葡萄(Vitis vinifera L.)是葡萄科(Vitaceae)葡萄属(Vitis L.)多年生木质藤本植物,可鲜食、制干、制汁、药用等,因其适应性强,经济效益高,广泛种植在世界各地[1-2]。新疆作为中国最大的葡萄产区,因其光热资源丰富,葡萄品质独特而闻名中外。在传统种植过程中,多以自根苗繁育,无法避免土壤干旱[3]、盐渍化[4]等生态脆弱的问题,这严重制约其产业发展。因此,采用抗性砧木成为解决这一问题的有效措施之一。5BB、1103P、SO4、贝达、山河1号等葡萄砧木具有较强抗旱、抗盐碱能力,且生根和嫁接状况良好[5-6]。但在实际生产中,砧木通过扦插等方式繁殖数量有限,且易感染病毒[7]。茎段快繁作为植物组织培养的关键技术之一,具有取材方便、不易污染、繁殖系数高的特点,可实现苗木脱毒、离体再生,已成为欧美地区葡萄栽培的主流方式[8- 9]。Yildirim 等[10]研究发现,MS 培养基比WPM、QL、DWK 培养基所培养的Öküzgözü 葡萄和Boğazkere葡萄生长效果好;蔡文博等[11]对夏黑、红地球、巨峰和玫瑰香进行增殖培养和生根诱导时发现,WPM培养基比1/2MS 培养基中的葡萄茎段萌发率高,且叶片长势好,生根量多,更利于组培苗扩繁。多种植物生长调节剂的相互作用对植株快繁会产生一定的积极作用。林茜等[12]对阳光玫瑰的诱导培养时发现,1.50 mg·L-1 6-BA 和0.20 mg·L-1 NAA 的相互作用可得到最高的萌芽率。农艳丰等[13]发现,在阳光玫瑰继代培养时添加1.50 mg·L-1 KT和0.10 mg·L-1 IAA 的无菌芽较为粗壮,叶色更深。苏玲等[14]在贵妃玫瑰的生根培养中发现,以1/2MS 培养基为基本培养基,并添加0.50 mg·L-1 IBA 时,其生根率达到最高,根系生长状况最优。中国大部分研究集中在鲜食和酿酒葡萄上,对砧木研究较少,且不同的葡萄品种对培养基中各成分的响应各不相同[15],探究不同的植物生长调节剂对繁殖系数的影响已成为建立高效茎段快繁体系的关键所在。笔者在本研究中旨在通过对5BB、1103P、SO4葡萄砧木在初代培养、继代培养、生根培养过程中不同培养基和植物生长调节剂的筛选,以期为葡萄砧木的快速繁殖和生产应用提供参考依据。
1 材料和方法
1.1 材料
供试材料为来自新疆农业大学三坪教学实践基地的5BB、1103P、SO4一年生苗,其中5BB砧木亲本为V.berlandieri×V.riparia,1103P砧木亲本为V.berlandieri‘Rességuier’×V. rupestris‘Du Lot’,SO4 砧木亲本为V.berlandieri×V.riparia‘No.4’[16],将其种植在新疆农业大学园艺学院特色果树研究中心室内阳台,进行盆栽苗培养,每个品种30盆。约60 d后,取直径为1~2 mm粗半木质化的新梢进行试验处理。
1.2 方法
1.2.1 外植体的消毒 将新梢剪成2~3 cm 长的单芽茎段,放在烧杯中,盖上纱布,用流水冲洗1 h,去除表面污渍。随后在超净工作台上,以75%乙醇浸泡30 s,用0.10%HgCl 溶液消毒8 min,随后用无菌水冲洗3~5次,去除外植体表面消毒剂残留,最后将茎段置于已灭菌的接种盘中备用。
1.2.2 初代培养 以B5、MS、WPM 培养基为基本培养基,同时添加2.00 mg·L-1 6-BA、0.20 mg·L-1 IBA、0.20 mg·L-1 GA3、30 g·L-1蔗糖、7 g·L-1琼脂,调节pH为5.80。在超净工作台上,剪去外植体两端浸染过消毒剂的部分,留取长1.50~2.00 cm 带腋芽的茎段,接种于培养基上,每瓶接1 个茎段,每种培养基10瓶,3次重复。培养30 d后统计污染率、死亡率和腋芽萌发率。培养条件为:培养温度(25±1)℃,光照度2000~2500 lx,光照时间12 h·d-1。
1.2.3 继代培养 外植体培养30 d后,待萌发的丛生芽生长至长度为5~6 cm时进行继代培养。剪切萌发的丛生芽,去除叶片后剪成单芽茎段(长10~15 mm)。继代培养选用三因素二次回归正交旋转组合试验设计,以初代培养所筛选的MS 培养基为基本培养基,并添加不同质量浓度的3种植物生长调节剂(6-BA、IBA、GA3)、30 g·L-1蔗糖、7 g·L-1琼脂,调节pH 为5.80。
参照高林等[17]的二次回归正交旋转组合试验设计,设6-BA 质量浓度为1~3.00 mg·L-1、IBA 质量浓度为0~0.50 mg·L-1、GA3质量浓度为0~1.00 mg·L-1,3 种激素各设5 个浓度水平进行组合试验。各水平编码值与浓度详见表1。
表1 6-BA、IBA 和GA3的编码与浓度
Table 1 Coding and concentration of 6-BA,IBA and GA3
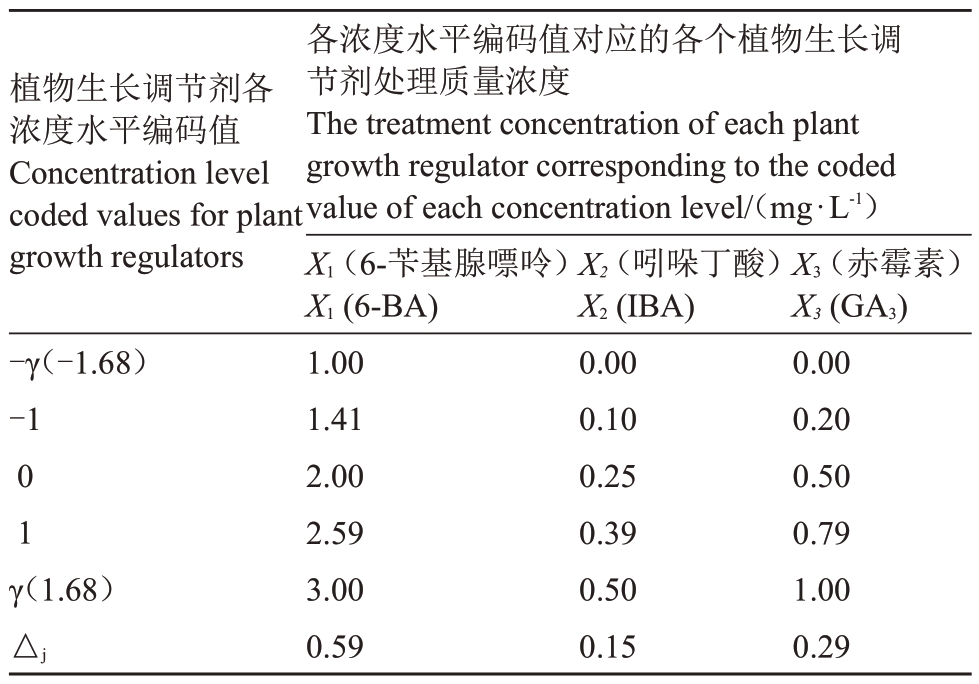
植物生长调节剂各浓度水平编码值Concentration level coded values for plant growth regulators-γ(-1.68)-101γ(1.68)△j各浓度水平编码值对应的各个植物生长调节剂处理质量浓度The treatment concentration of each plant growth regulator corresponding to the coded value of each concentration level/(mg·L-1)X1(6-苄基腺嘌呤)X1(6-BA)1.001.412.002.593.000.59 X2(吲哚丁酸)X2(IBA)0.000.100.250.390.500.15 X3(赤霉素)X3(GA3)0.000.200.500.791.000.29
