砂梨[Pyrus pyrifolia(Burm.f.)Nakai]主产于中国、韩国以及日本等国家[1],在我国主栽于长江流域及以南地区[2],成熟早、综合品质优良。翠冠是长江流域的主栽砂梨品种,但果实外观差、果皮粗糙且果锈多[2]。华酥梨果皮平滑有光泽,果点小而疏[3]。蔺经等[4]以华酥为母本、翠冠为父本进行了早熟砂梨新品种的选育,获得了成熟早、品质优且外观好的苏翠1 号,为生产推广和理论研究提供了极好的试材。但是,目前对苏翠1 号及其与父母本的果实比较的系统性研究还未开展。比较3个品种果实的经济性状,挖掘与优良性状形成相关的基因,对苏翠1号大面积生产推广和梨分子辅助育种工作具有重要的理论意义。
果实外观是衡量品种经济价值的重要指标。梨外观品质评价以果皮色泽为主[1]。砂梨成熟时果皮呈现翠绿/黄绿色、褐色或红色。另外,一些中间色梨在成熟时绿/黄绿色果面覆盖不规则果锈[5]。锈斑极大程度降低了梨的果实外观品质。研究表明,在果实快速膨大期,果实的表皮角质膜受力不均匀龟裂,失去保护的表皮细胞产生黄褐色木栓层积累覆盖于外果皮,形成果锈[6-7]。木栓层的主要成分为木栓质,木栓质是一种包括木栓聚酚结构域[suberin poly (phenolic) domain, SPPD]和木栓聚酯结构域[suberin poly (aliphatic) domain, SPAD]的复杂高分子聚合物[8-9],木质素是其主要组成部分[10]。木质素可增加细胞壁厚度和强度,其合成是影响果锈形成的关键因素。组成SPPD的物质主要是羟基化的肉桂酸,通常为阿魏酸、香豆酸和单木质醇,这些物质均来源于苯丙烷代谢途径[8,11],其含量受苯丙氨酸解氨酶(L-phenylalanine ammonia-lyase,PAL)、ω-羟基-亚麻酸O-阿魏酰基转移酶(ω-hydroxypalmitate O-feruloyl transferase,HHT)、4-香豆酸:辅酶A连接酶(4-coum-arate:coenzymeA ligase,4CL)、肉桂酸-4-羟化酶(cinnamate 4-hydroxylase,C4H)、肉桂酰辅酶A 还原酶(cinnamoyl-CoA reductase,CCR)等多种酶的影响[12]。Li等[13]研究发现,PAL使拟南芥维管间纤维和木质部细胞的木质素含量增加,细胞壁增厚。吕照清等[14]以黄花梨(褐色果皮)及其芽变绿黄花梨(绿色果皮)为材料,发现在果实发育的不同时期HHT在褐皮黄花梨中的表达均高于绿黄花梨。4CL通过催化羟基肉桂酰辅酶A脂的合成参与后续木质素和黄酮素的合成[15]。SPAD 是以含氧长链脂肪酸为主的三维聚酯网络,包括ω-羟基脂肪酸、α,ω-双羧基脂肪酸(简称α,ω-二羧酸)、中链含氧脂肪酸、未被取代的脂肪酸以及伯醇[16-17]。木质素、角质和蜡质的合成主要有2条途径:一条途径是C16和C18脂肪酸前体延伸为C20-C32的长链脂肪酸;另一条途径包括转化为脂肪酸衍生物所需的氧合反应。这些关键酶共同调控着木质素、角质和蜡质的合成[18]。黄酮类化合物是植物体内次生代谢的一类多酚代谢产物,多数存在于各种植物组织器官的细胞或表面,品种繁多,一般与糖结合以黄酮苷的形式存在,少部分以游离态存在[19-20]。其亚类化合物根据结构可以划分为黄酮、黄酮醇、黄烷酮、异黄酮、黄烷醇、儿茶素和花青素7 类[10]。黄酮类化合物具有清除自由基、抗炎及抗菌等多种作用[21]。黄酮醇在果树抗干旱、盐胁迫、低温、紫外线胁迫和植物激素胁迫等非生物胁迫中具有重要作用[22],特别是槲皮素和山奈酚具有抑制生长素运输的能力[23]。外源施用槲皮素引起了猕猴桃[24]和苹果[25]的果实硬度和可滴定酸的降低,显著降低了腐坏率和失重率,延缓了果实衰老进程,并提高了果实耐贮性。Mamat 等[26]研究发现,库尔勒香梨粗皮果实果皮中总酚、黄酮和异黄酮含量明显高于正常果实。黄酮类化合物以苯丙烷合成途径的香豆酰辅酶A 为底物,在查耳酮合酶(chalcone synthase,CHS)、查耳酮异构酶(chalcone isomerase,CHI)、黄烷酮3-羟化酶(flavanone 3-hydroxylase,F3H)、二氢黄酮醇还原酶(dihydroflavonol 4-reductase,DFR)、黄酮醇合成酶(flavonol synthase,FLS)、花色素还原酶(anthocyanidin reductase,ANR)等关键酶作用下合成各亚类化合物[20]。
在小麦[27]、番茄[28-30]、蓖麻[31]、玉米[32]、紫薯[33-34]、花生[35-36]等作物上,利用重测序和比较转录组技术比较近缘物种或不同物种及亚种间mRNA序列的差异,挖掘明显受到正向或负向选择的基因,揭示杂种优势形成的遗传机制。梨是高度杂合体,其遗传背景复杂,多数性状是由多基因控制的数量性状,对果实重要经济性状的遗传规律研究及主效基因的挖掘难度较大。Wang等[37]以翠冠和清香为研究对象,通过高通量测序筛选了与褐色果皮颜色形成相关的候选基因。Inoue 等[38]利用分子标记筛选到与绿色果皮性状紧密连锁的标记,并应用于育种生产中,获得了92%的准确率。衡伟等[39]利用mRNA差显法筛选了与砀山酥梨褐皮芽变品种相关的基因。
笔者在本研究中以苏翠1号及其母本华酥和父本翠冠的果实为材料,利用广靶代谢组学结合转录组测序的方法比较了子代和父母本之间与果皮性状有关的差异代谢产物及差异基因,并采用qRT-PCR进行验证,为实际生产工作提供初步的理论依据。
1 材料和方法
1.1 材料
试验于2020 年在江苏省农业科学院梨种质资源圃(南京,32.03°N,118.87°E),北亚热带季风气候,年平均降水量1100~1300 mm)进行。果园土质为黏壤土,亚表土层(20~50 cm)pH值5.8,有机质含量(w,后同)16.77 g·kg-1,碱解氮含量57.51 mg·kg-1,速效磷含量127.62 mg·kg-1,速效钾含量95.89 mg·kg-1。试验品种为6 年生苏翠1 号[Pyrus pyrifolia (Burm.f.) Nakai.‘Sucui 1’]、华酥[Pyrus pyrifolia (Burm.f.)Nakai.‘Huasu’]和翠冠[Pyrus pyrifolia(Burm.f.)Nakai.‘Cuiguan’]。株行距3.0 m×5.0 m,树体长势相对一致。3个品种分别于果实成熟时采样:苏翠1号7 月9 日,华酥7 月18 日,翠冠7 月23 日。每个品种取3株树,于每株树冠外围取3个果实,沿赤道部分用手术刀分离宽度1 cm、厚度0.1~0.2 cm的果皮,用药匙刮去背面果肉部分。每株的3个果皮切碎混合为1 个样,每品种3 份混品为3 组生物学重复,命名为苏翠1 号SP1~SP3、翠冠CP1~CP3 和华酥HP1-HP3,液氮速冻后-80 ℃保存。
1.2 代谢组学分析
样品在液氮中研磨至粉末状,称取100 mg,溶解于1.2 mL 70%(φ)甲醇提取液中充分提取。采用超高效液相色谱(ultra performance liquid chromatography,UPLC)(SHIMADZU Nexera X2, https://www.shimadzu.com.cn/)和串联质谱(tandem mass spectrometry, MS/MS)进行分析。利用MultiaQuant软件对所有物质质谱峰进行峰面积积分,并对其中同一代谢物在不同样本中的质谱出峰进行积分校正[40]。用R 软件(www.r-project.org/)的内置统计prcomp 函数,对数据进行归一化(unit variance scaling,UV)处理。对所有样本进行主成分(principal component analysis,PCA)及聚类分析(cluster analysis),对分组样本进行主成分及正交偏最小二乘法分析(orthogonal partial least squares-discriminant analysis,OPLS-DA)[41-43]。基于OPLS-DA结果,从获得的多变量分析OPLS-DA 模型的变量重要性投影(variable importance in projection,VIP),采 用Fold Change≥2 和Fold Change≤0.5 以及VIP≥1 为阈值,筛选苏翠1 号(SP)与翠冠(CP)和华酥(HP)成熟果实果皮差异代谢产物。用KEGG(kyoto encyclopedia of genes and genomes)数据库注释差异代谢物富集的代谢通路。
