葡萄花芽分化是生长发育过程中十分重要的阶段,花芽分化的数量及质量影响葡萄产量及经济效益[1]。葡萄的成花过程包括成花诱导(flower induction)、花的发端(flower initiation)即始原基分化、花序分化和花器官分化(inflorescence and flower organization)、花的成熟(flower maturation)和开花(anthesis)等过程[2]。葡萄花芽分化可大致分为两个过程,即生理分化和形态分化,成花诱导即为通常所称的生理分化,始原基分化、花序分化和花器官分化即通常所称的形态分化[2-3]。葡萄花芽形态分化跨越了两个生长季,花序分化一直持续到冬季休眠,第二季萌芽前转入花器官分化阶段[4]。
葡萄花芽分化是一个较为复杂的形态建成过程,受诸多因素影响,包括环境因素如光照、温度、水分和内部因素如营养水平、内源激素水平、基因调控等[4]。光照通过影响叶片光合作用和光合产物积累等间接影响葡萄花芽分化,光照与温度协同促进花芽分化[5]。葡萄花序原基诱导和分化通常要求温度在20 ℃以上,温度低于20 ℃始原基则发育形成卷须原基[6-8]。水分通过影响细胞分裂和膨大及树体光合作用、营养水平、内源激素水平及芽内微域环境直接或间接影响花芽分化[4]。葡萄中碳水化合物与花芽分化呈正相关,充足的糖和淀粉可促进葡萄花芽分化[9]。葡萄成花诱导及花序分化需要N 和P 元素供应[10-11]。高水平的P、K、Ca、Mg和低水平的N元素有利于葡萄花芽分化[12-13]。葡萄花芽分化还与内源激素密切相关,赤霉素和细胞分裂素是调控葡萄成花的主要激素[5]。赤霉素在葡萄花芽分化过程中抑制葡萄花序原基分化,细胞分裂素能够促进葡萄成花[14-15]。
葡萄花芽分化过程中,相关基因的表达与互作调控其成花。目前,葡萄中报道了很多与模式植物拟南芥同源的花芽分化相关基因,并且大多数基因功能相似[16]。FLOWERING LOCUS T(FT)、SUPPRESSOR OF CONSTANS 1(SOC1)和LEAFY(LFY)是重要的开花整合因子,参与调控成花时间[17-19]。在葡萄中,VvFT 基因在芽、叶片、花序、卷须及幼果等器官中均有表达,参与了花序与花器官分化及发育[20-21]。VvSOC1 基因在葡萄花序原基和苞片中的表达处于较高水平[22]。LEAFY(LFY)基因既是花分生组织特征基因,也是整合环境信号和内源信号的重要开花控制因子[23]。VvLEAFY基因在葡萄花序原基发育过程的表达水平较高,参与调控花序和花分生组织的发育[24]。APETALA1(AP1)及其旁系同源基因FRUITFULL(FUL)既是花分生组织特征基因,也作为花器官特征基因促进萼片和花瓣的发育[16]。葡萄中VvAP1 和VvFUL 基因在花序和花分生组织中表达[25]。APETALA2(AP2)、APETALA3(AP3)和AGAMOUS(AG)为花器官特征基因,调控花瓣、雄蕊、心皮及胚珠的发育[26-27]。VvAP2 基因在葡萄叶、茎、花序、花等器官中均有表达,其在花序和花中的表达量较高[28]。VvAG 基因在葡萄花序、果实、花瓣和雄蕊中表达[29]。FLOWERING LOCUS C(FLC)为成花抑制因子,FLC 基因通过抑制成花基因FT 和SOC1 的表达进而抑制成花[30]。刘丹等[31]基于EST数据库研究发现葡萄中VvFLC 抑制VvFT、VvSOC1和VvLFY基因的表达。
迄今,关于葡萄成花的相关生理及分子研究大部分集中于花芽分化关键时期,然而葡萄当季新梢上冬芽的花芽分化过程与果实的发育期重叠,在果实的生长发育期,尤其是花序原基分化后到果实采收期间成花相关的生理分子变化未见详细报道。阳光玫瑰葡萄是近年中国葡萄产业新兴推广的品种,风味品质独特,具有很好的发展前景,然而其花芽分化相关研究还鲜有报道。笔者在本研究中以阳光玫瑰葡萄为试材,研究葡萄生长期新梢上冬芽花芽分化的形态结构及可溶性糖、淀粉、矿质元素含量和成花关键基因表达的变化,揭示阳光玫瑰葡萄成花进程及花芽分化相关机制,以期在温光资源丰富的热区为葡萄的生产调控及其花芽分化的深入研究提供理论依据。
1 材料和方法
1.1 试验材料及试验设计
试验于2019年3月至2019年9月在广西南宁广西真诚农业有限公司武鸣东盟开发区示范基地进行。以4 年生阳光玫瑰葡萄为试材,果园管理采用当地常规一年两收栽培管理方式,树体生长良好。
在阳光玫瑰葡萄生长期,从新梢5 片展叶期开始每隔3 d 随机采新梢第5 节位冬芽20 个,3 次重复,采样当天即在体式解剖镜下徒手剥离冬芽鳞片观察花芽形态结构,统计并记录各芽所处的分化阶段,花芽分化阶段划分参照王海波等[32]和林玲[33]的研究,将阳光玫瑰冬芽花芽分化时期划分为叶芽期、平顶期、二分体期、始原基分化期、花序原基分化期、花序各级穗轴分化期。当超过75%的冬芽处于上述某一分化阶段时确定该次采样期进入该分化阶段,在花序原基分化期后,花序各级穗轴分化期每隔20 d 采样1 次继续观察冬芽花芽形态结构变化,至夏果果实采收时结束采样。