根据试验设计,优化后的水平编码值与激素质量浓度如表2所示。其中,X1、X2、X3分别代表6-BA、IBA、GA3的处理浓度。每个处理接种3 瓶,每瓶接种一个丛生芽,3组重复。30 d后统计不定芽的增殖情况,计算增殖系数(分别以Y1、Y2、Y3 代表5BB、1103P 和SO4 葡萄砧木的增殖系数)。培养条件与初代培养条件相同。
表2 增殖培养优化方案及结果
Table 2 Optimization scheme and results of proliferation culture

处理编号Treatment number各处理植物生长调节剂浓度水平的编码值Coded values of plant growth regulator concentration levels for each treatment增殖系数Multiplication coefficient X1 X2 X36-BA IBA 123456789111111-1-111-1-1 GA3 1-11-11-11-1101112 13141516 17181920 212223-1-1-1-11.68-1.680000000000000001.68-1.680000000000000001.68-1.68000000000各浓度水平编码值所对应的各个植物生长调节剂处理质量浓度The treatment concentration of each plant growth regulator corresponding to the coded value of each concentration level/(mg·L-1)6-苄基腺嘌呤6-BA 1.991.991.991.990.260.260.260.262.500.001.251.251.251.251.251.251.251.251.251.251.251.251.25吲哚丁酸IBA 0.800.800.200.200.800.800.200.200.500.501.000.000.500.500.500.500.500.500.500.500.500.500.50赤霉素GA3 0.800.200.800.200.800.200.800.200.500.500.500.501.000.000.500.500.500.500.500.500.500.500.50 Y1 1.671.441.671.561.671.221.781.442.892.332.111.781.781.561.000.670.780.780.560.560.330.441.11 Y2 1.221.441.671.561.331.561.221.671.561.782.221.221.561.671.000.561.001.220.781.001.000.781.00 Y3 1.332.001.562.221.671.331.441.221.331.561.001.111.221.780.670.781.000.561.000.781.001.000.56
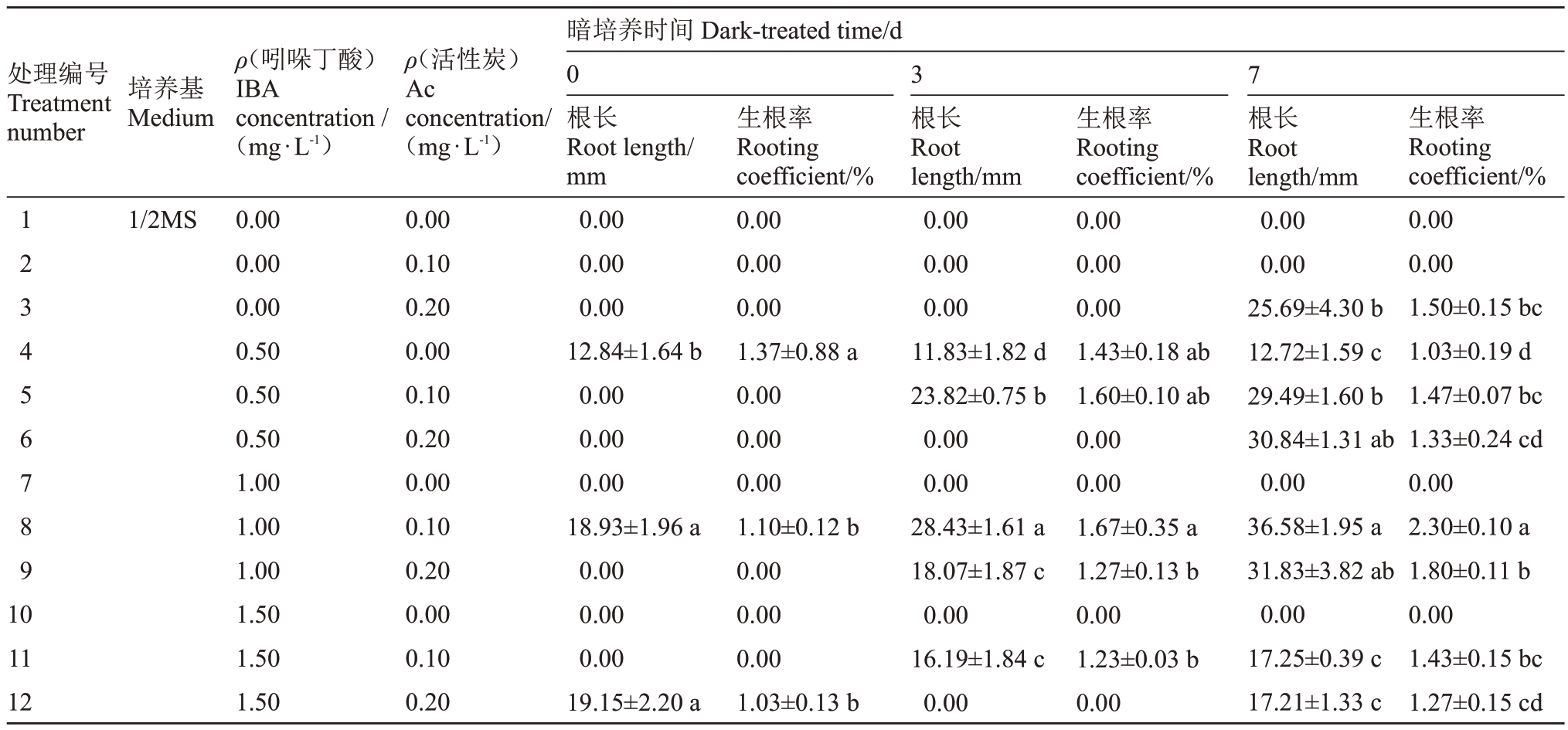
1.2.4 生根培养 将继代培养中长势相近的丛生芽切下,转接到生根培养基上。以1/2MS 培养基为基本培养基,添加不同质量浓度的IBA(0、0.50 mg·L-1、1.00 mg·L-1、1.50 mg·L-1)和Ac(0、0.10 g·L-1、0.20 g·L-1),并附加30 g·L-1蔗糖、7 g·L-1琼脂,调节pH 为5.80,共12 个处理,每个处理接种10 瓶,每瓶1 株,重复3组。接种后先进行一段时间的暗培养(0 d、3 d、7 d),随后进行12 h·d-1光周期培养,30 d后统计根长和生根系数。培养条件与初代培养条件相同。
生根系数=总生根数/总接种苗数。
1.3 数据分析
采用Microsoft Excel 2010 进行数据整理,DPS软件进行二次回归正交旋转组合试验设计,SPSS 20.0软件进行单因素方差分析(Duncan法,p<0.05)。
2 结果与分析
2.1 不同培养基对茎段初代培养的影响
由表3可知,不同培养基对3种葡萄砧木茎段初代培养的腋芽萌发率和平均株高存在显著差异(p<0.05)。5BB 砧木中,MS 培养基的腋芽萌发率达到100%,株高达到最大为25.20 mm,且茎段无污染死亡,B5 培养基和WPM 培养基的腋芽萌发率均为96.67%,但B5 培养基丛生芽的株高略大于WPM培养基,且WPM培养基的死亡率较高。1103P砧木的3种培养基的腋芽萌发率均达到100%,且茎段无污染死亡,但MS培养基的平均株高达到最大,为20.90 mm。SO4 砧木中的MS 和WPM 培养基的腋芽萌发率达到100%,且茎段无污染死亡,但MS 培养基的株高略高于WPM 为22.43 mm,B5 培养基的腋芽萌发率最低(96.67%),且污染率为3.33%。各砧木在不同培养基中的生长状况如图1所示。

图1 不同砧木初代培养的生长状况
Fig.1 Growth status of primary culture of different rootstocks
表3 不同培养基对葡萄砧木茎段初代培养腋芽萌发率的影响
Table 3 Effects of different medium on germination rate of axillary buds in primary culture of grape rootstock stem
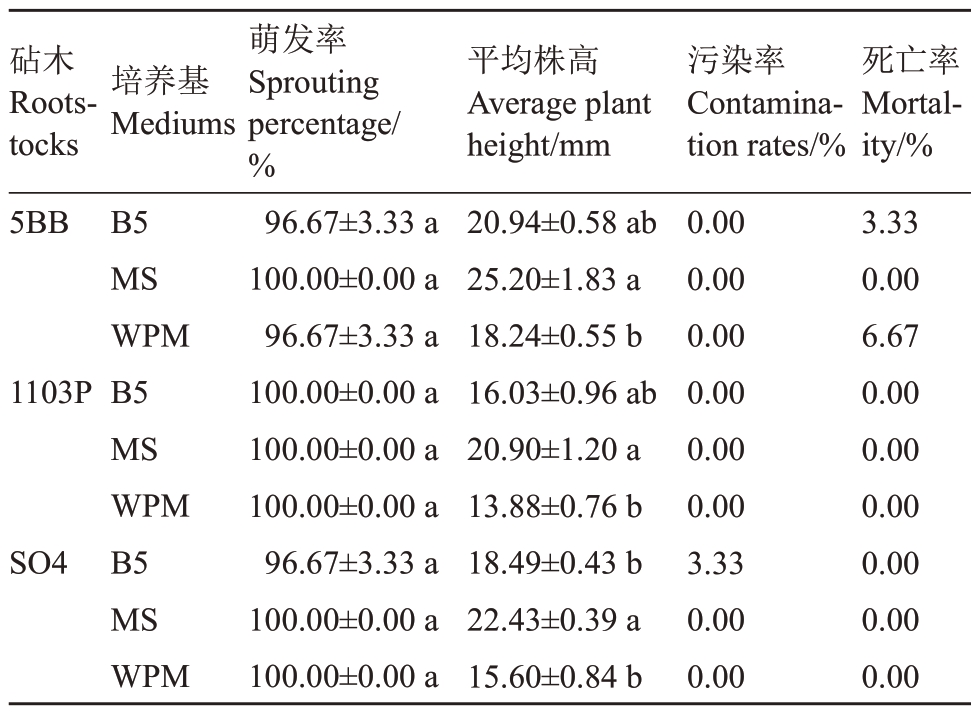
注:同列数据后不同小写字母表示相同品种不同培养基之间差异显著(p<0.05)。
Note: Different lowercase letters after the data in the same column indicate significant differences between different mediums of the same variety(p<0.05).
