枇杷(Eriobotrya japonica Lindl.)属蔷薇科(Rosaceae)苹果亚科(Maloideae)枇杷属(Eriobotrya)植物,原产于我国,常绿乔木,秋萌冬花,春末夏初成熟,是最早开始上市的水果之一[1]。我国枇杷栽培总面积约17 万hm2,年产量约100 万t,目前是世界上最大的枇杷生产国[2]。果肉酸甜适口,且具有润肺止咳、清热解毒等功效,制成的枇杷膏还可以治疗痤疮[3]。叶片也具有极高的药用价值,是难得的药食两用水果[4]。如用枇杷叶治疗糖尿病和皮肤病,并用其乙醇提取物来治疗炎症等[5]。因此,枇杷叶片具有重要的开发利用价值。
近年来,随着枇杷栽培面积的不断扩大,对枇杷病害的发生也越发重视,尤其是叶片病害,种类繁多,常见的有灰斑病、斑点病、角斑病等,这三种病害又统称为枇杷叶斑病[6]。这些病害导致树势早衰,影响果实产量和品质,成为限制枇杷产业快速发展的主要因素。因此,对叶斑病抗性机制进行研究,筛选抗叶斑病的枇杷种质,是枇杷产业发展的重要保障。
叶斑病是枇杷生产中一类严重的侵染性病害[7],其抗性可以作为枇杷遗传育种中的重要抗性指标之一。枇杷叶斑病的抗性表现为较复杂的数量性状遗传[8],其杂种后代抗叶斑病的强弱表现有母性遗传的趋势[9]。李金萍等[10]调查北京地区主要栽培的南方果树的病害发病情况,在确诊的11种病害中,由枇杷拟盘多毛孢(Pestalotiopsis eriobotrifolia)引起的灰斑病是危害最为严重的一种。该病害一年进行多次侵染,每个时期都会有发生,危害叶片,最终导致叶片僵化变小、易碎,造成早期大量落叶,影响新梢的生长,还能危害花和果实,造成花果的腐烂,导致枇杷产量严重下滑[11]。有关灰斑病的发病规律以及防治方法,前人做了大量的研究工作[12-14]。前人在多种作物(苹果、柑橘、杧果等)中研究发现植株的形态结构特征,包括叶片形态、气孔形态、结构和密度,叶片表皮覆盖物,叶片解剖结构等与抗病性相关[15-16],但对枇杷叶片的显微结构、气孔形态学指数与枇杷叶斑病抗性关系的研究还较少。因此,笔者在本研究中对11 份不同抗性的枇杷属植物叶片结构与叶斑病抗性的相关性进行了研究,旨在为枇杷抗性育种和合理利用野生枇杷资源提供理论依据。
1 材料和方法
1.1 试验材料
试验材料均采自华南农业大学枇杷属种质资源圃,针对收集保存的52份枇杷种质,包括15个枇杷野生种和37 个普通枇杷品种,2019—2021 年,进行了连续两年的田间调查评价和室内接种鉴定。其中田间调查评价按照《枇杷种质资源描述规范和数据标准》的分级标准[17],采用目测法和Photoshop 软件法[18]连续2年对上述52份枇杷种质进行叶斑病发病情况调查,枇杷叶斑病的病症对照《枇杷病虫害诊治图谱》进行识别[19],每株按照东南西北中5个方向进行调查,每个方向从上至下调查10 枚叶片,抗病性通过病情指数来评价。室内接种鉴定采用针刺法进行,具体操作方法参考文献[4]。最终,筛选出高抗种质3 份[齿叶枇杷(E. serrate)、香花枇杷(E. fragrans)、白梨]、抗病种质4份[薄叶枇杷(E.fulvicoma Chun&Liao)、台湾枇杷恒春变型(E.deflexa f.koshunensis)、茂木、白茂木]、感病种质2 份[广西枇杷(E.kwangsiensis)、大红袍]和高感种质2 份[大花枇杷(E.cavaleriei)、光荣本]共11份枇杷材料;叶片采集遵循新梢从上往下数的第4~6叶位成熟叶片作为试验材料。