在观察到叶芽期、平顶期、二分体期、始原基分化期、花序原基分化期的采样当天及花序各级穗轴分化期的每隔20 d 1次的采样当天,记录物候期,并采集碳水化合物、矿质元素含量测定和成花关键基因表达分析的样品。碳水化合物和矿质元素含量测定随机采集枝条第5节位叶片5枚,置于冰盒临时保存,回实验室后放入烘箱中108 ℃杀青20 min,后65 ℃烘干,将样品研磨成粉末后装袋保存,重复3次;成花关键基因表达分析随机采集新梢第5 节位冬芽20 个,芽切下后立即冻入液氮临时保存,后置于-80℃保存,3次重复。
1.2 测定方法
1.2.1 花芽分化过程芽形态结构观察 在体式解剖镜下剥去鳞片,观察葡萄花芽分化不同阶段芽的形态结构。
1.2.2 叶片可溶性总糖、淀粉含量测定 可溶性总糖及淀粉的提取及含量测定采用蒽酮乙酸乙酯-硫酸比色法。
1.2.3 叶片矿质元素含量测定 全N含量的提取与测定。用H2SO4-H2O2消煮法提取样品,使用连续流动分析仪进行测定。全P、K、Ca、Mg 含量的提取与测定。用H2NO2-H2O2消煮法提取样品,使用电感耦合等离子光谱仪进行测定。
1.2.4 芽内9个成花关键基因表达量测定 葡萄冬芽总RNA 的提取采用RNAprep Pure Plant Plus kit(TIANGEN)总RNA 提取试剂盒进行,用不含RNA酶的DNA 酶去除DNA。用PrimeScriptTM RTase 反转录试剂盒进行反转录,合成第一链cDNA,荧光定量采用TB Green®Premix Ex Taq ™Ⅱ(TaKaRa)试剂盒进行。以葡萄ACTIN 作为内参基因,引物序列参考Kobayashi 等[34],9 个成花关键基因VvFT、VvSOC1、VvLFY、VvAP1、VvFUL、VvAP2、VvAP3、VvAG和VvFLC的引物参考杨光等[35]的报道,具体引物序列见表1。试验中每个样品3次重复,同时设置3 个阴性对照,目的基因的表达量采用相对定量法(2-ΔΔCT)进行计算。
表1 引物序列
Table 1 Sequence of primers
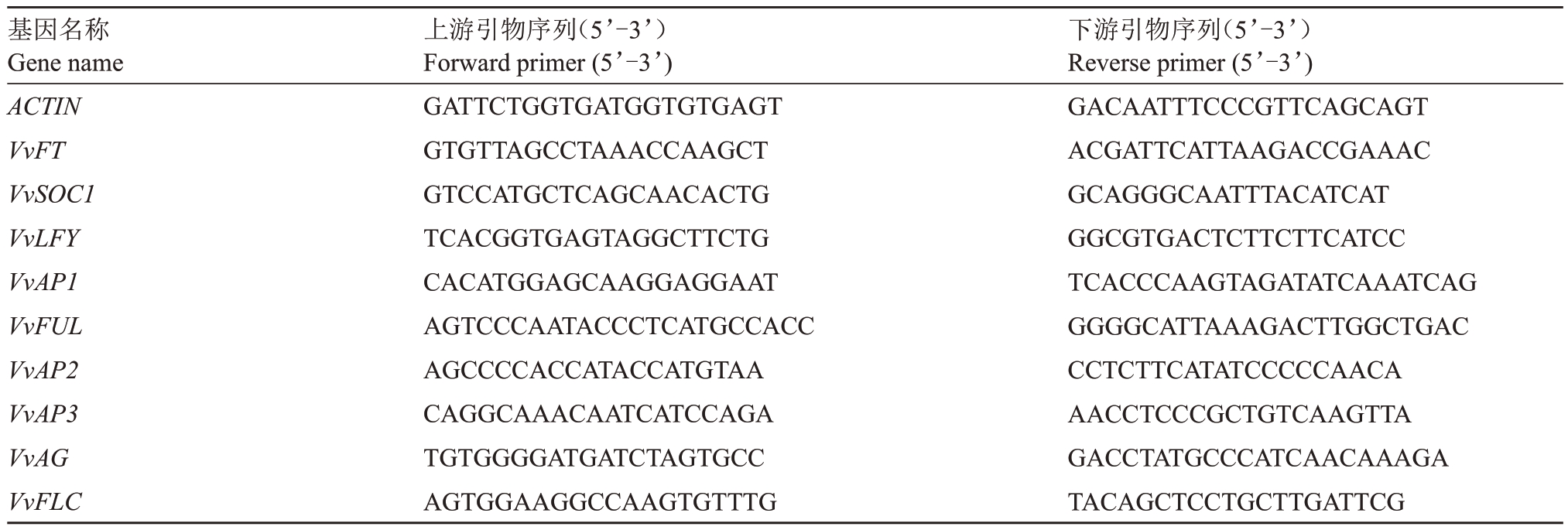
基因名称Gene name ACTIN VvFT VvSOC1 VvLFY VvAP1 VvFUL VvAP2 VvAP3 VvAG VvFLC上游引物序列(5’-3’)Forward primer(5’-3’)GATTCTGGTGATGGTGTGAGT GTGTTAGCCTAAACCAAGCT GTCCATGCTCAGCAACACTG TCACGGTGAGTAGGCTTCTG CACATGGAGCAAGGAGGAAT AGTCCCAATACCCTCATGCCACC AGCCCCACCATACCATGTAA CAGGCAAACAATCATCCAGA TGTGGGGATGATCTAGTGCC AGTGGAAGGCCAAGTGTTTG下游引物序列(5’-3’)Reverse primer(5’-3’)GACAATTTCCCGTTCAGCAGT ACGATTCATTAAGACCGAAAC GCAGGGCAATTTACATCAT GGCGTGACTCTTCTTCATCC TCACCCAAGTAGATATCAAATCAG GGGGCATTAAAGACTTGGCTGAC CCTCTTCATATCCCCCAACA AACCTCCCGCTGTCAAGTTA GACCTATGCCCATCAACAAAGA TACAGCTCCTGCTTGATTCG
1.3 数据分析
试验数据运用Excel 2010 进行整理及制图,运用SPSS软件进行显著性分析。
2 结果与分析
2.1 阳光玫瑰葡萄冬芽花芽分化过程形态结构观察及其对应的物候期
参考前人研究结果,根据阳光玫瑰葡萄冬芽分化过程中的形态结构变化对其花芽分化进程进行划分,将第5 节位冬芽花芽分化各阶段与葡萄树体所处物候期对应见图1,分为以下几个阶段:叶芽期,生长点还未开始分化,尖端突起;平顶期,开始分化的标志,生长点扁平;二分体时期,生长点开始分化为两个半球状;始原基分化期,生长点旁始原基形成;花序原基分化期,分化出花穗主轴,花序原基形成;花序各级穗轴分化期(花序原基分化后20~100 d),花序原基进一步分化和发育形成各级穗轴。

图1 阳光玫瑰葡萄各采样期冬芽分化形态结构及其对应的物候期
Fig.1 The morphology structure of winter bud differentiation of Shine Muscat grape in each sampling period and its corresponding phenological period
从阳光玫瑰葡萄各采样期冬芽分化进程对应的夏果物候期(表2)可以看出,阳光玫瑰葡萄在新梢6片展叶期时花芽分化处于平顶期即冬芽开始形态分化;葡萄盛花期时冬芽花芽分化进入始原基分化期,该阶段始原基可分化为卷须原基或花序原基,因此,盛花期是阳光玫瑰葡萄花芽形成的关键时期;在葡萄末花期时冬芽进入花序原基分化期;从花序原基分化期后到夏果采收期观察到的花芽形态变化可以看出,花序原基不断发育并逐渐分化出花序各级穗轴。
表2 阳光玫瑰葡萄各采样期的冬芽花芽分化阶段与其相应的物候期
