葡萄白粉病(grape powdery mildew)是一种世界性的真菌病害,在全球各葡萄种植区均有发生[1],病原菌为Erysiphe necator Schwein。葡萄白粉病可以危害葡萄的多个部位,其中最易被感染的是幼嫩器官组织[2],发病严重时,病斑可扩展至整张叶片使之变得卷曲随后造成干枯和脱落,阻碍果实生长,加重其他果实病害的发生,带来严重的产量损失。除了直接的病害损失,葡萄白粉病会影响叶片的光合作用,减少葡萄汁中糖和花青素含量,导致可溶性固形物含量降低,严重影响葡萄酒品质[3-5]。
目前栽培的绝大多数葡萄品种对葡萄白粉病菌没有遗传抗性,田间对葡萄白粉病表现为中到高度感病[6-8]。为有效地防控葡萄白粉病,化学杀菌剂在葡萄园中被大量使用,而吡唑醚菌酯作为一种广谱、高效的甲氧基丙烯酸酯类(QOI)杀菌剂,能够与病原菌的特定部位(细胞色素b 的泛醌氧化中心,即Qo部位)相结合以阻碍电子向细胞色素c1 转移,破坏线粒体正常供应ATP,进而抑制真菌孢子萌发和菌丝生长[9],在我国葡萄园病害中被频繁使用[10]。单一杀菌剂的大量频繁使用易导致抗药性的发生,目前已有多种植物病原真菌对吡唑醚菌酯敏感性下降或已产生抗药性。研究显示,海南杧果蒂腐病菌(Lasiodiplodia theobromae)[11]、桃炭疽病菌(Colletotrichum fructicola)[12]、草莓等果实灰霉病菌(Botrytis cinerea)均对吡唑醚菌酯存在抗药性[13]。美国学者相继检测到了对QoI类杀菌剂产生抗药性的葡萄白粉病菌菌株[14-17],Wong 等[18]测定了256 株葡萄白粉病菌对嘧菌酯的敏感性,田间嘧菌酯防治效果下降40%,证实葡萄白粉病菌对嘧菌酯的敏感性降低。另外,研究显示,与病原菌对QoI类杀菌剂产生抗药性相关的突变有3种(F129L,G137R,G143A),对于葡萄白粉病菌而言G143A 的突变被普遍报道[16-17],Miles 等[16]用荧光定量PCR 法检测了密歇根州的葡萄白粉病菌对吡唑醚菌酯的抗性频率,对于EC50值>1 μg·mL-1的抗性菌株,该方法可以检测到所有菌株均存在G143A的突变。
通过前期调研发现我国葡萄园经常使用含有吡唑醚菌酯的农药来防治葡萄白粉病,而国内葡萄白粉病菌对吡唑醚菌酯的敏感性尚未见研究报道。笔者在本研究中以5个葡萄种植区的葡萄白粉病样为研究对象,经单斑、纯化,采用孢子萌发法测定了供试菌株对吡唑醚菌酯的敏感性,同时采用荧光定量PCR 法检测了cytb 基因上G143A 的突变情况,并对两种检测方法的相关性进行比较,旨在进一步明确我国葡萄白粉病菌对QoI 类杀菌剂的抗性水平,为葡萄白粉病的科学防治提供理论依据。
1 材料和方法
1.1 材料
1.1.1 供试菌株 供试菌株为2020年间从北京、湖南、宁夏、云南、江苏5个葡萄种植地区采集、分离纯化得到的145株葡萄白粉病菌菌株。其中在当年未施用过化学杀菌剂的葡萄园共采集了51 个葡萄白粉病单斑病样,用于建立葡萄白粉病菌对吡唑醚菌酯的敏感基线。
1.1.2 供试药剂及试剂盒 吡唑醚菌酯(原药98%,巴斯夫欧洲公司)、水杨肟酸(SHAM)(原药99%,上海麦克林生化科技有限公司),吡唑醚菌酯用丙酮配制质量浓度为10 mg·mL-1的母液,水杨肟酸用甲醇配制质量浓度为10 mg·mL-1的母液。
