代谢组学于20世纪70年代由Devaux等[1]提出,主要研究生物体内源代谢物的种类、含量及其变化规律等。代谢物是生物体表型的基础,代谢水平可作为生物体对环境变化和遗传修饰的终端响应,通过其能更直观有效地了解生物学过程及其机制。植物代谢组学主要研究植物不同基因型及不同发育时期,或在受到某些刺激或胁迫前后代谢物的相应变化,包括其种类及含量,进而发现或验证一些基因及蛋白的功能,有助于进一步阐明多种植物天然产物的生物合成途径[2-3]。目前,代谢组学已应用于植物研究的多个方面,如不同芝麻品种间的营养差异[4]、四季豆的抗病性[5]、野生稻与栽培稻之间的代谢物差异[6]。
刺梨(Rosa roxburghii Tratt.)与无籽刺梨(R.sterilis S. D. Shi)均是蔷薇科蔷薇属多年生落叶果树,主要分布于中国西南地区,其中贵州省刺梨种质资源丰富。在笔者研究组收集的多个优异刺梨种质资源中,Rr-3 与多个刺梨基因型间的代谢物差异较小,更具代表性,且单果质量较大,有望成为贵农5号的补充栽培资源,以促进刺梨产量的提高[7]。此外,研究表明刺梨与无籽刺梨果实均富含生物活性物质,包括酚酸[8]、黄酮[9]、有机酸[10]、氨基酸[11-12]和维生素C[13]等。据《本草纲目拾遗》《中药大辞典》等记载,刺梨花、果、叶、根、籽可入药,有健胃、消食、滋补作用,根皮有止泻的功效等。刺梨还具抗癌、预防动脉粥样硬化和抗氧化[14-17]等功效。时京珍等[18]研究发现,刺梨与无籽刺梨的药理作用相似,均具有抗炎及镇痛作用。但两者间的营养成分及活性物质含量均有差异[19];刺梨的维生素C 及必需氨基酸含量高于无籽刺梨,被认为具较高营养价值[11];与刺梨相比,无籽刺梨具较高的总黄酮与总酚含量[20]。在刺梨与无籽刺梨代谢物研究中,刺梨具较高的儿茶素与杨梅素含量;而金刺梨酒石酸和槲皮素含量较高[21]。研究表明刺梨与无籽刺梨的主要香气物质也存在差异[22-23]。此前已有研究检测并鉴定了刺梨果实次生代谢物及中药化学成分[7];而无籽刺梨的次生代谢物研究多集中在酚酸、黄酮、萜类、氨基酸与挥发性物质等方面,其中药化学成分暂不清楚;因此,笔者在本研究中采用广泛靶向代谢组学技术检测和鉴定无籽刺梨果实的次生代谢物及中药化学成分。此外,研究表明,相较无籽刺梨,刺梨具更高抗氧化能力[24],但对刺梨及无籽刺梨中具抗氧化能力次生代谢物的研究主要集中在酚酸、黄酮、三萜及有机酸等分类方面[7,25-26],而研究表明鞣质也具有较高的抗氧化能力[27],因此笔者在本研究中以2 个刺梨基因型(Rr-5,Rr-3)与Rs 果实为试材进行代谢物分析,明确刺梨与无籽刺梨次生代谢物中的中药化学成分及抗氧化物质并分析其间的差异,为促进刺梨与无籽刺梨的合理应用提供理论参考。
1 材料和方法
1.1 材料
以无籽刺梨(R.sterilis S.D.Shi,简称Rs)与两个刺梨(R. roxburghii Tratt.)基因型[贵农3 号(Guinong 3,简称Rr-3)和贵农5 号(Guinong 5,简称Rr-5)]的成熟果实为试验材料。果实样品均采自贵州省贵阳市花溪区贵州大学刺梨种质资源圃(106°67.353′E,26°42.408′N),每个样本均来源于连续面积为3.35 hm2的刺梨圃中生长良好且树龄均为4~5 a(年)的植株。从各基因型的3 棵树的4 个方向的树冠边缘采集3 个果实作为生物学重复;每个生物学重复包括12 个果实。采集后立即去籽在液氮中快速冷冻,并于-80 ℃下储存备用。
1.2 样品前处理
首先将供试样品放置于冻干机(Scientz-100F)中真空冷冻干燥;后使用研磨仪(MM 400,Retsch)以30 Hz研磨1.5 min;称取100 mg粉末,溶解于1.2 mL 70%(φ)甲醇提取液中;溶解后的样品于4 ℃冰箱过夜,期间涡旋6 次;以12 000 r·min-1离心10 min后,取上清,用微孔滤膜(0.22 μm pore size)过滤样品,保存于进样瓶中,用于UPLC-MS/MS分析。
1.3 UPLC-MS/MS分析
液相条件-色谱柱:Agilent SB-C18 1.8 μm,2.1 mm×100 mm;流动相:A相为超纯水(加入0.1%甲酸),B相为乙腈(加入0.1%甲酸);洗脱梯度:0.00 min B相比例为5%,9.00 min内B相比例线性增加到95%并维持1 min,10.00~11.10 min,B相比例降为5%并平衡至14min;流速0.35 mL·min-1;柱温40 ℃;进样量4 μL。
