太秋甜柿是中晚熟甜柿品种,目前主要分布在中国南方地区,如浙江、江苏、福建、湖北等地[1-4]。太秋甜柿具有独特的风味,营养丰富,且保脆时间较长,因此,深受广大消费者的喜爱,也是中国的主要出口水果之一,有着良好的国际市场价值[5]。
炭疽病是柿树的主要病害,可侵染叶片、花和果实等部位,引起果实腐烂或枝条枯死等症状,也是柿贮藏期的主要病害之一[6],而因其具有较高的含糖量和蛋白质含量的特性,更易被炭疽菌侵染,造成果实的腐烂。目前,中国已报道的柿炭疽菌主要病原菌有哈锐炭疽菌(Colletotrichum horii)[7-8]、喀斯特炭疽菌(C. karstii)[9]、胶孢炭疽菌(C. gloeosporioi-des)[10]和果生刺盘孢(C. fructicola)[11],国外报道的有尖孢炭疽菌(C.acutatum)[12]和暹罗炭疽菌(C.siamense)[13]、其中山东省甜柿炭疽病主要以哈锐炭疽菌为主,福建省以果生刺盘孢为主,各地区炭疽病病原菌种群类型差异较大。近年来,浙江省金华市永康柿园内太秋甜柿炭疽病逐年增加,主要因为甜柿炭疽病的病原种类及规律不明确,甜柿病害发病症状相似,田间药剂使用混乱,导致甜柿果实炭疽病发生加重,影响了甜柿产量及质量。因此,弄清甜柿炭疽病炭疽菌的种类、生物学特性及发生条件,不仅可以了解甜柿炭疽病的发生及影响因素,而且可以为甜柿炭疽病提供生态防治措施。笔者在本试验中主要对浙江永康太秋甜柿果实炭疽病种类进行确定,着重研究甜柿炭疽病不同病原小种间生物学特性和发生规律的差异,以期为甜柿炭疽病发病规律的解析及病害的科学防治提供理论依据。
1 材料和方法
1.1 甜柿炭疽病的发生及病原菌分离、鉴定
1.1.1 炭疽病调查、采集及分离 2021年6—10月,针对浙江省金华市永康市太秋甜柿果实炭疽病进行病样采集,采集时间为每月1次,采摘太秋甜柿果实表面和萼片上发病症状较明显的样品,采集后的样品保存于保鲜袋内带回实验室后,采用组织分离法[11]进行甜柿炭疽病病原菌分离,得到的分离物培养3 d后在菌落边缘挑取单菌丝至PDA平板内进行纯化、备用。并观察所有分离病菌的菌落特征,统计太秋甜柿果实表面和萼片的炭疽病菌分离率。将纯化的菌种保存至浙江省农业科学院植物保护与微生物研究所。
1.1.2 分离物致病性测定 选取代表菌株进行致病性检测,选取健康、大小和成熟度一致的太秋甜柿果实,在果实或萼片进行接种,将纯化的供试菌株接种至PDA平板中,26 ℃培养7 d后,在菌落菌丝最外缘打取直径为5 mm的菌饼,将菌饼菌丝面朝寄主植物贴在果实表面或果蒂,再用含有无菌水的脱脂棉覆盖在菌饼上,对其进行保湿培养,放置于26 ℃16 h光照、8 h 黑暗的光照培养箱内进行保湿培养,接种方式为针孔洞伤口接种和无伤口直接接种,待5 d后观察接种情况并记录。
1.1.3 分离物分类地位确定 将分离纯化的代表菌株进行致病性测定,采用Ezup 柱式真菌基因组DNA 抽提试剂盒提取DNA,利用ITS、ACT、TUB2、GAPDH、CHS1 基因序列进行扩增[14-19],引物由杭州擎科梓熙生物技术有限公司合成并进行基因测序,结合NCBI-Blast进行序列比对。在NCBI 基因数据库中下载相关序列(表1),采用MEGA 7.0和Bayesian对ITS、ACT、TUB2、GAPDH、CHS-1联合序列构建系统发育树,采用最大似然法(maximum likelihood,ML)和贝叶斯法建树(Bayesian,Bpp),Bootstrap 1000次重复。
表1 系统发育分析序列登录号及来源
Table 1 Sequences of the isolates with GenBank accession number and source used for phylogenetic analyses

炭疽菌种类Colletotrichum species C.aenigma C.aeschynomenes C.alatae C.alienum C.aotearoa C.asianum C.acutatum C.camelliae C.clidemiae C.cordylinicola C.fructicola C.gloeosporioides C.horii C.musae C.nupharicola C.psidii C.salsolae C.siamense C.theobromicola C.tropicale C.karsti来源Source ICMP18608 ATCC201874 CBS304.67 ICMP18691 ICMP18532 ICMP18696 CBS129925 CS1-2 ICMP18658 ICMP18579 ICMP18613 CBS112999 ICMP12942 CBS116870 CBS469.96 CBS145.29 ICMP19051 ICMP12567 CBS124945 CBS124949 FJ38-4登录号Accession number ITS JX010244 JX010176 JX010190 JX010217 JX010220 JX010192 MH865676 MT940996 JX010265 JX010226 JX010167 JX010152 GQ329687 JX010146 JX010189 JX010219 JX010242 JX010250 JX010294 JX010264 MZ891695 ACT JX009443 JX009483 JX009471 JX009580 JX009544 JX009576 JQ949707 MT957873 JX009537 HM470235 JX009491 JX009531 JX009533 JX009433 JX009486 JX009515 JX009562 JX009541 JX009444 JX009489 