桃(Prunus persica L.),蔷薇科、李属植物[1],落叶小乔木,原产中国。桃的栽培历史已有4000 多年[2],品种多样,在世界各地均有分布[3]。温度是影响桃树生长发育以及决定其自然分布的主要环境因子[4],明确其响应低温的生理生化机制对培育和筛选桃抗寒品种,应对生长季低温及扩大栽培区域,具有重要的理论和实践意义。
在前期研究中,关于植物抗寒的生理机制已有不少报道。研究发现,植物体内存在一套负责清除活性氧的抗氧化系统[5],该系统是细胞抗寒生理生化的重要基础,当植物受到低温胁迫后,细胞内积累大量活性氧、自由基,这些伤害信号会引起植物对逆境胁迫的响应,形成氧化胁迫。氧化胁迫会破坏细胞的结构和功能,使代谢酶活性降低,影响细胞正常生长,严重时还会造成细胞死亡[6]。丙二醛(MDA)含量是常用的膜脂过氧化指标,通过测定MDA 含量可衡量细胞膜系统的损伤程度[7]。此外,脯氨酸也是植物的一种重要的抗逆相关物质,其含量在逆境条件下显著上升[8-9]。脯氨酸的大量积累有助于细胞保水,促进细胞恢复正常生长。因此,果树在寒冷胁迫过程中脯氨酸含量,也是衡量植物抗寒性的重要指标[10-11]。
代谢组学是20 世纪90 年代中期发展起来的新兴学科,作为系统生物学的重要组成部分,代谢组学能够揭示不同物种间、同一物种的不同组织之间以及同一物种的同一组织在不同逆境胁迫条件下的代谢物图谱的差异[12-13],从而有助于解释植物响应逆境的生理生化特征。目前,代谢组学已经广泛应用于植物逆境胁迫研究中[14]。Ding等[15]通过对番茄添加色胺类物质后,分析寒冷胁迫对番茄的伤害程度,表明外源施加物能够增强活性氧相关酶的活性,减少或者延缓寒冷胁迫对番茄的伤害。方彦等[16]对黑油菜在低温胁迫下根部代谢组进行分析,表明黑油菜在低温条件下通过调节糖、氨基酸和磷脂类代谢水平以适应逆境。朱建峰等[17]研究了盐胁迫下白榆种子萌发期的代谢组成分变化,暗示油菜素内酯和精胺可能是白榆种子萌发期适应盐胁迫的关键因子。牟丹等[18]对高加索三叶草响应不同降温模式的代谢组成分进行测定,发现γ-氨基丁酸、亮氨酸、组氨酸、赖氨酸、柠檬酸、矢车菊素和木犀草素含量均显著上升,提出上述物质可能是高加索三叶草适应低温胁迫的主要组分。
近年来,关于桃抗寒性形成的生理生化机制有了一定的研究,但大部分都集中在抗寒基因的发掘上。宋艳波等[19]对桃PpCBF2 基因进行了克隆,发现早红霞和大久保的PpCBF2 基因受低温诱导,且品种间的表达差异与品种抗寒性相关。申晴等[20]克隆了桃PpMYB3 基因,发现该基因主要在韧皮部表达,且抗寒性强的桃品种PpMYB3 基因表达量随温度降低呈下降趋势,而在抗寒性弱的品种中该基因的表达则随温度降低呈上升趋势,暗示PpMYB3 在桃抗寒性形成中起到负调控作用。左波等[21]对桃PpRBD2 基因进行克隆和表达分析,发现在不同时间的低温胁迫下,PpRBD2 基因在抗寒品种中的表达量比不抗寒品种更高,表明PpRBD2 基因的表达量与桃抗寒性呈正相关。
而在抗寒相关的代谢物研究方面,关于脯氨酸生物合成途径已经比较清楚。谷氨酸途径是高等植物合成脯氨酸的重要方式[22],首先,谷氨酸(Glu)在Δ1-二氢吡咯-5-羧酸合成酶(P5C synthetase,P5CS)催化下生成谷氨酸半醛(GSA),GSA 自动环化为Δ1-二氢吡咯-5-羧酸(Δ1-pyrroline-5-carboxylate,P5C),随后P5C又在Δ1-二氢吡咯-5-羧酸还原酶(P5Creductase,P5CR)的催化下生成L-脯氨酸。因此,P5CS和P5CR是谷氨酸途径中的2个关键酶,分别催化脯氨酸生物合成的第一步和最后一步反应,其中第一步反应为限速反应。
为揭示在桃上是否存在除脯氨酸之外的低温响应相关代谢物质,笔者在本研究中采用基于超高效液相色谱串联质谱(UPLC-MS/MS)的广泛靶向代谢组学技术,通过聚类分析(hierarchical cluster analysis,HCA)、差异倍数法(fold change,FC)等统计分析方法,鉴定低温胁迫处理条件下桃芽中的显著差异代谢物质,并验证关键代谢物与桃芽抗寒性的关系。研究有利于阐明桃抗寒的生理生化基础,为后续开发相应的调控措施提供理论支撑。
1 材料和方法
1.1 材料
用于抗寒处理以鉴定差异代谢物所用的桃品种为中农金辉,试验材料取自中国农业科学院郑州果树研究所桃圃,树龄6 a(年),取样时间为2021 年7月,所选试材为树冠外围中上部的1年生幼嫩枝条。
验证差异代谢物与抗寒性关系的材料为经笔者所在实验室组培快繁的2年生桃抗砧1号盆栽桃苗。