真菌基因组DNA 提取试剂盒(D1090-02,OMEGA公司)。
1.1.3 供试接种材料 选取温室盆栽夏黑葡萄的自上而下第2~4枚嫩叶,整张新鲜嫩叶在l%次氯酸钠溶液里消毒30 s后,用无菌水冲洗3次,用无菌滤纸片吸干叶片表面残留的水分,斜切叶柄插入1.5%水琼脂平板中备用,并在当天完成接种。
1.2 方法
1.2.1 供试菌株的分离与纯化 对田间采集到的葡萄白粉病叶在室内进行保湿培养,将叶片正面朝上放置于铺有湿滤纸的保鲜盒中,每张叶片需单独处理,置于人工气候箱中[温度(25±1)℃、湿度(60±10)%,12 h·d-1的光周期]培养1~2 d。待叶片长出新鲜的霉层,在体式显微镜下观察分生孢子状态。选择分生孢子新鲜、成串直立的单个病斑,剪下备用。
葡萄白粉病菌单斑纯化扩繁参考贾静怡等[19]的方法稍有改进,使用整张叶片进行接种扩繁。将剪下的单个病斑用小毛刷将霉层刷至300~600 μL 无菌水中,制成浓度105个孢子·mL-1的孢子悬浮液。取出先前已插入水琼脂的、经表面消毒的叶片,每个叶片滴2~4 滴孢子悬浮液,每滴20 μL,接种后3~5 min内吸干叶片表面残余液滴。使用封口膜封口,置于人工气候箱中培养[温度(25±1)℃、湿度(60±10)%,12 h·d-1的光周期]。待接种10~14 d叶片长出新鲜的霉层时备用。
1.2.2 含药培养基制备 采用系列稀释法,在预试试验的基础上,用无菌水稀释成适宜的有效成分浓度梯度,选用水琼脂做培养基,取600 μL 的药剂加入60 mL 的水琼脂中混匀倒板,制备成0.1、0.5、1、5、10、25、50、100 μg·mL-1系列质量浓度梯度的含药平板。所用供试培养基均加入终质量浓度为100 μg·mL-1的水杨肟酸(SHAM),以降低旁路呼吸的影响。以加入SHAM和无菌水为对照。
1.2.3 葡萄白粉病菌对吡唑醚菌酯的敏感性测定(1)孢子萌发法:将经纯化扩繁后的新鲜霉层上的分生孢子均匀抖落在含药水琼脂平板和对照培养基平板上。每个浓度梯度重复3 皿,置于25 ℃、相对湿度为60%±10%、12 h·d-1的光周期的人工气候箱中培养24 h(对照孢子萌发率在90%以上时),调查各处理的分生孢子萌发情况,当芽管长度超过孢子短半径的一半时视为萌发。每菌株3次重复试验。
(2)荧光定量PCR 法:采用真菌基因组DNA 提取试剂盒。使用核酸浓度测定仪测定DNA浓度,剔除不合格样品。采用Baudoin 等[15]开发的突变阻滞扩增系统(ARMS)引物组检测葡萄白粉病菌cytb基因上G143A 的突变。将葡萄白粉病菌DNA 稀释至5~10 ng·μL-1,荧光定量PCR反应体系为20 μL,具体操作程序如下:利用野生型等位基因引物(5'-TACGGGCAGATGAGCCTATGCGG-3')、突变型等位基因引物(5'- TACGGGCA GATGAGC CTATGCGC-3')与通用反向引物(5'-ACCTACTTAAAGCTTTAGAAG TTTCC-3')进行点突变检测。20 μL反应体系:TB GREEN 10 μL、RoX Reference Dye II 0.4 μL、10 μmol·L-1正反向引物各0.4 μL、RNase Free ddH2O 6.8 μL、DNA 模板2 μL。荧光定量PCR 反应选择可获得高特异性的两步法,反应条件:60 ℃反应前处理1 min;95 ℃预变性1 min;95 ℃变性45 s,60 ℃退火45 s,共40 个循环;60 ℃反应后处理1 min。抗药性检测反应结束后,检测每1株病菌DNA的扩增曲线和解离曲线,记录循环阈值(Ct值)。
