远缘杂交诱导胚发育以培养甜瓜双单倍体,可以拓宽葫芦科(Cucurbitaceae)作物尤其是甜瓜属作物获得纯合系的方法思路,提高甜瓜优异种质资源培育效率。前人虽然在远缘花粉授粉获得葫芦科作物单倍体方面已有过探索[1],但是这一方法并未得到深入研究。Sauton 等[2]使用辐射花粉授粉技术获得甜瓜单倍体;Ficcadenti 等[3]首次通过离体子房培养获得甜瓜单倍体;薛光荣等[4-6]通过对西瓜花药培养获得单倍体;此后人们通过雌核发育和雄核发育的方式已成功获得黄瓜(Cucumis sativus L.)[7-8]、南瓜(Cucurbita moschata Duchesne ex.Poir)[9-10]、西葫芦(Cucurbita pepo L.)[11]、西 瓜[Citrullus lanatus(Thunb.) Matsum.& Nakai][12-13]等葫芦科作物的单/双单倍体。虽然辐射花粉授粉获得甜瓜单倍体已是成熟的技术体系[14],但是依然存在着需要特殊设备[15]、诱导率低[16]、单倍体染色体加倍等问题[16-18],这极大制约了单倍体技术在甜瓜育种上的应用。因此,目前学者对甜瓜单倍体技术的探究大多聚焦在技术改进的层面。虽已有一些学者通过单倍体技术创制了具抗病性的甜瓜双/单倍体[19-21],但创制具有优良品质性状的双/单倍体仅见一篇报道[22]。薄皮甜瓜具有广适和抗病的优点,但品质和耐贮性却较差。薄皮甜瓜优良新品种的短缺,已成为阻碍我国薄皮甜瓜产业发展的重要因素[23]。超甜小麦酥为普通商用品种,由地方品种小麦酥多年定向选育,具有口感好、肉质脆、抗病性好和耐贮运的优点[24]。远缘花粉结合胚拯救创制双单倍体不需要特殊昂贵设备,并且关于远缘杂交诱导结合胚拯救培育甜瓜双单倍体的方法还未见报道。笔者在本研究中利用远缘杂交结合胚拯救培育超甜小麦酥甜瓜双单倍体,并详细介绍其植物学特性,为甜瓜双单倍体创制的理论研究和实践应用奠定一定基础。
1 材料和方法
1.1 材料
试验材料于2020 年春季种植在南京农业大学白马基地。母本材料为商品种超甜小麦酥,父本材料为远缘野生种非洲角。种子均由南京农业大学园艺学院葫芦科作物遗传与种质创新实验室提供,各种植10株,以提供雌蕊和花粉。
1.2 远缘授粉
上午8:00左右开始采集父本(非洲角)花粉;使用4朵远缘雄花对前一日去雄套袋的甜瓜雌花进行授粉。对Patial等[25]的远缘授粉结合激素2,4-D处理获得单倍体的操作稍加改进,具体操作:授粉后在子房靠近柱头处喷施10 mg·L-1 噻苯隆(thidiazuron,TDZ),于子房基部喷25 mg·L-1氯吡脲溶液(又称“坐果灵”),以促进瓜坐果和胚发育;授粉后3~4 h,再次喷施1次10 mg·L-1TDZ溶液,以强化刺激胚发育。
1.3 胚胎拯救和再生植株驯化
对子房授粉4周后采收果实。用75%乙醇对果实表面消毒,在超净工作台上将果实剖开,取相对饱满的种子接种到不添加任何激素的MS固体培养基上进行胚胎拯救[26],种子间隔为1 cm。10 d后,挑出黄绿色的种子继续转至MS固体培养基上进行成苗培养。植株进入三叶一心期,采用先将植株转至自然光下适应光照后,再逐渐开瓶适应湿度的方式进行驯化[27]。
1.4 再生植株鉴定
对再生植株DH70-1 和对照品种超甜小麦酥进行倍性鉴定和SSR分子标记鉴定。
倍性鉴定:以正常二倍体甜瓜叶片作为对照,从再生植株上取处于同时期约1 cm2幼嫩叶片,经细胞流式仪测定倍性[28]。由金迪未来生物科技(北京)有限公司代为测定。