Table 2 The differentiation stage of winter buds of Shine Muscat grape in each sampling period and its corresponding phenological period

采样批次Sampling batch 1 2 3 4 5 6 7 8 9 1 0花芽分化阶段Differentiation stage叶芽期Leaf bud stage平顶期Flat top stage二分体期Dichotomy stage始原基分化期Anlagen differentiation stage花序原基分化期Inflorescence primordium differentiation stage花序原基分化期后20 d Stage of 20 days after inflorescence primordium differentiation花序原基分化期后40 d Stage of 40 days after inflorescence primordium differentiation花序原基分化期后60 d Stage of 60 days after inflorescence primordium differentiation花序原基分化期后80 d Stage of 80 days after inflorescence primordium differentiation花序原基分化期后100 d Stage of 100 days after inflorescence primordium differentiation物候期Phenological period of sampling新梢5片展叶期Stage of 5 leaves spread新梢6片展叶期Stage of 6 leaves spread花穗分离期Flower spikes segregation stage盛花期Flower full-bloom stage末花期Flowering ending stage幼果期Young fruit stage果实膨大期Fruit enlargement stage果实软化期Fruit softening stage果实成熟期Fruit ripening stage果实采收期Fruit harvest period
2.2 阳光玫瑰葡萄冬芽花芽分化过程中叶片可溶性总糖和淀粉含量的变化
如图2 所示,在阳光玫瑰葡萄冬芽花芽分化过程中,叶片可溶性总糖和淀粉含量整体呈上升趋势,叶片中可溶性总糖含量在花芽分化平顶期即冬芽开始形态分化时显著升高,而后至始原基分化期其含量(w,后同)无显著变化,均保持在3.5 μg·g-1左右,从花序原基分化期开始至花序原基分化后100 d 期间可溶性总糖含量一直呈显著上升趋势。叶片淀粉含量从花芽分化平顶期时开始显著升高,在花序原基分化期后20 d达到最高值(141.38 μg·g-1),此时对应的物候期为幼果期,随后叶片淀粉含量有所下降,但均保持在100 μg·g-1以上。以上结果表明,阳光玫瑰葡萄花芽形态分化起始需要一定的可溶性总糖和淀粉,花芽分化过程对可溶性总糖和淀粉含量的需求整体呈上升趋势,保持较高水平的可溶性总糖和淀粉利于花序原基及其各级穗轴分化。
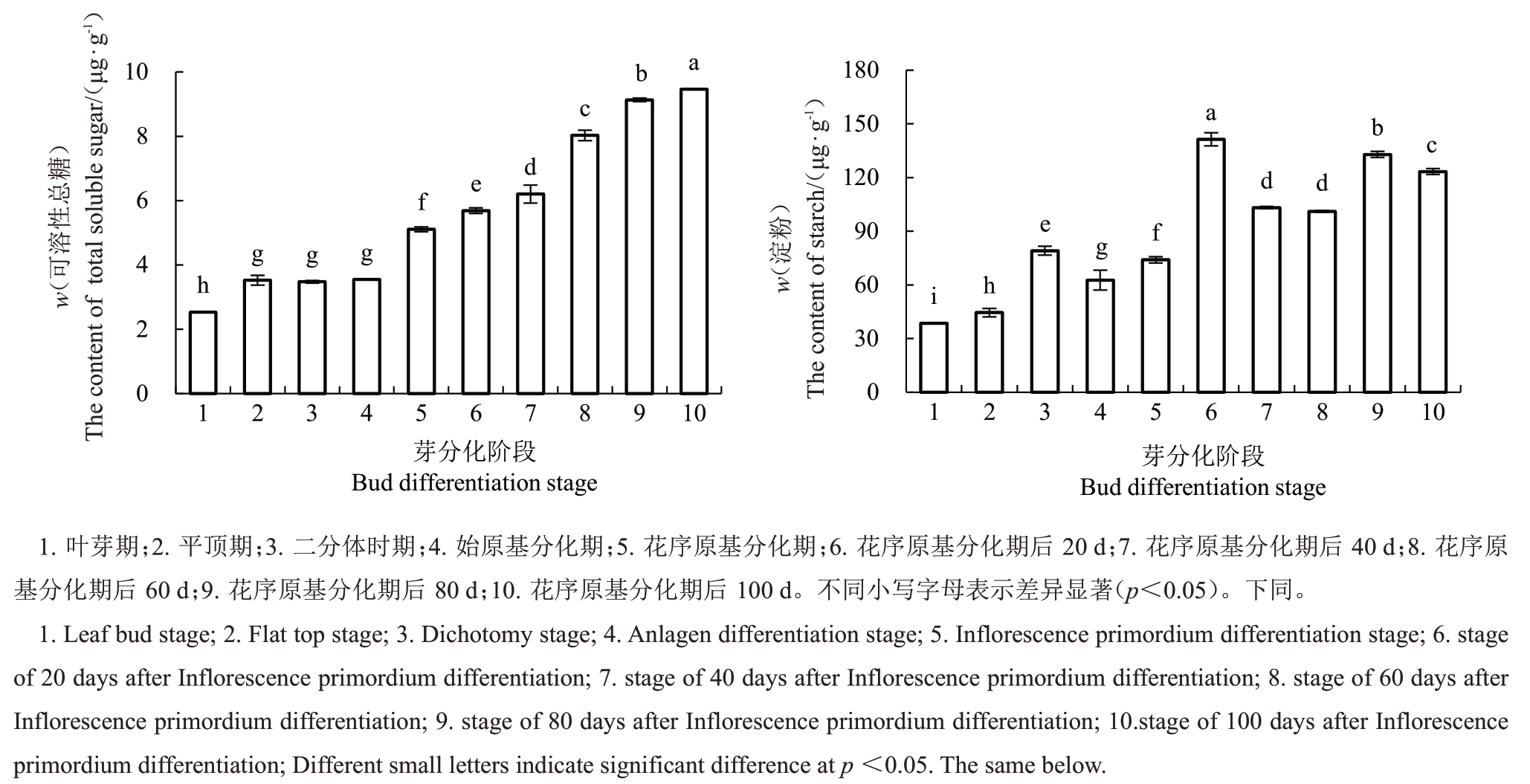
图2 阳光玫瑰葡萄冬芽花芽分化过程中可溶性总糖和淀粉的含量
Fig.2 The total soluble sugar and starch content during flower bud differentiation of Shine Muscat grape
2.3 阳光玫瑰葡萄冬芽花芽分化过程中叶片矿质元素N、P、K、Ca和Mg含量的变化
图3为阳光玫瑰葡萄冬芽花芽分化过程中叶片各矿质元素含量的变化。冬芽叶芽期到花序原基分化期叶片全N 含量维持在2.5~3.0 g·kg-1,其在花序原基分化后20 d 时最低,这可能与此时果实发育N需求增加有关,花序原基分化后40 d 时N 含量迅速上升并维持在3.0~4.0 g·kg-1,较高水平的N 可能有利于花序原基各级穗轴分化和发育。在冬芽花芽分化过程中叶片P、K 和Mg 含量变化趋势基本一致,平顶期叶片中P、K 和Mg 含量较叶芽期显著下降,此后出现两次波峰,P和Mg含量在花序原基分化期达到第一个波峰,K 在花序原基分化期后40 d 达到第一个波峰,三者均在花序原基分化期后100 d时达到第二个波峰。以上表明葡萄花芽分化起始对P、K和Mg需求较大,花序原基分化期后100 d时叶片P、K 和Mg 含量较高可能与采收期果实对矿质营养需求减少有关。在花芽分化过程中叶片Ca 含量整体呈上升趋势,叶芽期及平顶期Ca 含量相对较低,于二分体时期开始显著升高,此后Ca含量分别在花序原基分化后40 d 和花序原基分化后100 d 出现两个波峰,表明较高水平的Ca可能有利于促进花芽进入始原基分化阶段。叶片中P、K、Ca 和Mg 含量均在花序原基分化后60 d 即果实软化期显著下降,这可能与软化期果实发育也需要消耗较多的P、K、Ca和Mg等营养元素有关。