砧木Rootstocks 5BB萌发率Sprouting percentage/%96.67±3.33 a 100.00±0.00 a 96.67±3.33 a 100.00±0.00 a 100.00±0.00 a 100.00±0.00 a 96.67±3.33 a 100.00±0.00 a 100.00±0.00 a死亡率Mortality/%3.330.006.670.000.000.000.000.000.00培养基Mediums 1103P SO4 B5 MS WPM B5 MS WPM B5 MS WPM平均株高Average plant height/mm 20.94±0.58 ab 25.20±1.83 a 18.24±0.55 b 16.03±0.96 ab 20.90±1.20 a 13.88±0.76 b 18.49±0.43 b 22.43±0.39 a 15.60±0.84 b污染率Contamination rates/%0.000.000.000.000.000.003.330.000.00
2.2 不同植物生长调节剂对增殖系数的影响
2.2.1 二次回归方程的建立 由表2中5BB葡萄砧木的数据分析可得二次回归方程Y1=0.71-0.05 X1-0.07 X2+0.06 X3+0.55 X12+0.32 X22+0.22 X32+0.03 X1X2-0.06 X1X3+0.03 X2X3。由表4 中F 值可知,F1=4.456<F0.05(2,8)=4.46,回归方程失拟不显著,认为所选用的回归模型适当,未受其他因子的影响。回归显著性检验F2=5.448>F0.05(3,5)=5.41,回归显著,回归方程拟合较好,模型准确度可达95%。在剔除α=0.10 时的不显著项,得到优化后的方程Y1=0.71+0.55 X12+0.32 X22+0.22 X32。
表4 5BB 砧木增殖培养试验结果的方差分析
Table 4 Variance analysis of proliferation culture test results of 5BB Rootstocks
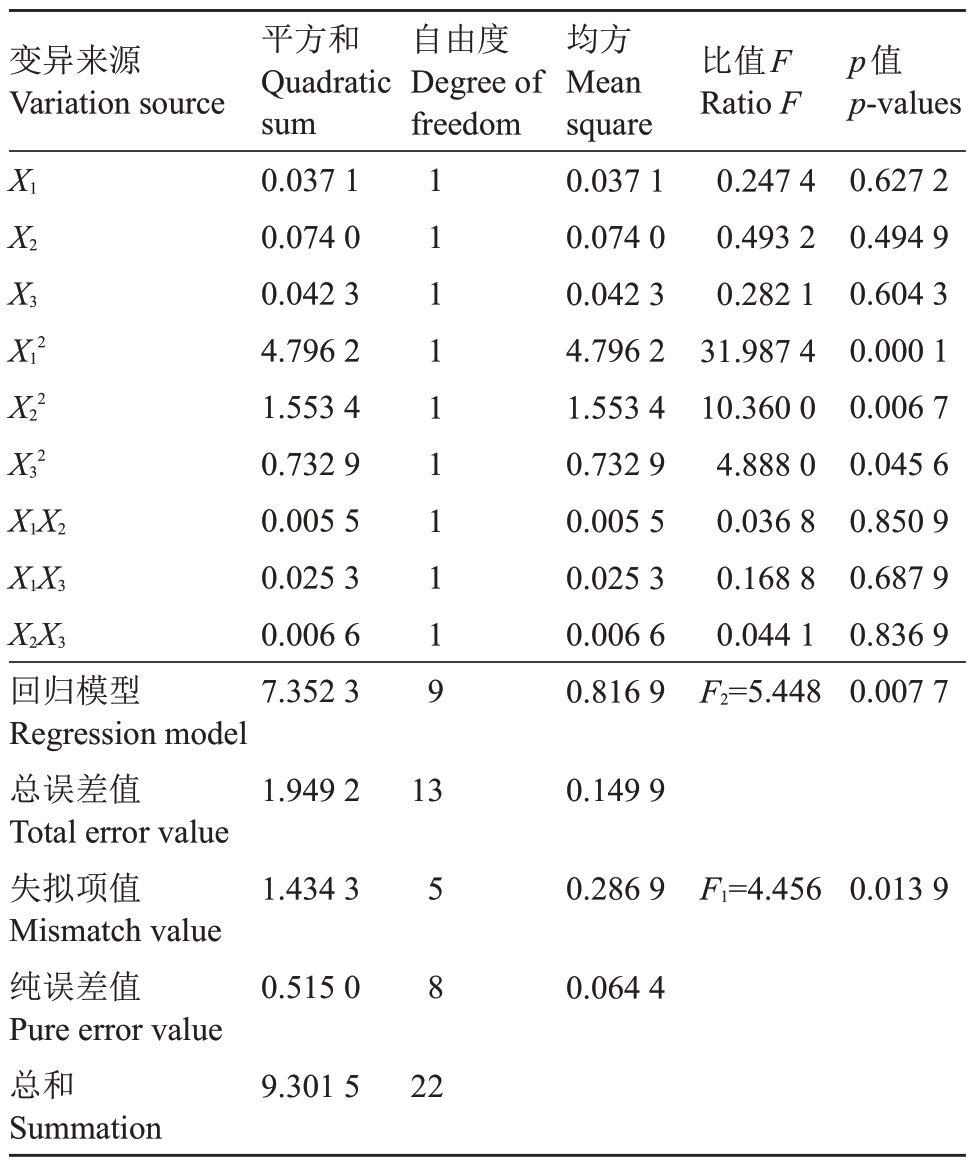
变异来源Variation source自由度Degree of freedom X1 X2 X3 X12 X22 X32 X1X2 X1X3 X2X3回归模型Regression model总误差值Total error value失拟项值Mismatch value纯误差值Pure error value总和Summation平方和Quadratic sum 0.03710.07400.04234.79621.55340.73290.00550.02530.00667.35231111111119均方Mean square 0.03710.07400.04234.79621.55340.73290.00550.02530.00660.8169比值F Ratio F 0.24740.49320.282131.987410.36004.88800.03680.16880.0441 F2=5.448 p值p-values 0.62720.49490.60430.00010.00670.04560.85090.68790.83690.00771.9492130.14991.43430.2869 F1=4.4560.01390.515058 0.06449.301522
由表2 中1103P 葡萄砧木的数据分析可得二次回归方程Y2=0.93+0.04 X1-0.16 X2-0.04X3+0.21 X12+0.23 X22+0.19 X32-0.07 X1X2+0.07 X1X3-0.01 X2X3。由表5 中F 值可知,F1=2.647<F0.05(9,14)=2.65,回归方程失拟不显著,认为所选用的回归模型适当,未受其他因子的影响。回归显著性检验F2=4.818>F0.05(9,5)=4.77,回归显著,回归方程拟合较好,模型准确度可达95%。在剔除α=0.10时的不显著项,得到优化后的方程Y2=0.93-0.16 X2+0.21 X12+0.23 X22+0.19 X32。
表5 1103P 砧木增殖培养试验结果的方差分析
Table 5 Variance analysis of proliferation culture test results of 1103P rootstocks

变异来源Variation source自由度Degree of freedom X1 X2 X3 X12 X22 X32 X1X2 X1X3 X2X3回归模型Regression model总误差值Total error value失拟项值Mismatch value纯误差值Pure error value总和Summation平方和Quadratic sum 0.01690.37130.02680.68190.80410.55910.04060.04060.00152.57101111111119均方Mean square 0.01690.37130.02680.68190.80410.55910.04060.04060.00150.2857比值F Ratio F 0.28456.26230.452111.501113.56259.42980.68500.68500.0255 F2=4.818 p值p-values 0.60270.02650.51310.00480.00280.00890.42280.42280.87590.01180.7708130.05930.48040.0961 F1=2.6470.07310.290458 0.03633.341822
由表2中SO4葡萄砧木的数据分析可得二次回归方程Y3=0.81+0.13 X1+0.01 X2+0.01X3+0.27 X12+0.13 X22+0.29 X32-0.10 X1X2-0.24 X1X3+0.01 X2X3。由表6 中F 值可知,F1=2.488<F0.05(7,21)=2.49,回归方程失拟不显著,认为所选用的回归模型适当,未受其他因子的影响。回归显著性检验F2=6.839>F0.05(3,4)=6.59,回归显著,回归方程拟合较好,模型准确度可达95%。在剔除α=0.10时的不显著项,得到优化后的方程Y3=0.81+0.13 X1+0.27 X12+0.13 X22+0.29 X32-0.24 X1X3。
表6 SO4 砧木增殖培养试验结果的方差分析
Table 6 Variance analysis of proliferation culture test results of SO4 rootstocks