SSR 鉴定:利用SSR 标记的共显性特征鉴定二倍体再生植株基因位点的纯合性。从葫芦科作物基因组数据库53对标记中筛选出3对在母本显示杂合条带的引物(表1)。利用CTAB 法提取超甜小麦酥和DH70-1 叶片的DNA,SSR 引物由金斯瑞生物科技公司合成。扩增反应体系总体积20µL:1µL 模板DNA,2×Taq Master Mix 10.0µL,各1 μL上、下游引物,7 µL ddH2O。扩增反应在PCR 仪中进行:94 ℃预变性5 min;94 ℃变性30 s,52 ℃退火30 s,72 ℃延伸30 s,28 个循环;72 ℃延伸10 min,最后4 ℃保存。产物经聚丙烯酰胺凝胶电泳检测。
表1 多态性标记引物名称及对应序列
Table 1 The names and corresponding sequences of primers with polymorphic markers
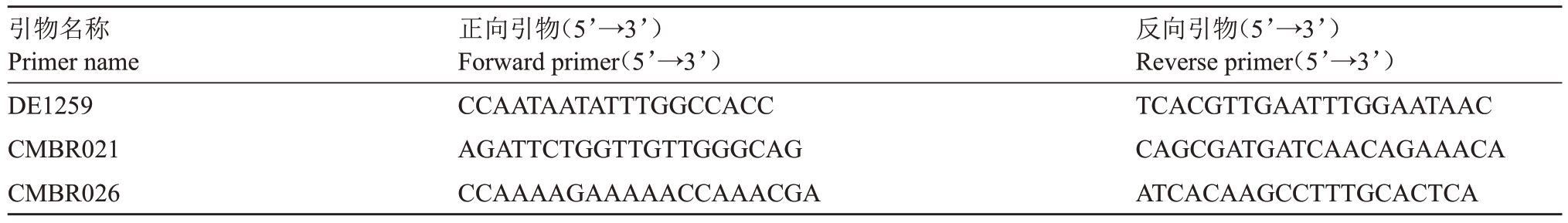
引物名称Primer name DE1259 CMBR021 CMBR026正向引物(5’→3’)Forward primer(5’→3’)CCAATAATATTTGGCCACC AGATTCTGGTTGTTGGGCAG CCAAAAGAAAAACCAAACGA反向引物(5’→3’)Reverse primer(5’→3’)TCACGTTGAATTTGGAATAAC CAGCGATGATCAACAGAAACA ATCACAAGCCTTTGCACTCA
1.5 再生植株自交留种及自交一代一致性评价
对DH70-1 进行自交留种,自交一代记为DH170-1,并对DH170-1 自交留种。每3 朵雄花授1朵雌花,留种3个瓜,35 d后采瓜。以品种超甜小麦酥为对照,计算结籽率,测量种子的长宽和发芽率;并对植株的典型性状(子叶长宽、叶片长宽、茎粗、节间长、花瓣长宽)进行测量。数据采用SPSS 软件进行LSD检验。
结籽率(%)=(饱满种子数/种子数)×100;
发芽率(%)=(发芽种子数/种子数)×100。
测量DH170-1 植株的子叶长宽、叶片长宽、茎粗、节间距和花瓣长宽。在一叶一心(第一片真叶完全展开)时测量子叶长宽;花期测量7、8、9、10 节位的长度和茎粗;18、19、20 节位的叶片长宽;每株随机选择3 朵雄花测量花瓣宽度。数据使用SPSS 软件进行单因素方差分析,并使用Excel 软件进行频数绘图。
1.6 DH170-1的果实性状的测定
以DH170-1 为材料,二倍体超甜小麦酥和超甜小麦酥S4为对照进行双单倍体果实性状的测定。其中超甜小麦酥S4为超甜小麦酥的自交4 代,其与原品种的果实形态(果实质量、纵横径和果皮果肉颜色)有较大的差别。参考Gocmen 等[22]的方法,待果实成熟时分3次采收果实,每次间隔2 d。测量果实的纵横径,计算其果形指数,称量单果质量。采用手持折光糖度计(WAY型)测定可溶性固形物含量。
采用蒽酮比色法、抗坏血酸滴定法测量果实可溶性糖、抗坏血酸含量[29]。试验均3次重复,数据采用SPSS软件进行LSD检验。
2 结果与分析
2.1 双单倍体材料的创制
2020年春季对超甜小麦酥远缘授粉10朵雌花,坐果7个,坐果率为70%。共接种179个相对饱满的种子,在接种10 d 后,如图1 所示,少量种子的顶部开始转变为黄绿色(图1-A),挑出黄绿色种子,转至MS固体培养基中,5 d后胚根突破种皮,然后子叶开始生长。在胚根长出5 d后,植株逐渐开始形成根系和真叶(图1-B),最后获得1株再生苗,命名为DH70-1,其中再生植株的诱导率为0.56%。待植株长出3片真叶后(图1-C),先闭瓶将植株转至棚中,适应光照与温度后,逐渐开盖驯化移栽至田间(图1-D)。

图1 再生苗形成
Fig.1 Regenerated seedlings development
A.转绿的胚;B.转绿的胚开始发芽形成根系和真叶;C.再生苗驯化前;D.再生苗驯化后。
