葡萄(Vitis vinifera L.)是一种富含各类维生素、膳食纤维等较高营养物质的浆果类水果[1],在世界五大洲均有种植,2018年全球葡萄总产量达7780万t,其中鲜食葡萄占36%,而中国鲜食葡萄产量已位居世界首位[2]。在植物的新陈代谢中,铁是植物生长必需的微量元素之一,参与叶绿素及呼吸酶的合成、氮代谢、活性氧代谢等[3]过程。褪黑素(N-乙酰基-5-甲氧基色胺,Melatonin,MT)是色氨酸吲哚类衍生物,为新型植物生长调节剂[4]。然而不合理的施肥方式、栽培管理措施导致设施土壤盐渍化[5-6],许多的微量元素不能被足量吸收,不能满足作物生长的需求,导致植物发生缺铁性失绿症,影响植物正常生长及发育[7-8]。近年来,外源供给能够提高植物细胞的抗逆性并缓解逆境胁迫,这已成为全球农业研究的热点[9]。探索分析其他领域中的外源供给物质和使用剂量及在葡萄试管苗缺铁胁迫上的生长表现和生理响应,为葡萄抗逆境胁迫的外源供给物质库添砖加瓦,成为研究的指南针。目前研究调控植物逆境的外源物质众多[10]。如:Noor 等[11]研究的茉莉酸酯等在短生长周期作物的抗逆境生物学响应,Mfarrej等[12]研究了外源NO和H2S对小麦内涝的缓解作用,外源曲霉提高多年生黑麦草的耐旱性和耐热性[13]及外源邻苯二甲酸对西瓜种子萌发、根系生理特性及矿质元素吸收的影响[14]等。但是在果树的抗逆境研究中外源物质的使用较少,存在某外源物质对提高葡萄的逆境抗性不明显。油菜素内酯(BR)、水杨酸(SA)、NO、外源硅、外源Ca2+和MT能够通过提高叶片中叶绿素的含量,降低作物叶片的黄化面积,促进抗氧化酶[15](SOD 等)的活性,降低超氧阴离子累积效应的损伤程度,促进葡萄果实中可溶性糖和可溶性蛋白的合成[16],提高在氨基酸上的代谢、合成水平,协助作物提高渗透调节物质(Pro、SS和SP)含量以抵御逆境,有效缓解作物(华山松[17]、番茄[18]、小白菜[19]、黄瓜[20-21]、葡萄[16,22-23]等)缺铁黄化症状,减轻逆境对植株生长的伤害,但未见明确描述外源MT 对葡萄试管苗缺铁逆境的响应。缺铁胁迫下植株会表现出叶片黄化的现象,影响单株的生物合成量及长势,外源MT 处理可以有效缓解许多作物的胁迫症状,使作物能够抵抗逆境并短时间脱离盐害或缺素环境。然而在葡萄试管苗的缺铁胁迫研究中,并未明确外源褪黑素对植株的生长表现影响和生理响应如何。笔者在本试验中以实验室组培苗繁殖技术提供的克瑞森无核葡萄试管苗为材料,在缺铁胁迫下添加外源褪黑素,观察各处理葡萄的生长情况,并从生理层面上研究褪黑素对缺铁胁迫的缓解效应,确定外源褪黑素对缺铁胁迫下的葡萄缓解效果是否显著,以期增添农业生产中葡萄抗缺铁胁迫调控的外源物质种类。
1 材料和方法
1.1 试验材料
试验材料为继代保存的克瑞森无核葡萄试管苗,采用改良B5 固体培养基扩繁,选择长势均匀且生长健壮的试管苗,剪成长1.0~1.5 cm的单芽茎段,转接至添加了不同处理的B5 培养基上,置于温度(27±1)℃,光照度3000 lx,光、暗期比2∶1的环境下培养。
1.2 试验处理
在前期试验的基础上,设置铁浓度为30 μmol·L-1(轻度胁迫),外源褪黑素处理浓度为140 μmol·L-1,试验处理如表1 所示。每个处理转接20 瓶试管苗,共3次重复,总计60瓶。
表1 试验处理