质谱条件-电喷雾离子源温度550 ℃,质谱电压5500 V(正模式)/-4500 V(负模式),帘气25 psi,碰撞诱导电离参数设置为高。在三重四级杆中,每个离子对根据优化的去簇电压和碰撞能进行扫描检测[28]。
1.4 中药化学成分及抗氧化能力
将刺梨与无籽刺梨果实中检测到的次生代谢物在中药系统药理学数据库分析平台(Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform,TCMSP)进行查询,筛选出中药化学成分并找出其对应的靶点及疾病。后根据生物口服利用度(OB≥5%)、药物相似性(DL≥0.14)进一步筛选关键活性成分[29]。将所有代谢物在系统药理学抗癌草药数据库中查询(CancerHSP,其中抗癌草药2439 种,抗癌成分3575 种),鉴定其中抗癌/肿瘤成分。在TCMSP 数据库的疾病名称菜单中输入“糖尿病”“心血管疾病”“高血压”“动脉粥样硬化”和“血栓性疾病”。然后下载每种抗病物质的相关成分。最后,将UPLC-MS/MS中鉴定出的代谢物与获得的抗病相关成分进行比较,鉴定出试样中抗5 种疾病相关的活性药物成分。
1,1-二苯基-2-三硝基苯肼(1,1-Diphenyl-2-picrylhydrazyl radical,DPPH)自由基清除活性、2,2-联氮-二(3-乙基-苯并噻唑-6-磺酸)二铵盐[2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate),ABTS]阳离子自由基清除活性和铁离子还原活性(ferric ion reducing antioxidant power,FRAP)测定参考Moure等[30]、Martinez-Villaluenga等[31]和Benzie 等[32]的方法且略有调整;其中Rr 的3 种抗氧化能力数据由Rr-3与Rr-5 求和后取平均值而得。并将代谢物相对含量与三种抗氧化能力进行相关性分析,鉴定出刺梨与无籽刺梨中主要的抗氧化代谢物。
1.5 数据分析
基于自建数据库MWDB(metware database),根据二级谱信息进行物质定性,获得不同样本的代谢物质谱分析数据后,对所有物质质谱峰进行峰面积积分,并对其中同一代谢物在不同样本中的质谱出峰进行积分校正[33]。采用多元数据统计分析,对样本进行主成分分析(principal component analysis,PCA)、聚类分析和正交偏最小二乘判别分析(orthogonal PLS-DA,OPLS-DA)。结合差异倍数值和OPLS-DA 模型的变量重要性投影(variable importance in projection,VIP)值筛选差异代谢物。筛选标准:选取fold change≥2 和fold change≤0.5,且VIP≥1。对所测抗氧化能力利用SPSS进行单因素方差分析,并对抗氧化能力与代谢物相对定量进行皮尔逊相关性分析。
2 结果与分析
2.1 次生代谢物总体分析
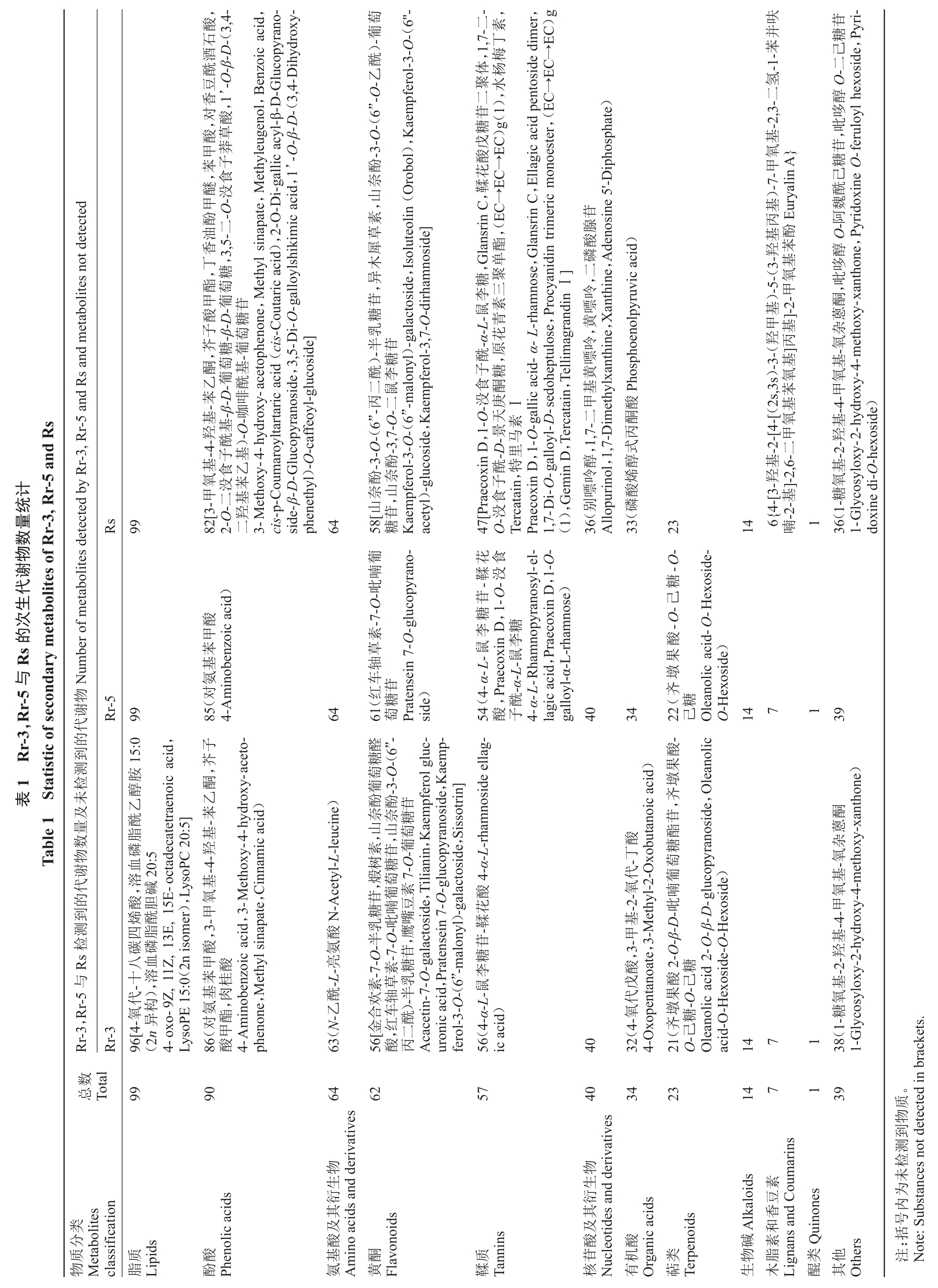
通过对质控样本(quality control sample,QC)质谱检测分析的总离子流图(图1-A)及多反应监测模式的代谢物检测多峰图(图1-B)进行展示分析,可以判断代谢物提取和检测的结果稳定,数据可靠且重复性良好。笔者在本研究中共检测到12类530种次生代谢物,其中包括99种脂质,90种酚酸,64种氨基酸及其衍生物,62种黄酮,57种鞣质,40种核苷酸及其衍生物,34种有机酸,23种萜类,14种生物碱,7种木脂素和香豆素,1种醌类和39种其他物质(表1)。

图1 刺梨及无籽刺梨代谢概况分析
Fig.1 Analysis on metabolism of R.roxburghii and R.sterilis
A.QC 样本质谱检测分析的总离子流图;B.多反应监测模式下代谢物检测多峰图谱;C.Rr-3,Rr-5 和Rs 的主成分分析得分图;D.Rr-3,Rr-5和Rs 次生代谢物相对定量聚类分析热图。
A.Total ion flow diagram of mass spectrometry detection and analysis of QC samples;B.Multi peak map of metabolite detection in multi reaction monitoring mode;C.Principal component analysis scores of Rr-3,Rr-5 and Rs;D.Cluster analysis heat map of relative quantity of Rr-3,Rr-5 and Rs secondary metabolites.
?
