我国桃种植面积和产量分别为89.0 万hm2和1599.3万t,均居世界第1位,我国有20个省份的桃种植面积超过1 万hm2,山东省位居首位,其中蒙阴县4.33万hm2,为中国桃第一大种植县[1]。桃褐腐病又名菌核病、果腐病、实腐病,是由子囊菌链核盘菌Monilinia spp.(无性态为Monilia)引起的一种重要桃病害,世界产桃区均可发生[2]。桃褐腐病主要危害果实,也可危害花、叶和枝梢。果实自幼果期至成熟期均可受害,若生长后期多雨常流行成灾,发病率超过80%甚至绝收[3],还可在运输、贮藏期发生,使果实丧失商品价值[4]。
在世界范围内,对核果和仁果类果树造成褐腐病的病原主要有6 种[5],分别为核果链核盘菌Monilinia laxa、果生链核盘菌Monilinia fructigena[6]、美澳型核果链核盘菌Monilinia fructicola[7]、梅生链核盘菌Monilinia mumecola (synonymy: Monilia mumecola)[8]、多子座链核盘菌Monilinia polystroma(synonymy: Monilia polystroma)[9]和云南链核盘菌Monilinia yunnanensis (synonymy: Monilia yunnanensis)[10]。目前,造成中国桃褐腐病病原菌的种主要为Monilinia fructicola、Monilia yunnanensis和Monilia mumecola,其中Monilinia fructicola 是优势种[3,10-11],对中国经济危害最大、地理分布最广,在中国各桃产区均有发生[12-16]。Monilia yunnanensis目前仅在中国有报道,为云南省的优势种,此外在陕西、北京、河北、甘肃及辽宁有分布[10-11,17]。
不同桃褐腐病病原菌在菌落形态上存在较大差异,可从菌落颜色、生长速率、孢子形态及产孢量等形态学进行初步鉴定[18-19]。但对一些种间形态差异较小、培养时出现性状变异等不典型菌株的鉴定,传统的形态学鉴定就会受到限制。近年来形态学观察结合分子生物学鉴定的方法被广泛应用于菌种的鉴定分类[20-22]。谈彬[23]采用形态学观察与分子生物学鉴定方法将12 个省市23 株桃褐腐病病原菌确定为Monilinia fructicola 和Monilia yunnanensis。纪兆林等[24]的研究表明,山东、辽宁等桃主产区的桃褐腐病病原菌菌落形态存在明显差异,并将桃褐腐病病原菌鉴定为Monilinia fructicola 和Monilia yunnanensis[2]。周芳[25]通过形态学和分子生物学鉴定明确了山西省81株桃褐腐病病原菌的分类地位,其中75株为Monilinia fructigena,15 株为Monilinia laxa,3 株为Monilinia fructicola。另外,致病力方面也存在较大差异,谈彬[23]证实桃褐腐病病原菌致病力差异与多聚半乳糖醛酸酶基因PG1相关。
山东省作为全国产桃大省,对桃褐腐病的研究报道主要集中在病害防治、桃褐腐病病原菌发病规律等方面,未见关于褐腐病病原菌种群鉴定及致病力分化方面的研究报道。为此,笔者在本研究中采集了山东省主要桃产区典型果实褐腐病样本,并通过对其进行形态观察及rDNA-ITS 序列鉴定,对其种群结构进行聚类分析,并对其在叶片上的致病力进行比较分析,为明确山东省各桃主产区桃褐腐病病原菌种类、深入研究其致病机制、制定适合不同产区的褐腐病防治技术提供理论依据。
1 材料和方法
1.1 材料
自山东省临沂、烟台、威海等8市不同地区采集具有桃褐腐病典型症状的发病果实、叶片、枝条,将发病样品用纸袋分装带回实验室进行分离纯化,4 ℃保存备用;致病力测定所用桃树叶片品种为金秋红蜜,采集自烟台市农业科学院试验农场内。
1.2 试剂及仪器
马铃薯葡萄糖琼脂(potato dextrose agar,PDA)培养基,生工生物工程(上海)股份有限公司;真菌基因组DNA提取试剂盒,天根生化科技(北京)有限公司;BioPhotometer Plus 核酸蛋白测定仪,Eppendorf中国有限公司;DM750显微镜,德国莱卡公司;SPX-250生化培养箱,上海跃进医疗器械有限公司;RXZ智能型人工气候箱,宁波江南仪器厂。
1.3 方法
1.3.1 桃褐腐病病原菌的分离纯化 采用单孢分离法进行分离。用灭菌挑针挑取发病部位外缘较新鲜的孢子堆,转接至新鲜PDA 平板上培养,并经科赫氏法则验证,最后将纯化好的菌落切小块保存于灭菌的25%甘油中,于4 ℃下保存备用(表1)。
表1 桃褐腐病病原菌采集地
Table 1 Collection locations of peach brown rot pathogens