A.Embryos that turned green;B.Embryos that turned green began to germinate to form roots and true leave;C.Regenerated seedlings before acclimation;D.Regenerated seedlings after acclimation.
2.2 双单倍体的综合鉴定
2.2.1 再生植株倍性鉴定 对DH70-1 和对照品种超甜小麦酥进行细胞流式仪分析。如图2 所示,DH70-1 首个呈正态分布的峰荧光强度为200,与对照品种超甜小麦酥一致,表明再生植株为二倍体。

图2 甜瓜再生植株的倍性鉴定
Fig.2 Ploidy identification of melon regenerated plants
A.DH70-1 再生苗细胞流式仪图;B.超甜小麦酥细胞流式仪图。
A.The flow cytometry diagram of the DH70-1 regenerated seedlings;B.The flow cytometry diagram of the Chaotianxiaomaisu.
2.2.2 再生植株SSR标记 从53对引物挑选出的3对 引 物DE1259、CMBR021 和CMBR026 对 甜 瓜DH70-1 呈多态性的标记。电泳图(图3)显示,3 个标记的杂合带均位于100~200 bp 之间,母本超甜小麦酥的3个标记均为杂合带型;而再生植株DH70-1与超甜小麦酥的条带不同,仅具有母本的部分条带。因此,说明再生植株的基因是纯合的。
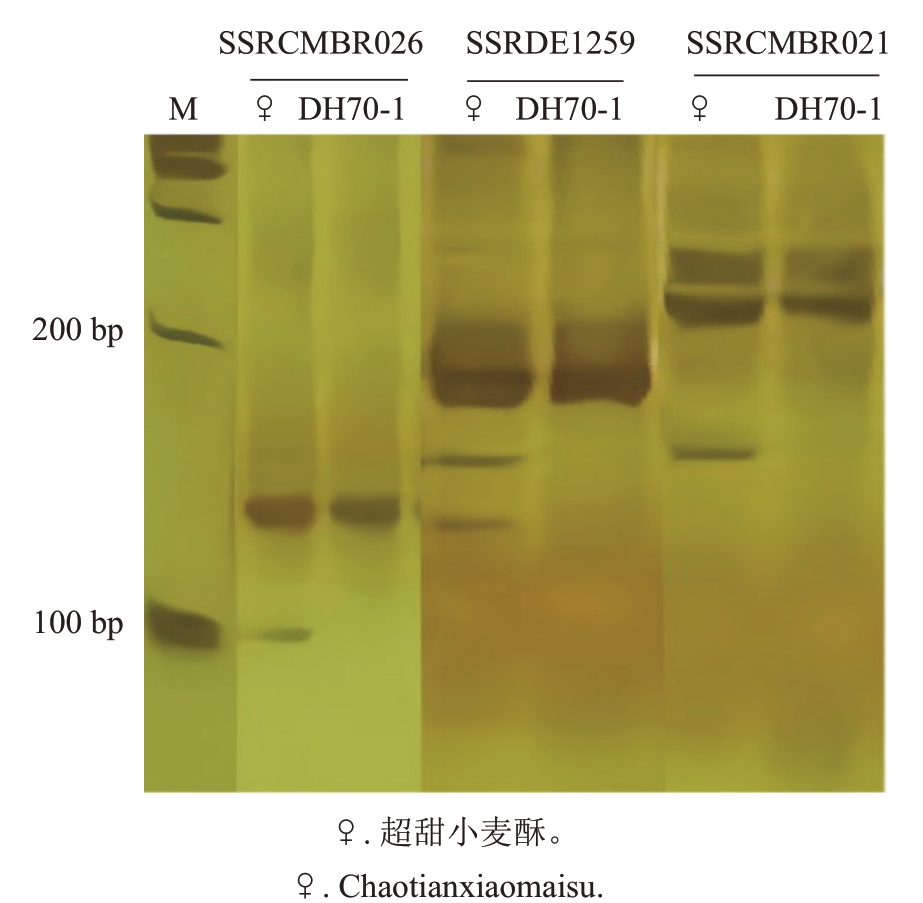
图3 SSR 电泳图谱
Fig.3 SSR electrophoresis pattern
2.3 双单倍体植物学特性
2.3.1 双单倍体与亲本的性状差异 DH70-1 自交后获得自交一代记为DH170-1。DH70-1自交种子的发芽率(85%)虽然比对照品种超甜小麦酥(94%)低,但差异未达到显著水平(表2),即表明DH70-1可以直接通过种子繁殖保存,这将有利于育种生产实践和理论研究。双单倍体的种子颜色呈浅黄色(图4-A),不同于对照的黄色;且种子的长度显著大于双单倍体(表2)。

图4 再生植株的植物学性状
Fig.4 Botanical traits of regenerated plants
A.左边为超甜小麦酥S4,中间为DH170-1,右边为超甜小麦酥;B.上方为超甜小麦酥种子,下方为DH 的种子;C.上方为DH170-1 雄花,下方为超甜小麦酥雄花。
A.Left is Chaotianxiaomaisu S4, middle is DH170-1, right is Chaotianxiaomaisu; B.Top is Chaotianxiaomaisu’seed, bottom is DH’s seed;C.Above is the male flower of DH170-1,and the below is the male flower of Chaotianxiaomaisu.
表2 DH70-1、DH170-1 和超甜小麦酥的种子特征及发芽率
Table 2 Seed characteristics and germination rates of DH70-1,DH170-1 and Chaotianxiaomaisu
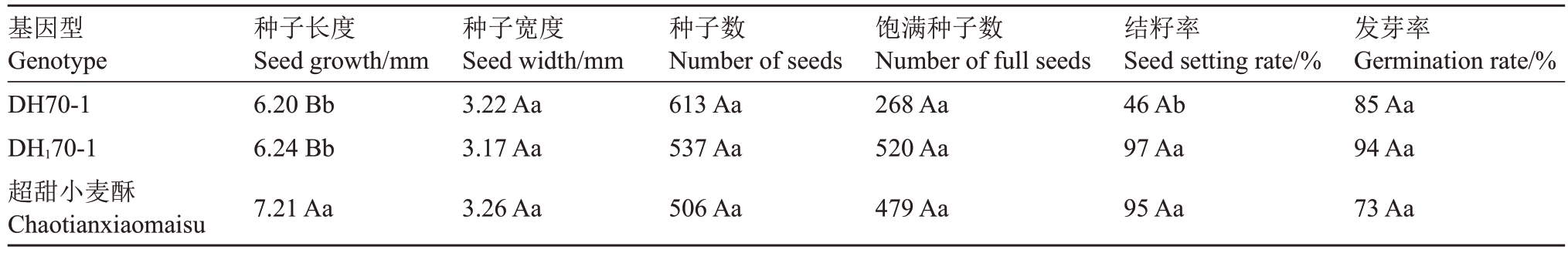
注:不同大写字母表示在p<0.01 水平上差异极显著;不同小写字母表示在p<0.05 水平上差异显著。下同。
Note:Different uppercase letters indicate that the difference is extremely significant at p<0.01; different lowercase letters indicate that the difference is significant at p<0.05.The same below.