1.2 低温胁迫处理方法
剪取树冠外围中上部幼嫩枝条,保鲜膜封住枝条以避免水分散失,将枝条带回实验室后剪成30 cm左右的枝段,保鲜膜包裹后放入冰箱开始胁迫处理,一次性放入试验所需的所有枝条,依据前人[23]研究确定胁迫温度为-14 ℃,并记录处理开始时间,分别在处理时间达到0、24、72 h时取出部分枝段,在枝段未解冻状态下去掉枝段上的叶子,选择枝段上大小一致且未受机械损伤的桃芽,用镊子与解剖针剥出后迅速放入液氮中,称量剥出桃芽的总质量,达到代谢物测定所需要的质量后停止取样,将所取桃芽平均分成2份,作为2个重复,用于代谢物测定。
1.3 体外喷施药剂对抗寒性的影响
首先,选择氨基嘌呤、腺苷、黄嘌呤核苷以及DL-同型半胱氨酸标准品,用蒸馏水配制不同浓度的溶液,浓度参考前人[24-26]研究,其中氨基嘌呤、腺苷和黄嘌呤核苷的质量浓度均为50 mg·L-1,DL-同型半胱氨酸质量浓度5 mg·L-1,标准品氨基嘌呤纯度为98%,腺苷纯度≥99%,黄嘌呤核苷纯度≥99%,DL-同型半胱氨酸纯度≥90%,均购自源叶生物科技有限公司(上海)。
取15株生长一致的中桃抗砧1号在光照培养箱中进行低温处理,为保证桃苗处于存活状态,选择-4 ℃进行低温处理,光照培养箱设定光照时间16 h,黑暗时间8 h。然后,分别用配制的不同标准品溶液进行喷施,每株每次喷施用量大约15 mL,每24 h喷施处理1次。最后,测定处理后1、3、7 d的叶片中脯氨酸含量,脯氨酸测定方法按照脯氨酸(Pro)含量检测试剂盒(索莱宝生物科技有限公司,北京)说明书操作。
1.4 代谢物提取流程
样品放置于冻干机(Scientz-100F,新芝,宁波)中进行真空冷冻干燥,研磨仪(MM 400,Retsch,Germany)30 Hz 研磨90 s 成粉末。称取100 mg,溶解于1.2 mL 70%甲醇提取液中,每30 min涡旋1次,每次持续30 s,共涡旋6 次,样品置于4 ℃冰箱过夜。12 000 r·min-1离心10 min 后吸取上清液,用微孔滤膜(0.22 μm)过滤样品,并保存于进样瓶中,用于UPLC-MS/MS分析。
1.5 色谱和质谱采集条件
数据采集系统主要包括超高效液相色谱和串联质谱。液相条件:①色谱柱:Agilent SB-C18 1.8 μm,2.1 mm×100 mm;②流动相:A相为超纯水(含0.1%的甲酸),B相为乙腈(含0.1%的甲酸);③洗脱梯度:0.0 min B 相比例为5%;9.0 min 内B 相比例线性增加到95%,并维持1 min;10.0~11.1 min,B 相比例降为5%,并以5%平衡至14 min;流速0.35 mL·min-1;柱温40 ℃;进样量4 μL。质谱所用的系统为AB4500 Q TRAP UPLC/MS/MS,采用三重四极杆线性离子阱。ESI 源操作参数如下:离子源,涡轮喷雾;源温度550 ℃;离子喷雾电压5500 V(正离子模式)/-4500 V(负离子模式);离子源气体Ⅰ、气体Ⅱ和帘气分别设置为50、60 和25.0 psi,碰撞诱导电离参数设置为高。
1.6 代谢物定性与定量
基于自建的MWDB(metware database)数据库,根据二级谱信息进行物质定性,分析时去除了同位素、含K+离子、Na+离子、NH4+离子的重复信号,以及本身是其他更大分子质量物质碎片离子的重复信号。
代谢物定量是利用三重四级杆质谱的多反应监测模式(multiple reaction monitoring,MRM)分析完成。MRM 模式中,四级杆首先筛选目标物质的前体离子(母离子),排除掉其他分子质量物质对应的离子以初步排除干扰;前体离子经碰撞室诱导电离后断裂形成很多碎片离子,碎片离子再通过三重四级杆过滤选择出所需要的一个特征碎片离子。获得不同样本的特征离子数据后,对所有物质质谱峰进行峰面积积分,并对其中同一代谢物在不同样本中的质谱出峰进行积分校正。
1.7 关键基因的定量表达分析
提取用代谢物溶液喷施处理后的中桃抗砧1号叶片RNA,反转录后得到cDNA。选择1 个桃抗寒相关基因PpCBF2 以及1 个脯氨酸合成关键基因PpP5CS,以cDNA 为模板,以PpCBF2- F/R、PpP5CS-F/R 为荧光定量引物,以β-actin-F/R 为内参引物进行qPCR 反应,根据2-△△CT法计算基因的相对表达量,试验3次重复,引物序列详见表1。RNA提取试剂盒购自北京华越洋生物科技有限公司(北京),反转录与SYBR-Green 试剂盒购自北京康润诚业生物科技有限公司(北京),qPCR 采用Roche LightCycler® 480 Ⅱ实时荧光定量PCR 系统,qPCR反应步骤为:95 ℃预变性5 min;95 ℃变性10 s,60 ℃退火10 s,72 ℃延伸10 s,40 ℃保持10 s,45个循环。