Table 1 Experimental treatments
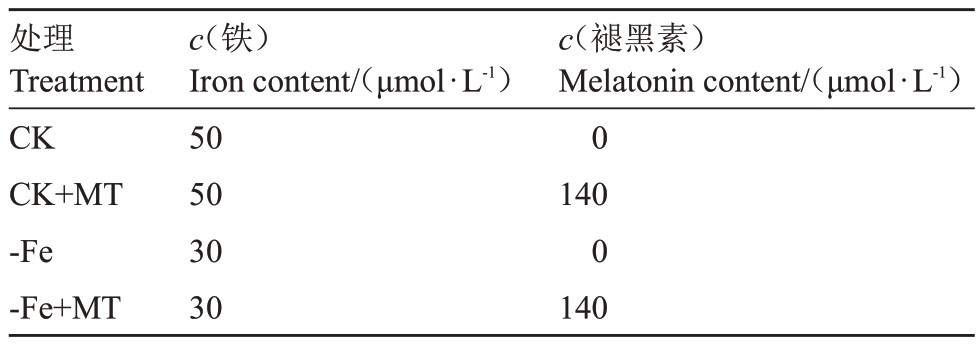
处理Treatment CK CK+MT-Fe-Fe+MT c(铁)Iron content/(μmol·L-1)50503030 c(褪黑素)Melatonin content/(μmol·L-1)01400140
1.3 测定指标与方法
处理后定期观察葡萄长势,接种后50 d时,采样测定相关指标。
将植株从三角瓶中小心取出,测定株高、茎粗、植株干鲜质量、叶面积及根系指标。叶面积和根系指标用Epson 根系扫描仪(STD 4800 型)扫描,用根系分析软件(Win RHIZO 5.0,Canada)进行分析。
叶绿素含量采用95%乙醇浸提法测定,叶片丙二醛含量采用硫代巴比妥酸比色法测定,脯氨酸含量用茚三酮法测定,SOD活性用氮蓝四唑光化学还原法测定,POD活性用愈创木酚法[24]测定;根系活力采用氯化三苯基四氮唑法[25]测定;可溶性蛋白含量采用考马斯亮蓝G-250 染色法[26]测定;可溶性糖含量采用蒽酮法[27]测定;硝酸还原酶活性采用磺胺比色法[28]测定。
1.4 统计分析
数据用IBM SPSS 24软件进行差异显著性分析(Ducan法),用Origin 9.1软件制图。
2 结果与分析
2.1 外源褪黑素对缺铁胁迫下葡萄生长的影响
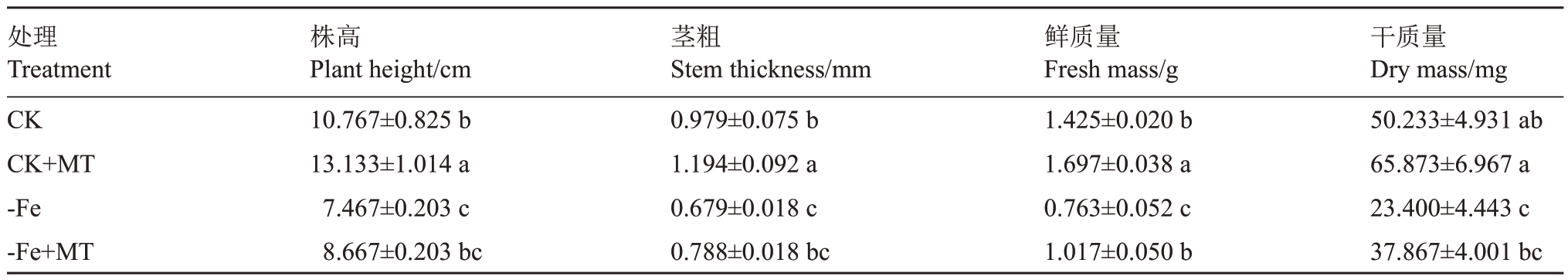
2.1.1 外源褪黑素对缺铁胁迫下葡萄植株形态及生物量的影响 各处理葡萄植株形态如图1所示,CK+MT处理植株叶片鲜绿,茎秆粗壮,长势最强;-Fe处理植株矮小,茎秆纤细,长势最弱,在幼叶部位出现了明显的黄化现象;然而较-Fe处理植株,-Fe+MT处理植株长势略显优势,茎秆较粗,黄叶数量显然减少。由表2可知,试管苗的株高和茎粗,对照组显著高于缺铁处理,但-Fe 和-Fe+MT 处理间差异不显著;CK+MT 株高较CK、-Fe 和-Fe+MT 分别高21.98%、75.88%、51.53%,茎粗分别高21.96%、75.85%、51.52%。全株鲜质量CK+MT较CK高19.09%,差异显著;-Fe+MT较-Fe高33.29%,差异显著;CK和-Fe+MT差异不显著。对照组植株干质量显著大于缺铁处理,-Fe干质量较CK低53.63%,但CK和CK+MT间、-Fe和-Fe+MT间差异不显著。可见,MT对植株生长具有一定促进作用,对于轻度缺铁胁迫下的植株有良好的保护作用,使植株胁迫症状得到缓解。

图1 不同处理葡萄的植株形态
Fig.1 Plant morphology of grapes under different treatments
表2 外源褪黑素对缺铁胁迫下葡萄地上部生长及生物量的影响
Table 2 Effect of exogenous melatonin on above-ground growth and biomass of grapes under iron deficiency stress
注:不同小写字母表示在p <0.05 差异显著。下同。
Note:Different small letters indicate significant difference at p <0.05.The same below.