基因型Genotype DH70-1 DH170-1超甜小麦酥Chaotianxiaomaisu种子长度Seed growth/mm 6.20 Bb 6.24 Bb 7.21 Aa种子宽度Seed width/mm 3.22 Aa 3.17 Aa 3.26 Aa种子数Number of seeds 613 Aa 537 Aa 506 Aa饱满种子数Number of full seeds 268 Aa 520 Aa 479 Aa结籽率Seed setting rate/%46 Ab 97 Aa 95 Aa发芽率Germination rate/%85 Aa 94 Aa 73 Aa
如表3 所示,DH70-1 与DH170-1 各性状间的差异均不显著;但是它们与对照品种相比在各性状上都呈显著差异,部分性状如花瓣、子叶长、叶宽、节间长的差异达到极显著水平。
表3 DH70-1、DH170-1 与超甜小麦酥的一些植物学性状比较
Table 3 Comparison of some botanical traits between DH70-1,DH170-1 and Chaotoanxiaomaisu
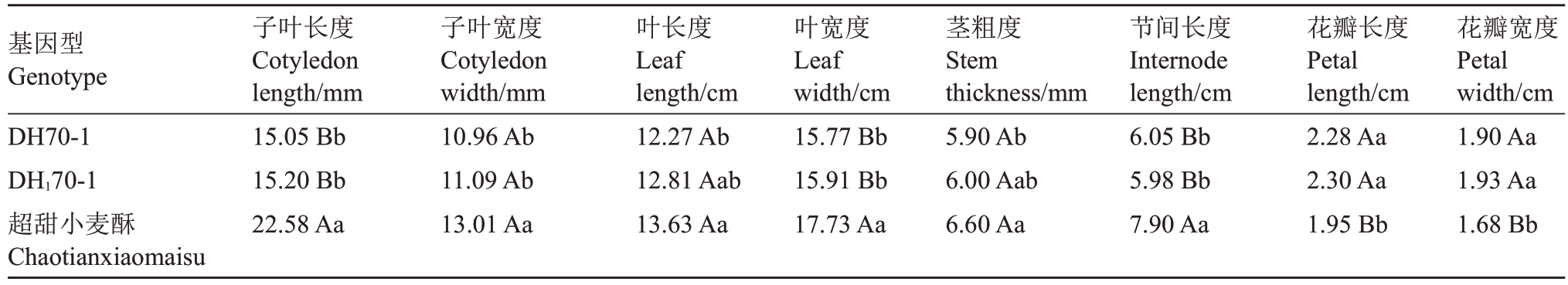
基因型Genotype DH70-1 DH170-1超甜小麦酥Chaotianxiaomaisu子叶长度Cotyledon length/mm 15.05 Bb 15.20 Bb 22.58 Aa子叶宽度Cotyledon width/mm 10.96 Ab 11.09 Ab 13.01 Aa叶长度Leaf length/cm 12.27 Ab 12.81 Aab 13.63 Aa叶宽度Leaf width/cm 15.77 Bb 15.91 Bb 17.73 Aa茎粗度Stem thickness/mm 5.90 Ab 6.00 Aab 6.60 Aa节间长度Internode length/cm 6.05 Bb 5.98 Bb 7.90 Aa花瓣长度Petal length/cm 2.28 Aa 2.30 Aa 1.95 Bb花瓣宽度Petal width/cm 1.90 Aa 1.93 Aa 1.68 Bb
2.3.2 双单倍体果实生理指标 如表4 所示,比较DH170-1与超甜小麦酥和超甜小麦酥S4果实的各数据可知,DH170-1的单果质量与后两者差异极显著;果实纵径和横径与超甜小麦酥差异极显著,但与超甜小麦酥差异不显著;DH170-1 与超甜小麦酥S4差异显著,但与超甜小麦酥差异不显著。观察三者的果实形态发现,超甜小麦酥果形呈梨形并且具有明显的果面沟,果皮和果肉颜色为绿色;而DH170-1为圆形,无果面沟分布,并且果实底色和果肉颜色都转变为白色,这与超甜小麦酥S4的果实形态相似(图4-B)。
表4 DH170-1、超甜小麦酥S4、超甜小麦酥间的果实性状比较
Table 4 Comparison of fruit traits between DH170-1,Chaotianxiaomaisu S4 and Chaotianxiaomaisu
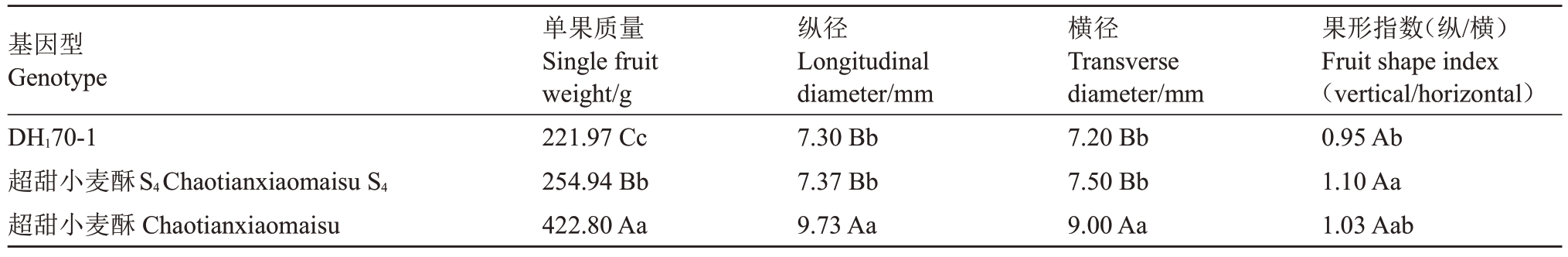
基因型Genotype DH170-1超甜小麦酥S4Chaotianxiaomaisu S4超甜小麦酥Chaotianxiaomaisu单果质量Single fruit weight/g 221.97 Cc 254.94 Bb 422.80 Aa纵径Longitudinal diameter/mm 7.30 Bb 7.37 Bb 9.73 Aa横径Transverse diameter/mm 7.20 Bb 7.50 Bb 9.00 Aa果形指数(纵/横)Fruit shape index(vertical/horizontal)0.95 Ab 1.10 Aa 1.03 Aab