1.3 RNA提取、文库构建
采用多糖多酚植物RNA超快提取试剂盒(庄盟生物ZOMANBIO),根据说明书步骤进行果皮样品总RNA 的提取。文库构建和测序工作交由北京诺禾致源科技股份有限公司完成,样品测序平台为Illumina。
1.4 测序质量控制
原始数据经过去除接头(adapterreads)、低质量reads、测序错误率检查后获得高质量的clean reads。用Tophat2(http://tophat.cbcb.umd.edu[44])/Hisat2(http://ccb.jhu.edu/software/hisat2[45])/STAR(http://code.google.com/p/rna-star[46])软件对RNA-Seq 测序数据进行比对分析,参考基因组为http://peargenome.njau.edu.cn[47]。获得的数据用于后续生物信息学分析。
1.5 差异基因及KEGG代谢通路分析
用StringTie 对转录本进行拼接与定量分析,基因表达水平用每百万个读序中的转录本数(fragments per kilobase of transcript sequence per millions base pairs sequenced,FPKM)衡量。利用edgeR对苏翠1 号、华酥和翠冠成熟果实果皮的基因表达水平进行分析。以padj<0.05 为阈值筛选差异表达的基因。采用KOBAS(2.0)[48]对差异表达基因进行KEGG 代谢通路富集分析。
1.6 代谢组和转录组联合分析
基于代谢组学和转录组差异基因分析结果,将落于同一代谢通路的差异基因及差异代谢物同时映射到KEGG 图上,进一步研究基因和代谢物间的关系。
1.7 实时荧光定量PCR验证
采用HiScript®ⅢRT SuperMix for qPCR 试剂盒(诺唯赞Vazyme)进行反转录合成cDNA,控制反应体系中模板RNA 的含量均为1 μg,以使合成的cDNA 间具有平行性。用Primer 3.0 软件设计引物(表1)。内参基因为GAPDH[13]。采用ChamQ Universal SYBR qPCR Master Mix 试剂盒(诺唯赞Vazyme)进行qRT-PCR 反应。扩增程序:50 ℃2 min,95 ℃2 min,94 ℃30 s,60 ℃30 s,72 ℃30 s,40 个循环。熔解曲线分析程序:95 ℃5 s,72 ℃1 min,95 ℃15 s。3 次技术重复,使用2-ΔΔCt法[49]对结果进行分析。
表1 实时荧光定量PCR 引物
Table 1 Oligo nucleotide sequences for qRT-PCR primers
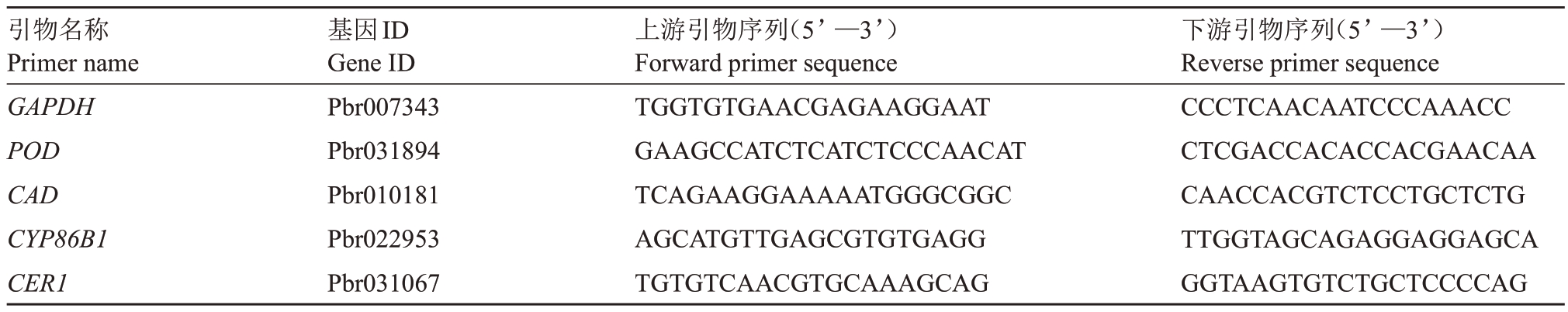
引物名称Primer name GAPDH POD CAD CYP86B1 CER1基因ID Gene ID Pbr007343 Pbr031894 Pbr010181 Pbr022953 Pbr031067上游引物序列(5’—3’)Forward primer sequence TGGTGTGAACGAGAAGGAAT GAAGCCATCTCATCTCCCAACAT TCAGAAGGAAAAATGGGCGGC AGCATGTTGAGCGTGTGAGG TGTGTCAACGTGCAAAGCAG下游引物序列(5’—3’)Reverse primer sequence CCCTCAACAATCCCAAACC CTCGACCACACCACGAACAA CAACCACGTCTCCTGCTCTG TTGGTAGCAGAGGAGGAGCA GGTAAGTGTCTGCTCCCCAG
1.8 数据处理
采用GraphPad Prism 8.0.2 软件进行数据整理,用SPSS 16.0 分析软件进行统计和相关性分析,用one-way ANOVA 方法对每个变量进行Turkey 检验(p<0.05)。
2 结果与分析
2.1 苏翠1号与华酥、翠冠果皮外观比较
在长江流域,苏翠1 号果实于7 月上旬成熟,其母本华酥果实7 月中旬成熟,父本翠冠果实成熟期较苏翠1 号晚14 d 左右,在7 月下旬成熟,分别于每个品种果实成熟时采摘样品。表型观察结果表明,在南京地区,苏翠1号表型上更接近母本华酥,与父本翠冠间产生了明显的表型差异:苏翠1 号果皮呈黄绿色,蜡质平滑,仅少数在梗洼处发生果锈;华酥果皮光滑细腻,呈黄绿色;翠冠果皮粗糙,大面积覆盖褐色果锈(图1)。

图1 苏翠1 号、华酥与翠冠成熟果实
Fig.1 Ripening fruits of Sucui 1,Huasu and Cuiguan
A.华酥(母本);B.苏翠1 号;C.翠冠(父本)。
A.Huasu(female parent);B.Sucui 1;C.Cuiguan(male parent).
2.2 质谱分析体系的建立
采用不同品种果皮混合样本作为质控样本,并在每10 个检测样本中插入1 个质控样本,对不同质控样本总离子流图进行重叠展示分析。结果表明,同一代谢产物在不同样品中及不同时间检测时,其保留时间和峰强度均一致,重叠性高,表明仪器稳定性高,数据重复性好。所得数据可用于进一步分析。对所有样本进行PCA,结果显示,不同品种果皮代谢产物的第一、第二主成分均分离明显,表明样品组间代谢组存在差异。对所有样品数据进行聚类及相关性分析,用皮尔逊相关系数r(pearsons correlation coefficient)作为生物学重复相关性的评估指标。结果表明,苏翠1号、华酥和翠冠不同样品间的相关性系数均大于0.98。这表明组内3个生物学样本的重复性较好,可用于进一步分析。
2.3 差异代谢物的筛选
3个品种果皮共检测到586种物质,基于OPLSDA 及VIP≥1 和Fold change≥2 和Fold change≤0.5,筛选翠冠与华酥及翠冠与苏翠1号间果皮的差异代谢产物。翠冠与华酥成熟期果实的果皮共有210种差异代谢产物,其中次生代谢产物占56%,初生代谢物中脂类占12%,而有机酸、可溶性糖等初级代谢产物占28%。翠冠与苏翠1 号共有156 种差异代谢产物,其中次生代谢产物占64%,初生代谢物中脂类占17%,而有机酸、可溶性糖等初级代谢产物占20%。次生代谢产物主要包括黄酮类、酚酸类及木质素和香豆素类。在翠冠与华酥和翠冠与苏翠1 号中,黄酮类分别占31%和34%,酚酸类占13%和17%,木质素和香豆素类占4%和5%(图2)。
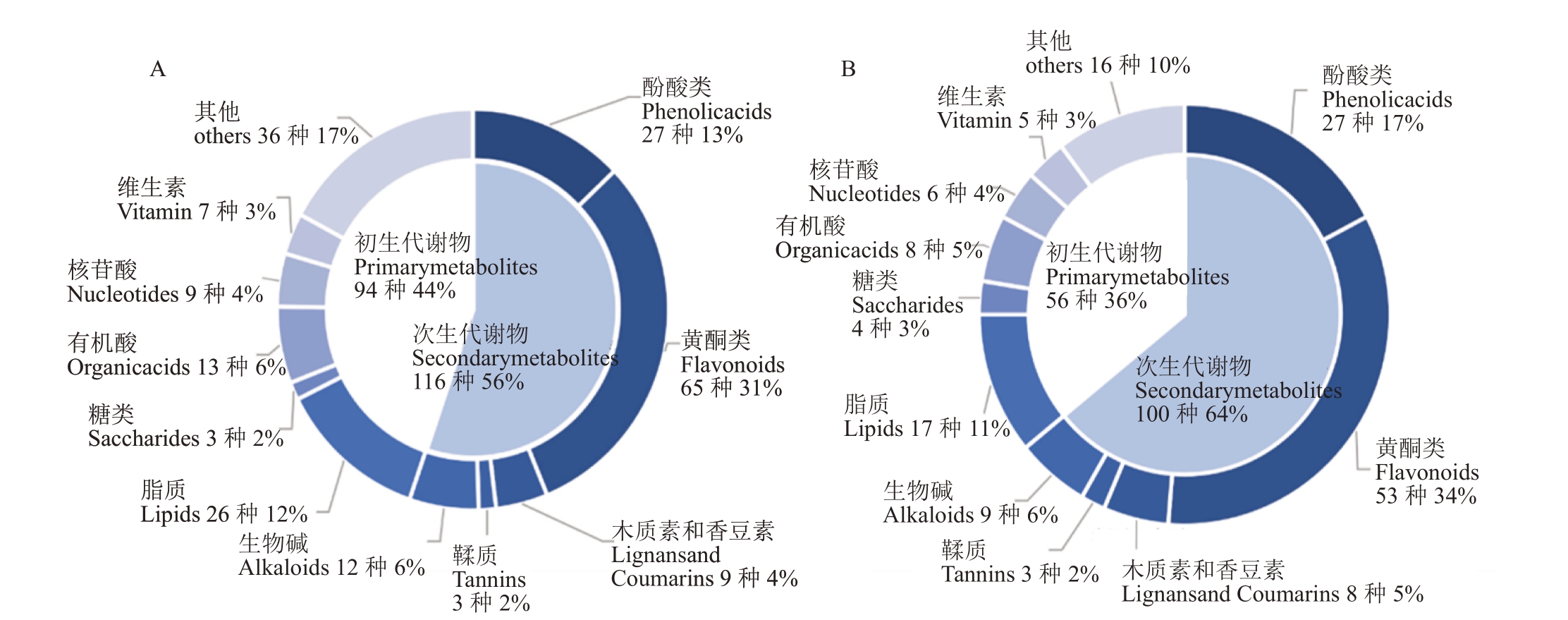
图2 果皮差异代谢产物种类与比例
Fig.2 Species and proportion of differentially expressed metabolites
A.华酥果皮vs 翠冠果皮;B.苏翠1 号果皮vs 翠冠果皮。下同。
A.Pericarp of Huasu(HP)vs Pericarp of Cuiguan(CP);B.Pericarp of Sucui 1(SP)vs Pericarp of Cuiguan(CP).The same below.