表1 实时荧光定量PCR 引物序列
Table 1 Primer sequences for quantitative real-time PCR
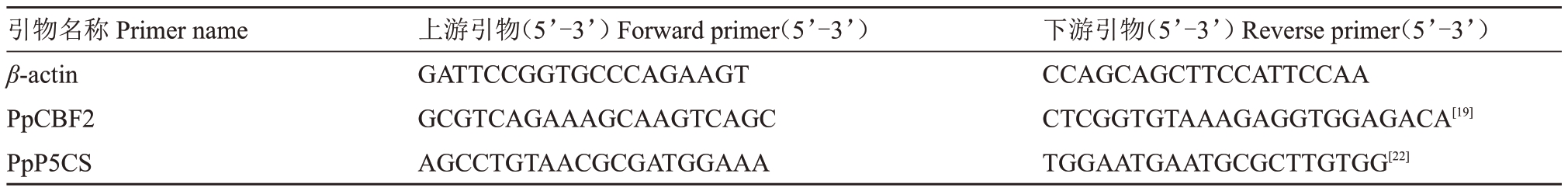
引物名称Primer name β-actin PpCBF2 PpP5CS上游引物(5’-3’)Forward primer(5’-3’)GATTCCGGTGCCCAGAAGT GCGTCAGAAAGCAAGTCAGC AGCCTGTAACGCGATGGAAA下游引物(5’-3’)Reverse primer(5’-3’)CCAGCAGCTTCCATTCCAA CTCGGTGTAAAGAGGTGGAGACA[19]TGGAATGAATGCGCTTGTGG[22]
1.8 数据分析
将原始数据导入代谢组学处理软件Analyst 1.6.3 进行处理,包括基线过滤、峰识别、积分、保留时间校正、峰对齐和归一化等。处理后的数据根据差异倍数值(fold change,FC)和显著性p 值进行差异性代谢物的筛选,以|log2FC|>0.6,p-value<0.05为筛选标准,log2FC>0.6 视为显著上调代谢物,log2 FC<-0.6 视为显著下调代谢物,其余视为差异不显著。
2 结果与分析
2.1 桃芽低温处理后代谢组成分的类别分析
将桃芽低温胁迫处理0、1、3 d 的样本分别定义为T1、T2、T3,然后将通过UPLC-MS/MS 检测平台检出的信号与数据库进行比对,发现在中农金辉桃芽的3 个样品中共检测到172 种有功能注释的代谢物,可分为10 类(表2),其中氨基酸及其衍生物占比最高,其次是酚酸类、黄酮、有机酸和脂质等。
表2 桃芽代谢物分类占比
Table 2 Classification proportion of total metabolites in bud
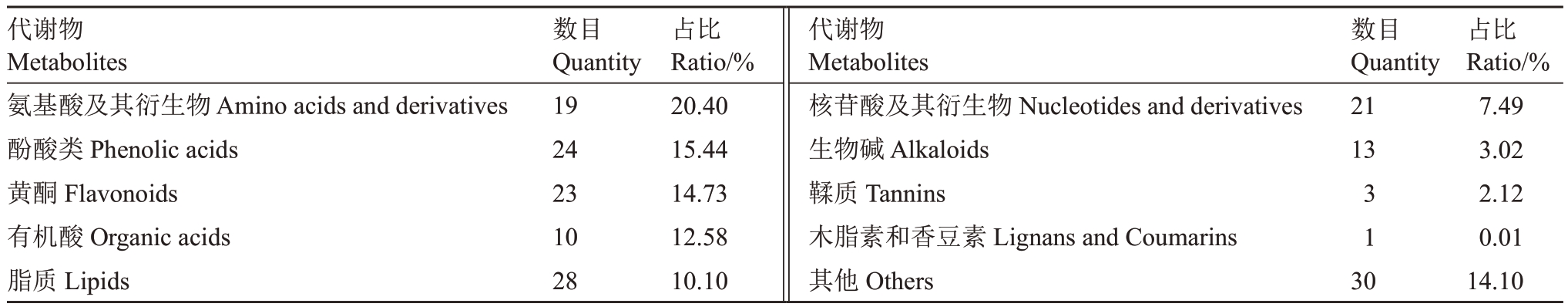
代谢物Metabolites氨基酸及其衍生物Amino acids and derivatives酚酸类Phenolic acids黄酮Flavonoids有机酸Organic acids脂质Lipids数目Quantity 19 24 23 10 28占比Ratio/%20.40 15.44 14.73 12.58 10.10代谢物Metabolites核苷酸及其衍生物Nucleotides and derivatives生物碱Alkaloids鞣质Tannins木脂素和香豆素Lignans and Coumarins其他Others数目Quantity 21 13 3 1 3 0占比Ratio/%7.49 3.02 2.12 0.01 14.10
2.2 桃芽低温处理后代谢组成分的含量分析
分析172 种代谢物成分的含量,发现中农金辉桃芽中占比大于1%的主要代谢物有32种(表3),其中氨基酸类衍生物7种、黄酮5种、酚酸类4种、脂质4 种、有机酸3 种、生物碱2 种、核苷酸及其衍生物2种、鞣质1种、其他类4种。水杨酸O-咖啡酸含量最高,达到5.58%,其次是L-天冬酰胺和2,5-二羟基苯甲酸-O-己糖苷含量等。
表3 桃芽中主要代谢物种类与占比
Table 3 Classes and proportion of main metabolites in buds

?
2.3 低温处理对桃芽代谢物的影响
为进一步分析低温对桃芽代谢物成分和含量的影响,研究绘制了不同处理间代谢物相对含量的火山图(图1)。

图1 芽不同处理时间的差异代谢物火山图
Fig.1 Volcano plot of differential metabolites of peach buds at different treatment times
在T1 vs T2 与T1 vs T3 中,共筛选到32 种差异代谢物,其中8种下调和24种上调(表4)。
表4 不同处理时间下芽差异代谢物
Table 4 Different metabolites in peach buds under different treatment periods

注:-.无差异。
Note:-.No difference.
?