1.2.4 数据处理 根据公式计算各处理的分生孢子相对萌发抑制率,采用Microsoft Excle2016软件,以药剂浓度对数数值为横坐标,相对分生孢子萌发抑制率的概率值为纵坐标,进行回归分析,计算各菌株的毒力回归方程、相关系数,根据回归方程计算EC50值。
R(%)=Ng/Nt×100。
R为孢子萌发率;Ng为孢子萌发数;Nt为调查的孢子总数。
I(%)=(R0-Rt)/R0×100。
I为孢子萌发相对抑制率;R0为空白对照孢子萌发率;Rt 为处理孢子萌发率。
对来自未施用过化学杀菌剂的葡萄园的51 个葡萄白粉病菌菌株的EC50值,采用SPSS软件进行正态性、方差齐性检验,将符合连续性正态分布的菌株的EC50值均值作为我国葡萄白粉病菌对吡唑醚菌酯的敏感基线。
抗性水平以葡萄白粉病菌对吡唑醚菌酯的敏感基线为参照,根据抗药类型分级标准划分抗性水平(RF)[16,20]。RF=供试菌株EC50值/敏感基线EC50值,RF≤2 时,为敏感菌株;2<RF≤10 时,为低抗菌株;10<RF≤100,为中抗菌株;当RF>100倍时,为高抗菌株。
采用Microsoft Excle2016软件记录和整理荧光定量PCR 数据。根据每一株葡萄白粉病菌DNA经过扩增得到的突变型引物和野生型引物的Ct值,使用下列公式计算每个分离株的总线粒体DNA 中G143A的百分比:100×{1/[1+2(突变型Ct-野生型Ct)]}[16]。若菌株中G143A等位基因百分比高于95%,视为阳性结果,即发生突变。
2 结果与分析
2.1 病原菌分离、纯化
2020 年从北京、湖南、宁夏、云南、江苏等葡萄种植地区采集葡萄白粉病病叶,经分离纯化获得145株菌株。
2.2 葡萄白粉病菌对吡唑醚菌酯的敏感基线
获得当年未施用过化学杀菌剂的51 个葡萄白粉病菌菌株的EC50 值,EC50 值范围分布在0.034~13.219 μg · mL- 1 之间,平均EC50 值为(1.661±0.296)μg·mL-1。使用SPSS软件Shapiro-Wilk方法进行正态性检验,发现51个葡萄白粉病菌菌株对吡唑醚菌酯的敏感性频率不符合正态分布(W=0.624,p<0.05),存在对吡唑醚菌酯敏感性较低的亚群体。经对比,其中43个菌株的敏感性频率分布呈现连续性单峰曲线且为正态分布(W=0.957,p=0.109>0.05),EC50值为0.034~3.328 μg·mL-1,平均EC50值为(1.387±0.107)μg·mL-1,取该值作为葡萄白粉病菌对吡唑醚菌酯的敏感基线。以EC50值为横坐标、菌株分布频数为纵坐标绘制频数分布直方图(图1)。
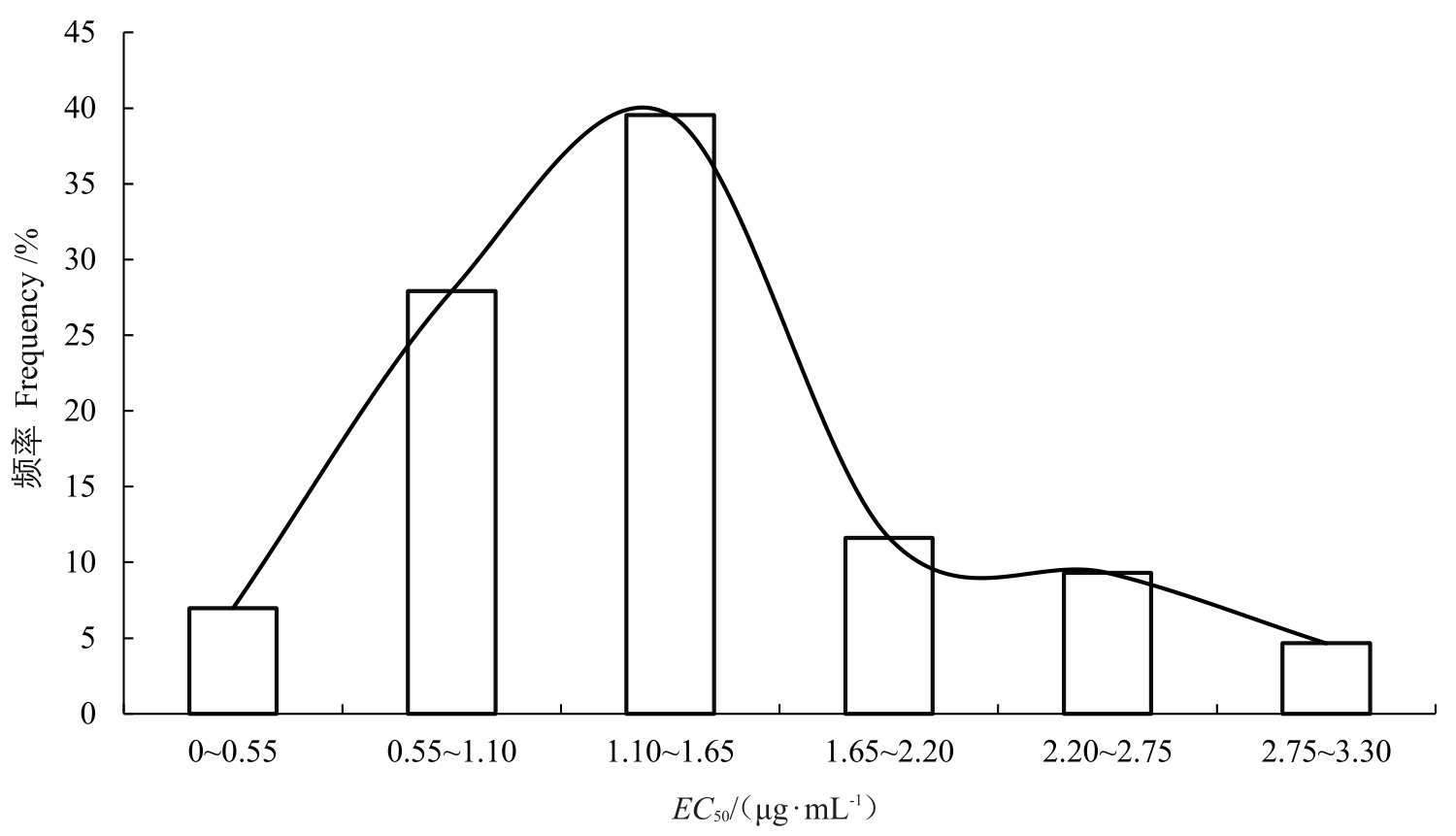
图1 43 株菌株EC50值连续性正态分布
Fig.1 The continuous normal distribution of EC50 values of 43 isolates
2.3 采用孢子萌发法测定葡萄白粉病菌对吡唑醚菌酯的抗性水平
