGTACCGCCATTTTGGG TCCTGACTAGCTCCTTGGCA目的Purpose qRT-PCR qRT-PCR qRT-PCR qRT-PCR qRT-PCR qRT-PCR qRT-PCR qRT-PCR qRT-PCR qRT-PCR qRT-PCR qRT-PCR
2 结果与分析
2.1 LcCDPK基因家族成员鉴定及其系统发育分析
为了鉴定荔枝CDPK 基因家族成员,笔者在本研究中使用拟南芥的CDPK氨基酸序列作为参考序列,然后在荔枝全基因组数据库中进行搜索比对,共筛选到21个候选基因。随后,对候选基因进行蛋白结构分析,以确定其具有Protein kinase 和EF-hand结构域(图1),最终得到19 个荔枝CDPK 基因家族成员,将其命名为LcCDPK1~LcCDPK19(表2)。19个LcCDPK 中,除LcCDPK13 外,其余基因的CDS为1491~2556 bp,编码496~851 个氨基酸(表2),蛋白质分子质量为55.65~95.41 ku,等电点为5.22~9.13(表2)。LcCDPK13比较特殊,其CDS为4047 bp,编码1348 个氨基酸,蛋白质分子质量为151.44 ku,等电点为8.70。利用Wolf psort 对LcCDPK 亚细胞定位进行预测,如表2 所示,LcCDPK1 和LcCDPK4 定位在叶绿体中;LcCDPK3、LcCDPK6 和LcCDPK17定位在细胞核中;LcCDPK13定位在质膜中;其余定位在细胞质中。CDPK 的N 末端含有N-myristoylation(豆蔻酰化位点),能促进蛋白间的相互作用。此研究用https://web.expasy.org/myristoylator/网站预测的19 个LcCDPK 基因中,有9 个在其N 末端具有豆蔻酰化位点,而其余10个不具有豆蔻酰化位点(表2)。

图1 LcCDPK 氨基酸序列多重序列比对
Fig.1 Multiple sequence alignment of LcCDPK protein in lychee
红色的直线表示Ser/Thr 蛋白激酶区域,黑色的直线分别表示4 个EF-hands 区域。
Red line indicate the Ser/Thr kinase domain,black lines indicate the four EF-hands.
表2 荔枝CDPK 基因家族成员特征信息
Table 2 The characteristics of 19 LcCDPK genes

基因名称Gene name基因编号Gene ID EF Hands等电点pIa起始位置Start终止位置End N-Myristoylation LcCDPK1 LcCDPK2 LcCDPK3 LcCDPK4 LcCDPK5 LcCDPK6 LcCDPK7 LcCDPK8 LcCDPK9 LcCDPK10 LcCDPK11 LcCDPK12 LcCDPK13 LcCDPK14 LcCDPK15 LcCDPK16 LcCDPK17 LcCDPK18 LcCDPK19 LITCHI001674 LITCHI002195 LITCHI006474 LITCHI006812 LITCHI007334 LITCHI008906 LITCHI010062 LITCHI011639 LITCHI014137 LITCHI015851 LITCHI019005 LITCHI019021 LITCHI022010 LITCHI022230 LITCHI023733 LITCHI023923 LITCHI027028 LITCHI027050 LITCHI029637 CDS长度CDS Length/bp 1806 1683 1689 1869 1503 1557 1608 1491 1581 1644 1866 1722 4047 1674 1611 2556 1542 1770 1641氨基酸长度Amino acids/aa 601 560 562 622 500 518 535 496 526 547 621 573 1348 557 536 851 513 589 546染色体Chromosome Chr5 Chr5 Chr11 Chr11 Chr11 Chr7 Chr8 Chr8 Chr2 Chr1 Chr15 Chr15 Chr10 Chr10 Chr13 Chr13 Chr3 Chr3 Chr9 1441444444444444344 8.67 5.80 9.13 8.87 5.27 6.61 5.86 5.59 6.10 6.14 5.48 5.34 8.70 6.55 5.46 6.57 5.58 5.22 6.33分子质量Molecular mass/ku 67.68 62.90 63.80 69.22 56.29 58.81 60.12 55.65 58.81 62.20 69.86 63.48 151.44 63.34 59.19 95.41 57.11 65.51 61.70 29 446 798 34 128 272 1 569 144 4 705 922 11 613 237 19 312 107 1 857 782 24 775 847 39 048 466 19 870 410 11 587 821 11 743 007 2 965 362 4 555 317 5 194 965 8 788 218 16 195 697 16 459 442 27 392 060 29 453 620 34 132 785 1 578 338 4 712 563 11 618 541 19 317 474 1 862 