我国是枇杷属植物的起源与分布中心,拥有丰富的枇杷属种质资源,我国有原产20 个种类(包括16个种和4个变种或变型),东南亚原产的则至少有6 个种[1]。丰富的野生枇杷资源是植物提取物产业的一个宝库。迄今,我国已对普通枇杷叶片等器官的三萜酸类物质的提取和药效做了大量的研究,原远[2]和龙婷[3]已对这些进展做了较系统的综述。
枇杷叶的化学成分是体现其可利用价值的重要指标依据。枇杷叶中含有三萜类,多酚类绿原酸和槲皮素-3-桑布双糖苷、甲基绿原酸、山奈黄素-和斛皮素-鼠李糖苷[4],倍半萜、挥发油类水杨酸甲酯、环己酮等[5]等有效活性成分。枇杷叶中三萜类化合物包括熊果酸、齐墩果酸和委陵菜酸为母体的衍生物等,其中熊果酸是含量最高,也是报道研究最多的五环三萜酸。还包括2α-羟基熊果酸、2α-羟基齐墩果酸、蔷薇酸、2α-羟基齐墩果酸甲酯等[6]。枇杷叶的药理作用包括镇咳祛痰平喘作用[7]、抗炎作用[8]、抗癌作用[9-10]、降血糖作用[11]等。
目前,对于普通枇杷枇杷叶主要药用成分,已做了较多的研究,发表了数以百计的相关论文[2-3]。对于三萜酸,已做了较为深入的研究,特别是基于对解放钟品种的基因组测序阐释了枇杷与苹果梨等在三萜酸代谢上的差异[12]。但是,对枇杷属普通枇杷之外的20多个野生种的三萜酸的研究,除了华南农业大学等单位已测试了部分野生种及其少数种间杂种后代的三萜酸含量,但至今尚未有全面的报道。
笔者以华南农业大学国家重点实验室野生枇杷种质资源圃内种植的25 种野生种质资源、6 种种间杂交后代以及3 种栽培品种及种内杂种共计34 种,以及莆田学院枇杷种质资源圃内保存的枇杷属植物种间杂种15种,即能够采到的国内外所有枇杷种类为材料(栽培枇杷品种则只取少数几个作为对照或参照),采用HPLC 方法分离三萜酸,测定枇杷叶片三萜酸类物质熊果酸(Ursolic acid,UA)、齐墩果酸(Oleanolic acid,OA)、山楂酸(Maslinic acid,MA)、科罗索酸(Corosolic acid,CA)含量,以期筛选出枇杷属野生植物三萜酸含量高于普通枇杷20%~30%及以上的种类,为野生枇杷叶片的开发利用奠定一定的基础。
1 材料和方法
1.1 材料
第一批试验所用枇杷属植物材料(表1)均取自华南农业大学枇杷属植物种植资源圃。包括中国枇杷(20 个种类或变型)S1(代号对应的种质见表1)、S2、S3、S4、S5、S6、S7、S10、S11、S12、S13、S16、S17、S18、S20、S21、S23、S24、S25、S27,东南亚枇杷(5种)S8、S9、S19、S22、S26,2 个栽培枇杷品种(S14、S15)及7 个种间杂交组合后代株系S28、S29、S30、S31、S32、S33、S34 的叶片。第二批种间杂种材料(表2)取自莆田学院枇杷属植物种质资源圃。包括4个杂交组合后代JD1(代号对应的种质见表2)、JT7、JT8、JT13、JT14、JT15、JT160、JT162、JTA1、JTA5、JTA7、JTA8、JTH1、JTH4、JTH12的叶片。枇杷属植物种材料叶片特征见图1。

图1 部分枇杷属野生种材料
Fig.1 Some wild species of Eriobotrya plants
表1 第一批试验所用枇杷属植物材料
Table 1 The first batch of Eriobotrya plants used in the experiment

编号Serial number S1 S2 S3 S4 S5 S6 S7 S8 S9 S10 S11 S12 S13 S14 S15 S16 S17 S18 S19 S20 S21 S22 S23 S24 S25 S26 S27 S28 S29 S30 S31 S32 S33 S34中文名Chinese name南亚枇杷南亚枇杷窄叶变型大花枇杷台湾枇杷台湾枇杷武葳山变型台湾枇杷恒春变型椭圆枇杷长叶柄枇杷椭圆枇杷贝特罗变种香花枇杷薄叶枇杷窄叶枇杷普通枇杷野生树早钟六号解放钟广西枇杷麻栗坡枇杷倒卵叶枇杷波宜兰枇杷栎叶枇杷大渡河枇杷栎叶枇杷老挝变种怒江枇杷小叶枇杷齿叶枇杷椭圆托叶枇杷腾越枇杷解放钟×大红袍解放钟×栎叶枇杷解放钟×恒春变型早钟六号×栎叶枇杷大红袍×武葳山变型解放钟×台湾枇杷栎叶枇杷×大红袍学名Scientific name E.bengalensis f.Hook.E.bengalensis f.angustifolia Vidal E.cavaleriei Rehd E.deflexa Nakai E.deflexa f.buisanensis Nakai E.deflexa f.koshunensis Nakai E.elliptica Lindl.E.petiolata Hook.E.elliptica var.petelottii Vidal E.fragrans Champ E.fulvicoma Chun&Liao E.henryi Nakai E.japonica Lindl.E.japonica Lindl.