1.2 叶片气孔形态观察
于晴天10:00左右进行叶片采集,从枇杷资源圃采集的叶片样品用冰盒保鲜带回实验室,用指甲油涂抹,待干后撕下,置于Olympus 荧光显微镜(20×)下观测叶片气孔的密度和大小,包括气孔长度和宽度、保卫细胞长度和宽度等数据指标[20]。每份材料采集新梢从上往下数的第4~6叶位成熟叶片6枚,每个叶片观察5个视野,计算气孔密度;同时随机测量10 个气孔,测量气孔长、宽度,保卫细胞长、短轴等指标,重复3次,取平均值。
1.3 叶片显微结构观察
各枇杷材料采集6 枚成熟叶片进行石蜡切片,切取每叶片主脉两侧0.5 cm×0.5 cm 正方形小方块[21],置于FAA固定液中固定24 h,采用常规石蜡切片方法进行切片,均使用番红-固绿对染的方法,经过脱水、透明、浸蜡、包埋、修蜡、切片、脱蜡、染色、封片等步骤制成永久装片,具体参考温国等[22]的方法。在Olympus 荧光显微镜(20×)下观察叶片切片结构,利用Image J软件测量,并记录叶片厚度、栅栏组织厚度、海绵组织厚度、上下表皮细胞厚度、上下角质层厚度、紧密度和疏松度等数据指标,每片叶观察12个视野,每个视野读取3个观察值,每项计算其平均数及偏差,叶片组织细胞结构紧密度(CTR)和叶片组织细胞结构疏松度(SR)按下面公式计算:
CTR(%)=栅栏组织厚度/叶片厚度×100;SR(%)=海绵组织厚度/叶片厚度×100[22-23]。
1.4 数据处理
采用Excel 进行数据处理,利用IBM SPSS Statistics 25.0 软件的方差分析和邓肯氏多重比较方法进行统计分析和相关性分析(Pearson法)。
2 结果与分析
2.1 叶片气孔与抗病性的关系
由表1 可知,11 份枇杷种质叶片下表皮之间的气孔密度有显著性差异,尤其是抗病种质与感病种质间的差异为极显著,气孔密度越大越不抗病,气孔密度越小,抗性越强。在11 份种质中,大花枇杷的气孔密度最大,达到887.47个·mm-2,香花枇杷最小,平均为249.33个·mm-2;气孔密度从大到小排序为大花枇杷>大红袍>光荣本>广西枇杷>白茂木>茂木>薄叶枇杷>台湾枇杷恒春变型>白梨>齿叶枇杷>香花枇杷。
表1 11 份枇杷种质叶片的气孔形态学指数
Table 1 Leaf stomatal morphological index of 11 loquat germplasm
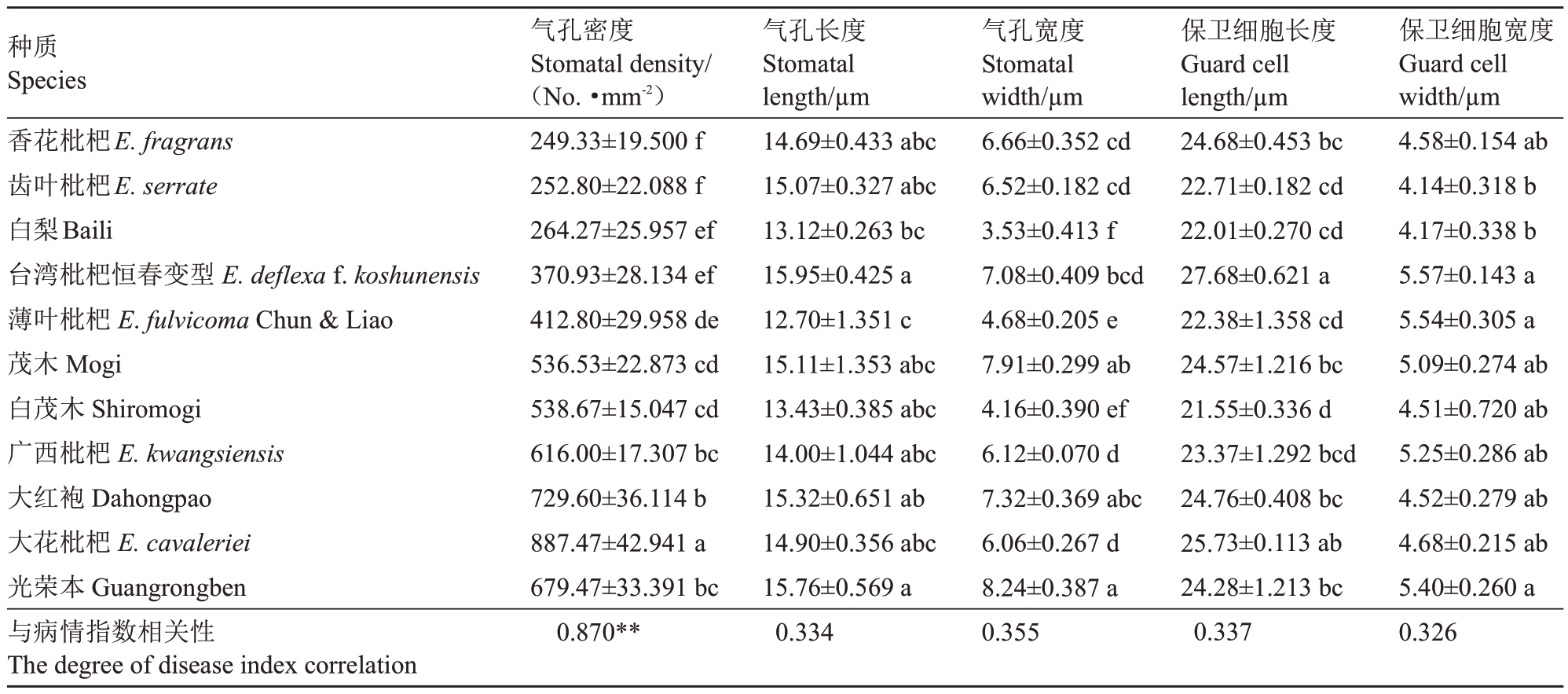
注:不同小写字母表示差异显著p<0.05。相关性数据均通过Pearson 法进行相关分析,**.表示极显著相关。下同。
Note:Different small letters mean significant difference at p<0.05.The correlation data were analyzed by pearson method,**.Extremly significant correlation.The same below.