对差异代谢产物进一步分析,结果表明,与华酥和苏翠1 号相比,在翠冠果皮中均上调的物质有49种,包括酚酸类12 种,如紫丁香苷(Syringin)、芥子酸吡喃葡萄糖苷(1-O-beta-D-Glucopyranosyl sinapate)等;黄酮类9种,如染料木苷(Genistein)、高圣草酚(Homoeriodictyol)、6-C-己糖基金圣草黄素O-阿魏酰己糖苷(6-C-hexosyl-chrysoeriol O-feruloylhexoside)等;木质素和香豆素类2 种,即东莨菪内酯(Scopoletin)和松脂醇(Pinoresinol);脂质15种,如溶血磷脂酰胆碱19:0(19:0 LysoPC 19:0)等;有机酸类2种,如2,3-二羟基苯甲酸(2,3-Dihydroxybenzoic acid)等;其他还包括生物碱、糖类和鞣质等共9种(表2)。在翠冠表皮中特异下调的物质有29种,包括黄酮类10 种,如乙酰基圣草酚O-己糖苷(Acetyl-eriodictyol O-hexoside);有机酸类2种,如安息香酸(Ethyl protocatechuate)等;木质素及香豆素类2种,即滨蒿內酯(Scoparone)、阿魏酸4-羟基香豆素(O-Feruloyl 4-hydroxylcoumarin);脂质1 种,即单酰甘油酯(酰基18:4)异构3(MAG(18:4)isomer3);其他包括生物碱、氨基酸类等共14 种(表2)。以上结果表明,果皮性状形成可能和黄酮、脂质、酚酸类代谢物关系密切。
表2 华酥、苏翠1 号与翠冠果皮差异代谢产物
Table 2 Metabolites with specific content changes in Cuiguan pericarp
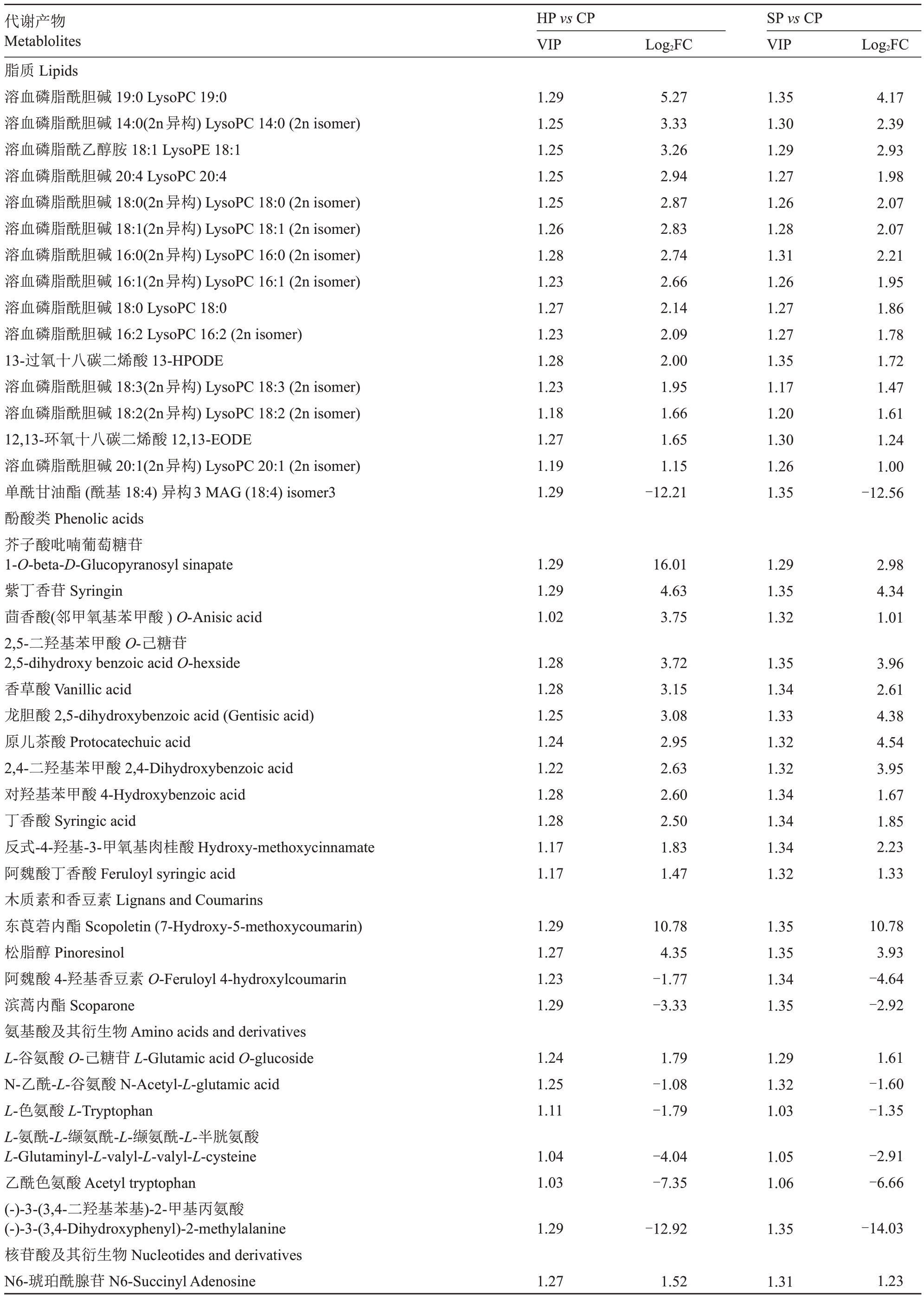
代谢产物Metablolites HP vs CP VIP Log2FC SP vs CP VIP Log2FC脂质Lipids溶血磷脂酰胆碱19:0 LysoPC 19:0溶血磷脂酰胆碱14:0(2n异构)LysoPC 14:0(2n isomer)溶血磷脂酰乙醇胺18:1 LysoPE 18:1溶血磷脂酰胆碱20:4 LysoPC 20:4溶血磷脂酰胆碱18:0(2n异构)LysoPC 18:0(2n isomer)溶血磷脂酰胆碱18:1(2n异构)LysoPC 18:1(2n isomer)溶血磷脂酰胆碱16:0(2n异构)LysoPC 16:0(2n isomer)溶血磷脂酰胆碱16:1(2n异构)LysoPC 16:1(2n isomer)溶血磷脂酰胆碱18:0 LysoPC 18:0溶血磷脂酰胆碱16:2 LysoPC 16:2(2n isomer)13-过氧十八碳二烯酸13-HPODE溶血磷脂酰胆碱18:3(2n异构)LysoPC 18:3(2n isomer)溶血磷脂酰胆碱18:2(2n异构)LysoPC 18:2(2n isomer)12,13-环氧十八碳二烯酸12,13-EODE溶血磷脂酰胆碱20:1(2n异构)LysoPC 20:1(2n isomer)单酰甘油酯(酰基18:4)异构3 MAG(18:4)isomer3酚酸类Phenolic acids芥子酸吡喃葡萄糖苷1-O-beta-D-Glucopyranosyl sinapate紫丁香苷Syringin茴香酸(邻甲氧基苯甲酸)O-Anisic acid 2,5-二羟基苯甲酸O-己糖苷2,5-dihydroxy benzoic acid O-hexside香草酸Vanillic acid龙胆酸2,5-dihydroxybenzoic acid(Gentisic acid)原儿茶酸Protocatechuic acid 2,4-二羟基苯甲酸2,4-Dihydroxybenzoic acid对羟基苯甲酸4-Hydroxybenzoic acid丁香酸Syringic acid反式-4-羟基-3-甲氧基肉桂酸Hydroxy-methoxycinnamate阿魏酸丁香酸Feruloyl syringic acid木质素和香豆素Lignans and Coumarins东莨菪内酯Scopoletin(7-Hydroxy-5-methoxycoumarin)松脂醇Pinoresinol阿魏酸4-羟基香豆素O-Feruloyl 4-hydroxylcoumarin滨蒿内酯Scoparone氨基酸及其衍生物Amino acids and derivatives L-谷氨酸O-己糖苷L-Glutamic acid O-glucoside N-乙酰-L-谷氨酸N-Acetyl-L-glutamic acid L-色氨酸L-Tryptophan L-氨酰-L-缬氨酰-L-缬氨酰-L-半胱氨酸L-Glutaminyl-L-valyl-L-valyl-L-cysteine乙酰色氨酸Acetyl tryptophan(-)-3-(3,4-二羟基苯基)-2-甲基丙氨酸(-)-3-(3,4-Dihydroxyphenyl)-2-methylalanine核苷酸及其衍生物Nucleotides and derivatives N6-琥珀酰腺苷N6-Succinyl Adenosine 1.29 1.25 1.25 1.25 1.25 1.26 1.28 1.23 1.27 1.23 1.28 1.23 1.18 1.27 1.19 1.29 5.27 3.33 3.26 2.94 2.87 2.83 2.74 2.66 2.14 2.09 2.00 1.95 1.66 1.65 1.15-12.21 1.35 1.30 1.29 1.27 1.26 1.28 1.31 1.26 1.27 1.27 1.35 1.17 1.20 1.30 1.26 1.35 4.17 2.39 2.93 1.98 2.07 2.07 2.21 1.95 1.86 1.78 1.72 1.47 1.61 1.24 1.00-12.56 1.29 1.29 1.02 16.01 4.63 3.75 1.29 1.35 1.32 2.98 4.34 1.01 1.28 1.28 1.25 1.24 1.22 1.28 1.28 1.17 1.17 3.72 3.15 3.08 2.95 2.63 2.60 2.50 1.83 1.47 1.35 1.34 1.33 1.32 1.32 1.34 1.34 1.34 1.32 3.96 2.61 4.38 4.54 3.95 1.67 1.85 2.23 1.33 1.29 1.27 1.23 1.29 10.78 4.35-1.77-3.33 1.35 1.35 1.34 1.35 10.78 3.93-4.64-2.92 1.24 1.25 1.11 1.79-1.08-1.79 1.29 1.32 1.03 1.61-1.60-1.35 1.04 1.03-4.04-7.35 1.05 1.06-2.91-6.66 1.29-12.92 1.35-14.03 1.27 1.52 1.31 1.23
续表Continued Table