变异来源Variation source自由度Degree of freedom X1 X2 X3 X12 X22 X32 X1X2 X1X3 X2X3回归模型Regression model总误差值Total error value失拟项值Mismatch value纯误差值Pure error value总和Summation平方和Quadratic sum 0.24700.00040.00221.13510.26211.30750.07800.44650.00153.51321111111119均方Mean square 0.24700.00040.00221.13510.26211.30750.07800.44650.00150.3904比值F Ratio F 4.32820.00720.037919.88764.592722.90801.36687.82290.0265 F2=6.839 p值p-values 0.05780.93360.84870.00060.05160.00040.26340.01510.87320.00340.7420130.05710.45160.0903 F1=2.4880.08610.290458 0.03634.255222
2.2.2 单因素效应分析 采用“降维法”对3种植物生长调节剂进行单因素效应分析,如表4 所示,X1、X2、X3的均方分别为0.0371、0.074、0.0423,由此可知,影响5BB 砧木增殖培养的3 种因素的主次顺序为:IBA>GA3>6-BA。如表5所示,X1、X2、X3的均方分别为0.0169、0.3713、0.0268,由此可知,影响1103P 砧木增殖培养的3 种因素的主次顺序为:IBA>GA3>6-BA。如表6 所示,X1、X2、X3的均方分别为0.2470、0.0022、0.0004,由此可知,影响SO4砧木增殖培养的3 种因素的主次顺序为6-BA>GA3>IBA。图2为各编码值下3种植物生长调节剂对增殖系数影响的变化趋势,3 种植物生长调节剂对3种葡萄砧木的增殖效果均呈现先降低后升高的变化趋势,且6-BA 的增殖效果普遍高于IBA 和GA3,当质量浓度均处于中间编码值时,即6-BA 为2.00 mg·L-1、IBA 为0.25 mg·L-1、GA3为0.50 mg·L-1时,各个品种的增殖效果均最优。

图2 单因素效应分析
Fig.2 Single factor effect analysis
2.2.3 两因素相互作用效应分析 由表4、表5 可知,5BB、1103P砧木的回归方程中,X1X2、X1X3和X2X3的交互作用在α=0.10 时不显著,即6-BA 与IBA、6-BA与GA3、IBA与GA3的交互作用在α=0.10时不显著;由表6可知,SO4砧木的回归方程中,X1X2和X2X3的交互作用在α=0.10 时不显著,而X1X3的交互作用在α=0.10时显著,即6-BA与IBA、6-BA与GA3的交互作用在α=0.10 时不显著,IBA 与GA3的交互作用在α=0.10时显著。
对3种植物生长调节剂之间相互作用的影响效果进行比较分析,由图3 可知,当6-BA 和IBA 均处于最高浓度水平时,5BB、SO4 的增殖系数达到最大;当6-BA 处于最高质量浓度水平,IBA 最低质量浓度水平处时,1103P增殖系数达到最大。
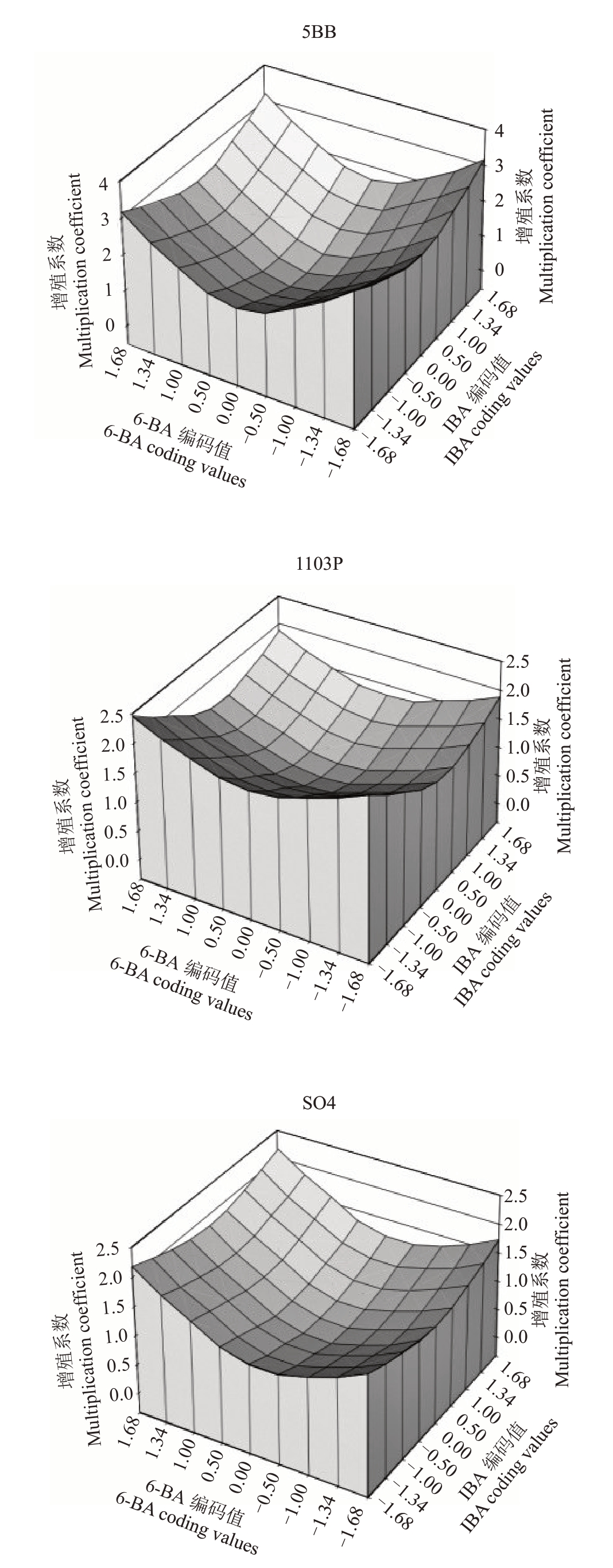
图3 6-BA 与IBA 的交互作用分析
Fig.36-BA and IBA interaction analysis
由图4可知,当6-BA 和GA3处于高质量浓度水平或低质量浓度水平时,对5BB、1103P 的增殖系数并无明显影响;当6-BA处于最高质量浓度水平,GA3最低质量浓度水平处时,SO4增殖系数达到最大。
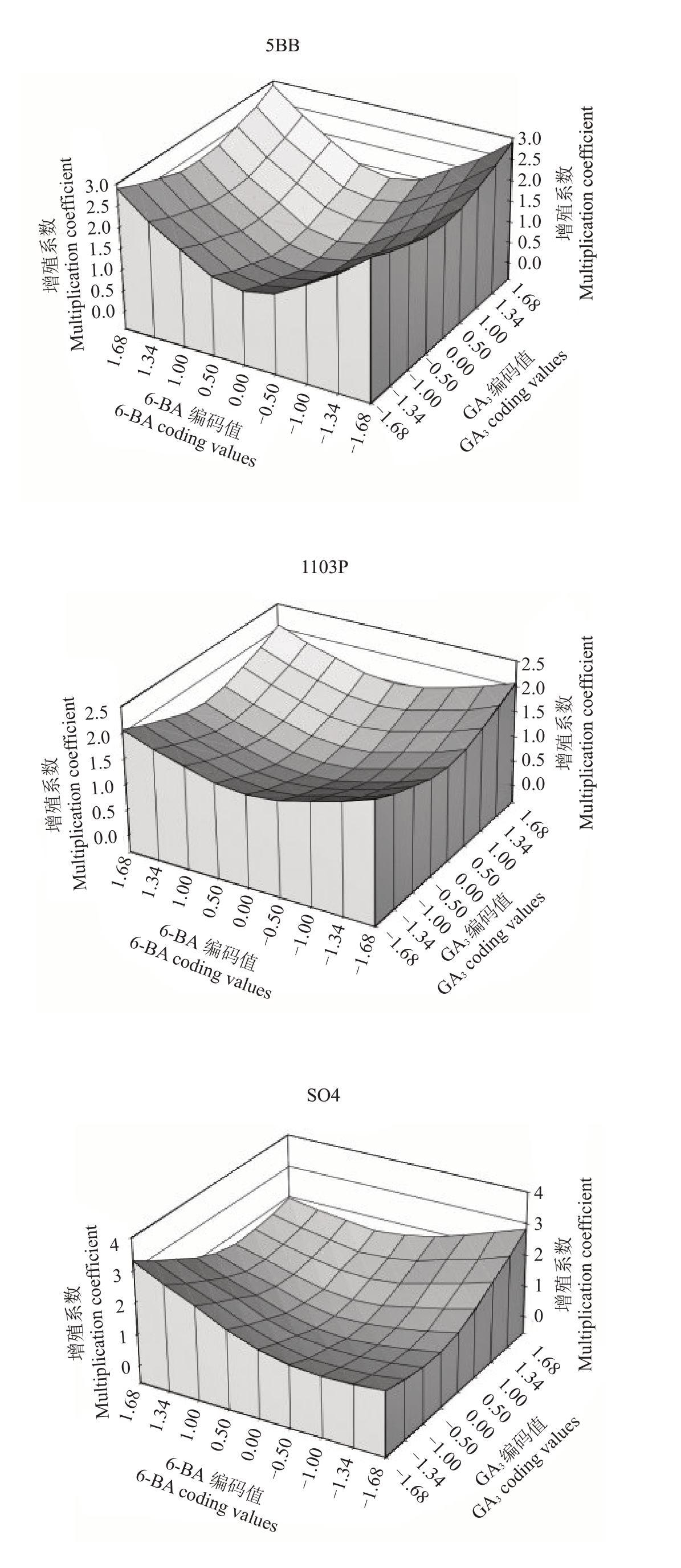
图4 6-BA 与GA3的交互作用分析
Fig.46-BA and GA3 interaction analysis
由图5可知,当IBA和GA3均处于最高质量浓度水平时,5BB、SO4的增殖系数达到最大;当IBA最低质量浓度水平,GA3处于最高质量浓度水平或最低质量浓度水平时,1103P增殖系数均可达到最大。
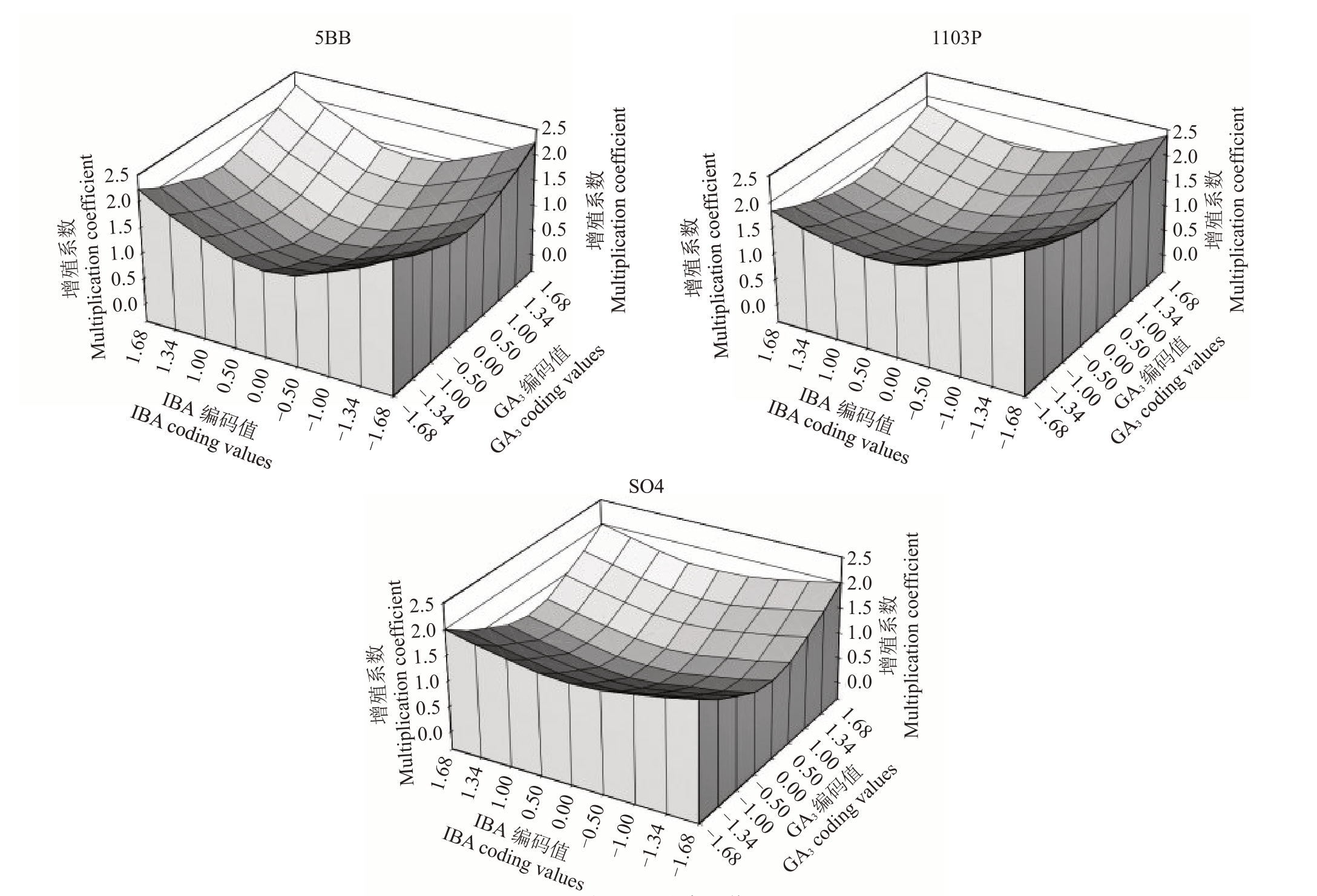
图5 IBA 与GA3的交互作用分析
Fig.5 IBA and GA3 interaction analysis
2.2.4 频率分析法模拟最优值 5BB砧木优化方案中各变量取值的频率分布如表7 所示,增殖系数大于1.35 的方案有112 个,其中95%的试验方案中,6-BA适宜的编码值范围为-0.45~4.45,IBA 适宜的编码值范围为-0.38~0.88,GA3 适宜的编码值范围为-0.73~1.73,取平均值为最适浓度所对应的编码值,即当6-BA 的质量浓度为2.00 mg·L-1、IBA 的质量浓度为0.25 mg·L-1、GA3的质量浓度为0.50 mg·L-1时,增殖培养的效果可达到最优(图6-A)。

图6 不同砧木继代培养的生长状况
Fig.6 Growth status of subculture of different rootstocks
表7 5BB 砧木优化方案中各变量取值的频率颁布
Table 7 Frequency promulgation of variable values in 5BB rootstock optimization scheme
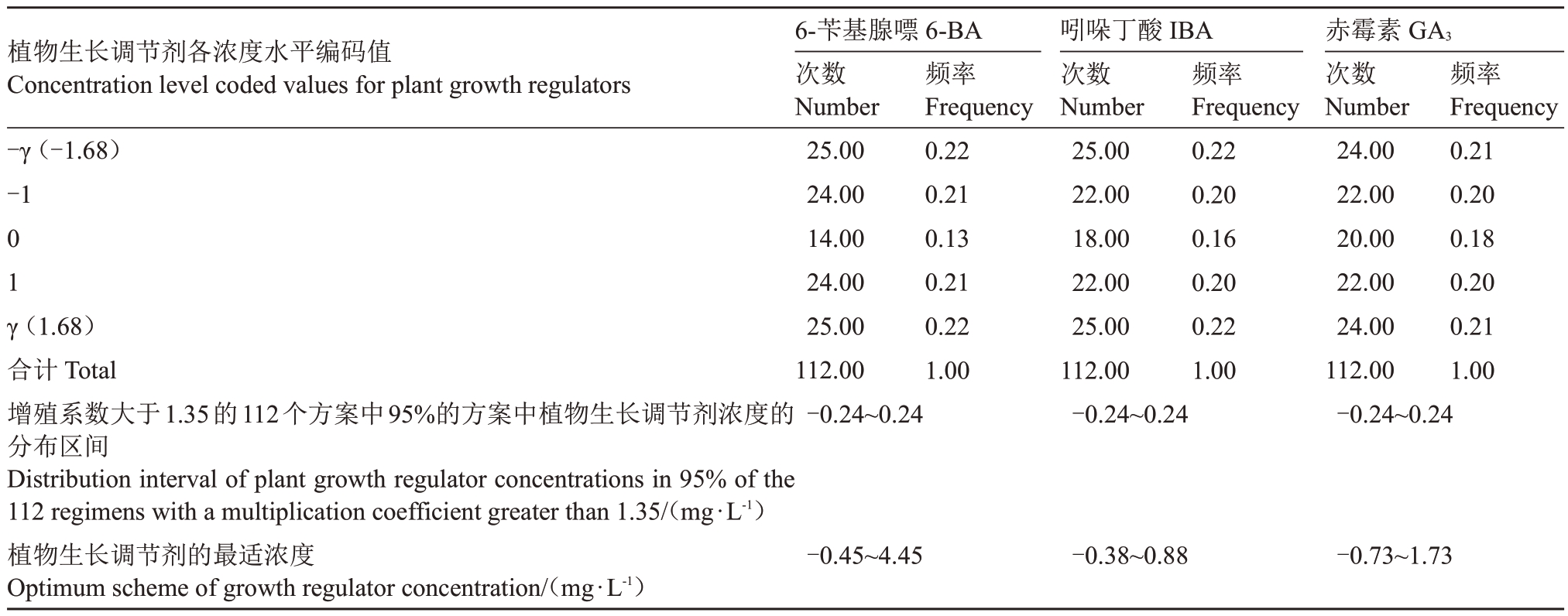
植物生长调节剂各浓度水平编码值Concentration level coded values for plant growth regulators-γ(-1.68)-101 γ(1.68)合计Total增殖系数大于1.35的112个方案中95%的方案中植物生长调节剂浓度的分布区间Distribution interval of plant growth regulator concentrations in 95% of the 112 regimens with a multiplication coefficient greater than 1.35/(mg·L-1)植物生长调节剂的最适浓度Optimum scheme of growth regulator concentration/(mg·L-1)6-苄基腺嘌6-BA次数Number 25.0024.0014.0024.0025.00112.00-0.24~0.24频率Frequency 0.220.210.130.210.221.00吲哚丁酸IBA次数Number 25.0022.0018.0022.0025.00112.00-0.24~0.24频率Frequency 0.220.200.160.200.221.00赤霉素GA3次数Number 24.0022.0020.0022.0024.00112.00-0.24~0.24频率Frequency 0.210.200.180.200.211.00-0.45~4.45-0.38~0.88-0.73~1.73
1103P 砧木优化方案中各变量取值的频率分布如表8 所示,增殖系数大于1.31的方案有114个,其中95%的试验方案中,6-BA 适宜的编码值范围为-0.51~4.51,IBA适宜的编码值范围为-0.27~0.77,GA3适宜的编码值范围为-0.73~1.73,取平均值为最适质量浓度所对应的编码值,即当6-BA的质量浓度为2.00 mg·L-1、IBA 的质量浓度为0.25 mg·L-1、GA3的质量浓度为0.50 mg·L-1时,增殖培养的效果可达到最优(图6-B)。
