核果类果树是我国重要的经济作物,尤其是桃和李,分布广泛,栽培面积大,在我国果树生产中占有重要地位。因此,培育具有优良性状的核果类果树新品种对提高经济价值意义重大。随着分子生物技术的快速发展,利用基因工程进行遗传改良已成为作物育种的重要途径之一。遗传转化是开展植物遗传改良的必要技术,同时也是验证基因功能、解析性状形成机制的关键技术[1]。而高效的离体再生体系是进行遗传转化的基础,也是限制大多数核果类果树遗传转化的“瓶颈”[2-3]。
李是蔷薇科(Rosaceae)李属(Prunus)植物,具有重要的经济价值,世界平均年产量约1260 万t(FAOSTAT(2021)),目前商业种植的主要有6 倍体的欧洲李(P.domestica)和2倍体的中国李(P.salicina)。李是核果类果树中再生及遗传转化技术较为成熟的树种。1991 年首次实现欧洲李转基因[4],此后也有人开展了中国李离体再生及遗传转化研究[5-8]。但目前李的离体再生体系中多以下胚轴和子叶为外植体,由于不同基因型间差异较大,因此极易导致再生体系不稳定、重复性差的问题。此外,基因型不同也使得再生体系的应用受到限制。组培再生的两个主要用途是品种扩繁和基因的遗传转化,而以下胚轴和子叶为外植体建立的再生体系无法应用于品种扩繁,同时由于外植体基因型不同也可能导致基因转化后表型功能的不一致。因此,选用基因型一致的材料为外植体建立稳定的李再生体系很有必要。
为获得稳定、高效、重复性好的再生体系,本研究以欧洲李叶片为外植体,探究了不定芽诱导、增殖、生长和生根的最适条件,初步建立了欧洲李叶片离体再生体系,为进一步利用欧洲李叶片开展遗传转化奠定基础,也为再生比较困难的核果类果树的品种改良、基因遗传转化和功能验证等生物技术育种提供参考。
1 材料和方法
1.1 材料
4 个欧洲李品种斯塔尔(Startovaya)、女神(Victoria)、法兰西(France)和兰蜜(Bluebyrd)当年生休眠枝条于2018年从新疆欧洲李种质资源圃采集,枝条水培后取展开的幼嫩叶片,经消毒后进行不定芽的诱导。获得无菌组培苗后,保存于河南农业大学园艺学院果树组织培养室。继代增殖培养基为MS+3%(ρ)蔗糖+0.7%(ρ)琼脂+100 mg·L-1肌醇+2.0 mg·L-1 6-苄基腺嘌呤(6-benzylaminopurine, 6-BA)+0.1 mg·L-1 3-吲哚丁酸(indole-3-butyric acid,IBA),pH=5.8,培养温度为(25±1)℃,光周期16 h/8 h(光照/黑暗),光照强度为36~45 μmol·m-2·s-1。
1.2 方法
1.2.1 叶片不定芽的诱导 取枝条水培后展开的幼嫩叶片,用0.5%(ρ)的次氯酸钠消毒10 min,无菌蒸馏水冲洗3遍,再用0.1%(ρ)氯化汞消毒6 min,无菌蒸馏水冲洗5~6 次,吸干叶片表面水分后接种于诱芽培养基,MS基础培养基添加不同质量浓度的6-BA(1.0、3.0和5.0 mg·L-1)和IBA(0.1、0.3和0.5 mg·L-1),每个处理接种40个外植体,3次重复。
取培养30 d 组培苗上展开的叶片,保留1 mm左右的叶柄,用手术刀垂直于叶片中脉轻划2~3刀,切断叶脉但保持叶片边缘相连,将切好的外植体接种于诱芽培养基上,叶片近轴端接触培养基:(1)以WPM 为基础培养基,添加不同质量浓度的TDZ(1.0、2.0、3.0、4.0、5.0 mg·L-1)和2,4-D(0.1、0.2、0.3、0.4、0.5、0.6、0.8、1.0 mg·L-1),暗培养10 d,研究不同浓度激素配比下欧洲李的再生情况,筛选出不定芽诱导的最适培养基;(2)按照叶片生长部位分顶端幼叶(第1~2 片叶)、中部成熟叶(第3~5 片叶)和基部老叶3种,分别接种于再生培养基(WPM+2.0 mg·L-1 TDZ+0.2 mg·L-12,4-D),暗培养10 d,研究不同生长状态叶片的再生情况;(3)取下叶片后用手术刀将叶片垂直于叶脉切成三部分:叶基部、叶中、叶尖,分别接种于再生培养基(WPM+2.0 mg·L-1 TDZ+0.2 mg·L-1 2,4-D)上,黑暗培养10 d,研究叶片不同部位的再生情况;(4)将外植体叶片接种于再生培养基(WPM+2.0 mg·L-1 TDZ+0.2 mg·L-12,4-D)上,分别黑暗培养0、7、14、21、28 d,研究暗培养时间对欧洲李再生的影响。以上试验每个处理均接种50 个外植体,3 次重复,光照条件下培养30 d 后统计再生率以及再生芽个数。
1.2.2 不定芽的增殖 将再生的不定芽接种到增殖培养基上进行培养,以MS为基础培养基,添加不同质量浓度的6-BA(1.0、2.0和3.0 mg·L-1)和IBA(0.1、0.2和0.3 mg·L-1),培养20 d后观察并统计增殖芽的状态及增殖倍数;每个处理接种20个不定芽,3次重复。
