温州蜜柑为细胞质雄性不育类型[1],果实无核,易剥皮,品质佳,易栽培,是中国很多柑橘产区重要的主栽品种。国庆1号温州蜜柑是华中农业大学早期从龟井温州蜜柑芽变选出的早熟品种。在生产中,为保持品种特性,柑橘一般采用无性繁殖,但长期无性繁殖会在其体内积累一种或多种病毒、类病毒及细菌性病害[2],导致出现一定程度的品种退化,逐渐表现出生活力降低、产量下降和品质衰退等现象,急需进行提纯复壮和品种改良。由于大多数病毒病不能通过种子传播,柑橘珠心胚苗能脱除大多数病毒,通常表现为生长势比亲本更旺盛,从而达到提纯复壮的目的[3-4]。
基于珠心系实生变异选择育种是柑橘的重要育种途径。日本育种家利用珠心系变异选育出大批熟期、果型、果皮色泽各异的温州蜜柑新品种,如兴津早生、三保早生等品种就是利用温州蜜柑的多胚性,从宫川温州蜜柑珠心系选育而来[3,5]。黄秀等[6]以由良温州蜜柑为母本与鸡尾葡萄柚等4个品种有性杂交,获得温州蜜柑种子用于珠心苗培育,以期选育符合市场需求的新品种。笔者在本研究中以国庆1号温州蜜柑为材料,采用离体播种方法培育珠心胚苗,以期为国庆1 号温州蜜柑提纯复壮、珠心胚变异发掘和相关基础研究提供珍贵的种质材料。
1 材料和方法
1.1 研究材料
国庆1 号温州蜜柑(Citrus unshiu Marc.,简称G1)嫁接于枳砧并定植于华中农业大学柑橘育种试验基地,其周围栽植有华农本地早橘等品种,树龄均10 a(年)以上。2021年1月因低温冻害影响,国庆1号温州蜜柑果实受冻而不可食用,全部采摘用于收集种子。
1.2 种子离体培养
将饱满种子置于1 mol·L-1 NaOH 溶液中浸泡15 min 去除果胶,用自来水冲洗干净后剥去外种皮;无菌条件下,将去除外种皮的种子置于3%(φ)NaClO 溶液中浸泡消毒15 min;用无菌蒸馏水清洗至少3次后,将种子转移至灭菌的三角瓶;用镊子去除内种皮后,将其接种于播种培养基(MT+25 g·L-1蔗糖,pH=5.8)并置于培养室光照培养。培养室条件:温度(25±1)℃;光照16 h。
1.3 倍性鉴定
待幼苗长至有3~5 枚真叶大小时,用流式细胞仪和根尖染色体压片2种方法检测植株倍性。流式细胞仪(Cyflow space,Sysmex,Japan)倍性检测参考解凯东等[7]的方法。根尖染色体压片参考夏强明[8]的方法并适当修改。取生长旺盛的根尖,饱和对二氯苯溶液室温下预处理3 h后,用新鲜的卡诺固定液(V 乙醇∶V 乙酸=3∶1)固定24 h,最后转移至75%乙醇溶液4 ℃保存备用。制片前,根尖用酶液(1%果胶酶(Sigma-aldrich)、2%纤维素酶Onozuka(R-10,Yakult)和1%果胶酶(Y-23,Yakult))37 ℃水浴处理90 min后,用火焰干燥法进行染色体制片,并用荧光显微镜(Imager.M2,Zeiss,Germany)镜检,染色体图像用ZEN软件采集。
四倍体群体发生频率/%=四倍体植株数/群体植株总数×100。
1.4 植株移栽
将倍性分析后的所有植株移栽至装有营养土(V 进口泥炭土∶V 基质土∶V 蛭石∶V 珍珠岩=4∶4∶1∶1)的营养钵并置于生长室炼苗。待幼苗长至7~8 枚真叶大小时,将其转移至温室,期间保证正常肥水管理。生长室条件:温度(25±1)℃;光照16 h。
1.5 SSR分子鉴定