347 24 780 830 39 057 334 19 873 267 11 593 634 11 748 387 2 976 577 4 559 219 5 198 136 8 801 045 16 204 228 16 465 948 27 399 369亚细胞定位Subcellular localization Chloroplast Cytoplasm Nucleus Chloroplast Cytoplasm Nucleus Cytoplasm Cytoplasm Cytoplasm Cytoplasm Cytoplasm Cytoplasm Plasma Membrane Cytoplasm Cytoplasm Cytoplasm Nucleus Cytoplasm Cytoplasm YNYYNNYNYYNNNNYYNNY
为更好地研究荔枝中CDPK 家族进化关系,笔者使用已知34个拟南芥AtCPK和31个水稻OsCPK氨基酸序列,以及笔者在本研究中所鉴定到的19个LcCDPK 氨基酸序列,共84 个CDPK 序列进行了进化分析。如图2 和图4-A 所示,19 个LcCDPK 可分为4个亚家族,其中Ⅰ~Ⅳ亚家族中分别含有6、5、4和4个LcCDPK基因。
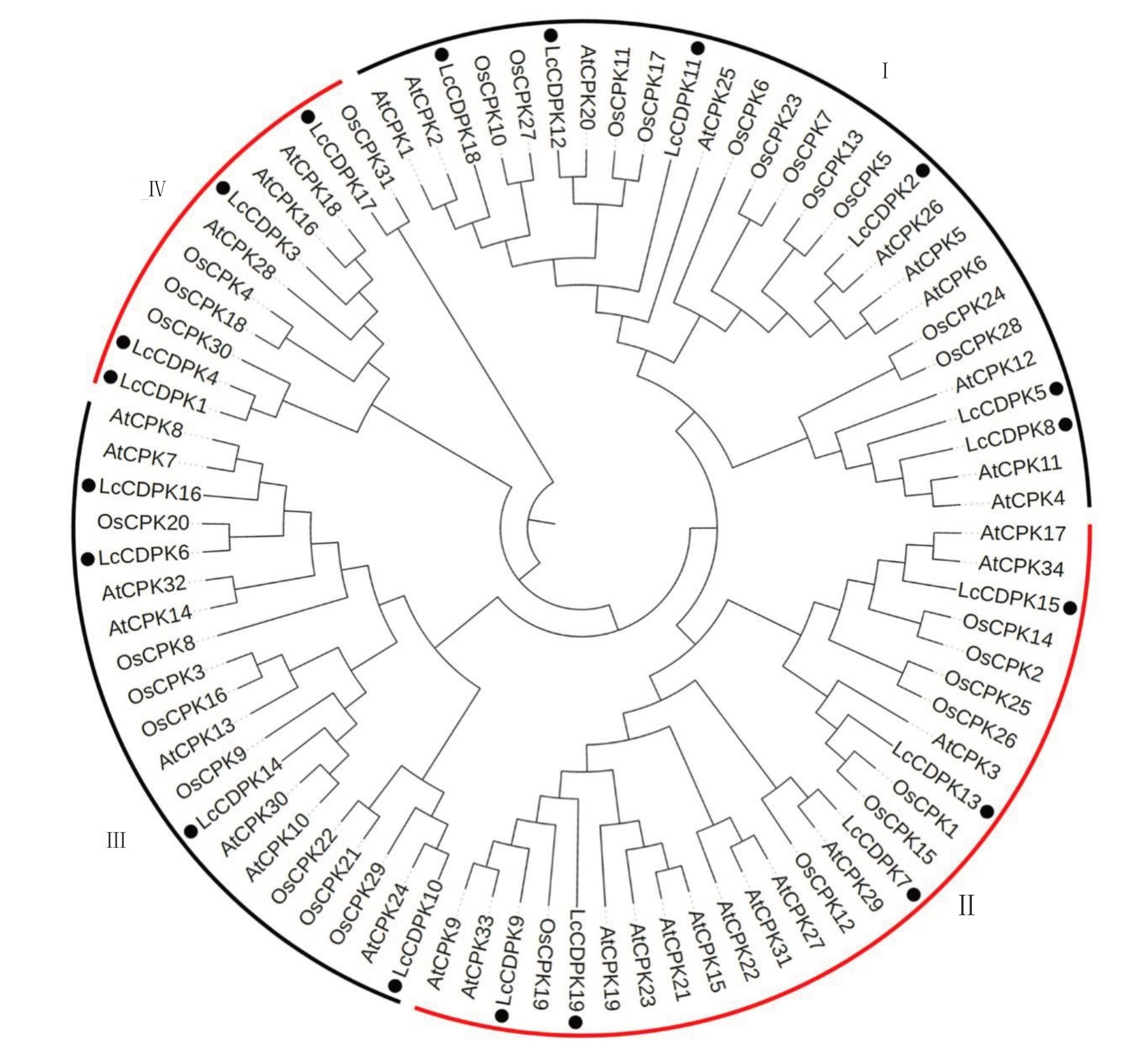
图2 LcCDPK 氨基酸序列的系统发育分析
Fig.2 Phylogenetic of LcCDPK sequences
利用荔枝、拟南芥和水稻CDPK 的全长氨基酸序列进行系统发育分析。CDPK 家族分为4 个主要亚组(Ⅰ~Ⅳ)。黑点表示荔枝CDPK。
The full-length amino acid sequences of CDPKs from Lychee(Lc),Arabidopsis(At)and rice(Os)were used for phylogenetic analysis.The CDPK family is divided into four major subgroups(Ⅰ-Ⅳ).Black dots indicate the lychee CDPK.
2.2 LcCDPK染色体分布
基于LcCDPK基因的物理位置(表2),笔者对这19 个LcCDPK 基因在15 条染色体上进行了定位(2n=30,图3)。结果发现,第11号染色体包含最多的LcCDPK基因,第4、6、12和14号染色体没有发现LcCDPK基因。多数LcCDPK位于染色体臂上。如图3 所示,LcCDPK1 和LcCDPK2 被定位在Chr5 上;LcCDPK3、LcCDPK4 和LcCDPK5 被定位在Chr11上;LcCDPK6 被定位在Chr7 上;LcCDPK7 和LcCDPK8被定位在Chr8上;LcCDPK9被定位在Chr2上;LcCDPK10 被定位在Chr1 上;LcCDPK11 和LcCDPK12 被定位在Chr15 上;LcCDPK13 和LcCDPK14被定位在Chr10上;LcCDPK15和LcCDPK16被定位在Chr13 上;LcCDPK17 和LcCDPK18 被 定 位 在Chr3 上;LcCDPK19 被定位在Chr9 上。在拟南芥中34 个CDPK 基因分布于5 条染色体上[36],在水稻中31 个CDPK 基因分布于12 条染色体上[37],表明CDPK基因在植物基因组中分布广泛。

图3 LcCDPK 染色体定位
Fig.3 Distribution of LcCDPK on lychee chromosomes
2.3 LcCDPK基因结构和保守Motif分析
为了探索LcCDPK 基因结构的保守性和多样性,笔者对LcCDPK 基因进行了结构分析,发现LcCDPK 分别含有7~19 个外显子,以及6~18 个内含子。如图4-B 所示,LcCDPK 的第Ⅰ亚家族成员中均含有7 个外显子和6 个内含子,表明同一亚家族中的LcCDPK 基因具有高度保守的基因结构。与之相似的是第Ⅱ亚家族,除LcCDPK13 含有19个外显子和18 个内含子外,其余家族成员均含有8 个外显子和7 个内含子。相反,在第Ⅲ和第Ⅳ亚家族中呈现较大的基因结构多样性,这两个家族中含有多个外显子和内含子,例如,在第Ⅲ亚家族中LcCDPK6 与LcCDPK10 含有8 个外显子和7 个内含子,LcCDPK14 仅含有7 个外显子和6 个内含子,LcCDPK16 含有13 个外显子和12 个内含子。第Ⅳ亚族中,LcCDPK1 与LcCDPK4 含有11 个外显子和10 个内含子,LcCDPK3 含有12 个外显子和11 个内含子,LcCDPK17 含有7 个外显子和6 个内含子。

图4 LcCDPK 基因家族成员进化树、基因结构和保守基序分布
Fig.4 Phylogenetic tree,gene structure and distribution of conserved motifs of Lychee CDPKs
A.利用LcCDPK 氨基酸全长序列,采用Neighbor-Joining(NJ)方法构建系统进化树;B.LcCDPK 基因家族成员的基因结构;C.LcCDPK 基因家族成员保守基序分布。
A.The phylogenetic tree was constructed using full-length protein sequences by the Neighbor-Joining(NJ)method;B.Gene structure of LcCDPK family members;C.Distribution of conserved motifs of LcCDPK.