处理Treatment CK CK+MT-Fe-Fe+MT干质量Dry mass/mg 50.233±4.931 ab 65.873±6.967 a 23.400±4.443 c 37.867±4.001 bc株高Plant height/cm 10.767±0.825 b 13.133±1.014 a 7.467±0.203 c 8.667±0.203 bc茎粗Stem thickness/mm 0.979±0.075 b 1.194±0.092 a 0.679±0.018 c 0.788±0.018 bc鲜质量Fresh mass/g 1.425±0.020 b 1.697±0.038 a 0.763±0.052 c 1.017±0.050 b
2.1.2 外源褪黑素对缺铁胁迫下葡萄根系和叶片生长的影响 由表3 可知,CK+MT 的总根长、平均根系直径、根系表面积、根系投影面积、根系总体积和叶面积显著高于其他处理,平均值最高,-Fe 最低。-Fe+MT 较CK 平均根系直径、根系表面积、根系投影面积、根系总体积和叶面积差异显著,分别下降8.60%、39.84%、42.70%、26.00%和26.80%;-Fe+MT的叶投影面积较-Fe显著上升147.78%。通过分析发现,加入外源MT,对CK 的根系建成和叶面积增加有促进效果,对-Fe地上地下的胁迫症状有缓解作用,保证植株的正常生长。
表3 外源褪黑素对缺铁胁迫下葡萄根系和叶片生长的影响
Table 3 Effect of exogenous melatonin on root and leaf growth of grapes under iron deficiency stress
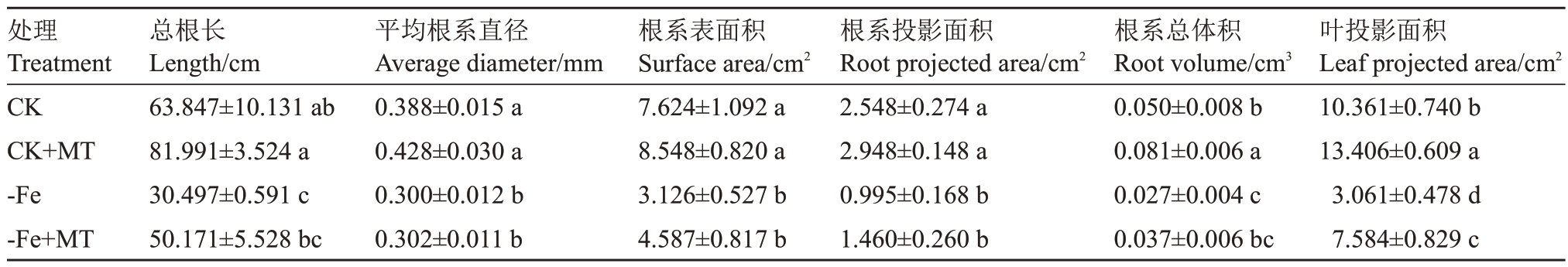
处理Treatment CK CK+MT-Fe-Fe+MT总根长Length/cm 63.847±10.131 ab 81.991±3.524 a 30.497±0.591 c 50.171±5.528 bc平均根系直径Average diameter/mm 0.388±0.015 a 0.428±0.030 a 0.300±0.012 b 0.302±0.011 b根系表面积Surface area/cm2 7.624±1.092 a 8.548±0.820 a 3.126±0.527 b 4.587±0.817 b根系投影面积Root projected area/cm2 2.548±0.274 a 2.948±0.148 a 0.995±0.168 b 1.460±0.260 b根系总体积Root volume/cm3 0.050±0.008 b 0.081±0.006 a 0.027±0.004 c 0.037±0.006 bc叶投影面积Leaf projected area/cm2 10.361±0.740 b 13.406±0.609 a 3.061±0.478 d 7.584±0.829 c
2.2 外源褪黑素对缺铁胁迫下葡萄生理指标的影响
2.2.1 外源褪黑素对缺铁胁迫下葡萄叶绿素含量的影响 叶绿素a、叶绿素b和类胡萝卜素含量,CK和CK+MT 之间、-Fe 和-Fe+MT 及CK 和-Fe+MT 之间差异均显著(表4)。叶绿素a、叶绿素b 和类胡萝卜素含量对比中,CK+MT 较CK 分别上升18.46%、142.94%、16.24%;-Fe+MT 较-Fe 分别上升55.07%、57.14%、63.77%;-Fe+MT 较CK 分别下降28.20%、33.94%、21.35%。而在总叶绿素含量中,CK与CK+MT 之间、-Fe 与-Fe+MT 之间差异不显著,-Fe+MT较CK下降了23.37%,差异显著。
表4 外源褪黑素对缺铁胁迫下葡萄叶绿素含量的影响
Table 4 Effect of exogenous melatonin on chlorophyll content of grapes under iron deficiency stress
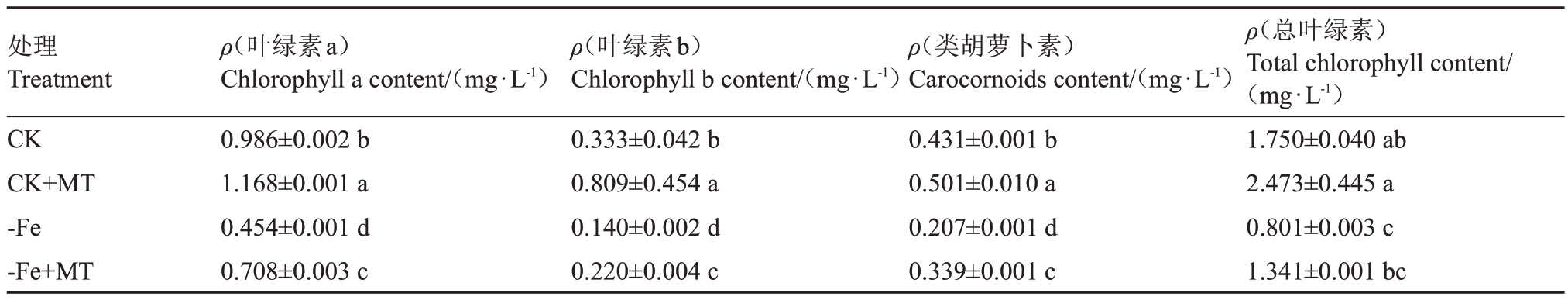
处理Treatment CK CK+MT-Fe-Fe+MT ρ(叶绿素a)Chlorophyll a content/(mg·L-1)0.986±0.002 b 1.168±0.001 a 0.454±0.001 d 0.708±0.003 c ρ(叶绿素b)Chlorophyll b content/(mg·L-1)0.333±0.042 b 0.809±0.454 a 0.140±0.002 d 0.220±0.004 c ρ(类胡萝卜素)Carocornoids content/(mg·L-1)0.431±0.001 b 0.501±0.010 a 0.207±0.001 d 0.339±0.001 c ρ(总叶绿素)Total chlorophyll content/(mg·L-1)1.750±0.040 ab 2.473±0.445 a 0.801±0.003 c 1.341±0.001 bc