MZ912857 TUB2 JX010389 JX010392 JX010383 JX010385 JX010421 JX010384 JQ950037 MT957841 JX010438 JX010440 JX010388 JX010445 JX010375 HQ596280 JX010397 JX010443 JX010403 JX010387 JX010447 JX010407 MZ912909 GAPDH JX010044 JX009930 JX009990 JX010018 JX009906 JX009915 JQ948717 MT957847 JX009989 JX009975 JX009998 JX010056 GQ329685 JX010050 JX009936 JX009967 JX009916 JX009940 JX010006 JX010007 MZ912753 CHS-1 JX009774 JX009799 JX009837 JX009754 JX009764 JX009753 JQ949047 MT957885 JX009877 JX009864 JX009772 JX009818 JX009748 JX009896 JX009834 JX009901 JX009863 JX009761 JX009869 JX009870 MZ912805
1.2 病菌生物学特性测定
1.2.1 不同培养基对菌丝生长和产孢的影响 将纯化的甜柿炭疽病代表菌株转至新的PDA 培养基上,培养5 d后,采用打孔器在菌落边缘打取直径为5 mm的菌饼,菌丝面一致朝下接种至供试培养基平板中央。供试培养基类型为PDA(马铃薯琼脂培养基)、CA(胡萝卜培养基)、CMA(玉米粉培养基)、RA(大米培养基)、OA(燕麦培养基)、GA(高粱培养基)、WA(水琼脂培养基)。置于25 ℃恒温培养箱中进行暗培养,每个处理3 次重复。5 d 后,采用十字交叉法测量菌落直径[20]。
产孢量的测定:培养14 d后,加入10 mL无菌水至供试平板,将培养基表面孢子完全洗脱后配制成孢子悬液,在光学显微镜下采用血球计数法统计每个平板的产孢总量。
1.2.2 温度对菌丝生长和产孢的影响 采用打孔器打取直径为5 mm 的菌饼,菌丝面一致朝下接种至新的PDA 平板中央,分别置于5、10、15、20、25、30、35、40 ℃恒温培养箱中进行暗培养,每个处理3次重复。5 d 后测量菌落直径,14 d 后测量产孢总量,测量方法同1.2.1。
1.2.3 光照对菌丝生长和产孢的影响 将菌饼(5 mm)接种至PDA 培养基,分别置于光照条件为全光(24 h光照)、全暗(24 h黑暗)、半光照(12 h光+12 h暗)下培养,每个处理3次重复。25 ℃恒温培养5 d 后测量菌落直径,14 d 后测量产孢总量,测量方法同1.2.1。
1.2.4 pH 值对菌丝生长和产孢的影响 以PDA 培养基为基础培养基,用0.1%HCl 溶液和NaOH 溶液将培养基的pH 值分别调至4.01、5.03、6.09、7.03、8.02、9.03、10.09,每个处理3 次重复,在各培养基中央接种直径5 mm的菌饼,密封处理后,置于25 ℃恒温培养箱进行暗培养,5 d 后测量菌落直径,14 d 后测量产孢总量,测量方法同1.2.1。
1.2.5 病菌致死温度的测定 将在PDA上培养14 d的病原菌菌板加无菌水配置为105个·mL-1的孢子悬液,吸取50 μL 孢子悬液移入PCR 管中,放入梯度PCR 仪中,分别置于40.0、40.6、41.9、43.9、46.2、47.9、49.2、50.0、51.0、52.0 ℃,恒温处理10 min 后吸取10 μL滴在PDA平板中央进行涂板,放置于25 ℃恒温培养箱内培养3 d后观察菌落生长情况,每个处理3次重复。
2 结果与分析
2.1 甜柿炭疽病的发生及调查
2021年6—10月对浙江省金华市永康市太秋甜柿(共5 次样品)果实上的病斑进行了采样及分离,如表2 所示,侵染太秋甜柿果实炭疽病病原类型主要有Ⅲ类,其中Ⅰ类主要危害甜柿表面,形成黑色密密麻麻的微凸起小黑点,为初期发生病害,分离概率为21/66,病原菌在PDA培养基上菌落灰绿色,分生孢子长圆形或圆柱形,两端圆,单孢,无色,大小(19.626~31.933)μm×(3.717~4.320)μm,代表菌株为TS32-G11-E(图1-A~C);Ⅱ类主要危害柿蒂,常见为柿蒂先发病变褐后侵染至果实,后期果实全部腐烂,整个生育期均有发病,分离概率为54/100,病原菌在PDA 培养基上菌落灰绿色,分生孢子椭圆形,单孢,无色,大小(5.164~7.173)μm ×(14.202~15.568)μm,代表菌株为T3S1-E1-B(图1-D~F);Ⅲ类主要为萼片,也可侵染果实,后期果实表面易产生橘色孢子堆,分离概率为16/45,病原菌在PDA培养基上菌落灰绿色,孢子椭圆形,单孢,无色,大小(4.018~4.699)μm×(11.547~12.768)μm,代表菌株为T2S12-G-A(图1-G、H和I)。经形态学描述初步将3类病原菌鉴定为刺盘孢属真菌(Colletotrichum sp.)。

图1 甜柿炭疽病发病症状、病原性状及致病性测定
Fig.1 Symptoms,pathogens and pathogenicity of sweet persimmon anthracnose
表2 永康市甜柿果实样品炭疽病病原菌分离比例
Table 2 The isolation frequency of Colletotrichum species isolated from sweet persimmon in Yongkang
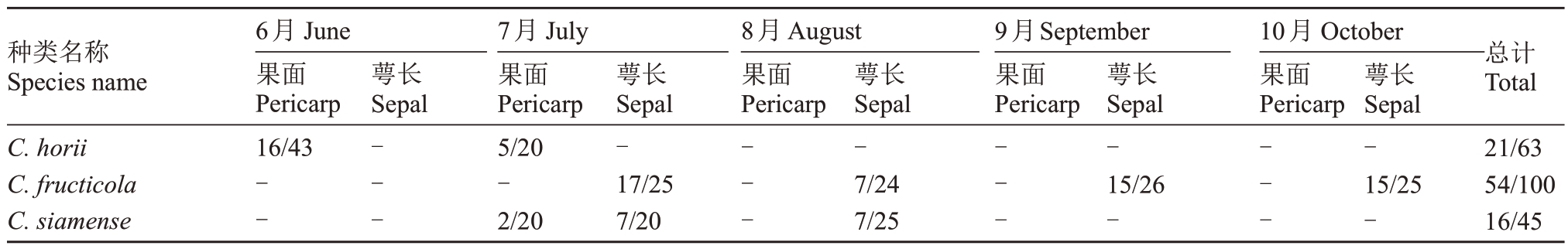
注:“-”表示未检出。
Note:“-”means no detection.