种质Species香花枇杷E.fragrans齿叶枇杷E.serrate白梨Baili台湾枇杷恒春变型E.deflexa f.koshunensis薄叶枇杷E.fulvicoma Chun&Liao茂木Mogi白茂木Shiromogi广西枇杷E.kwangsiensis大红袍Dahongpao大花枇杷E.cavaleriei光荣本Guangrongben与病情指数相关性The degree of disease index correlation气孔密度Stomatal density/(No.·mm-2)249.33±19.500 f 252.80±22.088 f 264.27±25.957 ef 370.93±28.134 ef 412.80±29.958 de 536.53±22.873 cd 538.67±15.047 cd 616.00±17.307 bc 729.60±36.114 b 887.47±42.941 a 679.47±33.391 bc 0.870**气孔长度Stomatal length/µm 14.69±0.433 abc 15.07±0.327 abc 13.12±0.263 bc 15.95±0.425 a 12.70±1.351 c 15.11±1.353 abc 13.43±0.385 abc 14.00±1.044 abc 15.32±0.651 ab 14.90±0.356 abc 15.76±0.569 a 0.334气孔宽度Stomatal width/µm 6.66±0.352 cd 6.52±0.182 cd 3.53±0.413 f 7.08±0.409 bcd 4.68±0.205 e 7.91±0.299 ab 4.16±0.390 ef 6.12±0.070 d 7.32±0.369 abc 6.06±0.267 d 8.24±0.387 a 0.355保卫细胞长度Guard cell length/µm 24.68±0.453 bc 22.71±0.182 cd 22.01±0.270 cd 27.68±0.621 a 22.38±1.358 cd 24.57±1.216 bc 21.55±0.336 d 23.37±1.292 bcd 24.76±0.408 bc 25.73±0.113 ab 24.28±1.213 bc 0.337保卫细胞宽度Guard cell width/µm 4.58±0.154 ab 4.14±0.318 b 4.17±0.338 b 5.57±0.143 a 5.54±0.305 a 5.09±0.274 ab 4.51±0.720 ab 5.25±0.286 ab 4.52±0.279 ab 4.68±0.215 ab 5.40±0.260 a 0.326
相关性分析表明,气孔密度与病情指数呈极显著正相关,相关系数为0.870,即气孔密度越大,抗病性越弱。而气孔长度、气孔宽度、保卫细胞长度和保卫细胞宽度与抗病性并无明显的相关性,且这些指标在各种质间的表现并无显著的差异,相关系数分别为0.334、0.355、0.337和0.326。
2.2 叶片显微结构与抗病性的关系
从图1 可以看出,11 份枇杷种质的叶片组织结构基本一致,皆分为上角质层、上表皮细胞、栅栏组织、海绵组织、下表皮细胞、下角质层6个部分,栅栏组织和海绵组织分化明显。栅栏组织是由多层柱状细胞组成,每层细胞排列整齐、紧密,而海绵组织细胞排列的形状并不规则。显微观察叶片横截面结果如表2显示,供试的11个枇杷种质间的叶片厚度、栅栏组织厚度、海绵组织厚度、上表皮厚度、下表皮厚度、上角质层厚度、下角质层厚度均存在差异。11份枇杷种质的叶片厚度大小排列为光荣本>大花枇杷>大红袍>广西枇杷>白茂木>台湾枇杷恒春变型>薄叶枇杷>茂木>白梨>齿叶枇杷>香花枇杷,叶片厚度与病情指数的相关系数为0.964,呈极显著正相关,即叶片厚度越厚,病情指数越大,抗病性就越弱。海绵组织厚度与抗病性呈显著正相关,相关系数为0.683;栅栏组织厚度也与抗病性呈正相关,但相关性不明显,相关系数仅有0.486。
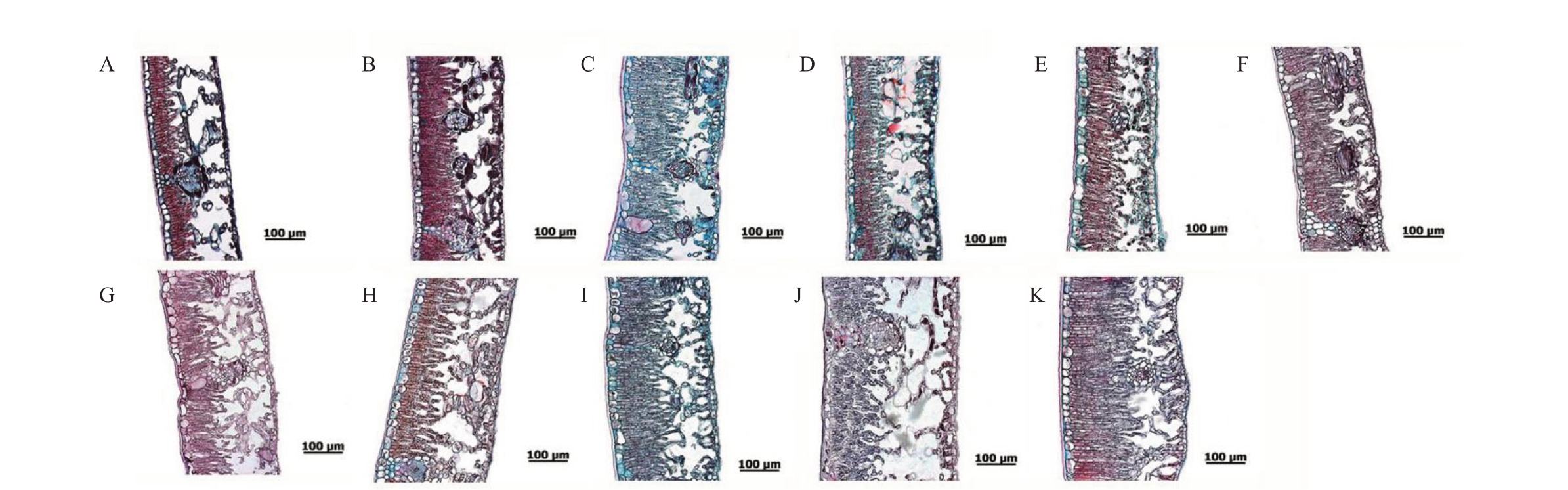
图1 11 份枇杷种质叶片显微结构比较
Fig.1 Comparison of Leaf Microstructure of 11 Loquat Germplasm