所有样本的主成分分析结果如图1-C 所示,每组的3 个重复样本明显聚集,组间明显分离。与PCA 结果一致,3 个样本的相对定量热图也具有明显差异(图1-D)。结果表明,刺梨和无籽刺梨的代谢物存在明显差异。因此,进一步分析刺梨与无籽刺梨的特有代谢物。分析表明,刺梨与无籽刺梨间存在较大差异,31 种代谢物仅在刺梨中检测到,包括10种单宁、8种酚酸、4种黄酮类化合物、4种核苷酸及其衍生物、1 种有机酸、1 种木脂素和香豆素以及3 种其他代谢物(表1)。齐墩果酸-O-己糖-O-己糖、对氨基苯甲酸、红车轴草素-7-O-吡喃葡萄糖苷和4-α-L-鼠李糖苷-鞣花酸这4种物质仅在无籽刺梨中检测到。这些物质可被作为区分刺梨与无籽刺梨的生物标志物。
2.2 中药化学成分鉴定
由于两类果实中具健康促进作用的次生代谢物尚不清楚。因此,笔者在中药系统药理学数据库与分析平台(TCMSP)中进一步查询,鉴定刺梨与无籽刺梨果实中药化学成分及关键活性成分。结果表明,530 种次生代谢物中有118 种是中药化学成分(包括25 种酚酸,16 种鞣质,12 种核苷酸及其衍生物,12 种氨基酸及其衍生物,12 种黄酮,11 种有机酸,9种其他,8种脂质,7种萜类,3种生物碱,3种木脂素与香豆素),其中91 种成分对应347 种靶点及359种疾病(表2)。在118种中药化学成分中,36种是关键活性成分,其中11 种代谢物(罗布麻酸、二十碳烯酸、西索林、尿苷5’-二磷酸、鸟苷、绿原酸、2α-羟基熊果酸、狭窄素、肉豆蔻酸、特卡坦、双子D)没有相应的靶点和疾病(表2)。因此,可对这些物质进行深入研究,探索其药用价值,以用于新药研发。更重要的是,丁香油酚甲醚、黄嘌呤、苯甲酸、特里马素Ⅰ、Tercatain、Glansrin C 和水杨梅丁素仅在一个或两个刺梨基因型中检测到,且Tercatain 和水杨梅丁素是关键活性成分。因此,这些物质可被认为是刺梨与无籽刺梨间药用价值差异的主要成分。以上结果表明,刺梨比无籽刺梨具更高的药用价值。
表2 530 种次生代谢物中的36 种关键活性成分
Table 2 36 key active components in 530 secondary metabolites

注:N/A 表示无。
Note:N/A means none.
ID Zmpn006881 pmp000269 pmn001606 pmn001514 pmn001426 pmf0359物质名称Metabolites name委陵菜酸Tormentic acid阿江榄仁酸Arjunic acid二十碳烯酸Eicosenoic acid双没食子酸Digallic acid蔷薇酸Rosamultic acid茵芋苷Skimmin生物口服利用度Oral Bioavailability(OB)/%11.40 26.73 28.64 61.85 11.40 38.35药物相似性Druglikeness(DL)0.71 0.72 0.20 0.26 0.71 0.32对应靶点Related targets numbers 1 N/A N/A 3 1 4相应疾病Related diseases numbers 1 N/A N/A 47 1 47 pme340019.630.79N/A N/A pme30077.940.32N/A N/A pme2246 pme2125 pme1178鹰嘴豆素7-O-葡萄糖苷Sissotrin尿苷5’-二磷酸Uridine 5’-diphosphate鞣花酸Ellagic acid棉籽糖Raffinose鸟苷Guanosine 43.06 11.79 21.43 0.43 0.66 0.21 20 4 N/A 64 7 N/A pme0460 pme0458 48.96 53.57 0.24 0.75 55 1 pme023015.980.1862 pmb0889 mws1521 mws1491 mws1397 44.90 7.15 41.90 17.89 0.15 0.16 0.14 0.75 6 1 7 3 1 1 34 32 53 12 mws11799.330.7432 mws107722.910.3934 mws088419.470.3535 mws0836 mws0396 mws0232 mws0191 mws0178 mws0055 mws0054 mws0042物质分类Metabolites classification萜类Terpenoids萜类Terpenoids脂质Lipids酚酸类Phenolic acids萜类Terpenoids木脂素和香豆素Lignans and coumarins黄酮Flavonoids核苷酸及其衍生物Nucleotides and derivatives鞣质Tannins其他类Others核苷酸及其衍生物Nucleotides and derivatives黄酮Flavonoids酚酸类Phenolic