槟子(Malus pumila × M. asiatica)为蔷薇科(Rosaceae)苹果属(Malus)落叶乔木,由绵苹果(M.pumila)和沙果(M.asiatica)杂交而来,在我国有着非常悠久的栽培历史,主要分布在北京、河北、山西、陕西等地,是我国重要的苹果属植物种质资源[1]。槟子品种多样、种类丰富,不同品种之间的果色、果个和风味也略有不同[2]。槟子,又名酸槟子,果形为尖顶,果色为紫红色,香气浓郁[3-4]。香气赋予槟子果实独特的风味品质,但关于其香气物质组分及合成代谢的研究还未见报道。
香气是一类具有水溶性和脂溶性特征的挥发性物质。目前,在植物界中已经鉴定出2000 多种挥发性物质,主要分为酯类、醛类、醇类、萜类、酮类及酸类等[5-6]。其中,苹果中已发现350 多种,主要以酯类物质为主[7-8]。果实中酯类物质的合成主要通过脂肪酸和氨基酸代谢途径,其中脂肪酸代谢途径又分为脂氧合酶(Lipoxygenase,LOX)和β-氧化途径[9-11]。脂氧合酶途径主要涉及四种催化酶,分别为脂氧合酶(LOX)、氢过氧化物裂解酶(HPL)、醇脱氢酶(ADH)和醇酰基转移酶(AAT)[12]。作为脂氧合酶途径的起始酶,LOX 催化亚麻酸、亚油酸等不饱和脂肪酸生成氢过氧化物,氢过氧化物在HPL作用下转化成相应的醛类物质,而这些醛类物质在ADH 催化下生成醇,最后由AAT 将醇类物质催化生成短链的酯类物质,这些酯类物质在果实香气品质形成过程中发挥重要作用[4,13-15]。目前,已在多种植物中克隆到LOX 基因,如番茄[16]、猕猴桃[17]、梨[18]等。南果梨中,茉莉酸甲酯能够诱导PuLOX1和PuLOX3 的表达[19];辣椒CaLOX2 在各个器官都有所表达,其表达受到茉莉酸甲酯、水杨酸及H2O2的诱导,并能够积极响应疫霉菌及低温、高盐等逆境胁迫[20];过表达MdLOX1a 能够提高苹果愈伤的醇类、酯类等物质含量,表明苹果MdLOX1a 有助于促进香气物质的合成[21]。
香气是槟子果实重要的风味特征之一,挖掘香气相关的重要基因,对槟子呈香机制的研究具有重要意义。笔者在槟子转录组数据的基础上,从槟子果实中克隆到脂氧合酶途径中的起始酶基因LOX2a,并通过生物信息学和分子生物学等技术手段,对LOX2a编码蛋白性质及表达情况进行了初步探究,为LOX2a基因在槟子果实中的功能研究奠定基础。
1 材料和方法
1.1 材料
试验材料于2020 年4—10 月取自北京市延庆区帮水峪村槟子种植园(115°56′29.472″E,40°19′42.445″N)生长状态良好的24年生槟子果树。槟子果实取样时间为花后30 d(5 月17 日)、60 d(6 月16日)、90 d(7月16日)、120 d(8月15日)和150 d(9月14 日),花于4 月中旬盛花期采集,茎、叶取自花后150 d结果枝新梢,于花后150 d取根部材料,种子取自花后150 d的果实。取样后立即运往实验室,液氮速冻后放于-80 ℃冰箱保存备用。
1.2 总RNA提取及反转录
使用华越洋多糖多酚植物RNA 提取试剂盒提取槟子各组织部位的RNA,具体操作参照说明书。反转录参照反转录试剂盒TransScript First-Strand cDNA Synthesis SuperMix 说明书(北京全式金生物技术有限公司)。
1.3 CTAB法粗提基因组
参照侯柄竹[22]的方法提取槟子基因组,加入20 μL ddH2O溶解基因组,放入-80 ℃冰箱备用。
1.4 槟子LOX2a编码区序列克隆
基于槟子转录组数据(SRA 登录号:PRJNA875845),利用SnapGene软件设计引物(表1),以反转录得到的cDNA为模板,使用高保真DNA聚合酶2× Phanta® Max Master Mix(南京诺唯赞生物科技股份有限公司)克隆目的片段。PCR 产物经1%(w)琼脂糖凝胶电泳检测后,用Axygen 胶回收试剂盒回收目的条带,并连接至克隆载体pEASY®-Blunt Simple Cloning Vector(北京全式金)上,转化大肠杆菌Trans5α,挑取单克隆测序(苏州金唯智生物科技有限公司)。
表1 PCR 引物信息
Table 1 Primers information for PCR
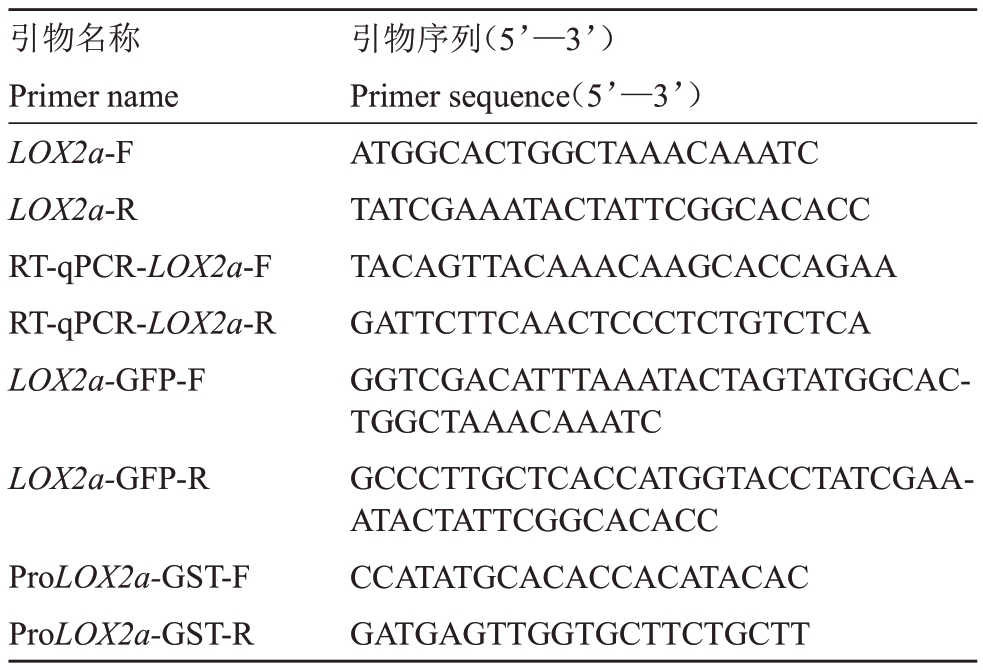
引物名称Primer name LOX2a-F LOX2a-R RT-qPCR-LOX2a-F RT-qPCR-LOX2a-R LOX2a-GFP-F LOX2a-GFP-R ProLOX2a-GST-F ProLOX2a-GST-R引物序列(5’—3’)Primer sequence(5’—3’)ATGGCACTGGCTAAACAAATC TATCGAAATACTATTCGGCACACC TACAGTTACAAACAAGCACCAGAA GATTCTTCAACTCCCTCTGTCTCA GGTCGACATTTAAATACTAGTATGGCACTGGCTAAACAAATC GCCCTTGCTCACCATGGTACCTATCGAAATACTATTCGGCACACC CCATATGCACACCACATACAC GATGAGTTGGTGCTTCTGCTT
1.5 槟子LOX2a生物信息学分析