基因组DNA 提取和SSR 分子标记分别参考Cheng等[9]和谢善鹏等[10]的方法。筛选的4对多态性SSR 引物(表1)由生工生物工程(上海)股份有限公司合成。PCR反应体系10 μL:2×PCR mix 5 μL,正、反向引物各0.25 μL(10 μmol·L-1),DNA 模板1 μL,无菌水3.5 μL。PCR 反应在ProFlex PCR 仪(ABI,USA)进行,扩增程序:95 ℃预变性5 min,95 ℃变性30 s,55 ℃退火30 s,72 ℃延伸10 s,35 个循环,72 ℃延伸5 min,4 ℃保存。PCR 产物由全自动毛细管电泳系统(QIAxcel Advanced,QIAGEN)电泳分离。
表1 SSR 引物序列
Table 1 Sequence of SSR primers
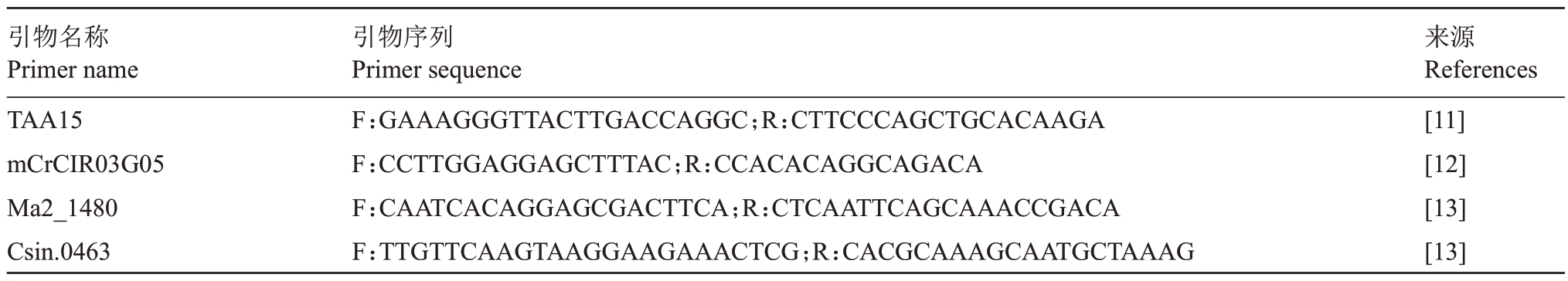
引物名称Primer name TAA15 mCrCIR03G05 Ma2_1480 Csin.0463引物序列Primer sequence F:GAAAGGGTTACTTGACCAGGC;R:CTTCCCAGCTGCACAAGA F:CCTTGGAGGAGCTTTAC;R:CCACACAGGCAGACA F:CAATCACAGGAGCGACTTCA;R:CTCAATTCAGCAAACCGACA F:TTGTTCAAGTAAGGAAGAAACTCG;R:CACGCAAAGCAATGCTAAAG来源References[11][12][13][13]
2 结果与分析
2.1 离体实生播种获得62株国庆1号温州蜜柑幼苗
温州蜜柑一般表现为雄性不育、果实无核,种子极少见;但若花期遇高温,育性能部分恢复,成熟果实可能偶尔有少量种子。基于该现象,笔者从国庆1 号温州蜜柑约12500 个成熟果实中,剥取获得106 粒种子(图1-A),其中80 粒为饱籽,26 粒为瘪籽(图1-B)。对所有种子离体播种培养,由于部分饱满种子污染和瘪籽未能萌发,最终有41 粒饱满种子萌发,萌发率为38.7%;经培养获得再生实生幼苗62 株,平均每粒种子再生植株1.5 株(图1-C~D)。

图1 国庆1 号温州蜜柑种子及实生幼苗形态
Fig.1 Morphology of seeds and seedlings from Satsuma mandarin Guoqing No.1
2.2 倍性分析发掘1株国庆1号温州蜜柑四倍体植株
用流式细胞仪对所有62 株实生幼苗进行倍性分析,其中61株为二倍体(图2-A),发掘获得1株四倍体植株(图2-B),四倍体发生频率1.61%。进一步用根尖压片法对获得的四倍体进行染色体数目分析,表明其染色体数为36 条(图2-D),是二倍体(图2-C)染色体数目的2 倍,验证了流式细胞仪倍性分析结果的可靠性。
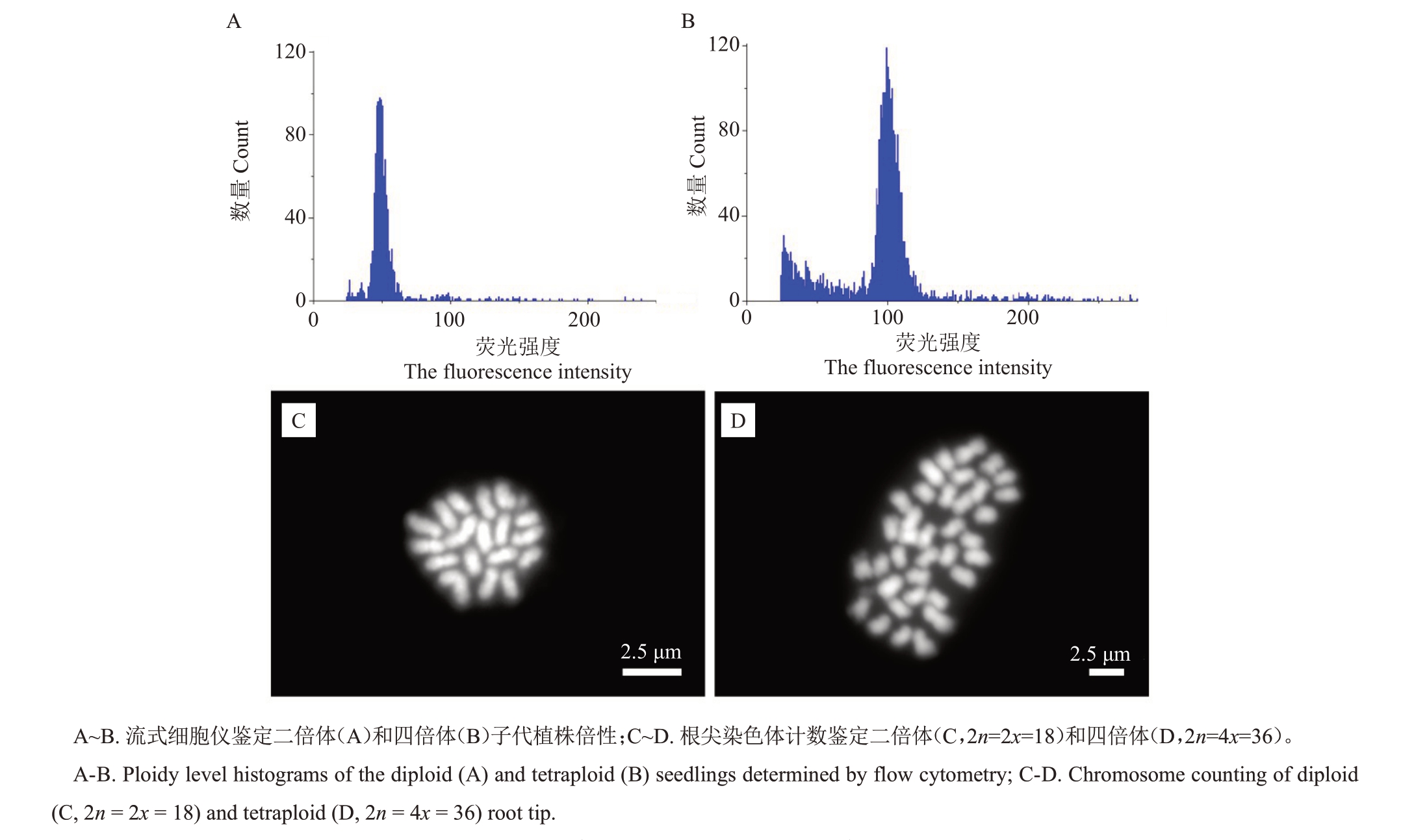
图2 国庆1 号温州蜜柑实生幼苗倍性分析
Fig.2 Ploidy level analysis of the regenerated seedlings from Satsuma mandarin Guoqing No.1 using flow cytometry and shoot tip chromosome counting