2.4 差异代谢物聚类分析
采用聚类分析方法,进一步分析3 个处理时间桃芽中代谢物的整体变化趋势(图2)。由图2 可以看出,差异代谢物在芽中的含量区分明显,种类最多的为脂质,其次是氨基酸及其衍生物、核苷酸及其衍生物、生物碱等。其中,长寿花糖苷与烟酸2种代谢物含量整体表现先下调再上调,但前期下调不显著(长寿花糖苷p-value = 0.8>0.05,烟酸p-value =0.73>0.05);同样的,L-精氨酸含量整体表现先下调再上调,但后期上调不显著(p-value=0.13>0.05);DL-同型半胱氨酸、苜蓿素这2种代谢物含量整体表现上调,但前期变化不显著(DL-同型半胱氨酸pvalue=0.19>0.05,苜蓿素p-value=0.07>0.05)。
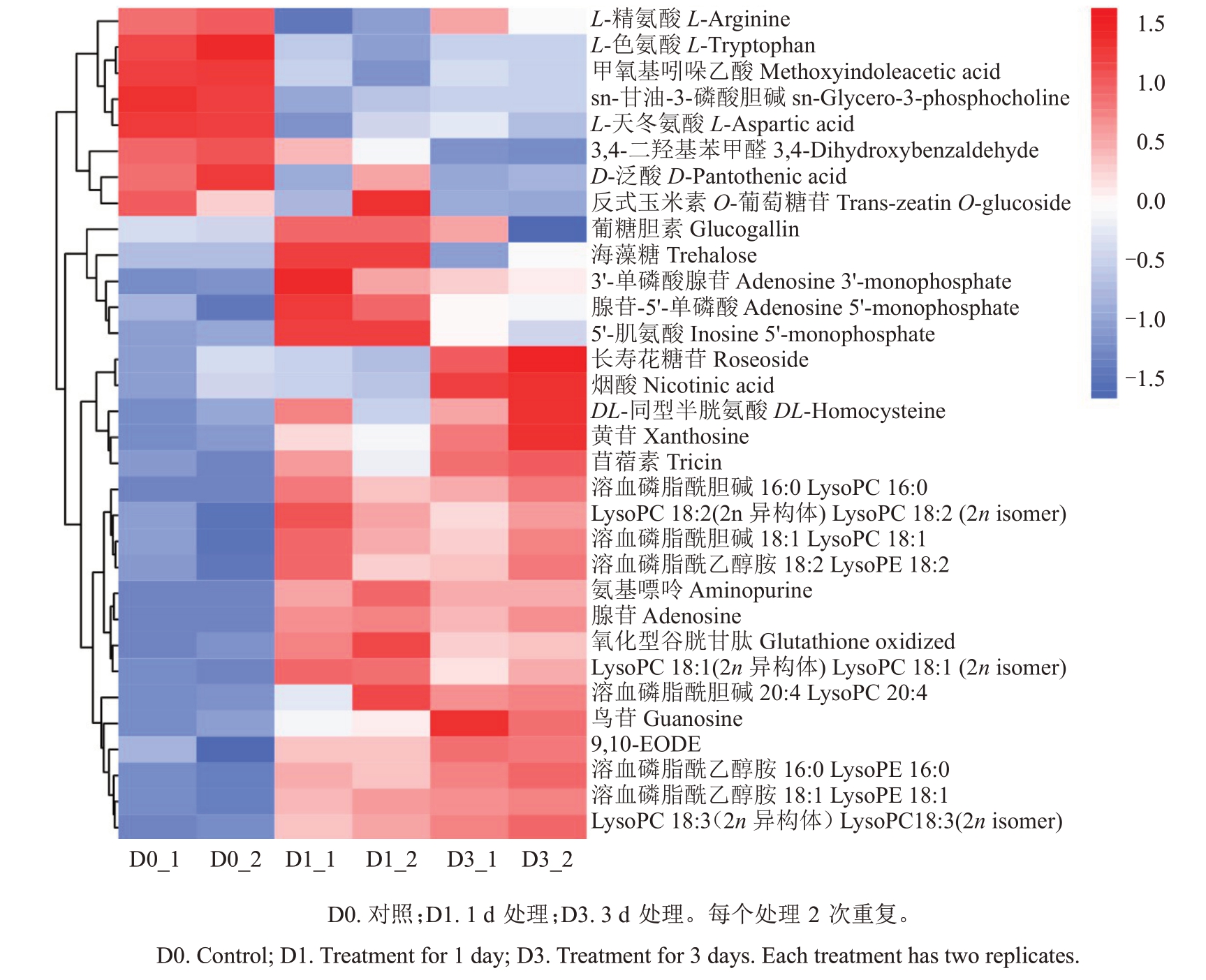
图2 不同处理时间桃芽差异代谢物聚类分析
Fig.2 Cluster analysis of differential metabolites in peach bud under different treatments
2.5 喷施特定代谢物对桃抗寒性的影响
为证实差异代谢物的功能,从32种差异代谢物中,选择经低温处理1 d 与3 d 后,与对照组(0 d)相比表现上调的4 种代谢物,配制成标准品溶液并喷施桃幼苗,测定低温处理后对照组与处理组叶片的脯氨酸含量,以探讨特定代谢物对桃抗寒性的调控作用(图3)。结果表明,低温胁迫处理后,不同代谢物处理的脯氨酸含量均上升,处理后第3 天出现峰值,在第7 天则有所下降。喷施清水的对照组在胁迫前后脯氨酸含量上升幅度较小,第3 天脯氨酸含量仅为0 d的2倍;而喷施其他物质如黄嘌呤核苷的第3天脯氨酸含量为0 d的6倍。
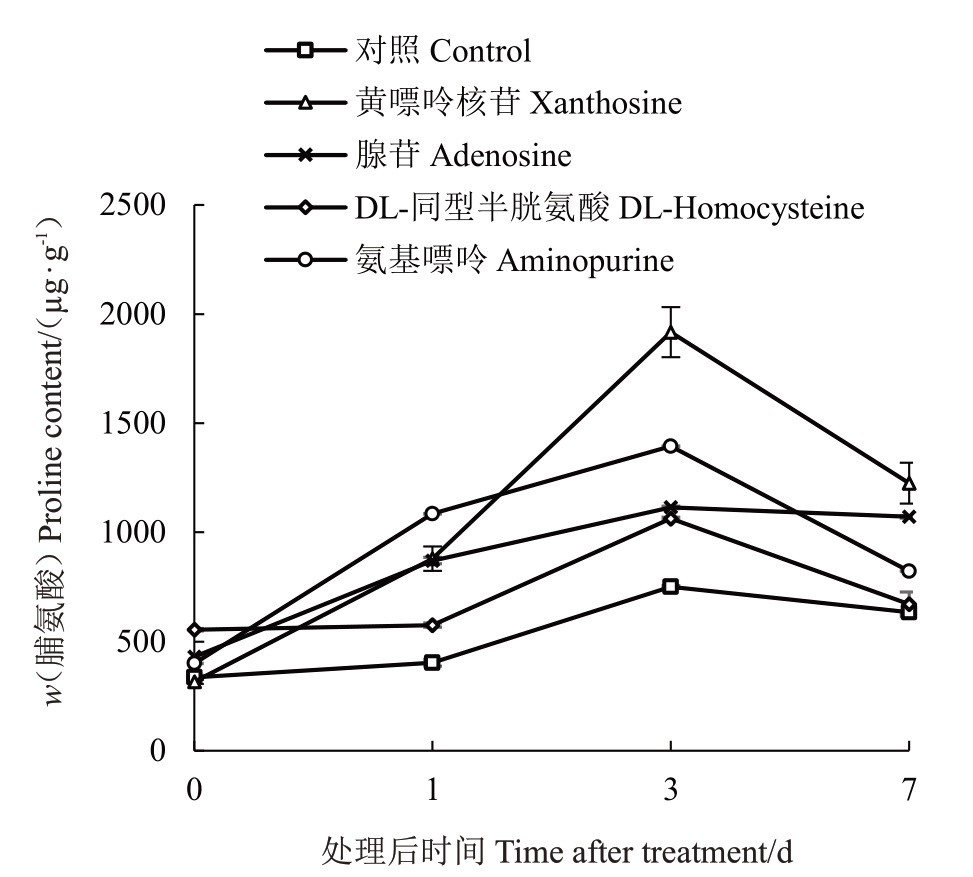
图3 不同代谢物溶液处理后叶片脯氨酸含量变化情况
Fig.3 Changes of proline content in leaves after treated with different metabolite solutions
2.6 喷施特定代谢物对桃抗寒相关基因表达量的影响
为进一步从转录水平验证代谢物喷施对桃幼苗抗寒性的影响,笔者选择了1 个低温响应相关基因PpCBF2[19]以及1个脯氨酸合成关键基因PpP5CS[22],测定其在低温处理后的相对表达量。
由图4-A 可见,喷施清水的对照组,PpCBF2 表达量随低温胁迫时间延长逐渐上升,在第7 天达到最高值,为0 d 的1246 倍;经DL-同型半胱氨酸与黄嘌呤核苷处理后的幼苗,其叶片中PpCBF2 的表达量同样随胁迫时间延长逐渐增加,但峰值出现在处理后第3 天,随后表达量有所下降,第3 天表达量分别达到0 d 的13 810 与3650 倍;经氨基嘌呤与腺苷处理后的幼苗,其叶片中PpCBF2 的表达量峰值出现在第1 天,分别为0 d 的1464 与1169 倍,自第3 天开始逐渐下降。该结果表明,桃幼苗确实受到了低温胁迫,但不同代谢物处理可能影响了桃幼苗的低温响应能力。
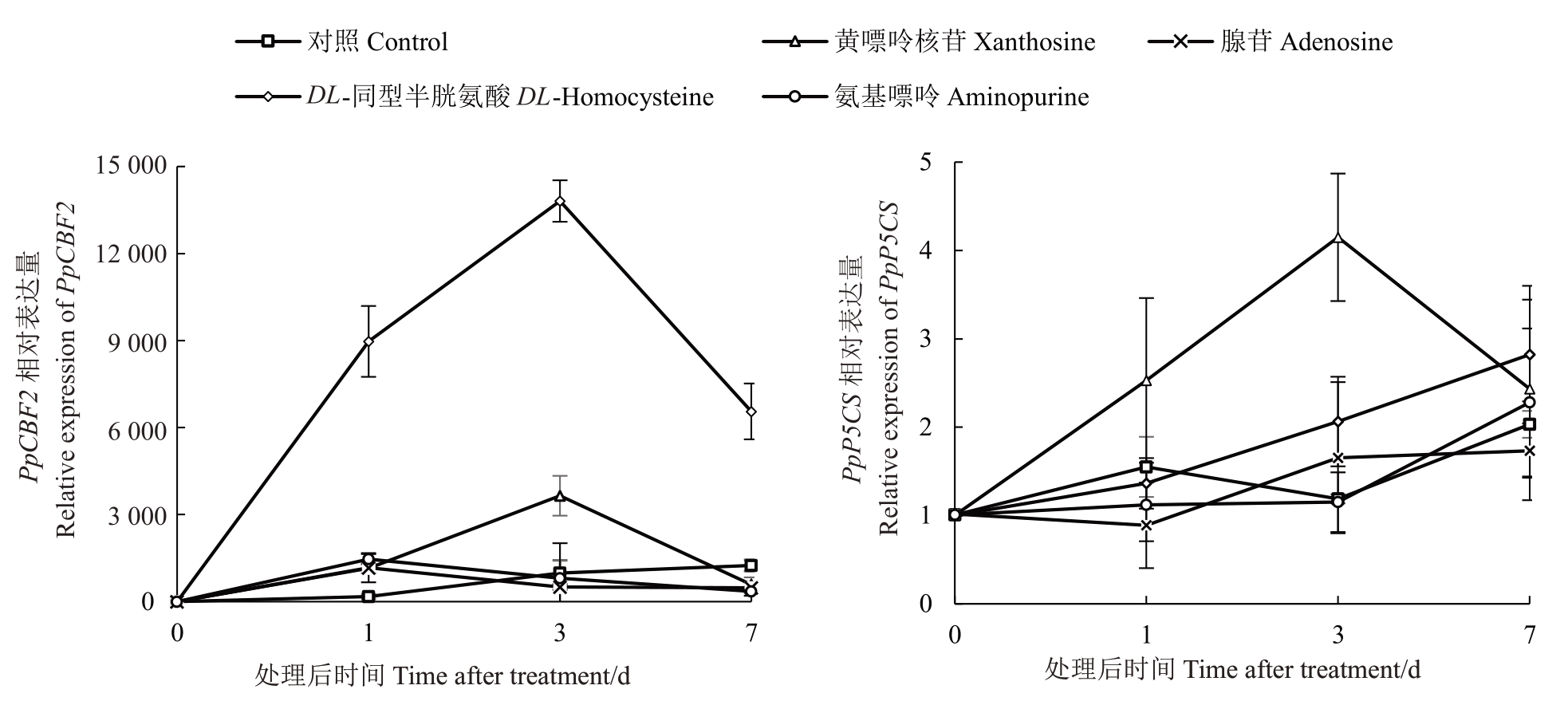
图4 不同代谢物溶液处理后叶片相关基因表达量变化情况
Fig.4 Changes of gene expression in leaves after treated with different metabolite solutions