利用NCBI Conserved domains(https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi)预 测LOX2a保守结构域;使用TMHMM 2.0(https://services.healthtech.dtu.dk/service.php? TMHMM-2.0)进行蛋白的跨膜结构域分析;利用ExPASy(https://web.expasy.org/protparam/)在线预测分子质量、等电点、蛋白质分子式、脂肪系数和不稳定系数;利用NPS(https://npsa- prabi.ibcp.fr/cgi- bin/npsa_automat.pl?page=npsa_gor4.html)和Swiss Model(http://swissmodel.expasy.org/)在线分析蛋白质二级和三级结构;利用MEGA 5.1 软件进行氨基酸序列的同源性比对并构建系统进化树;用Cell-PLoc 2.0(http://www.csbio.sjtu.edu.cn/bioinf/Cell-PLoc-2/)预测细胞定位;通过在线软件PlantCARE(http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)对LOX2a启动子的顺式作用元件进行分析。
1.6 实时荧光定量PCR(RT-qPCR)
以槟子不同组织部位和果实时期的cDNA为模板,利用Primer 5 软件设计荧光定量引物(表1),以苹果Actin 作为内参基因,使用Roche LightCycler®96 Real-Time PCR仪检测LOX2a基因在槟子各个组织部位和果实时期的表达情况。荧光定量反应体系按照全式金TransStart Top Green qPCR SuperMix说明书加样。采用三步法反应程序:94 ℃30 s;94 ℃5 s,60 ℃15 s,72 ℃10 s,40个循环。试验采取3次重复,用2-ΔΔCt计算基因的相对表达水平。
1.7 亚细胞定位
表达载体为pCAMBIA-Super1300-GFP,选定酶切位点SpeⅠ和KpnⅠ设计引物(表1),克隆携有载体同源臂的目的片段,利用同源重组酶ClonExpressⅡOne Step Cloning Kit(南京诺唯赞)将目的片段与表达载体相连。将构建好的重组质粒35S::LOX2a-GFP 转化农杆菌GV3101,侵染烟草下表皮,3 d后在激光共聚焦下观察绿色荧光在烟草细胞的位置。
1.8 圆片温育与激素处理
选取花后90 d 的槟子果实用于圆片温育,使用打孔器将果实制备成直径为1 cm 的圆柱体,用刀片切成厚度为1 mm 的薄片,将果实圆片放入温育平衡液中平衡30 min,温育平衡液包含50 mmol·L-1 MES(pH 5.5)、10 mmol·L-1 MgCl2、10 mmol·L-1 EDTA、5 mmol ·L-1 CaCl2、5 mmol ·L-1 Vc 和200 mmol·L-1 甘露醇。将平衡后的圆片分成5部分,试验组有4个,分别在平衡液中加入100 μmol·L-1脱落酸(ABA)、100 μmol·L-1 茉莉酸甲酯(MeJA)、100 μmol·L-1水杨酸(SA)和200 mmol·L-1赤霉素(GA3),激素配制参照表2。25 ℃振荡孵育,每隔1 h(共计5 h)取样2 g,液氮速冻,放于-80 ℃保存备用。
表2 激素配制方法
Table 2 Hormone preparation method
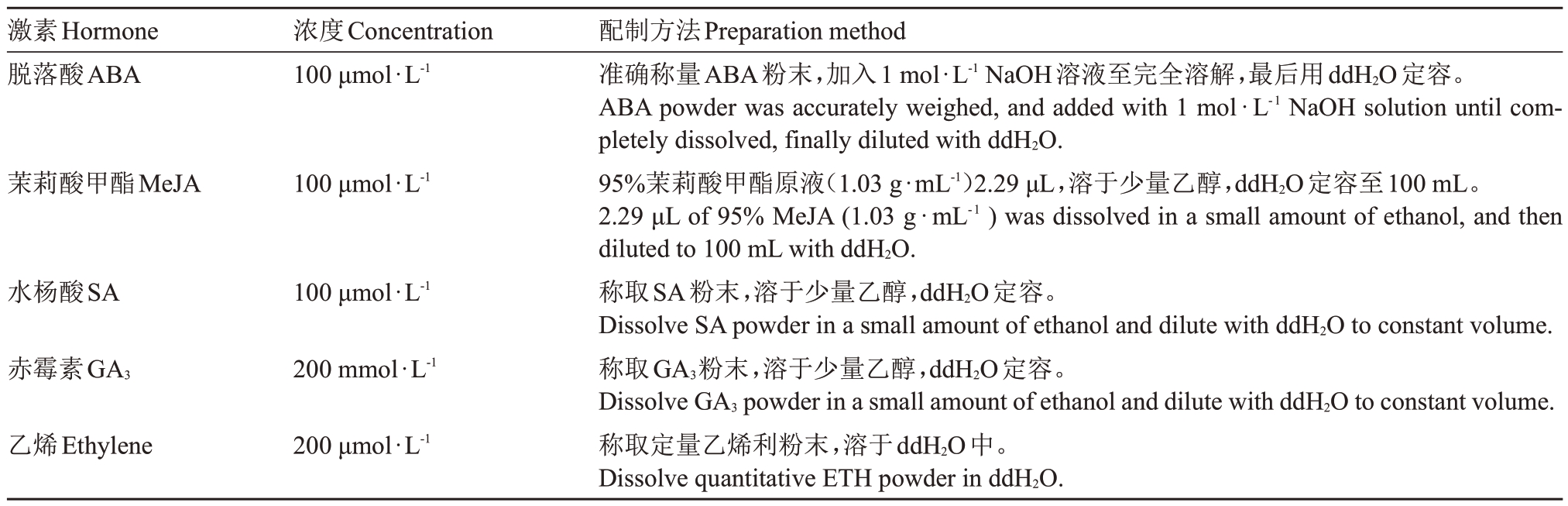
激素Hormone脱落酸ABA浓度Concentration 100 μmol·L-1茉莉酸甲酯MeJA 100 μmol·L-1水杨酸SA 100 μmol·L-1赤霉素GA3 200 mmol·L-1乙烯Ethylene 200 μmol·L-1配制方法Preparation method准确称量ABA粉末,加入1 mol·L-1 NaOH溶液至完全溶解,最后用ddH2O定容。ABA powder was accurately weighed, and added with 1 mol·L-1 NaOH solution until completely dissolved,finally diluted with ddH2O.95%茉莉酸甲酯原液(1.03 g·mL-1)2.29 μL,溶于少量乙醇,ddH2O定容至100 mL。2.29 μL of 95% MeJA(1.03 g·mL-1 ) was dissolved in a small amount of ethanol, and then diluted to 100 mL with ddH2O.称取SA粉末,溶于少量乙醇,ddH2O定容。Dissolve SA powder in a small amount of ethanol and dilute with ddH2O to constant volume.称取GA3粉末,溶于少量乙醇,ddH2O定容。Dissolve GA3 powder in a small amount of ethanol and dilute with ddH2O to constant volume.称取定量乙烯利粉末,溶于ddH2O中。Dissolve quantitative ETH powder in ddH2O.