1.2.3 不定芽的生长与生根 将增殖后的不定芽转移至生长培养基,以MS 为基础培养基,添加IBA(0.1 mg·L-1)和不同质量浓度的6-BA(0.1、0.2、0.4、0.6、0.8 和1.0 mg·L-1),培养30 d 后测量再生芽茎生长量以及第三节茎粗度,每个处理接种20 个不定芽,3次重复。
选取生长培养后2.0~3.0 cm、有3~4 片叶片且带少量愈伤组织的不定芽接种于生根培养基,以MS 为基础培养基,添加不同质量浓度的IBA(0、0.1、0.3、0.5、0.7、1.0、1.5 mg·L-1)。暗培养7 d 后转入光照培养,30 d 后记录生根率、生根系数、植株高度和根长。每个处理接种30 个不定芽,3 次重复。生根后的欧洲李组培苗移植至培养钵(V 基质∶V 蛭石=2∶1)生长。
1.3 结果观察与数据分析
污染率/%=(污染个数/接种叶片总数)×100;存活率/%=(存活个数/未污染个数)×100;再生率/%=再生叶片数/接种叶片总数×100;平均再生芽数=再生芽个数/再生外植体总数;增殖倍数=增殖后芽体数/增殖前芽体数;成活率/%=成活苗数量/移栽总数×100。利用SPSS软件对数据进行方差分析,用新复极差(SSR)法进行多重比较。
2 结果与分析
2.1 不同欧洲李品种再生芽的诱导
基因型是影响植物离体再生的重要因素,植物离体再生的难易程度与基因型密切相关。本试验对4 个欧洲李品种(斯塔尔、女神、法兰西、兰蜜)水培枝条的叶片进行不定芽诱导,结果发现:暗培养7 d后在接种叶片的叶柄和叶脉切口处产生了少量的白色愈伤组织,见光培养30 d 左右部分愈伤组织开始分化产生不定芽,随着培养时间的增加,未再生的愈伤组织生长变缓、褐化;最终从女神品种诱导获得不定芽,再生率5.4%,其他3 个品种无不定芽(表1)。因此,本试验后期以欧洲李女神品种为材料进行欧洲李叶片再生体系的探索。
表1 植物生长调节剂对水培枝条叶片诱导不定芽的影响
Table 1 Effects of plant growth regulators on adventitious bud induction from leaves of hydroponic branches

品种Varieties斯塔尔、女神、法兰西、兰蜜Startovaya,Victoria,France,Bluebyrd植物生长调节剂组合Plant growth regulators/(mg·L-1)6-BA 1 3斯塔尔、法兰西、兰蜜France,Startovaya,Bluebyrd 5女神Victoria 5 IBA 0.1 0.3 0.5 0.1 0.3 0.5 0.1 0.3 0.5 0.5再生率Regeneration rate/%0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 5.4
2.2 不同条件对不定芽诱导的影响
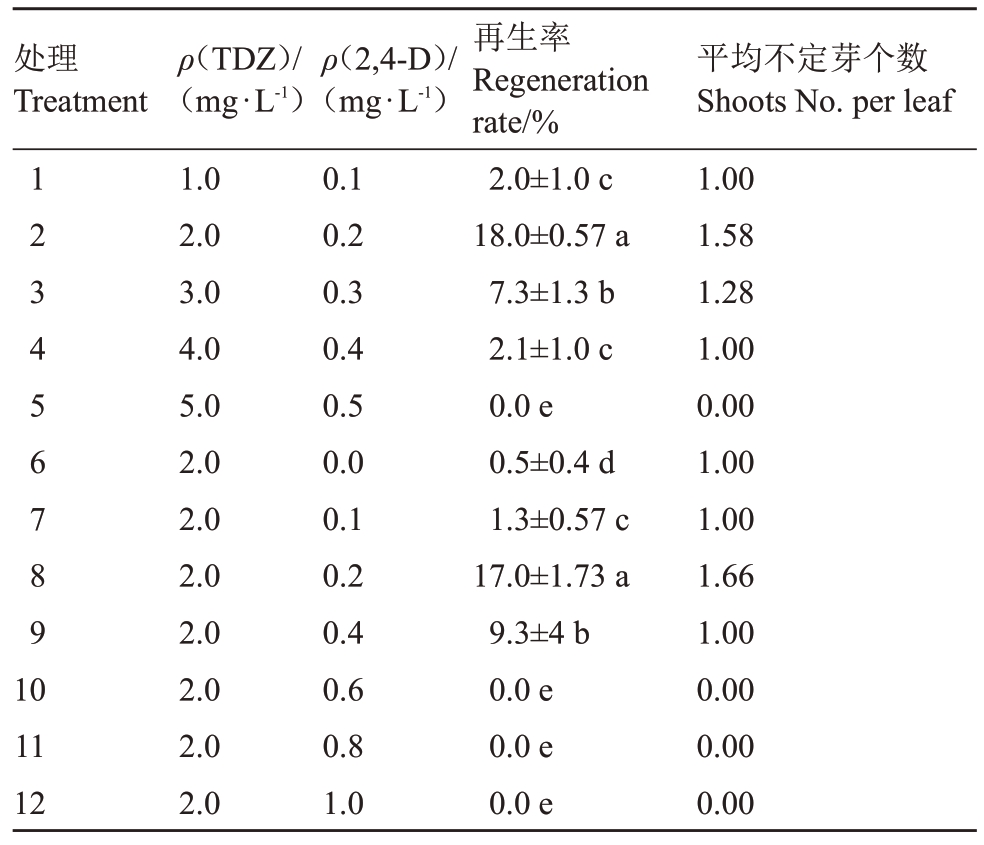
基础培养基是影响离体再生的重要因素之一,不同树种和外植体对基础培养基的要求不同,本试验在前期研究基础上筛选出WPM培养基为欧洲李女神组培苗叶片再生的基础培养基。植物生长调节剂的使用是叶片离体再生的关键因素,本试验以不同浓度的TDZ和2,4-D 组合进行不定芽的诱导,研究不同浓度配比下叶片的再生情况。结果如表2所示:以TDZ和2,4-D浓度比为1∶10,当TDZ和2,4-D质量浓度分别为2.0 mg·L-1和0.2 mg·L-1时再生率最高,达到18%,平均再生芽1.58个;当TDZ质量浓度为2.0 mg·L-1时,2,4-D的质量浓度由0到1 mg·L-1,在2,4-D 的质量浓度为0.2 mg·L-1时再生率最高为17%。以上结果表明:以WPM 为基础培养基,TDZ 2.0 mg·L-1和2,4-D 0.2 mg·L-1为欧洲李叶片诱导产生不定芽效率最高的激素配比。
表2 不同浓度的TDZ 和2,4-D 组合对欧洲李叶片再生的影响
Table 2 Effects of different concentration TDZ and 2,4-D on leaves regeneration of European plum
注:不同小写字母表示差异显著(p<0.05)。下同。
Note: Different small letters indicate significant difference according to SSR test(p<0.05).The same below.