由图4-B可见,喷施清水、腺苷、DL-同型半胱氨酸与氨基嘌呤处理的样品中PpP5CS表达量整体随胁迫时间延长而上升,在第7天达到峰值,其峰值分别为0 d 的2、1.7、2.8 与2.3 倍。而经黄嘌呤核苷处理的幼苗,其叶片中PpP5CS表达量在第3天达到峰值,其峰值达到0 d 的4 倍。喷施黄嘌呤核苷后,脯氨酸合成相关基因表达最为明显,与该处理的叶片中脯氨酸含量较高相一致。
3 讨 论
笔者在本研究中利用广泛靶向代谢组检测方法,一共检测到172 种代谢物,其中占比大于1%的主要代谢物有32 种,水杨酸O-咖啡酸的含量最高,达到5.58%,其次是L-天冬酰胺。
水杨酸作为一种广泛存在于植物体内的酚类激素,除了参与植物生长发育的多个过程以外,还能够提高植物非生物抗逆性,在植物对环境胁迫响应中发挥着重要作用。水杨酸可以通过调节活性氧及抗氧化酶活性来影响植物抗逆性[27],且水杨酸的作用具有双重性[28],即低浓度的水杨酸能够减轻幼苗所受氧化伤害的程度;高浓度则加重。此外,谷晓勇等[29]和Wildermuth等[30]认为植物体内水杨酸浓度的变化是水杨酸响应环境胁迫的重要途径,在逆境条件下,水杨酸生物合成途径中的基因被诱导表达,促进水杨酸积累进而增强植物抗逆性。结合前人研究结果,笔者推测当植物遭遇冷害时,体内的水杨酸信号通路被激活,从而诱导抗寒相关基因表达、改变植物体内水杨酸浓度,以及其他诸如抗氧化酶、蛋白质以及植物激素等渗透调节物质的变化,进而提高植物抗寒性。
植物在应对非生物胁迫和病菌侵害时,其体内的天冬酰胺合成酶基因表达量会提高,从而积累大量天冬酰胺以抵御逆境[31],天冬酰胺合成酶基因也因此被认为是潜在的抗逆基因。张晓磊[32]在对小黑麦天冬酰胺合成酶基因克隆及功能验证研究中发现,转天冬酰胺合成酶基因的拟南芥和烟草植株,其耐逆性能得到提高,表明该基因具有增强植物耐逆性的功能。因此,天冬酰胺作为植物体内运输新合成氮的重要载体,在植物体遭遇逆境胁迫时,其含量的积累有助于生物体抵御逆境。
在本实验筛选到的4 种差异代谢物中,黄嘌呤核苷、腺苷、氨基嘌呤均属于核苷酸及其衍生物,生产上对该类物质的应用也有较长的历史,研究表明,核苷酸类物质能够促进粮食作物对磷肥、钾肥的吸收,增加瓜果蔬菜的果实干质量、维生素C、总糖含量[26,33],改善果实外观和品质[34]等,增加菌类产品的氨基酸含量[35-36],提升菌类营养价值,增加产量和可溶性固形物含量。此外,核苷酸在农作物上也被发现可以作为一种植物生长调节剂,通过促进植物根系生长、提高根系吸收能力来增强植株抗逆性[37-38]。DL-同型半胱氨酸属于氨基酸及其衍生物,对于该类物质在农业生产中的应用也有研究,如高活性的酶解左旋游离氨基酸能够提高植物抗逆性、改善光合作用[39]等。本研究中鉴定出的DL-同型半胱氨酸与丝氨酸在胱硫键-β 合成酶作用下合成半胱氨酸,而半胱氨酸作为一种天然的抗褐变剂,能够维持细胞功能,增强抗氧化酶活性、减轻膜脂过氧化、保证细胞膜的完整性[40]。
综上,各类大量代谢物与差异代谢物,在植物体响应冷害过程中,参与不同的代谢途径,或作为渗透调节物质改变细胞液浓度,降低细胞冰点,以抵御低温伤害;或参与活性氧清除系统反应,清除代谢过程中产生的多余活性氧自由基,减少由于膜脂过氧化作用而对细胞造成的伤害。因此,了解各类代谢物的功能及其参与的生理生化反应,对提高植物应对逆境的能力具有重要作用。
4 结 论