编号Number THF-01编号Number THF-24 THF-02 THF-25 THF-03 THF-27 THF-04 THF-28 THF-05 THF-33 THF-06 THF-34 THF-07 THF-35 THF-08 THF-37 THF-09 THF-39 THF-10 THF-40 THF-11 THF-41 THF-12 THF-43 THF-13 THF-45 THF-14 THF-47 THF-15 THF-49 THF-16 THF-51 THF-17 THF-52 THF-18 THF-54 THF-19 THF-55 THF-20 THF-57采集地Collecting locations临沂市上峪村Shangyu Village,Linyi City临沂市文家峪村Wenjiayu Village,Linyi City临沂市龙王峪村Longwangyu Village,Linyi City临沂市大崮村Dagu Village,Linyi City临沂市常坪村Changping Village,Linyi City烟台市青山后村Qingshanhou Village,Yantai City淄博市上马庄村Shangmazhuang Village,Zibo City淄博市西官庄村Xiguanzhuang Village,Zibo City淄博市北山村Beishan Village,Zibo City烟台市后发坊村Houfafang Village,Yantai City烟台市后发坊村Houfafang Village,Yantai City济南市南庄村Nanzhuang Village,Jinan City济南市丁家庄村Dingjiazhuang Village,Jinan City烟台市杏吕村Xinglv Village,Yantai City烟台市大宋家村Dasongjia Village,Yantai City烟台市和尚庄村Heshangzhuang Village,Yantai City烟台市许家村Xujia Village,Yantai City烟台市北山后村Beishanhou Village,Yantai City威海市刘大庄村Liudazhuang Village,Weihai City潍坊市蟠龙峪村Panlongyu Village,Weifang City潍坊市石堆村Shidui Village,Weifang City采集部位Collection parts果实Fruit果实Fruit叶片Leaf果实Fruit果实Fruit果实Fruit叶片Leaf叶片Leaf果实Fruit果实Fruit枝条Branch果实Fruit叶片Leaf果实Fruit枝条Branch枝条Branch果实Fruit果实Fruit叶片Leaf叶片Leaf叶片Leaf采集地Collecting locations潍坊市老峒峪村Laotongyu Village,Weifang City泰安市龙岗村Longgang Village,Tai’an City泰安市高岭村Gaoling Village,Tai’an City泰安市上寨村Shangzhai Village,Tai’an City烟台市东解家村Dongxiejia Village,Yantai City烟台市迟家村Chijia Village,Yantai City烟台市大于家村Dayujia Village,Yantai City威海市宋家庄村Songjiazhuang Village,Weihai City威海市东宋格庄村Dongsonggezhuang Village,Weihai City威海市下初村Xiachu Village,Weihai City威海市大院村Dayuan Village,Weihai City济南市雪野村Xueye Village,Jinan City济南市官家村Guanjia Village,Jinan City济南市戴鱼池村Daiyuchi Village,Jinan City青岛市北蒋家村Beijiangjia Village,Qingdao City青岛市兴隆寨村Xinglongzhai Village,Qingdao City青岛市里兰埠村Lanlibu Village,Qingdao City青岛市孙贾城村Sunjiacheng Village,Qingdao City烟台市后沟村Hougou Village,Yantai City烟台市上车门村Shangchemen Village,Yantai City采集部位Collection parts果实Fruit果实Fruit果实Fruit果实Fruit枝条Branch果实Fruit果实Fruit果实Fruit叶片Leaf果实Fruit叶片Leaf果实Fruit果实Fruit枝条Branch叶片Leaf果实Fruit果实Fruit果实Fruit叶片Leaf叶片Leaf THF-21
1.3.2 桃褐腐病病原菌的形态学鉴定 将单孢分离纯化后的菌株分别接种到PDA 平板上,置于25 ℃全黑暗生化培养箱中培养5 d后,观察菌落形态和色泽,测量菌落直径,并在显微镜下观察分生孢子、分生孢子梗形态特征,观察10 个以上视野,每个视野观察50个孢子,对其进行形态学鉴定。