种类名称Species name 6月June果面Pericarp 16/43萼长Sepal 8月August果面Pericarp 9月September果面Pericarp 10月October果面Pericarp总计Total C.horii C.fructicola C.siamense 21/6354/10016/45-----7月July果面Pericarp 5/20-2/20萼长Sepal-17/257/20---萼长Sepal-7/247/25---萼长Sepal-15/26----萼长Sepal-15/25-
2.2 分离菌株致病性测定
病原菌致病性测定结果表明,3 个甜柿炭疽病代表菌株均可侵染甜柿果实,代表菌株T2S32-G12-E 在有伤口和无伤口接种条件下均可致病,有伤口病原可侵入果肉,使组织变黑腐烂,直接接种病原可在柿果实表皮侵染形成小黑点,随接种环境湿度升高而扩大,与田间症状一致(图1-J、L)。代表菌株T2S12-G-A 仅在有伤口的情况下侵染甜柿果实,果面变软塌陷,7 d 后在接种寄主组织上可产生橘色孢子液,与田间症状相似(图1-K)。代表菌株T3S1-E1-B 一般由果蒂侵染甜柿果实及萼片,接种发现该病原菌侵染速度很快,7 d 即可使整个甜柿果实腐烂,果蒂及萼片发病症状与田间相似(图1-M),因此该3 类病原菌均为甜柿果实炭疽菌的致病菌。
2.3 系统发育分析
如图2 所示,采用最大似然法和贝叶斯方法对代表菌株的5个基因进行序列扩增和联合构建系统发育树,结果表明,代表菌株T2S32-G12-E 与C.horii 聚在同一分支上,支持率达99%,贝叶斯概率达100%;代表菌株T3S1-E1-B与C.fructicola聚在同一分支上,支持率达99%,贝叶斯概率达100%;代表菌株T2S12-G-A 与C.siamense 聚在同一分支上,支持率达99%,贝叶斯概率达100%。因此,太秋甜柿果实上病原菌主要包含3 个不同的种,即哈锐炭疽菌(C.horii)、果生刺盘孢(C.fructicola)和暹罗刺盘孢(C.siamense),详细分离结果见表2。
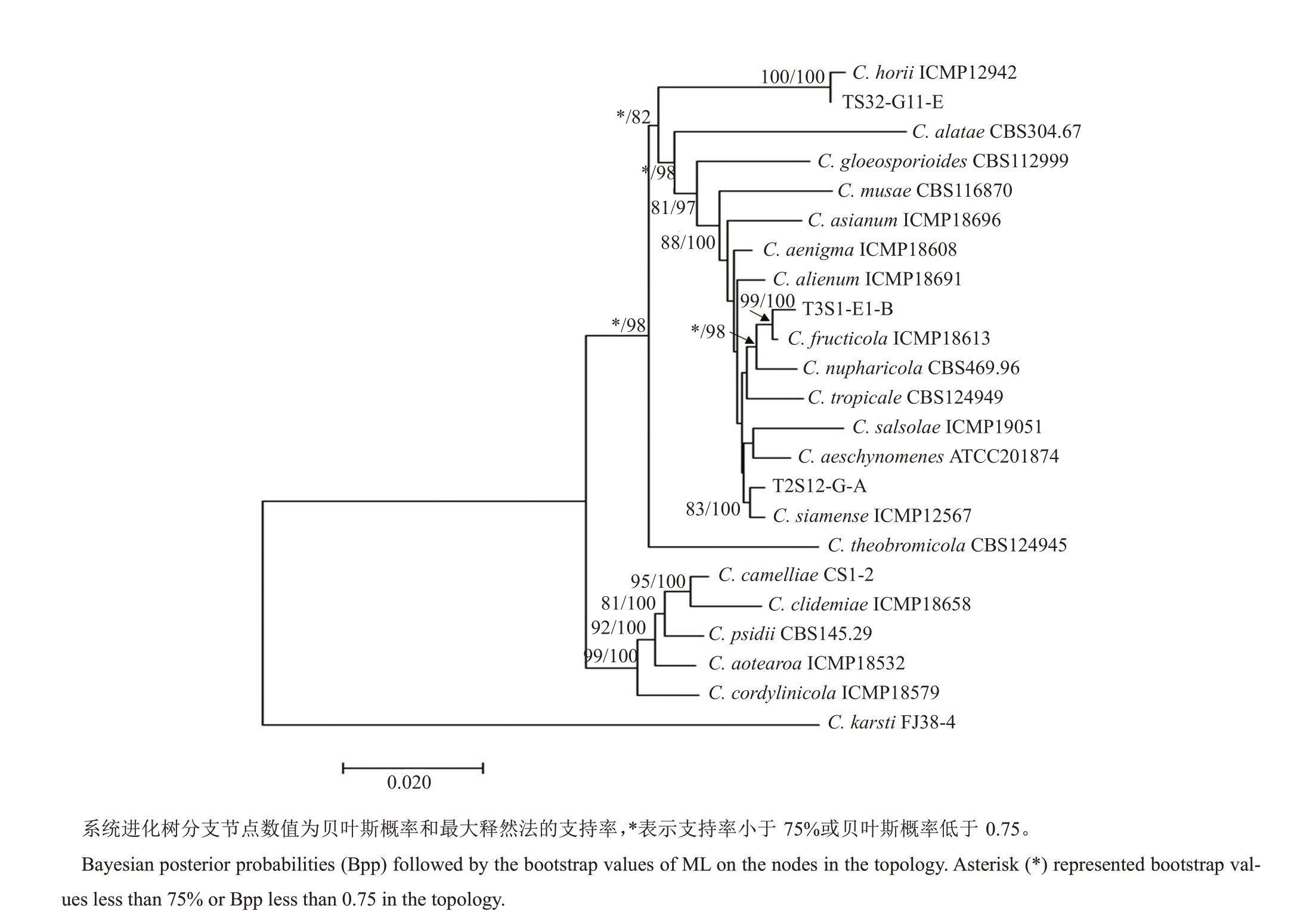
图2 甜柿炭疽病病原菌基于ITS-ACT-TUB2-GAPDH-CHS1 基因序列系统进化树
Fig.2 Phylogenetic relationship with Colletotrichum species based on the combined ITS-ACT-TUB2-GAPDH-CHS-1 sequences of the representative isolates
2.4 病菌生物学特性研究