如表5 所示,三者的可溶性糖含量差异并不显著;DH170-1 的抗坏血酸含量高于超甜小麦酥S4且差异达到显著水平,与超甜小麦酥相比差异不显著;然而DH170-1 可溶性固形物含量为11.03%,高于后两者,差异达到极显著水平。
表5 DH170-1、超甜小麦酥、超甜小麦酥S4果实生理指标比较
Table 5 Comparison of fruit physiological indicators of DH170-1,Chaotianxiaomaisu and Chaotianxiaomaisu S4
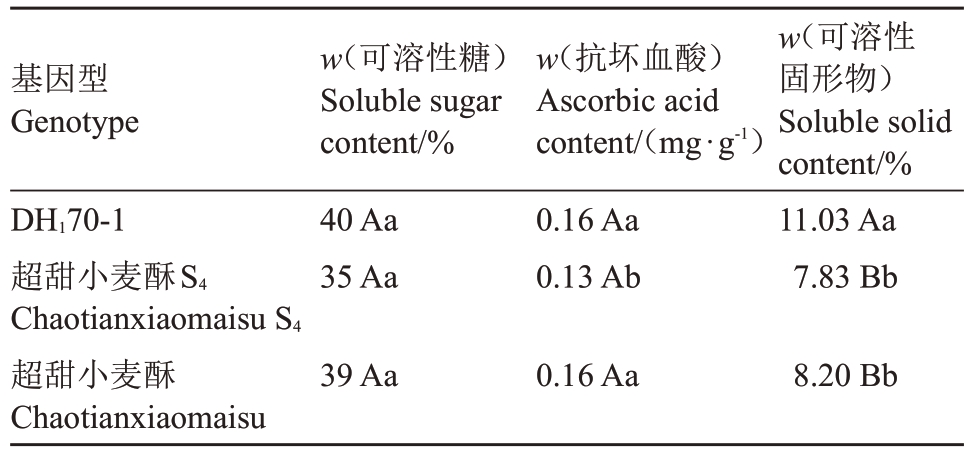
基因型Genotype DH170-1超甜小麦酥S4 Chaotianxiaomaisu S4超甜小麦酥Chaotianxiaomaisu w(可溶性糖)Soluble sugar content/%40 Aa 35 Aa w(抗坏血酸)Ascorbic acid content/(mg·g-1)0.16 Aa 0.13 Ab w(可溶性固形物)Soluble solid content/%11.03 Aa 7.83 Bb 39 Aa 0.16 Aa 8.20 Bb
2.4 自交一代性状一致性
随机选择DH170-1 株系内16 株,一叶一心测量子叶长宽,花期测量叶片长宽、茎长、茎粗、雄花宽。将各性状的数据制成频数分布图,发现各性状数据均符合正态分布(图5)。将各性状数据进行单因素方差分析(表6)。苗期性状“子叶长宽”的F 值分别为1.99、1.56,均小于F(15,32)0.01=2.65,因此不同株的子叶大小差异不显著。同样地,叶片长F=2.33<F(15,32)0.01=2.65、叶片宽F=1.84<F(15,32)0.01=2.65、茎长F=0.55<F(15,48)0.01=2.44、茎 粗F=1.31<F(15,48)0.01=2.44、雄花宽F=2.53<F(15,32)0.01=2.65,表明该株系内不同株的叶片长宽、茎粗、节间距、花瓣宽差异均不显著。经上述分析,即表明该株系内各株基因纯合,再次表明DH170-1的双单倍体特性。
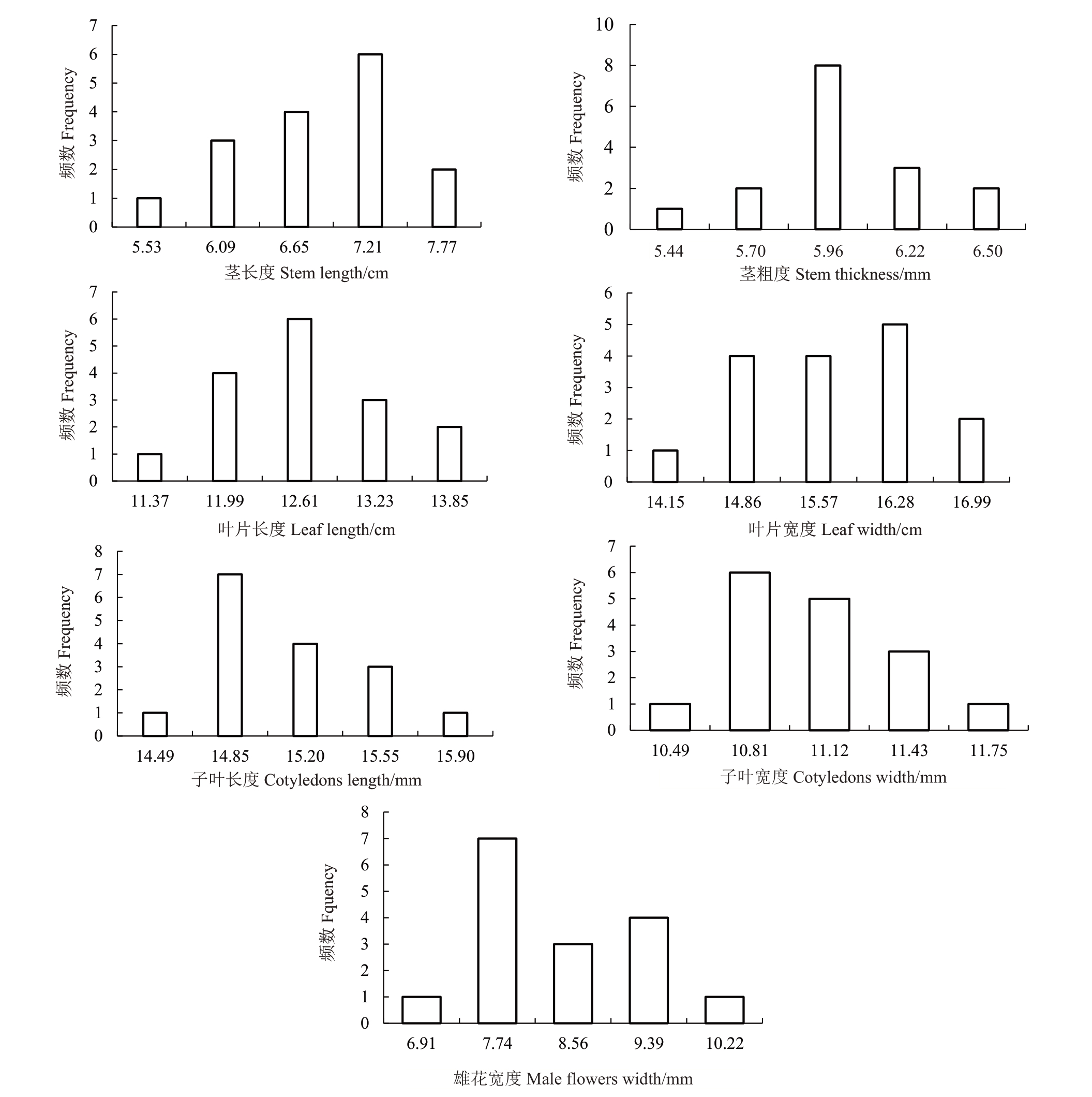
图5 DH170-1 各性状的频数分布
Fig.5 Frequency distribution of each trait in DH170-1
表6 株系DH170-1 七个性状的方差分析
Table 6 Analysis of variance of 7 traits of strain DH170-1
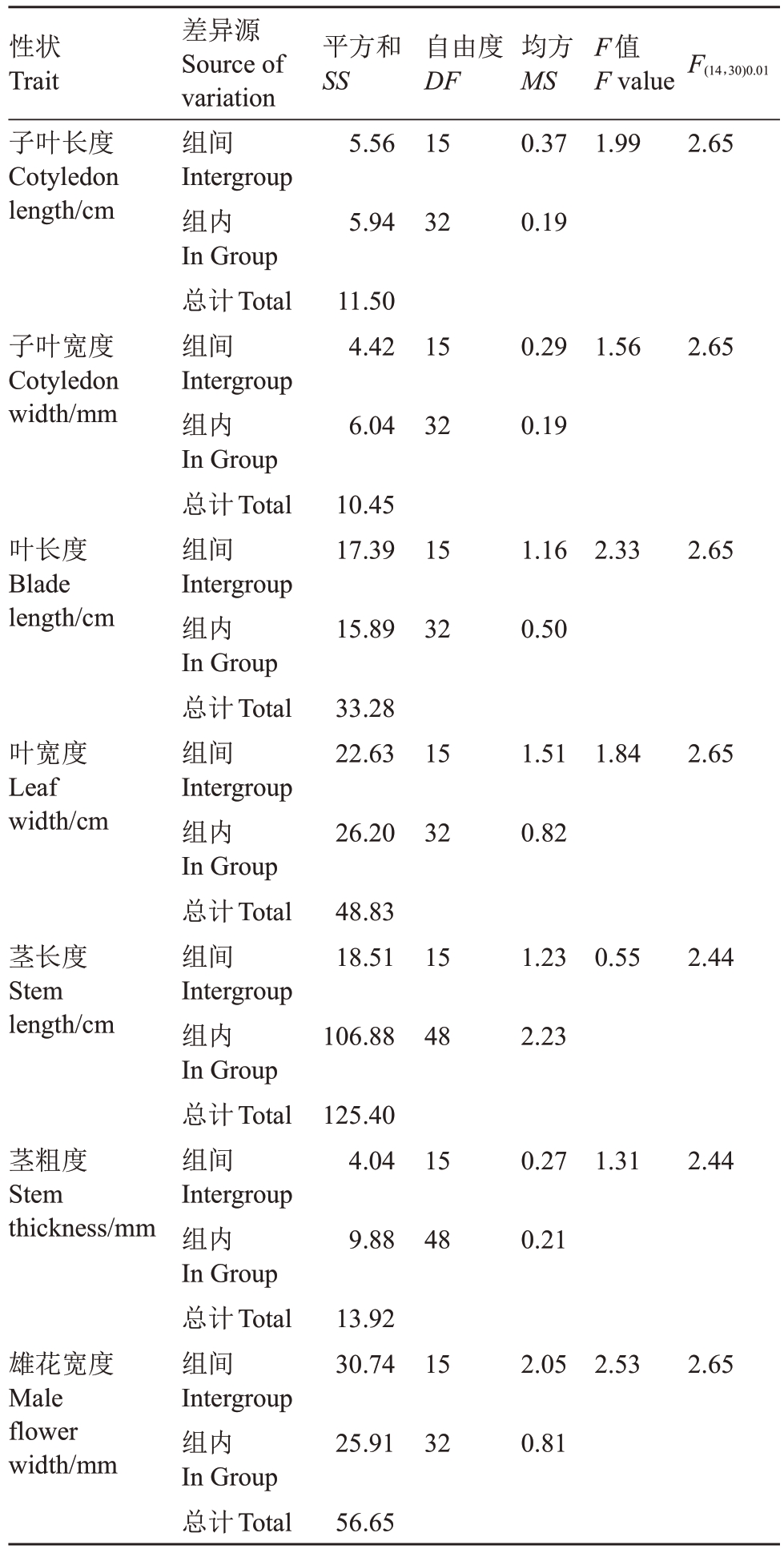
性状Trait子叶长度Cotyledon length/cm平方和SS 5.56自由度DF 15差异源Source of variation组间Intergroup组内In Group总计Total组间Intergroup组内In Group总计Total组间Intergroup组内In Group总计Total组间Intergroup组内In Group总计Total组间Intergroup组内In Group总计Total组间Intergroup组内In Group总计Total组间Intergroup组内In Group总计Total均方MS 0.37 F值F value 1.99 F(14,30)0.01 2.65 5.94 32 0.19子叶宽度Cotyledon