代谢产物Metablolites HP vs CP VIP Log2FC SP vs CP VIP Log2FC琥珀酰腺苷Succinyladenosine黄酮Flavonoids染料木苷Genistein 7-O-Glucoside(Genistin)6-C-己糖基金圣草黄素O-阿魏酰己糖苷6-C-hexosyl-chrysoeriol O-feruloylhexoside麦黄酮O-甘油Tricin O-glycerol麦黄酮O-葡萄糖二酸Tricin O-saccharic acid高圣草酚Homoeriodictyol丁香亭Syringetin橙皮素O-丙二酰基己糖苷Hesperetin O-malonylhexoside麦黄酮O-芥子酸Tricin O-sinapic acid花翠素3-O-葡萄糖苷Delphinidin 3-O-glucoside(Mirtillin)甲基槲皮素O-己糖苷methylQuercetin O-hexoside异鼠李素5-O-己糖苷Isorhamnetin 5-O-hexoside圣草酚O-丙二酰己糖苷Eriodictyol O-malonylhexoside槲皮素7-O-芸香糖苷Quercetin 7-O-rutinoside木犀草素8-C-己糖苷-O-己糖苷Luteolin 8-C-hexosyl-O-hexoside氧甲基金圣草黄素7-O-己糖苷O-methylChrysoeriol 7-O-hexoside氧甲基金圣草黄素5-O-己糖苷O-methylChrysoeriol 5-O-hexoside金圣草黄素C-己糖基-O-鼠李糖苷Chrysoeriol C-hexosyl-O-rhamnoside金圣草黄素7-O-芸香糖苷Chrysoeriol 7-O-rutinoside乙酰基圣草酚O-己糖苷Acetyl-eriodictyol O-hexoside其他类Others反式-13,14-二羟视黄醇All-trans-13,14-dihydroretinol烟酸Nicotinic acid古洛糖酸内酯L-Gulonic-γ-lactone吡哆素Pyridoxine鞣质Tannins没食子酸甲酯Methyl gallate生物碱Alkaloids甜菜碱Betaine可可碱Theobromine吲哚-5-甲酸Indole-5-carboxylic acid腐胺Putrescine葫芦巴碱Trigonelline胍丁胺Agmatine N-乙酰丁二胺N-Acetylputrescine糖类Saccharides D(+)-无水葡萄糖D(+)-Glucose D-(+)-蔗糖D-(+)-Sucrose D(+)-松三糖D(+)-Melezitose有机酸Organic acids 2,3-二羟基苯甲酸2,3-Dihydroxybenzoic acid壬二酸Azelaic acid 2-吡啶甲酸2-Picolinic acid 3,4-二羟基苯甲酸乙酯(安息香酸)Ethyl 3,4-Dihydroxybenzoate(Ethyl protocatechuate)1.29 1.28 1.29 1.14 1.29 15.61 1.33 1.64 1.10 1.09 1.29 1.28 1.22 1.25 1.28 1.24 1.02 1.04 1.15 1.24 1.23 1.26 1.27 5.27 4.84 3.98 3.50 2.74 2.23 1.53 1.25-1.08-1.08-1.97-2.34-2.42-3.05-3.32 1.34 1.35 1.35 1.34 1.31 1.33 1.33 1.29 1.31 1.31 1.24 1.24 1.24 1.35 1.35 3.83 12.01 3.49 3.22 2.43 2.82 1.42 1.15-2.13-2.21-2.03-1.10-1.17-3.32-3.74 1.29 1.29 1.29-10.47-10.78-13.34 1.20 1.23 1.35-2.01-1.95-14.06 1.00 1.15 1.22 1.28 3.32-1.12-1.38-2.93 1.35 1.31 1.35 1.33 13.42-2.09-2.02-2.20 1.00 1.76 1.14 2.32 1.26 1.26 1.25 1.04 1.15 1.26 1.27 2.41 1.96 1.67-1.17-2.39-2.71-2.81 1.21 1.33 1.32 1.32 1.25 1.27 1.28 1.03 2.17 1.48-3.40-2.80-2.79-2.92 1.29 1.19 1.05 17.63-1.11-2.11 1.35 1.22 1.22 17.63-1.06-2.47 1.23 1.23 1.17 1.29 3.06 1.59-1.41-17.34 1.32 1.30 1.31 1.35 4.69 1.87-2.18-15.07
2.4 转录组分析
2.4.1 测序质量 经去除杂质后分别得到53 976 172、52 264 180、47 828 208、63 030 088、53 523 576、52 947 240、47 757 364、49 945 586 和72 227 180 个序列,总碱基数为75.7 G,所有样品的测序总核苷酸均大于7 Gb。Phred 值大于20 的碱基比例(Q20)在97.57%~97.89%之间,Phred 值大于30 的碱基比例(Q30)在93.37%~94.01%之间;所有样品的GC含量均在46%左右,较为一致。对比砀山酥梨基因组,各样品比对率均达85%以上。
2.4.2 差异基因及其KEGG分析 对差异基因进行分析,以padj<0.05为标准,筛选出SP vs CP差异基因共16 165 个,其中上调7973 个,下调8632 个;HP vs CP 差异基因共12 841 个,其中上调5866 个,下调6975个(图3)。
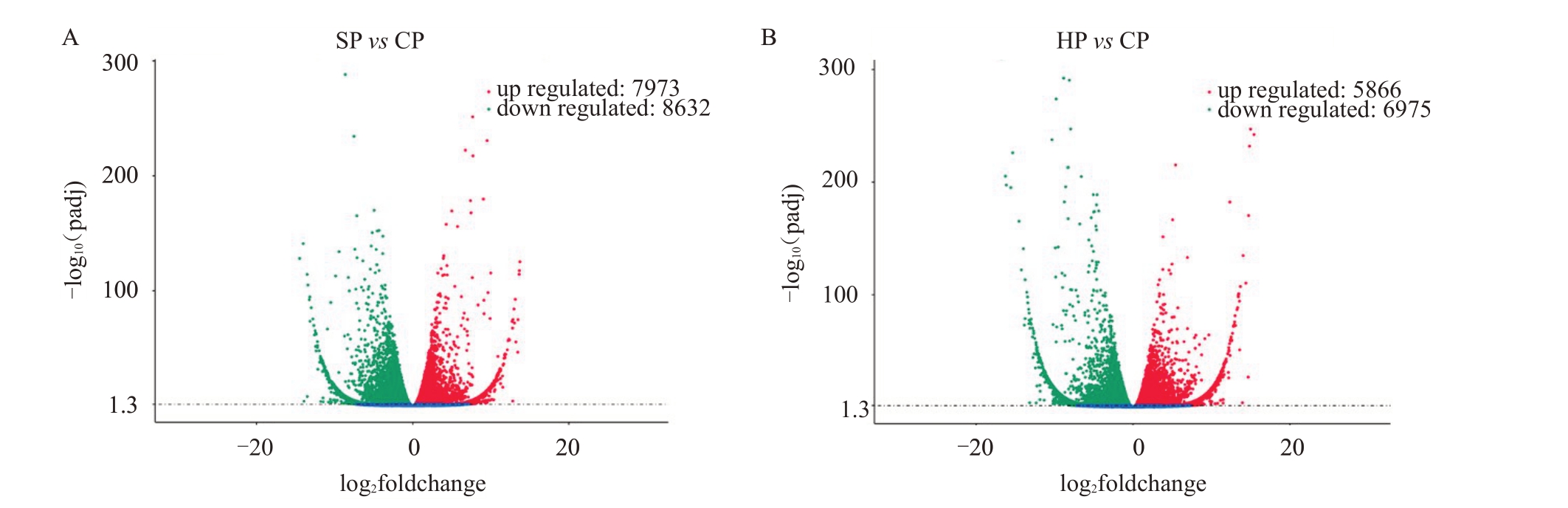
图3 差异表达基因的火山图
Fig.3 Volcano plot of the DEGs
进一步对差异基因进行KEGG 代谢通路注释,结果表明,在HP vs CP 中,角质、栓质和蜡质生物合成途径、苯丙烷类生物合成途径以及萜类骨架生物合成显著富集(图4-A)。SP vs CP 中,类黄酮合成,苯丙素代谢,苯丙素合成,苯丙胺酸、酪氨酸和色氨酸生物合成以及叶绿素代谢显著富集(图4-B)。
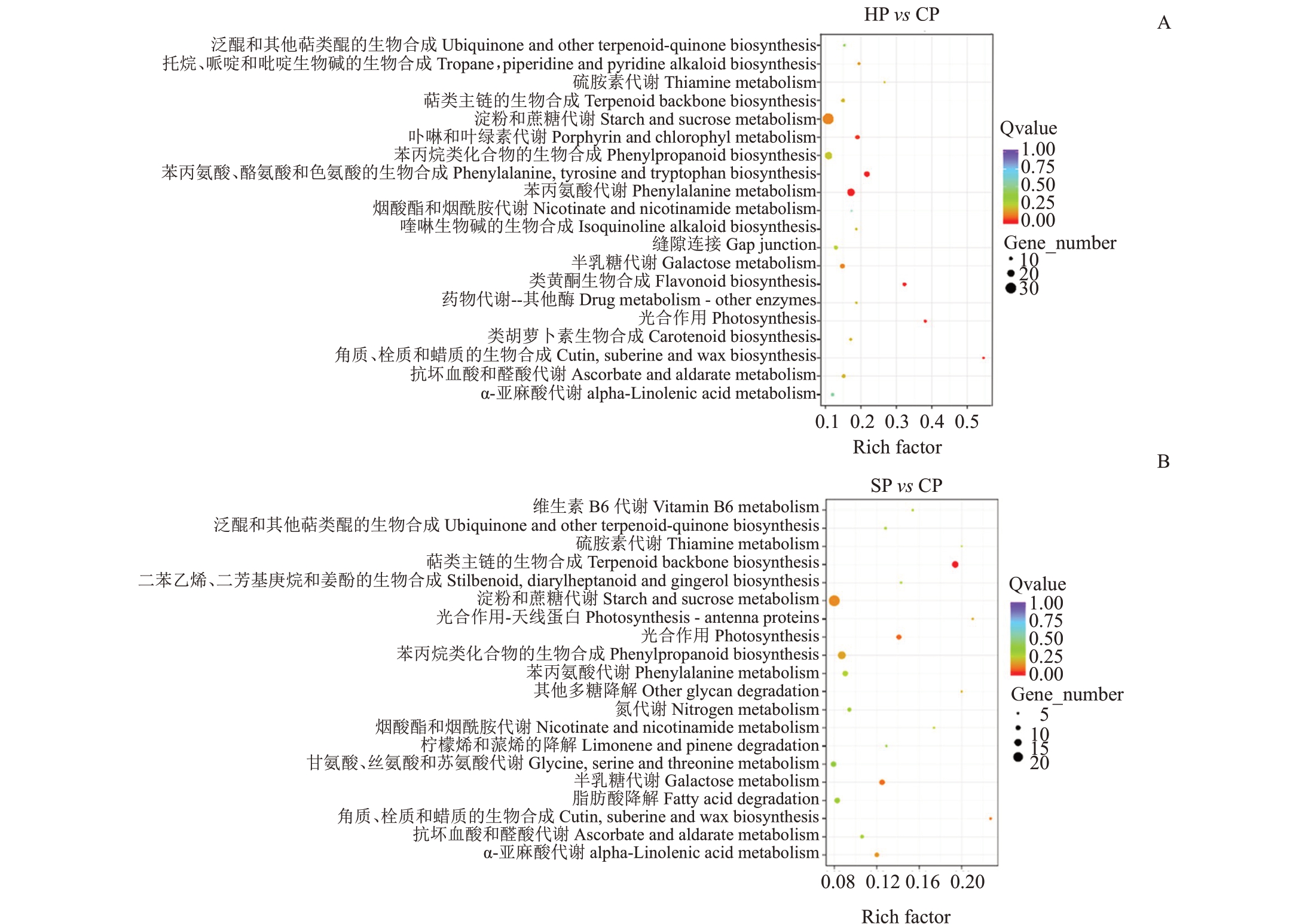
图4 差异基因KEGG 富集图
Fig.4 KEGG enrichment diagram of DEGs
2.5 代谢组和转录组联合分析
根据本试验的差异代谢物分析结果,最终筛选出3 条代谢途径,包括角质、栓质和蜡质生物合成、苯丙素生物合成以及类黄酮生物合成途径。将相同分组的差异基因(图5)及差异代谢物同时映射到KEGG 通路图上,展示同时具有差异代谢物和差异基因的通路,并对落于这些代谢通路的差异代谢产物和差异基因进行联合分析(图6)。
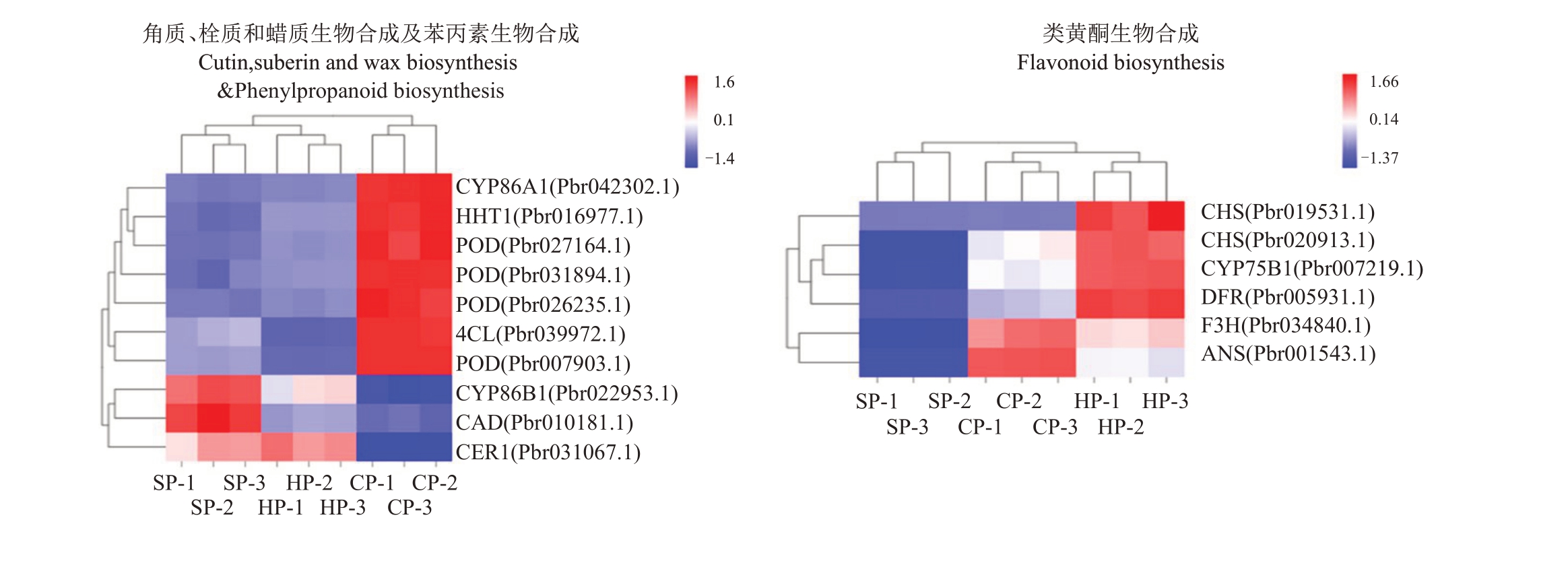
图5 翠冠与苏翠1 号、华酥果皮关键代谢途径差异基因表达分析
Fig.5 DEGs related to key metabolic pathways in Cuiguan(CP),Sucui 1(SP)and Huasu(HP)
SP-1/2/3:苏翠1 号果皮混合样品的3 个生物学重复;CP-1/2/3:翠冠果皮混合样品的3 个生物学重复;HP-1/2/3:华酥果皮混合样品的3 个生物学重复。
SP-1/2/3:Three biological repeats of mixed pericarp samples of Sucui 1;CP-1/2/3:Three biological repeats of mixed pericarp samples of Cuiguan;HP-1/2/3:Three biological repeats of mixed pericarp samples of Huasu.