acids核苷酸及其衍生物Nucleotides and derivatives脂质Lipids酚酸类Phenolic acids脂质Lipids黄酮Flavonoids黄酮Flavonoids木脂素和香豆素Lignans and coumarins核苷酸及其衍生物Nucleotides and derivatives鞣质Tannins脂质Lipids其他类Others生物碱Alkaloids酚酸类Phenolic acids黄酮Flavonoids黄酮Flavonoids黄酮Flavonoids 67.87 33.13 6.79 24.80 11.93 21.38 54.83 24.18 0.66 0.14 0.50 0.55 0.33 0.43 0.24 0.27 5 1 2 2 2 1 1 24 2 4 N/A 29 11 8 61 85 39 37 N/A 118 57 60 Lmzn006284萜类Terpenoids 18.560.74N/A N/A Lmhn002379 Lmdp003286 Hmln003605 HmdP001587 HJN109 Cmhn001271 N/A 11 N/A N/A 4 N/A表儿茶素Epicatechin儿茶素没食子酸Catechin gallate腺苷Adenosine石榴酸Punicic acid水杨苷Salicin亚油酸Linoleic acid表儿茶素没食子酸酯Epicatechin gallate樱桃苷Naringenin-7-O-glucoside东莨菪苷Scopolin腺苷-3’-5’-环单磷酸水合物Cyclic AMP原花青素B1 Procyanidin B1反油酸Elaidic acid核黄素Riboflavin甜菜碱Betaine绿原酸Chlorogenic acid橘皮素Tangeretin儿茶素Catechin表没食子酸儿茶素Epigallocatechin 2α-羟基熊果酸2α-hydroxyursolic acid Strictinin异金丝桃苷isohyperoside Myrianthic acid Tercatain齐墩果酸oleanolic acid水杨梅丁素Gemin D鞣质Tannins黄酮Flavonoids萜类Terpenoids鞣质Tannins萜类Terpenoids鞣质Tannins 12.93 8.31 14.85 26.67 29.02 27.39 0.44 0.77 0.76 0.21 0.76 0.56 N/A 2 N/A N/A 6 N/A
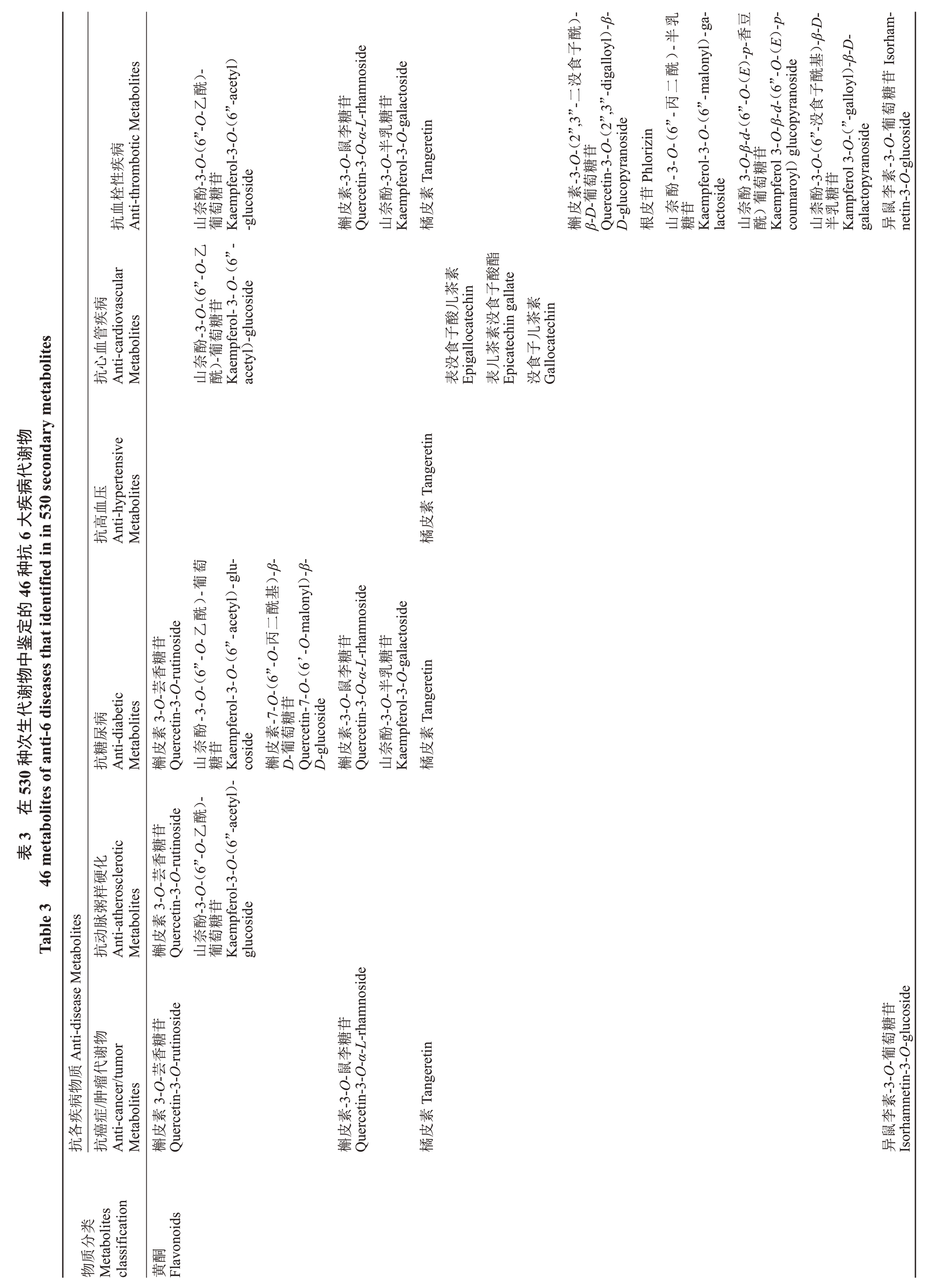
目前,世界范围内威胁人类健康的主要疾病包括癌症/肿瘤、糖尿病、高血压、心血管疾病、动脉粥样硬化和血栓性疾病。为鉴定刺梨与无籽刺梨果实中抗六大疾病的活性成分,笔者对检测的代谢物进行CancerHSP及TCMSP数据库查询。结果表明,刺梨及无籽刺梨果实中共有46种代谢物(包括15种黄酮,11种酚酸,8种鞣质,4种萜类,2种氨基酸及其衍生物,2 种核苷酸及其衍生物,2 种生物碱,2 种有机酸)至少对应一种疾病,其中迷迭香酸对应六大疾病,还有多种代谢物对应3~4种疾病(表3)。更重要的是,山奈酚-3-O-(6”-O-乙酰)-葡萄糖苷、山奈酚-3-O-(6”-丙二酰)-半乳糖苷仅在刺梨中检测到,且分别对应4种与2种疾病。
?

?
2.3 抗氧化能力分析及抗氧化物质鉴定
由图2-A 可知,Rr 的3 种抗氧化能力均显著高于Rs(p<0.05)。但其中主要的抗氧化物质并不清楚。分析表明,笔者在本研究中检测到的530 种次生代谢物中,145种物质与抗氧化能力显著相关,包括38种鞣质、27种氨基酸及其衍生物、23种酚酸、18种核苷酸及其衍生物、13种黄酮、5种有机酸、4种三萜、3 种木脂素与香豆素、3 种脂质、2 种生物碱和9种其他物质(包括维生素C)(图2-B)。仅在刺梨中检测到的物质有20 种,而1-O-没食子酰-α-L-鼠李糖、2-O-二没食子酰基-β-D-葡萄糖-β-D-葡萄糖、Glansrin C、1’-O-β-D-(3,4-二羟基苯乙基)-O-咖啡酰基-葡萄糖苷、3,5-二-O-没食子莽草酸和原花青素三聚单酯、Tercatain,特里马素Ⅰ和对香豆酰酒石酸这9 种代谢物与3 种抗氧化能力均显著相关(p<0.05)。而仅在无籽刺梨中检测到的4种代谢物均与抗氧化能力呈负相关。这一结果与测得的两类果实的抗氧化能力结果一致。因此,笔者认为刺梨具有更高的抗氧化能力,且富含更多具有抗氧化能力的次生代谢物。
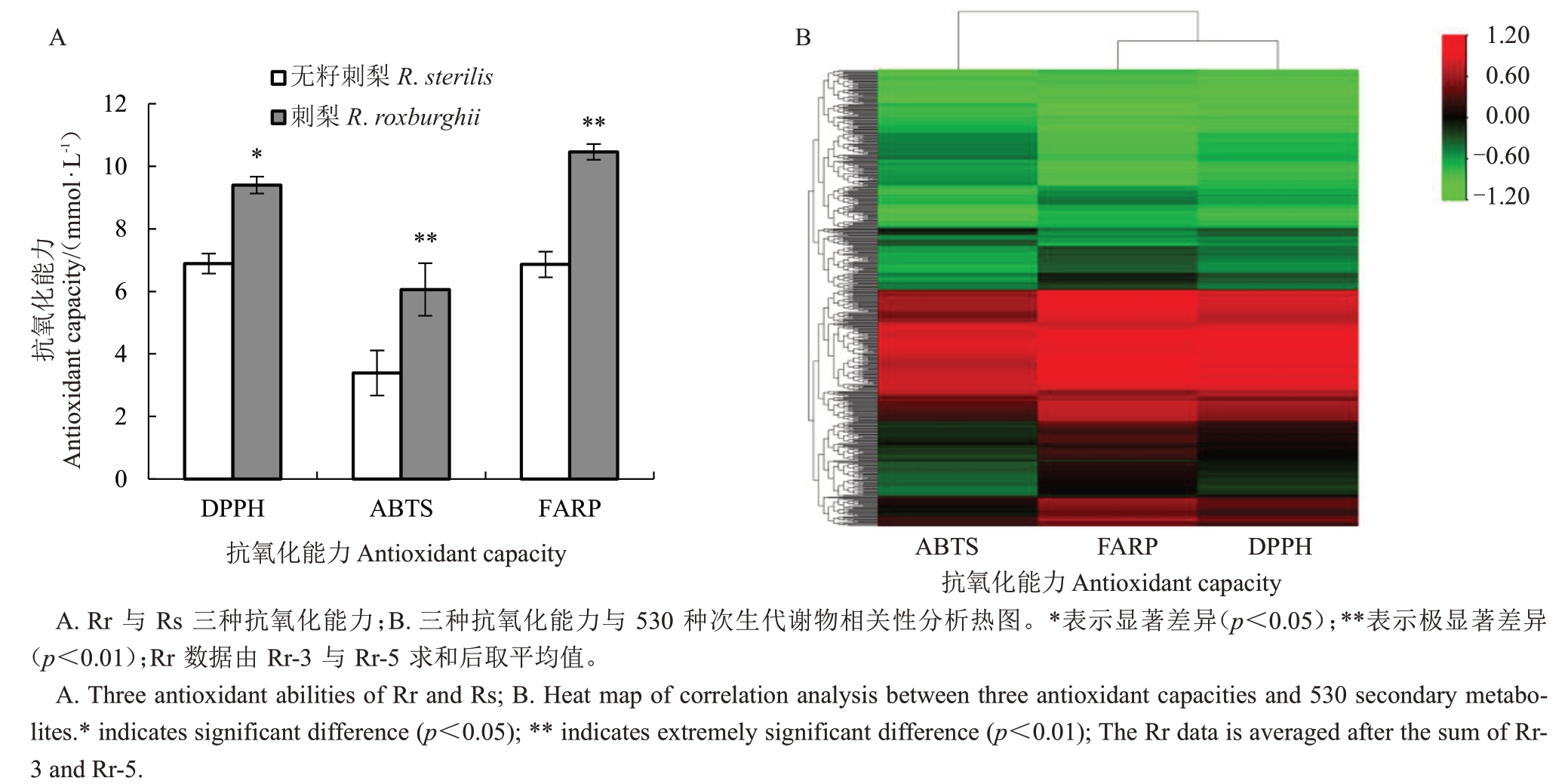
图2 刺梨与无籽刺梨抗氧化能力分析
Fig.2 Analysis on antioxidant capacity of R.roxburghii and R.sterilis