width/mm 11.50 4.42 15 0.29 1.56 2.65 6.04 32 0.19叶长度Blade length/cm 10.45 17.39 15 1.16 2.33 2.65 15.89 32 0.50叶宽度Leaf width/cm 33.28 22.63 15 1.51 1.84 2.65 26.20 32 0.82茎长度Stem length/cm 48.83 18.51 15 1.23 0.55 2.44 106.88 48 2.23茎粗度Stem thickness/mm 125.40 4.04 15 0.27 1.31 2.44 9.88 48 0.21雄花宽度Male flower width/mm 13.92 30.74 15 2.05 2.53 2.65 25.91 32 0.81 56.65
3 讨 论
远缘花粉杂交促进胚发育获得单倍体的方法在禾本科作物已较为成熟[30-31],且已经应用于育种。远缘杂交促进胚发育之所以能够获得单倍体,是因为远缘授粉后形成的杂合子核型高度不稳定,从而出现染色体消除的现象[32]。在葫芦科作物中,1954 年Hayase[1]以笋瓜(Cucurbita maxima L.)为母本,南瓜(C.moschata L.)为父本杂交获得了单倍体植株;与前人的做法不同的是:本研究中在授粉后使用了TDZ 激素,促进了胚的发育和胚细胞染色体加倍,从而直接获得了双单倍体。在黄瓜离体子房培养的研究中,在添加有TDZ 的培养基上获得的再生苗,其倍性发生了变异,获得了DH和同源四倍体[8,33-35],表明TDZ 具有诱导再生物染色体加倍的效果。因此,笔者认为在本研究中TDZ激素的刺激可能是单倍体自发加倍为二倍体的原因。在高宁宁等[36]的甜瓜单倍体的染色体加倍的研究中发现KT激素同样有可使单倍体转换为双单倍体的效果,这与本研究TDZ 的效果类似。有关远缘授粉杂交促进胚发育直接获得双单倍体的具体机制还有待深入研究。
在前人的研究中发现,外植体或植株经秋水仙素处理后会出现效率较低、出现混倍体、形态畸形、外植体或植株死亡、加倍为DH 植株的育性不良等问题[37]。如Lim 等[17-18]发现甜瓜单倍体外植体经秋水仙素处理获得再生植株的花粉活力表现出不同水平(<5%、5%~20%、>20%),且仅当花粉活力>20%时子代的种子才有较好的发芽率。而本研究通过远缘杂交的方法直接获得DH,没有秋水仙素诱导染色体加倍的过程,不存在药剂加倍处理的副作用;DH 的结籽率和种子的发芽率与对照品种类似,可以直接用于育种的理论和实践研究。本研究获得双单倍体的育性正常,可能是远缘杂交诱导胚培养双单倍体方法的本身优势。
本研究中,DH170-1 的果实颜色和果肉颜色相比于对照品种有很大的差异,这种变化可能是基因的纯合导致某些隐形的基因表达。此外,DH170-1果实的可溶性固形物含量相对于超甜小麦酥具有显著性提升,口感更甜。前人研究表明甜瓜群体糖度符合正态分布,为典型的数量性状[38],并且研究表明甜度为多基因控制的性状[39]。传统育种纯化含糖量QTL基因需要多代选择自交,不仅费时、耗力,且效率低下,已经逐渐不能满足现代育种的需要[40-42]。因此,使用远缘花粉诱导创制DH 可在一个世代纯化与含糖量相关的多个QTL,这对于创制高糖甜瓜新品种具有较高的育种价值。
4 结 论
远缘杂交诱导结合胚培养,获得了含糖量提高的薄皮甜瓜双单倍体,且该双单倍体可以通过种子扩繁保存。
[1] HAYASE H.Cucurbita-crosses V.Occurrence of a haploid twin pair from a F1 progeny of C. maxima × C. moschata[J].Japanese Journal of Breeding,1954,4(2):115-121.