乙烯利与1-MCP 处理槟子果实,具体操作过程:将槟子浸泡在200 μmol·L-1 乙烯利溶液中,20 min 后取出,自然晾干;将体积分数为1 μL·L-1的1-MCP溶液与果实放入密闭中熏蒸24 h。所有样品处理结束后放入0.02 mm 厚PE保鲜袋中,贮存条件为温度(20±1)℃、相对湿度90%。
1.9 数据分析
使用软件Excel、Origin 8.1 和Adobe Illustrator处理图表,使用SPSS Statistics v.20进行数据的差异显著性分析(p<0.05)。
2 结果与分析
2.1 槟子LOX2a基因的克隆
根据笔者实验室前期获得的槟子果实转录组数据,筛选到1 个与槟子果实采后总挥发性物质变化趋势高度相似的LOX家族基因(图1)。以槟子果实cDNA 为模板,使用特异性引物(表1)克隆该基因,得到大小约2700 bp的单一条带(图2-A),测序结果显示该基因含有2721 bp 的开放阅读框(open reading frame,ORF),并 与 苹 果LOX2a(GenBank:KC706483.1)基因序列相似度最高,因此将该基因命名为槟子LOX2a,已将该基因序列上传至NCBI(GenBank:ON952464)。槟子LOX2a 含有1 个Lipoxygenase 功能域,且无跨膜结构,为膜外蛋白(图2-B~C)。
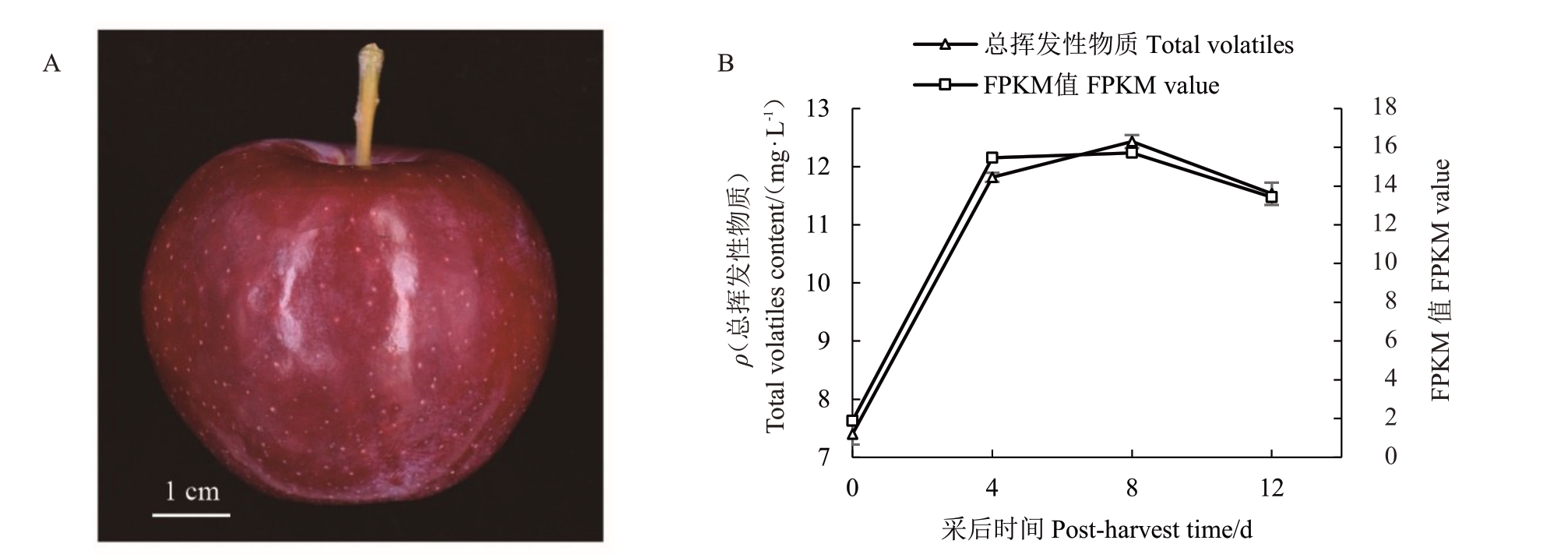
图1 槟子果实(A)及其采后总挥发性物质含量和LOX2a FPKM 值(B)的变化
Fig.1 Variation of total volatiles contents and LOX2a FPKM value(B)after harvest of Binzi fruit(A)
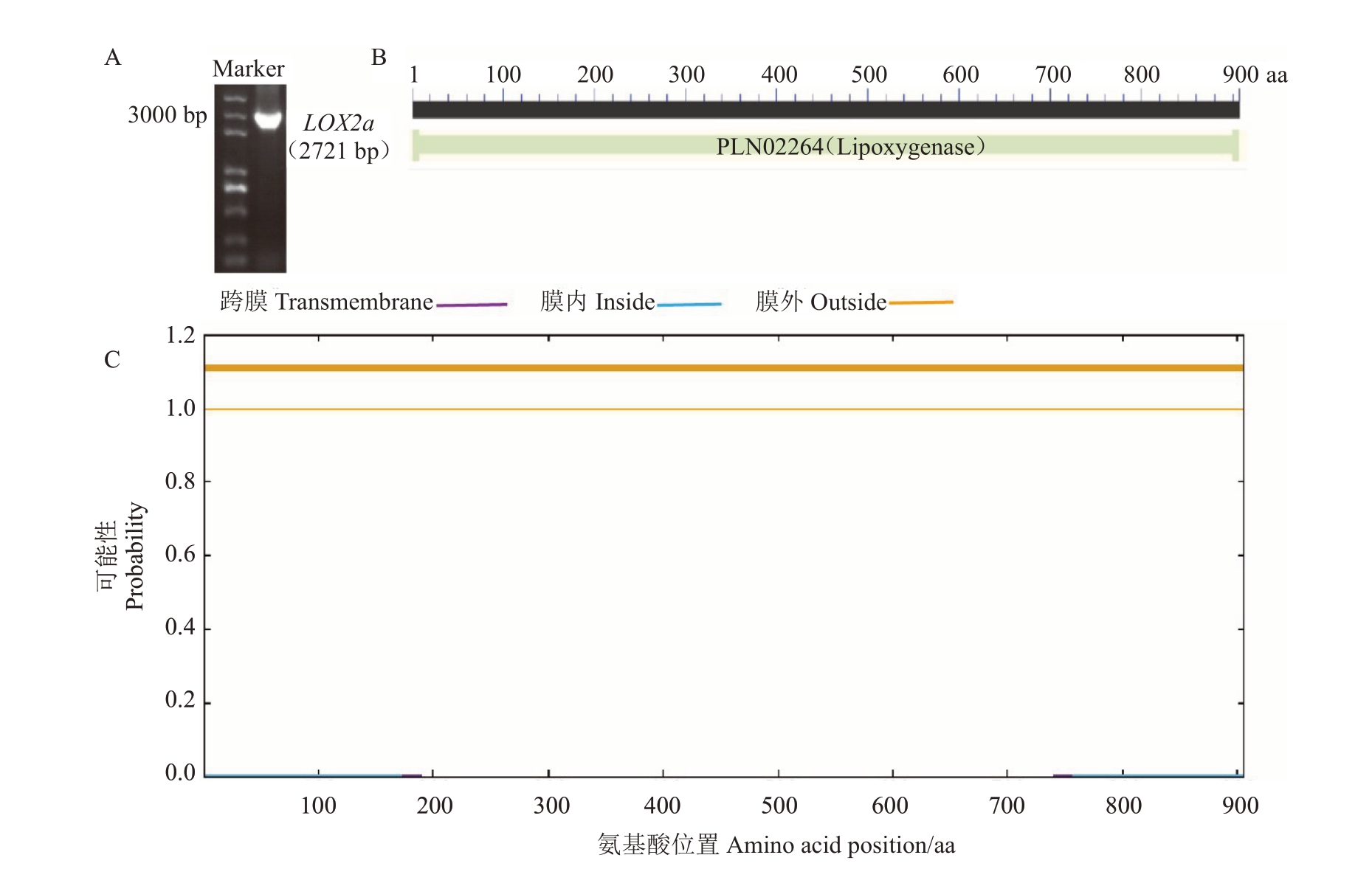
图2 槟子LOX2a 基因克隆及功能域和跨膜域分析
Fig.2 Cloning,functional domains,and transmembrane domains of Binzi LOX2a
A.槟子LOX2a 基因克隆;B.LOX2a 功能域分析;C.LOX2a 跨膜域分析。
A.Cloning of Binzi LOX2a;B.Functional domain of LOX2a;C.Transmembrane domain of LOX2a.