1.3.3 桃褐腐病病原菌的分子生物学鉴定 PDA平板上活化菌株5 d后,将菌丝刮下置于灭菌研钵中加液氮研磨,采用基因组DNA提取试剂盒提取病原菌基因组,于-20 ℃冰箱中保存,备用。委托生工生物工程(上海)股份有限公司利用rDNA-ITS序列引物ITS1(5’-TCCGTAGGTGAACCTGCGG-3’)/ITS4(5’-TCCTCCGCTTATTGATATGC-3’)进行菌株ITS 序列测定。测序所得序列与NCBI 中已知的基因序列进行BLAST 同源性检索,从NCBI 数据库中下载与待测菌株相似度99%以上的部分桃褐腐菌株及其序列,采用最大似然法和邻接法,运用MEGA 6.0软件构建系统发育树。
1.3.4 桃褐腐病病原菌菌丝生长速率测定及产孢量测定 将各桃褐腐病病原菌菌株于PDA平板上25 ℃培养5 d,采用十字交叉法测量菌落的直径,按照如下公式计算菌丝的平均生长速率:菌丝平均生长速率=(菌落直径-0.5 cm)/5 d;利用DPS 18.10 高级版,采用DMRT法进行显著性分析,采用欧氏距离中的非加权组平均法(UPGMA)对不同菌株生长速率进行聚类分析,将菌株按照生长速率分为不同等级。
测量产孢量时,于菌落边缘打取5 mm菌饼,接种于PDA 培养基上,每菌株3 次重复,于25 ℃暗培养7 d。用10 mL灭菌水将所有孢子洗下,搜集孢子悬浮液,加入10 颗小玻璃珠,振荡器振荡1 min,使孢子分散均匀。各菌株孢子悬浮液浓度用血球计数板计数。每个菌株3次重复,取平均值计算产孢量。
1.3.5 桃褐腐病病原菌致病力测定及聚类分析 将4 ℃下保存的菌种接种于PDA 平板上,25 ℃恒温培养5 d 后,用0.5 cm 的打孔器在菌落边缘打孔制备菌饼。用75%乙醇进行桃叶片表面消毒后用无菌水冲洗3次,待叶片表皮自然风干进行有伤接种,用三号昆虫针刺伤叶片背面表皮,每个菌株接种3 枚叶片,每枚叶片3个接种点,将上述菌饼带菌丝一面紧贴刺伤部位,空白PDA菌饼为对照。用无菌水浸湿的脱脂棉包裹叶柄保鲜叶片,在叶片表面喷无菌水保湿,放置于温度为25 ℃、相对湿度为70%、光照条件为16 h 光照/8 h 黑暗的人工气候箱中培养,观察并记录发病情况。采用十字交叉法测量病斑直径,利用DPS 18.10 高级版对发病程度进行差异显著性及聚类分析。
1.3.6 桃褐腐病病原菌菌丝生长速率及致病力相关性分析 利用Excel 对菌丝生长速率及致病力相关性进行分析,0<|r|≤0.3,无相关性;0.3<|r|<0.8,弱相关性;|r|≥0.8,强相关性。
2 结果与分析
2.1 桃褐腐病田间发病症状
褐腐病病原菌可侵染核果及仁果植物的叶片、枝梢和果实等多个部位。叶片受害后,自叶边缘开始变褐枯萎,干枯的病叶残留枝上久不脱落(图1-A);枝条受害后,产生菱形、不规则形的溃疡病斑,初为黄褐色,后变为深褐色,病斑凹陷、界限明显,不断向上下扩展蔓延,病斑处易形成流胶,可造成病部以上枝条干枯死亡甚至整个大枝枯死(图1-B)。果实受害后,果面最初形成褐色圆形水溃状小斑点,后迅速扩大造成全果腐烂,当分生孢子梗突破病斑表层后,呈现成丛的同心轮纹状的病症,即分生孢子梗和分生孢子(图1-C)。部分果实失水、干缩形成僵果,僵果落到地上,或悬挂枝上经久不落。

图1 桃褐腐病田间发病症状
Fig.1 Symptoms of the peach brown rot in the field
2.2 山东省不同地区桃褐腐病病原菌的分离鉴定
2.2.1 桃褐腐病病原菌的形态学鉴定 对典型桃褐腐病样本经单孢分离纯化后共获得41株菌株,从菌落形态观察主要分为三大类。以THF-01 为代表的菌株:菌落边缘较整齐,颜色呈灰黄色,产孢量丰富,为(14.49-20.11)×104个·cm-2(表2),孢子堆可形成同心圆环结构(图2-A1);菌落背面呈圆形边缘浅灰色,中间颜色较深,边缘及中心灰色菌圈间夹有白色菌圈(图2-B1);分生孢子梗成串珠状,不分枝或二叉状分枝,分枝呈锐角形,产孢梗较短(图2-C1);分生孢子无色单孢,串珠状排列,卵圆形或柠檬形,分生孢子大小范围为(15.3~18.9)μm×(13.4~14.8)μm,平均孢子大小为16.2~13.5 μm(图2-D1)。
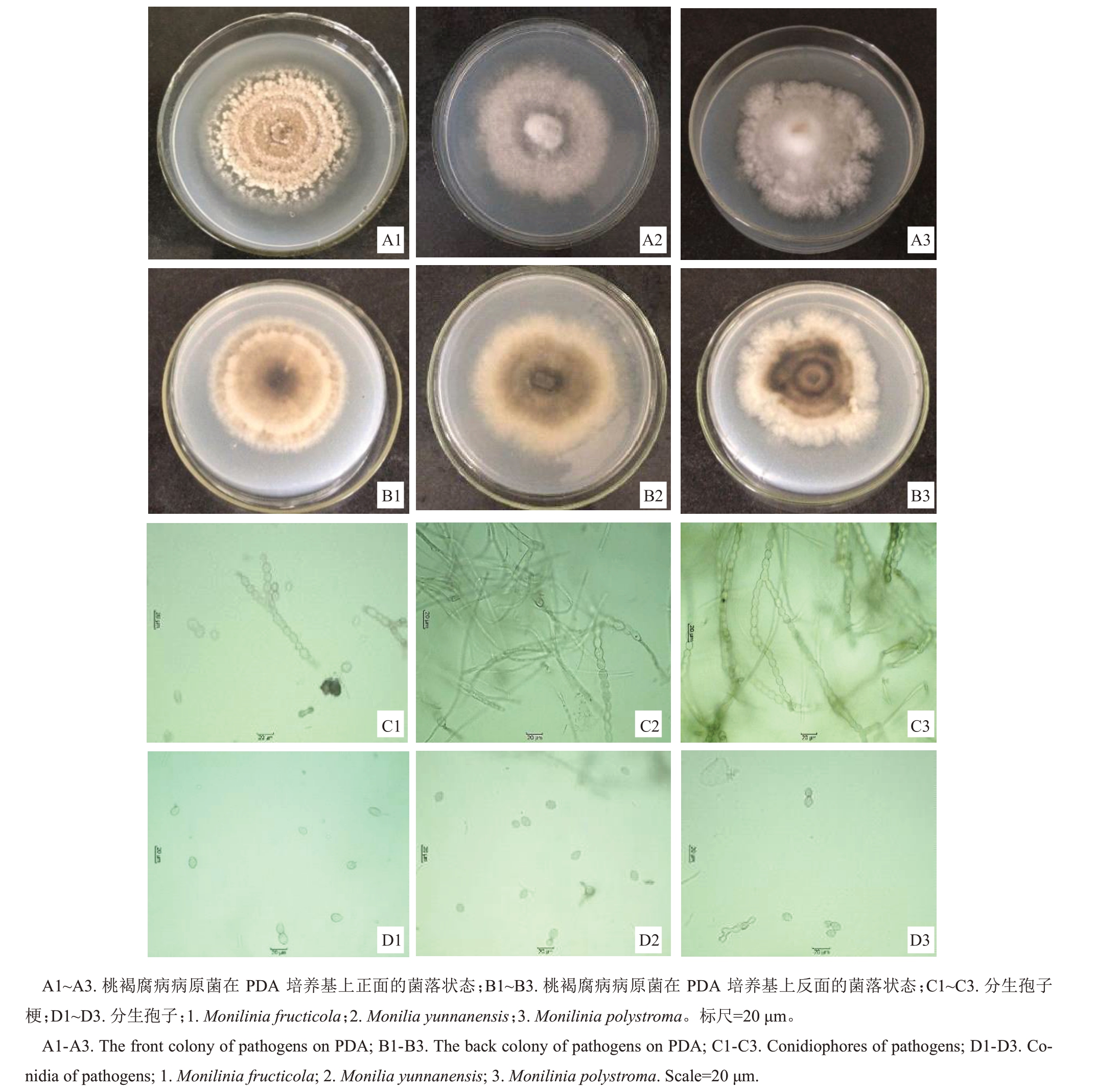
图2 桃褐腐病病原菌的培养性状及形态特征
Fig.2 Colony and morphological characteristics of peach brown rot pathogens
表2 桃褐腐病病原菌菌丝生长速率、产孢量及病斑长度
Table 2 Mycelial growth rates,spore outputs and lesion lengths of peach brown rot pathogens

注:不同大、小写字母分别表示各处理间在p<0.01、0.05 水平上的差异极显著和显著。
Note:The different capital and small letters indicate the significant difference at p<0.01 and p<0.05,respectively.
