琯溪蜜柚[Citrus grandis(L.)Osbeck. Guanxi pomelo]为亚热带芸香科常绿果树,是福建省平和县乃至全国具有代表性的亚热带水果之一,已有500多年的栽培历史,是当地农民脱贫致富的主要经济来源[1]。然而,琯溪蜜柚的汁胞粒化严重影响到了果实的生产和销售,对琯溪蜜柚的口碑和相关产业造成了冲击,给当地农民带来一定的经济损失。
琯溪蜜柚成熟过程中的汁胞粒化是一种生理病害,将粒化汁胞与正常汁胞比较,发现粒化汁胞变干、变硬,呈现浑浊的絮状物。汁胞粒化对果实汁胞的外观、质地和风味都有不利影响[2]。Shomer等[3]通过观察琯溪蜜柚汁胞细胞壁的超微结构,认为汁胞粒化是汁胞细胞次生壁木质化形成厚壁组织的结果。潘腾飞等[4]的研究发现琯溪蜜柚汁胞粒化指数与木质素含量呈显著正相关,表明琯溪蜜柚汁胞粒化和木质素合成关系密切。木质素是沉积在植物细胞壁中的酚类聚合物,主要有3 种类型[5],分别是由香豆醇合成的对羟基苯基木质素(H 型)、由松伯醇合成的愈创木基木质素(G 型)、由芥子醇合成的紫丁香基木质素(S 型)[6]。琯溪蜜柚粒化汁胞中发现的木质素主要为G型木质素[7]。研究发现肉桂酰辅A 还原酶(CCR)、肉桂醇脱氢酶(CAD)和过氧化物酶(POD)等和木质素合成直接相关[8]。此外,苯丙氨酸解氨酶(PAL)、肉桂酸-4-羟基化酶(C4H)、4-香豆酸辅酶A连接酶(4CL)、CAD、POD等酶活性的变化也与木质素含量相关[9]。目前,对琯溪蜜柚汁胞粒化与木质素代谢关系的报道多为对木质素代谢途径中个别基因家族的分析或木质素相关合成酶活性对木质素含量的影响,关于琯溪蜜柚汁胞粒化过程中木质素代谢途径的系列关键基因表达变化与汁胞粒化关系的相关研究尚未见报道,因此在基因、酶及其代谢物水平上对此展开系统研究是十分必要的。
琯溪蜜柚果实汁胞粒化现象通常发生在果实成熟和采后的贮藏过程中,有关采后贮藏汁胞粒化的研究较多,但对成熟果实的汁胞粒化研究较少。笔者在本研究中以不同生长发育时期的琯溪蜜柚汁胞为材料,通过转录组筛选差异表达的木质素合成关键基因分析相关酶活性和木质素含量、粒化率的变化,以期揭示琯溪蜜柚汁胞粒化过程的生理与分子调控机制。
1 材料和方法
1.1 试验材料
琯溪蜜柚果实样品由福建省漳州市平和县小溪镇旧楼村石角坛“平和琯溪蜜柚综合试验站科研基地”果园提供,果园海拔420 m,选取25年以上树龄、树势一致的健壮树体,正常管理。于2018 年花后135、165、195和215 d 4个时期分别随机采集大小一致、健康、无明显机械外伤的果实各9个立即运回实验室,并进行果实粒化率的测定和果实汁胞石蜡切片。每个时期每个果实各取汁胞10 g,混合后放入液氮中速冻,存于-80 ℃冰箱,用于后续木质素合成相关酶活性的测定和qRT-PCR验证试验。此外,每个果实各取约100 g 置于玻璃瓶内于烘箱60 ℃烘干,研钵研磨后装入15 mL离心管中,用于木质素含量测定。以上所有试验均设3次生物学重复。
1.2 蜜柚果实粒化率、汁胞木质素含量测定
琯溪蜜柚果实粒化率测定参考代亚兰等[10]的方法,分别测定花后4个时期的粒化汁胞质量和汁胞总质量。果实粒化率/%=粒化汁胞质量/汁胞总质量×100。
琯溪蜜柚果实汁胞木质素含量采用AB 法,即乙酰溴法测定[11]。
1.3 蜜柚汁胞木质素显微镜观察
参照秦永亭等[12]的方法,将琯溪蜜柚汁胞用石蜡包埋后,用石蜡切片机切成7 μm厚的切片,干燥后用番红染液染色2 h、中性树胶封片后,用LEICADMI 48倒置显微镜观察。木质化的细胞壁被番红染成红色。
1.4 蜜柚汁胞转录组木质素合成差异基因表达分析