平均不定芽个数Shoots No.per leaf 1.00 1.58 1.28 1.00 0.00 1.00 1.00 1.66 1.00 0.00 0.00 0.00处理Treatment 123456789 10 11 12 ρ(TDZ)/(mg·L-1)1.0 2.0 3.0 4.0 5.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0 ρ(2,4-D)/(mg·L-1)0.1 0.2 0.3 0.4 0.5 0.0 0.1 0.2 0.4 0.6 0.8 1.0再生率Regeneration rate/%2.0±1.0 c 18.0±0.57 a 7.3±1.3 b 2.1±1.0 c 0.0 e 0.5±0.4 d 1.3±0.57 c 17.0±1.73 a 9.3±4 b 0.0 e 0.0 e 0.0 e
不同生长部位的叶片再生率存在显著差异,其中中部成熟叶的再生率最高,为26.5%,平均每个外植体再生1.75 个不定芽,显著高于顶端幼叶和基部老叶(表3)。
表3 叶片生长部位、叶片不同部位及暗培养时长对欧洲李叶片再生的影响
Table 3 Effects of leaf growth position,different parts of leaves and dark-incubated time on adventitious shoot regeneration of European plum
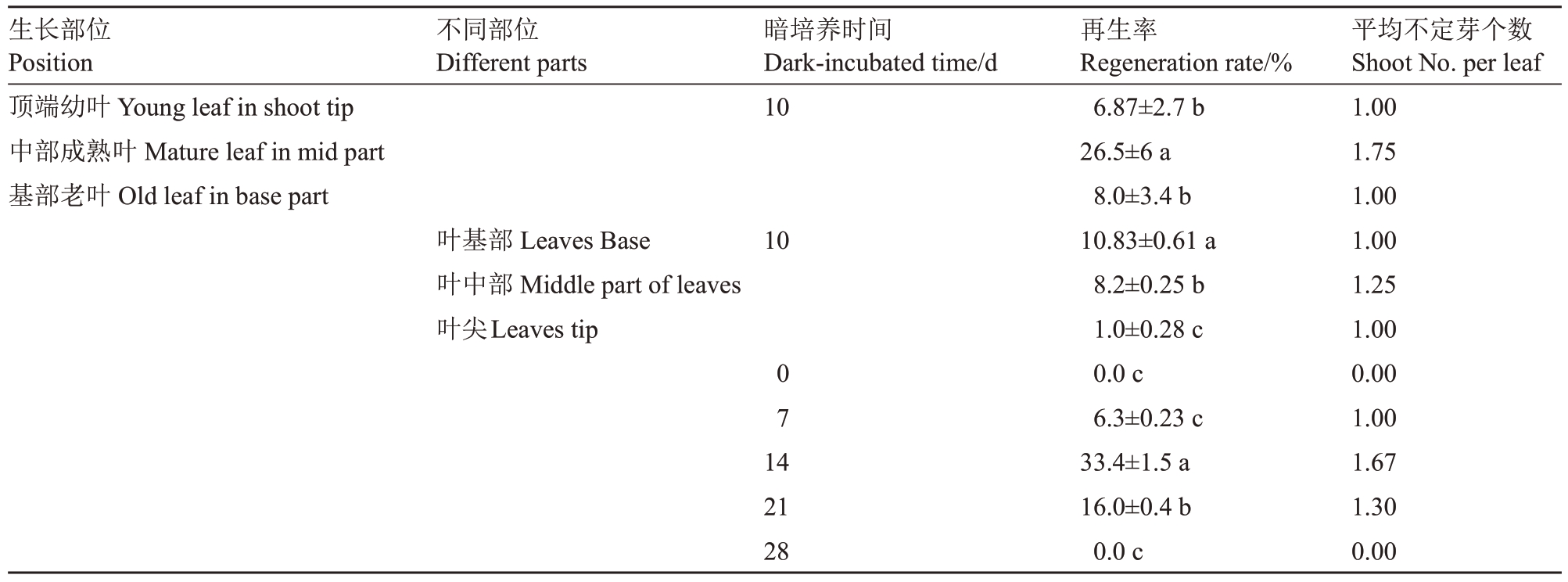
生长部位Position顶端幼叶Young leaf in shoot tip中部成熟叶Mature leaf in mid part基部老叶Old leaf in base part不同部位Different parts暗培养时间Dark-incubated time/d 10叶基部Leaves Base叶中部Middle part of leaves叶尖Leaves tip 10 07 14 21 28再生率Regeneration rate/%6.87±2.7 b 26.5±6 a 8.0±3.4 b 10.83±0.61 a 8.2±0.25 b 1.0±0.28 c 0.0 c 6.3±0.23 c 33.4±1.5 a 16.0±0.4 b 0.0 c平均不定芽个数Shoot No.per leaf 1.00 1.75 1.00 1.00 1.25 1.00 0.00 1.00 1.67 1.30 0.00
为了进一步确认外植体状态对再生效率的影响,本试验将叶片垂直于叶脉分成三部分:叶基部、叶中、叶尖,研究了叶片不同部位的再生效率。由表3 可知叶片不同部位均可以再生不定芽,其中靠近叶柄的基部再生率最高为10.83%,叶片中间部位的再生率为8.2%,叶尖的再生率最低仅1.0%。
黑暗培养对叶片的再生是必需的,本试验研究了不同暗培养时长对再生效率的影响。结果发现暗培养时间不同,叶片的再生率有显著性差异,随着培养时间的延长,再生效率逐渐提高,暗培养14 d时外植体再生率最高,为33.4%;当暗培养21 d,再生率降至16%;暗培养28 d 时,愈伤组织出现水渍化,见光后没有再生芽(表3)。以上结果表明,以中部膨大的完整叶为外植体,暗培养14 d时欧洲李女神叶片的再生效率最高。
2.3 不同浓度的6-BA和IBA组合对不定芽增殖的影响
为了获得大量芽体,本试验对获得的欧洲李不定芽进行增殖培养,以MS 为基础培养基添加不同浓度的6-BA 和IBA 组合;表4 结果显示随着6-BA的浓度升高,不定芽的增殖倍数逐渐升高,其中6-BA质量浓度为3 mg·L-1时增殖倍数最高,但高浓度6-BA导致不定芽出现叶片黄化、卷曲以及严重玻璃化的现象(图1-A);过低质量浓度的6-BA(1 mg·L-1)增殖倍数较低,不定芽叶片出现黄化(图1-C);当6-BA质量浓度为2 mg·L-1、IBA质量浓度为0.1 mg·L-1时,增殖倍数较高且不定芽状态较好(图1-B)。因此以MS+6-BA 2 mg·L-1+IBA 0.1 mg·L-1为不定芽增殖的最适培养基。