[2] SAUTON A,VAULX R D.Obtention de plantes haploïdes chez le melon (Cucumis melo L.) par gynogenèse induite par du pollen irradié[J].Agronomie,1987,7(2):141-148.
[3] FICCADENTI N,SESTILI S,ANNIBALI S,SCHIAVI M,DI MARCO M. In vitro gynogenesis to induce haploid plants in melon Cucumis melo L.[J].Journal of Genetics and Breeding,1999,53(3):255-257.
[4] 薛光荣,余文炎,费开伟,崔海南,孙瑞星.西瓜花药离体培养获得花粉植株[J].植物生理学通讯,1983,19(4):40-42.XUE Guangrong,YU Wenyan,FEI Kaiwei,CUI Hainan,SUN Ruixing. In vitro culture of watermelon anthers to obtain pollen plants[J].Plant Physiology Communications,1983,19(4):40-42.
[5] 薛光荣,余文炎,杨振英,孙瑞星.西瓜花粉植株后代的初步观察[J].科技通报,1987,3(4),39-41.XUE Guangrong,YU Wenyan,YANG Zhenying,SUN Ruixing.Preliminary observations on the progeny of watermelon pollen plants[J].Bulletin of Science and Technology,1987,3(4),39-41.
[6] 薛光荣,余文炎,杨振英,孙瑞星.西瓜花粉植株的诱导及其后代初步观察[J].遗传,1988,10(2):5-8.XUE Guangrong,YU Wenyan,YANG Zhenying,SUN Ruixing.Induction of watermelon pollen plants and preliminary observations on their progeny[J].Hereditas,1988,10(2):5-8.
[7] 付文苑,唐兵,邓英,李芳,文林宏.辐射花粉授粉诱导黄瓜单倍体及染色体加倍[J].分子植物育种,2019,17(21):7150-7155.FU Wenyuan,TANG Bing,DENG Ying,LI Fang,WEN Linhong.Induction of haploid by irradiated pollen pollination and chromosome doubling in cucumber[J].Molecular Plant Breeding,2019,17(21):7150-7155.
[8] DENG Y,TANG B,ZHOU X,FU WY,TAO L,ZHANG L,CHEN J F.Direct regeneration of haploid or doubled haploid plantlets in cucumber (Cucumis sativus L.) through ovary culture[J].Plant Cell,Tissue and Organ Culture,2020,142(2):253-268.
[9] ERTAN S K,AHMET B,MEHTAP O,TENZILE O.Induction of haploid embryo and plant regeneration via irradiated pollen technique in pumpkin (Cucurbita moschata Duchesne ex.Poir)[J].African Journal of Biotechnology,2009,8(21):5944-5951.
[10] SAIT K E,AHMET B,OZBAKIR O M.Production of callus mediated gynogenic haploids in winter squash (Cucurbita maxima Duch.) and pumpkin (Cucurbita moschata Duch.)[J].Czech Journal of Genetics and Plant Breeding,2018,54(1):9-16.
[11] BAKTEMUR G,YÜCEL N K,TAŞKIN H,ÇÖMLEKÇİOĞLU S,BÜYÜKALACA S.Effects of different genotypes and gamma ray doses on haploidization using irradiated pollen technique in squash[J].Turkish Journal of Biology,2014,38(3):318-327.
[12] ZOU T,SU H N,WU Q,SUN X W.Haploid induction via unfertilized ovary culture in watermelon[J].Plant Cell,Tissue and Organ Culture,2018,135(2):179-187.
[13] ZHU Y C,SUN D X,DENG Y,LI W H,AN G L,SI W J,GAO P,LIU J P.Regeneration of double haploid plants from unpollinated ovary cultures of watermelon[J].2019.DOl:https://doi.org/10.21203/rs.2.14098/v1.
[14] DONG Y Q,ZHAO W X,LI X H,LIU X C,GAO N N,HUANG J H,WANG W Y,XU X L,TANG Z H.Androgenesis,gynogenesis,and parthenogenesis haploids in cucurbit species[J].Plant Cell Reports,2016,35(10):1991-2019.
[15] KATOH N,HAGIMORI M,IWAI S.Production of haploid plants of melon by pseudofertilized ovule culture[J].Plant Tissue Culture Letters,1993,10(1):60-66.
[16] HOOGHVORST I,TORRICO O,HOOGHVORST S,NOGUÉS S. In situ parthenogenetic doubled haploid production in melon‘Piel de Sapo’for breeding purposes[J].Frontiers in Plant Science,2020,11:378.
[17] LIM W,EARLE E D.Effect of in vitro and in vivo colchicine treatments on pollen production and fruit set of melon plants obtained by pollination with irradiated pollen[J].Plant Cell,Tissue and Organ Culture,2008,95(1):115-124.