2.4.1 不同培养基和光照对菌丝生长和产孢的影响 3种炭疽病菌在供试7种培养基上均能生长(表3)。果生刺盘孢和暹罗刺盘孢菌丝生长最佳培养基为PDA、CMA 和GA,果生刺盘孢产孢最佳培养基为胡萝卜煎汁培养基,暹罗刺盘孢产孢最佳培养基为CMA培养基;哈锐炭疽菌菌丝生长最佳培养基为PDA和CA,产孢最佳培养基为OA培养基。
表3 不同培养基和光照处理对病原菌菌丝生长和产孢的影响
Table 3 Effects of carbon sources,nitrogen sources and light on the mycelia growth and spore production of C.horii,C.siamense and C.fructicola

注:表中数据为(平均值±标准误)(n=6),不同小写字母表示不同处理间差异显著(p<0.05),表中每个因素处理单独分析。下同。
Note:Values are (mean± standard error)(n=6), different small letter mean significant difference(p<0.05)and each factor in the table was analyzed separately.The same below.
因素Factor培养基Media source C.siamense 4.40±0.40 b 1.60±0.40 b 1.73±0.23 b 2.20±0.28 b 21.60±12.28 a 6.13±0.23 b 1.33±0.23 b 3.87±0.23 a 3.60±0.40 a 3.47±0.46 a光照Light处理Treatment PDA CA GA RA CMA OA WA 24 h L 12 h L+12 h D 24 h D菌落直径Colony diameter/mm(5 d)C.horii 51.33±1.03 a 43.83±2.14 b 42.50±2.17 b 42.17±0.41 b 42.17±2.04 b 39.17±0.75 c 32.67±1.21 d 48.50±1.76 b 46.67±2.66 b 52.00±2.19 a C.fructicola 70.33±0.82 a 57.00±3.16 c 61.33±1.37 b 59.33±2.25 b 61.67±0.82 b 56.00±1.26 c 51.83±2.32 d 67.17±0.98 b 66.83±1.17 b 70.50±3.62 a C.siamense 64.33±1.37 a 55.50±0.84 d 61.00±2.00 b 58.67±1.03 c 58.67±0.52 c 55.50±0.55 d 