A.香花枇杷;B.齿叶枇杷;C.白梨;D.台湾枇杷恒春变型;E.薄叶枇杷;F.茂木;G.白茂木;H.广西枇杷;I.大红袍;J.大花枇杷;K.光荣本。
A.E.fragrans;B.E.serrate;C.Baili;D.E.deflexa f.koshunensis;E.E.fulvicoma Chun&Liao;F.Mogi;G.Shiromogi;H.E.kwangsiensis;I.Dahongpao;J.E.cavaleriei;K.Guangrongben.
表2 11 份枇杷种质叶片解剖结构的比较
Table 2 Comparison of leaf anatomical structures of 11 loquat germplasm
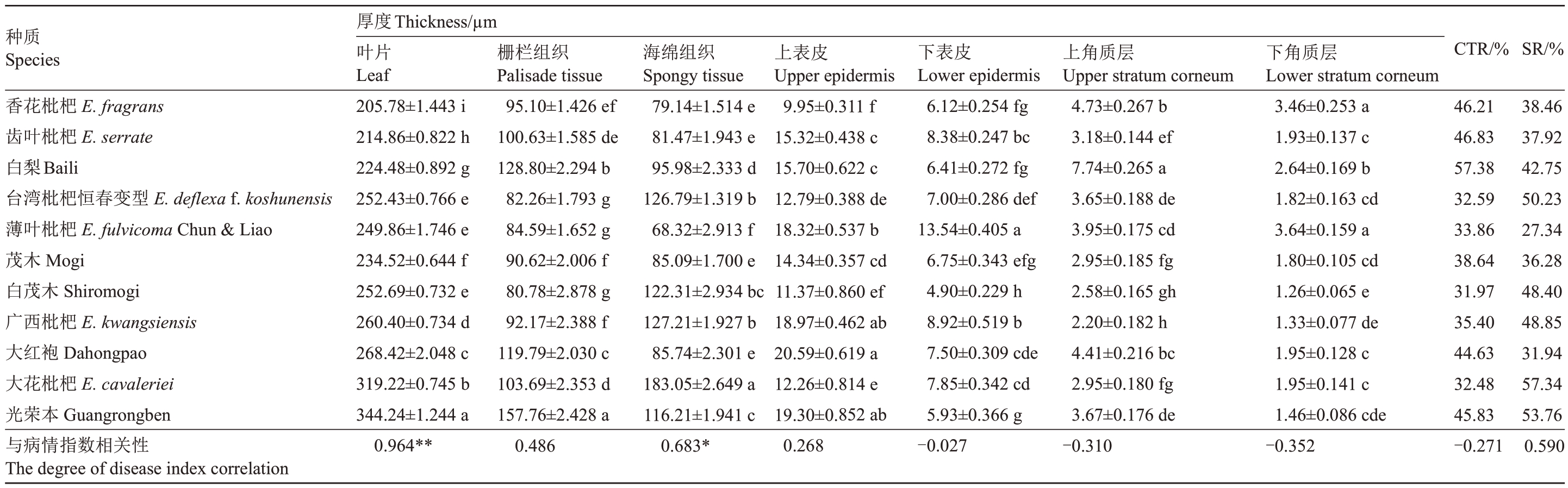
SR/%38.46 37.92 42.75 50.23 27.34 36.28 48.40 48.85 31.94 57.34 53.76 0.590 CTR/%46.21 46.83 57.38 32.59 33.86 38.64 31.97 35.40 44.63 32.48 45.83-0.271质下Lower stratumcorneum角层3.46±0.253a 1.93±0.137c 2.64±0.169b 1.82±0.163cd 3.64±0.159a 1.80±0.105cd 1.26±0.065e 1.33±0.077de 1.95±0.128c 1.95±0.141c 1.46±0.086cde-0.352角4.73±0.267b 7.74±0.265a层质3.18±0.144ef 3.65±0.188de 3.95±0.175cd 2.95±0.185fg 2.58±0.165gh 2.20±0.182h 4.41±0.216bc 2.95±0.180fg 3.67±0.176de-0.310下Lower epidermis表8.38±0.247bc皮6.12±0.254fg 6.41±0.272fg 7.00±0.286def 6.75±0.343efg 13.54±0.405 a 4.90±0.229h 8.92±0.519b 7.50±0.309cde 7.85±0.342cd 5.93±0.366g-0.027皮表上Upper epidermis 9.95±0.311f 15.32±0.438 c 15.70±0.622 c 12.79±0.388 de 18.32±0.537 b 14.34±0.357 cd 11.37±0.860 ef 18.97±0.462 ab 20.59±0.619 a 12.26±0.814 e 19.30±0.852 ab 0.268海Spongytissue织组上Upper stratumcorneum绵79.14±1.514 e 81.47±1.943 e 95.98±2.333 d 126.79±1.319b 68.32±2.913 f 85.09±1.700 e 122.31±2.934bc 127.21±1.927b 85.74±2.301 e 183.05±2.649a 116.21±1.941c 0.683*织组栏95.10±1.426 ef 82.26±1.793 g 84.59±1.652 g 90.62±2.006 f 80.78±2.878 g 92.17±2.388 f 0.486 Thickness/µm栅Palisadetissue 100.63±1.585de 128.80±2.294b 119.79±2.030c 103.69±2.353d 157.76±2.428a度片0.964**厚叶Leaf 205.78±1.443i 214.86±0.822h 224.48±0.892g 252.43±0.766e 249.86±1.746e 234.52±0.644f 252.69±0.732e 260.40±0.734d 268.42±2.048c 319.22±0.745b 344.24±1.244a E.fragrans E.serrate E.deflexa f.koshunensis Liao型性变关春相Baili恒E.fulvicoma Chun&E.kwangsiensis E.cavaleriei数种Species杷杷杷杷Shiromogi杷Dahongpao杷Guangrongben指枇枇枇枇Mogi木枇袍枇本情质花叶梨湾叶木茂西红花荣病香齿白台薄茂白广大大光与The degree of diseaseindexcorrelation