‘Zaozhong No.6’E.japonica‘Jiefangzhong’E.kwangsiensis Chun E.malipoensis Kuan E.obovata W.W.Smith E.poilanei Vidal E.prinoides Redh.&Wils E.prinoides var.dadunensis H.Z.Zhang E.prinoides var.laotica Vidal E.salwinensis Hand-Mazz E.seguinii Card E.serrate Vidal E.stipularis Craib E.tengyuehensis W.W.Smith E.japonica×E.japonica E.japonica×E.prinoides E.japonica×E.deflexa f.koshunensis E.japonica×E.prinoides E.japonica×E.deflexa f.buisanensis E.japonica×E.deflexa E.prinoides×E.japonica样品原产地Origin of sample越南河内Hanoi,Vietnam云南昆明Kunming,Yunnan广东连州Lianzhou,Guangdong台湾恒春Hengchun,Taiwan广东乳源Ruyuan,Guangdong台湾恒春Hengchun,Taiwan云南大围山Daweishan,Yunnan缅甸甘基Kan Gyi,Myanmar越南老街Lào Cai,Vietnam广东乳源Ruyuan,Guangdong广东信宜Xinyi,Guangdong云南澄江Dengjiang,Yunnan广东乳源Ruyuan,Guangdong福建福州Fuzhou,Fujian福建莆田Putian,Fujian广西象州Xiangzhou,Guangxi云南麻栗坡Malipo,Yunnan云南麻栗坡Malipo,Yunnan越南大叻Dalat,Vietnam云南石屏Shiping,Yunan四川汉源Hanyuan,Sichuan老挝石缸平原Thong Hai Hin,Laos云南贡山Gongshan,Yunnan广西凌云Lingyun,Guangxi云南景洪Jinghong,Yunnan越南大叻Dalat,Vietnam西藏樟木Zhangmu,Tibet广东广州Guangzhou,Guangdong广东广州Guangzhou,Guangdong广东广州Guangzhou,Guangdong广东广州Guangzhou,Guangdong广东广州Guangzhou,Guangdong广东广州Guangzhou,Guangdong广东广州Guangzhou,Guangdong
表2 第二批试验所用枇杷属植物材料
Table 2 The second batch of Eriobotrya plants used in the experiment
编号Serial number JFZ JD1 JT7 JT8 JT13 JT14 JT15 JT160 JT162 JTA1 JTA5 JTA7 JTA8 JTH1 JTH4 JTH12中文名Chinese name解放钟解放钟×大渡河枇杷1解放钟×椭圆枇杷7解放钟×椭圆枇杷8解放钟×椭圆枇杷13解放钟×椭圆枇杷4解放钟×椭圆枇杷15解放钟×椭圆枇杷160解放钟×椭圆枇杷162解放钟×台湾枇杷1解放钟×台湾枇杷5解放钟×台湾枇杷7解放钟×台湾枇杷8解放钟×恒春变型1解放钟×恒春变型4解放钟×恒春变型12学名Scientific name E.bengalensis f.Hook.E.japonica×E.prinoides