病原菌种类Pathogenic species美澳型核果链核盘菌Monilinia fructicola云南链核盘菌Monilia yunnanensis多子座链核盘菌Monilinia polystroma病斑长度Lesion length/cm 0.95±0.01 rsRS 1.63±0.01 gG 1.58±0.02 hiHI 2.01±0.01 eEF 2.16±0.02 dD 1.23±0.01 mM 0.88±0.01 vU 2.02±0.01 eE 2.32±0.01 bB 1.33±0.01 kK 1.56±0.01 iI 1.02±0.03 qQ 0.96±0.00 rR 0.00±0.00 aZ 0.93±0.01 tuST 1.15±0.01 oO 0.94±0.00 stRST 0.93±0.01 tuT 0.85±0.01 wV 1.59±0.03 hH 1.35±0.01 kK 1.27±0.01 lL 1.11±0.01 pP 1.98±0.03 fF 0.75±0.01 zY 1.26±0.01 lLM 2.23±0.01 cC 0.85±0.01 wUV 0.92±0.00 uT 0.77±0.01 yX 1.57±0.03 iHI 1.49±0.01 jJ 1.11±0.01 pP 0.00±0.00 aZ 0.81±0.02 xW 0.86±0.01 wUV 1.20±0.01 nN 2.35±0.06 bB 2.40±0.02 aA 2.42±0.01 aA 2.43±0.02 aA编号Number THF-01 THF-02 THF-03 THF-04 THF-05 THF-07 THF-08 THF-09 THF-12 THF-13 THF-15 THF-20 THF-21 THF-24 THF-25 THF-27 THF-28 THF-33 THF-34 THF-35 THF-37 THF-39 THF-40 THF-41 THF-43 THF-45 THF-47 THF-49 THF-51 THF-52 THF-54 THF-55 THF-57 THF-06 THF-10 THF-11 THF-18 THF-14 THF-16 THF-17 THF-19菌丝生长速率Mycelial growth rates/(cm·d-1)0.83±0.01 rsRS 0.90±0.00 gG 0.61±0.01 hiHI 0.97±0.01 eEF 1.01±0.01 dD 0.84±0.00 mM 1.03±0.00 vU 0.90±0.00 eE 1.01±0.01 bB 1.04±0.01 kK 0.68±0.03 iI 0.98±0.00 qQ 1.09±0.00 rR 0.96±0.00 aZ 0.52±0.00 tuST 1.03±0.00 oO 0.89±0.01 stRST 0.93±0.01 tuT 0.92±0.01 wV 0.72±0.01 hH 0.98±0.01 kK 0.81±0.03 lL 0.53±0.01 pP 0.86±0.01 fF 0.81±0.00 zY 0.99±0.01 lLM 0.96±0.01 cC 0.61±0.01 wUV 0.98±0.01 uT 0.67±0.00 yX 0.84±0.01 iHI 0.99±0.00 jJ 0.84±0.01 pP 0.79±0.01 aZ 0.75±0.00 xW 0.47±0.01 wUV 0.88±0.01 nN 1.00±0.00 bB 0.95±0.03 aA 0.94±0.04 aA 1.00±0.00 aA产孢量Spore output/(×104·cm-2)19.83±1.88 abABC 17.75±0.53 abcdefghABCDEFG 18.26±0.94 abcdefgABCDEFG 16.33±0.39 ghiEFGHI 18.04±1.45 abcdefgABCDEFG 19.60±1.75 abcABCD 16.86±1.44 efghCDEFGHI 18.24±1.08 abcdefgABCDEFG 16.02±0.56 ghiEFGHI 16.37±0.62 ghiEFGHI 18.06±1.55 abcdefgABCDEFG 20.04±0.58 aA 17.65±0.65 bcdefghABCDEFG 18.14±1.23 abcdefgABCDEFG 14.57±1.27 iHI 16.60±2.03 fghiDEFGHI 16.51±0.97 fghiEFGHI 16.92±1.05 efghBCDEFGHI 16.52±1.03 fghiEFGHI 19.28±1.10 abcABCDE 20.01±2.09 abAB 16.00±0.46 ghiEFGHI 18.72±0.66 abcdefABCDEFG 17.40±0.14 cdefghABCDEFGH 17.04±1.54 defghABCDEFGHI 18.16±1.57 abcdefgABCDEFG 15.73±1.09 hiGHI 14.49±0.93 iI 17.84±1.93 abcdefghABCDEFG 20.11±0.66 aA 19.36±1.13 abcABCDE 16.13±0.54 ghiEFGHI 18.92±0.86 abcdeABCDEF 0.42±0.16 kK 0.43±0.05 kK 0.36±0.09 kK 0.42±0.08 kK 9.10±0.81 jJ 8.69±1.47 jJ 8.43±0.76 jJ 9.47±1.01 jJ