从叶片结构疏松度(SR)来看,高抗种质(齿叶枇杷、香花枇杷和白梨)的叶片结构疏松度(SR)小于2 个高感种质(大花枇杷和光荣本),抗病种质和感病种质的SR 平均值分别为40.20%和47.97%,说明叶片中海绵组织比重较大,叶片结构相对疏松的枇杷种质较易感病。11份枇杷种质9项解剖结构指标与抗病性关联度强弱排序依次为叶片厚度、海绵组织厚度、栅栏组织厚度、下角质层厚度、上角质层厚度、CTR、上表皮厚度、SR、下表皮厚度,其中上角质层厚度、下角质层厚度和CTR 与病情指数呈负相关,相关系数分别为-0.310、-0.352、-0.271(p<0.05)。
3 讨 论
3.1 叶片气孔与叶斑病抗性的关系
气孔是植物与环境进行气体和水分交换的重要生理结构[24],对于从气孔入侵的病原菌来说,植物表皮气孔的数量、形态结构以及开闭状态等与抗病性是密切相关的。Wang 等[25]对感病品种纽荷尔与抗溃疡病品种宁波釐柑的叶片结构进行比较,发现纽荷尔的气孔密度显著大于宁波釐柑。温寿星等[26]研究发现,柑橘叶片中抗溃疡病品种的气孔密度显著小于感病品种。本研究结果表明,枇杷叶气孔密度与病情指数极显著相关,相关系数达到了0.870,表现出极显著相关,但气孔宽度、气孔长度、保卫细胞长度和宽度与病情指数相关性不显著。抗病种质的气孔密度均小于感病种质,气孔密度大为外界病原菌提供了更多的通道进入寄主植物,病原菌成功侵染的机会就越大[27],推测易感病枇杷种质在气孔密度方面比抗病种质高,更有利于病菌的侵入,该结果与郑伟等[13]、潘贞珍等[14]和温寿星等[26]分别在苹果和柑橘上的研究结果相一致。但在桉树[28]、华山松[29]和葡萄[30]上的研究结果却认为叶下表皮气孔密度与其抗病性无显著相关性,这可能与研究物种间的差异有关。
3.2 叶片结构与叶斑病抗性的关系
研究人员对多种作物的叶片解剖结构及其与抗病性的关系进行了研究,但对枇杷种质叶片解剖结构与枇杷叶斑病抗性关系的研究较为少见。栅栏组织发达被认为是植物抗逆性叶片的结构特征[31]。冯丽贞等[28]研究认为,叶片厚度越大、栅栏组织排列整齐以及层数相对较多的桉树品种,抗焦枯病的能力更强。李淼等[32]研究认为叶片厚度越大、表皮越厚的猕猴桃品种,抗溃疡病的能力就越强。周之珞[33]研究表明,叶片厚度、结构和海绵组织厚度与抗溃疡病具有一定的相关性,叶片越厚、结构排列整齐紧密的柑橘品种,对溃疡病菌的抗性更强。Ebrahim等[15]认为,叶片厚度与杧果畸形病存在负相关的关系。本试验对叶片结构与枇杷叶斑病抗性作了初步研究,从11份枇杷种质的病情指数和叶片显微结构可以看出,不同种质间叶片的显微结构特征差异显著,高抗种质香花枇杷、齿叶枇杷和白梨的叶片结构紧密度均高于高感种质(大花枇杷和光荣本),而且叶片CTR与病情指数呈负相关(r=-0.271),同时,上角质层厚度(r=-0.310)、下角质层厚度(r=-0.352)也与病情指数呈负相关,且高抗种质香花枇杷和白梨的上下角质层厚度均高于高感种质大花枇杷和光荣本,这说明叶片CTR和角质层厚度与叶斑病抗性有一定关系,而高感种质的叶片厚度、海绵组织厚度、叶片结构疏松度高于高抗种质,这说明枇杷种质叶片厚度、海绵组织厚度、叶片结构疏松度与叶斑病抗性有密切关系,叶片厚度越大、海绵组织厚度越厚、叶片结构越疏松的枇杷种质越容易受到叶斑病的侵染,其中叶片厚度与病情指数相关系数最大,相关系数达到了0.964,与叶斑病抗性表现出极显著负相关;海绵组织厚度与病情指数也表现出显著的相关性,相关系数为0.683。该结果与周之珞[33]认为叶片厚度越大越抗溃疡病的研究结果相反,但与张戈壁等[34]认为叶片厚度与病情指数有关系,叶片海绵组织厚度越大越不抗病的结论一致。而李敏[35]在对罗浮金柑、新生系3 号椪柑、纽荷尔脐橙、星露比葡萄柚4个品种的叶片厚度及海绵组织厚度的研究中并未发现其厚度与抗溃疡病之间存在明显的相关性。这可能与寄主植物的不同或病害种类的不同而引起的差异有关。
4 结 论