var.dadunensis E.japonica×E.elliptica E.japonica×E.elliptica E.japonica×E.elliptica E.japonica×E.elliptica E.japonica×E.elliptica E.japonica×E.elliptica E.japonica×E.elliptica E.japonica×E.deflexa E.japonica×E.deflexa E.japonica×E.deflexa E.japonica×E.deflexa E.japonica×E.deflexa f.koshunensis E.japonica×E.deflexa f.koshunensis E.japonica×E.deflexa f.koshunensis样品原产地Origin of sample福建莆田Putian,Fujian福建莆田Putian,Fujian福建莆田Putian,Fujian福建莆田Putian,Fujian福建莆田Putian,Fujian福建莆田Putian,Fujian福建莆田Putian,Fujian福建莆田Putian,Fujian福建莆田Putian,Fujian福建莆田Putian,Fujian福建莆田Putian,Fujian福建莆田Putian,Fujian福建莆田Putian,Fujian福建莆田Putian,Fujian福建莆田Putian,Fujian福建莆田Putian,Fujian
1.2 方法
1.2.1 样品采集及前处理 分别从每株树的东、南、西、北四个方向,分别采集嫩叶、成熟叶和老叶。其中嫩叶为刚抽梢的新叶,成熟叶为上一季度萌发的枝梢上的生长稳定的叶片,老叶为上一年或两年的叶片已转枯,即将脱落。叶片放置在烘箱40 ℃烘干,粉粹、60目孔径筛子过滤后保存待测。第1批试验所用枇杷属植物材料取嫩叶、成熟叶和老叶,第2批取成熟叶。
1.2.2试验主要试剂熊果酸( 批号C15O11Q126879,纯度≥98%)、齐墩果酸(批号J24HB174960,纯度≥ 98% )、山楂酸( 批号J20GB152065,纯度≥98%)、科罗索酸(批号BCBQ4293V,纯度≥98%)标准品;甲醇(色谱级);甲醇、冰乙酸、香草醛、高氯酸等均为分析级。
1.2.3 枇杷属植物总萜类的提取 分别取供试枇杷叶粉末2.000 g 于锥形瓶中,再加入100 mL的100%甲醇(分析级)、料液比为1∶50(g∶mL),提取温度为40 ℃,提取时间为40 min,处理好之后放置冰箱冷藏保存。
1.2.4 枇杷属植物总三萜类物质含量的测定 采用熊果酸作为总三萜类物质的测量标准,以标准物熊果酸含量绘制标准曲线,计算各样品的总三萜类物质含量。取冷藏的枇杷处理液20 μL,放置在37 ℃真空干燥箱下蒸干溶剂,随后加入0.2 mL新配置的5%香草醛-冰醋酸溶液作为显色剂,再加入0.8 mL高氯酸作为稳定剂,在水浴锅中60 ℃加热15 min后取出,置于冰块上冷却,待完全冷却后加入5 mL冰乙酸并摇晃均匀,静置10 min 后,用紫外分光光度计于548 nm 波长处测定不同含量熊果酸的吸光值,以空白(不添加样品的显色剂)为对照,测定各样品总萜类含量,以干质量计。
1.2.5 枇杷属植物4种主要三萜酸含量的测定 由于熊果酸和齐墩果酸是性质相似的同分异构体,分离检测较为困难。参考前人经验,试验中考察了不同色谱柱和流动相体系对熊果酸和齐墩果酸的分离效果。测试方法1:色谱柱Waters Sunfire C18,流动相0.1%磷酸水溶液-甲醇(14∶86)(均以体积比计,下同),流速1 mL·min-1,柱温30 ℃,检测波长208 nm[3];方法2:色谱柱Kromasil C18,流动相0.1%磷酸水溶液-乙腈,梯度洗脱,流速1 mL·min-1,柱温35 ℃,检测波长208 nm[13];方法3:色谱柱Hypersil ODS2,流动相乙腈-水-甲醇-乙酸铵(60∶26∶14∶0.55),流速1 mL·min-1,柱温25 ℃,检测波长215 nm[14];方法4:色谱柱Inertsil ODS-SP,流动相乙腈-醋酸铵-甲醇(67∶21∶12),流速0.4 mL·min-1,柱温为室温,检测波长210 nm[10]。方法5:色谱柱Sunfire C18,0.1%磷酸水溶液-甲醇(2∶98)为流动相流速0.6 mL·min-1,柱温为室温,检测波长210 nm[15]。