表8 1103P 砧木优化方案中各变量取值的频率颁布
Table 8 Frequency promulgation of variable values in 1103P Rootstock optimization scheme

植物生长调节剂各浓度水平编码值Concentration level coded values for plant growth regulators-γ(-1.68)-101 γ(1.68)合计Total增殖系数大于1.31的114个方案中95%的方案中植物生长调节剂浓度的分布区间Distribution interval of plant growth regulator concentrations in 95% of the 114 regimens with a multiplication coefficient greater than 1.31/(mg·L-1)植物生长调节剂的最适浓度Optimum scheme of growth regulator concentration/(mg·L-1)6-苄基腺嘌6-BA次数Number 25.0023.0018.0023.0025.00114.00-0.24~0.24频率Frequency 0.220.200.160.200.221.00吲哚丁酸IBA次数Number 25.0025.0020.0020.0024.00114.00-0.29~0.17频率Frequency 0.220.220.180.180.211.00赤霉素GA3次数Number 25.0023.0018.0023.0025.00114.00-0.24~0.24频率Frequency 0.220.200.160.200.221.00-0.51~4.51-0.27~0.77-0.73~1.73
SO4 砧木优化方案中各变量取值的频率分布如表9 所示,增殖系数大于1.22 的方案有111 个,其中95%的试验方案中,6-BA 适宜的编码值范围为-1.03~4.07,IBA适宜的编码值范围为-0.38~0.88,GA3适宜的编码值范围为-0.86~1.58,取平均值为最适质量浓度所对应的编码值,即当6-BA的质量浓度为1.52 mg·L-1、IBA 的质量浓度为0.25 mg·L-1、GA3的质量浓度为0.36 mg·L-1时,增殖培养的效果可达到最优(图6-C)。
表9 SO4 砧木优化方案中各变量取值的频率颁布
Table 9 Frequency promulgation of variable values in SO4 Rootstock optimization scheme
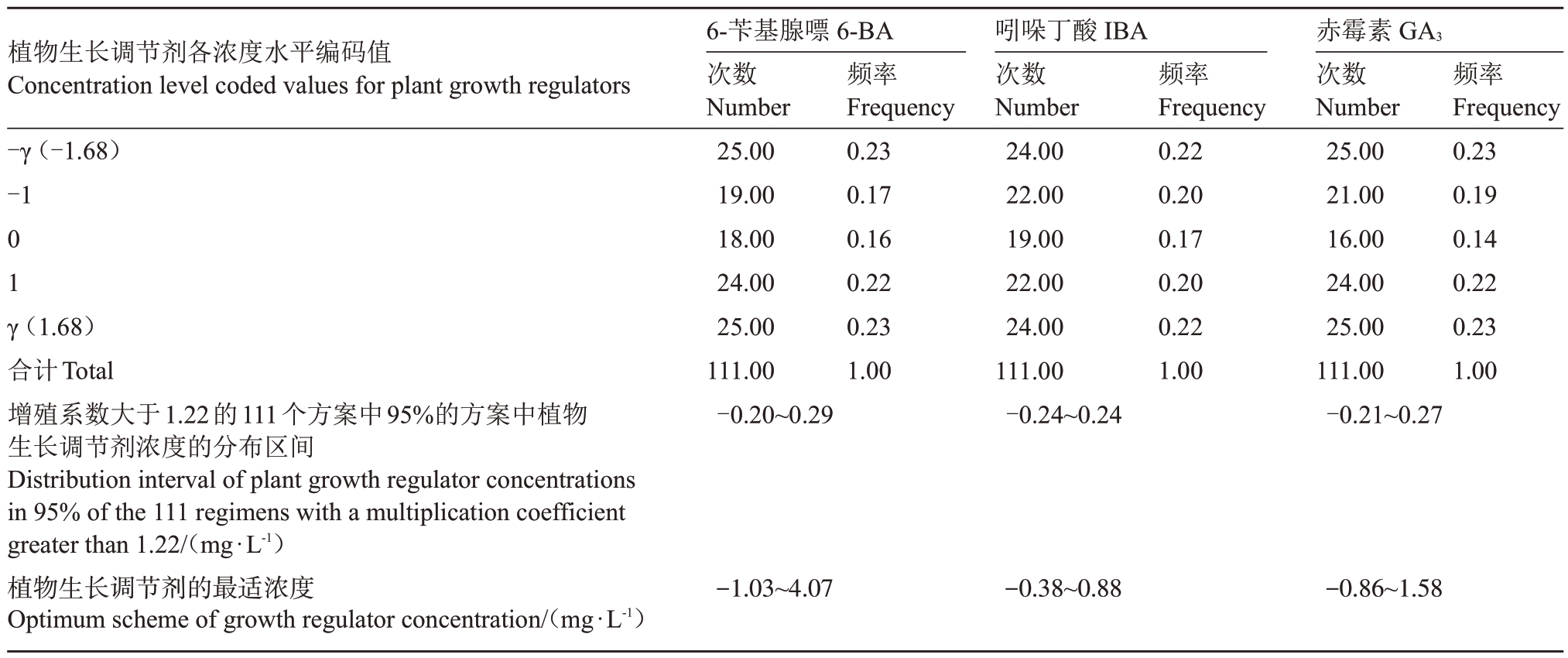
植物生长调节剂各浓度水平编码值Concentration level coded values for plant growth regulators-γ(-1.68)-101 γ(1.68)合计Total增殖系数大于1.22的111个方案中95%的方案中植物生长调节剂浓度的分布区间Distribution interval of plant growth regulator concentrations in 95% of the 111 regimens with a multiplication coefficient greater than 1.22/(mg·L-1)植物生长调节剂的最适浓度Optimum scheme of growth regulator concentration/(mg·L-1)6-苄基腺嘌6-BA次数Number 25.0019.0018.0024.0025.00111.00-0.20~0.29频率Frequency 0.230.170.160.220.231.00吲哚丁酸IBA次数Number 24.0022.0019.0022.0024.00111.00-0.24~0.24频率Frequency 0.220.200.170.200.221.00赤霉素GA3次数Number 25.0021.0016.0024.0025.00111.00-0.21~0.27频率Frequency 0.230.190.140.220.231.00-1.03~4.07-0.38~0.88-0.86~1.58
2.3 不同浓度IBA和Ac对生根培养的影响
由表10~表12可知,不同质量浓度的IBA和Ac在不同时间的暗处理下对不同砧木根长及生根系数的影响程度各不相同,各处理间存在显著差异(p<0.05),以暗培养7 d、0.10 g·L-1的Ac 更有利于根的生长。5BB和SO4砧木的根长和生根系数整体随着暗培养时间、IBA质量浓度的增加而增加,IBA质量浓度为1.50 mg·L-1时,5BB 砧木的生根系数可达2.60,生长状况如图7-A所示,SO4砧木的生根系数可达1.47,生长状况如图7-C所示。1103P砧木随着IBA 质量浓度的增加呈现先增加后减少的变化趋势,当IBA质量浓度为1.00 mg·L-1时,生根系数可达2.30,生长状况如图7-B所示。

图7 不同砧木生根培养的生长状况
Fig.7 Growth status of rooting culture of different rootstocks
表10 不同浓度IBA 和Ac 对5BB 生根培养的影响
Table 10 Effects of IBA and Ac at different concentrations on rooting culture of 5BB

注:同列数据后不同小写字母表示差异显著(p<0.05)。下同。
Note:Different lowercase letters after the same column of data show significant differences(p<0.05).The same below.
处理编号Treatment number培养基Medium ρ(吲哚丁酸)IBA concentration/(mg·L-1)ρ(活性炭)Ac concentration/(mg·L-1)3暗培养时间Dark-treated time/d 0根长Root length/mm 34.86±2.69 ab 39.47±3.17 a 0.000.0025.43±5.18 c 0.000.0029.61±0.91 bc 27.35±1.65 c 0.000.000.007123456789生根率Rooting coefficient/%0.50±0.06 e 1.00±0.12 d 0.50±0.06 e 1.77±0.15 bc 1.03±0.12 d 0.53±0.07 e 2.00±0.15 b 1.53±0.12 c 1.47±0.15 c 2.07±0.12 b 2.60±0.12 a 0.60±0.12 e 1/2MS 1011120.000.000.000.500.500.501.001.001.001.501.501.500.000.100.200.000.100.200.000.100.200.000.100.20生根率Rooting