采用孢子萌发法,依据建立的葡萄白粉病菌对吡唑醚菌酯的敏感基线,按照抗药类型分级标准,对我国葡萄白粉病菌抗药水平进行划分,结果如图2,测定的145株葡萄白粉病菌中,低抗菌株60株,中抗菌株6株,抗性频率分别为41.38%和4.14%,总的抗性菌株66株,总的抗性频率为45.52%;敏感菌株79株,占比54.48%。

图2 葡萄白粉病菌对吡唑醚菌酯不同抗性水平的菌株比例
Fig.2 Proportion of isolates with different resistance levels of E.necator to pyraclostrobin
2.4 采用荧光定量PCR 法检测葡萄白粉病菌对吡唑醚菌酯的抗性水平
采用荧光定量PCR 分子检测技术对葡萄白粉病菌cytb 基因上G143A 的突变进行检测。研究结果显示,测定的130株葡萄白粉病菌菌株(15株质检不合格去除)中,抗性菌株为75 株,抗性频率为57.69%;敏感菌株55株,占比42.31%。
2.5 生物学检测与Q-PCR(G143A)检测之间的关系
将孢子萌发法的检测结果和Q-PCR G143A 检测结果进行关联分析,随着RF 值范围的增加,具有G134A 突变的菌株比例逐渐增加。根据葡萄白粉病菌对吡唑醚菌酯的敏感基线及抗药性倍数,划分了RF值1倍、2倍、10倍、大于10倍4个范围(图3)。在RF 值<1 倍(0~1.387 μg·mL-1)的49 株病菌中,DNA发生G143A有9株,占18.37%;在RF值1~2倍(1.387~2.774 μg·mL-1)的19 株病菌中,DNA 发生G143A 有14 株占73.68%;在RF 值2~10 倍(2.774~13.87 μg·mL-1)的47 株病菌中,DNA发生G143A 有39株,占82.98%;在RF值10倍以上(13.87 μg·mL-1)的15 株病菌中,DNA 发生G143A 有13 株,占86.67%。同时检测了两种方法结果的相关性,将抗性菌株赋值为1,敏感菌株赋值为0,使用SPSS 中Pearson 相关性分析检测相关性,结果表明Pearson相关系数为0.503**,在0.01 水平(双侧)上显著相关。

图3 不同RF 值范围内G143A 突变频率
Fig.3 G143A mutation frequency in different RF value ranges
3 讨 论
葡萄白粉病(Erysiphe necator)是葡萄生产中最重要的真菌病害之一,可危害葡萄叶片、茎、果实等,引起叶片褪绿变黄,果实生长受损。随着保护地栽培、避雨栽培面积的扩大,白粉病的发生有加重趋势,部分葡萄品种抗病性较弱,白粉病发生严重,严重影响葡萄的产量和品质[21]。对于葡萄白粉病的防治,化学杀菌剂的使用是主要的技术手段。
吡唑醚菌酯作为甲氧基丙烯酸酯类杀菌剂的一种,具有广谱、高效、低毒、低残留的特点[22],在葡萄种植区常被作为推荐药剂用于白粉病、霜霉病等的田间防治。但由于其作用位点单一、长期大量使用易导致病原菌产生抗药性,增加防治难度。建立敏感基线是进行抗药性监测、制定抗性治理策略的重要内容。目前国内许多病原菌已经建立了对吡唑醚菌酯的敏感基线,如水稻纹枯病菌[23]、水稻稻瘟病菌[24]、梨树褐斑病菌[25]、苹果腐烂病菌[26]、辣椒炭疽病菌[27]、杧果蒂腐病菌[11]、葡萄白腐病菌[28]、火龙果溃疡病菌等[29]。病原菌的敏感性数值变化的范围越宽,在药剂选择压力存在的情况下产生抗药性的概率越大,这些信息对预测田间抗药性的发生和制定科学的病害管理方案具有重要意义。