转录组测序由深圳华大基因研究院完成,统计和评估转录组测序数据数量和质量以及组装效果。通过转录组测序,得到基因的表达水平。将log2>2,并且Q-value ≤0.001的基因定义为差异基因。根据4 个时期汁胞转录组测序中的差异基因KEGG(Kyoto Encyclopedia of Genes and Genomes)途径富集分析,木质素合成代谢途径属于苯丙烷次生代谢途径中的一部分,将所有木质素代谢途径关键基因进行表达量分析,利用TBtools 软件绘制热图,并从中挑选出差异表达基因。
1.5 蜜柚汁胞木质素生物合成差异基因引物设计
从转录组中筛选出13 个差异表达基因(CrPAL1、CrPAL3、CrC4H1、CrC4H2、Cr4CL、CrHCT1、CrCCR3、CrCAD3、CrCOMT4、CrPOD2、CrPOD6、CrPOD7、CrPOD8),利用NCBI 上的引物设计程序(https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi)、按照荧光定量引物设计原则设计基因的qRTPCR 引物,以Actin 为内参基因(表1)。引物合成由擎科生物公司完成。
表1 内参基因与13 个琯溪蜜柚汁胞木质素生物合成途径目的基因的引物
Table 1 Primers of internal reference gene and 13 target genes related to lingnin biosynthesis in Guanxi pomelo juice sacs

基因名称Gene name CrPAL1 CrPAL3 CrC4H1 CrC4H2 Cr4CL CrHCT1 CrCCR3 CrCAD3 CrCOMT4 CrPOD2 CrPOD6 CrPOD7 CrPOD8 Actin基因ID Gene ID 102607860 102619342 102577934 102578013 102623419 102611960 102615114 102630907 102629858 102612835 102619551 102625757 102626445正向引物(5’—3’)Forward primer(5’—3’)TTTACGGACCACTTGACGC GGAACAAGGCATTACACGG CAGTGTAGGAGGTTGGCTTTCT TTGGACGCTCAGACAAAGG GGATTTCAAGTTGCTCCAGC TACACGAGTTGAGGCAGTTTC GTGGTGGAGATTCTGGCTAAG AACAAGAACGGCAGCAGTT CAGTGCCTCAGTCTTGCCTA GACTGCTTCATCGTGGGAT ATCCCGAACCAAACCGTT GCACATCATTCAGGGCTCA GCAACCACCAGAAGAACAAC CCAAGCAGCATGAAGATCAA反向引物(5’—3’)Reversed primer(5’—3’)CGCCTTGTTCCTTGATACATC CACCAGACAGATTTGAAGGC TGTTGTTTCTATTGCCGCC GCGGCACAAGAAGAGGAAT TTGCCAGATGGTGCTTTC CCATCCAGAGCAGATTTCC GTGTAGGGATTGGAAGGTGAC GAACAAAGTGGAAGTGGTGG AGCCTCTCAACTTTGCCGT CTCCAGCCAGAACTACAACATC TGAGCCGACTAAAGTTGAAGG CTTTCTGTTGACCAGGTTCTTG TCTCCATCAGTCCCAGTGAG ATCTGCTGGAAGGTGCTGAG
1.6 qRT-PCR验证分析