笔者基于广泛靶向代谢组学技术鉴定了桃芽响应低温处理的相关代谢物,并选择部分代谢物进行了体外喷施验证。结果表明,低温诱导了低温响应基因PpCBF2 的表达,证实了低温处理的可靠性。与清水对照相比,外源喷施标准品溶液处理的桃苗叶片中脯氨酸含量显著上升;同时,脯氨酸合成关键基因PpP5CS 的表达与其表型变化具有相同趋势,进一步表明黄嘌呤核苷、腺苷、DL-同型半胱氨酸以及氨基嘌呤可增强植株抗寒性。
[1]华文.中国桃属植物分类[J].花卉,2015(3):19-21.HUA Wen. Classification of Chinese peach plants[J]. Flowers,2015(3):19-21.
[2]渠红岩,吴敏.古代的桃文献史料与当代的桃文化研究[J].韶关学院学报,2007(8):102-105.QU Hongyan,WU Min. Documents and historical materials relating to peach and contemporary peach research[J]. Journal of Shaoguan University,2007(8):102-105.
[3]WELLING A,PALVA E T. Molecular control of cold acclimation in trees[J].Physiologia Plantarum,2010,127(2):167-181.
[4]陈杰忠.果树栽培学各论(南方本第四版)[M].北京:中国农业出版社,2011.CHEN Jiezhong.Theories of fruit tree cultivation(Southern Edition,Fourth Edition)[M]. Beijing:China Agricultural Press,2011.
[5]王淑杰,王连君,王家民,杨晓光,马秀华.果树抗寒生理研究进展[J].北方园艺,1998(5):32-33.WANG Shujie,WANG Lianjun,WANG Jiamin,YANG Xiaoguang,MA Xiuhua. Advances in cold resistance physiology of fruit trees[J].Northern Horticulture,1998(5):32-33.
[6]柴茂峰. 拟南芥NAD(H)激酶在非生物胁迫中的功能分析[D].北京:中国农业大学,2005.CHAI Maofeng. Functions of NAD(H) kinases in Arabidopsis thaliana under abiotic stress[D].Beijing:China Agricultural University,2005.
[7]刘荣,刘清国,范建新,龚德勇,吴小波,韩树全,雷朝云.果树抗寒性生理生化及分子机理研究进展[J]. 分子植物育种,2017,15(5):2028-2034.LIU Rong,LIU Qingguo,FAN Jianxin,GONG Deyong,WU Xiaobo,HAN Shuquan,LEI Zhaoyun. Research advances in the cold-resistance physiology biochemistry and molecular mechanism in fruit crops[J]. Molecular Plant Breeding,2017,15(5):2028-2034.