2.3 SSR分子鉴定
将倍性分析后的所有实生幼苗移栽至温室。当幼苗长至一定大小后,选取4 对扩增效果好的多态性SSR分子标记对随机选取的38株二倍体植株和1株四倍体植株进行遗传鉴定(图3)。结果表明,四倍体植株带型与其亲本国庆1号温州蜜柑完全一致(图3-T4),表明该四倍体可能由国庆1 号温州蜜柑珠心细胞自然加倍形成,为双二倍体;而38 株二倍体植株中,35 株的条带与国庆1 号温州蜜柑完全一致,推测由国庆1号温州蜜柑珠心胚发育而来;其余3 株二倍体植株(I1、O2、S3)与国庆1 号温州蜜柑条带不一致,为合子胚来源,可能为国庆1号温州蜜柑与其附近其他柑橘品种的天然有性后代。

图3 国庆1 号温州蜜柑实生幼苗SSR 分子鉴定
Fig.3 SSR profiles of the seedlings of Satsuma mandarin Guoqing No.1
3 讨论
笔者在本研究中采用成熟种子离体播种,结合倍性分析和SSR 分子鉴定,发掘出1 株国庆1 号温州蜜柑四倍体植株;随机挑选的38株二倍体实生幼苗,3株为国庆1号温州蜜柑有性胚苗,其余35株为珠心胚苗。这些种质材料将助力国庆1号温州蜜柑提纯复壮、珠心胚变异育种及相关基础研究。
温州蜜柑一般表现为雄性不育、果实无核;但若对其授粉或花期遇高温(育性会部分恢复),其果实偶尔会产生种子。如黄秀等[6]发现自然条件下,由良温州蜜柑果实基本无核,而用其他品种的花粉对其人工授粉,可获得少量种子(单果种子数介于0.36~1.66 粒之间);邓秀新等[14]对移入25 ℃温室的国庆1 号温州蜜柑花粉育性进行统计,与对照相比表现出育性恢复现象,笔者在本研究中从约12500个国庆1 号温州蜜柑成熟果实获得106 粒种子的结果与前人研究基本一致。推测国庆1号温州蜜柑种子可能由其与周围栽植的华农本地早橘等其他品种异花授粉形成或由于其花粉育性部分恢复后自交形成。笔者在本研究中离体播种了80 粒饱籽和26 粒瘪籽,由于操作不当,部分饱籽污染,仅41粒饱籽萌发;而瘪籽均未萌发,可能与瘪籽营养不足或低温受冻导致种子活力下降等有关。
笔者采用4 对SSR 引物对发掘的1 株四倍体和38株二倍体实生幼苗进行遗传鉴定,发现四倍体带型与其二倍体亲本完全一致,与前人研究一致[15-17],推测其由国庆1 号温州蜜柑珠心细胞自然加倍形成。王江波等[18]用ISSR 分子标记对由种子萌发来的7 株芦柑实生苗进行遗传鉴定,其与母本条带完全一致均为珠心苗。笔者在本研究中随机挑选的38株二倍体植株中,35株二倍体植株条带与母本完全一致,为珠心胚苗;其余3株二倍体植株遗传背景与国庆1号温州蜜柑不一致,推测其为国庆1号温州蜜柑与周围其他柑橘品种异花授粉形成的有性胚苗。日本科学家最近报道宫川温州蜜柑四倍体与二倍体正反杂交时,均能产生饱满种子和三倍体后代,说明宫川温州蜜柑四倍体育性恢复,可以用作育种亲本[17]。笔者在本研究中发掘的国庆1号温州蜜柑四倍体,其开花结果习性有待观察评价,国庆1号温州蜜柑四倍体为细胞质雄性不育等相关基础研究和多倍体育种提供了珍贵的种质材料;培育的国庆1号温州蜜柑珠心胚苗将有助于该品种提纯复壮和二倍体水平珠心苗变异发掘。