使用通用RNA提取试剂盒(东盛生物)提取汁胞RNA,参照HiScrip® ⅡQ RT SuperMix for qPCR试剂盒说明书,以琯溪蜜柚果实汁胞RNA为模板合成双链cDNA。使用ChamQ Universal SYBR qPCR Master Mix 试剂盒在罗氏荧光定量PCR 仪上测定基因的表达量。
1.7 蜜柚汁胞木质素合成相关酶活性测定
分别参考PAL、C4H、4CL、CAD 和POD 检测试剂盒(上海优选)说明对琯溪蜜柚果实汁胞进行PAL、C4H、4CL、CAD和POD提取并测定其活性。
1.8 数据处理
采用2-△△Ct方法计算基因的相对表达量[13]。利用WPS 表格进行数据统计(2019),SPSS 19.0 进行差异显著性分析。
2 结果与分析
2.1 琯溪蜜柚果实粒化率及汁胞木质素含量的变化
在琯溪蜜柚果实不同生长发育期间,花后195 d之前的果实发育期未见汁胞粒化,但在花后195 d之后的果实成熟期汁胞粒化率明显增加(图1);随着蜜柚果实的成熟,汁胞木质素含量也呈现增加的趋势,在花后195和215 d汁胞中发现木质素含量显著增加(图1),这些结果说明在蜜柚果实生长发育期间,随着木质素的积累,果实汁胞粒化率也随之增加,果实汁胞粒化可能是木质素含量过度累积引起的。
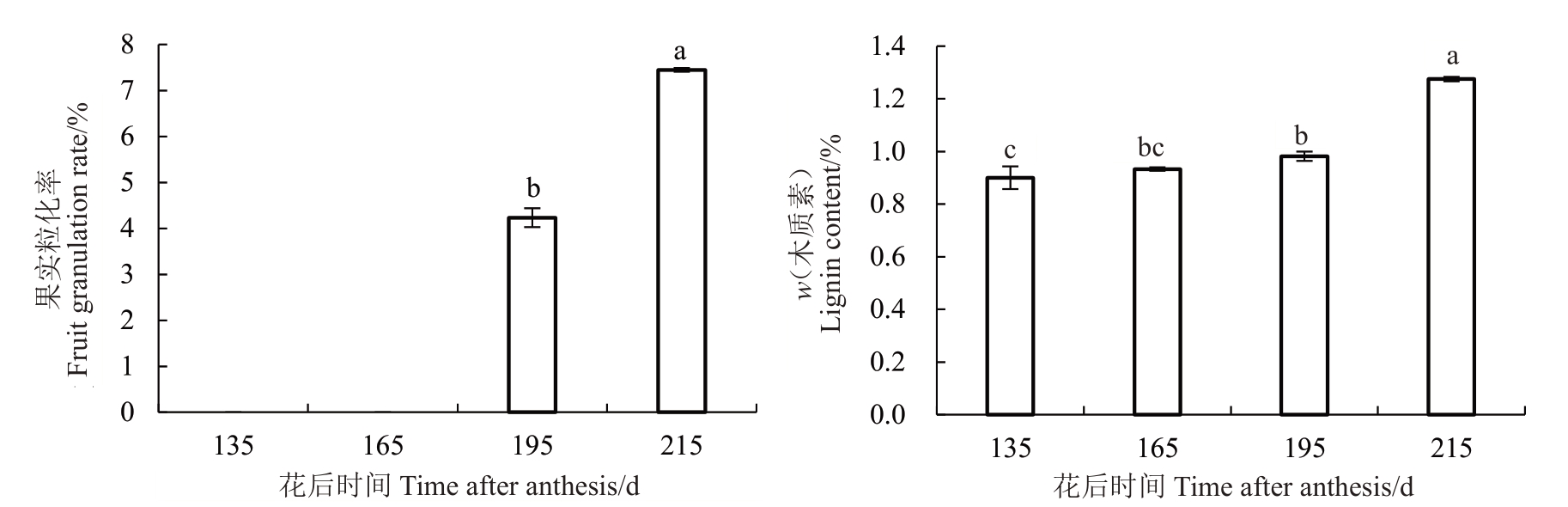
图1 琯溪蜜柚果实汁胞粒化率和汁胞木质素含量的变化
Fig.1 Changes of the rates of fruit juice sac granulation and lignin contents of Guanxi pomelo
2.2 蜜柚汁胞木质素显微观察
对FAA固定处理后的花后135、165、195和215 d的琯溪蜜柚果实汁胞进行石蜡包埋、切片及番红染色,图片由下至上是汁胞内部细胞到汁胞外表皮层。用20倍显微镜观察发现,花后135和165 d蜜柚果实汁胞颜色相似,无明显红色、无明显颜色变化(图2-A、B),说明这两个时期汁胞木质素含量较低;花后195 d汁胞内部细胞壁呈现红色(图2-C),汁胞外表皮层细胞壁颜色与花后135、165 d 汁胞相比无明显变化,但此时汁胞已出现木质化,木质素含量较花后135、165 d相比略微增加。花后215 d汁胞内部细胞和外表皮层的细胞壁均被染成红色(图2-D),且颜色较花后195 d 更深,说明在花后195 d 之后的果实成熟期汁胞细胞壁木质化程度增加,木质素含量显著上升。

图2 番红染色显示木质素在琯溪蜜柚汁胞细胞壁中的沉积
Fig.2 Safranin O staining showed lignin deposition in cell walls of Guanxi pomelo juice sacs
A.花后135 d 汁胞;B.花后165 d 汁胞;C.花后195 d 汁胞;D.花后215 d 汁胞;IC.汁胞内部细胞壁;EC.汁胞外表皮层细胞壁。
A.Juice sacs on 135 day after anthesis;B.Juice sacs on 165 day after anthesis;C.Juice sacs on 195 day after anthesis;D.juice sacs on 215 day after anthesis;IC.Internal cell walls of juice sacs;EC.Epidermic cell walls of juice sacs.
2.3 蜜柚汁胞转录组木质素合成差异基因表达分析