2.2 槟子LOX2a基因的生物信息学分析
通过ExPASy对槟子LOX2a氨基酸序列进行分析,结果表明,LOX2a 编码906 个氨基酸,分子质量为102 559.30,等电点为6.97,蛋白质分子式为C4612H7222N1254O1341S27,脂肪系数为87.49,不稳定系数为43.39。对LOX2a 蛋白二级、三级结构的预测结果如图3 所示,发现LOX2a 蛋白的二级结构主要由α 螺旋(α-helix)、延伸链(extended strand)和无规则卷曲(random coil)构成(图3-A)。LOX2a 蛋白的α螺旋包含302个氨基酸,占比为33.33%;延伸链包含141个氨基酸,占比为15.56%;无规则卷曲所含氨基酸最多,有463个,占比为51.10%。

图3 槟子LOX2a 二级结构和三级结构预测
Fig.3 Prediction of secondary and tertiary structures of Binzi LOX2a
A.槟子LOX2a 二级结构预测;B.槟子LOX2a 三级结构预测。
A.Prediction of secondary structure of Binzi LOX2a;B.Prediction of tertiary structure of Binzi LOX2a.
从NCBI下载与槟子LOX2a相似度较高的21条氨基酸序列,利用软件MEGA 5.1 以邻接法(Neighbor-Joining method)构建LOX2a 氨基酸序列的系统进化树。结果表明,LOX2a的同源序列中有11个属于蔷薇科,其中与苹果LOX2a同源性最高(图4)。

图4 槟子LOX2a 的系统进化分析
Fig.4 Phylogenetic analysis of Binzi LOX2a
2.3 槟子LOX2a亚细胞定位
利用在线软件Cell-PLoc 2.0 预测LOX2a 定位于细胞质(Cytoplasm)。为了探究LOX2a 的亚细胞定位,将携带35S::LOX2a GFP 重组质粒的农杆菌GV3101注射到烟草下表皮。通过激光共聚焦发现在烟草细胞质位置有绿色荧光,表明槟子LOX2a定位于细胞质(图5)。

图5 槟子LOX2a 亚细胞定位
Fig.5 Subcellular localization of Binzi LOX2a
2.4 槟子LOX2a的时空表达
为了研究LOX2a 在槟子各器官及果实发育期的表达模式,提取了根、茎、叶、花、种子和不同发育期果实的总RNA,并通过RT-qPCR检测了LOX2a基因的表达水平。结果表明,LOX2a 基因在槟子根部表达量最高,其次是茎、叶、花,表达量最低的是种子,根中表达量是种子的109.71倍,表明槟子LOX2a基因的表达具有组织特异性;LOX2a 基因在果实发育成熟过程中呈现出先降低后升高的趋势,尤其是在花后30 d 时表达量最高,在花后60~120 d 期间变化不明显,在成熟期(花后150 d)时表达量呈现出升高的趋势(图6)。

图6 槟子LOX2a 在不同组织部位和果实发育阶段的相对表达量
Fig.6 Relative expression level of Binzi LOX2a in different tissues and developmental stages of fruit
不同小写字母代表样本间的差异显著(p<0.05)。下同。
Different small letters indicate significant differences of the independent sample t-test(p<0.05).The same below.