[1] YAMAMOTO M,MATSUMOTO R,OKUDAI N,YAMADA Y. Aborted anthers of Citrus result from gene-cytoplasmic male sterility[J]. Scientia Horticulturae,1997,70(1):9-14.
[2] WANG Q C,VALKONEN J P T.Cryotherapy of shoot tips:Novel pathogen eradication method[J]. Trends in Plant Science,2008,14(3):119-122.
[3] 徐建国. 温州蜜柑在柑桔育种中的应用[J]. 浙江柑桔,1995(4):12-14.XU Jianguo. Application of Satsuma mandarin in citrus breeding[J]. Zhejiang Citrus,1995(4):12-14.
[4] SPIELMAN M,VINKENOOG R,SCOTT R J. Genetic mechanisms of apomixis[J]. Philosophical Transactions of the Royal Society of London. Series B:Biological Sciences,2003,358(1434):1095-1103.
[5] OMURA M,SHIMADA T.Citrus breeding,genetics and genomics in Japan[J].Breeding Science,2016,66(1):3-17.
[6] 黄秀,柯甫志,孙立方,王平,聂振朋,徐建国,孙建华.不同花粉授粉对温州蜜柑种子形成的影响[J].浙江农业科学,2021,62(11):2207-2210.HUANG Xiu,KE Fuzhi,SUN Lifang,WANG Ping,NIE Zhenpeng,XU Jianguo,SUN Jianhua. Effects of different pollen pollination on seed formation of Satsuma mandarin[J]. Journal of Zhejiang Agricultural Sciences,2021,62(11):2207-2210.
[7] 解凯东,彭珺,袁东亚,强瑞瑞,谢善鹏,周锐,夏强明,伍小萌,柯甫志,刘高平,GROSSER J W,郭文武.以本地早橘和槾橘为母本倍性杂交创制柑橘三倍体[J].中国农业科学,2020,53(23):4961-4968.XIE Kaidong,PENG Jun,YUAN Dongya,QIANG Ruirui,XIE Shanpeng,ZHOU Rui,XIA Qiangming,WU Xiaomeng,KE Fuzhi,LIU Gaoping,GROSSER J W,GUO Wenwu.Production of citrus triploids based on interploidy crossing with Bendizao and Man tangerines as female parents[J]. Scientia Agricultura Sinica,2020,53(23):4961-4968.