对4个生长发育时期琯溪蜜柚果实汁胞转录组17 个木质素生物合成途径中差异表达基因进行热图分析(图3),结果表明,其中1 个基因CrCCR1 和另外3 个基因CrPAL、Cr4CL2、Cr4CL7 分别在花后165和195 d的表达量与这2个时期的木质素含量的增加趋势不相一致。但值得注意的是,CrPAL1、CrPAL3、CrC4H1、CrC4H2、Cr4CL、CrHCT1、CrCCR3、CrCAD3、CrCOMT4、CrPOD2、CrPOD6、CrPOD7、CrPOD8 等13 个基因的表达量随着果实的发育成熟进程,在花后165、195和215 d差异表达(log2>2,Q-value ≤0.001)这些基因的表达水平在蜜柚花后215 d与195 d相比上调了2至9倍,并且和木质素含量的变化趋势相一致。研究认为这13 个基因可能是蜜柚汁胞木质素合成的关键基因,因此筛选这13个基因用于后续的qRT-PCR验证分析。

图3 不同发育时期琯溪蜜柚汁胞转录组木质素合成差异基因的相对表达量
Fig.3 Relative expression levels of differential genes in lignin biosynthesis pathway screening by transcriptome in Guanxi pomelo juice sacs at different developmental stages
2.4 蜜柚汁胞木质素合成差异基因qRT-PCR验证分析
对13 个木质素生物合成途径差异基因进行qRT-PCR 验证(图4)。随着果实的生长发育CrPAL1、CrPAL3、CrC4H1、CrC4H2、Cr4CL、CrCCR3、CrCAD3、CrPOD2和CrPOD7等9个基因qRTPCR 相对表达量都上调表达,尤其在花后195 至215 d的果实成熟期显著表达,且与转录组测序的结果一致,证明了这9 个基因转录组数据的可靠性。说明花后195 至215 d 是蜜柚果实汁胞粒化时期。另 外4 个 基 因CrHCT1、CrCOMT4、CrPOD6 和CrPOD8的qRT-PCR相对表达量变化与转录组数据不一致。

图4 (续) Fig.4(Continued)
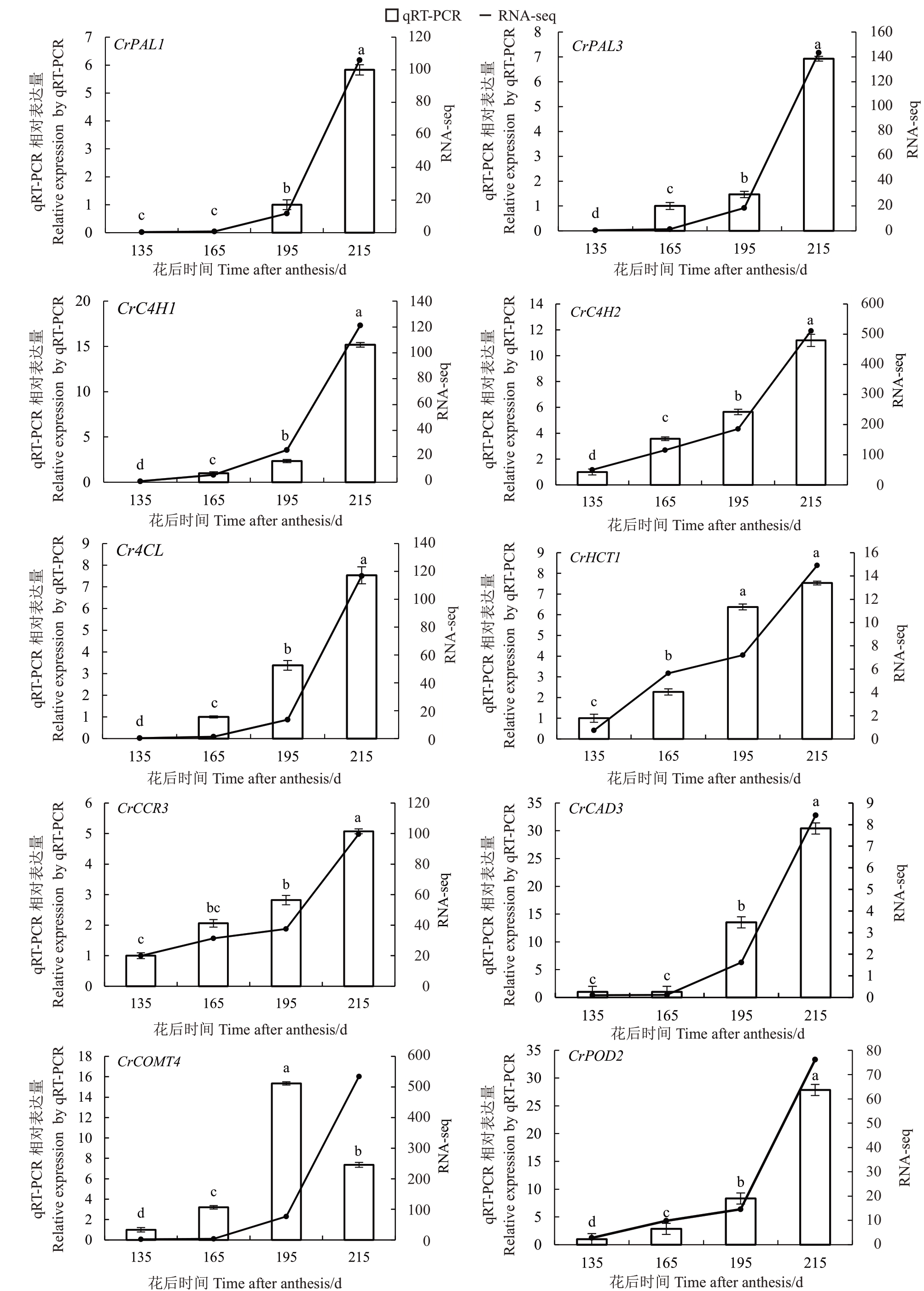
图4 木质素生物合成途径差异基因在琯溪蜜柚果实汁胞的qRT-PCR 验证
Fig.4 Verification of different genes related to lignin metabolism pathway in Guanxi pomelo juice sacs by qRT-PCR
2.5 蜜柚汁胞木质素生物合成途径相关酶活性变化分析
分别对花后135 至215 d 琯溪蜜柚果实汁胞中木质素生物合成途径相关酶PAL、C4H、4CL、CAD和POD 的活性进行测定,结果显示,酶活性随着果实的发育成熟呈现上升趋势,在花后215 d达到峰值(图5),与木质素含量及粒化率的变化规律基本一致,表明这些酶可能在蜜柚果实发育成熟阶段的汁胞木质素生物合成途径中起关键作用。
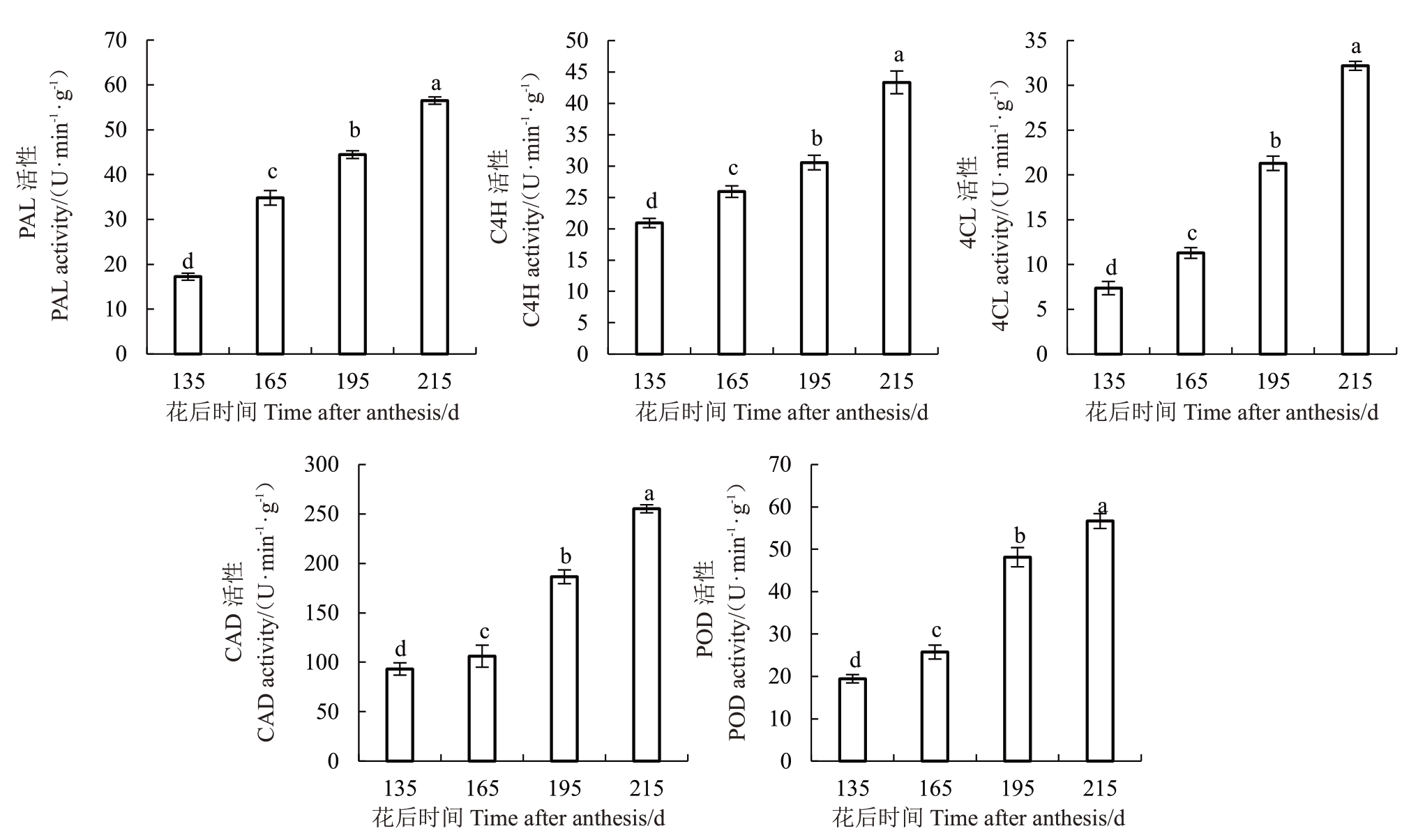
图5 琯溪蜜柚果实汁胞木质素合成相关酶活性的变化
Fig.5 Changes of enzyme activities related to lignin synthesis in the fruit juice sacs of Guanxi pomelo
3 讨 论
木质素是植物细胞中的苯丙烷代谢途径的代谢产物。木质素在果实汁胞中过量积累可能会导致汁胞粒化,会影响果实的口感和风味,目前在收获的柑橘果实中普遍存在汁胞粒化这一现象[14]。前人有研究表明木质素生物合成与汁胞粒化之间有着密切关联,粒化的汁胞通常发生木质素过度积累的现象[15],但总体研究还不够完整和系统。因此,笔者在基因转录、翻译和产物生成3 个水平展开木质素代谢与琯溪蜜柚汁胞粒化机制的系统研究。