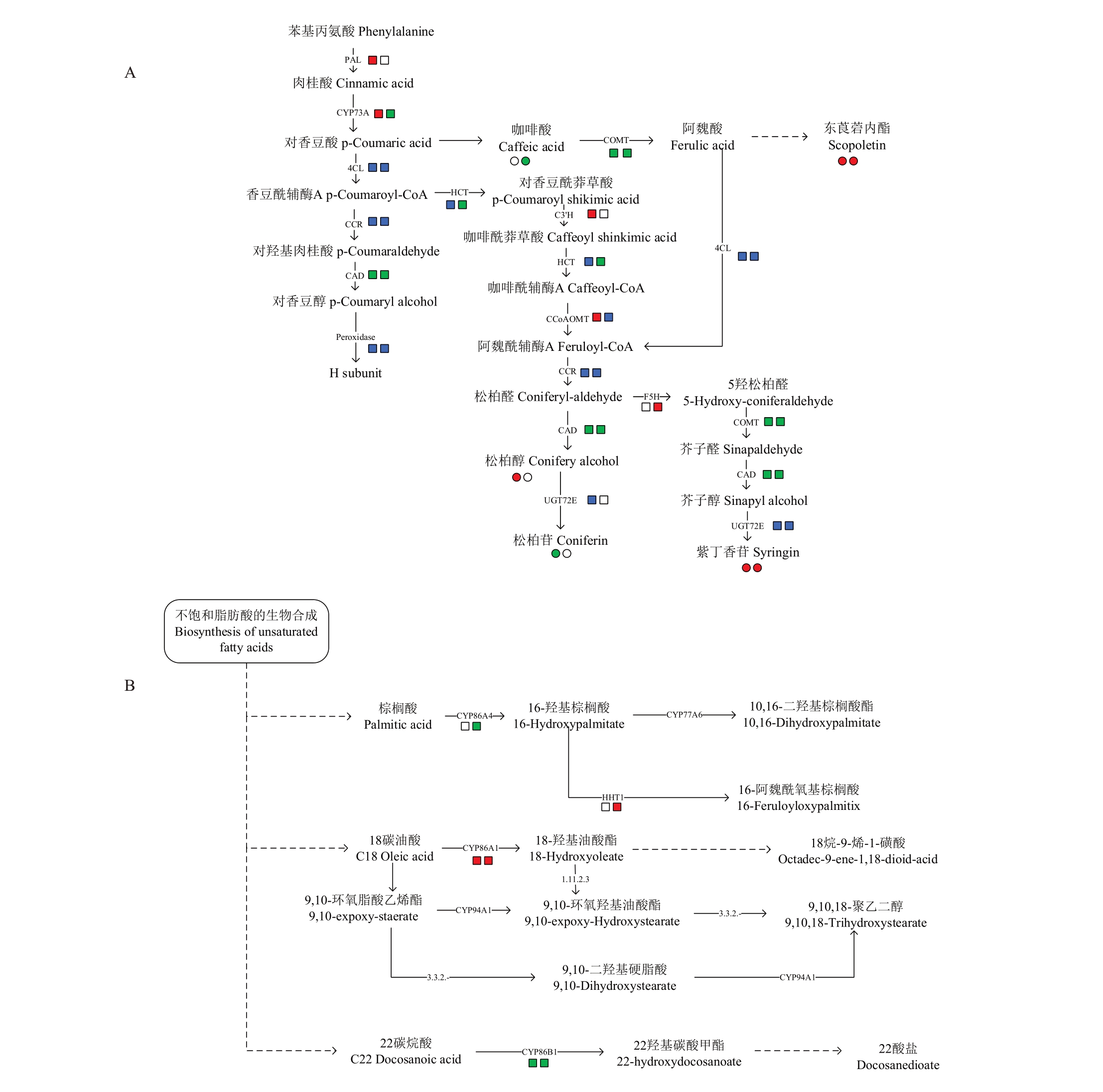
图6 差异代谢产物和差异基因联合分析
Fig.6 Joint analysis of differentially expressed metabolites and genes
A.苯丙素类的生物合成途径;B.角质、栓质和蜡质的生物合成途径;C.蜡质的生物合成途径(一般形式);D.类黄酮生物合成途径。SP-苏翠1 号果皮;CP-翠冠果皮;HP-华酥果皮。红色代表上调。绿色代表下调,蓝色代表有上调也有下调。圆圈注释物质,方格注释基因。在并列的两个注释图标中,左侧的代表该物质/基因在SP vs CP 中的含量/表达情况,右侧的代表该物质/基因在HP vs CP 中的含量/表达情况。虚线连接起始原料与终产物,省略中间过程。
A.The pathway of phenylpropanoid biosynthesis;B.The pathway of cutin,suberin and wax biosynthesis;C.The pathway of wax biosynthesis(general form); D.The pathway of Flavonoid biosynthesis.SP-Pericarp of Sucui 1; CP-Pericarp of Cuiguan; HP-Pericarp of Huasu.Red circle/squre means up-regulated metabolites/genes and green circle means down-regulated metabolites/genes in Cuiguan.Blue represents some up-regulated and some down-regulated.The circle annotates the metabolite and the square annotates the gene.In the two side-by-side annotation icons,the one on the left represents the content/expression of the metabolites/gene in SP vs CP,the right side represents the content/expression of the metabolites/gene in HP vs CP.Dotted line connects the starting material and the end product,omitting intermediate process.
角质、栓质和蜡质生物合成途径中包括4 个差异基因。与苏翠1 号和华酥比较,脂肪酸ω-羟化酶基因CYP86A1(Pbr042302.1)与HHT1(Pbr016977.1)在翠冠中显著上调。脂肪酸ω-羟化酶基因CYP86B1(Pbr022953.1)和乙醛脱羧酶基因CER1(Pbr031067.1)在翠冠显著下调。值得注意的是HHT1、CYP86A1 和CYP86B1 在苏翠1 号中相对表达量略高于华酥。SP vs HP 中CER1 均未差异表达。苯丙素类合成途径的4 个POD(Pbr007903.1、Pbr026235.1、Pbr027164.1、Pbr031894.1)和1 个4CL(Pbr039972.1)在翠冠中均差异上调,而SP vs HP 中未差异表达。苯丙素类合成途径的CAD(Pbr010181.1)在翠冠中下调。
苏翠1号、华酥和翠冠三者相比较,在类黄酮生物合成途径中,查耳酮合酶基因CHS(Pbr019531.1、Pbr020913.1)、二氢黄酮还原酶基因DFR(Pbr005931.1)、类黄酮3-羟化酶基因CYP75B1(Pbr007219.1)在翠冠中上调,黄烷酮3-氢化酶基因F3H(Pbr034840.1)、花色素苷合成酶基因ANS(Pbr001543.1)在华酥中上调。
2.6 荧光定量PCR验证
选择4 个基因进行验证:CAD(Pbr010181.1)、CER1(Pbr031067.1)、CYP86B1(Pbr022953.1)、POD(Pbr031894.1)。qRT-PCR 结果显示CAD、CER1、CYP86B1 在苏翠1 号和华酥中相对表达量较高,在翠冠中相对表达量低;POD 在翠冠中高表达,在苏翠1 号和华酥中相对表达量低(图7)。荧光定量PCR 数据与转录组数据趋势一致,验证了转录组测序的可靠性。

图7 苏翠1 号与翠冠和华酥基因的荧光定量PCR 验证
Fig.7 The qRT-PCR of DEGs in Sucui 1,Cuiguanand Huasu
不同小写字母表示在p <0.05 差异显著。
Different small letters indicate significant difference at p <0.05.