以THF-06 为代表的菌株:菌落边缘较整齐,菌丝呈暗灰色,气生菌丝贴近培养皿(图2-A2),产孢量稀少,为(0.36~0.43)×104个·cm-2(表2);菌落背面黑褐色,边缘呈灰白色,无明显同心轮纹状结构(图2-B2);分生孢子梗成串珠状,不分枝或二叉状分枝,分枝呈锐角形,产孢梗长短不一(图2-C2);分生孢子无色单孢,串珠状排列,卵圆形或柠檬形,分生孢子大小范围为(14.9~18.0)μm×(13.6~14.9)μm,平均孢子大小为16.6~13.4 μm(图2-D2)。
以THF-14 为代表的菌株:菌落边缘不整齐,呈不规则形或圆形,边缘有明显裂缺,菌丝灰白色,气生菌丝较发达(图2-A3),产孢量较少,为(8.43~9.47)×104个·cm-2(表2);菌落背面边缘灰白色,中间深灰色及浅灰色交替呈同心轮纹状(图2-B3);分生孢子梗成串珠状,不分枝或二叉状分枝,分枝呈锐角形,产孢梗较长(图2-C3);分生孢子无色单孢,串珠状排列,卵圆形或柠檬形,分生孢子大小范围为(15.6~18.2)μm×(14.4~15.8)μm,平均孢子大小为17.2~14.9 μm(图2-D3)。
2.2.2 桃褐腐病病原菌的分子生物学鉴定 从NCBI GenBank 下载相似性最高的同种和近似种褐腐病病原菌菌株以及桃树黑斑病菌(Alternaria alter-nata,GenBank 登录号为AF404664.1、OL958426.1)及炭疽病菌(Colletrichum gloeosporioides,GenBank登录号为JX010152.1,AY423476.1)序列,用MEGA 6.0 软件进行系统发育分析。采用最大似然法和邻接法构建系统发育进化树,自展值设为1000,基于rDNA-ITS序列构建的系统发育树结果表明,桃褐腐病病原菌菌株共分为三大类,第Ⅰ类包括THF-01等菌株,共33株,该类与Monilinia fructicola(GenBank登录号为MZ047241.1,EF207419.1)聚在一个分支上,相似性高达99%,将其鉴定为Monilinia fructicola;第Ⅱ类包括THF-06 等菌株,共4 株,该类与Monilia yunnanensis(GenBank 登录号为MW355895.1)聚在一起,相似性高达100%,将其鉴定为Monilia yunnanensis;第Ⅲ类包括THF-14等菌株,共4株,该类与Monilinia polystroma(GenBank 登录号为LT615178.1、LT615192.1)聚在一个分支上,相似性为99%,将其鉴定为Monilinia polystroma(图3)。
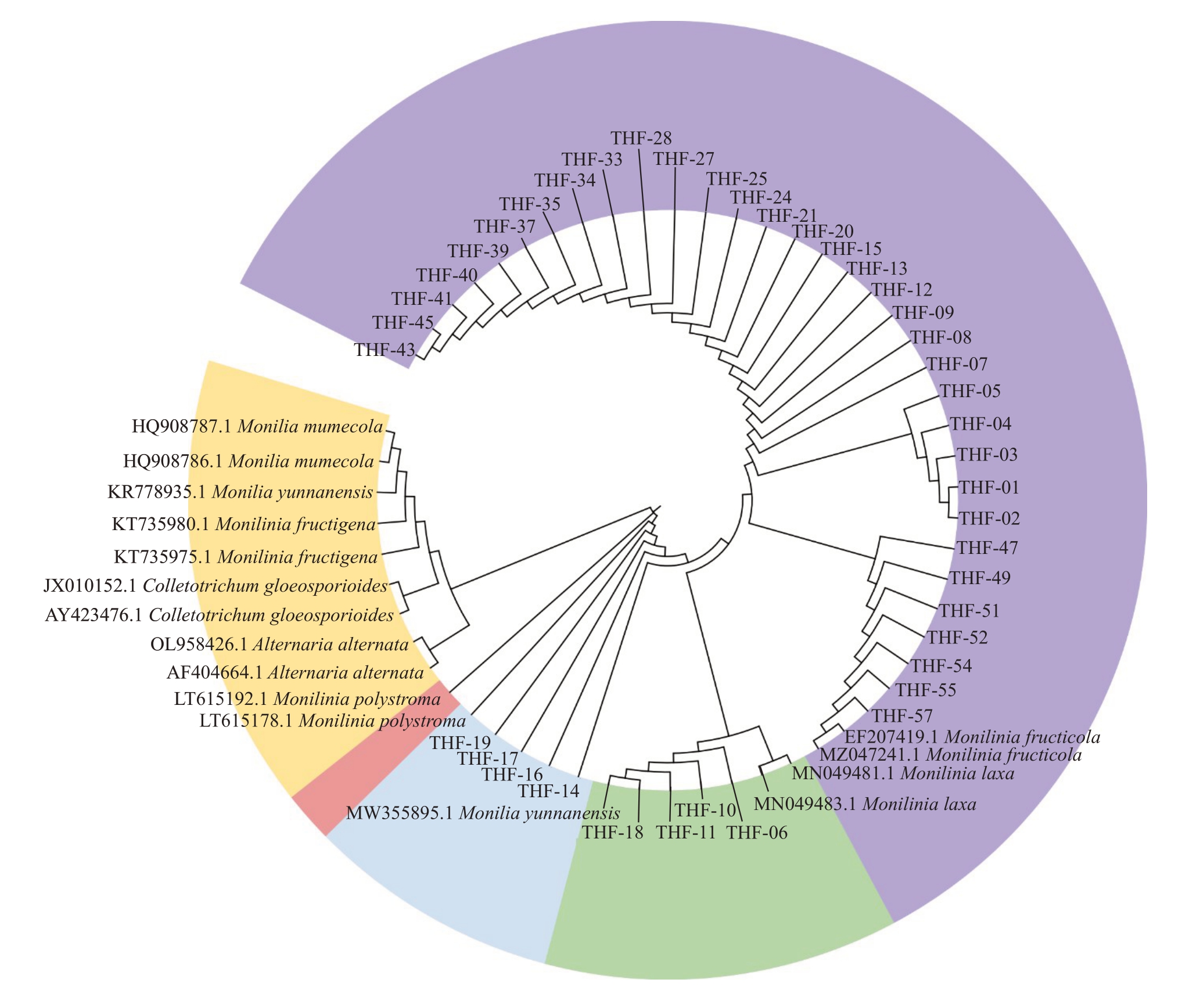
图3 桃褐腐病病原菌基于rDNA-ITS 序列的系统发育树
Fig.3 Phylogentic tree of peach brown rot pathogens based on the rDNA-ITS sequence
2.2.3 山东省不同地区桃褐腐病病原菌的分布 经形态学及分子生物学鉴定,确定山东省桃褐腐病病原菌为Monilinia fructicola、Monilia yunnanensis 和Monilinia polystroma,分别有33株、4株和4株,其分离频率分别为80.48%、9.76%和9.76%,具体分布如表1所示。Monilinia fructicola广泛分布于山东省烟台、青岛等沿海及临沂、济南等内陆大部分地区,Monilia yunnanensis 仅存在于烟台市,Monilinia polystroma分布于烟台市及青岛市(表3)。
表3 桃褐腐病病原菌在山东省的分布及数量
Table 3 Distribution and number of peach brown rot pathogens in Shandong province

菌种Strains Monilinia fructicola株数Number 33 Monilia yunnanensis Monilinia polystroma 44分布及数量Distribution and number烟台6株、济南5株、临沂5株、青岛4株、威海4株、淄博3株、潍坊3株、泰安3株6 strains from Yantai,5 strains from Jinan,5 strains from Linyi,4 strains from Qingdao,4 strains from Weihai,3 strains from Zibo,3 strains from Weifang,3 strains from Tai’an烟台4株4 strains from Yantai烟台3株、威海1株3 strains from Yantai,1 strain from Weihai