果实中木质素含量的变化与木质素生物合成途径相关基因的表达有关,其中PAL、C4H、4CL、CCR、CAD 和POD 等基因在木质素的合成过程中起着极为重要的调控作用,且木质素合成量与这些基因的表达量呈正相关[16-17]。笔者在本研究中通过琯溪蜜柚汁胞转录组测序筛选到17 个木质素生物合成途径的差异表达基因,其中13个基因的表达量与汁胞粒化率及木质素含量变化趋势一致,且在花后195至215 d 显著表达。qRT-PCR 验证结果显示,CrPAL1、CrPAL3、CrC4H1、CrC4H2、CrCCR3、CrCAD3、Cr4CL、CrPOD2、CrPOD7等9个基因在蜜柚果实发育和成熟期的表达量与转录组测序结果变化趋势一致,均随着果实的成熟呈显著上调表达趋势,同时相关酶活性也随之增强,并与木质素生物合成的增加及蜜柚汁胞粒化趋势相一致。这一研究结果表明以上9个基因是蜜柚汁胞木质素的合成调控的关键基因,它们可能参与了琯溪蜜柚汁胞粒化的过程。
PAL是苯丙烷代谢途径中木质素生物合成的第一个关键酶,它在木质素生物合成中非常重要[18],PAL催化苯丙氨酸脱氨为反式肉桂酸[19]。前人通过对5 种白梨PAL 基因家族比较分析,结果显示Pb-PAL1和PbPAL2的表达水平对梨果中石细胞和木质素的含量有影响,PbPAL1 和PbPAL2 的转录水平在木质化组织(根和茎)中高于在木质化程度较低的组织(叶、芽和花)[20]。笔者在本研究中发现CrPAL1、CrPAL3 在汁胞木质素含量及粒化率较高的成熟期果实中表达量也大幅度增加,PAL 酶活性与汁胞木质素含量测定结果及PAL 基因表达量一致,与汁胞粒化率也大致相符。C4H被证明参与了木质素生物合成途径中G 木质素单体的产生[21],小麦中发现C4H1基因的表达与木质素含量呈显著相关[22],竹笋贮藏期间C4H活性增加促进木质素的合成,导致竹笋组织木质化[23],在本研究中,蜜柚汁胞CrC4H1、CrC4H2 的表达量在成熟期显著上调表达。同时,C4H 酶活性也随着果实的发育成熟逐渐增强,并与汁胞中木质素含量及粒化率变化相一致。4CL 是CCR 上游的一个酶,4CL 的产物是CCR 的底物,而CCR被认为是木质素合成的关键酶[24]。青稞研究中发现4CL 是影响木质素合成的一个关键酶,4CL 活性的提高可以增强茎秆的抗倒伏能力[25]。在本研究中转录组测序与qRT-PCR 结果显示随着木质素含量的增加及汁胞粒化率的上升,Cr4CL 在蜜柚成熟过程中也逐渐上调表达,4CL 的酶活性也与Cr4CL的表达相一致,这也与芹菜发育阶段的观察结果相似[26]。前人在梨果实中木质素合成的有关研究中发现,PbCCR1、PbCCR2和PbCCR3的表达趋势与果核细胞的积累和木质素含量相关[27],笔者在本研究中发现CrCCR3 在汁胞中的表达趋势也与木质素含量、粒化率变化趋势相一致。此外,催化肉桂醛转化为肉桂醇[28],参与单体木质素生物合成最后一步的另一个关键酶是CAD[29]。SbCAD2 和OsCAD2 基因已被证明参与了茎秆木质素的生物合成[30- 31],PpCAD2过量表达促进了转基因番茄植株中木质素的沉积,木质素含量增加,CAD 酶活性更活跃[32]。笔者在本研究中发现,蜜柚汁胞中CAD活性在蜜柚成熟后期相应上升,且与木质素含量、木质素合成酶基因CrCAD3的表达以及粒化率相一致。POD则通过聚合单体木质素催化松柏醇发生脱氢聚合反应形成G 型木质素[33]。有报道对白桦POD 全基因组鉴定,发现BpPOD6、BpPOD21 和BpPOD37 在木质部中高度表达[34],AgPOD 在芹菜叶柄、叶片中表达水平与木质素积累模式一致,说明POD在木质素生物合成中起着重要作用[35]。贮藏水果蔬菜时,通常会通过抑制木质素合成相关酶POD 活性来减缓木质素积累[36],对贮藏菜薹进行乙烯处理,发现木质素含量下降的同时BcPOD 的表达也下降[37]。在本研究中CrPOD2以及CrPOD7基因表达与POD酶活性以及木质素含量和粒化率变化相符,表明CrPOD2 和CrPOD7参与了蜜柚汁胞粒化过程木质素的调控作用。本研究表明,以上琯溪蜜柚汁胞9 个木质素合成基因的表达及相关酶活性的动态变化与汁胞木质素含量和粒化率变化相一致,因此认为琯溪蜜柚汁胞粒化是由木质素代谢途径中的系列关键基因共同调控的结果。