吡唑醚菌酯可破坏线粒体正常供应ATP,导致细胞死亡,但旁路呼吸途径中交替氧化酶(AOX)的存在会降低QoI 类杀菌剂的抑制活性,而此过程对水杨肟酸(SHAM)敏感,试验含药平板中添加了100 μg·mL-1的SHAM 以减少影响。现阶段已有大量数据表明葡萄白粉病菌对QoI 类杀菌剂抗药性的产生是由于病菌cytb基因第143位碱基由G突变为A(G143A),导致相应蛋白质由甘氨酸(Gly)被丙氨酸(Ala)替代,根据此原理,笔者使用孢子萌发法及突变阻滞扩增系统2 种方法检测葡萄白粉病菌抗药性。
采用孢子萌发法测定了吡唑醚菌酯对未施用过化学杀菌剂的51 株葡萄白粉病菌菌株的EC50值并进行检测分析,其中43个菌株的敏感性频率分布呈现连续性单峰曲线且为正态分布,平均值为(1.387±0.107)μg·mL-1,将此作为葡萄白粉病菌对吡唑醚菌酯的敏感基线。其余8株葡萄白粉病菌由于不符合正态分布规律且EC50值较大,不作为敏感基线数据参考,其EC50值分布如下:3.657、4.009、4.615、4.743、5.324、6.495、11.966、13.219 μg·mL-1,暗示未使用过吡唑醚菌酯的葡萄白粉病菌群体中可能已经存在抗药性的遗传分化。
Q-PCR(G143A)检测结果与孢子萌发法结果相互验证,但又不完全相同。采用孢子萌发法测定的结果显示,145株病菌总的抗性频率为45.52%,QPCR(G143A)检测结果显示除去15株不合格样品,剩余的130株病菌总抗药性频率为57.69%。结合抗药性倍数(RF)结果,在RF<1 倍、RF>2 倍时,QPCR检测结果与孢子萌发检测结果差异较小,不超过20%,在1 倍<RF<2 倍时差异较大,RF<2 倍抗药性分级标准为“敏感”,在Q-PCR 检测结果中,此范围内超70%菌株存在G143A 突变,与Miles 等[16]的部分检测结果一致。敏感表型的菌株在Q-PCR检测中被判定为抗性菌株,以及在RF>2倍时,20%以上的菌株未检测到G143A 突变,这些现象说明G143A位点突变不是影响抗性的唯一机制,还有其他机制影响葡萄白粉病菌对此药剂的敏感性,不排除可能有其他的位点突变导致了抗药性产生,Gisi等[30]认为cytb 基因F129L、G137R 突变也会导致病原菌对QoI 类杀菌剂产生低到中等程度的抗药性,但这种情况不如G143A突变普遍,供试葡萄白粉病菌是否存在另外的突变位点还需后续进一步确认。2 种检测方法结果虽存在差异,但结果显著相关。因此上述2 种方法均可用于葡萄白粉病菌对吡唑醚菌酯抗药性的检测。
4 结 论
目前我国葡萄白粉病菌对吡唑醚菌酯的抗药频率已较高,两种检测方法菌株总抗性频率已在50%左右,抗药风险水平较高。为有效防控葡萄白粉病菌,延缓杀菌剂的抗药性,今后应加强葡萄白粉病菌对吡唑醚菌酯的抗性水平监测,通过混配、轮换用药等多种方式优化杀菌剂的使用,并与农艺措施、生物防治相协调,制定综合的葡萄白粉病抗药治理策略。