coefficient/%0.63±0.67 c 0.37±0.33 d 0.000.000.93±0.67 b 0.000.001.33±0.15 a 0.97±0.09 b 0.000.000.00根长Root length/mm 37.87±2.77 bc 46.12±0.74 a 0.0012.42±0.59 de 10.94±0.31 e 0.0015.29±0.61 d 35.47±0.72 c 40.12±2.83 b 0.000.000.00生根率Rooting coefficient/%0.43±0.07 cd 0.27±0.03 e 0.000.40±0.06 de 0.57±0.07 c 0.000.53±0.09 cd 1.60±0.06 a 1.10±0.06 b 0.000.000.00根长Root length/mm 42.08±1.26 b 59.31±2.61 a 8.43±4.23 d 26.54±0.69 c 25.65±0.43 c 12.39±0.58 d 12.61±0.69 d 41.23±0.44 b 41.57±0.65 b 13.02±0.40 d 27.26±0.58 c 12.46±0.57 d
表11 不同浓度IBA 和Ac 对1103P 生根培养的影响
Table 11 Effects of IBA and Ac at different concentrations on rooting culture of 1103P
处理编号Treatment number培养基Medium ρ(吲哚丁酸)IBA concentration/(mg·L-1)ρ(活性炭)Ac concentration/(mg·L-1)3暗培养时间Dark-treated time/d 0根长Root length/mm 0.000.000.0012.84±1.64 b 0.000.000.0018.93±1.96 a 0.000.000.0019.15±2.20 a 71234567891/2MS 1011120.000.000.000.500.500.501.001.001.001.501.501.500.000.100.200.000.100.200.000.100.200.000.100.20生根率Rooting coefficient/%0.000.000.001.37±0.88 a 0.000.000.001.10±0.12 b 0.000.000.001.03±0.13 b根长Root length/mm 0.000.000.0011.83±1.82 d 23.82±0.75 b 0.000.0028.43±1.61 a 18.07±1.87 c 0.0016.19±1.84 c 0.00生根率Rooting coefficient/%0.000.000.001.43±0.18 ab 1.60±0.10 ab 0.000.001.67±0.35 a 1.27±0.13 b 0.001.23±0.03 b 0.00根长Root length/mm 0.000.0025.69±4.30 b 12.72±1.59 c 29.49±1.60 b 30.84±1.31 ab 0.0036.58±1.95 a 31.83±3.82 ab 0.0017.25±0.39 c 17.21±1.33 c生根率Rooting coefficient/%0.000.001.50±0.15 bc 1.03±0.19 d 1.47±0.07 bc 1.33±0.24 cd 0.002.30±0.10 a 1.80±0.11 b 0.001.43±0.15 bc 1.27±0.15 cd
表12 不同浓度IBA 和Ac 对SO4 生根培养的影响
Table 12 Effects of IBA and Ac at different concentrations on rooting culture of SO4

处理编号Treatment number培养基Medium ρ(吲哚丁酸)IBA concentration/(mg·L-1)ρ(活性炭)Ac concentration/(mg·L-1)3暗培养时间Dark-treated time/d 0根长Root length/mm 0.000.000.000.000.0020.35±2.69 b 0.0025.29±2.61 a 0.000.0020.13±1.64 b 0.0071234567891/2MS 1011120.000.000.000.500.500.501.001.001.001.501.501.500.000.100.200.000.100.200.000.100.200.000.100.20生根率Rooting coefficient/%0.000.000.000.000.000.70±0.12 a 0.000.67±0.03 a 0.000.000.87±0.20 a 0.00根长Root length/mm 0.000.000.000.000.0024.44±2.91 bc 0.0027.78±2.22 ab 32.56±5.34 a 0.0021.49±2.50 c 0.00生根率Rooting coefficient/%0.000.000.000.000.000.77±0.03 c 0.001.07±0.07 b 1.33±0.17 a 0.000.97±0.07 b 0.00根长Root length/mm 0.000.000.009.16±0.82 c 33.48±5.41 b 30.57±4.16 b 0.000.0035.27±2.23 b 7.19±1.04 cd 42.63±2.52 a 0.00生根率Rooting coefficient/%0.000.000.001.20±0.15 b 0.83±0.03 c 1.27±0.07 ab 0.000.000.67±0.09 c 0.70±0.12 c 1.47±0.15 a 0.00
3 讨论
3.1 不同培养基对茎段初代培养的影响
培养基是外植体赖以生存和发展的营养基质,其无机物、有机物含量的差异会对芽的诱导造成不同的影响[18]。杨光等[19]对状元红葡萄研究时发现,相比MS 培养基和B5 培养基,1/2MS 培养基可获得更高的茎段腋芽萌发率,这可能是因为1/2MS 培养基中无机盐含量较少,低盐反而促进了芽的萌发。B5 培养基为高硝酸钾培养基,铵盐较少,对试管苗的生长抑制作用较小,有利于芽的萌发[20]。Mozafari等[21]研究时发现,MS 培养基比WPM 培养基更适合Khoshnav、Farkhi、Bidaneh Sefid葡萄外植体的培养,更利于芽的生长。这与本研究结果一致,即MS 培养基更利于茎段腋芽的萌发。
3.2 不同植物生长调节剂对增殖系数的影响