4 结 论
在琯溪蜜柚果实发育成熟过程中,汁胞粒化率随着木质素含量的积累而提高,尤其在成熟后期提高迅速。蜜柚汁胞中CrPAL1、CrPAL3、CrC4H1、CrC4H2、Cr4CL、CrCCR3、CrCAD3、CrPOD2 和CrPOD7 是木质素合成的关键调控基因。这9 个基因的上调表达,调控木质素合成途径中重要酶PAL、C4H、4CL、CAD和POD活性的增强,从而促进汁胞木质素含量积累,引起汁胞粒化发生。笔者在分子和生理水平上系统地研究了琯溪蜜柚果实发育过程中汁胞木质素生物合成途径关键基因表达与汁胞粒化的关系,为今后琯溪蜜柚的品种改良和分子育种提供了理论基础。
[1] 程琛,张世祺,林伟杰,陈欢欢,林锋,朱东煌,陈立松,李延,郭九信.福建省平和县蜜柚园土壤铜素(Cu)状况及其影响因素研究[J].果树学报,2018,35(3):301-310.CHENG Chen,ZHANG Shiqi,LIN Weijie,CHEN Huanhuan,LIN Feng,ZHU Donghuang,CHEN Lisong,LI Yan,GUO Jiuxin. Soil copper (Cu) nutrient status and its influencing factors in pomelo orchards in Pinghe county,Fujian province[J].Journal of Fruit Science,2018,35(3):301-310.
[2] THEANJUMPOL P,WONGZEEWASAKUN K,MUENMANEE N,WONGSAIPUN S,KRONGCHAI C,CHANGRUE V,BOONYAKIAT D,KITTIWACHANA S. Non-destructive identification and estimation of granulation in‘Sai Num Pung’tangerine fruit using near infrared spectroscopy and chemometrics[J].Postharvest Biology and Technology,2019,153:13-20.
[3] SHOMER I,CHALUTZ E,VASILIVER R,LOMANIEC E,BERMAN M. Scierification of juice sacs in pummelo (Citrus grandis)fruit[J].Canadian Journal of Botany,1989,67(3):625-632.
[4] 潘腾飞,朱学亮,潘东明,郭志雄,佘文琴,陈桂信.‘琯溪蜜柚’贮藏期间汁胞粒化与木质素代谢的关系[J]. 果树学报,2013,30(2):294-298.PAN Tengfei,ZHU Xueliang,PAN Dongming,GUO Zhixiong,SHE Wenqin,CHEN Guixin. Relationship between granulation and lignin metabolism in‘Guanximiyou’pummelo fruit during storage[J].Journal of Fruit Science,2013,30(2):294-298.