图3 阳光玫瑰葡萄冬芽花芽分化过程中N、P、K、Ca、Mg 元素的含量
Fig.3 The total N,P,K,Ca and Mg content during flower bud differentiation of Shine Muscat grape
2.4 阳光玫瑰葡萄冬芽花芽分化过程中芽内9个成花关键基因表达量的变化
FT、SOC1 及LEAFY 是成花调控重要整合因子。本研究结果(图4)表明,成花诱导关键基因VvFT 和VvSOC1 在阳光玫瑰葡萄冬芽花芽形态分化起始阶段表达量较高。在花芽分化过程中VvFT 基因表达水平变化较大,在平顶期达到最高值后显著下调,在花序原基分化后20 d 出现第二次波峰后下降,在花序原基分化后40 d 时最低;VvSOC1 基因表达水平在花芽分化开始后显著上调,在二分体时期达到最大值后显著下降,在花序原基分化后40 d 后一直处于较低水平;VvLFY 基因的表达水平在花芽分化前期较低,在花序原基分化期出现一次小波峰,其后在花序原基分化后80 d再次出现一次大的波峰。以上结果表明,VvFT 和VvSOC1 基因主要参与调控葡萄花芽形态分化起始和始原基分化,VvLFY 基因主要参与始原基分化及花序各级穗轴分化。VvFT 基因在平顶期及VvSOC1 在二分体期的高表达利于促进花芽进入始原基分化阶段。

图4 阳光玫瑰葡萄冬芽花芽分化过程中VvFT、VvSOC1、VvLFY、VvAP1、VvFUL、VvAP2、VvAP3、VvAG、VvFLC 基因的表达趋势
Fig.4 The variation of expression levels of VvFT,VvSOC1,VvLFY,VvAP1,VvFUL,VvAP2,VvAP3,VvAG and VvLFY in the flower bud differentiation process of winter buds of Shine Muscat grape
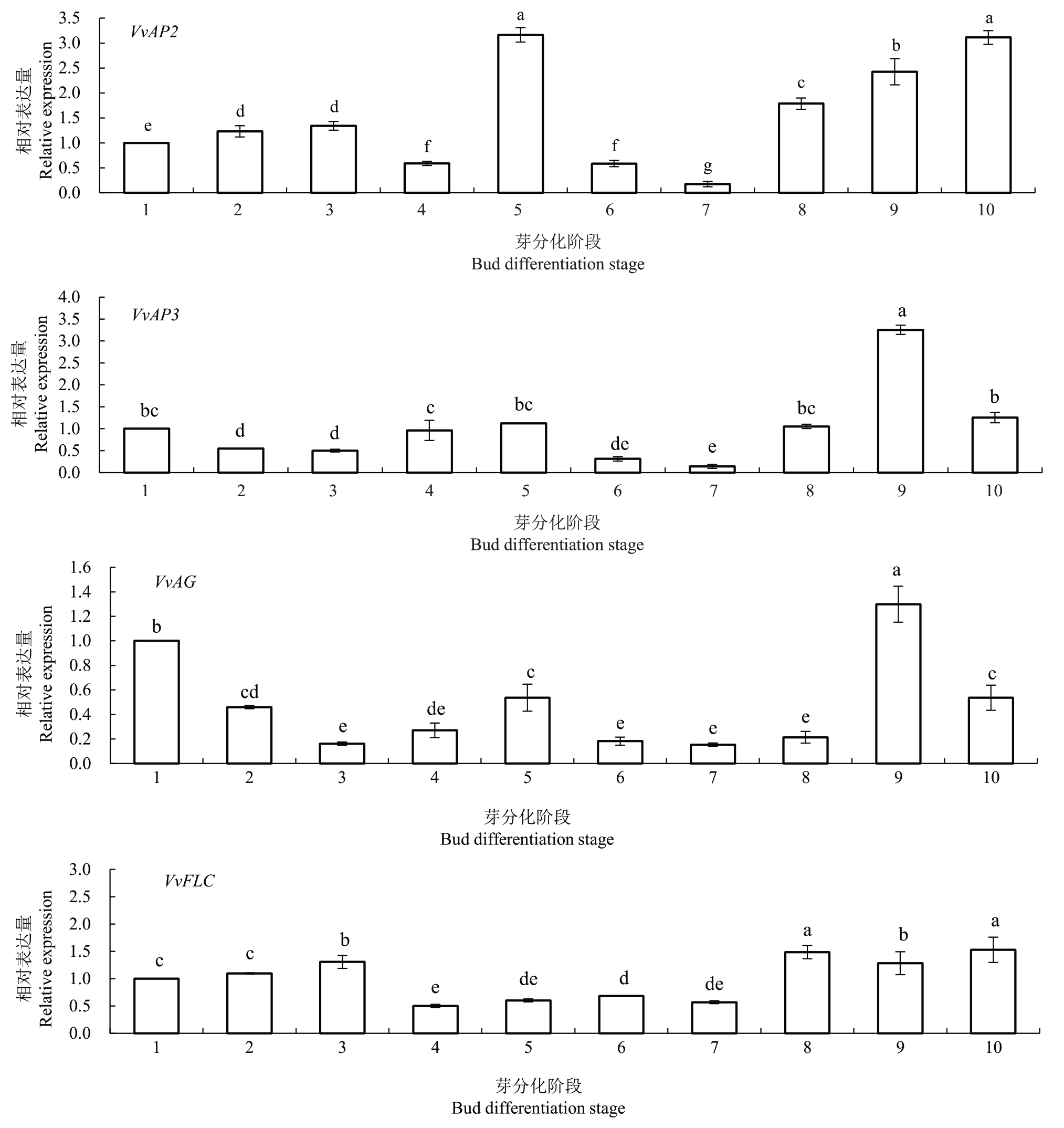
续图Continued Figure
AP1及其同源旁系基因FUL为花分生组织特征基因。VvAP1基因在阳光玫瑰葡萄冬芽花芽分化前期表达水平较低,在花序原基分化期显著上升,此后其表达水平呈波动变化,分别在花序原基分化后20 d和100 d两次达到最高值,VvAP1的高表达可能有利于促进花序各级穗轴分化和发育。VvFUL 基因在花芽分化前期表达水平较低,在花序原基分化期表达上升,其后呈现波动变化,至花序原基分化后80 d时表达水平达最高,表明VvFUL基因主要参与花序原基及花序各级穗轴分化。
AP2、AP3 和AG 是花器官特征基因。VvAP2 基因在花序原基分化期达到最高表达水平后显著下调,在花序原基分化后40 d至100 d期间呈显著上升趋势,VvAP2 基因在花序原基分化期的高表达利于促进花序原基及花序各级穗轴分化。VvAP3 和VvAG 基因在冬芽花芽分化过程中的表达趋势一致,VvAP3 和VvAG 基因在花芽分化起始时表达显著下调,在花序原基分化后80 d时表达水平最高,表明VvAP3和VvAG基因与花序原基及其各级穗轴分化相关。
FLC是开花抑制因子。在阳光玫瑰葡萄花芽分化过程中,VvFLC 基因在始原基分化期至花序原基分化期后40 d 的表达水平显著低于其他时期,在花序原基分化60~100 d 的表达保持在相对较高水平,表明VvFLC 基因可能参与抑制葡萄花芽分化起始及花序各级穗轴分化。
3 讨 论
葡萄花芽分化进程与树体生长发育期相对应,不同葡萄品种间花芽分化阶段对应的树体物候期不同。研究表明,维多利亚葡萄冬芽在新梢盛花期开始花芽分化,红宝石和红地球葡萄冬芽在末花期才开始花芽分化[36-38]。夏黑葡萄冬芽在新梢长至7~8片叶时开始花芽分化,坐果期才进行花序原基分化[33,39-40]。本研究发现,阳光玫瑰葡萄在新梢6片展叶期时开始花芽分化,在末花期进行花序原基分化,可见阳光玫瑰葡萄冬芽花芽分化进程早于上述葡萄品种,这可能与品种特性及产区气候条件有关。
碳水化合物是葡萄花芽分化重要的能量和营养物质,影响着葡萄成花的全过程[41]。吴月燕等[9]研究发现,无核鸡心葡萄叶片中淀粉和糖类含量与花芽分化呈正相关,充足的糖和淀粉可以促进葡萄花芽分化。赵静等[42]研究夏黑葡萄冬芽成花过程中叶片碳氮物质代谢变化,发现叶片中可溶性总糖与淀粉含量增加可以缩短夏黑花芽分化进程。本研究中,阳光玫瑰葡萄叶片中可溶性总糖含量从平顶期开始显著升高,花芽分化过程中可溶性总糖含量不断升高。葡萄叶片中淀粉含量也从平顶期开始显著升高,在花序原基分化期后20 d 时达到最大值,花序原基分化期后淀粉处于相对较高水平,与林玲[33]的研究结论一致。以上结果表明,可溶性总糖和淀粉含量增加促进了阳光玫瑰葡萄花芽分化起始,较高水平可溶性总糖与淀粉有利于葡萄花序原基及其各级穗轴分化。