2.3 山东省不同地区桃褐腐病病原菌菌丝生长速率分析
对桃褐腐病病原菌菌丝生长速度进行测定,结果显示生长速率在0.47~1.09 cm·d-1之间(表2)。采用UPGMA对不同菌株生长速率进行聚类分析(图4),当欧式距离取0.7时,41个供试菌株的生长速率被划分为快速、中速和慢速3种类型。生长速率为快速类型的菌株有17株,占测定菌株的41.46%,菌株生长速率为0.95~1.09 cm·d-1,平均生长速率为1.00 cm·d-1;生长速率为中速类型的菌株为9 株,占测定菌株的21.95%,菌株生长速率为0.79~0.94 cm·d-1,平均生长速率为0.87 cm·d-1;生长速率为慢速类型的菌株有15株,占测定菌株的36.59%,菌株生长速率为0.47~0.75 cm·d-1,平均生长速率为0.62 cm·d-1。结果表明,山东省桃褐腐病病原菌以快、慢生长速率类型为主。
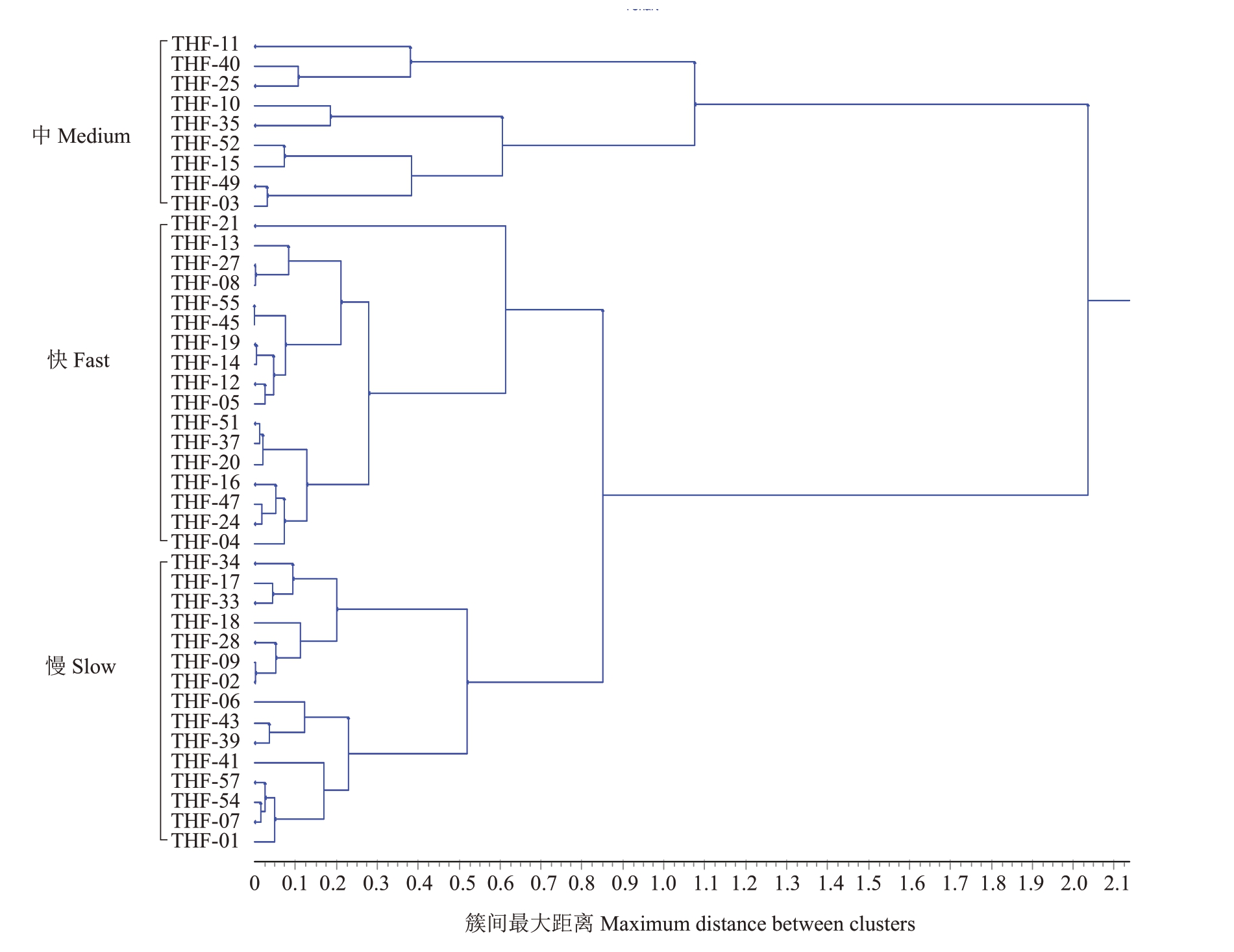
图4 欧氏距离非加权组平均法(UPGMA)对桃褐腐病病原菌菌丝生长速率的聚类分析
Fig.4 Cluster analysis of mycelial growth rates of peach brown rot pathogens with unweighted pair-group method with arithmetic means
2.4 山东省不同地区桃褐腐病病原菌致病力分析
将桃褐腐病病原菌有伤接种到离体桃叶片,测量其病斑大小,结果显示不同菌株对桃叶片的致病力存在明显差异(表2)。采用UPGMA 对不同桃褐腐病病原菌菌株的致病力进行了聚类分析(图5-B),当欧式距离取0.4时,41个供试菌株被划分为强致病力、中等致病力、弱致病力三种类型。强致病力的菌株共14 株,占测定菌株的34.15%,病斑长度为1.11~2.43 cm;中等致病力的菌株共25 株,占测定菌株的60.97%,病斑长度为0.75~1.02 cm;弱致病力的菌株共2 株,占测定菌株的4.88%,无病斑产生(表2,图5-A)。对接种发病的病斑进行再次分离,经柯赫氏法则及分子生物学鉴定确定为原病菌菌株。

图5 桃褐腐病病原菌致病力分析
Fig.5 Cluster analysis of pathogenicity of peach brown rot pathogens
2.5 桃褐腐病病原菌菌丝生长速率及产孢量与致病力相关性分析
对桃褐腐病病原菌菌株的生长速率与致病力进行相关性分析(图6),其相关系数r为0.2975,0<|r|≤0.3,显示菌丝生长速率与致病力之间无相关性;对桃褐腐病病原菌菌株的产孢量与致病力进行相关性分析(图7),其相关系数r 为0.0300,0<|r|≤0.3,显示产孢量与致病力之间无相关性。
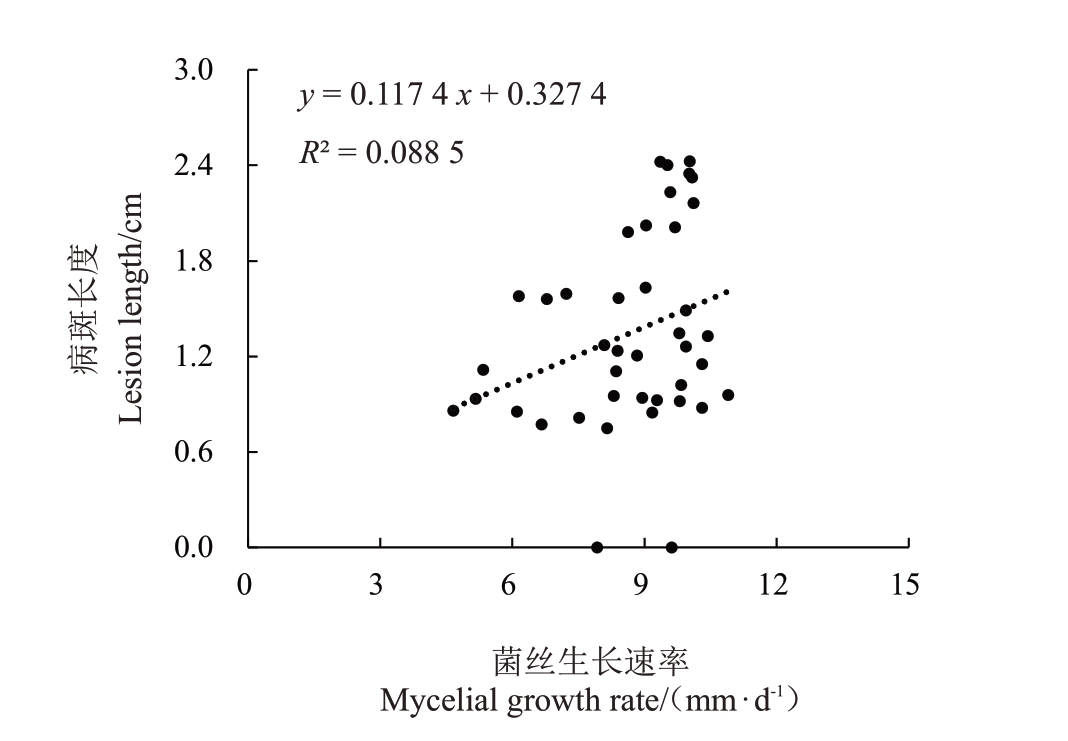
图6 桃褐腐病病原菌菌丝生长速率及致病力相关性分析
Fig.6 Linear analysis of the relationship between pathogenicity and mycelial growth rates of peach brown rot pathogens
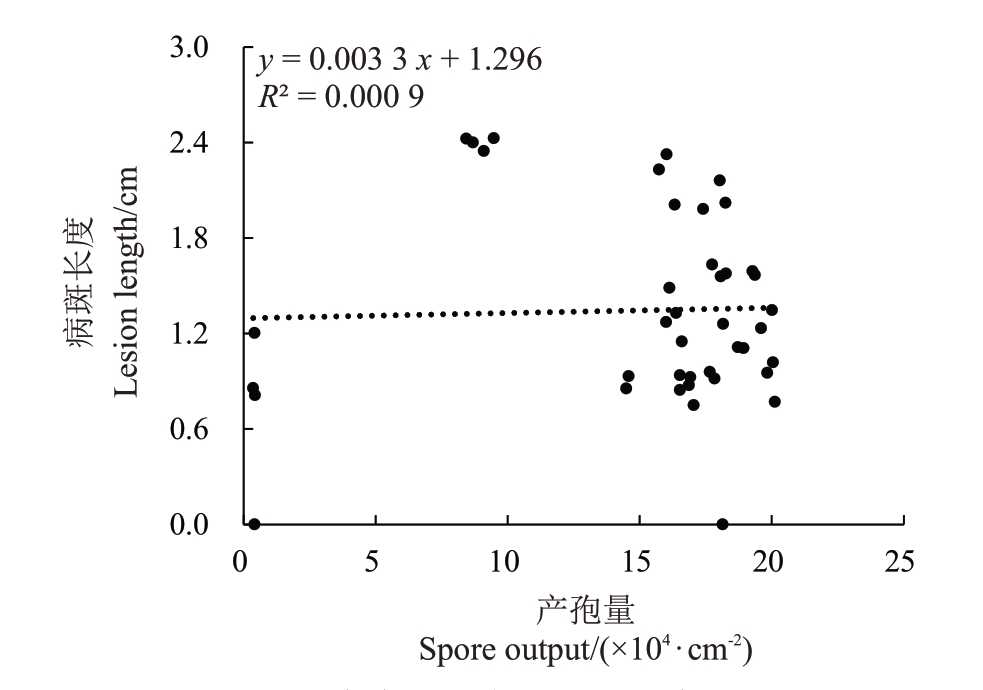
图7 桃褐腐病病原菌产孢量及致病力相关性分析
Fig.7 Linear analysis of the relationship between pathogenicity and spore output of peach brown rot pathogens
3 讨论
山东省桃种植面积和产量均为中国第一,桃已成为山东省主导的水果产业,是全国第一产桃大省。桃褐腐病病原菌可在花期至收获贮藏期造成严重危害,严重威胁桃产业的健康发展[3-4,26]。而不同产区的桃褐腐病病原菌在形态及致病力方面均存在较大差异[2,23],因此针对不同桃褐腐病病原菌种类制定适合不同产区的防治技术对于桃褐腐病病原菌的防控至关重要。
笔者在本研究中对山东桃褐腐病病原菌进行菌落形态观察及ITS 序列比对分析,确定了山东省桃褐腐病病原菌主要为Monilinia fructicola,而Monilia yunnanensis 和Monilinia polystroma 占比较低。在形态学观察鉴定时发现,3 种桃褐腐病病原菌在PDA培养基上的菌落形态存在较大差异,分生孢子梗长短不一,存在显著差异,分生孢子形态无明显差异,但产孢量存在显著差异:Monilinia fructicola产孢量最多,Monilinia polystroma 产孢量次之,Monilia yunnanensis 产孢量最少。其中Monilinia fructicola形态与周芳[25]、Hu等[10]及尹良芬[3]对Monilinia fructicola的菌落形态描述一致,且该菌种在山东省占比最大,这与纪兆林等[24]对中国桃产区桃褐腐病病原菌的鉴定结果一致,在山东省青岛及泰安地区分离到的桃褐腐病病原菌均鉴定为Monilinia fructicola,且占比最大,而周芳[25]对山西省桃褐腐病病原菌调查时发现Monilinia fructigena 在山西省桃褐腐病病原菌中为优势菌群,这也证实了不同地区桃褐腐病病原菌菌群结构差异较大,进行广泛深入的菌群结构调查是有效防治桃褐腐病病原菌的重要研究基础。