[8]曹建东,陈佰鸿,王利军,毛娟,赵鑫.葡萄抗寒性生理指标筛选及其评价[J].西北植物学报,2010,30(11):2232-2239.CAO Jiandong,CHEN Baihong,WANG Lijun,MAO Juan,ZHAO Xin. Cold resistance indexes identification and comprehensive evaluation of grape varieties[J]. Acta Botanica Boreali-Occidentalia Sinica,2010,30(11):2232-2239.
[9]马媛.新疆间作果园微气候特征及果树抗寒性研究[D].乌鲁木齐:新疆农业大学,2012.MA Yuan. Microclimate characteristic and cold resistance of fruit tree in Xinjiang intercropping orchard[D]. Urumqi:Xinjiang Agricultural University,2012.
[10]SAGISAKA S,ARAKI T.Amino acid pools in perennial plants at the wintering stage and at the beginning of growth[J]. Plant and Cell Physiology,1983,28(3):479-494.
[11]陈钰,郭爱华,姚延梼.自然降温条件下杏品种蛋白质、脯氨酸含量与抗寒性的关系[J].山西农业科学,2007(6):53-55.CHEN Yu,GUO Aihua,YAO Yantao. The relationship of soluble protein and dissociated proline of almonds and their tolerance to coldness under drop in temperature[J].Journal of Shanxi Agricultural Sciences,2007(6):53-55.
[12]NICHOLSON J K,LINDON J C,HOLMES E.‘Metabonomics’:Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data[J]. Xenobiotica,1999,29(11):1181-1189.
[13]FIEHN O,KOPKA J,DÖRMANN P,ALTMANN T,TRETHEWEY R N,WILLMITZER L. Metabolite profiling for plant functional genomics[J]. Nature Biotechnology,2000,18(11):1157-1161.
[14]张凤,陈伟.代谢组学在植物逆境生物学中的研究进展[J].生物技术通报,2021,37(8):1-11.ZHANG Feng,CHEN Wei. Research progress of metabolomics in plant stress biology[J]. Biotechnology Bulletin,2021,37(8):1-11.
[15]DING F,LIU B,ZHANG S X. Exogenous melatonin ameliorates cold-induced damage in tomato plants[J].Scientia Horticulture,2017,219:264-271.
[16]方彦,曾秀存,马骊,孙柏林,武军艳,董云,刘丽君,金姣姣,孙万仓,李学才.低温胁迫下冬油菜陇油7 号根部代谢组学分析[J].干旱地区农业研究,2021,39(4):80-85.FANG Yan,ZENG Xiucun,MA Li,SUN Bailin,WU Junyan,DONG Yun,LIU Lijun,JIN Jiaojiao,SUN Wancang,LI Xuecai.Metabolomics profiling of Brassica campestris Longyou 7 roots under cold stress[J]. Agricultural Research in the Arid Areas,2021,39(4):80-85.
[17]朱建峰,张会龙,杨秀艳,武海雯,张华新.盐胁迫下白榆种子萌发期代谢组学分析[J/OL]. 分子植物育种:1-15[2022-08-18]. http://kns. cnki. net/kcms/detail/46. 1068. S. 20210609.1326.016.html.ZHU Jianfeng,ZHANG Huilong,YANG Xiuyan,WU Haiwen,ZHANG Huaxin.Metabonomic analysis of seed germination period of Ulmus pumila under salt stress[J/OL]. Molecular Plant Breeding:1-15. [2022-08-18]. http://kns. cnki. net/kcms/detail/46.1068.S.20210609.1326.016.html.
[18]牟丹,杨帆,卡着才让,祁学忠,顾智辉,谢久祥,李希来.高加索三叶草响应不同降温模式低温胁迫的代谢组学分析[J].草地学报,2021,29(9):1877-1884.MOU Dan,YANG Fan,Kazhuo Cairang,QI Xuezhong,GU Zhihui,XIE Jiuxiang,LI Xilai. Metabonomic analysis of caucasian clover in response to low-temperature stress of different cooling modes[J].Acta Agrestia Sinica,2021,29(9):1877-1884.
[19]宋艳波,赵小慧,王科杰,李耀霞,陈双建,梅霞,李宏,吴国良.桃PpCBF2 基因克隆及其响应低温表达特性研究[J].中国农业大学学报,2020,25(9):36-45.SONG Yanbo,ZHAO Xiaohui,WANG Kejie,LI Yaoxia,CHEN Shuangjian,MEI Xia,LI Hong,WU Guoliang. Cloning of PpCBF2 gene in peach and its response to low temperature expression[J]. Journal of China Agricultural University,2020,25(9):36-45.
[20]申晴,殷亚蕊,武军凯,王海静,张立彬.桃MYB3 基因的克隆及表达分析[J].河北果树,2020(2):8-10.SHEN Qing,YIN Yarui,WU Junkai,WANG Haijing,ZHANG Libin. Cloning and expression analysis of peach MYB3 gene[J].Hebei Fruits,2020(2):8-10.
[21]左波,安明燕,郝艳,武军凯,王海静,肖啸,刘建珍,张立彬.桃PpRBD2 基因的克隆与表达分析[J/OL].分子植物育种:1-16. [2022-08-18]http://kns. cnki. net/kcms/detail/46. 1068. S.20210326.1601.017.html.ZUO Bo,AN Mingyan,HAO Yan,WU Junkai,WANG Haijing,XIAO Xiao,LIU Jianzhen,ZHANG Libin. Cloning and expression analysis of PpRBD2 gene in peach[J/OL]. Molecular Plant Breeding:1-16. [2022-08-18]http://kns. cnki. net/kcms/detail/46.1068.S.20210326.1601.017.html.
[22]全先庆,张渝洁,单雷,毕玉平.高等植物脯氨酸代谢研究进展[J].生物技术通报,2007(1):14-18.QUAN Xianqing,ZHANG Yujie,SHAN Lei,BI Yuping. Advances in proline metabolism researches of higher plants[J].Biotechnology Bulletin,2007(1):14-18.
[23]郭学民,刘建珍,肖啸,张立彬,高荣孚.桃、杏和樱桃树抗寒性研究进展[J].河北科技师范学院学报,2016,30(1):1-9.GUO Xuemin,LIU Jianzhen,XIAO Xiao,ZHANG Libin,GAO Rongfu. Advancement in cold hardiness researches on peach,apricot and cherry[J]. Journal of Hebei Normal University of Science&Technology,2016,30(1):1-9.