48.50±0.55 e 68.33±2.34 a 64.00±1.67 b 64.67±2.07 b产孢量Spore production/(×105)(14 d)C.horii 0.93±0.23 d 2.27±0.46 c 2.93±0.46 b 0.00±0.00 e 0.20±0.23 e 4.13±0.23 a 0.00±0.00 e 0.40±0.00 c 2.27±0.23 a 1.07±0.23 b C.fructicola 33.33±4.62 e 1129.33±4.39 a 405.33±26.03 b 70.00±8.49 de 166.00±6.62 c 112.00±8.00 cd 14.67±2.31 e 65.33±6.11 a 34.67±6.11 b 45.33±2.31 c
2.4.2 光照对菌丝生长和产孢的影响 在光照试验中,3个甜柿炭疽病菌受光照条件的影响不同,哈锐炭疽菌和果生刺盘孢菌在全黑暗培养条件下菌丝生长最快,而暹罗刺盘孢在全光照条件下菌丝生长最快。哈锐炭疽菌在光暗交替的条件下可刺激产孢,产孢量达2.27×105个·mL-1,明显高于全光照和全黑暗培养条件。全光照条件可以刺激果生刺盘孢菌产生孢子,14 d 可达6.53×106个·mL-1。而暹罗刺盘孢受光照条件的影响无明显差异。
2.4.3 温度和pH 对菌丝生长和产孢的影响 由图3-A可知,3种甜柿炭疽病菌菌丝生长和产孢适宜温度均为30~35 ℃,最适生长温度30 ℃,果生刺盘孢和暹罗刺盘孢最适产孢温度为35 ℃,哈锐炭疽菌最适产孢温度为25 ℃;pH 值4~10 均适宜甜柿炭疽病菌菌丝生长(图3-B),果生刺盘孢在pH 值5 和9 时产孢最佳,暹罗刺盘孢和哈锐炭疽菌在pH值9和10时产孢最佳(表4)。
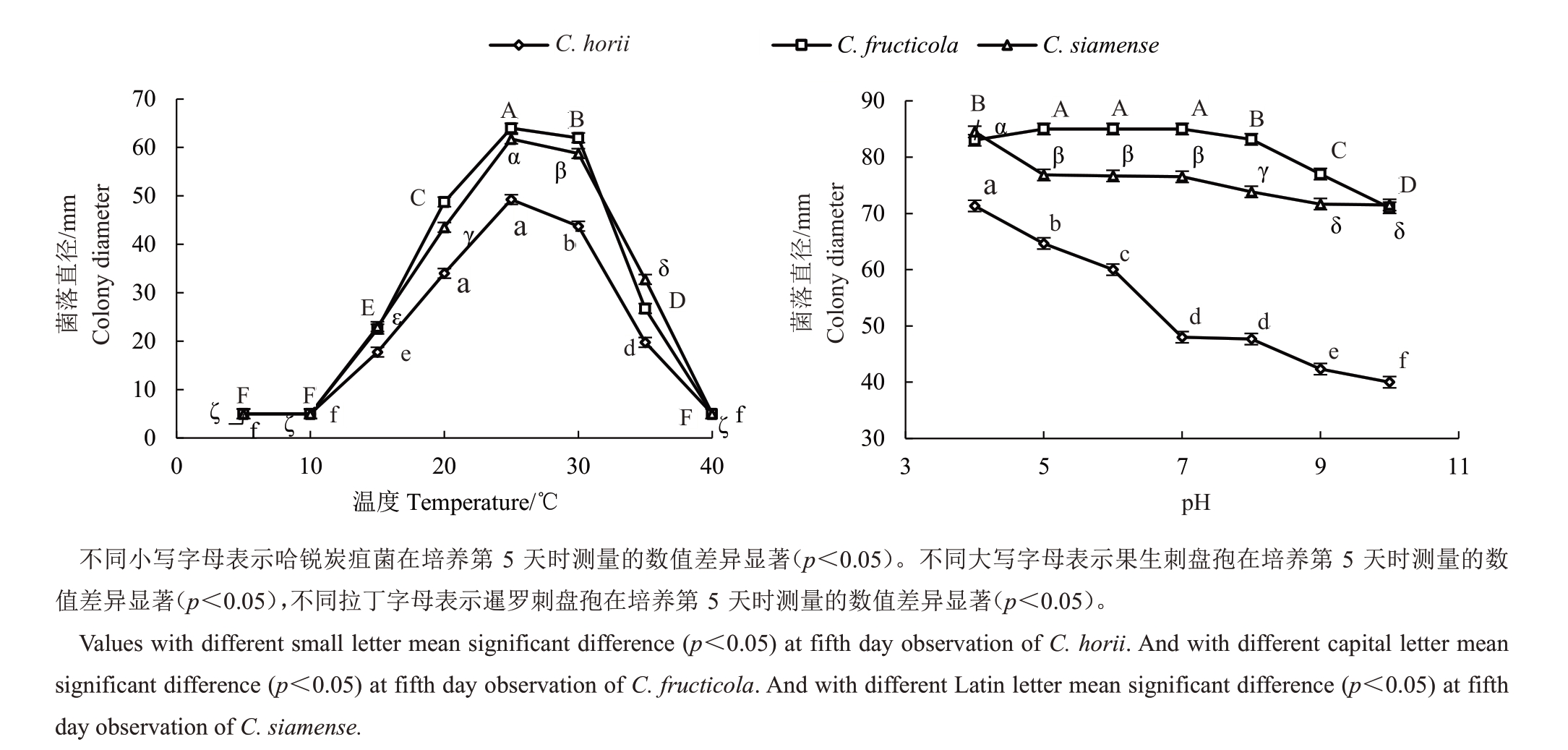
图3 温度(A)和pH(B)对甜柿炭疽病病原菌菌丝生长的影响
Fig.3 Effects of temperature(A)and pH(B)on the mycelia growth of C.horii,C.siamense and C.fructicola
表4 不同温度和pH 对甜柿炭疽病病原菌产孢的影响
Table 4 Effects of temperature and pH on the spore production of C.horii,C.siamense and C.fructicola
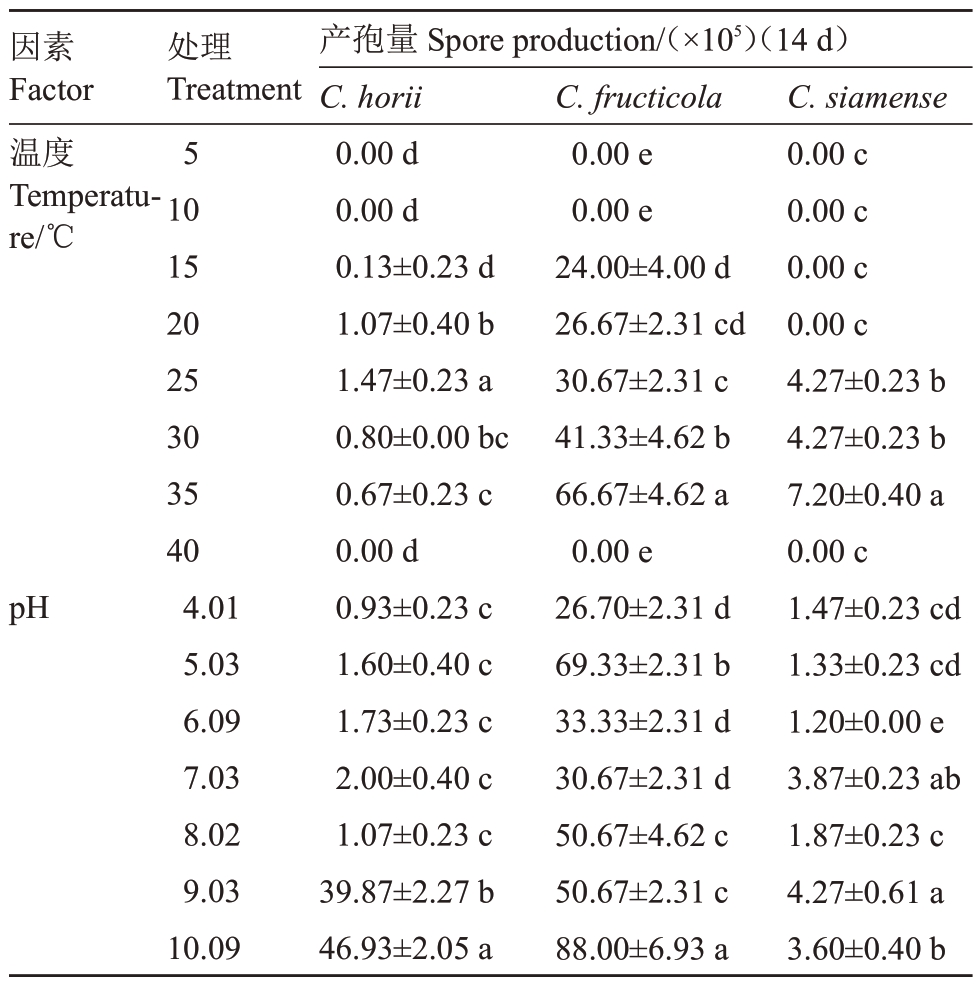