2.4 差异代谢物筛选及差异代谢通路分析
通过对代谢物数据进行多元统计分析,基于OPLS-DA 结果(图3-A,C)及获得的多变量分析OPLS-DA 模型的VIP,同时结合差异倍数值来筛选差异代谢物。由火山图可见,Rr-3 与Rs 间有272 种差异代谢物(图3-B),Rr-5 与Rs 间有242 种差异代谢物(图3-D),而两个比较组间筛选出171 种共有的差异代谢物,包括37 种鞣质、34 种酚酸、21 种核苷酸及其衍生物、20 种黄酮、18 种氨基酸及其衍生物、13 种脂质、8 种有机酸、7 种萜类、4 种木脂素和香豆素、1 种生物碱与8 种其他物质(图3-E)。其中111 种代谢物在刺梨中的相对定量相较于Rs 来说是上调的,其中有87 种抗氧化物质及31 种中药化学成分(分类如下:12 种鞣质,7 种核苷酸,4 种有机酸,4种酚酸,2种氨基酸,1种其他,1种萜类),且其中8 种差异代谢物是关键活性成分,分别是双没食子酸、腺苷、鸟苷、腺苷-3’-5’-环单磷酸水合物、鞣花酸、水杨梅丁素、Tercatain 和Myrianthic acid;其中23 种物质既是抗氧化物质也是中药化学成分(丙二酸,胞嘧啶,组胺,延胡索酸,胸腺嘧啶,L-谷氨酸,黄嘌呤,吡哆素,没食子酸,没食子酸甲酯,芥子醇,尿嘧啶核苷,鸟苷,鞣花酸,双没食子酸,迷迭香酸,水杨梅丁素,英国栎鞣花酸,特里马素Ⅰ,Glansrin C,Pterocarinin A,Glansrin A,Platycaryanin A)。而Rs 中上调的差异代谢物有53 种,其中仅10种差异代谢物(3种酚酸,2种黄酮,2种有机酸,1种脂质,1 种木脂素与香豆素,1 种其他物质)是中药化学成分,仅3 个关键活性成分,分别是石榴酸、绿原酸和樱桃苷。

图3 两个刺梨基因型分别与无籽刺梨差异代谢物分析
Fig.3 Analysis of differential metabolites in two R.roxburghii and R.sterilis
根据2个刺梨基因型与无籽刺梨比较筛选出的差异代谢物进一步进行差异富集通路分析。结果表明,Rr-3与Rs间272种差异代谢物共注释到73条代谢通路;Rr-5与Rs间242种差异代谢物共注释到66条代谢通路;在两个刺梨与无籽刺梨的差异代谢物富集的差异代谢通路中,前5 条高富集程度的代谢通路是嘌呤代谢、嘧啶代谢、玉米素生物合成、碳代谢和氨基酸生物合成(图4)。这一结果表明上述5条差异代谢通路可能被认为是刺梨与无籽间主要的差异代谢通路。

图4 刺梨与无籽刺梨差异代谢通路分析
Fig.4 Analysis on differential metabolic pathways of R.roxburghii and R.sterilis
3 讨 论
笔者首次鉴定并比较分析了两类果实的中药化学成分及抗氧化物质。前人研究主要集中在两类果实挥发性物质[20,23-25,34]、酚酸[7]、氨基酸[11-12]和黄酮[9]类代谢物中,但其中药化学成分及抗氧化物质尚不清楚。而刺梨与无籽刺梨在市场上常被混用,研究表明无籽刺梨与刺梨及不同刺梨基因型间的营养价值、药用价值及抗氧化能力存在显著差异[8,19,24]。因此,笔者基于广泛靶向代谢组学技术对刺梨及无籽刺梨果实的次生代谢物进行检测,鉴定并比较分析其中药化学成分及抗氧化物质差异。
在不同刺梨基因型代谢物研究中,检测到723种次生代谢物,鉴定出172 种中药化学成分及41 种抗氧化物质[7]。笔者在本研究中共检测到530 种次生代谢物,31种物质仅在刺梨中检测到,4种仅在无籽刺梨中检测到;鉴定出118种中药化学成分(关键活性成分36种)及145种抗氧化物质,其中数量较多的均是鞣质与酚酸类代谢物,表明酚类物质在刺梨与无籽刺梨果实的中药活性及抗氧化能力中起重要作用。此外,笔者还发现91 种中药化学成分对应347 个靶点及359 种疾病。进一步分析发现其中46种物质对6 大疾病中的一种或多种疾病具抗性,而迷迭香酸对6 种疾病均有抗性。由于其采样、送检时间的不同,迈维数据库有所更新,因此两次试验检测到的次生代谢物数量存在差异,而笔者检测到的部分代谢物在上述研究中均未检测到,丰富了两类果实中次生代谢物的种类及数量。并深入挖掘了其中抗六大疾病的次生代谢物与中药化学成分及其靶点与疾病,首次鉴定了无籽刺梨果实的中药化学成分。