图1 增殖后不定芽的生长状态
Fig.1 The growth of adventitious shoots after proliferation
A.6-BA 质量浓度为3 mg·L-1时的不定芽;B.6-BA 和IBA 质量浓度分别为2 mg·L-1 和0.1 mg·L-1时的不定芽;C.6-BA 质量浓度为1 mg·L-1时的不定芽。
A.The growth of adventitious shoots at the concentration of 6-BA is 3 mg·L-1;B.The growth of adventitious shoots at the concentration of 6-BA is 2 mg·L-1 and IBA is 0.1 mg·L-1;C.The growth of adventitious shoots at the concentration of 6-BA is 1 mg·L-1.
表4 不同浓度的6-BA 和IBA 对不定芽增殖的影响
Table 4 Effects of 6-BA and IBA on adventitious shoot proliferation
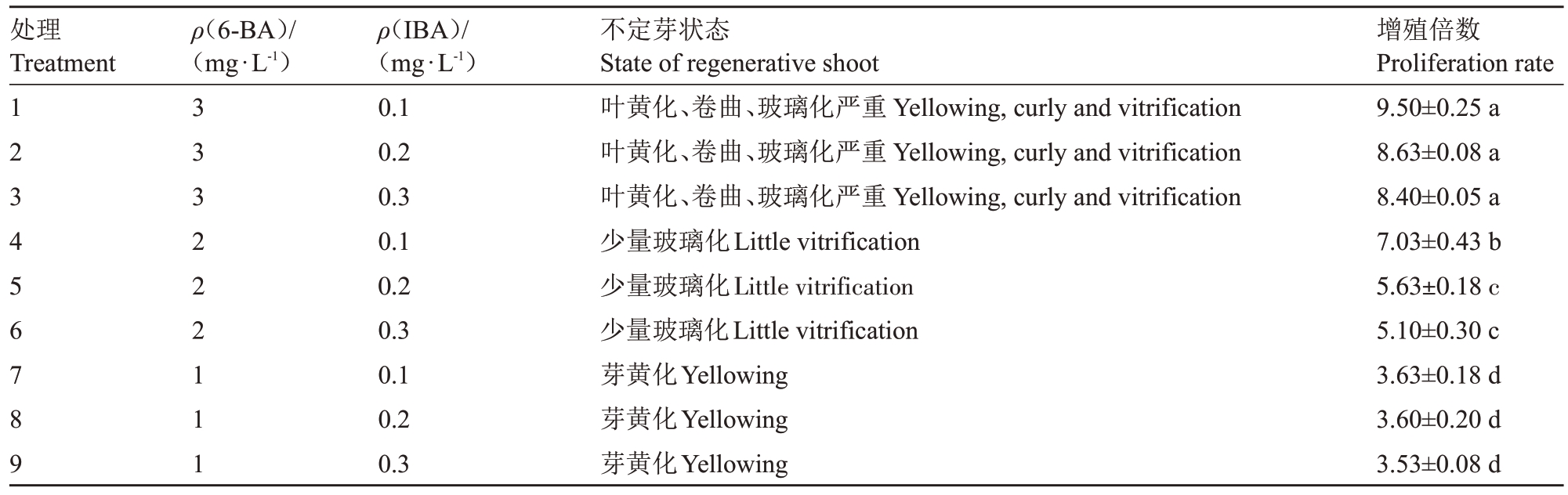
处理Treatment ρ(6-BA)/(mg·L-1)123456789 333222111 ρ(IBA)/(mg·L-1)0.1 0.2 0.3 0.1 0.2 0.3 0.1 0.2 0.3不定芽状态State of regenerative shoot叶黄化、卷曲、玻璃化严重Yellowing,curly and vitrification叶黄化、卷曲、玻璃化严重Yellowing,curly and vitrification叶黄化、卷曲、玻璃化严重Yellowing,curly and vitrification少量玻璃化Little vitrification少量玻璃化Little vitrification少量玻璃化Little vitrification芽黄化Yellowing芽黄化Yellowing芽黄化Yellowing增殖倍数Proliferation rate 9.50±0.25 a 8.63±0.08 a 8.40±0.05 a 7.03±0.43 b 5.63±0.18 c 5.10±0.30 c 3.63±0.18 d 3.60±0.20 d 3.53±0.08 d
2.4 不同浓度的6-BA对不定芽生长的影响
经增殖培养的不定芽不适合直接生根,需要进行生长培养;本试验以MS为基础培养基,在IBA为0.1 mg·L-1时添加不同浓度的6-BA。结果发现,6-BA 质量浓度从0~0.2 mg·L-1时,茎生长量及第三节茎粗逐渐增加,在0.2 mg·L-1时最高(表5);之后随着6-BA浓度的增加,又逐渐降低,且再生芽出现黄化、小叶、水渍等现象(图2)。因此不定芽生长的最适培养基为MS+6-BA 0.2 mg·L-1+IBA 0.1 mg·L-1。

图2 不同浓度6-BA 下不定芽的生长状态
Fig.2 The growth of adventitious shoots under different concentration of 6-BA
图中所标数字分别对应表5 中的处理编号。
The number in figure correspond to the treatment number in Table 5.