Monilia yunnanensis 和Monilinia polystroma 占比较低可能与寄主选择性及针对不同病原菌抗病性差异有关。仅在烟台地区采集的Monilia yunnanensis 为Hu 等[10]首次在苹果和梨上发现的1 个新记录种,其菌落形态特征与Hu等[10]及纪兆林等[24]的描述一致,产孢稀少,气生菌丝紧贴培养基表面,难与培养基表面分离。Monilia yunnanensis 最早发现于云南,为云南的优势种,后来在北京、陕西及辽宁沈阳等部分地区也采集到[10-11,17],而笔者在本研究中首次发现证实在山东省桃果实上也分离得到了Monilia yunnanensis,这也暗示Monilia yunnanensis传播范围在逐步扩大,山东省乃至全国桃种植区褐腐病病原菌的种类日趋多样化,对桃主产区进行褐腐病病原菌的全面分离鉴定对其防控是十分必要的。
41 株桃褐腐病病原菌菌株的菌丝生长速率也存在较大差异,菌丝生长速率从0.47~1.09 cm·d-1不等,聚类分析证实,生长速率可被划分为慢、中、快三大类,即使同一菌种不同菌株间菌丝生长速率也存在差异,这些差异或与地理气候特点有关,或是菌株适应了当地的气候条件被赋予了一定的生物学特性。这暗示褐腐病病原菌存在丰富的生理生化多样性,而这也为该病的防控带来了一定的困难[24]。同时,菌丝生长速率的差异暗示可能在侵染寄主时存在致病力的差异,在对致病力进行测定时发现41 株桃褐腐病病原菌确实在致病力方面存在较大差异,而致病力差异与菌丝生长速率差异类似,在不同菌种间不存在特异性。对其菌丝生长速率及致病力进行相关性分析后证实二者无相关性,这也暗示在实际侵染寄主时菌丝扩展速率并不能与致病力及危害性直接相关。41 株桃褐腐病病原菌的产孢量也存在较大差异,研究证实真菌的致病毒素在桃树发病过程中发挥重要作用[27],而毒素可由孢子萌发产生,产孢量多意味着产生毒素多,对寄主植物的致病力越强[28],但本研究中证实不同桃褐腐病病原菌的产孢量与致病力无相关性。致病力差异还与多种因素有关,例如细胞壁降解酶活性及主要致病因子多聚半乳糖醛酸酶基因PG1 与桃褐腐病病原菌致病性密切相关[23]。因此,在不同桃褐腐病病原菌致病力差异机制方面应进行更深入全面的研究。
笔者在本研究中对山东桃褐腐病病原菌种类进行了较全面的鉴定及抗病性分析,初步确定了桃褐腐病病原菌的种类及占比。但仍存在部分地区菌株数较少的限制,今后有必要扩大和增加不同产区褐腐病病原菌菌株数量,进行更多地区桃褐腐病病原菌的生物学特性研究,并结合分子生物学研究,对其致病力机制进行深入探索,完善不同产区病菌群体的多样性分析,为不同产区桃褐腐病病原菌的有效防控提供理论基础。
4 结论
引起山东省桃褐腐病病原菌有美澳型核果链核盘菌(Monilinia fructicola)、云南链核盘菌(Monilia yunnanensis)及多子座链核盘菌(Monilia polystroma),三者占比分别为80.48%、9.76%、9.76%,其中美澳型核果链核盘菌为优势种。3种桃褐腐病病原菌的菌丝生长速率、产孢量均与致病力无相关性。
[1] 王力荣.我国桃产业现状与发展建议[J].中国果树,2021(10):1-5.WANG Lirong. Current situation and development suggestions of peach industry in China[J].China Fruits,2021(10):1-5.
[2] 纪兆林,谈彬,朱薇,董京萍,朱峰,徐敬友,童蕴慧.我国不同产区桃褐腐病病原鉴定与分析[J]. 微生物学通报,2019,46(4):869-878.JI Zhaolin,TAN Bin,ZHU Wei,DONG Jingping,ZHU Feng,XU Jingyou,TONG Yunhui. Identification and analysis of the peach brown rot pathogens from different peach-growing areas in China[J].Microbiology China,2019,46(4):869-878.
[3] 尹良芬.中国核果/仁果类果树褐腐病菌种群结构及Cyt b 基因的遗传进化研究[D].武汉:华中农业大学,2015.YIN Liangfen.Characterization of populations of Monilinia spp.on stone and pome fruits in China and genetic evolution of the Cyt b genes[D]. Wuhan:Huazhong Agricultural University,2015.