2.5 槟子LOX2a基因在激素诱导下表达分析
为了研究LOX2a 基因的表达调控,对槟子LOX2a基因的启动子进行了克隆,得到长度为1839 bp的启动子序列(图7)。使用在线软件PlantCARE 对LOX2a基因启动子的顺式作用元件进行预测,发现启动子上有多个不同类型的响应元件,比如光响应元件(photo responsive element)、激素响应元件(hormone response element)和胁迫响应元件(stress related component)。其中,激素响应元件包括ABA响应元件、MeJA响应元件、GA响应元件和SA响应元件(表3)。结果表明,LOX2a基因的表达很可能受到激素的调控。
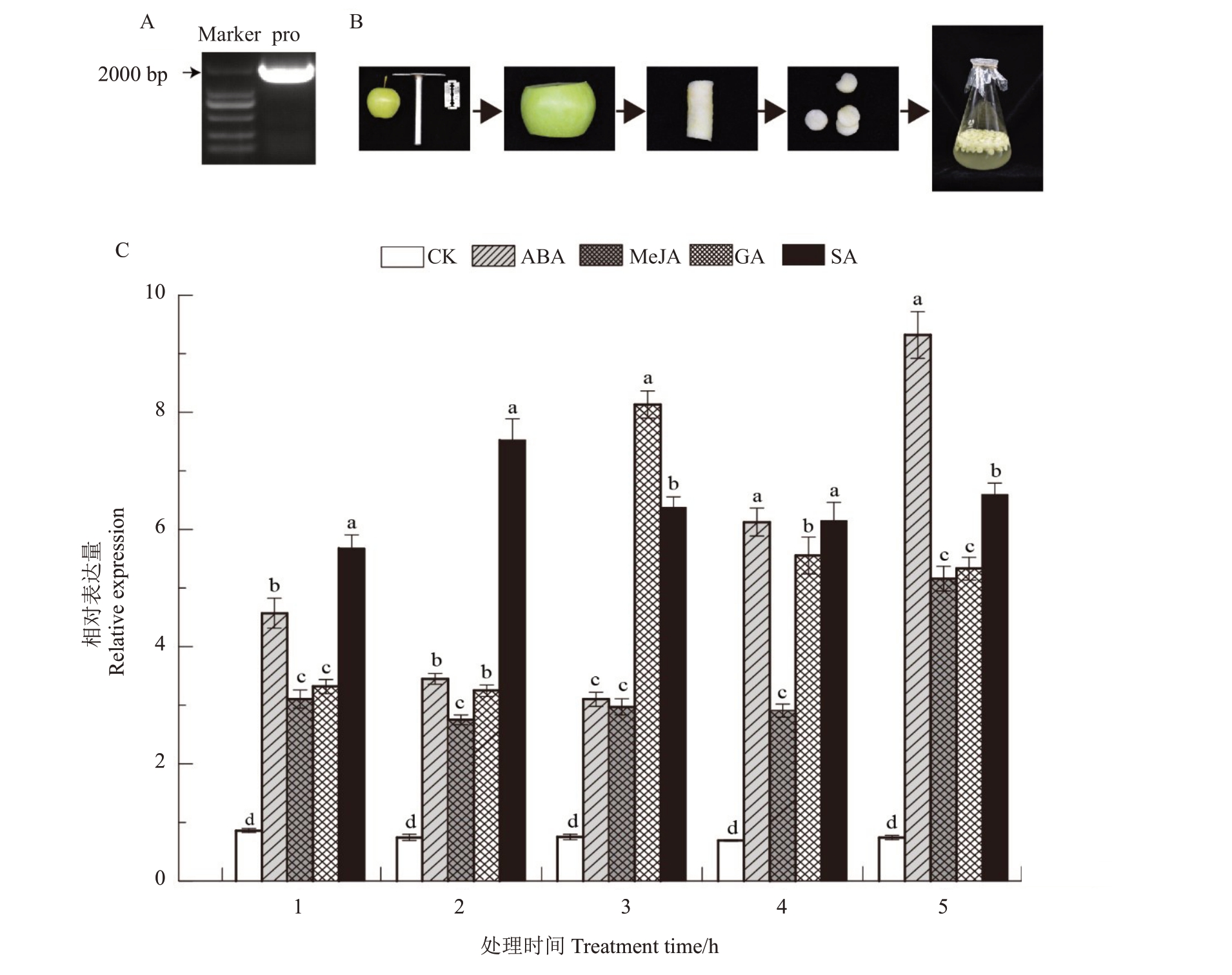
图7 不同外源激素处理槟子果实后的LOX2a 基因表达水平变化
Fig.7 Expression levels of LOX2a in Binzi fruit with treatment of different exogenous hormone
A.Pro.克隆出的LOX2a 启动子;B.圆片温育实验操作流程;C.外源激素处理槟子果实后的LOX2a 基因表达水平变化。
A.Pro.The promoter of LOX2a;B.The operational process of fruit disc tissue incubation;C.Expression levels of LOX2a in Binzi fruit with treatment of different exogenous hormone.
表3 槟子LOX2a 启动子顺式作用元件
Table 3 The cis-acting regulatory elements in promoter of Binzi LOX2a
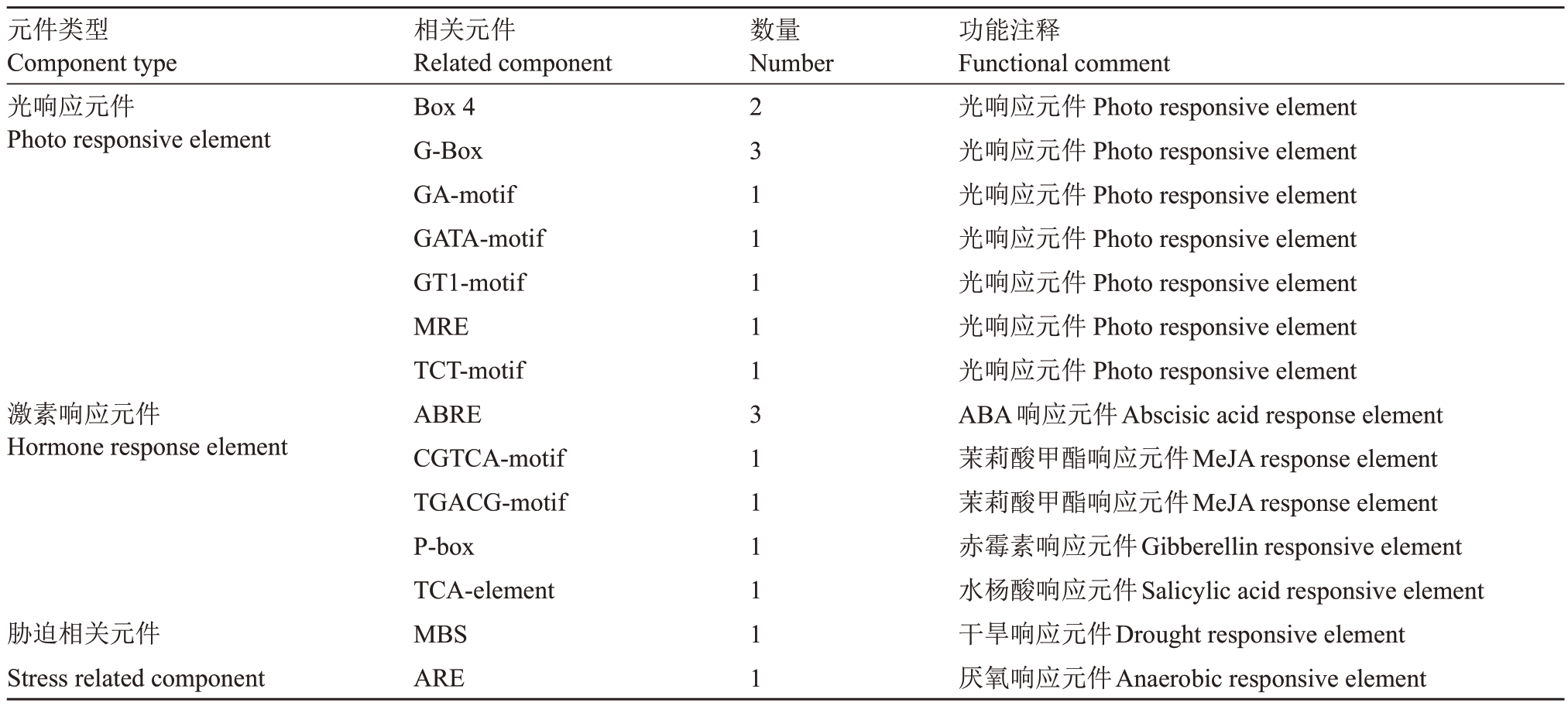
元件类型Component type光响应元件Photo responsive element数量Number激素响应元件Hormone response element胁迫相关元件Stress related component相关元件Related component Box 4 G-Box GA-motif GATA-motif GT1-motif MRE TCT-motif ABRE CGTCA-motif TGACG-motif P-box TCA-element MBS ARE 23111113111111功能注释Functional comment光响应元件Photo responsive element光响应元件Photo responsive element光响应元件Photo responsive element光响应元件Photo responsive element光响应元件Photo responsive element光响应元件Photo responsive element光响应元件Photo responsive element ABA响应元件Abscisic acid response element茉莉酸甲酯响应元件MeJA response element茉莉酸甲酯响应元件MeJA response element赤霉素响应元件Gibberellin responsive element水杨酸响应元件Salicylic acid responsive element干旱响应元件Drought responsive element厌氧响应元件Anaerobic responsive element
为了验证激素是否调控LOX2a基因表达,设计了槟子果实圆片温育试验,用ABA、MeJA、GA和SA四种激素处理果实圆片,通过实时荧光定量PCR检测LOX2a基因表达水平的变化(图7-B~C)。结果表明,4种激素处理后的LOX2a基因表达水平都明显高于对照组,并且呈现出不同的表达趋势。ABA实验组的LOX2a基因表达呈现出先下降后升高的趋势,尤其是在5 h 表达量最高;MeJA 处理后提高了LOX2a基因的表达水平,但在处理后的1~4 h变化不明显,5 h时表达水平最高;GA处理3 h时,LOX2a基因的表达量最高,随着处理时间延长其表达量逐渐降低;SA 试验组的LOX2a 基因表达水平在2 h 时达到最高,随后下降并维持在较为恒定的表达水平。在1种激素处理1 h后,SA组的LOX2a基因表达水平最高,其次是ABA,最后是MeJA和GA。综上结果表明,槟子LOX2a基因表达受到ABA、MeJA、GA和SA四种激素的调控,并且响应ABA和SA的调控更为明显。
乙烯是促进苹果果实发育成熟的重要激素之一。为了探究乙烯对槟子LOX2a基因表达的调控,本研究开展了乙烯及其抑制剂1-MCP 处理槟子果实的实验。结果如图8 所示,乙烯促进槟子果实成熟,而1-MCP的处理结果则相反;与对照相比,乙烯处理后LOX2a基因表达水平升高,1-MCP处理组的LOX2a 基因表达量低于对照组。以上结果表明,LOX2a基因表达受到乙烯的诱导。