[5] HU S,KAMIMURA N,SAKAMOTO S,NAGANO S,TAKATA N,LIU S,GOEMINNE G,VANHOLME R,UESUGI M,YAMAMOTO M,HISHIYAMA S,KIM H,BOERJAN W,RALPH J,MASAI E,MITSUDA N,KAJITA S. Rerouting of the lignin biosynthetic pathway by inhibition of cytosolic shikimate recycling in transgenic hybrid aspen[J].The Plant Journal,2022,110(2):358-376.
[6] VANHOLME R,DE MEESTER B,RALPH J,BOERJAN W.Lignin biosynthesis and its integration into metabolism[J]. Current Opinion in Biotechnology,2019,56:230-239.
[7] 潘露露.授粉对‘琯溪蜜柚’果心汁胞次生壁木质素合成代谢的影响[D].福州:福建农林大学,2017.PAN Lulu. Pollination on‘Guanxi pomelo’core juice sac effect of secondary cell wall synthesis of lignin metabolism[D].Fuzhou:Fujian Agriculture and Forestry University,2017.
[8] YAO T,FENG K,XIE M,BARROS J,TSCHAPLINSKI TJ,TUSKAN G A,MUCHERO W,CHEN J G. Phylogenetic occurrence of the phenylpropanoid pathway and lignin biosynthesis in plants[J].Frontiers in Plant Science,2021,12:704697.
[9] HU D,LIU X B,SHE H Z,GAO Z,YI Z L.The lignin synthesis related genes and lodging resistance of Fagopyrum esculentum[J].Biologia Plantarum,2017,61(1):138-146.
[10] 代亚兰,赵秋月,刘若南,刘林婷,庄木来,李延,王平.琯溪蜜柚成熟期间汁胞纤维素含量及其合成酶基因表达分析[J].果树学报,2021,38(9):1435-1443.DAI Yalan,ZHAO Qiuyue,LIU Ruonan,LIU Linting,ZHUANG Mulai,LI Yan,WANG Ping. Analysis of cellulose content and synthase gene expression in juice sacs secondary wall during granulation of Guanxi pomelo[J]. Journal of Fruit Science,2021,38(9):1435-1443.
[11] FUKUSHIMA R S,KERLEY M S. Use of lignin extracted from different plant sources as standards in the spectrophotometric acetyl bromide lignin method[J]. Journal of Agricultural and Food Chemistry,2011,59(8):3505-3509.
[12] 秦永亭,秦双立,倪杰,王士珍.组织学中石蜡切片制作的注意事项[J].基层医学论坛,2019,23(20):2894-2895.QIN Yongting,QIN Shuangli,NI Jie,WANG Shizhen. Precautions for the production of paraffin sections in histology[J].The Medical Forum,2019,23(20):2894-2895.
[13] LIVAK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔCt method[J].Methods,2001,25(4):402-408.
[14] SHI M Y,LIU X,ZHANG H P,HE Z Y,YANG H B,CHEN J J,FENG J,YANG W H,JIANG Y W,YAO J L,DENG C H,XU J.The IAA-and ABA-responsive transcription factor CgMYB58 upregulates lignin biosynthesis and triggers juice sac granulation in pummelo[J].Horticulture Research,2020,7(1):139.
[15] CHEN C Y,NIE Z P,WAN C P,GAN Z Y,CHEN J Y. Suppression on postharvest juice sac granulation and cell wall modification by chitosan treatment in harvested pummelo (Citrus grandis L. Osbeck) stored at room temperature[J]. Food Chemistry,2020,336:127635.
[16] JIA N,LIU J Q,SUN Y F,TAN P H,CAO H,XIE Y Y,WEN B T,GU T Y,LIU J M,LI M M,HUANG Y T,LU J,JIN N,SUN L C,XIN F J,FAN B. Citrus sinensis MYB transcription factors CsMYB330 and CsMYB308 regulate fruit juice sac lignification through fine-tuning expression of the Cs4CL1 gene[J].Plant Science,2018,277:334-343.
[17] WANG Y X,TENG R M,WANG W L,WANG Y,SHEN W,ZHUANG J. Identification of genes revealed differential expression profiles and lignin accumulation during leaf and stem development in tea plant (Camellia sinensis (L.) O. Kuntze)[J].Protoplasma,2019,256(2):359-370.
[18] 黄杰恒.干旱胁迫下油菜抗倒伏相关性状动态变化及木质素关键基因表达特性分析[D].重庆:西南大学,2013.HUANG Jieheng. Dynamic changes of rapeseed lodging resistance under drought stress-related traits and critical analysis of gene expression characteristics of lignin[D].Chongqing:Southwest University,2013.
[19] BAGAL U R,LEEBENS-MACK J H,LORENZ W W,DEAN J F D. The phenylalanine ammonia lyase (PAL) gene family shows a gymnosperm- specific lineage[J]. BMC Genomics,2012,13(S3):139-146.
[20] LI G H,WANG H,CHENG X,SU X Q,ZHAO Y,JIANG T S,JIN Q,LIN Y,CAI Y P. Comparative genomic analysis of the PAL genes in five Rosaceae species and functional identification of Chinese white pear[J].PeerJ,2019,7(W1):e8064.
[21] ZIEBELL A,GJERSING E,HINCHEE M,KATAHIRA R,SYKES R W,JOHNSON D K,DAVIS M F. Downregulation of p-coumaroyl quinate/shikimate 3′-hydroxylase(C3′H)or cinnamate-4-hydrolylase (C4H) in Eucalyptus urophylla ×Eucalyptus grandis leads to increased extractability[J]. BioEnergy Research,2016,9(2):691-699.