3 讨 论
梨是我国第二大落叶果树树种,近20 a来,在国家梨产业技术体系等政策的扶持下,育种工作积极展开。品质育种至关重要,果皮物质含量与耐贮性和果锈发生有关。笔者在本研究中选取遗传上直接相关的3 个品种开展工作,对揭示光洁果皮性状的形成机制更有针对性,也为后续分子辅助育种工作的开展提供参考。
本研究发现,苏翠1 号中,角质、栓质和蜡质生物合成途径的HHT1(Pbr016977.1)、CYP86A1(Pbr042302.1)和苯丙素类合成途径的4CL(Pbr039972.1)、POD(Pbr007903.1、Pbr026235.1、Pbr027164.1、Pbr031894.1)基因显著下调,苯丙素类合成途径的CAD(Pbr010181.1)和角质、栓质和蜡质生物合成途径的CYP86B1(Pbr022953.1)、CER1(Pbr031067.1)基因显著上调。黄酮类合成途 径 的CHS(Pbr019531.1、Pbr020913.1)、DFR(Pbr005931.1)、CYP75B1(Pbr007219.1)、F3H(Pbr034840.1)、ANS(Pbr001543.1)在苏翠1 号中均下调。
在梨和苹果上的研究表明,木栓层是果锈的重要组分,其形成与木质素和酚类物质的含量有关[18,50]。SPPD 包括阿魏酸、香豆酸和木质素醇等羟基化肉桂酸,主要来自于苯丙氨酸代谢途径。苯丙氨酸(phenylalanine)首先在PAL 等酶催化下,形成对-香豆酸(p-coumaric acid);接着在4CL 与CCR 催化下形成香豆醛(p-coumaraldehyde)、松柏醛(coniferyl-aldehyde)、5-羟基松柏醛(5-hydroxy-coniferaldehyde)和芥子醛(sinapaldehyde);这些衍生物在CAD 催化下转化为对应的醇类物质香豆醇(p-coumaryl alcohol)、松柏醇(coniferyl alcohol)和芥子醇(sinapyl alcohol)[51]。木质素主要由这3种不同的单体组成[52]。POD 催化木质素单体脱氢,使木质素单体分子组装为高分子聚合物,组成SPPD。苯丙氨酸代谢途径中产生大量的酚酸类物质,代谢组比较结果显示,翠冠中酚酸类物质远高于其他两个品种,其在苯丙氨酸合成中积累了更多的前体物质,果皮表面木质素次生代谢积累较苏翠1号和华酥更多。
木质素合成过程的最后一步中,CAD催化肉桂醇的衍生物向其对应的醇转化形成木质素单体。前人研究表明,CAD 在果实发育早期相对表达量较低,中期相对表达量最高,果实成熟时相对表达量逐渐下降,CAD 与木质素含量呈现正相关[53]。但在本研究中,尽管苏翠1 号与华酥中的CAD 相对表达量高于翠冠,却没有使二者出现大面积果锈。前人在水稻[54]和番茄[55]上的研究发现,抑制4CL 表达可以显著降低植株茎秆、叶片的木质素含量。本研究中4CL 在苏翠1 号与华酥中均处于低表达状态,可能导致后续木质素单体合成缺乏可用底物。POD 基因也只在翠冠中上调表达,可能使其清除自由基的能力更强,催化单体木质素组装为高分子聚合物向木栓位点堆积。因此,本研究推测关键基因4CL 和POD的低表达量使苏翠1号木质素聚合物组装受抑制,SPPD 关键组分合成受阻,从而使苏翠1 号呈现光洁无锈的优良果皮性状。相关基因功能有待进一步研究。
组学分析表明,相较于无/极少锈组,翠冠角质的生物合成受到抑制,栓质的生物合成受到促进。果皮蜡质的合成可以分为3个阶段:第一步,在质体中合成C16-C18 的脂肪酸前体,前体接着在内质网中延伸至C20~C34 的超长链脂肪酸(very-longchain fatty acids,VLCFAs),这些VLCFAs 在最后一步经过初级醇代谢途径和烷烃代谢途径形成烷烃、醇、醛和酯类等各种蜡质产物[56]。脂肪酸的延伸是角质、栓质和蜡质合成的重要上游反应,超长链的脂肪酸类化合物是角质、栓质和蜡质的主要成分[57]。VLCFAs 在烷烃代谢途径中首先被还原成醛,而后再进行脱羰反应生成烷烃,羟化反应后这些烷烃又生成二级醇和酮输送到植物表皮角质膜。CER1 是控制超长链烷烃合成的核心成分,编码醛脱羰酶,催化醛脱羰形成烷烃,其突变体中烷烃的含量显著减少,在拟南芥上的研究显示CER1 基因过表达将导致烷烃含量增加最终导致角质积累,使渗透性降低[56,58]。本研究推测CER1 的下调或许减缓了翠冠中角质单体的产生,使其角质层机械强度降低,易受果实膨大的内部张力破裂,进而引起表皮细胞木栓化堆积。
角质、栓质和蜡质的生物合成途径中,HHT1 在翠冠中表达量远高于另外2种,而在苏翠1号中的表达量又略高于华酥,与三者外在的性状表现趋势一致,表明该基因参与了梨果锈的形成,与果锈程度呈正相关,这与前人结果一致。对拟南芥种子和根的研究[59]中发现,HHT 是将阿魏酸转移到SPPD 的关键酶,在马铃薯上的研究也发现HHT 促进了木栓化[60]。CYP86 主要参与脂肪酰基-CoA ω 位点的羟基化形成ω-羟基酸,其中一些被氧化成α,ω二羧酸并进一步参与角质和木栓的生物合成,对木质素合成起正向调控作用[16]。CYP86A1 参与C12~C18 短链和一些较长脂肪酸的羟基化,CYP86B1 参与C22~C24超长链脂肪酸的羟基化[61]。敲除拟南芥的CYP86A1 或CYP86B1,对应的羟基酸与α,ω 二羧酸积累显著减少,引起木栓质含量减少[16,62]。Molina等[63]敲除拟南芥种子CYP86B1 后,发现超长链饱和α,ω-双官能团脂肪族单体几乎完全消失,栓质组分发生变化。在本试验的极少/无锈组中,CYP86B1上调表达没有导致极少/无锈组木质素含量的增加。CYP86A1在极少/无锈组中被抑制,推测其极低表达量有助于苏翠1号获得光洁无锈的性状。CYP86A1可能是木质素合成的关键基因。结合代谢组结果,翠冠中C16~C20 磷脂酰胆碱含量高于另2 种,推测梨果锈木栓质SPAD的组装中C12~C18的α,ω二羧酸是关键前体物质。CYP86A1极低表达量对苏翠1号果锈形成的抑制力更强,对光洁果皮的贡献更大。
苯丙素合成途径为酚类次生代谢物的合成提供前体。类黄酮生物合成途径是苯丙烷途径的一部分。香豆酰辅酶A在CHS作用下生成查耳酮,接着被CHI催化为柚皮素。形成的柚皮素与查耳酮一起作为前体物质通过不同分支途径进入其他不同黄酮类物质的合成代谢支路[20]。F3H或F3H催化柚皮素变为二氢黄酮醇,二氢黄酮醇是黄酮醇和花青素合成的共同前体物质。F3H 属于P450 的CYP75B 家族[64]。CYP75 家族在以花青素、飞燕草素为主导的花果颜色形成中起着决定性作用[65]。DFR、CYP75B1 和ANS 均位于花青素合成途径中。ANS沉默的烟草中黄酮醇含量显著增加[66]。代谢组结果显示苏翠1号中黄酮醇类如山奈酚、槲皮素,含量高于翠冠和华酥。苏翠1号中DFR、CYP75B1、F3H和ANS 均下调,在花色苷合成途径中没有消耗过多的二氢黄酮醇底物,因此积累了更多的黄酮醇。黄酮醇在果树干旱胁迫[67]、盐胁迫[68]、紫外线胁迫[69]、低温胁迫[64]等非生物胁迫中发挥重要作用[22]。梨中的黄酮醇主要存在于叶片、果皮中[70]。黄酮醇类化合物具有抗氧化作用,在猕猴桃[24]和苹果[25]上的研究证实槲皮素可以延缓果实衰老、提高耐贮性。由此本研究推测,高黄酮醇的果皮使苏翠1 号果实具有良好的耐贮性。
随着黄酮类化合物抗氧化、抗肿瘤、抗癌、降血压、降血脂等功能的被发现,国内外进行了许多关于黄酮类化合物药理功能的研究[71]。而之前的研究多集中于传统药用植物中[72-74]。本研究发现梨果皮中含有丰富的黄酮类化合物,便于日后开展对梨中黄酮类代谢物的药理性研究,开发果皮副产品,进一步提高果实商品价值。
4 结 论
通过转录组分析,获得了3 个早熟梨品种果实的基因表达谱数据,结合差异代谢物分析了果皮优异性状形成的物质及分子基础。结果显示苯丙素合成途径关键酶基因POD、4CL以及角质/栓质和蜡质合成途径上的HHT1、CYP86A1在苏翠1号中被抑制表达,可能导致苏翠1 号果锈的减少。类黄酮合成途径中CHS、DFR、CYP75B1、F3H、ANS 在苏翠1 号中均下调,可能使苏翠1 号具有良好的耐贮性。分析了苏翠1号果皮优良性状成因,为苏翠1号的大面积推广提供理论依据。
[1] 卢晓鹏.砂梨(Pyrus pyrifolia Nakai)果实石细胞、褐色果皮和柠檬酸形成机制研究[D].武汉:华中农业大学,2011.LU Xiaopeng.Mechenism researches on the formation of stone cell,russet skin and citric acid of pear (Pyrus pyrifolia Nakai)fruit[D].Wuhan:Huazhong Agricultural University,2011.
[2] 蔺经,杨青松,李晓刚,盛宝龙,王中华,常有宏.套袋微环境对‘翠冠’梨果实外观品质的影响[J].西北农林科技大学学报(自然科学版),2009,37(10):133-139.LIN Jing,YANG Qingsong,LI Xiaogang,SHENG Baolong,WANG Zhonghua,CHANG Youhong.Effect of microenvironment of bagging on appearance quality of‘Cuiguan’pears[J].Journal of Northwest A & F University (Natural Science Edition),2009,37(10):133-139.
[3] 方成泉,陈欣业,林盛华,徐汉英,蒲富慎,米文广.梨新品种:华酥[J].园艺学报,2000,27(3):231.FANG Chengquan,CHEN Xinye,LIN Shenghua,XU Hanying,PU Fushen,MI Wenguang.A new pear variety:Huasu[J].Acta Horticulturae Sinica,2000,27(3):231.
[4] 蔺经,盛宝龙,李晓刚,杨青松,王中华,李慧,王宏,常有宏.早熟砂梨新品种‘苏翠1 号’[J].园艺学报,2013,40(9):1849-1850.LIN Jing,SHENG Baolong,LI Xiaogang,YANG Qingsong,WANG Zhonghua,LI Hui,WANG Hong,CHANG Youhong.A new Pyrus pyrifolia cultivar‘Sucui 1’[J].Acta Horticulturae Sinica,2013,40(9):1849-1850.
[5] 滕元文,沈玉英,周先章.砂梨果皮锈斑成因及解决对策[J].中国南方果树,2005,34(3):52-54.TENG Yuanwen,SHEN Yuying,ZHOU Xianzhang.Cause of russet spots of sand pear and its solution[J].South China Fruits,2005,34(3):52-54.
[6] 施泽彬.砂梨果皮性状形成机制研究[D].南京:南京农业大学,2011.SHI Zebin.The mechanisms of fruit appearance characters formation in sand pear(Pyrus pyrifolia Nakai)[D].Nanjing:Nanjing Agricultural University,2011.
[7] KHANAL B P,GRIMM E,KNOCHE M.Russeting in apple and pear:A plastic periderm replaces a stiff cuticle[J/OL].AoB Plants,2013,5:pls048.DOI:10.1093/aobpla/pls048.
[8] BERNARDS M A,SUMMERHURST D K,RAZEM F A.Oxidases,peroxidases and hydrogen peroxide:The suberin connection[J].Phytochemistry Reviews,2004,3(1/2):113-126.
[9] GRAÇA J,SANTOS S.Suberin:a biopolyester of plants'skin[J].Macromolecular Bioscience,2007,7(2):128-135.
[10] 张鹏飞.‘翠冠’梨果锈形成机制及关键过氧化物酶基因挖掘[D].扬州:扬州大学,2020.ZHANG Pengfei.Mechanism of russet formation and mining of key PRX genes in‘Cuiguan’pear fruit[D].Yangzhou:Yangzhou University,2020.
[11] POLLARD M,BEISSON F,LI Y H,OHLROGGE J B.Building lipid barriers:Biosynthesis of cutin and suberin[J].Trends in Plant Science,2008,13(5):236-246.
[12] 王新卫.黄花梨及其绿皮芽变果皮发育特性和差异表达基因的克隆与功能分析[D].南京:南京农业大学,2012.WANG Xinwei.Fruit skin development,cloning and functional analysis of differentially expressed genes in pear‘Huanghua’(Pyrus pyrifolia Nakai) and its green skin bud sport[D].Nanjing:Nanjing Agricultural University,2012.