图8 乙烯与1-MCP 对槟子LOX2a 基因表达的影响
Fig.8 Effects of ethylene and 1-MCP on Binzi LOX2a gene expression
3 讨 论
目前,果树育种方向主要在提高果实产量、增强植物抗逆性等方面,对果实香气等风味品质的关注相对较少[23-24]。但是,香气通过赋予果实独特的风味特征,在很大程度上吸引了消费者的购买欲望[25-28]。槟子作为我国传统的果树资源,果实色泽艳丽、香气浓郁,但是关于其香气形成的研究还未见报道。因此,挖掘出香气相关的基因,对解析槟子香气浓郁的分子机制具有重要作用。
脂氧合酶LOX是植物香气代谢中脂氧合酶途径的起始酶,在植物香气积累中扮演重要角色[4]。本研究以槟子转录组数据为基础,克隆出槟子LOX2a基因。槟子LOX2a 有且只有一个Lipoxygenase 功能域,且系统发育分析结果表明,其与苹果、梨、山荆子、草莓等蔷薇科植物的进化关系较近,说明LOX2a在基因进化过程中结构和功能较为保守。
关于LOX 基因表达模式已有一些研究,LOXs基因在植物器官(根、茎、叶、花、果实、种子等)和细胞器(叶绿体、细胞质等)中具有广泛分布的特点[29]。辣椒LOX2a 基因在叶片中表达量最高,花中表达量最低[20]。马铃薯StLOXH1 在叶片中表达最高,而StLOXH3主要在根中表达[30]。研究发现,苹果MdLOX1a基因在果皮中表达最高,在根、茎、叶等器官有少量表达[21]。槟子LOX2a 基因表达模式表明,其在根部表达量最高,其次是茎、叶、花,种子中表达量最低。研究表明,脂氧合酶LOX在调控植物生长发育、成熟衰老以及逆境胁迫中发挥重要作用[31]。番茄中过表达tomLOXD 能够提高抗逆基因LeHSP90、LePR1、LePR6 和LeZAT 的表达量[32]。根作为植物“隐藏”的敏感器官,在响应逆境胁迫过程中发挥重要作用[33-34]。槟子LOX2a基因在根部表达水平是果实的近20倍,表明LOX2a不仅与果实香气合成有关,而且可能参与了响应逆境胁迫的生理过程。苹果果实在发育早期主要以醛类物质为主,成熟时以酯类物质为主[35-36]。通过对槟子果实不同发育成熟时期的荧光定量PCR检测,发现LOX2a在花后30 d时表达量最高,随后下降并维持在一定水平,在150 d 时表达量升高。结果表明,成熟期LOX2a基因的表达可能促进了果实酯类香气物质的积累,而花后30 d时表达量最高,意味着LOX2a可能不仅参与香气合成,而且可能参与了诸如果实生长发育等过程。
LOX作为植物中一类具有多种生理功能的酶,其基因的表达受到茉莉酸、生长素、乙烯等激素的调控[19,37-38]。通过对茶树进行茉莉酸甲酯处理,发现有3 个CsLOX 基因表达水平发生上调,其中CsLOX1基因表达量上调117 倍[39]。乙烯能够促进苹果Md-LOX基因的表达和挥发性酯类物质的积累,并且在乙烯处理21 d 后,促进效果仍然明显[37]。本研究发现槟子LOX2a基因启动子区域含有脱落酸、茉莉酸甲酯、赤霉素和水杨酸等激素响应元件,推测LOX2a 基因表达可能受到这4 种激素的诱导。为此,结合圆片温育和外源激素处理实验,发现脱落酸、茉莉酸甲酯、赤霉素和水杨酸处理后,LOX2a基因表达明显受到诱导,而且响应ABA 和SA 的调控更为明显。此外,在LOX2a基因启动子区域上还存有与光和胁迫相关的作用元件,表明LOX2a基因的表达可能受到多种因素的诱导。槟子与苹果一样,是典型的呼吸跃变型果实,而且乙烯在苹果果实成熟过程中发挥重要作用[37]。本研究通过乙烯及其抑制剂1-MCP处理槟子果实,发现LOX2a基因的表达受到乙烯的诱导。香气物质代谢与激素密不可分,研究发现茉莉酸甲酯[19]、脱落酸[40]、乙烯[7]、赤霉素[41]等多种激素参与了植物香气物质的积累,但关于激素通过哪些主效基因来调控香气物质代谢的研究相对较少,需要进一步开展相关的研究工作。综上,LOX2a 基因可能通过多种激素信号转导途径参与槟子果实的香气代谢及其他生物过程。
4 结 论
笔者以香气浓郁的槟子果实为试验材料,以转录组数据为基础,克隆出槟子LOX2a 基因,对LOX2a基因的功能域、系统发育进化、时空表达及启动子顺式作用元件等内容进行分析,并通过圆片温育探究了外源激素对LOX2a 基因的诱导表达情况。证明了LOX2a基因是典型的LOX家族基因,定位在细胞质,在根部表达量最高,其表达受到脱落酸、茉莉酸甲酯、赤霉素、水杨酸和乙烯的调控,推测LOX2a基因可能通过多种激素信号转导途径参与槟子果实的香气代谢过程,为解析槟子香气浓郁的分子机制奠定了研究基础。
[1] 王中英.太谷槟子[J].山西大学学报(自然科学版),1960(1):114-128.WANG Zhongying.Taigu Binzi[J].Journal of Shanxi University(Natural Science Edition),1960(1):114-128.