[24]吴振强,李珊,罗宁.农用核苷酸的开发进展[J].中国土壤肥料,2004(4):3-6.WU Zhenqiang,LI Shan,LUO Ning. The review for nucleotide developmental progress in agriculture[J]. Soil and Fertilizer Sciences in China,2004(4):3-6.
[25]SHUMAILA K.外源L-甲硫氨酸对水培生菜生长、生理和抗氧化系统的影响[D].北京:中国农业科学院,2019.SHUMAILA K. Effect of exogenous L-Methionine on growth,physiology,and antioxidants system of lettuce in hydroponics[D].Beijing:Chinese Academy of Agricultural Sciences,2019.
[26]宁正祥,李玲,胡立侃.核苷酸在柑桔生产上的应用及其作用机理研究[J].中国南方果树,1986(1):2-6.NING Zhengxiang,LI Ling,HU Likan. Study on the application of nucleotides in citrus production and their mechanism of action[J]. South China Fruits,1986(1):2-6.
[27]ZAID A,MOHAMMAD F,WANI S H,SIDDIQUE K M H.Salicylic acid enhances nickel stress tolerance by up-regulating antioxidant defense and glyoxalase systems in mustard plants[J].Ecotoxicology and Environmental Safety,2019,180:575-587.
[28]孙歆,曾富春,胡攀.水分胁迫下水杨酸对大麦幼苗抗氧化能力的影响[J].四川农业大学学报,2011,29(2):160-163.SUN Xin,ZENG Fuchun,HU Pan. Effects of salicylic acid on the antioxidant activity of barley seedlings under water stress[J].Journal of Sichuan Agricultural University,2011,29(2):160-163.
[29]谷晓勇,刘扬,刘利静.植物激素水杨酸生物合成和信号转导研究进展[J].遗传,2020,42(9):858-869.GU Xiaoyong,LIU Yang,LIU Lijing.Progress on the biosynthesis and signal transduction of phytohormone salicylic acid[J].Hereditas,2020,42(9):858-869.
[30]WILDERMUTH M C,DEWDNEY J,WU G,AUSUBEL F M.Isochorismate synthase is required to synthesize salicylic acid for plant defence[J].Nature,2001,414(6863):562-565.
[31]薛爽.杉木天冬酰胺合成酶基因的非生物胁迫响应及遗传转化分析[D].福州:福建农林大学,2017.XUE Shuang. Responses of asparagus synthase gene of Chinese Fir to abiotic stress and genetic transformation analysis[D]. Fuzhou:Fujian Agriculture and Forestry University,2017.
[32]张晓磊.小黑麦天冬酰胺合成酶基因克隆及功能验证[D].石河子:石河子大学,2013.ZHANG Xiaolei. Cloning and functional analysis of asparagine synthetase gene from triticale[D]. Shihezi:Shihezi University,2013.
[33]陈日远,关佩聪,刘厚诚,余日焕.核苷酸及其组合物对冬瓜产量形成及其生理效应的研究[J].华南农业大学学报,2000,21(3):9-12.CHEN Riyuan,GUAN Peicong,LIU Houcheng,YU Rihuan.Studies on the yield formation and physiological effects of nucleotide and its combinations in wax wourd(Benincasa hispida Cogn.)[J].Journal of South China Agricultural University,2000,21(3):9-12.
[34]姜小文,张秋明,王灿辉,袁世珍.喷施核苷酸有机营养剂改进椪柑果实品质的效果[J].中国南方果树,2001,30(3):12-13.JIANG Xiaowen,ZHANG Qiuming,WANG Canhui,YUAN Shizhen. Effect of nucleotide organic nutrient spraying on improving the fruit quality of Ponkan[J].South China Fruits,2001,30(3):12-13.
[35]邵吉安,孙云祥,石兆魁,沈建国.核苷酸复配剂在农业上的应用[J].土壤,1992,24(4):3.SHAO Ji’an,SUN Yunxiang,SHI Zhaokui,SHEN Jianguo.Application of nucleotide compounding agents in agriculture[J].Soils,1992,24(4):3.
[36]张爵玉,郑泳苗,彭生.核苷酸制剂对猴头菌产量和品质的影响[J].仲恺农业技术学院学报,1995(2):29-32.ZHANG Jueyu,ZHENG Yongmiao,PENG Sheng.The effect of hericium erinaceus yield and quality applying nucieotide preparation[J]. Journal of Zhongkai University of Agriculture and Engineering,1995(2):29-32.
[37]王熹,施一平,金子渔,孙玉昆,钱元任.核酸降解物在水稻生产上的应用及其作用机理的研究:I.核酸降解产物的增产效果及其有效组分的初步研究[J].植物化学与生物物理学报,1975,7(1):31-40.WANG Xi,SHI Yiping,JIN Ziyu,SUN Yukun,QIAN Yuanren.Studies on the application of RNA hydrolysate to rice production and its mechanism of action:Ⅰ.the effect of RNAhydrolysate and its effective components on the yield of rice grain[J]. Acta Biochimica et Biophysica Sinica,1975,7(1):31-40.
[38]刘兴宇.植物生长调节剂核苷酸及其应用[J].农药市场信息,2007(21):1.LIU Xingyu.Plant growth regulators nucleotides and their applications[J].Pesticide Market News,2007(21):1.
[39]申明,成学慧,谢荔,李翠,孙新娥,张治平,汪良驹.氨基酸叶面肥对砂梨叶片光合作用的促进效应[J].南京农业大学学报,2012,35(2):81-86.SHEN Ming,CHENG Xuehui,XIE Li,LI Cui,SUN Xin’e,ZHANG Zhiping,WANG Liangju.Effects of amino-acid fertilizers on photosynthesis in leaves of pear cultivars[J]. Journal of Nanjing Agricultural University,2012,35(2):81-86.
[40]刘海,陈爽爽.半胱氨酸和异抗坏血酸处理对荔枝果实保鲜效果和氧代谢的影响[J].现代农业科技,2011(15):365-367.LIU Hai,CHEN Shuangshuang. Effect of cysteine and isoascorbic acid treatment on the preservation effect and oxygen metabolism of litchi fruit[J].Modern Agricultural Sciences and Technology,2011(15):365-367.