2.2.2 外源褪黑素对缺铁胁迫下葡萄MDA 和渗透调节物质含量的影响 由表5 可知,-Fe 处理的MDA、Pro、可溶性糖和可溶性蛋白含量明显高于其他处理。在CK 与CK+MT 和-Fe 与-Fe+MT 之间,MDA、可溶性糖和可溶性蛋白含量差异不显著,-Fe+MT 较CK 分别上升9.68%、3.08%、9.38%,差异显著;CK+MT脯氨酸含量较CK显著下降36.56%,-Fe 较CK 显著上升29.37%。综上可知,MDA、Pro、可溶性糖和可溶性蛋白含量由高到低均为:CK+MT>-Fe>-Fe+MT>CK。
表5 外源褪黑素对缺铁胁迫下葡萄MDA 和渗透调节物质含量的影响
Table 5 Effect of exogenous melatonin on the content of MDA and osmoregulatory substances in grapes under iron deficiency stress deficiency stress
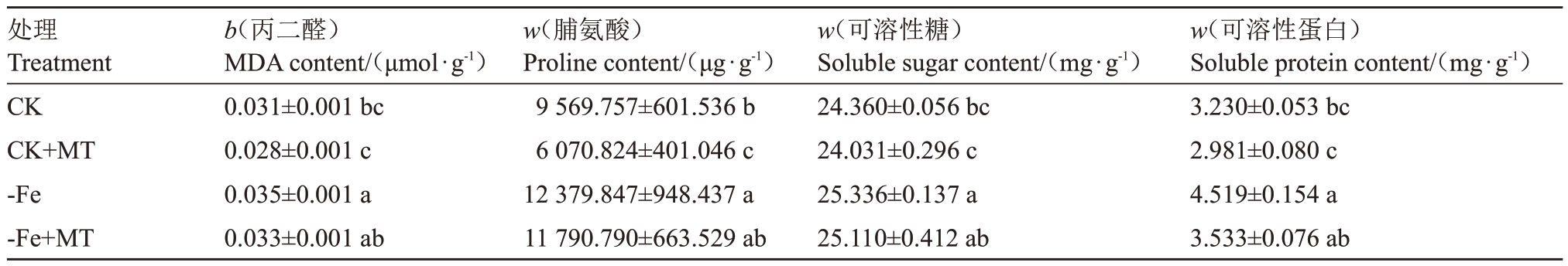
处理Treatment CK CK+MT-Fe-Fe+MT b(丙二醛)MDA content/(μmol·g-1)0.031±0.001 bc 0.028±0.001 c 0.035±0.001 a 0.033±0.001 ab w(脯氨酸)Proline content/(μg·g-1)9569.757±601.536 b 6070.824±401.046 c 12379.847±948.437 a 11790.790±663.529 ab w(可溶性糖)Soluble sugar content/(mg·g-1)24.360±0.056 bc 24.031±0.296 c 25.336±0.137 a 25.110±0.412 ab w(可溶性蛋白)Soluble protein content/(mg·g-1)3.230±0.053 bc 2.981±0.080 c 4.519±0.154 a 3.533±0.076 ab
2.2.3 外源褪黑素对缺铁胁迫下葡萄叶片酶活性和根系活力的影响 从表6 可知,-Fe 与-Fe+MT、CK与CK+MT 的叶片SOD 活性差异不显著,CK+MT较-Fe 的下降5.12%,差异显著(p<0.05);CK 与CK+MT 的POD 活性差异不显著,-Fe 较-Fe+MT 的显著下降36.56%。-Fe 较-Fe+MT 和CK 较CK+MT的根系活力差异显著,其中CK+MT 的根系活力值最高,-Fe的最低,CK与CK+MT较-Fe与-Fe+MT的都高。-Fe的NR活性最低,较CK、CK+MT和-Fe+MT呈现显著下降的趋势,分别下降了47.51%、53.2%和21.49%;在MT处理后,CK+MT与-Fe+MT较CK与-Fe,NR活性整体有明显的上升趋势,同比于未施MT的处理CK与-Fe分别上升了12.15%、27.37%。NR活性由高到低为:CK+MT>CK>-Fe+MT>-Fe。
表6 外源褪黑素对缺铁胁迫下葡萄叶片酶活性和根系活力的影响
Table 6 Effect of exogenous melatonin on enzyme activity and root vigor of grape leaves under iron deficiency stress

处理Treatment CK CK+MT-Fe-Fe+MT SOD活性SOD activity/(U·g-1)244.395±0.346 bc 240.631±2.956 c 253.863±1.366 a 251.607±4.120 ab POD活性POD acitivity/(U·min-1·g-1)407.001±22.904 ab 427.333±32.739 a 209.556±13.844 c 330.333±20.764 b硝酸还原酶活性NR activity/(μg·g-1·h-1)1005.555±14.699 b 1127.778±30.932 a 527.778±33.7932 d 672.222±38.889 c根系活力Root viability/(μg·g-1·h-1)240.468±3.3446 b 294.644±10.085 a 134.018±7.573 d 182.573±6.4074 c
3 讨论
3.1 缺铁胁迫下葡萄响应外源褪黑素处理的生长效应