通过方法5 得到较优色谱图,对测定条件进行优化,最终确定为:色谱柱Sunfire C18,以0.1%磷酸水溶液-甲醇为流动相进行梯度洗脱,程序为:在0到5 min 内,甲醇占比由97%升到99.5%,在5~25 min时,甲醇的含量保持在99.5%;流速0.300 mL·min-1,进样量8 μL,柱温20 ℃;检测波长208 nm。
绘制熊果酸、齐墩果酸、山楂酸、科罗索酸标准品溶液曲线。取冷藏的枇杷处理液,用0.22 μm 微孔滤膜过滤后上机测试,进而获得枇杷属植物4 种主要三萜酸含量。
2 结果与分析
2.1 枇杷属植物不同成熟度叶片三萜酸含量分析
由图2 可知,大部分种类各成熟度叶片之间总三萜酸含量(w,后同)无显著差异。老叶中,总三萜酸集中分布在30~40 mg·g-1之间,以解放钟为参照,仅有S3、S5高于解放钟;成熟叶中,总三萜酸集中分布在30~40 mg·g-1之间,大多数枇杷种类含量高于解放钟;嫩叶中,总三萜酸集中分布在30~40 mg·g-1之间,野生枇杷与栽培枇杷及杂交后代均有5 个种类含量高于解放钟。
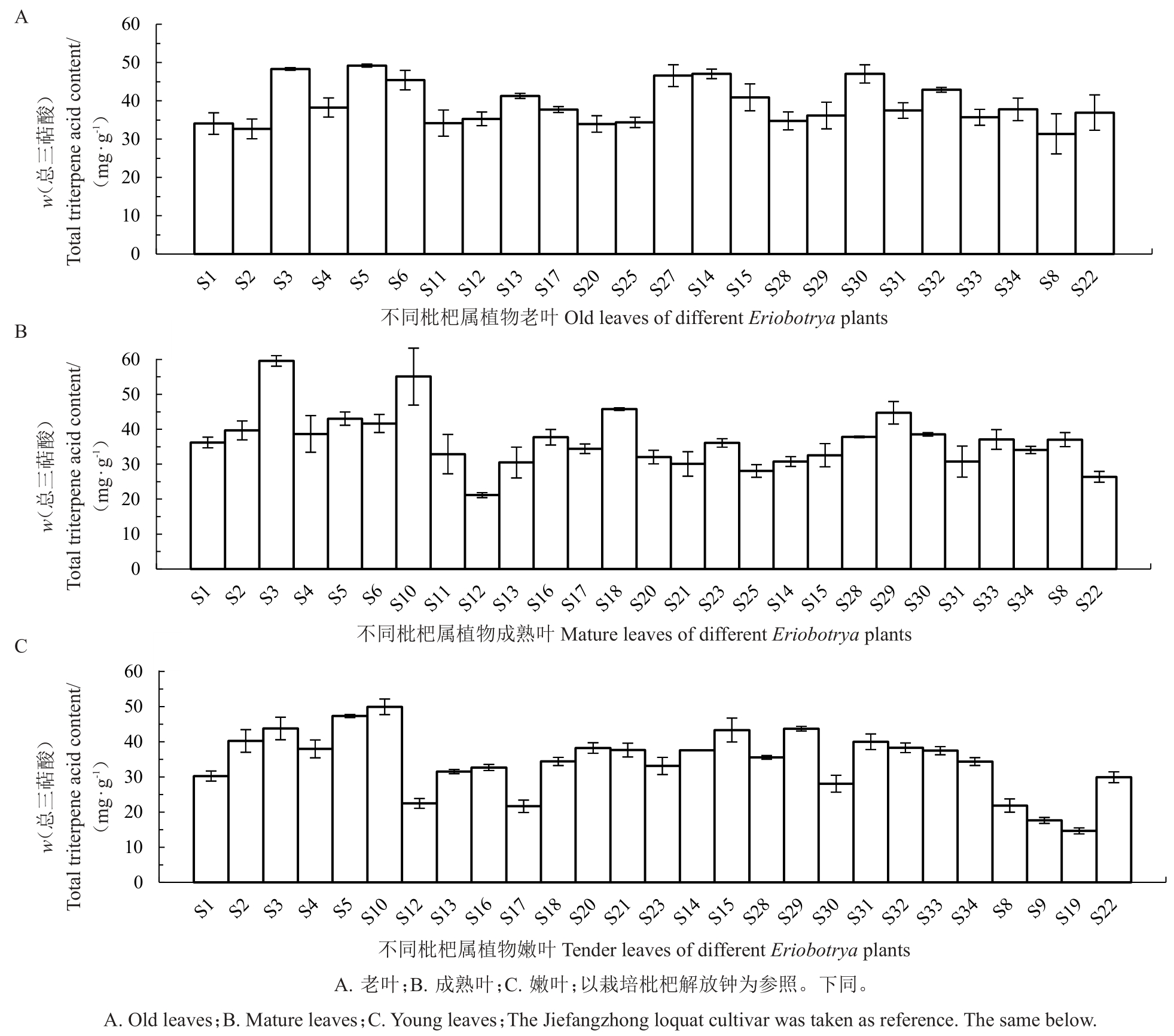
图2 不同成熟度叶片总三萜酸含量
Fig.2 Total triterpene acid content in leaves with different maturity
从图3 可知,老叶中山楂酸含量在1~11 mg·g-1之间,大多数种类集中在2~4 mg·g-1之间,以栽培枇杷解放钟为参照,S14、S18、S28、S30 含量高于解放钟;成熟叶中山楂酸含量在1~6 mg·g-1之间,大多数种类集中在2~4 mg·g-1之间,以栽培枇杷解放钟为参照,S1、S2、S30、S33含量高于解放钟;嫩叶中山楂酸含量在0.5~4 mg·g-1之间,大多数种类集中在0.5~2 mg·g-1之间,以栽培枇杷解放钟为参照,S2、S4、S10、S14、S25含量高于解放钟。
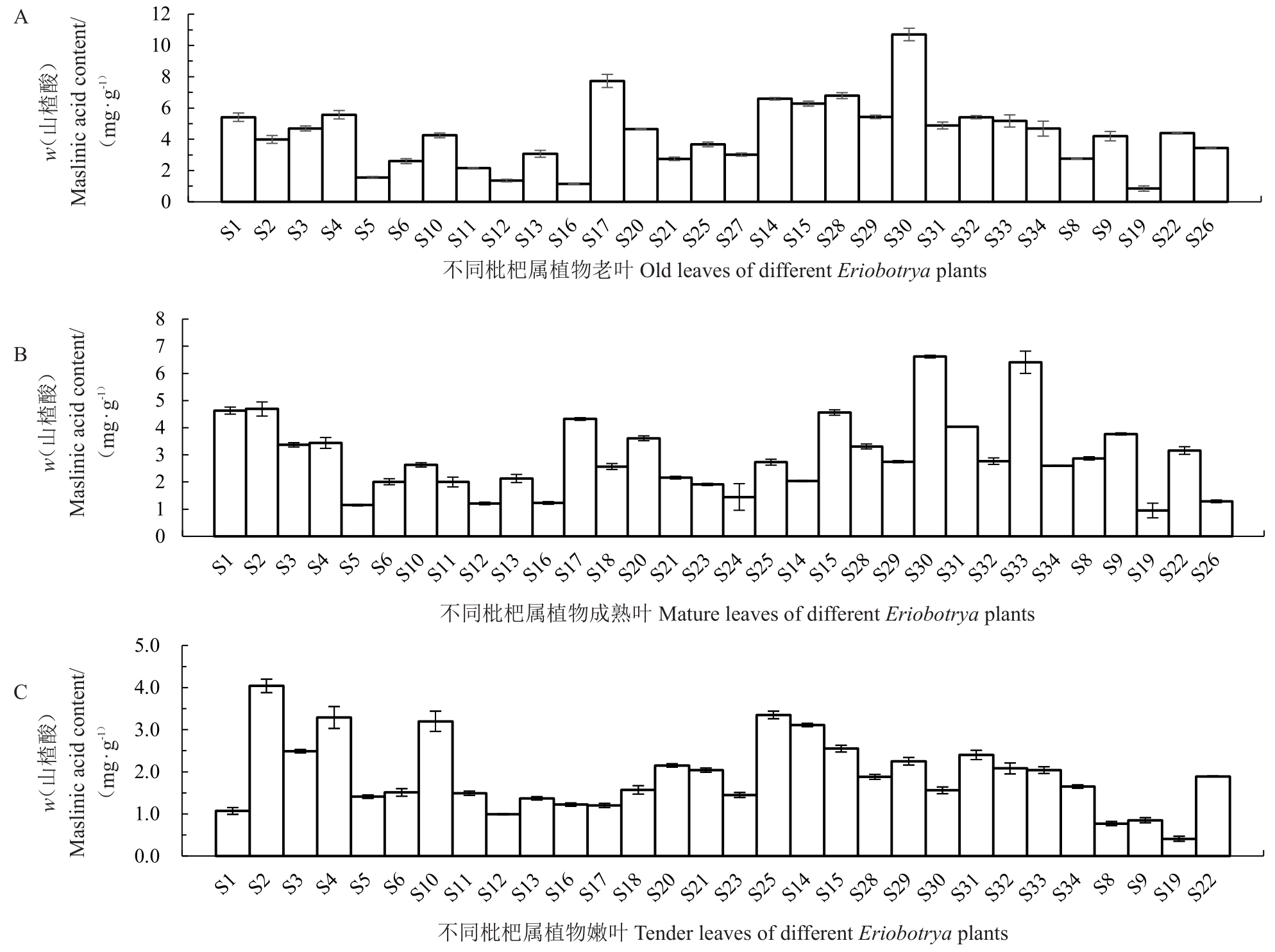
图3 不同成熟度叶片山楂酸含量
Fig.3 The MA content in leaves with different maturity
从图4可知,老叶中科罗索酸含量在2~35 mg·g-1之间,大多数种类集中在2~10 mg·g-1之间,以栽培枇杷解放钟为参照,S14、S18含量高于解放钟;成熟叶中科罗索酸含量在4~20 mg·g-1之间,大多数种类集中在4~10 mg·g-1之间,以栽培枇杷解放钟为参照,仅有S17含量高于解放钟;嫩叶中科罗索酸含量在1~15 mg·g-1之间,大多数种类集中在1~5 mg·g-1之间,以栽培枇杷解放钟为参照,仅有S25含量高于解放钟。

图4 不同成熟度叶片科罗索酸含量
Fig.4 The CA content in leaves with different maturity
从图5可知,老叶中齐墩果酸含量在1~5.5 mg·g-1之间,大多数种类集中在1~3 mg·g-1之间,以栽培枇杷解放钟为参照,有一半材料含量高于解放钟;成熟叶中齐墩果酸含量在1~3 mg·g-1之间,大多数种类集中在1~2.5 mg·g-1之间,以栽培枇杷解放钟为参照,7 个种类含量高于解放钟;嫩叶中齐墩果酸含量在0.5~3.5 mg·g-1之间,大多数种类集中在1~2 mg·g-1之间,以栽培枇杷解放钟为参照,有23 个种类含量高于解放钟。
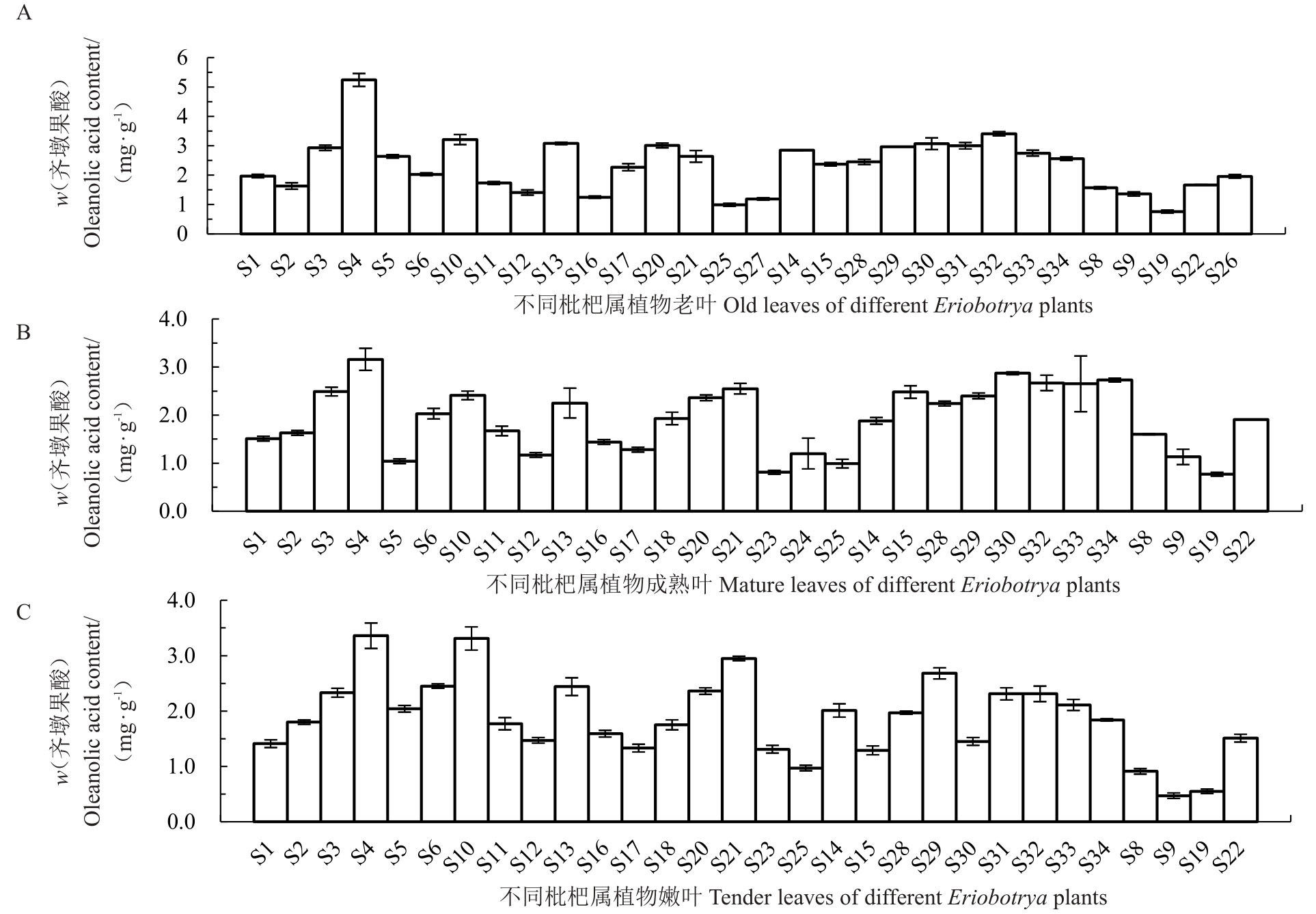
图5 不同成熟度叶片齐墩果酸含量
Fig.5 The MA content in leaves with different maturity
从图6 可知,老叶中熊果酸含量在5~28 mg·g-1之间,大多数种类集中在5~13 mg·g-1之间,以栽培枇杷解放钟为参照,有一半材料含量高于解放钟;成熟叶中熊果酸含量在5~20 mg·g-1之间,大多数种类集中在5~12 mg·g-1之间,以栽培枇杷解放钟为参照,12 个种类含量高于解放钟;嫩叶中熊果酸含量在4~15 mg·g-1之间,大多数种类集中在4~12 mg·g-1之间,以栽培枇杷解放钟为参照,大部分种类含量均高于解放钟。
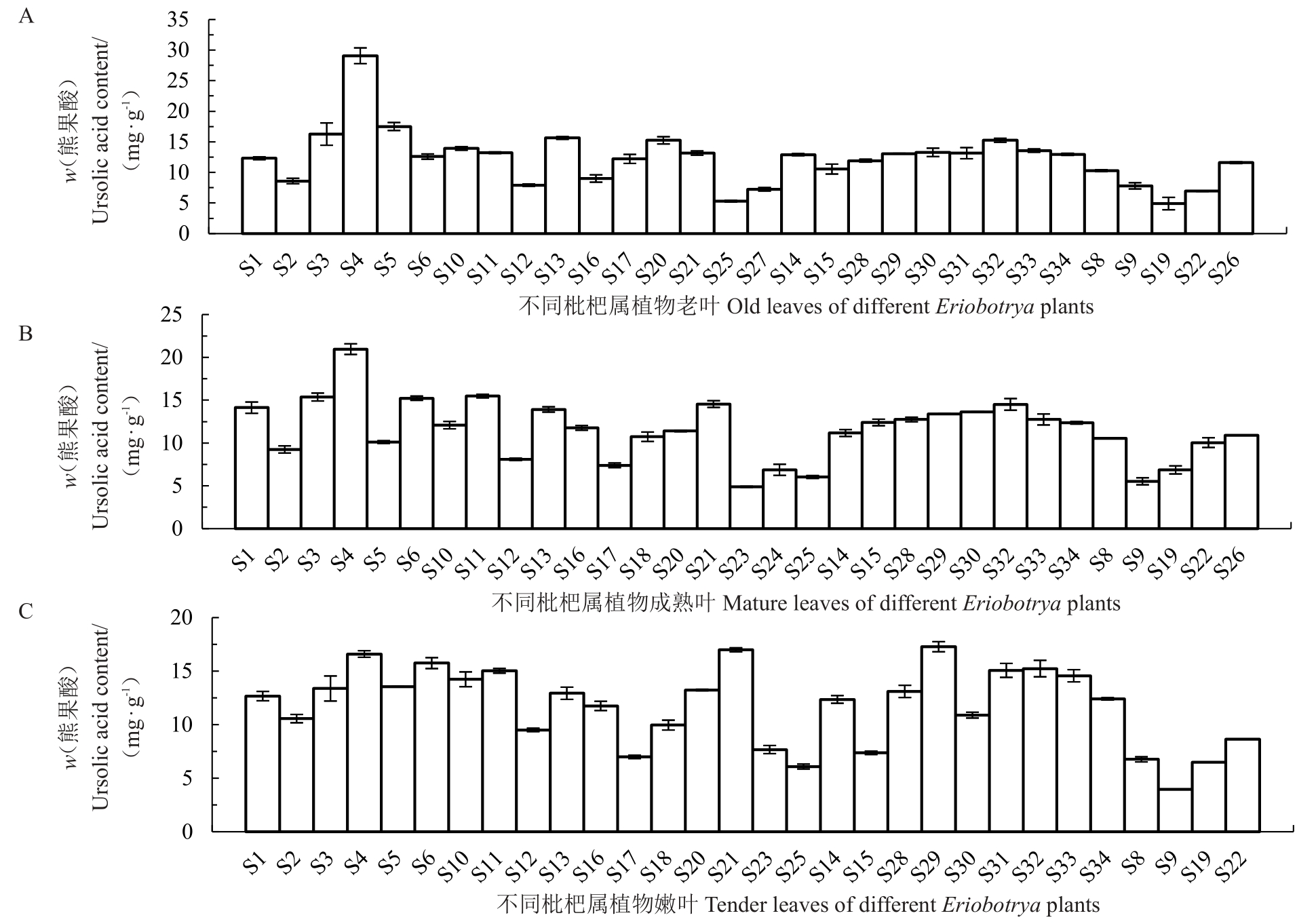