因素Factor温度Temperature/℃pH处理Treatment 5101520 25303540 4.015.036.097.038.029.0310.09产孢量Spore production/(×105)(14 d)C.horii 0.00 d 0.00 d 0.13±0.23 d 1.07±0.40 b 1.47±0.23 a 0.80±0.00 bc 0.67±0.23 c 0.00 d 0.93±0.23 c 1.60±0.40 c 1.73±0.23 c 2.00±0.40 c 1.07±0.23 c 39.87±2.27 b 46.93±2.05 a C.fructicola 0.00 e 0.00 e 24.00±4.00 d 26.67±2.31 cd 30.67±2.31 c 41.33±4.62 b 66.67±4.62 a 0.00 e 26.70±2.31 d 69.33±2.31 b 33.33±2.31 d 30.67±2.31 d 50.67±4.62 c 50.67±2.31 c 88.00±6.93 a C.siamense 0.00 c 0.00 c 0.00 c 0.00 c 4.27±0.23 b 4.27±0.23 b 7.20±0.40 a 0.00 c 1.47±0.23 cd 1.33±0.23 cd 1.20±0.00 e 3.87±0.23 ab 1.87±0.23 c 4.27±0.61 a 3.60±0.40 b
2.4.4 病菌致死温度 将孢子悬液置40.0、40.6、41.9、43.9、46.2、47.9、49.2、50.0、51.0、52.0 ℃恒温处理5、10、20 min后涂板。由表5可知,恒温处理10 min,哈锐炭疽菌和暹罗刺盘孢的致死温度为49.2 ℃,果生刺盘孢的致死温度为47.9 ℃。
表5 3 种炭疽菌孢子致死温度的测定
Table 5 Lethal temperature measurements of Colletotrichum horii,C.siamense and C.fructicola
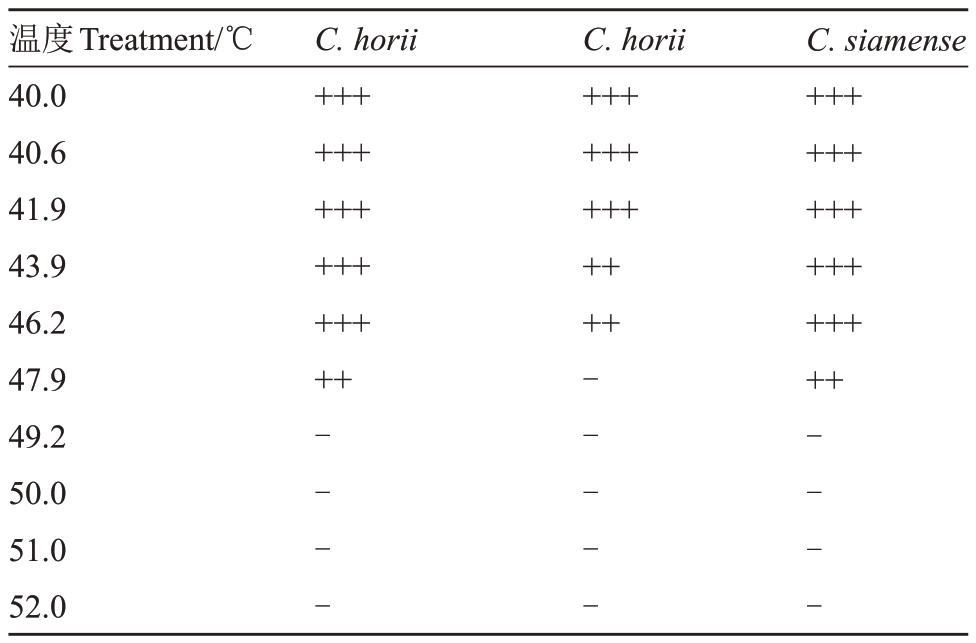
注:“+++”表示菌落占平板的>60%~100%;“++”表示菌落占平板的>20%~60%;“++”表示菌落占平板的0%~20%;“-”表示平板表面无菌落形成。
Note:“+++”means>60%-100% colonies were observed on PDA plate.“++”means>20%-60% colonies were observed on PDA plate.“+”means>0%-20% colonies were observed on PDA plate.“-”means none colony was observed on PDA plate.