研究结果表明:叶片厚度和气孔密度与抗病性呈显著正相关,其中叶片厚度与抗病性相关系数最大(0.964),海绵组织厚度与抗病性也表现出显著相关;叶片厚度、气孔密度和海绵组织厚度可作为枇杷抗病性鉴定的辅助指标。
[1] 邱武陵,章恢志.中国果树志:龙眼·枇杷卷[M].北京:中国林业出版社,1996.QIU Wuling,ZHANG Huizhi.Annals of fruit trees in China:Loquat·longan[M].Beijing:China Forestry Press,1996.
[2] HUANG X,WANG H K,QU S C,LUO W J,GAO Z H.Using artificial neural network in predicting the key fruit quality of loquat[J].Food Science&Nutrition,2021,9(3):1780-1791.
[3] SHAN Y X,DENG C J,HU W S,CHEN J W,CHEN X P,ZHENG S Q,QIN Q P.First insight into diversity of leaf color of loquat(Eribotrya)and its potential value on taxonomy[J].Genetic Resources and Crop Evolution,2019,66(1):143-163.
[4] 姜琬.重庆枇杷灰斑病菌鉴定、抗性评价及防控研究[D].重庆:西南大学,2014.JIANG Wan.Study on identification,resistance evaluation and control of Pestalotiopsis eriobotrifolia in Chongqing[D].Chongqing:Southwest University,2014.
[5] 张丹华.枇杷主要叶斑病病原真菌鉴定及多样性分析[D].重庆:西南大学,2017.ZHANG Danhua.Identification of pathogens causing main leaf spot diseases on loquat and analysis of fungal diversity[D].Chongqing:Southwest University,2017.
[6] 江旭升,杨勇胜,李庆宏,魏椿,陈树红,罗国伟.贵阳地区枇杷种质叶斑病的抗性调查[J].贵州农业科学,2016,44(8):39-44.JIANG Xusheng,YANG Yongsheng,LI Qinghong,WEI Chun,CHEN Shuhong,LUO Guowei.Resistance investigation of loquat leaf spot disease in Guiyang area[J].Guizhou Agricultural Science,2016,44(8):39-44.
[7] 杨秀娟,陈福如,占志雄,阮宏椿.叶斑病胁迫对枇杷叶片活性氧代谢和叶绿素含量的影响[J].江西农业大学学报,2005,27(5):667-669.YANG Xiujuan,CHEN Furu,ZHAN Zhixiong,RUAN Hongchun.Effects of leaf spot diseases stress on activated oxygen metabolism and chlorophyll content in loquat leaves[J].Acta Agriculturae Universitatis Jiangxiensis,2005,27(5):667-669.
[8] KIM J,HARIKRISHNAN R,KIM M,JANG I,KIM D,HONG S,BALASUNDARAM C,HEO M.Enhancement of Eriobotrya japonica extracts on non-specific immune response and disease resistance in kelp grouper Epinephelus bruneus against Vibrio carchariae[J].Fish&Shellfish Immunology,2011,31(6):1193-1200.