图6 不同成熟度叶片熊果酸含量
Fig.6 The UA content in leaves with different maturity
根据中国药典[16]规定,熊果酸与齐墩果酸含量不得低于0.7%。结合图7可知,除老叶批次的S19、S25,成熟叶批次的S23,老叶批次的S9 外,其余批次样品均符合药典规定,是合格的中药材。其中台湾枇杷S4是高含量种类,老叶和成熟叶含量达到了药典规定3倍以上,嫩叶达2倍以上。含量达药典规定2 倍以上的分别有19 种(老叶批次)、16 种(成熟叶批次)、17种(嫩叶批次)。

图7 不同成熟度叶片熊果酸与齐墩果酸含量之和
Fig.7 The UA and OA content in leaves with different maturity
由图8 可知,老叶中四三萜酸总含量在8~55 mg·g-1之间,大多数种类集中在15~30 mg·g-1之间,以栽培枇杷解放钟为参照,S4、S14、S18 含量高于解放钟;成熟叶中四三萜酸总含量在10~38 mg·g-1之间,大多数种类集中在10~25 mg·g-1之间,以栽培枇杷解放钟为参照,仅有S31含量高于解放钟;嫩叶中四三萜酸总含量在8~30 mg·g-1之间,大多数种类集中在15~25 mg·g-1之间,以栽培枇杷解放钟为参照,有15个种类含量高于解放钟。

图8 不同成熟度叶片4 种主要三萜酸总含量
Fig.8 The four triterpene acid content in leaves with different maturity
2.2 枇杷属种间杂种后代三萜酸含量分析
根据中国药典[16]规定,枇杷叶齐墩果酸与熊果酸含量不得低于0.7%,即不低于7 mg·g-1。本研究的结果显示(表3),齐墩果酸与熊果酸在不同枇杷属植物种间杂种叶片中的含量为4.74~19.65 mg·g-1,其中12 种种间杂种齐墩果酸与熊果酸含量高于药典标准,但低于母本解放钟的21.56 mg·g-1。除JT13、JT14 和JT160 外,其他种间杂种的齐墩果酸与熊果酸含量均超过药典规定的标准;其中,JTH4、JT8、JT162、JD1 达到药典规定的1.5~2 倍之间,JTH12、JTA1、JTH1、JTA5、JTA7、JT15 和JTA8 达到药典规定的2~3倍之间。
表3 母本解放钟与不同种间杂种材料三萜酸含量分析
Table 3 Analysis of triterpene acid content between female parent Jiefangzhong and different interspecific hybrids
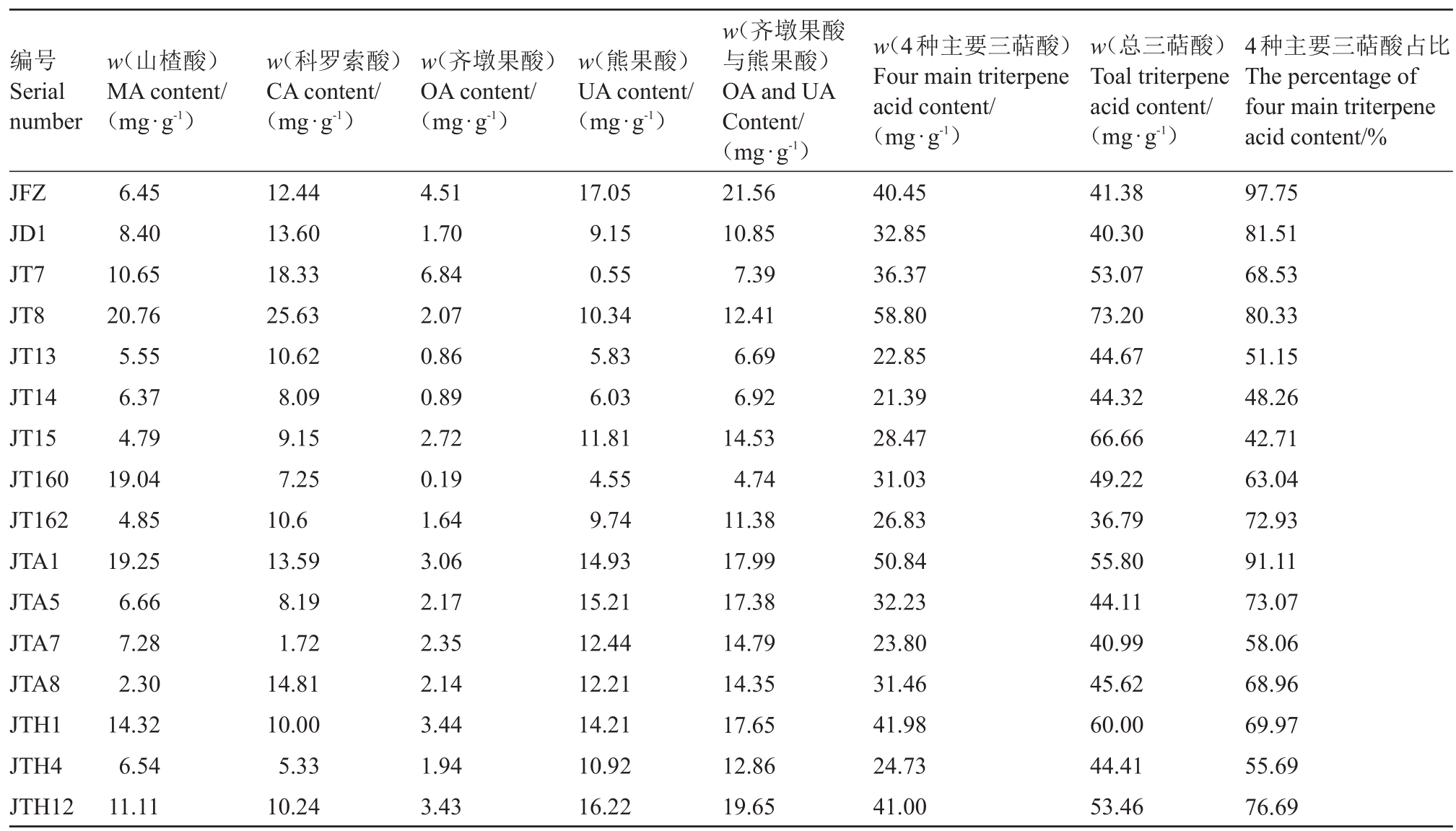
编号Serial number w(山楂酸)MA content/(mg·g-1)w(科罗索酸)CA content/(mg·g-1)w(齐墩果酸)OA content/(mg·g-1)w(熊果酸)UA content/(mg·g-1)w(4种主要三萜酸)Four main triterpene acid content/JFZ JD1 JT7 JT8 JT13 JT14 JT15 JT160 JT162 JTA1 JTA5 JTA7 JTA8 JTH1 JTH4 JTH12 6.45 8.40 10.65 20.76 5.55 6.37 4.79 19.04 4.85 19.25 6.66 7.28 2.30 14.32 6.54 11.11 12.44 13.60 18.33 25.63 10.62 8.09 9.15 7.25 10.6 13.59 8.19 1.72 14.81 10.00 5.33 10.24 4.51 1.70 6.84 2.07 0.86 0.89 2.72 0.19 1.64 3.06 2.17 2.35 2.14 3.44 1.94 3.43 17.05 9.15 0.55 10.34 5.83 6.03 11.81 4.55 9.74 14.93 15.21 12.44 12.21 14.21 10.92 16.22 w(齐墩果酸与熊果酸)OA and UA Content/(mg·g-1)21.56 10.85 7.39 12.41 6.69 6.92 14.53 4.74 11.38 17.99 