随后使用LcCDPK 的氨基酸序列进行了保守Motif 分析。一共鉴定出5 个保守的Motifs,Motif1~Motif5(图4-C)。19 个LcCDPK 均包含这5 个Motif。值得注意的是,LcCDPK16 较为特殊,拥有两组Motif1、Motif2和Motif4。
2.4 LcCDPK顺式作用元件分析
顺式作用元件在植物生长发育和抗逆境胁迫中均执行着不同的功能。植物激素例如水杨酸(SA)、茉莉酸(JA)、乙烯(ET)和脱落酸(ABA)等通过诱导转录因子与其相应的顺式作用元件相互作用,在植物生长和逆境胁迫中发挥着重要作用[47]。为了探索荔枝中LcCDPK 基因的转录调控,笔者在本研究中利用Plant CARE 数据库对LcCDPK 基因家族成员起始密码子上游2000 bp 启动子区域的潜在顺式作用元件进行了分析。如图5 所示,8 个潜在的顺式作用元件被鉴定,包含激素响应相关顺式作用元件(ABRE、CGTCA-motif 和TCA-element)、光响应顺式作用元件(AE-box 和Gbox)以及逆境胁迫顺式作用元件(ARE、MBS 和TC-rich repeats)。LcCDPK18 启动子只包含两个顺式作用元件(TC-rich repeats 和AE-box),其余LcCDPK 家族成员均含有多个顺式作用元件(图5)。TC-rich repeats 是一种植物防卫和胁迫的顺式作用元件[48],分析发现在LcCDPK2 和LcCDPK6 的启动子中均包含这一顺式作用元件(图5)。TCAelement 是一种植物响应水杨酸(SA)应对植物病原胁迫的重要顺式元件[49],分析发现LcCDPK1、LcCDPK5、LcCDPK10、LcCDPK12、LcCDPK14、LcCDPK17 和LcCDPK19 这7 个基因的启动子中均包含这一顺式作用元件。

图5 LcCDPK 顺式作用元件分析
Fig.5 Analysis of cis-regulatory elements in the promoters of LcCDPK genes
A.LcCDPK 基因家族成员启动子顺式作用元件种类和位置;B.LcCDPK 基因家族成员启动子顺式作用元件种类和数量。
A. The locations of cis-acting elements in promoters of LcCDPK family members; B. Types and numbers of cis-acting elements in promoters of LcCDPK family members.
2.5 LcCDPK组织特异性分析
CDPK 在植物不同发育阶段均发挥着重要作用[9]。为了研究LcCDPK 基因在荔枝发育中的表达模式,笔者在本研究中利用RNA-Seq 数据对LcCDPK 基因在9 个不同荔枝组织(根、叶、雄花花药、雌花子房、胚、外种皮、假种皮、果皮和种子)中进行了表达分析(图6)。结果发现,虽然19 个LcCDPK 基因在9 个组织中均有表达,但在不同组织中表达却存在巨大差异。聚类分析发现,19 个LcCDPK 分为3 组(图6)。第Ⅰ组包含8 个基因(LcCDPK16、LcCDPK7、LcCDPK4、LcCDPK19、LcCDPK5、LcCDPK9、LcCDPK6 和LcCDPK18),主要在根和叶组织中大量表达,而在假种皮中呈现低表达。第Ⅱ组包含9 个 基 因(LcCDPK14、LcCDPK17、LcCDPK1、LcCDPK8、LcCDPK11、LcCDPK12、LcCDPK10、LcCDPK15 和LcCDPK3),大部分基因在雄花花药中大量表达。第Ⅲ组包含两个基因(LcCDPK13 和LcCDPK2),在根、雌花子房和果皮中高表达,而雄花花药中显示低表达。归纳发现,LcCDPK16、LcCDPK7、LcCDPK4、LcCDPK9、LcCDPK6、LcCDPK18、LcCDPK14、LcCDPK17、LcCDPK11 和LcCD-PK13在根中表现显著的高表达,可能与植物根系生理有关;LcCDPK19、LcCDPK9、LcCDPK6 和LcCDPK3在叶中表现显著高表达,可能与叶片中的营养、抗病等生理功能有关;LcCDPK1、LcCDPK11、LcCDPK12和LcCDPK10在雄花花药中表现显著高表达,可能与生殖生理有关。

图6 LcCDPK 基因组织表达分析
Fig.6 Heatmap of lychee LcCDPK gene expressions in nine tissues
LcCDPK 基因在9 个不同荔枝组织中的表达。1.根;2.叶;3.外种皮;4.胚;5.雄花花药;6.雌花子房;7.果皮;8.假种皮;9.种子。
The heatmap of CDPK genes was constructe using R.Color key represents the relative transcript abundance of the LcCDPK genes in nine lychee tissues.1.Root;2.Leaf;2.Episperm;4.Embryo;5.Male flowers anther;6.Female flowers ovary;7.Pericarp;8.Aril;9.Seed.