[2] 李育农.苹果属植物种质资源研究[M].北京:中国农业出版社,2001.LI Yunong.Study on germplasm resources of apple plants[M].Beijing:China Agriculture Press,2001.
[3] 陆秋农,贾定贤.中国果树志·苹果卷[M].北京:中国农业科技出版社,中国林业出版社,1999:187.LU Qiunong,JIA Dingxian.Chinese fruit tree·Apple[M].Beijing:China Agricultural Science and Technology Press,China Forestry Press,1999:187.
[4] 安常娥,王怀忠.槟子树栽培技术要点[J].现代农村科技,2022(1):46-47.AN Chang’e,WANG Huaizhong.Key points of Binzi tree cultivation technology[J].Xiandai Nongcun Keji,2022(1):46-47.
[5] ESPINO-DIAZ M,SEPULVEDA D R,GONZALEZ-AGUILAR G,OLIVAS G I.Biochemistry of apple aroma[J].Food Technology and Biotechnology,2016,54(4):375-394.
[6] 陈发兴,郑少泉,蒋际谋.果实香气成分和生物合成代谢研究进展[J].福建果树,2010(2):26-30.CHEN Faxing,ZHENG Shaoquan,JIANG Jimou.Advances in fruit aroma composition and biosynthetic metabolism[J].Fujian Fruits,2010(2):26-30.
[7] QI W Y,WANG H J,ZHOU Z,YANG P,WU W B,LI Z M,LI X.Ethylene emission as a potential indicator of Fuji apple flavor quality evaluation under low temperature[J].Horticultural Plant Journal,2020,6(4):231-239.
[8] YAN D,SHI J R,REN X L,TAO Y S,MA F W,LI R,LIU X R,LIU C H.Insights into the aroma profiles and characteristic aroma of‘Honeycrisp’apple (Malus × domestica)[J/OL].Food Chemistry,2020,327:127074.https://doi.org/10.1016/j.foodchem.2020.127074.
[9] ECHEVERRIA G,GRAELL J,LOPEZ M L,LARA I.Volatile production,quality and aroma-related enzyme activities during maturation of‘Fuji’apples[J].Postharvest Biology and Technology,2004,31:217-227.
[10] DEFILIPPI B G,KADER A A,DANDEKAR A M.Apple aroma:Alcohol acyltransferase,a rate limiting step for ester biosynthesis,is regulated by ethylene[J].Plant Science,2005,168:1199-1210.
[11] RUGKONG A,MCQUINN R,GIOVANNONI J J,ROSE J K C,WATKONS C B.Expression of ripening-related genes in cold-stored tomato fruit[J].Postharvest Biology and Technology,2011,61(1):1-14.
[12] 徐坤范,王明林,艾希珍.日光温室黄瓜发育过程中主要芳香物质和脂肪酸含量的变化[J].西北植物学报,2009,29(2):390-396.XU Kunfan,WANG Minglin,AI Xizhen.Changes of main aromatic compounds and fatty acids contents of cucumber fruits during development in solar-greenhouse[J].Acta Botanica Boreali-Occidentalia Sinica,2009,29(2):390-396.
[13] CONTRERAS C H,BEAUDRY R.Lipoxygenase-associated apple volatiles and their relationship with aroma perception during ripening[J].Postharvest Biology and Technology,2013,82:28-38.
[14] WANG S S,SAITO T,OHKAWA K,OHARA K,SUKTAWEE S,IKEURA H,KNODO S.Abscisic acid is involved in aromatic ester biosynthesis related with ethylene in green apples[J].Journal of Plant Physiology,2018,221:85-93.
[15] 王欢欢,马越,白冰,王宇滨,赵晓燕,张超.番茄果实呈香组分及其代谢途径研究进展[J].中国瓜菜,2018,31(12):1-4.WANG Huanhuan,MA Yue,BAI Bing,WANG Yubin,ZHAO Xiaoyan,ZHANG Chao.Progress of aroma volatiles of tomato fruit and their metabolic pathway[J].China Cucurbits and Vegetables,2018,31(12):1-4.
[16] CHEN G P,HACKETT R,WALKER D,TAYLOR A,LIN Z F,GRIERSON D.Identification of a specific isoform of tomato lipoxygenase (TomloxC) involved in the generation of fatty acidderived flavor compounds[J].Plant Physiology,2004,136(1):2641-2651.
[17] ZHANG B,CKEN K S,BOWEN J,ALLAN A,ESPLEY R,KARUNAIRETNAM S,FERGUSON I.Differential expression within the LOX gene family in ripening kiwifruit[J].Journal of Experimental Botany,2006,57(14):3825-3836.
[18] LI M,LI L T,DUNWELL J M,QIAO X,LIU X,ZHANG S L.Characterization of the lipoxygenase (LOX) gene family in the Chinese white pear (Pyrus bretschneideri) and comparison with other members of the Rosaceae[J].BMC Genomics,2014,15(1):444.
[19] LUO M L,ZHOU X,HAO Y,SUN H J,ZHOU Q,SUN Y Y,JI S J.Methyl jasmonate pretreatment improves aroma quality of coldstored‘Nanguo’pears by promoting ester biosynthesis[J/OL].Food Chemistry,2021,338:127846.https://doi.org/10.1016/j.foodchem.2020.127846.
[20] 贾庆利,巩振辉,李大伟.辣椒定位于叶绿体的13-脂氧合酶基因(CaLOX2)克隆及表达分析[J].农业生物技术学报,2012,20(10):1126-1134.JIA Qingli,GONG Zhenhui,LI Dawei.Cloning and expression characterization of chloroplast-targeted 13-lipoxygenase gene(CaLOX2) in Capsicum annuum L.[J].Journal of Agricultural Biotechnology,2012,20(10):1126-1134.
[21] 岳璇璇,王庆鹏,房鸿成,胡甲飞,苏梦雨,王楠,张宗营,陈学森.苹果脂氧合酶基因MdLOX1a 的同源克隆与功能验证[J].植物遗传资源学报,2020,21(3):734-742.YUE Xuanxuan,WANG Qingpeng,FANG Hongcheng,HU Jiafei,SU Mengyu,WANG Nan,ZHANG Zongying,CHEN Xuesen.Homologous cloning and expression analysis of apple lipoxygenase gene MdLOX1a[J].Journal of Plant Genetic Resources,2020,21(3):734-742.
[22] 侯柄竹.ABA 调控草莓果实成熟的细胞信号转导分子机制[D].北京:中国农业大学,2018.HOU Bingzhu.Molecular mechanisms for ABA signaling transduction in regulation of strawberry (Fragaria × ananassa) fruit ripening[D].Beijing:China Agricultural University,2018.