17.38 14.79 14.35 17.65 12.86 19.65(mg·g-1)40.45 32.85 36.37 58.80 22.85 21.39 28.47 31.03 26.83 50.84 32.23 23.80 31.46 41.98 24.73 41.00 w(总三萜酸)Toal triterpene acid content/(mg·g-1)41.38 40.30 53.07 73.20 44.67 44.32 66.66 49.22 36.79 55.80 44.11 40.99 45.62 60.00 44.41 53.46 4种主要三萜酸占比The percentage of four main triterpene acid content/%97.75 81.51 68.53 80.33 51.15 48.26 42.71 63.04 72.93 91.11 73.07 58.06 68.96 69.97 55.69 76.69
总三萜酸在不同枇杷属植物种间杂种叶片中的含量为36.79 ~73.20 mg·g-1;其中,JT8 总三萜酸的含量最高,JT162 的含量最低;JT8、JT15、JTH1和JTA1 的三萜酸含量超过母本解放钟的30%,分别达到1.77、1.61、1.45 和1.35 倍。山楂酸、科罗索酸、齐墩果酸、熊果酸四种三萜酸在不同枇杷属植物种间杂种叶片中的含量为21.39~58.80 mg·g-1;其中,JT8 四种三萜酸的含量最高,JT14 的含量最低;仅JT8 中四种三萜酸的含量超过母本解放钟的30%,达到1.45 倍;有意思的是,在母本解放钟中这4 种主要三萜酸含量占总三萜酸的含量达到97.75%,而枇杷属植物种间杂种叶片中4 种三萜酸的含量比例为42.71%~91.11%,均低于母本解放钟。
不同枇杷属植物种间杂种叶片山楂酸含量在2.30~20.76 mg·g-1之间;其中JT8 的山楂酸含量最高,JTA6的山楂酸含量最低;有7份种间杂种材料山楂酸含量超过母本解放钟的30%,包括JT7、JT8、JT160、JTA1、JTH1、JTH12和JD1;其中,JT8、JT160、JTH1、JTA1 的山楂酸含量均超过解放钟的2 倍,特别是JT8更是达到解放钟的3.22倍。种间杂种叶片科罗索酸含量在1.72~25.63 mg·g-1之间;其中JT8的科罗索酸含量最高,JTA7的科罗索酸含量最低;有2份种间杂种材料科罗索酸含量超过母本解放钟的30%,包括JT7 和JT8,分别达到解放钟的1.47 倍和2.06 倍。种间杂种叶片齐墩果酸含量在0.19~6.84 mg·g-1之间。仅JT7 的齐墩果酸含量超过母本解放钟,达到1.52倍;JT14、JTA5的齐墩果酸含量不低于解放钟的70%;种间杂种叶片熊果酸含量在0.55~16.22 mg·g-1之间,均低于母本解放钟;其中,JTH1、JTH12、JTA1和JTA5的熊果酸含量不低于解放钟的80%。
结果表明,多数供试种间杂种的熊果酸与齐墩果酸含量均能超过药典规定标准的1.5倍,可以作为药材枇杷叶的来源植物。JT8、JT15、JTH1 和JTA1是高三萜酸含量材料,JT8 是最佳的山楂酸和科罗索酸来源材料,JT7 是优质的科罗索酸和最佳的齐墩果酸来源材料,JT160、JTH1、JTA1 是优质的山楂酸来源材料,而最佳的熊果酸来源材料仍是母本解放钟。
3 讨 论
中药材的质量受到产地和品种、采收期、保存条件的影响。关于枇杷叶的质量控制标准,中国药典(2005 年版)仅以水浸出物测定法检测,使用热浸法,测定浸出物质量,不得少于10.0%[7]。而中国药典[16]以齐墩果酸和熊果酸为测定指标,规定其干燥品中总含量不得低于0.7%。不少学者对枇杷叶的质量控制方法及指标做了大量研究,初步提出了以熊果酸和齐墩果酸等主要药效活性成分含量和重金属、农药残留量标准作为质量控制指标的建议[17]。
根据中国药典[16]关于枇杷叶质量标准的规定,除了椭圆枇杷贝特罗变种嫩叶、波宜兰老叶以及三个椭圆枇杷与解放钟的种间杂交后代解放钟×椭圆枇杷13、14和160号外,所有批次样品的熊果酸和齐墩果酸含量均达到药典规定,可作为合格中药材,具有进一步进行药用开发的潜力。