表5 不同浓度的6-BA 对不定芽生长的影响
Table 5 Effects of different concentration 6-BA on the growth of regenerated shoots

处理Treatment 1234567 ρ(6-BA)/(mg·L-1)0.0 0.1 0.2 0.4 0.6 0.8 1.0 ρ(IBA)/(mg·L-1)0.1 0.1 0.1 0.1 0.1 0.1 0.1苗生长状态Shoot growth status小叶、部分黄化Leaflet and little yellowing茎粗Thick stem健壮Robust良好Grow well良好Grow well部分黄化、茎细Little yellowing and thin部分水渍、小叶Little watery and leaflet茎生长量Stem elongation/cm 1.3±0.10 d 2.2±0.10 c 3.1±0.26 a 2.6±0.10 b 2.9±0.15 a 2.0±0.20 c 2.0±0.20 c茎粗Stem thickness/mm 0.91±0.07 d 1.31±0.05 a 1.21±0.03 ab 1.09±0.14 b 1.07±0.13 bc 1.17±0.05 ab 0.91±0.09 d
2.5 不同浓度的IBA对不定芽生根的影响
选取生长培养的不定芽接种至MS加不同浓度IBA 的培养基上进行生根培养,37 d 后统计生根情况,结果如表6所示:未加IBA的培养基中没有根的分化,说明IBA对不定芽的生根是必需的;IBA质量浓度在0~0.5 mg·L-1范围内随着IBA 浓度的升高生根率逐渐增加,IBA 质量浓度为0.5 mg·L-1时生根率最高达74%,平均根长14.7 cm;之后再提高IBA浓度,其生根率及平均根长均下降;因此MS+IBA 0.5 mg·L-1为最适生根培养基。对生根后的组培苗进行驯化移栽,移栽成活率达83.3%。
表6 不同浓度的IBA 对不定芽生根的影响
Table 6 Effects of different concentration 6-BA on rooting of regenerated shoots
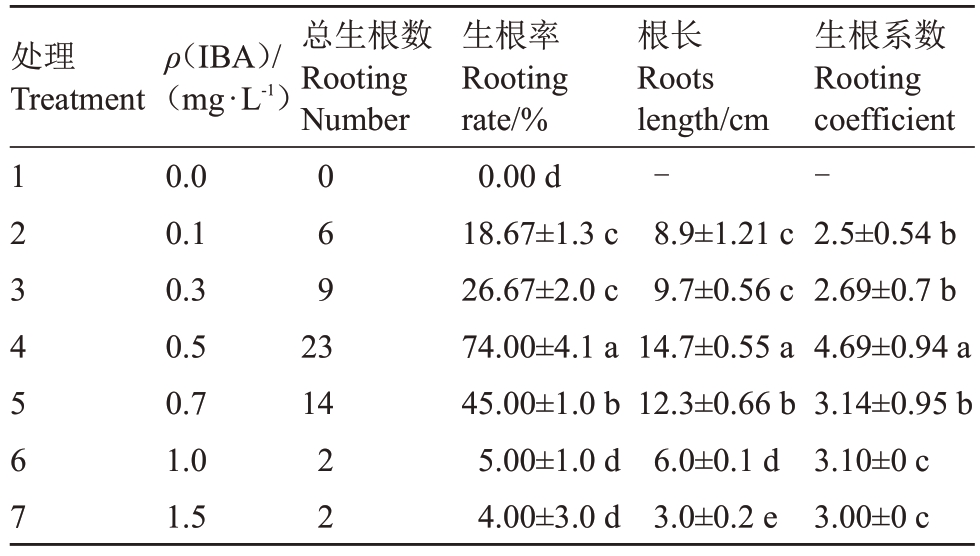