2.6 荔枝高抗霜疫病材料的鉴定
荔枝霜疫病,是由荔枝霜疫霉菌侵染荔枝所引起的、发生在荔枝上的一种最为严重的病害[41]。该病流行于荔枝的整个生长发育过程中,尤其在嫩叶、花期和果实成熟期,甚至在采后运输贮藏过程中也能发生严重的危害[50]。为探索LcCDPK家族成员在荔枝霜疫病胁迫下的表达模式,笔者在本研究中以276份荔枝自然群体的叶片和成熟果实为材料筛选高抗霜疫病荔枝品种(表3)。其中2021年使用荔枝叶片所鉴定的材料有256 份,病情指数最大值为100,最小值为2.22,平均值为68.28;2022 年使用叶片所鉴定的材料有242 份,病情指数最大值为100,最小值为2.59,平均值为68.47。使用荔枝成熟果实鉴定荔枝抗霜疫病材料只有2021 年的147 份材料,病情指数最大值为100,最小值为0,平均值为57.64(表3)。综合分析荔枝叶片和成熟果实病情指数的条形图和箱线图(图7),结果表明裕荣1号(YR1)荔枝在荔枝叶片和成熟果实的病情指数均小于25,按照前人的荔枝抗病分级标准[41],裕荣1号(YR1)是一种高抗荔枝霜疫病的荔枝品种。
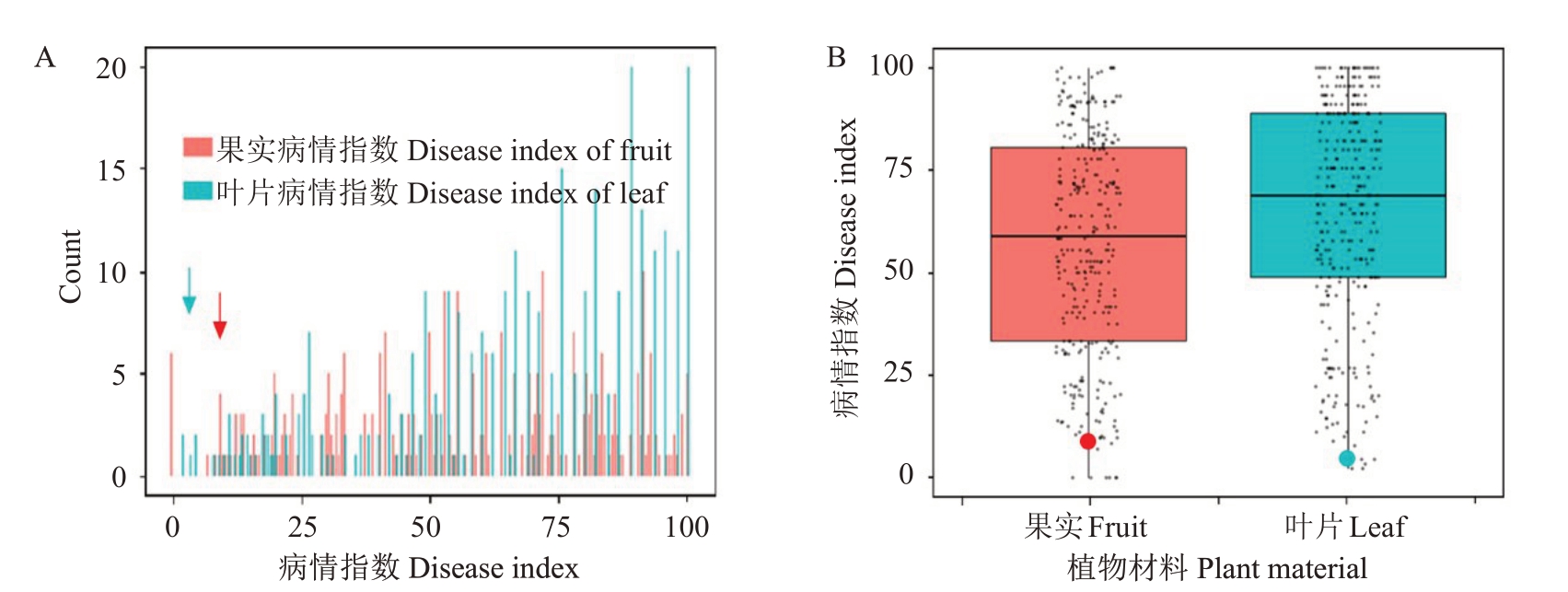
图7 荔枝抗霜疫病材料的筛选鉴定
Fig.7 Screening and Identification of lychee cultivar YR1 with high-resistance to lychee downy mildew
A.276 份荔枝叶片和成熟果实对霜疫病抗性的病情指数频率分布;B.荔枝叶片和成熟果实病情指数的表型分析。分别以276 份荔枝自然群体的叶片和成熟果实为材料筛选出对霜疫病高抗品种裕荣1 号(YR1)。青色箭头和圆点均表示裕荣1 号(YR1)叶片的病情指数,红色箭头和圆点均表示裕荣1 号(YR1)成熟果实病情指数。黑色小点表示每个材料的病情指数。荔枝叶片病情指数包含2021 和2022 年2 个生物学重复,荔枝果实病情指数包含2021 年1 个生物学重复。
A. Frequency distributions of DI based on the means of the traits of 276 lychee accessions; B. Phenotypic analysis of DI values in lychee leaves and mature fruits.Leaves and mature fruits of 276 lychee germplasm materials were used as materials to screen the high resistance cultivar YR1.The cyan arrow and dot indicated the DI values of YR1 leaves,and the red arrow and dot indicated the DI values of YR1 mature fruits.The data of lychee leaf DI with two biological repeat,and the data of lychee fruit DI with one biological repeat.