有研究者发现,在轻度缺铁胁迫下,葡萄顶叶表现失绿或黄白色,光合代谢受到抑制;MT能调节细胞壁延伸的物理过程以诱导植物根系生长,使根系投影面积,总根长和根系总体积增加[29]。本试验中,缺铁胁迫下,葡萄呈现长势不良、株高不均,叶片黄化,茎秆细小,根系发育不良等状况;添加MT后,植株生长状况有较好的恢复,各项指标明显优于缺铁处理。外源褪黑素可能通过参加必需物质的合成途径、调节植物激素的合成量和充当各类酶的辅基,促进蛋白质的表达[30],改善葡萄根系的生长环境,促进正常处理的植株生长,提高葡萄对逆境的抗性。
3.2 缺铁胁迫下葡萄响应外源褪黑素处理的生理效应
植物生长发育的基础是光合作用,叶片是植物光合作用的主要器官,光合色素是评价植株光合特性的重要指标之一,光合色素的含量直接影响光合作用的强弱以及植物的生长[31]。试验表明:在CK与-Fe 均加入外源褪黑素后,叶绿素a、叶绿素b、类胡萝卜素和总叶绿素含量都有明显回升,对-Fe处理的缓解效果最明显,说明外源褪黑素可能会加速受损的叶绿素分解,促进合成吲哚胺延缓叶绿素分解,增强了叶绿素的从头合成[32-34],对缺铁胁迫下葡萄叶片的叶绿素结构有很好的保护作用。
MDA 是细胞膜脂质过氧化程度的重要诊断指标,其含量体现植物对缺铁的反应程度大小[35];而植物遭受逆境胁迫,细胞膜氧化程度升高,细胞的膜透性增强。与徐宁等[4]研究的外源褪黑素对硝酸盐的缓冲机制相似,逆境使细胞内部的氧化能力提高,过氧化物质和超氧离子单位面积含量等急剧升高,机体将会打开防御机制,对逆境进行主动或被动调节,抗氧化防御系统(SOD-POD 抗氧化防御系统)被激活,会引起POD活性下降,SOD活性上升[36],主要清除细胞体内的超氧自由基和过氧化产物[35]。本试验中,缺铁胁迫下,葡萄的SOD活性、Pro和MDA含量较CK 处理显著升高,POD 活性下降;加入外源MT后,POD活性有所上升,膜脂过氧化产物MDA含量和SOD 活性快速下降。说明缺铁胁迫下添加MT,可激活SOD-POD 抗氧化防御系统,减轻盐胁迫下过量的自由基对植物细胞的盐害作用[37] ,提升了葡萄的抗逆性能。
Pro、SP和SS是植物逆境渗透调节的重要物质,反映植物对逆境的抵抗能力。在轻度缺铁胁迫下,CK与-Fe相比,-Fe处理下叶片的渗透调节物质含量显著高于CK,而CK 与-Fe 添加MT 后,能有效降低胁迫处理(-Fe)的Pro、可溶性蛋白和可溶性糖含量,CK 的降幅不大。外源褪黑素可能通过促进氨基酸转运、调节蛋白质和糖类的合成,提高葡萄的抗逆境能力[37],提高了渗透调节的能力,保护细胞膜的完整性[4,38],减少内含物质的丢失,也是为SOD-POD防御机制开启第二道防线,有效地缓解了缺铁胁迫对细胞膜的损伤。
根系活力表示植株的根系细胞活性,即对土壤营养元素的吸收能力[39]。且NR 是一类关于氮元素在植物体内相互转化的活性酶,其作用是催化NO3-转化为NO2-来进行氮代谢的调控[40],其活性反映氮代谢的强弱,活性越强,硝酸盐的累积量就越少,植物氮代谢能力越强。本试验中,-Fe处理的NR活性较低,但较其他处理试管苗的硝酸盐累积量高,-Fe+MT处理NR活性值上升。缺铁胁迫可能导致NR活性值降低,从而降低了植物对硝酸盐的利用率[40],导致植株对氮元素的吸收减少而体内累积硝酸盐,根系的生长和吸收能力受阻,导致植株长势不良。硝酸盐的累积又会导致根系活力的降低,细胞膜结构被破坏,植物体电解质渗透率提高,细胞膜稳定指数越来越低,活性氧代谢加强,MDA含量上升,叶绿素分解加剧,在双胁迫的压力下,植株长势矮小,茎秆纤细。再加入MT 后,由于自身亲水,进入细胞通畅,进一步提高了幼苗叶片中可溶性糖、可溶性蛋白、脯氨酸和游离氨基酸含量,从维持细胞膜结构的稳定和叶片中渗透调节物质的运输能力,同时缓解缺铁胁迫和硝酸盐累积胁迫[4,41]。
4 结论
在轻度缺铁胁迫下,加入MT后,葡萄试管苗根系体积和叶面积增大,植株生长状态良好;叶片光合色素含量、POD 活性、NR 活性和根系活力显著上升,MDA含量、Pro含量、SOD活性、SS含量和SP含量显著下降。140 μmol·L-1 MT 处理能有效缓解葡萄试管苗的缺铁症状,保护植株在生理方面免受伤害,保证植株的正常生长发育。
[1] 关棣锴,聂继云,李志霞,李静,程杨,闫震.葡萄及其制品营养与功能的研究进展[J].保鲜与加工,2017,17(6):142-146.GUAN Dikai,NIE Jiyun,LI Zhixia,LI Jing,CHENG Yang,YAN Zhen. Research progress of nutrition and its function of grape and derived products[J]. Storage and Process,2017,17(6):142-146.