[22] 陈键.普通小麦肉桂酸-4-羟基化酶基因克隆及表达模式分析[D].安徽:安徽农业大学,2020.CHEN Jian.Cloning and expression pattern analysis of cinnamic acid-4-hydroxylase gene in common wheat[D]. Anhui:Anhui Agricultural University,2020.
[23] 罗自生. GA3 处理对采后竹笋木质化及内源激素水平的影响[J].园艺学报,2005,32(3):454-457.LUO Zisheng.Effects of GA3 treatment on lignification and endogenous hormone levels of postharvest bamboo shoots[J].Acta Horticulturae Sinica,2005,32(3):454-457.
[24] XIE M,ZHANG J,TSCHAPLINSKI T J,TUSKAN G A,CHEN J G,MUCHERO W. Regulation of lignin biosynthesis and its role in growth-defense tradeoffs[J]. Frontiers in Plant Science,2018,9:1427.
[25] 王凯,赵小红,姚晓华,姚有华,白羿雄,吴昆仑.茎秆特性和木质素合成与青稞抗倒伏关系[J]. 作物学报,2019,45(4):621-627.WANG Kai,ZHAO Xiaohong,YAO Xiaohua,YAO Youhua,BAI Yixiong,WU Kunlun. Relationship of stem characteristics and lignin synthesis with lodging resistance of hulless barley[J].Acta Agronomica Sinica,2019,45(4):621-627.
[26] 段奥其.赤霉素和NAC 转录因子调控芹菜木质素合成的分子机制[D].南京:南京农业大学,2020.DUANAoqi.Molecular mechanism of Gibberellin and NAC transcription factors in regulation lignin biosynthesis in celery[D].Nanjing:Nanjing Agricultural University,2020.
[27] ZHANG J,LI J,XUE C,WANG R,ZHANG M,QI K,FAN J,HU H,S ZHANG,WU J.The variation of stone cell content in 236 germplasms of sand pear (Pyrus pyrifolia) and identification of related candidate genes[J]. Horticultural Plant Journal,2021,7(2):108-116.
[28] CHENG X,LI M L,LI D H,ZHANG J Y,JIN Q,SHENG L L,CAI Y P,YI L. Characterization and analysis of CCR and CAD gene families at the whole-genome level for lignin synthesis of stone cells in pear (Pyrus bretschneideri) fruit[J]. Biology Open,2020,9(5):1602-1613.
[29] PONNIAH S K,SHANG Z H,AKBUDAK M A,SRIVASTAVA V,MANOHARAN M. Down-regulation of hydroxycinnamoyl coa:shikimate hydroxycinnamoyl transferase,cinnamoyl coa reductase,and cinnamyl alcohol dehydrogenase leads to lignin reduction in rice (Oryza sativa L. ssp. japonica cv. Nipponbare)[J].Plant Biotechnology Reports,2017,11(1):17-27.
[30] LEE C J,PARK S U,KIM S E,LIM Y H,JI C Y,KIM Y H,KIM H S,KWAK S S. Overexpression of IbLfp in sweetpotato enhances the low-temperature storage ability of tuberous roots[J].Plant Physiology and Biochemistry,2021,167:577-585.
[31] KIM S J,KIM M R,BEDGAR D L,MOINUDDIN S G A,CARDENAS C L,DAVIN L B,KANG C L,LEWIS N G.Functional reclassification of the putative cinnamyl alcohol dehydrogenase multigene family in Arabidopsis[J]. Proceedings of the National Academy of Sciences of the United States of America,2004,101(6):1455-1460.
[32] LI M T,CHENG C X,ZHANG X F,ZHOU S P,LI L X,YANG S L. Overexpression of Pear (Pyrus pyrifolia) CAD2 in tomato affects lignin content[J].Molecules,2019,24(14):2595.
[33] SANTIAGO R,BARROS-RIOS J,MALVAR R. Impact of cell wall composition on maize resistance to pets and diseases[J].International Journal of Molecular Science,2013,14(4):6960-6980.
[34] CAI K W,LIU H X,CHEN S,LIU Y,ZHAO X Y,CHEN S.Genome-wide identification and analysis of class III peroxidases in Betula pendula[J].BMC Genomics,2021,22(1):314.
[35] JIA X L,WANG G L,XIONG F,YU X R,XU Z S,WANG F,XIONG A S.De novo assembly,transcriptome characterization,lignin accumulation and anatomic characteristics:Novel insights into lignin biosynthesis during celery leaf development[J].Scientific Reports,2015,5(1):8259.
[36] XIE G F,YANG C,FEI Y,MA L Z. Physiological and proteomic analyses of 1-MCP treatment on lignification in fresh common bean (Phaseolus vulgaris L.) during storage[J/OL].Postharvest Biology and Technology,2020,160:111041. DOI:10.1016/j.postharvbio.2019.111041.
[37] 宋康华,贾志伟,常金梅,孙曼丽,张鲁斌.低温下乙烯对采后菜薹木质化及相关基因表达的影响[J]. 园艺学报,2019,46(4):775-783.SONG Kanghua,JIA Zhiwei,CHANG Jinmei,SUN Manli,ZHANG Lubin. Lignification induced by ethephon and related gene expression in Postharvest flowering chinese cabbage at low temperature[J].Acta Horticulturae Sinica,2019,46(4):775-783.