温度Treatment/℃40.040.641.943.946.247.949.250.051.052.0 C.horii+++++++++++++++++C.horii+++++++++++++C.siamense+++++++++++++++++-------------
3 讨论
3.1 甜柿炭疽病病原种类及发生情况
炭疽病是柿果实的主要病害之一,引起柿果实腐烂或表面病斑,极大影响了甜柿果实的商品价值。刺盘孢属真菌作为柿炭疽病的主要病原菌,其种类较多且遗传差异较大,因不同的气候条件导致不同地区柿炭疽病的病原种类不一致。目前中国山东省报道的引起甜柿炭疽病的病原主要以哈锐炭疽菌为主,福建省的果实炭疽病主要以果生刺盘孢为主[8,14]。结果表明,引起浙江省金华市永康市太秋甜柿炭疽病的主要有3大类,分别为哈锐炭疽菌、果生刺盘孢和暹罗刺盘孢,其中哈锐炭疽菌为前期炭疽病主要病原菌,一般在6—7 月发生,后期主要是果生刺盘孢引起的炭疽病,一般在8—10月均有发生,在7—8 月份为该病原侵染高峰期。哈锐炭疽菌引起的炭疽病主要为早期果斑病,结合致病性测定结果分析,该病原在有伤口的时候可以快速引起甜柿的果腐病,但在无伤口的情况下也可直接在果面上形成小黑斑,在环境条件适宜时,扩展成片果腐和果斑病,因此在甜柿果实初期应主要对哈锐炭疽菌引起的果斑病进行防治,以防病原菌扩散形成初侵染来源。室内致病性测定结果表明,果生刺盘孢和暹罗刺盘孢都不能直接侵染甜柿,必须在有伤口的情况下才能侵染甜柿,这与田间采集在萼片上分离的病原菌数量多于果面上有直接关系,因此,适当给甜柿果实套袋,减少果面的损伤可以降低果生刺盘孢和暹罗刺盘孢引起的甜柿果实炭疽病。另外,果生刺盘孢菌在甜柿的萼片上分离率高于果面,因此,在萼片生长期是果生炭疽菌防治的主要时期。
3.2 不同甜柿炭疽病病原菌生物学特性比较分析
炭疽菌是一类重要的植物病原真菌,具有丰富的形态学及遗传多样性,笔者在本研究中针对3种甜柿炭疽菌的生物学特性进行了比较研究,结果表明,3 种甜柿炭疽病菌(C.horii、C.siamense 和C. fructicola)菌丝生长和产孢适宜温度均为30~35 ℃,最适生长温度30 ℃。哈锐炭疽菌最适产孢温度为25 ℃,该结果说明哈锐炭疽菌引起的炭疽病为偏低温病害,适宜低温可刺激该病原菌大量产孢,为后期侵染提供初侵染来源,这与浙江省6 月份柿果实初期即受到哈锐炭疽菌危害直接相关。产孢试验结果表明,果生刺盘孢和暹罗刺盘孢最适产孢温度为35 ℃,这与果生刺盘孢和暹罗刺盘孢为甜柿生长中后期主要病害有关,也与浙江省气候气温相关[21]。果生刺盘孢和暹罗刺盘孢菌丝生长最佳培养基为PDA、CMA 和GA,果生刺盘孢产孢最佳培养基为胡萝卜煎汁培养基,暹罗刺盘孢产孢最佳培养基为CMA 培养基;哈锐炭疽菌菌丝生长最佳培养基为PDA和CA,产孢最佳培养基为OA培养基。说明不同炭疽菌小种在菌丝生长所需养分存在差异,且培养基的成分直接影响病原菌的产孢情况[22]。pH 值4~10 均适宜甜柿炭疽病菌菌丝生长,并且无显著差异,说明该病害的发生与环境pH无关。果生刺盘孢在pH 值5 和9 时产孢最佳,暹罗刺盘孢和哈锐炭疽菌在pH 9和10时产孢最佳,说明在较差环境下可以刺激各炭疽病菌产孢;各甜柿炭疽病菌菌丝生长对光照均不敏感,但对其产孢的影响较大,持续光照有利于果生刺盘孢分生孢子的形成和产生,产孢量显著高于暗培养条件,在光暗交替的条件下可刺激哈锐炭疽菌产孢,暹罗刺盘孢产孢对光照不敏感,这一研究结果表明适当的遮阳处理可以减低该病害的传播速度[23]。果生炭疽菌病菌分生孢子的致死温度为51 ℃5 min,哈锐炭疽菌分生孢子的致死温度为50 ℃5 min,暹罗刺盘孢菌分生孢子的致死温度为52 ℃5 min。上述研究结果将为该病害的发生规律及病害防治提供理论依据。
4 结论
通过对浙江金华永康太秋甜柿炭疽菌种类的调查、致病性测定和系统发育分析,明确了太秋甜柿的炭疽病病原主要有哈锐炭疽菌、果生刺盘孢和暹罗刺盘孢,其中暹罗刺盘孢引起太秋甜柿炭疽病为国内首次报道。对病原菌生物学特性研究,首次比较了3个不同太秋甜柿炭疽病病原小种的最适培养温度、pH值、光照条件和孢子致死温度,为太秋甜柿炭疽病科学防治提供了科学依据。
[1] 艾呈祥,王洁,肖军,解小锋,龚榜初.太秋甜柿的生物学特性及栽培技术[J].落叶果树,2021,53(2):40-42.AI Chengxiang,WANG Jie,XIAO Jun,XIE Xiaofeng,GONG Bangchu. Biological characteristics and cultivation techniques of Taishuu sweet persimmon[J]. Deciduous Fruits,2021,53(2):40-42.
[2] 辛守鹏,李明,宋航,夏志卉,史志高.‘太秋’甜柿在淮安地区引种栽培初报[J].北方果树,2019(2):52-53.XIN Shoupeng,LI Ming,SONG Hang,XIA Zhihui,SHI Zhigao.Introduction and cultivation of‘Taiqiu’sweet persimmon in Huai′an area[J].Northern Fruits,2019(2):52-53.
[3] 徐严,骆夏辉,蔡建国,郑维威,谭李梅,曾蓓,唐志敏.‘太秋’甜柿在湘南地区的引种表现及栽培技术要点[J].中国果树,2021(6):68-70.XU Yan,LUO Xiahui,CAI Jianguo,ZHENG Weiwei,TAN Limei,ZENG Bei,TANG Zhimin. Introduction and cultivation technique of‘Taiqiu’non-astringent persimmon in southern Hunan[J].China Fruits,2021(6):68-70.
[4] 卢义华. 太秋甜柿在福建永定县的引种表现及栽培技术[J].中国南方果树,2013,42(3):107-109.LU Yihua. Introduction and cultivation techniques of Taiqiu sweet persimmon in Yongding county, Fujian province[J]. South China Fruits,2013,42(3):107-109.