前人研究表明,刺梨果实中总酚、总黄酮及总三萜与抗氧化能力显著相关[35-36],其中2种有机酸、5种鞣花酸及其衍生物、11 种黄酮类化合物和1 种三萜类化合物,是促进刺梨果实提取物抗氧化和酪氨酸酶抑制活性的主要化合物[25]。刺梨具较高抗氧化能力很大程度上归因于有机酸与酚类化合物,如没食子酸、儿茶素、表儿茶素和表没食子酸,由于羟基数量较多,它们可以作为良好的电子供体发挥自由基清除作用[26]。刺梨果实中主要的抗氧化物质的研究结果表明11种酚酸、9种黄酮及21种萜类物质与抗氧化能力显著相关[7]。上述研究表明酚酸类、类黄酮类及萜类物质是刺梨果实中主要的抗氧化物质。笔者在本研究中发现145种次生代谢物分别与至少一种抗氧化能力显著相关,其中鞣质类物质数量最多,其次是氨基酸及其衍生物与酚酸类物质。鞣质化学结构复杂,是具较高抗氧化能力的酚类物质,测定的总酚含量中包含鞣质[27]。此前的研究均表明刺梨总酚含量与抗氧化能力显著相关,与本研究结果一致。且笔者进一步挖掘出刺梨与无籽刺梨果实中主要的抗氧化物质。更重要的是,仅在刺梨果实中检测到的代谢物中有20种抗氧化物质{1-O-没食子酰-α-L-鼠李糖,异木犀草素,4-[3-羟基-2-[4-[(2s,3s)-3-(羟甲基)-5-(3-羟基丙基)-7-甲氧基-2,3-二氢-1-苯并呋喃-2-基]-2,6-二甲氧基苯氧基]丙基]-2-甲氧基苯酚,山奈酚-3-O-(6”-O-乙酰)-葡萄糖苷,Praecoxin D,2-O-二没食子酰基-β-D-葡萄糖-β-D-葡萄糖,Glansrin C,黄嘌呤,鞣花酸戊糖苷二聚体,吡哆醇O-二己糖苷,1’-O-β-D-(3,4-二羟基苯乙基)-O-咖啡酰基-葡萄糖苷,3,5-二-O-没食子莽草酸,原花青素三聚单酯,二磷酸腺苷,(EC→EC→EC)g(1),Tercatain,特里马素Ⅰ,水杨梅丁素,山奈酚-3,7-O-二鼠李糖苷,对香豆酰酒石酸},7种中药化学成分(丁香油酚甲醚,黄嘌呤,苯甲酸,特里马素Ⅰ,Tercatain,Glansrin C,水杨梅丁素)及2 种抗六大疾病物质(Tercatain,水杨梅丁素)。而仅在无籽刺梨中检测到的代谢物既没有中药化学成分也没有抗氧化物质。以上结果进一步说明刺梨具更高的药用价值及抗氧化能力。黄嘌呤、特里马素Ⅰ、Tercatain、Glansrin C、水杨梅丁素这5 种物质是刺梨特有的抗氧化物质及中药化学成分,且Tercatain 和水杨梅丁素也是抗六大疾病活性物质。因此,这5 种物质在刺梨与无籽刺梨果实中药活性及抗氧化能力的差异中均起重要作用。
在两个刺梨基因型与无籽刺梨的差异代谢物分析中,Rr-3 与Rs 间有272 种差异代谢物,Rr-5 与Rs间有242 种差异代谢物。上述差异代谢物映射到KEGG富集通路,富集程度较高的是嘌呤代谢、嘧啶代谢、玉米素生物合成、碳代谢、氨基酸生物合成。嘌呤可参与植物氮代谢、黄酮合成及激素合成过程,帮助植物应对不良环境[37];嘌呤和嘧啶核苷酸参与植物的许多生化过程,包括发芽开花结实等,它们是核酸合成的基石,是合成初生代谢物以及次生代谢物的前体[38];此外,植物嘌呤和嘧啶代谢的重要过程是初级代谢、次级代谢和基因表达所必需的,是细胞生长和生化过程的基础[39]。而映射到这两条通路中的差异代谢物均在刺梨中上调,因此,笔者认为嘌呤代谢及嘧啶代谢通路的差异是刺梨与无籽刺梨在种子上差异的原因之一,并推断刺梨比无籽刺梨具有更高的对逆境胁迫的耐受性。
差异代谢物中有171 种是Rr-3、Rr-5 与Rs 共同差异的物质,其中,在两个刺梨基因型均上调的差异代谢物有111 种,包括87 种抗氧化物质(31 种鞣质,15 种核苷酸及其衍生物,12 种酚酸,10 种鞣质,7 种黄酮,5种其他,2种脂质,2种萜类,2种有机酸,1种木脂素与香豆素),31 种中药化学物质,8 种关键活性成分,23种物质既是抗氧化物质又是中药化学物质(丙二酸,胞嘧啶,组胺,延胡索酸,胸腺嘧啶,L-谷氨酸,黄嘌呤,吡哆素,没食子酸,没食子酸甲酯,芥子醇,尿嘧啶核苷,鸟苷,鞣花酸,双没食子酸,迷迭香酸,水杨梅丁素,英国栎鞣花酸,特里马素Ⅰ,Glansrin C,Pterocarinin A,Glansrin A,Platycaryanin A);无籽刺梨中上调的有53种物质,包括10种中药化学成分,3种关键活性成分。综上,与无籽刺梨相比,刺梨具更高的中药活性及抗氧化能力,其差异主要源于两类果实中酚类物质数量与含量的不同。
4 结 论
笔者在本研究中首次对刺梨及无籽刺梨果实次生代谢物中的具中药活性与抗氧化能力的次生代谢物进行鉴定与差异分析。结果表明,在两个刺梨基因型与无籽刺梨果实中共检测到530 种次生代谢物,其中包括118种中药化学成分与145种抗氧化物质,酚类物质是刺梨及无籽刺梨果实中最主要的抗氧化物质与中药化学成分;刺梨不论是在特有物质中还是在差异代谢物中均具较多数量的抗氧化物质及中药化学成分,从而具更高的中药活性及抗氧化能力,而酚类物质的数量及含量是导致刺梨与无籽刺梨抗氧化能力及中药活性差异的主要原因。
[1]DEVAUX P G,HORNING M G,HORNING E C.Benzyloxime derivatives of steroids. A new metabolic profile procedure for human urinary steroids[J]. Analytical Letters,1971,4(3):151-160.