处理Treatment总生根数Rooting Number 1234567 ρ(IBA)/(mg·L-1)0.0 0.1 0.3 0.5 0.7 1.0 1.5 069 23 14 22生根率Rooting rate/%0.00 d 18.67±1.3 c 26.67±2.0 c 74.00±4.1 a 45.00±1.0 b 5.00±1.0 d 4.00±3.0 d根长Roots length/cm-8.9±1.21 c 9.7±0.56 c 14.7±0.55 a 12.3±0.66 b 6.0±0.1 d 3.0±0.2 e生根系数Rooting coefficient-2.5±0.54 b 2.69±0.7 b 4.69±0.94 a 3.14±0.95 b 3.10±0 c 3.00±0 c
2.6 欧洲李叶片再生体系流程
综上所述,本试验以欧洲李女神为材料,最终建立了以组培苗叶片为外植体的欧洲李再生体系。具体流程如图3所示:取水培枝条展开的幼嫩叶片(图3-A),经消毒后接种到诱芽培养基进行再生芽的诱导(图3-B),约40 d 后长出不定芽(图3-C);对不定芽进行增殖培养20 d,获得大量芽体(图3-D);增殖后的不定芽接种于茎伸长培养基,30 d 培养后茎生长3.1 cm(图3-E);对茎伸长生长后的不定芽进行生根培养,37 d 左右生根(图3-F);生根后的欧洲李组培苗移植至营养钵可正常生长(图3-G),同时以该组培苗叶片为外植体进一步探索欧洲李离体再生条件。
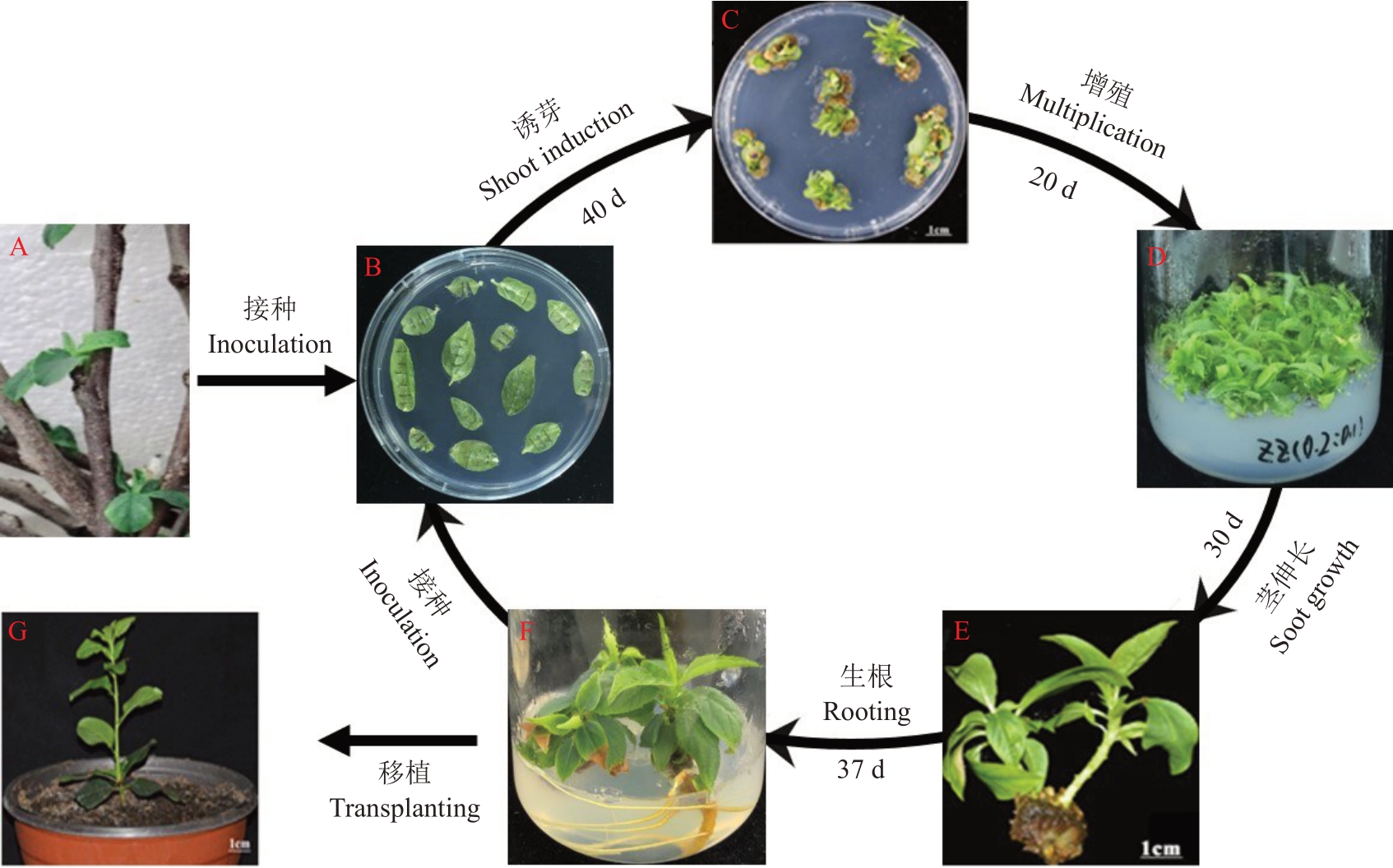
图3 欧洲李女神叶片再生体系流程
Fig.3 Procedure for adventitious shoot regeneration of European plum Victoria
A.水培欧洲李女神枝条;B.接种叶片诱导再生芽;C.再生不定芽;D.不定芽增殖;E.不定芽茎伸长生长;F.不定芽生根;G.移植后的欧洲李植株。
A.Water cultured branches; B.The leaves in vitro cultured for adventitious shoot induction; C.Adventitious shoots regeneration from leaves;D.Adventitious shoots proliferation;E.Stems elongation of adventitious shoots;F.Root formed from adventitious shoot;G.The plum plantlet transplanted to pots.