表3 荔枝自然群体病情指数的描述性统计
Table 3 Descriptive statistics explained by the population structure for disease index(DI)values in lychee accessions
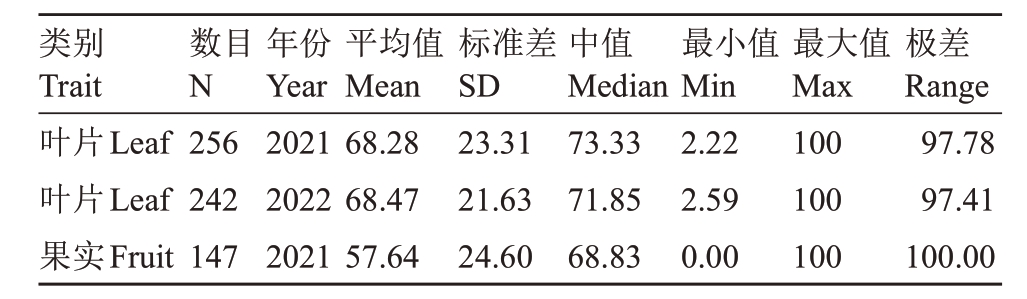
类别Trait叶片Leaf叶片Leaf果实Fruit数目N 256 242 147年份Year 2021 2022 2021平均值Mean 68.28 68.47 57.64标准差SD 23.31 21.63 24.60中值Median 73.33 71.85 68.83最小值Min 2.22 2.59 0.00最大值Max 100 100 100极差Range 97.78 97.41 100.00
2.7 荔枝霜疫病胁迫下的LcCDPK 家族成员表达分析
在筛选到的高抗荔枝霜疫病材料裕荣1 号(YR1)的基础上,笔者利用RNA-Seq分析了荔枝霜疫病胁迫下的LcCDPK 家族成员表达模式(图8-A)。与对照相比较,LcCDPK17,LcCDPK19 和LcCDPK5在荔枝霜疫病处理后显著上调表达(图8-A);LcCDPK3 和LcCDPK8 在病处理下显著下调表达(图8-A)。为了进一步检验这一结果,挑选这5个基因在荔枝霜疫病处理后分时间点取样进行qRTPCR 验证(图8-B~F)。与RNA-Seq 结果一致的是(图8-B~F),在荔枝霜疫病胁迫后,LcCDPK19 在病原菌胁迫24 h 后达到最高点,表达量是Mock 处理的6 倍左右(图8-F);LcCDPK17 在病处理后16 h 达到最大值(图8-E);LcCDPK5 在病处理后24 h 达到最大值,表达量大约是Mock 处理的3.5 倍(图8-E)。另外,LcCDPK3 和LcCDPK8 在病处理后迅速下调表达,均低于Mock处理(图8-B,8-D)。这些结果表明这些基因可能在荔枝抗霜疫病过程中发挥重要作用。

图8 LcCDPKs 在荔枝霜疫病胁迫下的表达分析
Fig.8 Expression pattern analysis of LcCDPKs under the infection of lychee downy mildew
A.热图表示荔枝霜疫病与Mock 处理荔枝叶片24 h 后LcCDPK 基因表达量的比值。RNA-seq 所使用材料为裕荣1 号(YR1)荔枝叶片,不同颜色表示表达量比值的差异,红色表示上调表达,绿色表示下调表达;B~F.基于A 中RNA-seq 所得到的候选基因进行qRT-PCR 验证。荔枝Actin 作为内参,所用数据为3 个生物学重复的(平均值±SD)。
A.The heatmap exhibit the ratio of the expression levels of lychee LcCDPK genes between lychee downy mildew treatment and Mock condition.RNA-seq data using leaf samples of the high-resistant lychee cultivar YR1.B-F.Relative expression levels of five lychee LcCDPKs in response to a simulated lychee downy mildew stress.The lychee Actin was used as the internal control.The data represent(the mean±SD)of three replicates.
3 讨 论
目前,CDPK 基因家族成员已在众多植物中被鉴定并被广泛研究。在模式植物拟南芥中共有34个CDPK 基因被鉴定;在禾本科中,水稻中有31 个CDPKs[37]、小麦中有20 个CDPKs[32]、玉米中有40 个CDPKs[38];在葫芦科中,黄瓜中有19个CDPKs[51]、甜瓜中有18 个CDPKs[52];在水果中,柑橘基因组含有29个CDPKs[53]、菠萝有17个CDPKs[54]。笔者利用生物信息学等方法,从荔枝中鉴定出19个LcCDPK基因家族成员。荔枝妃子笑主要含15 条假染色体序列,基因组大小约470 Mb[39]。这说明CDPK 基因家族成员数量与物种基因组大小没有线性关系,推测可能是在荔枝进化过程中,由于自然选择的压力,有些LcCDPK 基因功能丧失,逐渐演化消亡或演变成其他基因。
在植物中CDPK具有明显的结构特征。笔者在本研究中发现19 个LcCDPK 成员N 端可变区的长短不一;催化区蛋白激酶区域同源性较高,含有典型的Ser/Thr 蛋白激酶催化保守序列;调控区含有1~4个EF-hands 结构。综合前人研究进展,推测CDPK基因可能来自于蛋白激酶和CaM 基因的融合。然而,基因结构分析发现LcCDPK 各成员的内含子及外显子数量差异比较大。这可能是LcCDPK基因家族成员在植物中承担各种不同功能角色的重要原因。
CDPKs在植物的生长发育及应对生物、非生物胁迫中均发挥着重要的作用[55]。CDPKs 在不同组织中基因表达差异可能暗示着其功能的分化。在对荔枝9 个不同组织的表达模式分析时发现,19 个LcCDPK 基因在9 个组织中均有表达,但在不同组织中表达情况却存在巨大差异。第Ⅰ组成员主要在根和叶中大量表达,这暗示它们可能在根和叶中发挥重要作用。第Ⅱ组大部分基因在雄花花药中大量表达,这些基因可能与雄花的发育有关。例如,牵牛花PnCDPK1 是花形态建成生殖生长信号转导中的重要组成部分,转录水平在叶芽转变成花芽后迅速升高[56]。第Ⅲ组包含两个成员(LcCDPK13和LcCDPK2),在根、雌花子房和果皮中高表达。这两个基因在不同组织中可能发挥不同的功能。
荔枝霜疫病是由荔枝霜疫霉菌侵染所引起的、发生在荔枝上的一种严重病害。为了探讨LcCDPK对荔枝霜疫病的应答,笔者在本研究中以276 份荔枝自然群体叶片和成熟果实为材料,筛选出荔枝高抗品种裕荣1号(YR1)。基因表达数据表明,LcCDPK17、LcCDPK19和LcCDPK5在荔枝霜疫病处理后显著上调表达。顺式作用分析表明,LcCDPK17 和LcCDPK19启动子区域分别包含两个TCA-element;LcCDPK5启动子区域包含一个TCA-element。前人研究表明TCA-element是一种植物响应水杨酸(SA)应对植物病原胁迫的重要顺式作用元件[49]。这3个基因可能参与荔枝SA 通路响应病原微生物的胁迫。TC-rich repeats 是一种参与植物防卫和胁迫的顺式作用元件[48]。基因表达分析表明,LcCDPK8 在荔枝霜疫病处理下显著下调表达。顺式作用元件分析表明,LcCDPK8 启动子区域包含3 个TC-rich repeats,这表明LcCDPK8可能负调控植物病原微生物胁迫。
笔者在全基因组水平对荔枝CDPK家族基因进行了鉴定和详细的生物信息学分析。另外,在筛选到高抗霜疫病品种裕荣1 号(YR1)的基础上,对荔枝CDPK 家族成员进行了荔枝霜疫病胁迫相关研究,为进一步研究荔枝CDPK 基因家族功能提供了基础。
4 结 论
从荔枝基因组中共鉴定出19 个LcCDPK 基因家族成员,分为4 个亚家族,分布于11 条染色体上。在276份荔枝自然群体中鉴定出一份高抗霜疫病荔枝材料裕荣1 号。荔枝霜疫病胁迫表达分析表明LcCDPK5、LcCDPK17、LcCDPK19、LcCDPK3 和LcCDPK8可能在荔枝抗病过程中发挥着重要作用。