[2]SUMNER L W,LEI Z,NIKOLAU B J,SAITO K. Modern plant metabolomics:advanced natural product gene discoveries,improved technologies,and future prospects[J]. Natural Product Reports,2015,32(2):212-229.
[3]张晓磊,张瑞英.代谢组学及其在农作物研究中的应用[J].生物技术通讯,2018,29(3):446-450.ZHANG Xiaolei,ZHANG Ruiying.Metabolomics and its application in the crop research[J].Letters in Biotechnology,2018,29(3):446-450.
[4]WANG D D,ZHANG L X,HUANG X R,WANG X,YANG R N,MAO J,WANG X F,WANG X P,ZHANG Q,LI P W.Identification of nutritional components in black sesame determined by widely targeted metabolomics and traditional Chinese medicines[J].Molecules,2018,23(5):1180.
[5]CHEN L M,WU Q C,HE T J,LAN J J,DING L,LIU T F,WU Q Q,PAN Y M,CHEN T T. Transcriptomic and metabolomic changes triggered by Fusarium solani in common bean(Phaseolus vulgaris L.)[J].Genes,2020,11(2):177.
[6]CHU C,DU Y M,YU X T,SHI J,YUAN X L,LIU X M,LIU Y H,ZHANG H B,ZHANG Z F,YAN N.Dynamics of antioxidant activities,metabolites,phenolic acids,flavonoids,and phenolic biosynthetic genes in germinating Chinese wild rice (Zizania latifolia)[J].Food Chemistry,2020,318:126483.
[7]JIANG L L,LU M,RAO T Z,LIU Z Y,WU X M,AN H M.Comparative analysis of fruit metabolome using widely targeted metabolomics reveals nutritional characteristics of different Rosa roxburghii genotypes[J].Foods,2022,11(6):850.
[8]HE J Y,ZHANG Y H,MA N,ZHANG X L,LIU M H,FU W M. Comparative analysis of multiple ingredients in Rosa roxburghii and R. sterilis fruits and their antioxidant activities[J].Journal of Functional Foods,2016,27:29-41.
[9]ZHU J Z,ZHANG B,WANG B X,LI C,FU X,HUANG Q.Invitro inhibitory effects of flavonoids in Rosa roxburghii and R.sterilis fruits on α-glucosidase:Effect of stomach digestion on flavonoids alone and in combination with acarbose[J]. Journal of Functional Foods,2019,54:13-21.
[10]安华明,刘明,杨曼,樊卫国.刺梨有机酸组分及抗坏血酸含量分析[J].中国农业科学,2011,44(10):2094-2100.AN Huaming,LIU Ming,YANG Man,FAN Weiguo. Analysis of main organic acid compositions in Rosa roxburghii Tratt.[J].Scientia Agricultura Sinica,2011,44(10):2094-2100.
[11]鲁敏,安华明,赵小红.无籽刺梨与刺梨果实中氨基酸分析[J].食品科学,2015,36(14):118-121.LU Min,AN Huaming,ZHAO Xiaohong.Analysis of amino acids in Rosa sterilis and Rosa roxburghii fruits[J]. Food Science,2015,36(14):118-121.
[12]林陶,李婕羚,付远洪,杨卫灵.无籽刺梨与野生刺梨果实的氨基酸含量及组成[J].山东化工,2017,46(18):76-79.LIN Tao,LI Jieling,FU Yuanhong,YANG Weiling. Determination of amino acids in Rosa sterilis and wild Rosa roxburghii by HPLC[J].Shandong Chemical Industry,2017,46(18):76-79.
[13]LI L L,LU M,AN H M. Expression profiles of the genes involved in L-ascorbic acid biosynthesis and recycling in Rosa roxburghii leaves of various ages[J].Acta Physiologiae Plantarum,2017,39(2):1-9.
[14]REN B,CHEN C,LI C,FU X,YOU L,LIU R H. Optimization of microwave-assisted extraction of sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities[J].Carbohydr Polym,2017,173:192-201.
[15]WU H Y,LI M M,YANG X R,WEI Q,SUN L Z Y,ZHAO J C,SHANG H M.Extraction optimization,physicochemical properties and antioxidant and hypoglycemic activities of polysaccharides from roxburgh rose (Rosa roxburghii Tratt.) leaves[J]. International Journal of Biological Macromolecules,2020,165(PtA):517-529.
[16]XU J W,VIDYARTHI S K,BAI W B,PAN Z L.Nutritional constituents,health benefits and processing of Rosa Roxburghii:A review[J].Journal of Functional Foods,2019,60:103456.
[17]WU P H,HAN S C H,WU M H.Beneficial effects of hydroalcoholic extract from Rosa roxburghii Tratt. fruit on hyperlipidemia in high-fat-fed rats[J]. Acta Cardiologica Sinica,2020,36(2):148-159.
[18]时京珍,陈秀芬,彭冬.两种刺梨对小鼠炎症等的比较研究[J].贵州医药,1996,22(5):268-269.SHI Jingzhen,CHEN Xiufen,PENG Dong. Comparative study on the effects of two kinds of Rosa roxburghii on inflammation in mice[J].Guizhou Medicine,1996,22(5):268-269.