继代培养是试管苗增殖扩繁的重要环节之一,除营养物质外,还可通过多种植物生长调节剂协同作用共同促进试管苗生长,从而提高增殖效果[22]。植物生长调节剂浓度范围过高或过低都会影响外植体的生长,而利用二次回归正交旋转组合设计,可进一步优化各个植物生长调节剂的浓度范围,从而更准确地计算出适宜不同品种所需的浓度范围[23]。Alizadeh等[24]对4个遗传不同的葡萄砧木Dogridge、SO4、H-144和3309C研究时发现,6-BA和NAA的联合使用更利于促进芽的增殖,即当6-BA质量浓度为2.00 mg·L-1,NAA 质量浓度为0.20 mg·L-1更利于芽的增殖,并且在一定程度上可以缩短培养周期。Batukaev 等[25]研究时发现,0.50 mg·L-1 6-BA 和2.00 mg·L-1 KT有利于提高复杂抗性葡萄品种芽的增殖效果。Kinfe等[26]研究时发现,不同质量浓度的BAP 和IBA 能促进芽的增殖,当6-BA 质量浓度为1.00 mg·L-1,IBA质量浓度为0.10 mg·L-1时,Chenin blanc 葡萄和Canonannon 葡萄的增殖效果最好,在6-BA 质量浓度为2.00 mg·L-1,IBA 质量浓度为0.10 mg·L-1时,Ugni blanc 葡萄的增殖效果最好。Khan等[27]对King’s Ruby葡萄研究时发现,在1.00 mg·L-1 6-BA和0.10 mg·L-1 GA3组合优化的培养基中,芽数量和长度最大。6-BA和KT是较为稳定的细胞分裂素,可以促进细胞分裂和扩大,在增殖培养阶段,NAA 增殖效果略强于IBA,GA3可以打破休眠从而促进细胞生长[28]。由此可知,对于不同品种,选择合适的植物生长调节剂及其浓度是提高增殖系数的关键所在[29]。
3.3 不同浓度IBA和Ac对生根培养的影响
生根培养是植株成苗的关键,除了受植物生长调节剂的影响外,还与环境等多种因素的影响有关[30]。本研究表明,适宜5BB、SO4葡萄砧木生根培养的IBA 质量浓度为1.50 mg·L-1,适宜1103P 葡萄砧木生根培养的IBA 质量浓度为1.00 mg·L- 1。Amiri等[31]对Sultanine葡萄研究时发现,在1/2MS培养基中添加1.00 mg·L-1 IBA 生根率最高,可达95%。IBA作为常见的生长素对诱导不定根的生长具有一定的特异性[32],同时,在生根培养基中加入一定质量浓度的Ac会增加根长和生根频率,对提高根系质量至关重要[33]。笔者在本研究中发现,添加Ac的处理中,试管苗的生根情况均优于未添加Ac的处理。Lazo等[34]对火焰无核的研究中发现,在1/2MS培养基中添加2.00 mg·L-1 IBA、0.20 g·L-1Ac,根系可获得较好的发育。由此可知,适量的活性炭可通过吸附作用在创造黑暗环境的同时促进试管苗生根[35]。
4 结论
本研究表明,最适宜3 种葡萄砧木初代培养的培养基均为MS 培养基,萌发率均可达100%。最适宜5BB 和1103P 葡萄砧木的6-BA 质量浓度为2.00 mg·L-1、IBA 质量浓度为0.25 mg·L-1、GA3质量浓度为0.50 mg·L-1;最适宜SO4葡萄砧木的6-BA质量浓度为1.52 mg·L-1、IBA质量浓度为0.25 mg·L-1、GA3质量浓度为0.36 mg·L-1。最适宜3 种葡萄砧木生根培养的暗培养时间均为7 d,Ac 质量浓度均为0.10 g·L-1,最适宜5BB、SO4 葡萄砧木生根培养的IBA 质量浓度为1.50 mg·L-1,5BB 葡萄砧木的生根系数可达2.60,SO4葡萄砧木的生根系数可达1.47;最适宜1103P葡萄砧木生根培养的IBA质量浓度为1.00 mg·L-1,生根系数可达2.30。
[1] 贺普超.葡萄学[M].北京:中国农业出版社,1994:12-15.HE Puchao. Enology[M]. Beijing:China Agricultural Press,1994:12-15.
[2] 李小红,李运景,马晓青,郭军,刘海礁,郑国清,陶建敏.我国葡萄产业发展现状与展望[J].中国南方果树,2021,50(5):161-166.LI Xiaohong,LI Yunjing,MA Xiaoqing,GUO Jun,LIU Haijiao,ZHENG Guoqing,TAO Jianmin. Development status and prospect of grape industry in China[J]. South China Fruits,2021,50(5):161-166.
[3] 由佳辉,高林,王海鸥,卢倩倩,周龙,李树德.干旱胁迫对9 个葡萄砧木品种生理指标的影响[J].经济林研究,2020,38(3):180-189.YOU Jiahui,GAO Lin,WANG Haiou,LU Qianqian,ZHOU Long,LI Shude. Effects of drought stress on physiological indexes of nine grape rootstock varieties[J]. Non-wood Forest Research,2020,38(3):180-189.
[4] 鲁倩君,陈丽靓,马媛媛,刘迎,赵云文,赵宝龙,孙军利.盐碱胁迫对不同葡萄砧木光合及叶绿素荧光特性的影响[J].果树学报,2022,39(5):773-783.LU Qianjun,CHEN Liliang,MA Yuanyuan,LIU Ying,ZHAO Yunwen,ZHAO Baolong,SUN Junli. Effects of saline-alkali stress on photosynthetic and chlorophyll fluorescence characteristics of different grape rootstocks[J]. Journal of Fruit Science,2022,39(5):773-783.
[5] 侯毅兴,阿克居里德孜·努尔改里得,薛靖,周龙,李树德.鲜食葡萄砧穗组合生理指标及亲和力分析[J].中外葡萄与葡萄酒,2022(2):33-37.HOU Yixing,Akejulidezi·Nuergailide,XUE Jing,ZHOU Long,LI Shude.Analysis on physiological indexes and affinity of different stock-scion combinations of table grapes[J]. Sino-Overseas Grapevine&Wine,2022(2):33-37.
[6] 郑秋玲,刘万好,刘笑宏,谭洋洋,肖慧琳,王建萍,宋志忠,宫磊,唐美玲,卢建声.4 种砧木对‘赤霞珠’葡萄果实品质及抗氧化活性的影响[J].中国果树,2021(9):36-41.ZHENG Qiuling,LIU Wanhao,LIU Xiaohong,TAN Yangyang,XIAO Huilin,WANG Jianping,SONG Zhizhong,GONG Lei,TANG Meiling,LU Jiansheng. Effects of four rootstocks on fruit quality and antioxidant activity of‘Cabernet Sauvignon’grape[J].China Fruits,2021(9):36-41.