[1] KANCHISWAMY C N,MALNOY M,OCCHIPINTI A,MAFFEI M E.Calcium imaging perspectives in plants[J].International Journal of Molecular Sciences,2014,15(3):3842-3859.
[2] LECOURIEUX D,RANJEVA R,PUGIN A. Calcium in plant defence-signalling pathways[J]. New Phytologist,2006,171(2):249-269.
[3] YANG T B,POOVAIAH B W. Calcium/calmodulin-mediated signal network in plants[J]. Trends in Plant Science,2003,8(10):505-512.
[4] BOUDSOCQ M,WILLMANN M R,MCCORMACK M,LEE H,SHAN L B,HE P,BUSH J,CHENG S H,SHEEN J.Differential innate immune signalling via Ca2+ sensor protein kinases[J].Nature,2010,464(7287):418-422.
[5] DELORMEL T Y,BOUDSOCQ M. Properties and functions of calcium-dependent protein kinases and their relatives in Arabidopsis thaliana[J].New Phytologist,2019,224(2):585-604.
[6] ZHANG L,LU Y T. Calmodulin-binding protein kinases in plants[J].Trends in Plant Science,2003,8(3):123-127.
[7] KLIMECKA M,MUSZYŃSKA G. Structure and functions of plant calcium-dependent protein kinases[J].Acta Biochimica Polonica,2007,54(2):219-233.
[8] LIU H L,CHE Z J,ZENG X R,ZHOU X Q,SITOE H M,WANG H,YU D Y. Genome-wide analysis of calcium-dependent protein kinases and their expression patterns in response to herbivore and wounding stresses in soybean[J].Functional&Integrative Genomics,2016,16(5):481-493.
[9] CHENG S H,WILLMANN M R,CHEN H C,SHEEN J.Calcium signaling through protein kinases.The Arabidopsis calciumdependent protein kinase gene family[J]. Plant Physiology,2002,129(2):469-485.
[10] CHEN S,LIU G S,WANG Y Y,SUN Y H,CHEN J. Cloning of a calcium-dependent protein kinase gene NtCDPK12,and its induced expression by high-salt and drought in Nicotiana tabacum[J].Agricultural Sciences in China,2011,10(12):1851-1860.
[11] LI Y J,FEI X W,DAI H F,LI J Y,ZHU W J,DENG X D. Genome-wide identification of calcium-dependent protein kinases in Chlamydomonas reinhardtii and functional analyses in nitrogen deficiency-induced oil accumulation[J]. Frontiers in Plant Science,2019,10:1147.
[12] LIU Y,XU C J,ZHU Y F,ZHANG L N,CHEN T Y,ZHOU F,CHEN H,LIN Y J. The calcium-dependent kinase OsCPK24 functions in cold stress responses in rice[J]. Journal of Integrative Plant Biology,2018,60(2):173-188.
[13] BOUDSOCQ M,DROILLARD M J,REGAD L,LAURIÈRE C. Characterization of Arabidopsis calcium-dependent protein kinases:activated or not by calcium[J]. Biochemical Journal,2012,447(2):291-299.
[14] HETHERINGTON A,TREWAVAS A. Calcium-dependent protein kinase in pea shoot membranes[J].FEBS Letters,1982,145(1):67-71.
[15] HARPER J F,SUSSMAN M R,SCHALLER G E,PUTNAMEVANS C,CHARBONNEAU H,HARMON A C. A calciumdependent protein kinase with a regulatory domain similar to calmodulin[J].Science,1991,252(5008):951-954.
[16] GUTERMUTH T,LASSIG R,PORTES M T,MAIERHOFER T,ROMEIS T,BORST J W,HEDRICH R,FEIJ J A,KONRAD K R. Pollen tube growth regulation by free anions depends on the interaction between the anion channel SLAH3 and calciumdependent protein kinases CPK2 and CPK20[J].The Plant Cell,2013,25(11):4525-4543.
[17] ZHAO L N,SHEN L K,ZHANG W Z,ZHANG W,WANG Y,WU W H.Ca2+-dependent protein kinase11 and 24 modulate the activity of the inward rectifying K+channels in Arabidopsis pollen tubes[J].The Plant Cell,2013,25(2):649-661.