[19]LI H,FANG W Y,WANG Z,CHEN Y.Physicochemical,biological properties,and flavour profile of Rosa roxburghii Tratt.,Pyracantha fortuneana,and Rosa laevigata Michx fruits:A comprehensive review[J].Food Chemistry,2022,366:130509.
[20]张丹,韦广鑫,王文,冯飞,曾凡坤.安顺普定刺梨与无籽刺梨营养成分及香气物质比较研究[J]. 食品工业科技,2016,37(12):149-154.ZHANG Dan,WEI Guangxin,WANG Wen,FENG Fei,ZENG Fankun. Comparative research on basic ingredients and volatile aroma compounds of Rosa roxburghii Tratt. and Rosa sterilis S.D. Shi[J]. Science and Technology of Food Industry,2016,37(12):149-154.
[21]HOU Z Q,YANG H Z,ZHAO Y,XU L,ZHAO L,WANG Y T,LIAO X J. Chemical characterization and comparison of two chestnut rose cultivars from different regions[J]. Food Chemistry,2020,323:126806.
[22]张丹,韦广鑫,曾凡坤.贵州不同产地无籽刺梨的基本营养成分及香气物质比较[J].食品科学,2016,37(22):166-172.ZHANG Dan,WEI Guangxin,ZENG Fankun. Proximate nutritional composition and volatile aroma compounds of Rosa sterilis S.D.Shi fruits from different growing areas of Guizhou province[J].Food Science,2016,37(22):166-172.
[23]NIU Y W,WANG R L,XIAO Z B,SUN X X,WANG P P,ZHU J C,CAO X Y. Characterization of volatile compounds of Rosa roxburghii Tratt. by gas chromatography-olfactometry,quantitative measurements,odor activity value,and aroma intensity[J].Molecules,2021,26(20):6202.
[24]吴洪娥,金平,周艳,朱立,周洪英.刺梨与无籽刺梨的果实特性及其主要营养成分差异[J].贵州农业科学,2014,42(8):221-223.WU Hong’e,JIN Ping,ZHOU Yan,ZHU Li,ZHOU Hongying.Characteristics and main nutrition components of R. roxburghii and R. sterilis fruits[J]. Guizhou Agricultural Science,2014,42(8):221-223.
[25]ZENG F F,GE Z W,LIMWACHIRANON J,LI L,FENG S M,WANG Y S,LUO Z S.Antioxidant and tyrosinase inhibitory activity of Rosa roxburghii fruit and identification of main bioactive phytochemicals by UPLC-Triple-TOF/MS[J]. International Journal of Food Science&Technology,2017,52(4):897-905.
[26]HUANG D S,LI C,CHEN Q,XIE X,FU X,CHEN C,HUANG QI,HUANG Z B,DONG H.Identification of polyphenols from Rosa roxburghii Tratt.pomace and evaluation of in vitro and in vivo antioxidant activity[J]. Food Chemistry,2022,377:131922.
[27]刘佳,黄思洋,陈钰沁,朱艳玲,赵明智,付娟姬,杨伶俐.大黄中总鞣质的提取方法研究[J].昆明学院学报,2021,43(6):109-113.LIU Jia,HUANG Siyang,CHEN Yuqin,ZHU Yanling,ZHAO Mingzhi,FU Juanji,YANG Lingli. Study on extraction process of total tannin in rhubarb[J]. Journal of Kunming University,2021,43(6):109-113.
[28]CHEN W,GONG L,GUO Z L,WANG W S,ZHANG H G,LIU X Q,YU S B,XIONG L Z,LUO J. A novel integrated method for large-scale detection,identification,and quantification of widely targeted metabolites:Application in the study of rice metabolomics[J].Molecular Plant,2013,6(6):1769-1780.
[29]LI H Y,LV Q Y,LIU A. Comparative metabolomics study of Tartary [Fagopyrum tataricum (L.) Gaertn]and common (Fagopyrum esculentum Moench) buckwheat seeds[J]. Food Chemistry,2022,371:131125.
[30]MOURE A,FRANCO D,SINEIRO J,DOMı′NGUEZ H,JOSÉNÚÑEZ M,LEM J M. Antioxidant activity of extracts from Gevuina avellana and Rosa rubiginosa defatted seeds[J].Food Research International,2001,34(2/3):103-109.
[31]MARTINEZ- VILLALUENGA C,PENAS E,CISKA E,PISKUL M K,KOZLOWSKA H,VIDAL- VALVERDE C,FRIAS J.Time dependence of bioactive compounds and antioxidant capacity during germination of different cultivars of broccoli and radish seeds[J].Food Chemistry,2010,120(3):710-716.
[32]BENZIE I F F,STRAIN J J. The ferric reducing ability of plasma(FRAP)as a measure of“antioxidant power”:The FRAP assay[J].Analytical Biochemistry,1996,239(1):70-76.
[33]FRAGA C G,CLOWERS B H,MOORE R J,ZINK E M.Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography-mass spectrometry,XCMS,and chemometrics[J]. Analytical Chemistry,2010,82(10):4165-4173.
[34]LIU M H,ZHANG Q,ZHANG Y H,LU X Y,FU W M,HE J Y.Chemical analysis of dietary constituents in Rosa roxburghii and Rosa sterilis fruits[J].Molecules,2016,21(9):1204.
[35]周广志,鲁敏,安华明.刺梨果实发育过程中主要活性物质含量及其抗氧化性分析[J].食品科学,2018,39(22):20-25.ZHOU Guangzhi,LU Min,AN Huaming.Analysis of bioactive substance contents and antioxidant activities in Rosa roxburghii fruits during development[J].Food Science,2018,39(22):20-25.
[36]周广志,鲁敏,安华明.刺梨及其近缘种质叶片主要活性物质含量及抗氧化性分析[J].核农学报,2019,33(8):1658-1665.ZHOU Guangzhi,LU Min,AN Huaming.Analysis of main active substances and antioxidant activities in leaves of Rosa roxburghii and the two related species[J]. Journal of Nuclear Agricultural Sciences,2019,33(8):1658-1665.
[37]WATANABE S,MATSUMOTO M,HAKOMORI Y,TAKAGI H,SHIMADA H,SAKAMOTO A.The purine metabolite allantoin enhances abiotic stress tolerance through synergistic activation of abscisic acid metabolism[J]. Plant,Cell & Environment,2014,37(4):1022-1036.
[38]STASOLLA C,KATAHIRA R,THORPE T A,ASHIHAR H.Purine and pyrimidine nucleotide metabolism in higher plants[J].Journal of Plant Physiology,2003,160(11):1271-1295.
[39]ZRENNER R,STITT M,SONNEWALD U,BOLDT R. Pyrimidine and purine biosynthesis and degradation in plants[J].Annual Review of Plant Biology,2006,57:805-836.