[2] 刘俊,晁无疾,亓桂梅,刘寅喆,汉瑞峰.蓬勃发展的中国葡萄产业[J].中外葡萄与葡萄酒,2020(1):1-8.LIU Jun,CHAO Wuji,QI Guimei,LIU Yinzhe,HAN Ruifeng.Booming development of Chinese grape industry[J]. Sino-Overseas Grapevine&Wine,2020(1):1-8.
[3] GAO F,DUBOS C.Transcriptional integration of plant responses to iron availability[J].Journal of Experimental Botany,2021,72(6):2056-2070.
[4] 徐宁,孙晓慧,曹娜,王闯,李光亚,薛淼云.外源褪黑素对硝酸盐胁迫下番茄幼苗生长及渗透调节物质的影响[J].中国瓜菜,2020,33(9):23-27.XU Ning,SUN Xiaohui,CAO Na,WANG Chuang,LI Guangya,XUE Miaoyun. Effect of exogenous melatonin on tomato seedlings growth and osmotic adjustment substance under nitrate stress[J]. China Cucurbits and Vegetables,2020,33(9):23-27.
[5] 李小艳.设施土壤障碍分析与平衡肥施用效果研究[J].农学学报,2022,12(6):39-43.LI Xiaoyan.Barrier analysis of greenhouse soil and effect of balanced fertilizer application[J]. Journal of Agriculture,2022,12(6):39-43.
[6] 陈智坤.陕西省设施农业土壤环境质量与退化成因研究[D].杨凌:西北农林科技大学,2021.CHEN Zhikun. Study on environmental quality and degradation causes of greenhouse agricultural soil in Shaanxi province[D].Yangling:Northwest A&F University,2021.
[7] 陈亚铎,宋艳红,李刚,赵霞,刘丽锋,周厚成.草莓FaFIT 在拟南芥中异源表达促进根系铁吸收[J]. 果树学报,2022,39(9):1562-1572.CHEN Yaduo,SONG Yanhong,LI Gang,ZHAO Xia,LIU Lifeng,ZHOU Houcheng. Heterologous expression of strawberry FaFIT promotes iron uptakes in roots in Arabidopsis thaliana[J].Journal of Fruit Science,2022,39(9):1562-1572.
[8] 任延靖,柳红.外源物质干预对逆境胁迫下植物生长代谢的影响研究进展[J].青海农技推广,2021(3):15-23.REN Yanjing,LIU Hong. Research progress on the effect of exogenous substance intervention on plant growth and metabolism under adversity stress[J]. Qinghai Agro-Technology Extension,2021(3):15-23.
[9] 李颖,鱼小军,赵一珊,王琳,彭珍,范丽花,王玉霞,马晓东.水杨酸和脱落酸浸种对低温下扁蓿豆种子萌发和幼苗生长的影响[J].草地学报,2021,29(1):174-181.LI Ying,YU Xiaojun,ZHAO Yishan,WANG Lin,PENG Zhen,FAN Lihua,WANG Yuxia,MA Xiaodong. Effects of seed soaking with salicylic acid and abscisic acid on seed germination and seedling growth of Medicago ruthenica at low temperature[J].Acta Agrestia Sinica,2021,29(1):174-181.
[10] 苗卫东,王萌,高换超,井朋伟,李少东,樊秀彩.外源褪黑素对低温胁迫下不同葡萄品种抗氧化酶活性和AsA-GSH 循环的影响[J].江苏农业科学,2021,49(23):133-138.MIAO Weidong,WANG Meng,GAO Huanchao,JING Pengwei,LI Shaodong,FAN Xiucai. Influence of exogenous melatonin on antioxidant enzyme activities and ASA-GSH cycle of different grape cultivars under low temperature stress[J]. Jiangsu Agricultural Sciences,2021,49(23):133-138.
[11] NOOR J,ULLAH A,SALEEM M H,TARIQ A,ULLAH S,WAHEED A,OKLA M K,AL-HASHIMI A,CHEN Y L,AHMED Z,AHMED I. Effect of jasmonic acid foliar spray on the morpho-physiological mechanism of salt stress tolerance in two soybean varieties (Glycine max L.)[J]. Plants-Basel,2022,11(5):14.
[12] MFARREJ M F B,WANG X,SALEEM M H,HUSSAIN I,RASHEED R,ASHRAF M A,IQBAL M,CHATTHA M S,ALYEMENI M N. Hydrogen sulphide and nitric oxide mitigate the negative impacts of waterlogging stress on wheat(Triticum aestivum L.)[J].Plant Biology,2022,24(4):670-683.
[13] LI X N,ZHAO C C,ZHANG T,WANG G Y,AMOMBO E,XIE Y,FU J M. Exogenous aspergillus aculeatus enhances drought and heat tolerance of perennial ryegrass[J/OL].FrontiersinMicrobiology,2021,12:593722.DOI:10.3389/fmicb.2021.593722.
[14] LI M Y,XIAO J C,LEI F Y,ZHENG K M,LU W,MA J Y,HE M L,ZHENG Y X. Effects of exogenous phthalic acid on seed germination,root physiological characteristics,and mineral element absorption of watermelon[J]. Horticulturae,2022,8(3):235.