[1]FILIPENKO I,SHTIN L.Inheritance of resistance to downy and powdery mildew in hybrids European vines with V. amuresis[J].Plant Breed Abstract,1975,45(10):677.
[2]GROVE G G. Perennation of Uncinula necator in vineyards of eastern Washington[J].Plant Disease,2004,88(3):242-247.
[3]CALONNEC A,CARTOLARO P,POUPOT C,DUBOURDIEU D,DARRIET P. Effects of Uncinula necator on the yield and quality of grapes (Vitis vinifera) and wine[J]. Plant Pathology,2004,53(4):434-445.
[4]GADOURY D M,SEEM R C,FICKE A,WILCOX W F.The epidemiology of powdery mildew on concord grapes[J]. Phytopathology,2001,91(10):948-955.
[5]STUMMER B E,FRANCIS I L,MARKIDES A J,SCOTT E S.The effect of powdery mildew infection of grape berries on juice and wine composition and on sensory properties of Chardonnay wines[J].Australian Journal of Grape and Wine Research,2003,9(1):28-39.
[6]GAFORIO L,GARCÍA-MUÑOZ S,CABELLO F,MUÑOZORGANERO G. Evaluation of susceptibility to powdery mildew(Erysiphe necator)in Vitis vinifera varieties[J].Vitis,2011,50(3):123-126.
[7]STAUDT G. Evaluation of resistance to grapevine powdery mildew (Uncinula necator [Schw.]Burr.,anamorph Oidium tuckeri Berk.) in accessions of Vitis species[J]. Vitis,1997,36(3):151-154.
[8]DRY I B,FEECHAN A,ANDERSON C,JERMAKOW A M,BOUQUET A,ADAM-BLONDON A F,THOMAS M R. Molecular strategies to enhance the genetic resistance of grapevines to powdery mildew[J].Australian Journal of Grape and Wine Research,2010,16(S1):94-105.