矿质元素是植物生长发育过程中的重要营养成分,与其生长各个阶段息息相关。研究发现,N含量影响着葡萄花序原基的形成和发育,缺N 使得葡萄花序原基及花序的数量减少[10]。成花诱导及花序分化需要P 的供应,P 的缺失将会影响葡萄成花[11,43]。本研究中,在花序原基分化期后20 d 时叶片中全N含量显著降低,分析其原因可能是此时正处于夏果幼果期,且新梢还在继续生长,葡萄果实和叶片生长发育过程对N 需求量较大,导致此时叶片中N 含量显著下降,此时,及时补充氮源利于形成强大的光合叶幕,以满足树体生长、果实发育及冬芽花芽分化的氮素营养需求。赵文东等[11]研究发现,巨峰葡萄叶片P 含量在花芽分化初期较低,在花芽分化过程中呈先下降后上升再下降趋势。李双岑[13]研究发现,在红地球葡萄花芽分化的过程中芽内P、K含量在花序原基分化时迅速升高,Ca、Mg和N含量随花芽分化进程不断升高。本研究中,叶片中P、K 和Mg 含量在平顶期时显著下降,此后均出现两次波峰,P和Mg含量在花序原基分化期达到第一个波峰,K在花序原基分化期后40 d达到第一个波峰,P、K和Mg均在花序原基分化期后100 d时达到第二个波峰,这与赵文东等[11]及李双岑[13]的研究结果基本一致,叶片中P、K 和Mg 含量在平顶期时显著下降,可能是由于花芽分化起始对P、K 和Mg 需求较大,此时易移动元素P、K和Mg从叶片中移动至芽内供应冬芽需求,使得叶片含量显著降低。本研究中,叶片Ca 含量随花芽分化进程而整体不断增加,Ca元素的积累有助于花芽分化,该结论与李双岑[13]一致。本研究发现,P、K、Ca和Mg含量在花序原基分化期后60 d时均显著下降,此时正处于果实软化期,推测冬芽内矿质元素含量变化一定程度上受果实发育时营养竞争影响,生产上应该在夏果果实软化期前予以磷、钾、钙、镁肥的补充,以保证果实发育及花序原基分化正常进行。
葡萄花芽分化过程受内部基因的调控。前人研究发现,在葡萄成花诱导至花序原基分化过程中,基因FT、SOC1、LEAFY、AP1、FUL和FLC表达上调,参与调控成花诱导、始原基和花序原基分化。FT基因是成花时间的关键决定基因[17,44],正调控其下游基因SOC1 的表达[45-46]。葡萄中VvFT 基因在花分生组织诱导形成过程中表达上调[35]。VvSOC1 基因在葡萄花序原基和苞片中的表达处于较高水平[22]。花分生组织特征基因LEAFY 可通过激活AP1 基因来促进花序向花分生组织转变[21,23]。VvLEAFY基因在葡萄花序原基发育过程中的表达处于较高水平[24]。VvAP1 与VvFUL 基因在葡萄花芽分化早期的分生组织中就有表达[25]。FLC基因在花序原基诱导和分化过程中表达上调[47],葡萄中VvFLC 基因会抑制VvFT 和VvSOC1 的表达,负调控花序原基的形成[48]。本研究中,9个成花关键基因在成花诱导至花序原基分化过程中的表达情况与前人研究基本一致[31,48-50]。在阳光玫瑰葡萄冬芽分化平顶期即花芽形态分化起始时,基因VvFT、VvSOC1、VvAP1 和VvAP2 的表达显著上调;在花序原基分化期,基因VvFT、VvSOC1、VvLFY、VvAP1、VvFUL、VvAP2、VvAP3和VvAG表达均显著上调;VvFLC基因在始原基分化期前表达水平相对较高,在始原基分化期表达显著下调。以上结果表明,基因VvFT 和VvSOC1参与并促进了形态分化起始及始原基和花序原基分化,而VvFLC基因可能会抑制花芽进入形态分化阶段,基因VvAP1、VvAP2、VvLFY、VvFUL、VvAP3 和VvAG 可能均参与诱导花序原基分化。前人对葡萄花芽分化相关分子研究多集中在成花诱导至花序原基分化阶段,然而在花序原基分化至花序各级穗轴分化过程中的研究还未见详细报道。笔者在本试验中探究了这9个成花关键基因在花序原基分化至花序各级穗轴分化过程中的表达情况,研究发现,基因VvFT 和VvSOC1 在花序原基分化后的表达水平相对较低,基因VvLFY、VvFUL、VvAP3和VvAG在花序原基分化期后80 d表达显著上调并达到表达高峰。基因VvAP1 和VvAP2 在花序原基分化期后20 d 之后表达持续上调,在花序原基分化期后100 d时达表达波峰。VvFLC 基因在花序原基分化期后60 d 之后表达水平相对较高。以上结果表明,在花序原基分化期后基因VvFT 和VvSOC1 对花序各级穗轴的分化及发育的调控减弱,基因VvLFY、VvAP1、VvFUL、VvAP2、VvAP3 和VvAG 可能均参与促进花序各级穗轴的分化及发育,而VvFLC则可能抑制花序各级穗轴的分化。
4 结 论
研究探明了广西南宁地区阳光玫瑰葡萄生长期冬芽花芽分化进程,确定了阳光玫瑰葡萄花芽分化各阶段对应的物候期,发现南宁地区阳光玫瑰葡萄花芽分化进程比多数已报道的葡萄品种开始得早。在进入花序各级穗轴发育期后叶片可溶性总糖和淀粉含量较高,叶片P、K、Ca、Mg 元素含量均在花序原基分化期后60 d时显著降低。阳光玫瑰葡萄冬芽在花序原基分化期后对有机营养和主要矿质元素的需求增高,生产上在阳光玫瑰葡萄果实膨大期和软化期前后应注意适当补充磷、钾、钙、镁肥,以促进果实发育及花序各级穗轴分化正常进行。VvFT 和VvSOC1 基因参与诱导始原基及花序原基分化,Vv-LFY、VvAP1、VvFUL、VvAP2、VvAP3 和VvAG 基因可能均参与了花序原基分化及花序各级穗轴的分化及发育。VvFLC 基因在始原基前及花序原基分化后期的高表达可能会抑制始原基及花序各级穗轴的分化。