3 讨 论
植物离体再生的难易程度与基因型密切相关,不同品种再生效率也不相同。目前已报道的李离体再生多为欧洲李品种,中国李则较难建立离体再生体系。但无论是再生体系较为成熟的欧洲李还是再生较难的中国李,不同品种之间的再生效率均存在很大差异。Tian 等[5]报道的3 个中国李品种Early Golden,Shiro 和Redheart 再生中,Early Golden 再生率最高为28.3%,Redheart 则不产生再生芽。同样Mikhilov等[15]报道的2个欧洲李品种Etude和Startovaya的再生率分别为2%~7%和15%~17%。本试验对4 个欧洲李品种(斯塔尔、女神、法兰西、兰蜜)水培枝条的叶片进行不定芽诱导,仅从品种女神中获得再生不定芽,说明基因型是影响植物离体再生的一个主要因素。
外植体类型是影响植物离体再生的重要因素之一,不同树种最适外植体类型不同。如杏离体再生最常用的外植体是幼胚子叶[9-10]。叶片、茎尖和幼胚等在桃离体再生中均有应用,但以胚和子叶为外植体时再生效率较高[3,11-14]。目前已报道的李再生体系中,也多以种子来源的下胚轴和子叶为外植体[7,15-18]。以种子来源的组织为外植体,虽然更易获得再生不定芽,但同时面临着由基因型差异而导致的再生体系不稳定、重复性低等问题[19]。与下胚轴和子叶相比,以叶片为外植体,不仅有利于建立稳定的离体再生体系,同时还具有取材方便、试材充足、成本低等优势。但目前以叶片为外植体进行再生芽诱导的报道较少且再生率较低。Meerja等[15]以欧洲李品种Vanette、Stanley 和Veeblue 的叶片为外植体在3 种不同基础培养基上进行再生芽诱导时,再生率最高为8.3%。叶片的生理状态也是影响再生效率的关键因素之一,研究发现杏、苹果和甜樱桃等顶端展开的幼叶再生能力最高,主要是因为幼嫩叶片细胞分化程度不高,脱分化和再分化的能力强[20-22]。而在本试验中,欧洲李女神组培苗中部的成熟叶再生率最高,达到33.4%;同一叶片基部的再生率高于中部和叶尖,但均低于完整叶片;可能是因为不同部位的叶片及叶片不同部位内源激素水平及酶活不同,在外源激素及其他环境因素的作用下,表现出不同的再生能力。与前人研究结果相比,本试验较高的再生率可能与材料的基因型及培养条件的优化有关。
外植体消毒是导致植物离体再生系统稳定性差的原因之一。外植体材料的来源不同,受细菌侵染程度不同,因此灭菌所需时间及成活难易程度也不相同。不合适的消毒方法通常会造成消毒不彻底导致的高污染率和过度消毒引起的外植体死亡。耿文娟等[23]在野生欧洲李组织培养过程中发现,用0.1%的升汞处理田间或温室的欧洲李枝条10 min,会造成材料褐化、死亡严重。本研究中,以组培苗叶片为外植体进行离体再生系统的建立,减少了室外取材需要外植体消毒的步骤,大大提高了该再生系统的稳定性。同时,本试验通过对不定芽增殖、生长和生根条件的优化,增殖倍数和生根率均较高,可获得大量欧洲李组培苗,保证了离体再生过程中所需外植体材料,使得该再生系统取材不受环境条件限制。本试验所建立的欧洲李叶片离体再生系统较为稳定。
植物生长调节剂在植物离体再生过程中起着关键作用,不同植物、不同外植体对植物生长调节剂的种类及用量要求不尽相同,细胞分裂素和生长素类物质是目前最常用的植物生长调节剂。李属果树离体再生研究中常用的细胞分裂素有6-BA 和TDZ[1]。有报道发现TDZ能诱导大量物种尤其是木本植物不定芽的产生,提高其再生效率[21-22]。Canli等[7]发现在中国李子叶诱导不定芽的过程中,TDZ比BA 效果更明显。李再生过程中常用的生长素类物质有IBA 和2,4-D[24-29]。Yao 等[26]的研究表明TDZ 和2,4-D 组合对欧洲李Tardicots 再生不定芽的诱导具有较好的效果。因此,以TDZ和2,4-D 为组合开展了欧洲李叶片再生不定芽的诱导,筛选出最佳的不定芽诱导培养基:WPM+TDZ 2.0 mg·L-1+2,4-D 0.2 mg·L-1。
4 结 论
以欧洲李叶片为外植体,对不定芽的诱导、增殖、生长和生根条件进行了优化探索,最终以欧洲李女神叶片为外植体建立了完整的再生体系:再生率最高为33.4%,增殖倍数为7.03,生根率为74%,移栽成活率为83.3%,为进一步利用欧洲李叶片开展遗传转化奠定了基础。
[1] 李坤坤,徐昌杰.蔷薇科果树离体再生与遗传转化研究进展[J].园艺学报,2017,44(9):1633-1644.LI Kunkun,XU Changjie.Advances in regeneration and genetic transformation of rosaceae fruit trees[J].Acta Horticulturae Sinica,2017,44(9):1633-1644.
[2] SMIGOCKI A C,HAMMERSCHLAG F A.Regeneration of plants from peach embryo cells infected with a shooty mutant strain of agrobacterium[J].Journal of the American Society for Horticultural Science,1991,116(6):1092-1097.
[3] GENTILE A,MOTICELLI S,DAMIANO C.Adventitious shoot regeneration in peach [Prunus persica (L.)Batsch] [J].Plant Cell Reports,2002,20(11):1011-1016.
[4] PETR C,SCORZA R,DARDICK C.Genetic engineering of plum.(Prunus domestica L.) for plant improvement and genomics research in rosaceae[J].Genetics and Genomics of Rosaceae,2009,6(1):277-299.
[5] TIAN L,YAN W,SIBBALD J S.Regeneration of Prunus salicina Lindl.(Japanese plum) from hypocotyls of mature seeds[J].In Vitro Cellular & Developmental Biology-Plant,2007,43(4):343-347.
[6] URTUBIA C,DEVIA J,CASTRO A,ZAMORA P,AGUIRRE C,TAPIA E,BARBA P,DELLORTO P,MOYNIHAN M R,PETRIC C,SCORZA R,PRIETO H. Agrobacterium-mediated genetic transformation of Prunus salicina[J].Plant Cell Reports,2008,27(8):1333-1340.
[7] CANLI F A,TIAN F.Regeneration of adventitious shoots from mature stored cotyledons of Japanese plum (Prunus salicina Lindl.)[J].Scientia Horticulturae,2009,120(1):64-69.