笔者在本研究中筛选到台湾枇杷为最高熊果酸和齐墩果酸含量种类,成熟叶达药典规定3倍以上,老叶为4 倍以上。在不同成熟度的叶片材料中,嫩叶批次总含量达药典规定2~3 倍之间的有17 种,成熟叶批次中有16 种,老叶中有20 种。筛选到了14个三个成熟度含量都在药典2~3 倍之间的种类,一类是大于解放钟含量30%以上的种类:台湾枇杷、大渡河枇杷、香花枇杷、解放钟×大红袍、解放钟×栎叶枇杷、解放钟×恒春变型、大红袍×武葳山变型、解放钟×台湾枇杷。第二类是大于解放钟含量20%以上的种类:大花枇杷、台湾枇杷武葳山变型、栎叶枇杷老挝变种、台湾枇杷恒春变型、早钟6 号×栎叶枇杷、大红袍×武葳山变型。此外,第二批实验又筛选到7 个熊果酸和齐墩果酸含量达药典规定2~3 倍之间的种间杂种后代,包括解放钟×台湾枇杷1、5、7 和8 号、解放钟×台湾枇杷恒春变型1 和12 号、解放钟×椭圆枇杷15号。
除熊果酸和齐墩果酸外,山楂酸和科罗索酸也是枇杷叶的主要三萜类物质,本研究中这4 种三萜酸可占到解放钟枇杷叶片总三萜酸含量的90%以上。从枇杷愈伤组织[18]分离得到了包括山楂酸在内的三萜类物质,发现其具有抗肿瘤作用。此外,山楂酸等还具有促进动脉血管舒张[19]等作用。科罗索酸可用于医治糖尿病和防治肥胖症[20],可作用于胃癌细胞[21],还可改善高血压、异常脂质代谢、氧化应激[22],以及炎症状态[23-24]等。笔者在本研究中还筛选到高山楂酸来源材料解放钟×椭圆枇杷8号和160号、解放钟×台湾枇杷恒春变型1 号和解放钟×台湾枇杷1 号,以及高科罗索酸来源材料解放钟×椭圆枇杷7号和8号。特别是解放钟×椭圆枇杷8号,山楂酸和科罗索酸含量均为种间杂种中最高,分别可达解放钟的3.22倍和2倍。
以解放钟为亲本,第一批供试的杂交组合后代的熊果酸含量均高于解放钟含量;但杂交后代的科罗索酸含量却都低于解放钟。第二批供试的杂交组合后代材料中,10种材料山楂酸含量、5种材料科罗索酸含量和1 种材料的齐墩果酸含量高于解放钟,所有材料熊果酸含量均低于解放钟;此外,杂交组合后代材料中4种三萜酸的含量占总三萜酸含量的比例均低于母本解放钟。亲子代之间的三萜酸含量的传递关系值得深入研究。
本研究结果表明的多种枇杷属野生种及其杂种后代株系叶片的三萜酸含量高于普通枇杷,作为纯天然无污染的药用枇杷叶来源潜力巨大。以野生枇杷叶片为中药的药源,避免从栽培枇杷园里取叶片导致枇杷减产;以种间杂种后代优系进行造林,更易繁殖推广开来,林药两用。这些大量的纯天然无污染枇杷叶,不但可以用以传统中药的药源,而且可以作为优质枇杷茶产品的原料来源。
4 结 论
笔者在研究中以枇杷属25个种类/变型、7个野生-栽培枇杷杂交组合的21个后代株系为试料,以3个栽培品种及种内杂种作为对照,采用HPLC 分析枇杷叶片的熊果酸、齐墩果酸、山楂酸和科罗索酸等4 种主要三萜酸物质含量,发现多种枇杷属野生种及其杂种后代株系叶片的三萜酸含量高于普通枇杷,作为纯天然无污染的药用枇杷叶来源潜力巨大。
[1]林顺权,刘月学.枇杷属植物图谱[M].北京:科学出版社,2016.LIN Shunquan,LIU Yuexue. Collection of illustration for Eriobotrya plants[M].Beijing:Science Press,2016.
[2]原远.枇杷叶活性提取物及其抗肺癌效能研究[D].广州:华南农业大学,2014.YUAN Yuan. Determination of Eriobotrya leaf extract and anticancer activity[D]. Guangzhou:South China Agricultural University,2014.
[3]龙婷.枇杷属植物三萜酸类物质含量比较分析[D].广州:华南农业大学,2017 LONG Ting. Comparative analyses of triterpenoid acids content in Eriobotrya[D]. Guangzhou:South China Agricultural University,2017.
[4]JUNG H A,PARK J C,CHUNG H Y,KIM J,CHOI J S.Antioxidant flavonoids and chlorogenic acid from the leaves of Eriobotrya japonica[J]. Archives of Pharmacal Research,1999,22(2):213.