[15] 吕馨宁,王玥,贾润普,王胜男,姚玉新.不同温度下褪黑素处理对‘阳光玫瑰’葡萄采后品质的影响[J]. 中国农业科学,2022,55(7):1411-1422.LÜ Xinning,WANG Yue,JIA Runpu,WANG Shengnan,YAO Yuxin. Effects of melatonin treatment on quality of stored shine muscat grapes under different storage temperatures[J]. Scientia Agricultura Sinica,2022,55(7):1411-1422.
[16] 刘良松,杨宏艳,赵冬,冯峻,赵香云,罗正平,董云祥,马焕成. 油菜素内酯预处理对低温胁迫下华山松幼苗生理特性的影响[J].西部林业科学,2020,49(2):99-105.LIU Liangsong,YANG Hongyan,ZHAO Dong,FENG Jun,ZHAO Xiangyun,LUO Zhengping,DONG Yunxiang,MA Huancheng. Effects of brassinolide pretreatment on physiological characteristics of Pinus armandii seedlings under low temperature stress[J].Journal of West China Forestry Science,2020,49(2):99-105.
[17] 王光正,吕剑,毛娟,陈佰鸿,冯致,马宗桓.油菜素内酯对低温胁迫下番茄幼苗生理指标的影响[J]. 甘肃农业大学学报,2021,56(4):69-75.WANG Guangzheng,LÜ Jian,MAO Juan,CHEN Baihong,FENG Zhi,MA Zonghuan.Effects of brassinolide on physiological characteristics of tomato seedlings under low temperature stress[J].Journal of Gansu Agricultural University,2021,56(4):69-75.
[18] 黄程,文小梅,唐殷,郭磊周,江世杰,代其林.外源水杨酸对盐胁迫下小白菜幼苗生理的影响[J].江苏农业科学,2020,48(7):147-151.HUANG Cheng,WEN Xiaomei,TANG Yin,GUO Leizhou,JIANG Shijie,DAI Qilin. Influence of exogenous salicylic acid on physiology of Chinese cabbage seedlings under salt stress[J].Jiangsu Agricultural Sciences,2020,48(7):147-151.
[19] 崔庆,吴春燕,宋述尧,王雪科,王状,姜雨欣,刘希元,张晓明.外源NO 缓解黄瓜幼苗低温伤害的效果[J].江苏农业科学,2022,50(6):116-119.CUI Qing,WU Chunyan,SONG Shuyao,WANG Xueke,WANG Zhuang,JIANG Yuxin,LIU Xiyuan,ZHANG Xiaoming. Effects of exogenous NO on alleviating low temperature injury of cucumber seedlings[J]. Jiangsu Agricultural Sciences,2022,50(6):116-119.
[20] 吴帼秀,李胜利,李阳,李严曼,毕焕改,艾希珍.H2S 和NO 及其互作对低温胁迫下黄瓜幼苗光合作用的影响[J].植物生理学报,2020,56(10):2221-2232.WU Guoxiu,LI Shengli,LI Yang,LI Yanman,BI Huangai,AI Xizhen. Effects of hydrogen sulfide, ntric oxide and their interaction on photosynthesis of cucumber seedlings under chilling stress[J].Plant Physiology Journal,2020,56(10):2221-2232.
[21] 郑凯翔,王旺田,刘文瑜,张芮,王宝强,陈正军,毛琳.外源硅调控葡萄生理特性对低温胁迫的响应[J]. 分子植物育种,2020,18(3):1013-1019.ZHENG Kaixiang,WANG Wangtian,LIU Wenyu,ZHANG Rui,WANG Baoqiang,CHEN Zhengjun,MAO Lin. Exogenous silicon regulated the physiological characteristics of grapes responses to low temperature stress[J]. Molecular Plant Breeding,2020,18(3):1013-1019.
[22] 李萍,单守明,李映龙,刘成敏,平吉成.叶面喷施氨基酸钙对盐碱地‘北玺’葡萄光合作用和果实品质的影响[J].中国果树,2021(12):22-26.LI Ping,SHAN Shouming,LI Yinglong,LIU Chengmin,PING Jicheng.Effects of calcium amino acid on photosynthesis and berry quality in‘Beixi’grapevine cultivate in saline-alkali soil[J].China Fruits,2021(12):22-26.
[23] 王译,韩莹琰,郝敬虹,刘超杰,范双喜.褪黑素对高温胁迫下生菜抗氧化酶系统的影响[J].北京农学院学报,2022,37(2):45-49.WANG Yi,HAN Yingyan,HAO Jinghong,LIU Chaojie,FAN Shuangxi.Effects of melatonin on antioxidant enzyme of lettuce under high temperature stress[J]. Journal of Beijing University of Agriculture,2022,37(2):45-49.
[24] 朱秀云,梁梦,马玉. 根系活力的测定(TTC 法)试验综述报告[J].广东化工,2020,47(6):211-212.ZHU Xiuyun,LIANG Meng,MA Yu.A review report on the experiments for determination of root activity by TTC method[J].Guangdong Chemical Industry,2020,47(6):211-212.
[25] 蒋希瑶,黄俊杰,周英杰,牛宁,刘慧英,刁明.不同浓度外源褪黑素对NaHCO3 胁迫下番茄幼苗生长和生理指标的影响[J].北方园艺,2022(9):1-9.JIANG Xiyao,HUANG Junjie,ZHOU Yingjie,NIU Ning,LIU Huiying,DIAO Ming. Effects of exogenous melatonin on growth and physiological indexes of tomato seedlings under NaHCO3 stress[J].Northern Horticulture,2022(9):1-9.