[1]林玲,曹慕明,郭荣荣,谢蜀豫,韩佳宇,盘丰平,陈国品,王世平,白先进,王博.桂南一年两收栽培夏黑葡萄花芽分化进程调控[J].南方农业学报,2017,48(1):57-65.LIN Ling,CAO Muming,GUO Rongrong,XIE Shuyu,HAN Jiayu,PAN Fengping,CHEN Guopin,WANG Shiping,BAI Xianjin,WANG Bo. Regulation of flower bud differentiation process of Summer Black grape cultivated in two-crop-a-year mode in southern Guangxi[J]. Journal of Southern Agriculture,2017,48(1):57-65.
[2]袁志友,李宪利,高东升. 影响葡萄花芽分化的主要因素[J].河北林果研究,2000,15(4):367-372.YUAN Zhiyou,LI Xianli,GAO Dongsheng.Factors influencing floral initiation in grapevines[J]. Hebei Journal of Forestry and Orchard Research,2000,15(4):367-372.
[3]李宪利,袁志友,李凌浩,韩兴国.葡萄的成花过程及其影响因素[J].果树学报,2002,18(5):330-335.LI Xianli,YUAN Zhiyou,LI Linghao,HAN Xingguo. Process of flower formation and influencing factors in grapevines[J].Journal of Fruit Science,2002,18(5):330-335.
[4]LI-MALLET A,RABOT A,GENY L. Factors controlling inflorescence primordia formation of grapevine:Their role in latent bud fruitfulness?A review[J]. Botany (Botanique),2016,94(3):147-163.
[5]VASCONCELOS M C,GREVEN M,WINEFIELD C S,TROUGHT M C T,RAW V.The flowering process of Vitis vinifera:A review[J].American Journal of Enology and Viticulture,2009,60(4):411-434.
[6]BUTTROSE M S. Fruitfulness in grape-vines:The response of different cultivara to light,temperature and daylength[J]. Vitis,1970,9(2):121-125.
[7]DUNN G M,MARTIN S R. Do temperature conditions at budburst affect flower number in Vitis vinifera L. cv. Cabernet Sauvignon?[J].Australian Journal of Grape&Wine Research,2000,6(2):116-124.
[8]PÉREZ F J,KÜHN N,VERGARA R. Expression analysis of phytochromes A,B and floral integrator genes during the entry and exit of grapevine-buds from endodormancy[J]. Journal of Plant Physiology,2011,168(14):1659-1666.
[9]吴月燕,李培民,吴秋峰.葡萄叶片内碳水化合物及蛋白质代谢对花芽分化的影响[J].浙江万里学院学报,2002,15(4):54-57.WU Yueyan,LI Peimin,WU Qiufeng. Effects of carbohydrate and protein metabolism on flower bud differentiation in grape leaves[J].Journal of Zhejiang Wanli University,2002,15(4):54-57.
[10]GUILPART N,METAY A,GARY C.Grapevine bud fertility and number of berries per bunch are determined by water and nitrogen stress around flowering in the previous year[J]. European Journal of Agronomy,2014,54:9-20.