[5]王义潮,巩江,高昂,贾旭,曹梦晔,陈巧利,赵婷,侯晓艺,倪士峰. 枇杷叶挥发油气相色谱-质谱研究[J]. 安徽农业科学,2011,39(5):2637-2638.WANG Yichao,GONG Jiang,GAO Ang,JIA Xu,CAO Mengye,Chen Qiaoli,ZHAO Ting,Hou Xiaoyi NI Shifeng .Study on the volatile oil from leaves of Eriobotrya japonica(Thunb.)Lindl.by GC-MS[J].Journal of Anhui Agricultural Sciences,2011,39(5):2637-2638.
[6]吕寒,习超鹏,陈剑,李维林.不同生长季节枇杷叶中三萜酸成分的含量变化[J].中国中药杂志,2009,34(18):2353-2355.LÜ Han,XI Chaopeng,CHEN Jian,LI Weilin.Changes of triterpenoid acids in loquat leaves during different growing seasons[J].Chinese Journal of Traditional Chinese Medicine,2009,34(18):2353-2355.
[7]郭娜.福建地产枇杷叶的化学品质评价[D].福州:福建中医药大学,2014.GUO Na. Evaluation for quality of Chemical ingredients in Authentic loquat leaves from Fujian[D]. Fuzhou:Fujian university of traditional Chinese medicine,2014.
[8]葛金芳,李俊,姚宏伟,胡成穆,彭磊,金涌,吕雄文,张磊,余世春. 枇杷叶三萜酸的抗炎作用[J]. 安徽医科大学学报,2007(2):174-178.GE Jinfang,LI Jun,YAO Hongwei,HU Chengmu,PENG Lei,JIN Yong,LÜ Xiongwen,ZHANG Lei,YU Shichun. Anti-inflammatory effects of triterpenoid acid in loquat leaves[J].Acta Universitatis Medicinalis Anhui,2007(2):174-178.
[9]CHA D S,SHIN T Y,EUN J S,KIM D K,JEON H L.Anti-metastatic properties of the leaves of Eriobotrya japonica[J]. Archives of Pharmacal Research,2011,34(3):425-436.
[10]UTO T,SAKAMOTO A,TUNG N H,FUJIKI T,KISHIHARA K,OISO S,KARIYAZONO H,MORINAGA O,SHOYAM Y.Anti-proliferative activities and apoptosis induction by triterpenes derived from Eriobotrya japonica in human leukemia cell lines[J]. International Journal of Molecular Sciences,2013,14(2):4106-4120.
[11]DE TOMMASI N,DE SIMONE F,CIRINO G,CICALA C,PIZZA C. Hypoglycemic effects of sesquiterpene glycosides and polyhydroxylated triterpenoids of Eriobotrya japonica[J].Planta Medica,1991,57(5):414-416.
[12]SU W B,JING Y,LIN S K,YUE Z,...,LIU Z H.Polyploidy underlies co-option and diversification of biosynthetic triterpene pathways in the apple tribe[J].Proceedings of the National Academy of Sciences of the United States of America,2021,118(20):e2101767118.DOI:10.1073/PNAS.2101767118.
[13]邢雅丽,毕良武,赵振东,韩路路,夏田娟.中心复合设计法优化熊果酸和齐墩果酸的HPLC 测试条件[J].林产化学与工业,2014,34(4):37-41.XING Yali,BI Liangwu,ZHAO Zhendong,HAN Lulu,XIA Tianjuan. Optimization of the HPLC condition of ursolic acid and oleanolic acid by Central Composite Design[J]. Chemistry and Industry of Forest Products,2014,34(4):37-41.
[14]陈亚楠,毛运芝,冉慧,刘世尧.不同地方品种皱皮木瓜有机酸检测与差异分析[J].果树学报,2019,36(9):1171-11-84.CHEN Yanan,MAO Yunzhi,RAN Hui,LIU Shiyao. Detection and differential analysis of fruit organic acids among different local wrinkled papaya varieties(Chaenomeles speciosa)by GCMS[J].Journal of Fruit Science,2019,36(9):1171-11-84.
[15]尚姝,方方,刘园.高效液相色谱法测定枇杷润喉糖中11 个成分的含量[J].药学与临床研究,2022,30(01):31-34.SHANG Shu,FANG Fang,LIU Yuan.Determination of 11 main components in Loquat Throat Candy by High Performance Liquid Chromatography[J]. Pharmaceutical and Clinical Research,2022,30(01):31-34.
[16]国家药典委员会.中华人民共和国药典(一部)[M].北京:中国医药科技出版社,2020.National Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China,(Part I) [M]. Beijing:China Medical Science and Technology Press,2020.
[17]韩少奇.枇杷叶质量评价的研究[D].广州:广东药学院,2011.HAN Shaoqi. Studies on the quality assessment of Eriobotryae Folium[D]. Guangzhou:Guangdong Pharmaceutical University,2011.
[18]TANIGUCHI S,IMAYOSHI Y,KOBAYASHI E,TAKAMATSU Y,ITO H,HATANO T,SAKAGAMI H,TOKUDA H,NISHINO H,SUGITA D,S SHIMURA,YOSHIDA T. Production of bioactive triterpenes by Eriobotrya japonica Calli[J].Phytochemistry,2002,59(3):315-323.
[19]RODRIGUEZ-RODRIGUEZ R,PERONA J S,HERRERA M D,RUIZ-GUTIERREZ V.Triterpenic compounds from‘Orujo’olive oil elicit vasorelaxation in aorta from spontaneously hypertensive rats[J].Journal of Agricultural&Food Chemistry,2006,54(6):2096.
[20]MIZUSHINA Y,IKUTAA,ENDOH K,OSHIGE M,KASAI N,KAMIYA K,SATAKE T,TAKAZAWA H,MORITA H,TOMIYASU H,YOSHIDA H,SUGAWARA F,SAKAGUCHI K.Inhibition of DNA polymerases and DNA topoisomerase II by triterpenes produced by plant callus[J]. Biochemical & Biophysical Research Communications,2003,305(2):365-373.
[21]LEE M S,CHA E Y,THUONG P T,KIM J Y,AHN M S,SUL J Y. Down-regulation of human epidermal growth factor receptor 2/neu oncogene by corosolic acid induces cell cycle arrest and apoptosis in NCI-N87 human gastric cancer cells[J].Biological&Pharmaceutical Bulletin,2010,33(6):931-937.
[22]YAMAGUCHI Y,YAMADA K,YOSHIKAWA N,NAKAMURA K,HAGINAKA J,KUNITOMO M.Corosolic acid prevents oxidative stress,inflammation and hypertension in SHR/NDmcrcp rats,a model of metabolic syndrome[J].Life Sciences,2006,79(26):2474.
[23]RYU S Y,OAK M H,YOON S K,CHO D I,YOO G S,KIM T S,Kim K M. Anti-allergic and anti-inflammatory triterpenes from the herb of Prunella vulgaris[J]. Planta Medica,2000,66(4):358-360.
[24]SRIDHAR C,RAO K V,SUBBARAJU G V. Flavonoids,triterpenoids and a lignan from Vitex altissima[J]. Phytochemistry,2005,66(14):1707-1712.