[13] LI G H,WANG H,CHENG X,SU X,ZHAO Y,JIANG T,JIN Q,LIN Y,CAI Y.Comparative genomic analysis of the PAL genes in five Rosaceae species and functional identification of Chinese white pear[J/OL].PeerJ,2019,7:e8064.DOI:10.7717/peerj.8064.
[14] 吕照清,任丹丹,周贺,乔玉山.‘黄花’梨及其芽变‘绿黄花’梨HHT 基因克隆与表达分析[J].西北植物学报,2016,36(6):1105-1109.LÜ Zhaoqing,REN Dandan,ZHOU He,QIAO Yushan.Cloning and expression of HHTGene in‘Huanghua’pear and its bud mutant‘Lühuanghua’pear(Pyrus pyrifolia Nakai)[J].Acta Botanica Boreali-Occidentalia Sinica,2016,36(6):1105-1109.
[15] DIXON R A,PAIVA N L.Stress-induced phenylpropanoid metabolism[J].The Plant Cell,1995,7(7):1085-1097.
[16] HÖFER R,BRIESEN I,BECK M,PINOT F,SCHREIBER L,FRANKE R.The Arabidopsis cytochrome P450 CYP86A1 encodes a fatty acid Omega-hydroxylase involved in suberin monomer biosynthesis[J].Journal of Experimental Botany,2008,59(9):2347-2360.
[17] 高丽.CYP86A1 在拟南芥响应干旱胁迫中的作用研究[D].兰州:兰州大学,2018.GAO Li.The role of CYP86A1 in response of Arabidopsis thaliana to drought stress[D].Lanzhou:Lanzhou University,2018.
[18] 李晓峰,李雪,贾兵,刘莉,叶振风,衡伟,朱立武.‘砀山酥梨’褐皮芽变木质素含量及相关酶活性与CCoAOMT 表达量分析[J].园艺学报,2012,39(5):828-836.LI Xiaofeng,LI Xue,JIA Bing,LIU Li,YE Zhenfeng,HENG Wei,ZHU Liwu.Analysis of enzyme activity and lignin content and expression of CCoAOMT gene in the pericarp of‘Dangshan suli’and its russet mutant[J].Acta Horticulturae Sinica,2012,39(5):828-836.
[19] MUTHA R E,TATIYA A U,SURANA S J.Flavonoids as natural phenolic compounds and their role in therapeutics:An overview[J/OL].Future Journal of Pharmaceutical Sciences,2021,7(1):25.DOI:10.1186/s43094-020-00161-8.
[20] 邹丽秋,王彩霞,匡雪君,李滢,孙超.黄酮类化合物合成途径及合成生物学研究进展[J].中国中药杂志,2016,41(22):4124-4128.ZOU Liqiu,WANG Caixia,KUANG Xuejun,LI Ying,SUN Chao.Advance in flavonoids biosynthetic pathway and synthetic biology[J].China Journal of Chinese Materia Medica,2016,41(22):4124-4128.
[21] KARAK P.Biological activities of flavonoids:an overview[J].International Journal of Pharmaceutical Sciences and Research,2019,10:1567-1574.
[22] 靳俊婷,索晓静,丁红元,郭春磊,王东升,张京政,曹飞.黄酮醇在果树中的功能的研究进展[J].河北果树,2021(4):1-3,15.JIN Junting,SUO Xiaojing,DING Hongyuan,GUO Chunlei,WANG Dongsheng,ZHANG Jingzheng,CAO Fei.Research progress of function of flavonols in fruit trees[J].Hebei Fruits,2021(4):1-3,15.
[23] ZHAI R,ZHAO Y X,WU M,YANG J,LI X,LIU H,WU T,LIANG F,YANG C,WANG Z,MA F,XU L.The MYB transcription factor PbMYB12b positively regulates flavonol biosynthesis in pear fruit[J].BMC Plant Biology,2019,19(1):85.
[24] 杜芳梦,张丽媛,何玲,郭宇欢,徐乐艺,齐馨.槲皮素处理对猕猴桃贮藏的影响[J].陕西农业科学,2017,63(7):28-32.DU Fangmeng,ZHANG Liyuan,HE Ling,GUO Yuhuan,XU Leyi,QI Xin.Effect of quercetin treatment on storage of kiwifruit[J].Shaanxi Journal of Agricultural Sciences,2017,63(7):28-32.
[25] 王龙.外源槲皮素对苹果果实衰老的影响及其生理机制[D].保定:河北农业大学,2003.WANG Long.Effects and physiological mechanism of exogenous quercetin on fruit senescence in apples[D].Baoding:Hebei Agricultural University,2003.
[26] MAMAT A,TUSONG K,XU J.Identification of metabolic pathways related to rough-skinned fruit formation in Korla pear[J/OL].Scientia Horticulturae,2021,288:110414.DOI:10.1016/j.scienta.2021.110414.
[27] LI A L,LIU D C,WU J,ZHAO X,HAO M,GENG S,YAN J,JIANG X,ZHANG L,WU J,YIN L,ZHANG R,WU L,ZHENG Y,MAO L.mRNA and small RNA transcriptomes reveal insights into dynamic homoeolog regulation of allopolyploid heterosis in nascent hexaploid wheat[J].The Plant Cell,2014,26(5):1878-1900.
[28] DAI Q,GENG L L,LU M J,JIN W,NAN X,HE P A,YAO Y.Comparative transcriptome analysis of the different tissues between the cultivated and wild tomato[J/OL].PLoS One,2017,12(3):e0172411.DOI:10.1371/journal.pone.0172411
[29] KOENIG D,JIMÉNEZ-GÓMEZ J M,KIMURA S,FULOP D,CHITWOOD D H,HEADLAND L R,KUMAR R,COVINGTON M F,DEVISETTY U K,TAT A V,TOHGE T,BOLGER A,SCHNEEBERGER K,OSSOWSKI S,LANZ C,XIONG G,TAYLOR-TEEPLES M,BRADY S M,PAULY M,WEIGEL D,USADEL B,FERNIE AR,PENG J,SINHA N R,MALOOF J N.Comparative transcriptomics reveals patterns of selection in domesticated and wild tomato[J].Proceedings of the National Academy of Science of the United States of America,2013,110(28):2655-2662.
[30] ZHAO X M,LIU Y,LIU X,JIANG J.Comparative transcriptome profiling of two tomato genotypes in response to potassium-deficiency stress[J/OL].International Journal of Molecular Sciences,2018,19(8):2402.DOI:10.3390/ijms19082402.
[31] LIU T M,TANG S W,ZHU S Y,TANG Q,ZHENG X.Transcriptome comparison reveals the patterns of selection in domesticated and wild ramie [Boehmeria nivea (L.) Gaud][J].Plant Molecular Biology,2014,86(1/2):85-92.
[32] ZHENG H X,YANG Z,WANG W Q,GUO S,LI Z,LIU K,SUI N.Transcriptome analysis of maize inbred lines differing in drought tolerance provides novel insights into the molecular mechanisms of drought responses in roots[J].Plant Physiology and Biochemistry,2020,149:11-26.
[33] LIU Y H,KUI L W,DENG C,WARRAN B,WANG L,YU B,YANG H,WANG J,ESPLEY R V,ZHANG J,WANG D,ALLAN A C.Comparative transcriptome analysis of white and purple potato to identify genes involved in anthocyanin biosynthesis[J/OL].PLoS ONE,2015,10(6):e0129148.DOI:10.1371/journal.pone.0129148.
[34] LIU F,YANG Y J,GAO J W,MA C,BI Y.A comparative transcriptome analysis of a wild purple potato and its red mutant provides insight into the mechanism of anthocyanin transformation[J/OL].PLoS ONE,2018,13(1):e0191406.DOI:10.1371/journal.pone.0191406.
[35] HUANG J Y,XING M H,LI Y,CHENG F,GU H,YUE C,ZHANG Y.Comparative transcriptome analysis of the skin-specific accumulation of anthocyanins in black peanut (Arachis hypogaea L.)[J].Journal of Agricultural and Food Chemistry,2019,67(4):1312-1324.
[36] 李佳伟,马钰聪,杨鑫雷,王梅,崔顺立,侯名语,刘立峰,胡梦蝶,蒋晓霞,穆国俊.花生种皮色素合成相关通路的转录组-代谢组学联合分析[J].植物遗传资源学报,2022,23(1):240-254.LI Jiawei,MA Yucong,YANG Xinlei,WANG Mei,CUI Shunli,HOU Mingyu,LIU Lifeng,HU Mengdie,JIANG Xiaoxia,MU Guojun.Transcriptomics-metabolomics combined analysis highlight the mechanism of testa pigment formation in peanut(Arachis hypogaea L.)[J].Journal of Plant Genetic Resources,2022,23(1):240-254.
[37] WANG Y Z,DAI M S,ZHANG S J,SHI Z.Exploring candidate genes for pericarp russet pigmentation of sand pear (Pyrus pyrifolia) via RNA-Seq data in two genotypes contrasting for pericarp color[J].PLoS ONE,2014,9(1):e83675.
[38] INOUE E,KASUMI M,SAKUMA F,ANZAI H,AMANO K,HARA H.Identification of RAPD marker linked to fruit skin color in Japanese pear(Pyrus pyrifolia Nakai)[J].Scientia Horticulturae,2006,107(3):254-258.
[39] 衡伟,程秀云,贾兵,刘普,刘莉,叶振风,朱立武.mRNA 差异显示法筛选‘砀山酥梨’褐皮芽变相关差异表达基因[J].中国农业科学,2013,46(15):3172-3179.HENG Wei,CHENG Xiuyun,JIA Bing,LIU Pu,LIU Li,YE Zhenfeng,ZHU Liwu.Differential expression genes of‘Dangshansuli’pear russet mutant screened with DDRT-PCR[J].Scientia Agricultura Sinica,2013,46(15):3172-3179.
[40] FRAGA C G,CLOWERS B H,MOORE R J,ZINK E M.Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography-mass spectrometry,XCMS,and chemometrics[J].Analytical Chemistry,2010,82(10):4165-4173.
[41] CHEN W,GONG L,GUO Z L,WANG W S,ZHANG H Y,LIU X Q,YU S,XIONG L Z,LUO J.A novel integrated method for large-scale detection,identification,and quantification of widely targeted metabolites:Application in the study of rice metabolomics[J].Molecular Plant,2013,6(6):1769-1780.
[42] ZHU G T,WANG S C,HUANG Z J,ZHANG S,LIAO Q,ZHANG C,LIN T,QIN M,PENG M,YANG C,CAO X,HAN X,WANG X,VAN DER KNAAP E,ZHANG Z,CUI X,KLEE H,FERNIE A R,LUO J,HUANG S.Rewiring of the fruit metabolome in tomato breeding[J].Cell,2018,172(1/2):249-261.
[43] QIU W W,SU W Q,CAI Z Y,DONG L,LI C,XIN M,FANG W,LIU Y,WANG X,HUANG Z,REN H,WU Z.Combined analysis of transcriptome and metabolome reveals the potential mechanism of coloration and fruit quality in yellow and purple Passiflora edulis Sims[J].Journal of Agricultural and Food Chemistry,2020,68(43):12096-12106.