[7] JAMWAL M,SINGH B,SHARMA N,KUMAR R,ARTI S,SHARMA A,WALI V K,PARMAR A M. In vitro regeneration of grape(Vitis vinifera L.)cv.Perlette[J].World Journal of Agricultural Sciences,2013,9(2):161-166.
[8] PEDRO T S,PEIRO R,VILLANOVA J,OLMOS A,GISBERT C. In vitro propagation of Vitis vinifera L. cv. Monastrell[J].Electronic Journal of Biotechnology,2017,27:80-83.
[9] GARCÍA-GONZÁLES R,QUIROZ K,CARRASCO B,CALIGARI P.Plant tissue culture:current status,opportunities and challenges[J].Ciencia Einvestigación Agraria,2010,37(3):5-30.
[10] YILDIRIM H,OZDEMIR G. Influence of BAP concentrations and nutrient medium composition on in vitro regeneration of‘Öküzgözü’and‘Boğazkere’(Vitis vinifera L.)cultivars[J].Erwerbs-Obstbau,2018,60(1):55-59.
[11] 蔡文博,段虹,王军,朱元娣.4 个鲜食葡萄品种组培快繁体系的建立[J].核农学报,2019,33(2):248-254.CAI Wenbo,DUAN Hong,WANG Jun,ZHU Yuandi.Establishment of in vitro rapid micropropagation of four table grape cultivars[J].Journal of Nuclear Agriculture,2019,33(2):248-254.
[12] 林茜,高营营,覃换玲,黄天琨,赵宇,王钟霞,陈淑媛.‘阳光玫瑰’葡萄组培脱毒快繁技术研究[J].果树学报,2021,38(3):435-443.LIN Qian,GAO Yingying,QIN Huanling,HUANG Tiankun,ZHAO Yu,WANG Zhongxia,CHEN Shuyuan. Study on rapid propagation technology of virus-free seedlings by tissue culture in‘Shine Muscat’grape[J]. Journal of Fruit Science,2021,38(3):435-443.
[13] 农艳丰,王利园,李健.‘阳光玫瑰’葡萄嫩茎的组织培养研究[J].西南师范大学学报(自然科学版),2021,46(6):52-56.NONG Yanfeng,WANG Liyuan,LI Jian. On tissue culture of‘Sunshine Rose’grape stems[J]. Journal of Southwest Normal University(Natural Science Edition),2021,46(6):52-56.
[14] 苏玲,宫磊,尹向田,杨立英,杨阳.贵妃玫瑰葡萄组培技术探究[J].安徽农业科学,2020,48(8):51-53.SU Ling,GONG Lei,YIN Xiangtian,YANG Liying,YANG Yang.Study on tissue culture technology of the‘Guifeimeigui’grape[J].Journal ofAnhuiAgricultural Sciences,2020,48(8):51-53.
[15] YERBOLOVA L S,RYABUSHKINA N A,OLEICHENKO S N,KAMPITOVA G A,GALIAKPAROV N N. The effect of growth regulators on in vitro culture of some Vitis vinifera L.cultivars[J]. World Applied Sciences Journal,2013,23(1):76-80.
[16] 战吉宬,李德美.酿酒葡萄品种学[M].北京:中国农业大学出版社,2010:134-138.ZHAN Jicheng,LI Demei. Wine grape varieties[M]. Beijing:China Agricultural University Press,2010:134-138.
[17] 高林,冯琳骄,褚佳瑶,由佳辉,艾克热木·伊力哈木,周龙,陆彪.石生茶藨茎段快繁体系的建立[J].经济林研究,2021,39(4):69-78.GAO Lin,FENG Linjiao,CHU Jiayao,YOU Jiahui,Aikeremu·Yilihamu,ZHOU Long,LU Biao.Establishment of rapid propagation system of Ribes Saxatile Pall. stem segment[J]. Economic Forest Research,2021,39(4):69-78
[18] 王蒂,陈劲枫.植物组织培养[M].2 版.北京:中国农业出版社,2013:27-29.WANG Di,CHEN Jinfeng. Plant tissue culture[M]. 2nd ed.Beijing:China Agricultural Press,2013:27-29.
[19] 杨光,金桂花,董俊,张青,龚娜.‘状元红’葡萄的脱毒与快繁技术研究[J].浙江农业学报,2014,26(1):72-76.YANG Guang,JIN Guihua,DONG Jun,ZHANG Qing,GONG Na. Study on virus-free and rapid propagation techniques of‘Zhuangyuanhong’grape[J]. Acta Agriculturae Zhejiangensis,2014,26(1):72-76.
[20] 曹孜义.实用植物组织培养技术教程[M].兰州:甘肃科学技术出版社,1996:22-32.CAO Ziyi. Practical plant tissue culture technology tutorial[M].Lanzhou:GansuScienceandTechnologyPublishing,1996:22-32.
[21] MOZAFARI A A,GHORAISHI O,GHADERI N,JAVADI. T.Micropropagation of grape cultivars(Vitis vinifera L.)on different basal media supplemented with benzyl adenine[J]. Agriculturae Conspectus Scientificus,2016,81(3):23-129.
[22] 骆萌,王杰,李玉玲,宋士任,赵丽萍,许文平.‘新郁’葡萄离体再生体系的建立[J].北方园艺,2016(9):109-113.LUO Meng,WANG Jie,LI Yuling,SONG Shiren,ZHAO Liping,XU Wenping. Establishment of regeneration system of‘Xinyu’grapevine in vitro[J].Northern Horticulture,2016(9):109-113.
[23] 徐国前,栾丽英,焦旭亮,张振文.测定葡萄与葡萄酒总酚的二次回归正交旋转优化设计[J].果树学报,2011,28(3):531-535.XU Guoqian,LUAN Liying,JIAO Xuliang,ZHANG Zhenwen.Optimizing a simple determination method for total phenolics in grape and wine through quadratic regression rotational combination design[J].Journal of Fruit Science,2011,28(3):531-535.
[24] ALIZADEH M,SINGH S K,PATE V B,DESHMUKH P S.Comparative performance of in vitro multiplication in four grape (Vitis spp.) rootstock genotypes[J]. International Journal of Plant Production,2010,4(1):41-50.
[25] BATUKAYEV A A,PALAEVA D O,BATUKAVE M S,SOBRALIEVA E A.In vitro reproduction and ex vitro adaptation of complex resistant grape varieties[C]//International Conference on Smart Solutions for Agriculture(Agro-SMART 2018).Paris:Atlantis Press,2018:895-899.
[26] KINFE B,FEYSS A,BEDADA G. In vitro micropropagation of grape vine (Vitis vinifera L.) from nodal culture[J]. African Jouranl of Biotechnology,2017,16(43):2083-2091.
[27] KHAN N,AHMED M,HAFIZ I,ABBASI N,EJAZ S,ANJUM M. Optimizing the concentrations of plant growth regulators for in vitro shoot cultures,callus induction and shoot regeneration from calluses of grapes[J]. Journal International Des Sciences De La Vigne Et Du Vin,2015,49(1):37-45.
[28] 巩振辉,申书兴.植物组织培养[M].2 版.北京:化学工业出版社,2013:41-42.GONG Zhenhui,SHEN Shuxing. Plant tissue culture[M]. 2nd ed.Beijing:Chemical Industry Press,2013:41-42.
[29] 齐向丽,师校欣,杜国强.‘红国王’葡萄组织培养快速繁殖[J].分子植物育种,2020,18(3):982-987.QI Xiangli,SHI Xiaoxin,DU Guoqiang. Rapid propagation of‘Hongguowang’grape tissue culture in vitro[J]. Molecular Plant Breeding,2020,18(3):982-987.
[30] 董媛媛,柴慈江,夏瑞,黄洁萍,岑芬.环境、茎段状态与无糖培养基对葡萄试管苗生根的影响[J].天津农学院学报,2021,28(1):17-20.DONG Yuanyuan,CHAI Cijiang,XIA Rui,HUANG Jieping,CEN Fen. Effects of environment,stem section state and sugarfree medium on rooting of grape plantlet in vitro cultured in soil supporting medium[J]. Journal of Tianjin Agricultural University,2021,28(1):17-20.
[31] AMIRI S,MOHAMMADI R,AKBARI R.The effects of cytokinin and auxin interactions on proliferation and rooting of seedless grapevine (Vitis vinifera L.)‘Sultanine’[J]. Erwerbs-Obstbau,2019,61(1):85-92.
[32] ABIDO A I A,ALY M A M,HASSANEN S A,MOHAMED G.In vitro propagation of grapevine(Vitis vinifera L.)Muscat of Alexandria cv. for conservation of endangerment[J]. Middle-East Journal of Scientific Research,2013,13(3):328-337.
[33] OLAH R. The use of activated charcoal in grapevine tissue culture[J].Vitis Geilweilerhof,2017,56(4):161-171.
[34] LAZO M F,TRONCOSO R R,TIZNADO M E,MATINEZ M A,ARISPURO V,OSUMA M A,DOMINGUE R M. Surface disinfection procedure and in vitro regeneration of grapevine(Vitis vinifera L.)axillary buds[J].Springerplu,2016,5:453.
[35] ARIFUZZAMAN M,SULTANA S,HOSSAIN M S,SAIFULLA H. Paraphernalia of growth regulators during in vitro micropropagation of grapevine (Vitis vinifera L.) from shoot tips and nodal segments[J]. Scholars Journal of Agriculture and Veterinary Sciences,2016,3(4):326-331.