[9]BARTLETT D W,CLOUGH J M,GODWIN J R,HALL A A,PARR- DOBRZANSKI B. The strobilurin fungicides[J]. Pest Management Science,2002,58(7):649-662.
[10]高琪,李兴红,刘梅.我国葡萄园病害发生危害及防治用药情况调查[J].中国果树,2021(9):97-102.GAO Qi,LI Xinghong,LIU Mei.Investigation on the occurrence of diseases and the use of fungicides in vineyards in China[J].China Fruits,2021(9):97-102.
[11]贺瑞,赵磊,符瑞,陈芷岑,林晓翠,杨叶.海南杧果蒂腐病菌对吡唑醚菌酯的抗药性测定[J].植物保护,2018,44(4):188-193.HE Rui,ZHAO Lei,FU Rui,CHEN Zhiqin,LIN Xiaocui,YANG Ye. Resistance of Botryodiplodia theobromae caused mango stem end rot to pyraclostrobin in Hainan[J].Plant Protection,2018,44(4):188-193.
[12]USMAN H M,TAN Q,KARIM M M,ADNAN M,YIN W X,ZHU F X,LUO C X. Sensitivity of C. fructicola and C. siamense of peach in China to multiple classes of fungicides and characterization of pyraclostrobin-resistant isolates[J].Plant Disease,2021,105(11):3459-3465.
[13]COSSEBOOM S D,HU M G. Identification and characterization of fungicide resistance in Botrytis populations from small fruit fields in the Mid-Atlantic United States [J]. Plant Disease,2021,105(9):2366-2373.
[14]WILCOX W,BURR J,RIEGEL D,WONG F. Practical resistance to QoI fungicides in New York populations of Uncinula necator associated with quantitative shifts in pathogen sensitivities[J].Phytopathology,2003,93:90.
[15]BAUDOIN A,OLAYA G,DELMOTTE F,COLCOL J F,SIEROTZKI H. QoI resistance of Plasmopara viticola and Erysiphe necator in the mid-Atlantic United States[J]. Plant Health Progress,2008,9(1):25-33.
[16]MILES L A,MILES T D,KIRK W W,SCHILDER A M C.Strobilurin (QoI) resistancein populations of Erysiphe necator on grapes in Michigan[J].Plant Disease,2012,96(11):1621-1628.
[17]RALLOS L E E,JOHNSON N G,SCHMALE D G,PRUSSIN A J,BAUDOIN A B. Fitness of Erysiphe necator with G143A-based resistance to quinone outside inhibitors[J]. Plant Disease,2014,98(11):1494-1502.
[18]WONG F P,WILCOX W F. Sensitivity to azoxystrobin among isolates of Uncinula necator:Baseline distribution and relationship to myclobutanil sensitivity[J]. Plant Disease,2002,86(4):394-404.
[19]贾静怡,张玮,燕继晔.葡萄白粉病抗性鉴定方法优化及品种抗性评价[J].植物保护,2021,47(1):160-164.JIA Jingyi,ZHANG Wei,YAN Jiye. Optimization of grapevine powdery mildew resistance identification methods and evaluation of variety resistance[J]. Plant Protection,2020,47(1):160-164.
[20]COLCOL J F,RALLOS L E,BAUDOIN A B. Sensitivity of Erysiphe necator to demethylation inhibitor fungicides in Virginia[J].Plant Disease,2012,96(1):111-116.
[21]CALONNEC A,CARTOLARO P,POUPOT C,DUBOURDIEU D,DARRIET P. Effects of Uncinula necator on the yield and quality of grapes(Vitis vinifera)and wine[J].Plant Pathology,2004,53(4):434-445.
[22]秦小芳,张泽优,吴小刚,林纬,黎起秦,袁高庆.21 种杀菌剂对6 种蔬菜病原菌的离体抑制效果[J]. 中国瓜菜,2022,35(1):91-95.QIN Xiaofang,ZHANG Zeyou,WU Xiaogang,LIN Wei,LI Qiqin,YUAN Gaoqing. Inhibitory effects of 21 fungicides on 6 vegetable pathogens in vitro[J].China Cucurbits and Vegetables,2022,35(1):91-95.