[11]赵文东,孙凌俊,徐静,高圣华,张治东.薄膜温室葡萄花芽分化规律的研究[J].果树学报,2006,22(1):9-12.ZHAO Wendong,SUN Lingjun,XU Jing,GAO Shenghua,ZHANG Zhidong. Studies on the floralbud differentiation of grapes grown in greenhouse[J]. Journal of Fruit Science,2006,22(1):9-12.
[12]黄璐.巨峰葡萄不同芽节位夏季花芽分化规律及相关影响因子的研究[D].南宁:广西大学,2015.HUANG Lu. Study on the sunmer differentiation of bud in different nodes and its influence factors in Kyoho grape[D]. Nanning:Guangxi University,2015.
[13]李双岑.红地球花芽分化规律及其影响因子研究[D].银川:宁夏大学,2016.LI Shuangchen. Study on flower bud differentiation of Red Globe and its influencing factors[D].Yinchuan:Ningxia University,2016.
[14]MULLINS M G,BOUQUET A,WILLIAMS L E. Biology of the grapevine[M]. Cambridge,UK:Cambridge University Press,1992.
[15]SRINIVASAN C,MULLINS M. Flowering in Vitis:Conversion of tendrils into inflorescences and bunches of grapes[J]. Planta,1979,145:187-192.
[16]丰景,卢江. 葡萄成花分子生物学研究进展[J]. 热带作物学报,2018,39(8):1673-1681.FENG Jing,LU Jiang.Research progress of grape flowering molecular biology[J]. Chinese Journal of Tropical Crops,2018,39(8):1673-1681.
[17]DÍAZ-RIQUELME J,MARTÍNEZZAPATER J M,CARMONA M J. Transcriptional analysis of tendril and inflorescence development in grapevine(Vitis vinifera L.)[J/OL].PLoS ONE,2014,9(3):e92339.DOI:10.1371/journal.pone.0092339.
[18]PARCY F.Flowering:A time for integration[J].The International Journal of Developmental Biology,2005,49(5/6):585-593.
[19]LIU L,XUAN L J,JIANG Y P,YU H.Regulation by FLOWERING LOCUS T and TERMINAL FLOWER 1 in flowering time and plant architecture[J/OL]. Small Structures,2021,2(4):2000125.DOI:10.1002/sstr.202000125.
[20]王建楼,卢龙,鲁井云,秦旭冬,邢佳毅,胡建芳.VvFT 和VvTFL1A 基因在葡萄花发育中的表达特性研究[J].中国农业大学学报,2013,18(6):132-137.WANG Jianlou,LU Long,LU Jingyun,QIN Xudong,XING Jiayi,HU Jianfang.Expressing characteristics of genes VvFT and VvTFL1A in floral organs of grapevine in grapevine flower[J]. Journal of China Agricultural University,2013,18(6):132-137.
[21]杨光,曹雪,房经贵,黄振喜,陶建敏,王晨.葡萄花发育基因的亚细胞定位和表达分析[J]. 中国农业科学,2011,44(3):641-650.YANG Guang,CAO Xue,FANG Jinggui,HUANG Zhenxi,TAO Jianmin,WANG Chen. Sub-cellular localization and expression analysis of genes involved in grapevine floral development[J].Scientia Agricultura Sinica,2011,44(3):641-650.
[22]SREEKANTAN L,THOMAS M R. VvFT and VvMADS8,the grapevine homologues of the floral integrators FT and SOC1,have unique expression patterns in grapevine and hasten flowering in Arabidopsis[J]. Functional Plant Biology,2006,33(12):1129-1139.
[23]BLÁZQUEZ M A,WEIGEL D. Integration of floral inductive signals in Arabidopsis[J].Nature,2000,404(6780):889-892.
[24]CARMONA M J,CUBAS P,CA L M,MARTINEZ-ZAPATER J M. Flowering transition in grapevine (Vitis vinifera L.)[J]. Canadian Journal of Botany,2007,85(8):701-711.
[25]CALONJE M,CUBAS P,MARTÍNEZZAPATER J M. Floral meristem identity genes are expressed during tendril development in grapevine[J]. Plant Physiology,2004,135(3):1491-1501.
[26]HONMA T,GOTO K. Complexes of MADS-box proteins are sufficient to convert leaves into floral organs[J]. Nature,2001,409(6819):525-529.
[27]THEISSEN G. Development of floral organ identity:Stories from the MADS house[J]. Current Opinion in Plant Biology,2001,4(1):75-85.
[28]王晨,刘洪,房经贵,曹雪,杨光,章镇.基于EST 数据库的葡萄APETALA2 基因cDNA 克隆及其表达分析[J]. 果树学报,2010,27(2):207-212.WANG Chen,LIU Hong,FANG Jinggui,CAO Xue,YANG Guang,ZHANG Zhen. Cloning and expression analysis of APETALA2 gene from grapevine(Vitis vinifera)based on EST[J].Journal of Fruit Science,2010,27(2):207-212.
[29]宗成文.葡萄花发育相关基因的克隆与表达特性研究[D].南京:南京农业大学,2007.ZONG Chengwen. Cloning and expression of flower development related genes from grape (Vitis vinifera × V. labrusca)[D].Nanjing:Nanjing Agricultural University,2007.
[30]LI D,LIU C,SHEN L,WU Y,CHEN H Y,ROBERTSON M,HELLIWELL C A,ITO T,MEYEROWITZ E,HAO YU H. A repressor complex governs the integration of flowering signals in Arabidopsis[J].Developmental Cell,2008,15(1):110-120.
[31]刘丹,慕茜,李晓鹏,刘更森,李玉,张彦苹,房经贵.基于EST数据库的葡萄成花途径的预测及分析[J].园艺学报,2014,41(1):26-34.LIU Dan,MU Xi,LI Xiaopeng,LIU Gengsen,LI Yu,ZHANG Yanping,FANG Jingguo.Forecasting analysis of the grape flowering pathway based on the grape EST database[J]. Acta Horticulturae Sinica,2014,41(1):26-34.
[32]王海波,赵君全,王孝娣,史祥宾,王宝亮,郑晓翠,刘凤之.新梢内源激素变化对设施葡萄花芽孕育的影响[J].中国农业科学,2014,47(23):4695-4705.WANG Haibo,ZHAO Junquan,WANG Xiaodi,SHI Xiangbin,WANG Baoliang,ZHENG Xiaocui,LIU Fengzhi.The influence of changes of endogenous hormones in shoot on the grapes flower bud differentiation in greenhouse[J].Scientia Agricultura Sinica,2014,47(23):4695-4705.