[5] 艾呈祥,秦志华,辛力.2013 年中国柿产业发展报告[J].中国果菜,2014,34(2):10-13.AI Chengxiang,QIN Zhihua,XIN Li.2013 China persimmon industry development report[J].China Fruit&Vegetable,2014,34(2):10-13.
[6] XIE L,ZHANG J Z,CAI L,HYDE K D. Biology of Colletotrichum horii,the causal agent of persimmon anthracnose[J]. Mycology,2010,1(4):242-253.
[7] JEON J Y,HASSAN O,CHANG T,LEE D W,SHIN G S,OH N K.Anthracnose of persimmon(Diospyros kaki)caused by Colletotrichum horii in Sangju,Korea[J]. Plant Disease,2017,101(6):1035-1035.
[8] 余贤美,侯长明,王洁,王海荣,安淼,艾呈祥.山东牛心柿炭疽病菌的分离鉴定及致病性[J].林业科学,2015,51(4):126-133.YU Xianmei,HOU Changming,WANG Jie,WANG Hairong,AN Miao,AI Chengxiang. Identification of pathogen causing beef heard persimmon anthranose in Shandong and its pathogenicity[J].Scientia Silvae Sinicae,2015,51(4):126-133.
[9] WANG J,AI C X,YU X M,AN M,SUN S,GAO R. First report of Colletotrichum karstii causing anthracnose on persimmon leaves in China[J].Plant Disease,2016,100(2):532-532.
[10] PALOU L,MONTESINOS- HERRERO C,TARAZONA I,TABERBERV.Postharvest anthracnose of persimmon fruit caused by Colletotrichum gloeosporioides first reported in Spain[J].Plant Disease,2013,97(5):691.
[11] 张童,姚立萍,胡玉慈,唐鑫彪,何淑敏,陈清西,文志丰.甜柿炭疽病病原菌的鉴定及其防治药剂的筛选[J].福建农林大学学报(自然科学版),2020,49(5):589-596.ZHANG Tong,YAO Liping,HU Yuci,TANG Xinbiao,HE Shumin,CHEN Qingxi,WEN Zhifeng. Identification of anthracnose of sweet persimmon and screening of its control agents[J].Journal of Fujian Agriculture and Forestry University (Natural Science Edition),2020,49(5):589-596.
[12] WILLIAMSON S M,SUTTON T B. First report of anthracnose caused by Colletotrichum acutatum on persimmon fruit in the United States[J].Plant Disease,2010,94(5):634-638.
[13] CHANG T,HASSAN O,JEON J Y,SHIN J S,OH N K,LIM T H. First report of anthracnose of persimmon (Diospyros kaki L.f.) caused by Colletotrichum siamense in Korea[J]. Plant Disease,2018,102(2):443-444.
[14] 余贤美,侯长明,王洁,安淼,王海荣,艾呈祥.次郞甜柿炭疽病菌的分离鉴定及其rDNA-ITS 序列分析[J]. 经济林研究,2014,32(1):45-50.YU Xianmei,HOU Changming,WANG Jie,AN Miao,WANG Hairong,AI Chengxiang. Isolation,identification and rDNAITS sequence analysis of Colletotrichum sp.[J]. Non-wood Forest Research,2014,32(1):45-50.
[15] WHITE T J,BRUNS T D,LEE S B,TAYLOR J W,INNIS M A,GELFAND D H,SNINSKY J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics[J].PCR Protocols,1990,18(1):315-322.
[16] STEPHENSON S A,GREEN J R,MANNERS J M,MACLEAN D J. Cloning and characterisation of glutamine synthetase from Colletotrichum gloeosporioides and demonstration of elevated expression during pathogenesis on Stylosanthes guianensis[J].Current Genetics,1997,31(5):447-454.
[17] GLASS N L,DONALDSON G C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes[J].Applied and Environmental Microbiology,1995,61(4):1323-1330.
[18] TEMPLETON M D,RIKKERINK E H A,SOLON S L,CROWHURST R N. Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata[J].Gene,1992,122(1):225-230.
[19] CARBONE I,KOHN L M.A method for designing primer sets for speciation studies in filamentous ascomycetes[J].Mycologia,1999,91(3):553-556.
[20] 方中达.植病研究方法[M].北京:中国农业出版社,1998:122-125.FANG Zhongda. Plant pathology research protocols[M]. Beijing:China Agriculture Press,1998:122-125.
[21] 张敬泽,胡东维.影响柿树炭疽菌孢子萌发、附着胞形成及致病性的环境因子[J].植物病理学报,2004,34(2):154-161.ZHANG Jingze,HU Dongwei. Effects of environment factors on conidia germination,appressoria formation and pathogenicity of the persimmon anthracnose pathogens Colletotrichum gloeosporioides[J]. Acta Phytopathologica Sinica,2004,34(2):154-161.
[22] 白庆荣,韩双,赵莹,李海洋,梁峻玮,高洁.萱草叶枯病菌生物学特性及对药剂敏感性研究[J].园艺学报,2013,40(12):2513-2519.BAI Qingrong,HAN Shuang,ZHAO Ying,LI Haiyang,LIANG Junwei,GAO Jie. Biological characteristics and fungicide sensitivity of Kabatiella microsticta causing daylily leaf streak[J].Acta Horticulturae Sinica,2013,40(12):2513-2519.
[23] 廖旺姣,黄乃秀,邹东霞,覃世杰,蒋晓萍.八角炭疽病菌哈锐炭疽菌生物学特性[J].中国森林病虫,2018,37(3):10-12.LIAO Wangjiao,HUANG Naixiu,ZOU Dongxia,QIN Shijie,JIANG Xiaoping.Biological characteristics of pathogens of Colletotrichum horri for Illicium verum anthracnose[J]. Forest Pest and Disease,2018,37(3):10-12.