[9] 郑少泉.中国枇杷育种成就与展望[C].杭州:全国枇杷学术年会.2005.ZHENG Shaoquan.The achievement and advance of loquat breeding in China[C].Hangzhou:National Loquat Academic Annual Meeting.2005.
[10] 李金萍,郭喜红,侯峥嵘,王璐,解晓军,尹哲.北京地区南方果树病害调查初报[J].中国植保导刊,2015,35(8):21-25.LI Jinping,GUO Xihong,HOU Zhengrong,WANG Lu,XIE Xiaojun,YIN Zhe.Preliminary investigation on southern fruit trees diseases in Beijing[J].China Plant Protection,2015,35(8):21-25.
[11] 周蓓.若干枇杷属植物种及种间杂种叶斑病抗性鉴定、天牛危害与根系调查[D].广州:华南农业大学,2017.ZHOU Bei.Investigationon leaf spot disease with its identification of resistance,beetles damage and rootsystemin several Eriobotrya species and interspecific hybrids[D].Guangzhou:South China Agricultural University,2017.
[12] 李敏,段硕,李中安,周彦,周常勇,谭锦,彭耀武.叶片微形态结构特征与柑橘溃疡病抗性的关系[J].中国南方果树,2013,42(2):1-5.LI Min,DUAN Shuo,LI Zhong’an,ZHOU Yan,ZHOU Changyong,TAN Jin,PENG Yaowu.Analysis of relationship between citrus canker resistance and leaf micro-morphological characteristics[J].South China Fruits,2013,42(2):1-5.
[13] 郑伟,吴亚维,王彬,宋莎,罗昌国.苹果叶片结构与白粉病抗性的相关性初步研究[J].西南农业学报,2017,30(9):2108-2112.ZHENG Wei,WU Yawei,WANG Bin,SONG Sha,LUO Changguo.Preliminary study on correlation between leaf structure and powdery mildew resistance in apple[J].Southwest China Journal of Agricultural Sciences,2017,30(9):2108-2112.
[14] 潘贞珍,黄运鹏,黄桂香,杨翠红,何新华.3 个柑橘品种叶片结构和生化物质与柑橘溃疡病抗性的相关性研究[J].中国果树,2020(4):31-36.PAN Zhenzhen,HUANG Yunpeng,HUANG Guixiang,YANG Cuihong,HE Xinhua.Correlation research of leaf structure and biochemical substances of three varieties of citrus with the resistance to citrus canker[J].China Fruits,2020(4):31-36.
[15] EBRAHIM S,USHA K,SINGH B.Plant architectural traits and their role in defense mechanism against malformation in mango(Mangifera Indica L.)[J].Scientia Horticulturae,2012,139:25-31.
[16] MAMRUTHA H M,MOGILI T,LAKSHMI K J,RAMA N,KOSMA D,KUMAR M U,JENKS M A,NATARAJA K N.Leaf cuticular wax amount and crystal morphology regulate post-harvest water loss in mulberry (Morus species)[J].Plant Physiology and Biochemistry,2010,48(8):690-696.
[17] 郑少泉.枇杷种质资源描述规范和数据标准[M].北京:中国农业出版社,2006.ZHENG Shaoquan.Descriptors and data standard for loquat(Eriobotrya spp.)[M].Beijing:China Agriculture Publishing House,2006.
[18] 崔华威,杨艳丽,黎敬涛,罗文富,苗爱敏,胡振兴,韩小女.一种基于Photoshop 的叶片相对病斑面积快速测定方法[J].安徽农业科学,2009,37(22):10760-10762.CUI Huawei,YANG Yanli,LI Jingtao,LUO Wenfu,MIAO Aimin,HU Zhenxing,HAN Xiaonü.A faster method for measuring relative lesion area on leaves based on software photoshop[J].Journal of Anhui Agricultural Sciences,2009,37(22):10760-10762.
[19] 陈福如,陈元洪,翁启勇.枇杷病虫害诊治[M].福州:福建科学技术出版社,2010.CHEN Furu,CHEN Yuanhong,WENG Qiyong.Loquat pest diagnosis[M].Fuzhou:Fujian Science & Technology Publishing House,2010.
[20] 范晓懂.大气CO2倍增下水分亏缺对青椒生长、气孔特征及生理生化过程的影响[D].邯郸:河北工程大学,2021.FAN Xiaodong.Effects of water deficit on growth,stomatal traits and of physiological and biochemical processes of green peppers under doubling CO2 concentrations[D].Handan:Hebei University of Engineering,2021.
[21] DUAN Y D,HAO S X,LUO R,LU Y F,LI G,ZHANG J,TIAN J,YAO Y C.Antioxidant defense against rust infection in the leaf tissue of Malus crabapple[J].Acta Physiologiae Plantarum,2019,41(5):1-13.