[8] 夏强明.基于2n 雌配子有性群体定位柑橘着丝粒及其序列特征分析[D].武汉:华中农业大学,2020.XIA Qiangming. Localization and sequence analysis of citruscentromeres based on a sexual population derived from 2n megagametophytes[D]. Wuhan:Huazhong Agricultural University,2020.
[9] CHENG Y J,GUO W W,YI H L,PANG X M,DENG X X.An efficient protocol for genomic DNA extraction from Citrus species[J].Plant Molecular Biology Reporter,2003,21(2):177-178.
[10] 谢善鹏,解凯东,夏强明,周锐,张成磊,郑浩,伍小萌,郭文武.柑橘6 个地方品种资源四倍体高效发掘及分子鉴定[J].果树学报,2022,39(1):1-9.XIE Shanpeng,XIE Kaidong,XIA Qiangming,ZHOU Rui,ZHENG Chenglei,ZHENG Hao,WU Xiaomeng,GUO Wenwu.Efficient exploration and SSR identification of 53 doubled diploid seedlings from six local Citrus cultivars and germplasm resources[J].Journal of Fruit Science,2022,39(1):1-9.
[11] KIJAS J M H,THOMAS M R,FOWLER J C S,ROOSE M L.Integration of trinucleotide microsatellites into a linkage map of Citrus[J].Theoretical and Applied Genetics,1997,94(5):701-706.
[12] CUENCA J,FROELICHER Y,ALEZA P,JUÁREZ J,NAVARRO L,OLLITRAULT P.Multilocus half-tetrad analysis and centromere mapping in citrus: Evidence of SDR mechanism for 2n megagametophyte production and partial chiasma interference in mandarin‘Fortune’[J].Heredity,2011,107(5):462-470.
[13] XU Q,CHEN L L,RUAN X A,CHEN D J,ZHU A D,CHEN C L,BERTRAND D,JIAO W B,HAO B H,LYON M P,CHEN JJ,GAO S,XING F,LAN H,CHANG J W,GE X H,LEI Y,HU Q,MIAO Y,WANG L,XIAO S X,BISWAS M K,ZENG W F,GUO F,CAO H B,YANG X M,XU X W,CHENG Y J,XU J,LIU J H,LUO O J,TANG Z H,GUO W W,KUANG H H,ZHANG H Y,ROOSE M L,NAGARAJAN N,DENG X X,RUAN Y J. The draft genome of sweet orange (Citrus sinensis)[J].Nature Genetics,2013,45(1):59-66.
[14] 邓秀新,章文才,叶文明.温州蜜柑雄性不育性的恢复研究[J].浙江柑桔,1987(3):5-7.DENG Xiuxin,ZHANG Wencai,YE Wenming. Application of Satsuma mandarin in citrus breeding[J]. Zhejiang Citrus,1987(3):5-7.
[15] 洪柳,刘永忠,邓秀新.椪柑成熟种子胚培养获得四倍体植株[J].园艺学报,2005,32(4):688-690.HONG Liu,LIU Yongzhong,DENG Xiuxin.Obtaining of Ponkan (Citrus reticulate Blanco) tetraploid by culturing embryos of mature seeds[J].Acta Horticulturae Sinica,2005,32(4):688-690.
[16] ALEZA P,FROELICHER Y,SCHWARZ S,AGUSTÍ M,HERNÁNDEZ M,JUÁREZ J,LURO F,MORILLON R,NAVARRO L,OLLITRAULT P. Tetraploidization events by chromosome doubling of nucellar cells are frequent in apomictic citrus and are dependent on genotype and environment[J]. Annals of Botany,2011,108(1):37-50.
[17] SUDO M,YASUDA K,YAHATA M,SATO M,TOMINAGAA,MUKAI H,MA G,KATO M,KUNITAKE H. Morphological characteristics,fruit qualities and evaluation of reproductive functions in autotetraploid Satsuma mandarin (Citrus unshiu Marcow)[J].Agronomy,2021,11(12):2441.
[18] 王江波,潘东明,钟凤林,郭志雄,陈跃飞,尤有利.ISSR 分子标记在芦柑提纯复壮研究中的应用[J]. 亚热带农业研究,2010,6(1):52-55.WANG Jiangbo,PAN Dongming,ZHONG Fenglin,GUO Zhi xiong,CHEN Yuefei,YOU Youli. ISSR molecular marker and its application to the research on purification and rejuvenation of citrus[J].Subtropical Agriculture Research,2010,6(1):52-55.