[44] TRAPNELL C,ROBERTS A,GOFF L,ERTEA G,KIM D,KELLEY D R,PIMENTEL H,SALZBERG S L,RINN J L,PACHTER L.Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks[J].Nature Protocols,2012,7(3):562-578.
[45] KIM D,LANGMEAD B,SALZBERG S L.HISAT:a fast spliced aligner with low memory requirements[J].Nature Methods,2015,12(4):357-360.
[46] DOBIN A,DAVIS C A,SCHLESINGER F,DRENKOW J,ZALESKI C,JHA S,BATUT P,CHAISSON M,GINGERAS T R.STAR:ultrafast universal RNA-seq aligner[J].Bioinformatics,2012,29(1):15-21.
[47] WU J,WANG Z,SHI Z,ZHANG S,MING R,ZHU S,KHAN M A,TAO S,KORBAN S S,WANG H,CHEN N J,NISHIO T,XU X,CONG L,QI K,HUANG X,WANG Y,ZHAO X,WU J,DENG C,GOU C,ZHOU W,YIN H,QIN G,SHA Y,TAO Y,CHEN H,YANG Y,SONG Y,ZHAN D,WANG J,LI L,DAI M,GU C,WANG Y,SHI D,WANG X,ZHANG H,ZENG L,ZHENG D,WANG C,CHEN M,WANG G,XIE L,SOVERO V,SHA S,HUANG W,ZHANG S,ZHANG M,SUN J,XU L,LI Y,LIU X,LI Q,SHEN J,WANG J,PAULL RE,BENNETZEN J L,WANG J,ZHANG S.The genome of the pear (Pyrus bretschneideri Rehd.) [J].Genome Resarch,2013,23,396-408.
[48] XIE C,MAO X Z,HUANG J J,DING Y,WU J M,DONG S,KONG L,GAO G,LI C Y,WEI L P.KOBAS 2.0:A web server for annotation and identification of enriched pathways and diseases[J].Nucleic Acids Research,2011,39(S2):316-322.
[49] LIVAK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method[J].Methods,2001,25(4):402-408.
[50] 张智涛.大果水晶梨褐色突变体木栓层形成及其相关酶研究[D].秦皇岛:河北科技师范学院,2011.ZHANG Zhitao.Study on the cork layer formation of the brown mutant of suisho pear(Pyrus pyrifolia)and its related enzymes[D].Qinhuangdao:Hebei Normal University of Science&Technology,2011.
[51] 刘月,杨健,王龙,王苏珂,苏艳丽,李秀根,薛华柏.木质素合成途径及其调控梨褐皮性状研究进展[J].果树学报,2018,35(S):17-25.LIU Yue,YANG Jian,WANG Long,WANG Suke,SU Yanli,LI Xiugen,XUE Huabai.Advances in studies on lignin synthesis pathway and its regulation of pear brown peel characteristic[J].Journal of Fruit Science,2018,35(S):17-25.
[52] 王慧.‘翠冠’梨果锈的形成与防锈技术的研究[D].南京:南京农业大学,2017.WANG Hui.Study on fruit russet formation of‘Cuiguan’pear and its control measurement[D].Nanjing:Nanjing Agricultural University,2017.
[53] QI K J,SONG X F,YUAN Y Z,BAO J,GONG X,HUANG X,KHANIZADEH S,ZHANG S,TAO S. CAD genes:Genomewide identification,evolution,and their contribution to lignin biosynthesis in pear(Pyrus bretschneideri)[J/OL].Plants(Basel,Switzerland),2021,10(7):1444.DOI:10.3390/plants10071444.
[54] AMBAVARAM M M R,KRISHNAN A,TRIJATMIKO K R,PEREIRA A.Pereira,Coordinated activation of cellulose and repression of lignin biosynthesis pathways in rice[J].Plant Physiology,2011,155(2):916-931.
[55] 杨冲.调控番茄木质素合成的机理研究[D].武汉:华中农业大学,2019.YANG Chong.Study on the mechanism of S1MYB4 regulating tomato lignin synthesis[D].Wuhan:Huazhong Agricultural University,2019.
[56] 曹文雪.甘蓝蜡质合成基因BoCER1 的克隆和功能验证[D].北京:中国农业科学院,2021.CAO Wenxue.Cloning and functional verification of the wax synthesis gene BoCER1 in cabbage(Brassica oleracea var. capitata L.)[D].Beijing:ChineseAcademy ofAgricultural Sciences,2021.
[57] 李浩男,王宏,贾晓东,吕照清,蔡斌华,乔玉山.‘黄花’及其芽变‘绿黄花’梨成熟期果皮代谢物鉴定与比较分析[J].果树学报,2015,32(6):1118-1127.LI Haonan,WANG Hong,JIA Xiaodong,LÜ Zhaoqing,CAI Binhua,QIAO Yushan.Identification and comparison of differential metabolites from the skin of mature fruit in‘Huanghua’pear and its bud mutant‘Lühuanghua’pear(Pyrus pyrifolia Nakai)[J].Journal of Fruit Science,2015,32(6):1118-1127.
[58] BOURDENX B,BERNARD A,DOMERGUE F,PASCAL S,LÉGER A,ROBY D,PERVENT M,VILE D,HASLAM R P,NAPIER J A,LESSIRE R,JOUBÈS J.Overexpression of Arabidopsis ECERIFERUM1 promotes wax very-long-chain alkane biosynthesis and influences plant response to biotic and abiotic stresses[J].Plant Physiology,2011,156(1):29-45.
[59] GOU J Y,YU X H,LIU C J.A hydroxycinnamoyltransferase responsible for synthesizing suberin aromatics in Arabidopsis[J].Proceedings of the National Academy of Sciences of the United States of America,2009,106(44):18855-18860.
[60] LOTFY S,JAVELLE F,NEGREL J.Distribution of hydroxycinnamoyl-CoA:ω-hydroxypalmitic acid O-hydroxycinnamoyltransferase in higher plants[J].Phytochemistry,1995,40(2):389-391.
[61] WANG G L,XU J,LI L C,GUO Z,SI Q,ZHU G,WANG X,GUO W.GbCYP86A1-1 from Gossypium barbadense positively regulates defence against Verticillium dahliae by cell wall modification and activation of immune pathways[J].Plant Biotechnology Journal,2020,18(1):222-238.
[62] VISHWANATH S J,DELUDE C,DOMERGUE F,ROWLAND O.Suberin:biosynthesis,regulation,and polymer assembly of a protective extracellular barrier[J].Plant Cell Reports,2015,34(4):573-586.
[63] MOLINA I,LI-BEISSON Y,BEISSON F,OHLROGGE J B,POLLARD M.Identification of an Arabidopsis feruloyl-coenzyme a transferase required for suberin synthesis[J].Plant Physiology,2009,151(3):1317-1328.
[64] 孙欣,韩键,房经贵,上官凌飞,王西成,宋长年,李晓颖.葡萄浆果着色分子机理的重要研究进展[J].植物生理学报,2012,48(4):333-342.SUN Xin,HAN Jian,FANG Jinggui,SHANGGUAN Lingfei,WANG Xicheng,SONG Changnian,LI Xiaoying.Important research progress of coloring molecular mechanisms in grape berry[J].Plant Physiology Journal,2012,48(4):333-342.
[65] XIAO Y,WEN J,MENG R,MENG Y,ZHOU Q,NIE Z L.The expansion and diversity of the CYP75 gene family in Vitaceae[J].PeerJ,2021,9:e12174.
[66] 王贝贝.四翅滨藜类黄酮合成关键酶编码基因的克隆及功能分析[D].兰州:兰州大学,2021.WANG Beibei.Cloning and functional analysis of genes encoding key enzymes of flavonoid synthesis from Atriplex canescens[D].Lanzhou:Lanzhou University,2021.
[67] 刘晓.苹果黄酮醇合成酶基因MdFLS1 的功能研究[D].泰安:山东农业大学,2018.LIU Xiao.Functional identification of flavonol synthase gene MdFLS1 in apple[D].Taian:Shandong Agricultural University,2018.
[68] 赵宝泉,邢锦城,温祝桂,徐海东,王进左,李玉明,董静,洪立洲.林木盐胁迫响应机制研究进展[J].现代农业科技,2020(21):159-165.ZHAO Baoquan,XING Jincheng,WEN Zhugui,XU Haidong,WANG Jinzuo,LI Yuming,DONG Jing,HONG Lizhou.Research progress on salt stress response mechanism of forest trees[J].Modern Agricultural Science and Technology,2020(21):159-165.
[69] 李甦,魏薇,朱燕蕾,杜娟.紫外线(UVB)胁迫下葡萄叶片和果皮结构变化探究[J].现代园艺,2017(6):5-7.LI Sheng,WEI Wei,ZHU Yanlei,DU Juan.Investigation of structural changes in grapevine leaves and skins under ultraviolet(UVB)stress[J].Xiandai Horticulture,2017(6):5-7.
[70] 张小双,郑迎春,曹玉芬,田路明,董星光,张莹,齐丹,霍宏亮.‘早酥’和‘南果梨’16 个部位多酚物质组成及含量分析[J].中国农业科学,2017,50(3):545-555.ZHANG Xiaoshuang,ZHENG Yingchun,CAO Yufen,TIAN Luming,DONG Xingguang,ZHANG Ying,QI Dan,HUO Hongliang.The composition and content of polyphenols in 16 parts of‘Zaosu’and‘Nanguoli’[J].Scientia Agricultura Sinica,2017,50(3):545-555.
[71] 曹纬国,刘志勤,邵云,陶燕铎.黄酮类化合物药理作用的研究进展[J].西北植物学报,2003,23(12):2241-2247.CAO Weiguo,LIU Zhiqin,SHAO Yun,TAO Yanduo.A progress in pharmacological research of flavonoids[J].Acta Botanica Boreali-Occidentalia Sinica,2003,23(12):2241-2247.
[72] 于娜,范红艳.苦参黄酮类化合物药理作用的研究进展[J].吉林医药学院学报,2021,42(4):304-307.YU Na,FAN Hongyan.Research progress on pharmacological effects of flavonoids from Sophora flavescens[J].Journal of Jilin Medical University,2021,42(4):304-307.
[73] 蔡红蝶,陶伟伟,宿树兰,郭盛,朱悦,郭建明,钱大玮,丛旭东,唐仁茂,段金廒.黄蜀葵花中黄酮类化合物抗抑郁活性及其上调大鼠海马组织中BDNF 的作用机制研究[J].药学学报,2017,52(2):222-228.CAI Hongdie,TAO Weiwei,SU Shulan,GUO Sheng,ZHU Yue,GUO Jianming,QIAN Dawei,CONG Xudong,TANG Renmao,DUAN Jin’ao.Antidepressant activity of flavonoid ethanol extract of Abelmoschus manihot corolla with BDNF up-regulation in the Hippocampus[J].Acta Pharmaceutica Sinica,2017,52(2):222-228.
[74] 郭萌,张晴,闫丽萍,毛明清,房丽娜.黄酮类化合物为主要活性成分的单味药和复方中药及其药理作用[J].沈阳医学院学报,2018,20(6):558-561.GUO Meng,ZHANG Qing,YAN Liping,MAO Mingqing,FANG Lina.Flavonoids as the main active ingredients of single herbs and compound Chinese medicines and their pharmacological activity[J].Journal of Shenyang Medical College,2018,20(6):558-561.