[18] ZOU J J,LI X D,RATNASEKERA D,WANG C,LIU W X,SONG L F,ZHANG W Z,WU W H. Arabidopsis CALCIUMDEPENDENT PROTEIN KINASE8 and CATALASE3 function in abscisic acid-mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress[J]. The Plant Cell,2015,27(5):1445-1460.
[19] XU J,TIAN Y S,PENG R H,XIONG A S,ZHU B,JIN X F,GAO F,FU X Y,HOU X L,YAO Q H.AtCPK6,a functionally redundant and positive regulator involved in salt/drought stress tolerance in Arabidopsis[J].Planta,2010,231(6):1251-1260.
[20] FRANZ S,EHLERT B,LIESE A,KURTH J,CAZAL A C,ROMEIS T. Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana[J]. Molecular Plant,2011,4(1):83-96.
[21] MORI I C,MURATA Y,YANG Y Z,MUNEMASA S,WANG Y F,ANDREOLI S,TIRIAC H,ALONSO J M,HARPER J F,ECKER J R,KWAK J M,SCHROEDER J I.CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anionand Ca2+-permeable channels and stomatal closure[J/OL]. PLoS Biology,2006,4(10):e327.DOI:10.1371/journal.pbio.0040327.
[22] MATSCHI S,WERNER S,SCHULZE W X,LEGEN J,HILGER H H,ROMEIS T.Function of calcium-dependent protein kinase CPK28 of Arabidopsis thaliana in plant stem elongation and vascular development[J]. The Plant Journal,2013,73(6):883-896.
[23] SAIJO Y,HATA S,KYOZUKA J,SHIMAMOTO K,IZUI K.Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants[J]. The Plant Journal,2000,23(3):319-327.
[24] ABBASI F,ONODERA H,TOKI S,TANAKA H,KOMATSU S. OsCDPK13,a calcium-dependent protein kinase gene from rice,is induced by cold and gibberellin in rice leaf sheath[J].Plant Molecular Biology,2004,55(4):541-552.
[25] ALMADANIM M C,GONCALVES N M,ROSA M T G,ALEXANDRE B M,CORDEIRO A M,RODRIGUES M,SAIBO N J M,SOARES C M,ROMÃO C V,OLIVEIRA M M,ABREU I A. The rice cold-responsive calcium-dependent protein kinase OsCPK17 is regulated by alternative splicing and post- translational modifications[J]. Biochimica et Biophysica Acta(BBA)-Molecular Cell Research,2018,1865(2):231-246.
[26] WEI S Y,HU W,DENG X M,ZHANG Y Y,LIU X D,ZHAO X D,LUO Q C,JIN Z Y,LI Y,ZHOU S Y,SUN T,WANG L Z,YANG G X,HE G Y. A rice calcium-dependent protein kinase OsCPK9 positively regulates drought stress tolerance and spikelet fertility[J].BMC Plant Biology,2014,14(1):1-13.
[27] CAMPO S,BALDRICH P,MESSEGUER J,LALANNE E,COCA M,SAN SEGUNDO B.Overexpression of a calcium-dependent protein kinase confers salt and drought tolerance in rice by preventing membrane lipid peroxidation[J]. Plant Physiology,2014,165(2):688-704.
[28] ASANO T,HAKATA M,NAKAMURA H,AOKI N,KOMATSU S,ICHIKAWA H,HIROCHIKA H,OHSUGI R. Functional characterization of OsCPK21,a calcium-dependent protein kinase that confers salt tolerance in rice[J].Plant Molecular Biology,2011,75(1):179-191.
[29] MONAGHAN J,MATSCHI S,SHORINOLA O,ROVENICH H,MATEI A,SEGONZAC C,MALINOVSKY F G,RATHJEN J P,MACLEAN D,ROMEIS T,ZIPFE C. The calcium-dependent protein kinase CPK28 buffers plant immunity and regulates BIK1 turnover[J]. Cell Host & Microbe,2014,16(5):605-615.
[30] GRAVINO M,SAVATIN D V,MACONE A,DE LORENZO G.Ethylene production in Botrytis cinerea- and oligogalacturonideinduced immunity requires calcium-dependent protein kinases[J].The Plant Journal,2015,84(6):1073-1086.
[31] SCHULZ P,HERDE M,ROMEIS T. Calcium-dependent protein kinases:hubs in plant stress signaling and development[J].Plant Physiology,2013,163(2):523-530.
[32] LI A L,ZHU Y F,TAN X M,WANG X,WEI B,GUO H Z,ZHANG Z L,CHEN X B,ZHAO G Y,KONG X Y,JIA J Z,MAO L. Evolutionary and functional study of the CDPK gene family in wheat(Triticum aestivum L.)[J].Plant Molecular Biology,2008,66(4):429-443.
[33] ROMEIS T,LUDWIG A A,MARTIN R,JONES J D G. Calcium-dependent protein kinases play an essential role in a plant defence response[J]. The EMBO Journal,2001,20(20):5556-5567.
[34] SZCZEGIELNIAK J,BORKIEWICZ L,SZURMAK B,LEWANDOWSKA- GNATOWSKA E,STATKIEWICZ M,KLIMECKA M,CIEŚLA J,MUSZYŃSKA G. Maize calciumdependent protein kinase (ZmCPK11):Local and systemic response to wounding,regulation by touch and components of jasmonate signaling[J].Physiologia Plantarum,2012,146(1):1-14.
[35] YANG D H,HETTENHAUSEN C,BALDWIN I T,WU J Q.Silencing Nicotiana attenuata calcium-dependent protein kinases,CDPK4 and CDPK5,strongly up-regulates wound-and herbivory-induced jasmonic acid accumulations[J]. Plant Physiology,2012,159(4):1591-1607.
[36] HARMON A C,GRIBSKOV M,GUBRIUM E,HARPER J F.The CDPK superfamily of protein kinases[J]. New Phytologist,2001,151(1):175-183.
[37] RAY S,AGARWAL P,ARORA R,KAPOOR S,TYAGI A K.Expression analysis of calcium-dependent protein kinase gene family during reproductive development and abiotic stress conditions in rice(Oryza sativa L.ssp.indica)[J].Molecular Genetics and Genomics,2007,278(5):493-505.