[33]林玲.一年两收栽培模式下摘心和生长调节剂对‘夏黑’葡萄花芽分化规律影响的研究[D].南宁:广西大学,2017.LIN Ling. Study on effect of sunmer prunning and growth regulation the flower bud differentiation of grape cv. Summer Black under cultivation moodel of two- crop- a- year[D]. Nanning:Guangxi University,2017.
[34]KOBAYASH S,ISHIMARU M.,HIRAOKA K,HONDA C.Myb-related genes of the Kyoho grape (Vitis labruscana) regulate anthocyanin biosynthesis[J].Planta,2002,215(6):924-933.
[35]杨光,岳林旭,王晨,谭洪花,曹雪,房经贵.葡萄9 个重要花发育相关基因在藤稔葡萄夏芽成花过程中的表达分析[J].果树学报,2010,27(6):892-897.YANG Guang,YUE Linxu,WANG Chen,TAN Honghua,CAO Xue,FANG Jinggui. Expression of nine important floral genesduring flower differentiation and development of the summer[J].Journal of Fruit Science,2010,27(6):892-897.
[36]石雪晖,蔡桂华,杨国顺,王先荣,刘昆玉,钟晓红,熊兴耀,倪建军,郭光银.红地球葡萄二次果花序分化过程初探[J].中外葡萄与葡萄酒,2010(11):26-28.SHI Xuehui,CAI Guihua,YANG Guoshun,WANG Xianrong,LIU Kunyu,ZHONG Xiaohong,XIONG Xingyao,NI Jianjun,GUO Guangyin. Study on inflorescence differentiation for the second fruiting of grapevine cv. Red Globe[J]. Sino-Overseas Grapevine&Wine,2010(11):26-28.
[37]石雪晖,甘霖,杨国顺,刘昆玉,钟晓红,燕中炎,王先荣,郭光银,倪建军,周乃泉.优无核等20 个葡萄品种引种初报[J].湖南农业大学学报(自然科学版),2002,28(6):487-491.SHI Xuehui,GAN Lin,YANG Guoshun,LIU Kunyu,ZHONG Xiaohong,YAN Zhongyan,WANG Xianrong,GUO Guangyin,NI Jianjun,ZHOU Naiquan.Introduction of twenty grape varieties[J].Journal of Hunan Agricultural University(Natural Sciences),2002,28(6):487-491.
[38]蔡桂华.欧亚种葡萄二次果花序分化机制研究[D].长沙:湖南农业大学,2007.CAI Guihua. Study on mechanism of secondary differentiation of Eurasian grapes[D]. Changsha:Hunan Agricultural University,2007.
[39]朱维,林玲,谢蜀豫,韩佳宇,曹慕明,郭荣荣,白先进,王博.摘心对一年两收栽培‘夏黑’葡萄不同节位冬芽花芽分化的影响[J].果树学报,2020,37(2):226-234.ZHU Wei,LIN Ling,XIE Shuyu,HAN Jiayu,CAO Muming,GUO Rongrong,BAI Xianjin,WANG Bo. Effects of pinching on flower differentiation in winter buds at different nodes of‘Summer Black’grape under two-crop-a-year cultivation[J].Journal of Fruit Science,2020,37(2):226-234.
[40]王海波,王孝娣,赵君全,史祥宾,王宝亮,郑晓翠,刘凤之.设施促早栽培下耐弱光能力不同的葡萄品种冬芽的花芽分化[J].园艺学报,2016,43(4):633-642.WANG Haibo,WANG Xiaodi,ZHAO Junquan,SHI Xiangbin,WANG Baoliang,ZHENG Xiaocui,LIU Fengzhi. Studies on the flower bud differentiation of grape cultivars with different tolerant ability of low light in greenhouse[J].Acta Horticulturae Sinica,2016,43(4):633-642.
[41]LEBON G,WOJNAROWIEZ G,HOLZAPFEL B,FONTAINE F,VAILLANT-GAVEAU N,CLÉMENT C. Sugars and flowering in the grapevine (Vitis vinifera L.)[J]. Journal of Experimental Botany,2008,59(10):2565-2578.
[42]赵静,吕秀兰,聂萧,王进,刘芳.夏黑葡萄冬芽二花成花过程中叶碳氮物质代谢变化[J].黑龙江农业科学,2016(12):82-86.ZHAO Jing,LÜ Xiulan,NIE Xiao,WANG Jin,LIU Fang.Changes of carbon and nitrogen metabolism during the flowering of grape Summer Black winter bud[J].Heilongjiang Agricultural Sciences,2016(12):82-86.
[43]SKINNER P W,MATTHEWS M A. Reproductive development in grape (Vitis vinifera L.) under phosphorus-limited conditions[J].Scientia Horticulturae,1989,38(1/2):49-60.
[44]SREEKANTAN L,THOMAS M R. VvFT and VvMADS8,the grapevine homologues of the floral integrators FT and SOC1,have unique expression patterns in grapevine and hasten flowering in Arabidopsis[J]. Functional Plant Biology,2006,33(12):1129-1139.
[45]ABE M,KOBAYASHI Y,YAMAMOTO S. FD,a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex[J].Science,2005,309(5737):1052-1056.
[46]YOO S K,CHUNG K S,KIM J. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis[J].Plant Physiology,2005,139(2):770-778.
[47]SIMPSON G G. The autonomous pathway:epigenetic and posttranscriptional gene regulation in the control of Arabidopsis flowering time[J].Current Opinion in Plant Biology,2004,7(5):570-574.
[48]DÍAZ-RIQUELME J,GRIMPLET J,MARTÍNEZ-ZAPATER J M,CARMONA M J. Transcriptome variation along bud development in grapevine(Vitis vinifera L.)[J/OL].BMC Plant Biology,2012,12(1):1-13.https://doi.org/10.1186/1471-2229-12-181.
[49]刘丹,孙欣,慕茜,吴伟民,章镇,房经贵.葡萄花芽发育相关基因在不同节位芽中的表达分析[J]. 中国农业科学,2015,48(10):2007-2016.LIU Dan,SUN Xin,MU Xi,WU Weimin,ZHANG Zhen,FANG Jinggui. Analysis of expression levels of floral genes in the buds on different branch nodes of grapevine[J]. Scientia Agricultura Sinica,2015,48(10):2007-2016.
[50]慕茜,刘更森,孙欣,李玉,陶然,王晨,房经贵.‘藤稔’葡萄冬季休眠后期花芽发育相关基因表达的分析[J]. 园艺学报,2013,40(5):828-838.MU Qian,LIU Gengsen,SUN Xin,LI Yu,TAO Ran,WANG Chen,FANG Jinggui.Analysis of expression levels of floral genes during the late dormancy stage of‘Fujiminori’grapevine[J].Acta Horticulturae Sinica,2013,40(5):828-838.