[23] TIEMAN D,ZHU G T,RESENDE M F R,LIN T,NGUYEN C,BIES D,RANBLA J L,BELTRAN K S O,TAYLOR M,ZHANG B,IKEDA H,LIU Z Y,FISHER J,ZEMACH I,MONFORTE A,ZAMIR D,GRANELL A,KIRST M,HUANG S W,KLEE H.A chemical genetic roadmap to improved tomato flavor[J].Science,2017,355(6323):391-394.
[24] CAO X M,WEI C Y,DUAN W Y,CAO Y,KUANG J F,LIU M C,CHEN K S,KLEE H,ZHANG B.Transcriptional and epigenetic analysis reveals that NAC transcription factors regulate fruit flavor ester biosynthesis[J].Plant Journal,2021,106(3):785-800.
[25] LEWINSOHN E,SCHALECHE F,WILKINSON J,MATSUI K,TADMOR Y,NAM K H,AMAR O,LASTOCHKIN E,LARKOV O,RAVID U,HIATT W,GEPSTEIN S,PICHERSKY E.Enhanced levels of the aroma and flavor compound SLinalool by metabolic engineering of the terpenoid pathway in tomato fruits[J].Plant Physiology,2001,127(3):1256-1265.
[26] DEFILIPPI B G,MANRIQUEZ D,LUENGWILAI K.Chapter 1 aroma volatiles: Biosynthesis and mechanisms of modulation during fruit ripening[J].Advances in Botanical Research,2009,50:1-37.
[27] 宋丽娟,李雄伟,陈琳,柴明良,高中山.果实香气合成与遗传控制研究概述[J].果树学报,2008,25(5):708-713.SONG Lijuan,LI Xiongwei,CHEN Lin,CHAI Mingliang,GAO Zhongshan.A review on fruit aroma synthesis and its genetic control[J].Journal of Fruit Science,2008,25(5):708-713.
[28] 李旺雄,唐中祺,程鸿,孔维萍,罗石磊.不同甜瓜品种贮藏期间品质变化和风味物质分析[J].中国瓜菜,2021,34(9):50-55.LI Wangxiong,TANG Zhongqi,CHENG Hong,KONG Weiping,LUO Shilei.Analysis of quality changes and flavor substances of different melon varieties during storage[J].China Cucurbits and Vegetables,2021,34(9):50-55.
[29] 林馨颖,王鹏杰,陈雪津,郭永春,谷梦雅,郑玉成,叶乃兴.茶树LOX 基因家族的鉴定及其在白茶萎凋过程的表达分析[J].茶叶科学,2021,41(4):482-496.LI Xinying,WANG Pengjie,CHEN Xuejin,GUO Yongchun,GU Mengya,ZHENG Yucheng,YE Naixing.Identification of LOX gene family in camellia sinensis and expression analysis in the process of white tea withering[J].Journal of Tea Science,2021,41(4):482-496.
[30] ROYO J,VANCANNEYT G,Perez A G,SANZ C,STORMANN K,ROSAHL S,SANCHEZ-SERRANA J J.Characterization of three potato lipoxygenases with distinct enzymatic activities and different organ-specific and wound-regulated expression patterns[J].Journal of Biological Chemistry,1996,271(35):21012-21019.
[31] SIEDOW J N.Plant lipoxygenase:Structure and function[J].Annual Review Plant Physiology and Plant Molecular Biology,1991,42(1):145-188.
[32] HU T Z,ZENG H,HU Z L,QU X X,CHEN G P.Over-expression of the tomato 13- lipoxygenase gene TomloxD,increases generation of endogenous jasmonic acid and resistance to Cladosporium fulvum,and high temperature[J].Plant Molecular Biology Reporter,2013,31(5):1141-1149.
[33] HENDRICKS J J,NADELHOFFER K J,ABER J D.Assessing the role of fine roots in carbon and nutrient cycling[J].Trends in Ecology&Evolution,1993,8(5):174-178.
[34] 黄爱梅,方毅,孙俊,李锦隆,胡丹丹,钟全林,程栋梁.武夷山不同海拔毛竹细根功能性状研究[J/OL].生态学报,2023(1):1-10.[2022-08-17].http://kns.cnki.net/kcms/detail/11.2031.Q.20220826.1112.036.html.HUANG Aimei,FANG Yi,SUN Jun,LI Jinglong,HU Dandan,ZHONG Quanlin,CHENG Dongliang.Fine root traits of Phyllostachys edulis at different altitudes in Wuyi Mountain[J/OL].Acta Ecologica Sinica,2023(1):1-10[2022-08-17].http://kns.cnki.net/kcms/detail/11.2031.Q.20220826.1112.036.html.
[35] [CONTRERAS C H,TJELLSTROM H,BEAUDRY R M.Relationships between free and esterified fatty acids and LOX-derived volatiles during ripening in apple[J].Postharvest Biology and Technology,2015,112:105-113.
[36] LIU X J,HAO N N,FENG R F,MENG Z P,LI Y N,ZHAO Z Y.Transcriptome and metabolite profiling analyses provide insight into volatile compounds of the apple cultivar‘Ruixue’and its parents during fruit development[J].BMC Plant Biology,2021,21(1):231.
[37] YANG X T,SONG J,DU L N,CHARLES F,CAMPBELLPALMER L,FILLMORE S,PAUL W,ZHANG Z Q.Ethylene and 1-MCP regulate major volatile biosynthetic pathways in apple fruit[J].Food Chemistry,2016,194:325-336.
[38] WU Q,TAO X Y,AI X Z,LUO Z S,MAO L C,YING T J,LI L.Effect of exogenous auxin on aroma volatiles of cherry tomato(Solanum lycopersicum L.) fruit during postharvest ripening[J].Postharvest Biology and Technology,2018,146:108-116.
[39] ZHU J Y,WANG X W,GUO L X,XU Q S,ZHAO S Q,LI F D,YAN X M,LIU S R,WEI C L.Characterization and alternative splicing profiles of the lipoxygenase gene family in tea plant(Camellia sinensis)[J].Plant Cell Physiology,2018,59(9):1765-1781.
[40] WU Q,TAO X Y,AI X Z,LUO Z S,MAO L C,YING T J,LI L.Contribution of abscisic acid to aromatic volatiles in cherry tomato(Solanum lycopersicum L.)fruit during postharvest ripening[J].Plant Physiology&Biochemistry,2018,130:205-214.
[41] 贺凌霄,薛刚,孙聚涛,张智强,丁永乐,杨铁钊,徐世晓,程昌合.外源赤霉素对烤烟叶面腺毛和香气物质的影响[J].河南农业科学,2021,50(1):52-59.HE Lingxiao,XUE Gang,SUN Jutao,ZHANG Zhiqiang,DING Yongle,YANG Tiezhao,XU Shixiao,CHENG Changhe.Effects of exogenous gibberellin on flue-cured tobacco leaf trichome and aromatic substances[J].Journal of Henan Agricultural Sciences,2021,50(1):52-59.