[38] KONG X P,LV W,JIANG S S,ZHANG D,CAI G H,PAN J W,LI D Q.Genome-wide identification and expression analysis of calcium-dependent protein kinase in maize[J]. BMC Genomics,2013,14(1):433.
[39] HU G B,FENG J T,XIANG X,…,LI J G.Two divergent haplotypes from a highly heterozygous lychee genome suggest independent domestication events for early and late-maturing cultivars[J].Nature Genetics,2022,54(1):73-83.
[40] ZHAO L,WANG K,WANG K,ZHU J,HU Z Y. Nutrient components,health benefits,and safety of litchi (Litchi chinensis Sonn.):A review[J]. Comprehensive Reviews in Food Science and Food Safety,2020,19(4):2139-2163.
[41] SUN J H,CAO L L,LI H L,WANG G,WANG S J,LI F,ZOU X X,WANG J B.Early responses given distinct tactics to infection of Peronophythora litchii in susceptible and resistant litchi cultivar[J].Scientific Reports,2019,9(1):1-14.
[42] SUN J H,GAO Z Y,ZHANG X C,ZOU X X,CAO L L,WANG J B. Transcriptome analysis of Phytophthora litchii reveals pathogenicity arsenals and confirms taxonomic status[J/OL].PLoS One,2017,12(6):e0178245. DOI:10.1371/journal.pone.0178245.
[43] 陈迪飞,魏秀清,许玲,许家辉,曾黎辉.莲雾PG 基因家族全基因组鉴定及表达分析[J].果树学报,2022,39(4):548-563.CHEN Difei,WEI Xiuqing,XU Ling,XU Jiahui,ZENG Lihui.Genome-wide identification and expression analysis of PG gene family in wax apple[Syzygium samarangense(Bl.)Merr.et Perry][J].Journal of Fruit Science,2022,39(4):548-563.
[44] 马青龄,梁贝贝,杨莉,胡威,刘勇,刘德春.枳WOX 基因家族全基因组鉴定及表达分析[J].果树学报,2022,39(5):712-729.MA Qingling,LIANG Beibei,YANG Li,HU Wei,LIU Yong,LIU Dechun. Genome-wide identification and expression analysis of WOX gene family in Trifoliate orange[J]. Journal of Fruit Science,2022,39(5):712-729.
[45] XING M Y,ZHENG L,DENG Y Z,XU D D,XI P G,LI M H,KONG G H,JIANG Z D.Antifungal activity of natural volatile organic compounds against litchi downy blight pathogen Peronophythora litchii[J/OL]. Molecules,2018,23(2):358. DOI:10.3390/molecules23020358.
[46] LIU H L,LI Y G,HU Y L,YANG Y H,ZHANG W B,HE M,LI X M,ZHANG C Y,KONG F J,LIU X,HOU X L.EDS1-interacting J protein 1 is an essential negative regulator of plant innate immunity in Arabidopsis[J]. The Plant Cell,2021,33(1):153-171.
[47] SANTNER A,ESTELLE M. Recent advances and emerging trends in plant hormone signalling[J]. Nature,2009,459(7250):1071-1078.
[48] SAZEGARI S,NIAZI A,AHMADI F S.A study on the regulatory network with promoter analysis for Arabidopsis DREB-genes[J].Bioinformation,2015,11(2):101-106.
[49] MOU S L,LIU Z Q,GUAN D Y,QIU A L,LAI Y,HE S L.Functional analysis and expressional characterization of rice ankyrin repeat-containing protein,OsPIANK1,in basal defense against Magnaporthe oryzae attack[J/OL]. PLoS One,2013,8(3):e59699.DOI:10.1371/journal.pone.0059699.
[50] JIANG L Q,YE W W,SITU J J,CHEN Y B,YANG X Y,KONG G H,LIU Y Y,TINASHE R J,XI P G,WANG Y C,JIANG Z D. A Puf RNA-binding protein encoding gene PlM90 regulates the sexual and asexual life stages of the litchi downy blight pathogen Peronophythora litchii[J]. Fungal Genetics and Biology,2017,98:39-45.
[51] XU X W,LIU M,LU L,HE M,QU W Q,XU Q,QI X H,CHEN X H. Genome-wide analysis and expression of the calcium-dependent protein kinase gene family in cucumber[J]. Molecular Genetics and Genomics,2015,290(4):1403-1414.
[52] ZHANG H F,WEI C H,YANG X Z,CHEN H J,YANG Y C,MO Y L,LI H,ZHANG Y,MA J X,YANG J Q,ZHANG X.Genome-wide identification and expression analysis of calcium-dependent protein kinase and its related kinase gene families in melon (Cucumis melo L.)[J/OL]. PLoS One,2017,12(4):e0176352.DOI:10.1371/journal.pone.0176352
[53] SHU B,JUE D W,ZHANG F,ZHANG D J,LIU C Y,WU Q S,LUO C. Genome-wide identification and expression analysis of the citrus calcium-dependent protein kinase (CDPK) genes in response to arbuscular mycorrhizal fungi colonization and drought[J].Biotechnology&Biotechnological Equipment,2020,34(1):1304-1314.
[54] ZHANG M,LIU Y H,HE Q,CHAI M N,HUANG Y M,CHEN F Q,WANG X M,LIU Y Q,CAI H Y,QIN Y.Genome-wide investigation of calcium-dependent protein kinase gene family in pineapple:evolution and expression profiles during development and stress[J/OL]. BMC Genomics,2020,21(1):72.https://doi.org/10.1186/s12864-020-6501-8.
[55] LI X,ZHAO L M,ZHANG H,LIU Q C,ZHAI H,ZHAO N,GAO S P,HE S Z. Genome-Wide identification and characterization of CDPK family reveal their involvements in growth and development and abiotic stress in sweet potato and its two diploid relatives[J]. International Journal of Molecular Sciences,2022,23(6):3088.
[56] JAWORSKI K,PAWEŁEK A,KOPCEWICZ J,SZMIDT-JAWORSKA A. The calcium-dependent protein kinase (PnCDPK1) is involved in Pharbitis nil flowering[J]. Journal of Plant Physiology,2012,169(16):1578-1585.