[22] 温国,孙皓浦,党江波,蒋朋飞,王金英,杨垚,郭启高,梁国鲁.多倍体与二倍体枇杷叶片特征及抗旱性初步分析[J].果树学报,2019,36(8):968-979.WEN Guo,SUN Haopu,DANG Jiangbo,JIANG Pengfei,WANG Jinying,YANG Yao,GUO Qigao,LIANG Guolu.A preliminary study on leaf characteristics and drought resistance of polyploid and diploid loquat[J].Journal of Fruit Science,2019,36(8):968-979.
[23] 田丽波,商桑,杨衍,司龙亭,李丹丹.苦瓜叶片结构与白粉病抗性的关系[J].西北植物学报,2013,33(10):2010-2015.TIAN Libo,SHANG Sang,YANG Yan,SI Longting,LI Dandan.Relationship between the leaf structure of bitter melon and resistance to powdery mildew[J].Acta Botanica Boreali-Occide Ntalia Sinica,2013,33(10):2010-2015.
[24] ZINSOU V,WYDRA K,AHOHUENDO B,SCHREIBER L.Leaf waxes of cassava (Manihot esculenta Crantz) in relation to ecozone and resistance to Xanthomonas blight[J].Euphytica,2006,149(1):189-198.
[25] WANG Y,FU X Z,LIU J H,HONG N.Differential structure and physiological response to canker challenge between‘Meiwa’kumquat and‘Newhall’navel orange with contrasting resistance[J].Scientia Horticulturae,2011,128(2):115-123.
[26] 温寿星,黄镜浩,陈瑾,蔡子坚,包榕,张凌媛.叶片结构与柑橘溃疡病抗性的初步研究[J].中国农学通报,2009,25(13):66-69.WEN Shouxing,HUANG Jinghao,CHEN Jin,CAI Zijian,BAO Rong,ZHANG Lingyuan.Preliminary studies on leaves structure in resistant and susceptible cultivars of citrus[J].Chinese Agricultural Science Bulletin,2009,25(13):66-69.
[27] 易龙,夏宜林,赖爱萍,苏华楠,钟八莲.脐橙品种‘赣南早’与‘纽荷尔’对柑橘溃疡病的抗性比较[J].果树学报,2016,33(4):466-472.YI Long,XIA Yilin,LAI Aiping,SU Huanan,ZHONG Balian.A comparative study on resistance between‘Gannanzao’and‘Newhall’navel orange varieties to citrus canker[J].Journal of Fruit Science,2016,33(4):466-472.
[28] 冯丽贞,刘玉宝,郭素枝,黄榕辉,郭文硕.桉树叶片的解剖结构与其对焦枯病抗性的关系[J].电子显微学报,2008,27(3):229-234.FENG Lizhen,LIU Yubao,GUO Suzhi,HUANG Ronghui,GUO Wenshuo.Relationship between the leaf anatomical characteristics of Eucalyptus and its resistance to dieback[J].Journal of Chinese Electron Microscopy Society,2008,27(3):229-234.
[29] 蒙进芳,王曙光,普晓兰.华山松针叶表皮结构与抗疱锈病关系的初步研究[J].中南林学院学报,2006,26(2):43-46.MENG Jinfang,WANG Shuguang,PU Xiaolan.The relation of the blister rust resistance and the structures of the needle epidermis in the Pinus armandii Franch.[J].Journal of Central South Forestry University,2006,26(2):43-46.
[30] 刘天明,李华.葡萄对霜霉病抗性机制初探[J].植物保护,1995,21(6):12-15.LIU Tianming,LI Hua.A preliminary study on the mechanism of resistance of grapevines to Plasmopara viticola[J].Plant Protection,1995,21(6):12-15.
[31] RAJSNEROVA P,KLEM K,HOLUB P,NOVOTNA K,VECEROVA K,KOZACIKOVA M,RIVAS-UBACH A,SARDANS J,MAREK M V,PENUELAS J,URBAN O.Morphological,biochemical and physiological traits of upper and lower canopy leaves of European beech tend to converge with increasing altitude[J].Tree Physiology,2015,35(1):47-60.
[32] 李淼,檀根甲,李瑶,承河元,李珂.猕猴桃品种叶片组织结构与抗溃疡病的关系[J].安徽农业科学,2002,30(5):740-742.LI Miao,TAN Genjia,LI Yao,CHENG Heyuan,LI Ke.Study on the leaf tissue structure of kiwifruit cultivars in relation to bacterial canker disease resistance[J].Journal of Anhui Agricultural Sciences,2002,30(5):740-742.
[33] 周之珞.建阳橘柚在广西引种主要生物学性状评价研究[D].南宁:广西大学,2018.ZHOU Zhiluo.Evaluation main biological characters of Jianyang tangelo introduced in Guangxi[D].Nanning:Guangxi University,2018.
[34] 张戈壁,张素英.脐橙品种溃疡病抗性与叶片生理结构的相关性[J].中国南方果树,2015,44(2):57-59.ZHANG Gebi,ZHANG Suying.Correlation between resistance to canker and leaf physiological structure of Navel orange varieties[J].South China Fruits,2015,44(2):57-59.
[35] 李敏.柑橘抗溃疡病机制的初步研究[D].重庆:西南大学,2013.LI Min.Preliminary research on citrus canker resistance and susceptibility[D].Chongqing:Southwest University,2013.