[26] 侯雯,杜卓,王丽,李林,张凯,路运才.外源褪黑素对低温胁迫下玉米幼苗生长和生理特性的影响[J]. 中国糖料,2020,42(2):33-37.HOU Wen,DU Zhuo,WANG Li,LI Lin,ZHANG Kai,LU Yuncai. Effects of exogenous melatonin on maize seedlings growth and physiological traits under low temperature stress[J]. Sugar Crops of China,2020,42(2):33-37.
[27] 杜永成,王玉波,范文婷,盖志佳,于敦爽,谷维,张俐俐,马凤鸣.不同氮素水平对甜菜硝酸还原酶和亚硝酸还原酶活性的影响[J].植物营养与肥料学报,2012,18(3):717-723.DU Yongcheng,WANG Yubo,FAN Wenting,GAI Zhijia,YU Dunshuang,GU Wei,ZHANG Lili,MA Fengming. Effect of nitrogen fertilization on nitrate reductase and nitrite reductase activities of sugar beet[J]. Journal of Plant Nutrition and Fertilizers,2012,18(3):717-723.
[28] CHEN S C,JOHNSON B K,YU T,NELSON B N,WALKER E D. Elizabethkingia anophelis:Physiologic and transcriptomic responses to iron stress[J]. Frontiers in Microbiology,2020,11:804.
[29] MIR A R,FAIZAN M,BAJGUZ A,SAMI F,SIDDIQUI H,HAYAT S. Occurrence and biosynthesis of melatoninand its exogenous effect on plants[J].Acta Societatis Botanicorum Poloniae,2020,89(2):1-23.
[30] ZHANG J,LI H B,XU B,LI J,HUANG B R.Exogenous melatonin suppresses dark-induced leaf senescence by activating the superoxide dismutase-catalase antioxidant pathway and downregulating chlorophyll degradation in excised leaves of perennial ryegrass (Lolium perenne L.) [J]. Frontiers in Plant Science,2016,7:1500.
[31] DUY D,STUEBE R,WANNER G,PHILIPPAR K. The chloroplast permease PIC1 regulates plant growth and development by directing homeostasis and transport of iron[J].Plant Physiology,2011,155(4):1709-1722.
[32] SZAFRANSKA K,REITER R J,POSMYK M M.Melatonin improves the photosynthetic apparatus in pea leaves stressed by paraquat via chlorophyll breakdown regulation and its accelerated de novo synthesis[J].Frontiers in Plant Science,2017,8:878.
[33] ZHENG X D,TAN D X,ALLAN A C,ZUO B X,ZHAO Y,REITER R J,WANG L,WANG Z,GUO Y,ZHOU J Z,SHAN D Q,LI Q T HAN Z H,KONG J. Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress[J].Scientific Reports,2017,7(1):1-12.
[34] 赵小红,罗庆熙,饶玲.外源褪黑素在低温胁迫下对黄瓜幼苗抗冷性的影响[J].北方园艺,2017(14):55-59.ZHAO Xiaohong,LUO Qingxi,RAO Ling. Effects of exogenous melatonin on chilling tolerance of cucumber seedlings under low temperature stress[J].Northern Horticulture,2017(14):55-59.
[35] ITURBE-ORMAETXE I,ESCUREDO P R,ARRESE-IGOR C,BECANA M.Oxidative damage in pea plants exposed to water deficit or paraquat[J]. Plant Physiology,1998,116(1):173-181.
[36] 皇甫列翔.褪黑素促进盐胁迫下水稻种子萌发及调控叶片衰老和产量相关性状的分子机制研究[D].扬州:扬州大学,2021.HUANGFU Liexiang. Molecular mechanism of melatonin in promoting rice seed germination under salt stress,and in regulating leaf senescence and yield related traits[D].Yangzhou:Yangzhou University,2021.
[37] 苗宇,张浩阳,张丽佳,刘美君,王爽.少量NaCl 缓解KCl 胁迫对紫花苜蓿幼苗根系和叶片光合活性的影响[J].草业科学,2022,39(5):930-939.MIAO Yu,ZHANG Haoyang,ZHANG Lijia,LIU Meijun,WANG Shuang.Effects of small amounts of NaCl on alleviating damage caused to the photosynthetic activity of alfalfa seedling roots and leaves by KCl stress[J].Pratacultural Science,2022,39(5):930-939.
[38] FLOWERS T J,MUNNS R,COLMER T D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes[J].Annals of Botany,2015,115(3):419-431.
[39] 梁亮.硝酸还原酶活性对小白菜硝酸盐积累及相关代谢调节的研究[D].南京:南京农业大学,2008.LIANG Liang. Studies on the effect of nar on nitrate accumulation and metabolism regulation in pakchoi[D]. Nanjing:Nanjing Agricultural University,2008.
[40] 岳丽娟.铁胁迫对豌豆幼苗铁代谢、光合作用及抗氧化系统的影响[D].兰州:兰州大学,2009.YUE Lijuan.The effect of iron stress on iron metabolism,photosynthesis and antioxidant system in Pisum sativum seedlings[D].Lanzhou:Lanzhou University,2009.
[41] TURK H,ERDAL S,GENISEL M,ATICI O,DEMIR Y,YANMIS D.The regulatory effect of melatonin on physiological,biochemical and molecular parameters in cold-stressed wheat seedlings[J].Plant Growth Regulation,2014,74(2):139-152.