[8] 孙源蔚,李果果,姚延兴,李国怀.中国李Nubiana 胚性愈伤组织诱导及植株再生[J].果树学报,2011,28(1):42-45.SUN Yuanwei,LI Guoguo,YAO Yanxing,LI Guohuai.Embryonic callus induction and plantlet regeneration in Nubiana(Prunus salicina)[J].Journal of Fruit Science,2011,28(1):42-45.
[9] PETRI C,WANG H,BURGOS L,NAVARRO J S,ALBURQUERQUE N.Production of transgenic apricot plants from hypocotyl segments of mature seeds[J].Scientia Horticulturae,2015,197(1):144-149.
[10] 王鸿,郝燕,王玉安,陈建军,王发林.杏植株再生与遗传转化研究进展[J].果树学报,2011,28(5):875-879.WANG Hong,HAO Yan,WANG Yu’an,CHEN Jianjun,WANG Falin.Advances in apricot regeneration and genetic transformation[J].Journal of Fruit Science,2011,28(5):875-879.
[11] HAMMERSCHLAG F A,BAUCHAN G,SCORZA R.Regeneration of peach plants from callus derived from immature embryos[J].Theoretical and Applied Genetics,1985,70(3):248-251.
[12] ROSA M,AMPARO P S,LORENZO G F,BELTRAN J P,CAAS L A.Transgenic peach plants (Prunus persica L.) produced by genetic transformation of embryo sections using the green fluorescent protein(GFP)as an in vivo marker[J].Molecular Breeding,2004,14(4):419-427.
[13] PADILLA I M G,GOLIS A,GENTILE A,DAMIANO C,SCORZA R.Evaluation of transformation in peach Prunus persica explants using green fluorescent protein (GFP) and betaglucuronidase (GUS) reporter genes [J].Plant Cell,Tissue and Organ Culture,2006,84(3):309-314.
[14] SABBADINI S,PANDOLFINI T,GIROLOMINI L,MOLESINI B,NAVACCHI O.Peach(Prunus persica L.)[J].Methods in Molecular Biology,2015,1224(1):205-215.
[15] MEERJA F A,YUAN S,SUBRAMAMIAN J.European plum in vitro regeneration from different organs[J].Tree and Forestry Science and Biotechnology,2011,5(1):61-64.
[16] PRTRI C,WEBB K,HILY J M,DARDICK C,SCORZA R.High transformation efficiency in plum (Prunus domestica L.):A new tool for functional genomics studies in Prunus spp.[J].Molecular Breeding,2008,22(4):581-591.
[17] YANCHEVA S.Effect of the carbohydrate source on improvement of adventitious regeneration in plum (Prunus domestica L.)[J].Biotechnology & Biotechnological Equipment,2003,17(1):59-64.
[18] WANG H,CESAR P,BURGOS L,ALBURQUERQUE N.Phosphomannose-isomerase as a selectable marker for transgenic plum (Prunus domestica L.)[J].Plant Cell Tissue and Organ Culture,2013,113(2):189-197.
[19] PETRI C,NURIA A,FAIZE M,SCORZA R,DARDICK C.Current achievements and future directions in genetic engineering of European plum (Prunus domestica L.)[J].Transgenic Research,2018,27(3):225-240.
[20] PEREZ-TORNERO O,EGEA J,VANOOSTEND A,BURGOS L.Assessment of factors affecting adventitious shoot regeneration from in vitro cultured leaves of apricot[J].Plant Science,2000,158(1/2):61-70.
[21] JAMES D J,PASSEY A J,RUGINI E.Factors affecting high frequency plant regeneration from apple leaf tissues cultured in vitro[J].Journal of Plant Physiology,1988,132(2):148-154.
[22] 李洪雯,刘建军,陈克玲,关斌,王建辉,何建,何礼.欧洲甜樱桃砧木'SL64'离体叶片再生体系研究[J].果树学报,2014,31(6):74-77.LI Hongwen,LIU Jianjun,CHEN Keling,GUAN Bin,WANG Jianhui,HE Jian,HE Li.Studies on efficient plant regeneration system from in vitro leaves of sweetcherry rootstock‘SL64’[J].Journal of Fruit Science,2014,31(6):74-77.
[23] 耿文娟,吴玉霞,袁海英,廖康,周龙,许正.野生欧洲李组织培养技术[J].经济林研究,2009,27(1):45-48.GENG Wenjuan,WU Yuxia,YUAN Haiying,LIAO Kang,ZHOU Long,XU Zheng.Tissue culture techniques of Prunus domestica L.[J].Non-wood Forest Research,2009,27(1):45-48.
[24] GELLA R,ERREA P.Application of in vitro therapy for ilarvirus elimination in three Prunus-Arten[J].Journal of Phytopathology,2010,146(8):445-449.
[25] LU C Y.The use of thidiazuron in tissue culture[J]. In vitro Cellular&Developmental Biology Plant,1993,29(2):92-96.
[26] YAO Y X,SUN Y W,LI G G,LI G H.Regeneration of plants from in vitro culture of petioles in Prunus domestica Lindl.(European plum)[J].Biotechnology&Biotechnological Equipment,2011,25(3):2458-2463.
[27] HILY J M,RAVELONANDRO M,DAMSTEEGT V,BASSETT C,PETRI C,LIU Z R,SCORZA R.Plum pox virus coat protein gene intron-hairpin-RNA (ihpRNA) constructs provide resistance to plum pox virus in Nicotiana benthamiana and prunus domestica[J].Journal of the American Society for Horticultural Science,2007,132(6):850-858.
[28] PETRI C,HILY J M,VANN C,DARDICK C,SCORZA R.A high-throughput transformation system allows the regeneration of marker-free plum plants(Prunus domestica)[J].Annals of Applied Biology,2011,159(2):302-315.
[29] ALBURQUERQUE N,FAIZE L,BURGOS L.Silencing of Agrobacterium tumefaciens oncogenes ipt and iaaM induces resistance to crown gall disease in plum but not in apricot[J].Pest Management Science,2017,73(10):2163-2173.