[4] 李世访,陈策.桃褐腐病的发生和防治[J].植物保护,2009,35(2):134-139.LI Shifang,CHEN Ce. Incidence and management of the peach fruit brown rot[J].Plant Protection,2009,35(2):134-139.
[5] 朱小琼,段维军,胡勐郡,国立耘.核果和仁果褐腐病病原菌研究进展[J].菌物学报,2022,41(3):331-348.ZHU Xiaoqiong,DUAN Weijun,HU Mengjun,GUO Liyun. Research progress on brown rot pathogens of stone and pome fruit[J].Mycosystema,2022,41(3):331-348.
[6] TRAN T T,LI H,NGUYEN D Q,SIVASITHAMPARAM K,JONES M G K,WYLIE S J. Spatial distribution of Monilinia fructicola and M.laxa in stone fruit production areas in Western Australia[J].Australasian Plant Pthology,2017,46(3):339-349.
[7] MA Y F,HUANG L D,ABUDUAINI A,ZHOU H Y,WANG Y B,SUO F Y. Complete mitochondrial genome of plant pathogen Monilinia fructicola (Sclerotiniaceae,Helotiales)[J]. Mitochondrial DNA Part B,2019,4(1):791-792.
[8] HARADA Y,NAKAO S,SASAKI M,SASAKI Y,ICHIBASHI Y,SANO T. Monilia mumecola,a new brown rot fungus on Prunus mume in Japan[J]. Journal of General Plant Pathology,2004,70(6):297-307.
[9] VAN LEEUWEN G C M,BAAYEN R P,HOLB I J,JEGER M J. Distinction of the Asiatic brown rot fungus Monilia polystroma sp.nov.from M.fructigena[J].Mycological Research,2002,106(4):444-451.
[10] HU M J,COX K D,SCHNABEL G,LUO C X. Monilinia species causing brown rot of peach in China[J/OL]. PLoS One,2011,6(9):e24990.doi:10.1371/journal.pone.0024990
[11] YIN L F,CHEN S N,CHEN G K,SCHNABEL G,DU S F,CHEN C,LI G Q,LUO C X. Identification and characterization of three Monilinia species from plum in China[J].Plant Disease,2015,99(12):1775-1783.
[12] CHEN F,LIU X,CHEN S,SCHNABEL E,SCHNABEL G.Characterization of Monilinia fructicola strains resistant to both propiconazole and boscalid[J].Plant Disease,2013,97(5):645-651.
[13] ZHU X Q,CHEN X Y,LUO Y,GUO L Y. First report of Monilinia fructicola on peach and nectarine in China[J].Plant Pathology,2010,54(4):575-575.
[14] 樊锦艳,朱小琼,国立耘,骆勇.褐腐病菌三种分子鉴定方法的比较[J].植物保护学报,2007,34(3):289-295.FAN Jinyan,ZHU Xiaoqiong,GUO Liyun,LUO Yong.Comparison of three molecular identification methods for Monilinia spp. on stone and pome fruit[J]. Journal of Plant Protection,2007,34(3):289-295.
[15] 郝晓娟,周芳,李新凤,王美琴,王建明,李俊林.山西省欧李褐腐病病原菌鉴定[J].西北农业学报,2015,24(4):101-104.HAO Xiaojuan,ZHOU Fang,LI Xinfeng,WANG Meiqin,WANG Jianming,LI Junlin. Pathogen identification of brown rot of Cerasus humilis (Bge) Sok,in Shanxi[J]. Acta Agriculturae Boreali-Occidentalis Sinica,2015,24(4):101-104.
[16] 罗朝喜.果树褐腐病的研究现状及其展望[J].植物病理学报,2017,47(2):145-153.LUO Chaoxi. Advances and prospects on researches of brown rot disease on fruits[J]. Acta Phytopathologica Sinica,2017,47(2):145-153.
[17] ZHAO Y Z,WANG D,LIU Z H. First report of brown rot on Crataegus pinnatifida var. major caused by Monilia yunnanensis in China[J].Plant Disease,2013,97(9):1249-1249.
[18] SONODA R M,OGAWA J M,MANJI B T. Use of interactions of cultures to distinguish Monilinia laxa from Monilinia fructicola[J].Plant Disease,1982,66(4):325-326.
[19] LANE C R.A synoptic key for differentiation of Monilinia fructicola,M. fructigena and M. laxa,based on examination of cultural characters[J].EPPO Bulletin,2002,32(3):489-493.
[20] IOOS R,FREY P. Genomic variation within Monilinia laxa,M. fructigena and M. fructicola,and application to species identification by PCR[J]. European Journal of Plant Pathology,2000,106(4):373-378.
[21] MA Z,LUO Y,MICHAILIDES T J. Nested PCR assays for detection of Monilinia fructicola in stone fruit orchards and Botryosphaeria dothidea from Pistachios in California[J]. Journal of Phytopathology,2003,151(6):312-322.
[22] CỘTÉ M J,PRUD′HOMME M,MELDRUM A J,TARDIF M C.Variations in sequence and occurrence of SSU rDNA group I introns in Monilinia fructicola isolates[J]. Mycologia,2004,96(2):240-248.
[23] 谈彬.桃褐腐病病原鉴定与多样性研究及其PG 基因的克隆与表达[D].扬州:扬州大学,2019.TAN Bin. Identification and diversity of peach brown rot pathogens and cloning and expression of PG gene[D]. Yangzhou:Yangzhou University,2019.
[24] 纪兆林,朱薇,谈彬,董京萍,徐敬友,宋宏峰.我国不同产区桃褐腐病菌生物学特性研究[J].中国南方果树,2018,47(5):101-107.JI Zhaolin,ZHU Wei,TAN Bin,DONG Jingping,XU Jingyou,SONG Hongfeng. Study on biological characteristics of peach brown rot pathogen in different production areas in China[J].South China Fruits,2018,47(5):101-107.
[25] 周芳.山西省褐腐病菌种群结构及致病力研究[D].太谷:山西农业大学,2015.ZHOU Fang. Population structure and pathogenicity of Monilinia in Shanxi province[D].Taigu:Shanxi Agricultural University,2015.
[26] 彭福田. 山东省桃产业存在问题与对策建议[J]. 落叶果树,2019,51(2):1-3.PENG Futian. Problems and countermeasures of peach industry in Shandong province[J].Deciduous Fruits,2019,51(2):1-3.
[27] GARCIA-BENITEZ C,MELGAREJO P,SANDIN-ESPANA P,SEVILLA-MORAN B,DE CAL A. Degrading enzymes and phytotoxins in Monilinia spp. [J]. European Journal of Plant Pathology,2019,154(2):305-318.
[28] WANG C X,LI C,LI B H,LI G F,DONG X L,WANG G P,ZHANG Q M. Toxins produced by Valsa mali var. mali and their relationship with pathogenicity[J]. Toxins,2014,6(3):1139-1154.