[23]刘世江,丁怡,赵琪君,李明,文小东,宋星陈,李荣玉.贵州省水稻纹枯病菌对丙环唑和吡唑醚菌酯的敏感性[J].福建农业学报,2019,34(11):1294-1301.LIU Shijiang,DING Yi,ZHAO Qijun,LI Ming,WEN Xiaodong,SONG Xingchen,LI Rongyu. Sensitivities of Rhizoctonia so-lani to Propiconazole and Pyraclostrobin on rice plants in Guizhou province[J].Fujian Journal of Agricultural Sciences,2019,34(11):1294-1301.
[24]梁梦琦.长江中下游稻区稻瘟病菌对稻瘟灵和吡唑醚菌酯的抗性监测[D].北京:中国农业科学院,2018.LIANG Mengqi. Monitoring sensitivity of Magnaporthe oryzae to isoprothiolane and pyraclostrobin in middle and lower reaches of Yangtze River[D].Beijing:Chinese Academy of Agricultural Sciences,2018.
[25]毕秋艳,赵建江,张诗琪,韩秀英,吴杰,路粉,王文桥.河北省梨树褐斑病菌对不同机制杀菌剂的敏感性及其与戊唑醇敏感性的关系[J].植物病理学报,2021,51(2):248-257.BI Qiuyan,ZHAO Jianjiang,ZHANG Shiqi,HAN Xiuying,WU Jie,LU Fen,WANG Wenqiao.Sensitivity of Septoria piricola Desm from pear in Hebei Province to different classes of fungicides and correlation coefficient with tebuconazole[J].Acta Phytopathologica Sinica,2021,51(2):248-257.
[26]王帅.苹果树腐烂病菌对吡唑醚菌酯的抗药性风险评估及生物源杀菌剂的室内活性评价[D].杨凌:西北农林科技大学,2018.WANG Shuai. Resistance risk assessment of Valsa mali to pyraclostrobin and antifungal activity of biological fungicides[D].Yangling:Northwest A&F University,2018.
[27]高杨杨,禾丽菲,李北兴,林琎,慕卫,刘峰.山东省辣椒上尖孢炭疽复合种对吡唑醚菌酯的敏感基线及吡唑醚菌酯增效配方筛选[J].农药学学报,2017,19(6):701-707.GAO Yangyang,HE Lifei,LI Beixing,LIN Qin,MU Wei,LIU Feng. Baseline sensitivity to pyraclostrobin of Colletotrichum acutatum species complex collected from chili in Shandong Province and the screening of synergistic formula[J]. Chinese Journal of Pesticide Science,2017,19(6):701-707.
[28]李宝燕,栾炳辉,石洁,汪少丽,田园园,聂乐兴,王英姿.胶东地区葡萄白腐病菌对吡唑醚菌酯的敏感性及与其他4 种药剂的敏感性比较[J].农药学学报,2020,22(6):959-966.LI Baoyan,LUAN Binghui,SHI Jie,WANG Shaoli,TIAN Yuanyuan,NIE Lexing,WANG Yingzi. Sensitivity of Coniella diplodiella to pyraclostrobin in Jiaodong Area and comparison with four other fungicides[J]. Chinese Journal of Pesticide Science,2020,22(6):959-966.
[29]李希,汤敬诚,谢昌平,仇芳,李晶,李睿,王佳楠.海南省火龙果溃疡病菌对吡唑醚菌酯敏感性分析[J].果树学报,2022,39(1):104-111.LI Xi,TANG Jingcheng,XIE Changping,QIU Fang,LI Jing,LI Rui,WANG Jianan.Sensitivity surveillance and analysis of pitaya canker disease pathogen to pyraclostrobin in Hainan province,China[J].Journal of Fruit Science,2022,39(1):104-111.
[30]GISI U,SIEROTZKI H,COOK A,MCCAFFERY A. Mechanisms influencing the evolution of resistance to Qo inhibitor fungicides[J].Pest Management Science,2002,58(9):859-867.