[18] LIM W,EARLE E D.Enhanced recovery of doubled haploid lines from parthenogenetic plants of melon(Cucumis melo L.)[J].Plant Cell,Tissue and Organ Culture,2009,98(3):351-356.
[19] LOTFI M,ALAN A R,HENNING M J,JAHN M M,EARLE E D.Production of haploid and doubled haploid plants of melon(Cucumis melo L.) for use in breeding for multiple virus resistance[J].Plant Cell Reports,2003,21(11):1121-1128.
[20] KUZUYA M,HOSOYA K,YASHIRO K,TOMITA K,EZURA H.Powdery mildew (Sphaerotheca fuliginea) resistance in melon is selectable at the haploid level[J].Journal of Experimental Botany,2003,54(384):1069-1074.
[21] SARI N,SOLMAZ I,YETISIR H,EKIZ H,YUCEL S.New fusarium wilt resistant melon (Cucumis melo var. cantalupensis)varieties developed by dihaploidization:Sari F1,Yetisir F1,Solmaz F1,Emin F1 and Yucel F1[C]// IV International Symposium on Cucurbits,2010,871:267-272.
[22] GOCMEN M,AYDIN E,SIMSEK İ,SARI N,SOLMAZ İ,GOKSEVEN A.Characterization of some agronomic traits and β-carotene contents of orange fleshed altinbas melon dihaploid lines[J].Ekin Journal of Crop Breeding and Genetics,2017,3(1):12-18.
[23] 林茂,睢祥琨,梁邦军,周贤达,王凤辰,王浩波.薄皮甜瓜新品种‘青酥’的选育[J].中国瓜菜,2016,29(10):28-30.LIN Mao,SUI Xiangkun,LIANG Bangjun,ZHOU Xianda,WANG Fengchen,WANG Haobo.Breeding of a new oriental melon cultivar‘Qingsu’[J].China Cucurbits and Vegetables,2016,29(10):28-30.
[24] 张松.介绍几个瓜类良种[J].农村百事通,2010(6):31.ZHANG Song.Introduction of several good melon varieties[J].Nongcun Baishitong,2010(6):31.
[25] PATIAL M,CHAUDHARY H K,SHARMA N,SUNDARESHA S,KAPOOR R,PAL D,PRAMANICK K K,SHUKLA A K,KUMAR J.Effect of different in vitro and in vivo variables on the efficiency of doubled haploid production in Triticum aestivum L.using Imperata cylindrica-mediated chromosome elimination technique[J].Cereal Research Communications,2020,49(1):133-140.
[26] 苏芃,HIEN N H,管苇,崔利,陈劲枫.辐射花粉授粉技术诱导黄瓜单倍体[J].江苏农业学报,2015,31(1):134-137.SU Peng,HIEN N H,GUAN Wei,CUI Li,CHEN Jinfeng.Induction of haploid cucumbers by irradiated pollen pollination[J].Jiangsu Journal of Agricultural Sciences,2015,31(1):134-137.
[27] 谢冰,王秀峰,樊治成.西葫芦组培苗驯化和移栽影响因素分析[J].中国瓜菜,2006,19(4):4-7.XIE Bing,WANG Xiufeng,FAN Zhicheng.Analysis of main factors affecting the acclimatizing and transplanting of regenerated plantlets of summer squash (Cucurbita pepo L.) in vitro culture[J].China Cucurbits and Vegetables,2006,19(4):4-7.
[28] 汪艳,肖媛,刘伟,李婷婷,胡锐,乔志仙.流式细胞仪检测高等植物细胞核DNA 含量的方法[J].植物科学学报,2015,33(1):126-131.WANG Yan,XIAO Yuan,LIU Wei,LI Tingting,HU Rui,QIAO Zhixian.Operation skills of flow cytometer for detecting nuclear DNA contents in higher plant cells[J].Plant Science Journal,2015,33(1):126-31.
[29] 李合生.植物生理生化实验原理和技术[M].北京:高等教育出版社,2000:184-188.LI Hesheng.Principles and techniques of plant physiological and biochemical experiments[M].Beijing:Higher Education Press,2000:184-188.
[30] 孙敬三,刘辉,路铁刚,王兴安,任真,王景林.利用小麦×玉米获得小麦单倍体[J].植物学报,1992,34(11):817-821.SUN Jingsan,LIU Hui,LU Tiegang,WANG Xingan,REN Zhen,WANG Jinglin.The production of haploid wheat plants via wheat × maize to hybridization[J].Acta Botanica Sinica,1992,34(11):817-821.
[31] 孙敬三,路铁刚,辛化伟.通过和玉米杂交诱导硬粒小麦单倍体[J].植物学报,1995,37(6):452-457.SUN Jingsan,LU Tiegang,XIN Huawei.Induction of haploid durum wheat plants through pollination of maize pollen[J].Acta Botanica Sinica,1995,37(6):452-457.
[32] 孙敬三,陈纯贤,路铁刚.禾本科植物染色体消除型远缘杂交的研究进展[J].植物学通报,1998,15(1):1-7.SUN Jingsan,CHEN Chunxian,LU Tiegang.Advances in karyotypically-unstable wide crosses (chromosome elimination system) in gramineae[J].Chinese Bulletin of Botany,1998,15(1):1-7.
[33] 周霞,张璐,周俊国,邓英,王怡,娄群峰,陈劲枫.黄瓜未授粉子房离体培养获得胚囊再生植株[J].园艺学报,2020,47(3):455-466.ZHOU Xia,ZHANG Lu,ZHOU Junguo,DENG Ying,WANG Yi,LOU Qunfeng,CHEN Jinfeng.Embryonary sac regenerated plants were obtained in cucumber via in vitro unpollinated ovary culture[J].Acta Horticulturae Sinica,2020,47(3):455-466.
[34] DIAO W P,JIA Y Y,SONG H,ZHANG X Q,LOU Q F,CHEN J F.Efficient embryo induction in cucumber ovary culture and homozygous identification of the regenetants using SSR markers[J].Scientia Horticulturae,2009,119(3):246-251.
[35] 刁卫平,贾媛媛,江彪,鲍生有,娄群峰,陈劲枫.黄瓜未授粉子房培养获得同源四倍体[J].园艺学报,2008,35(12):1781-1786.DIAO Weiping,JIA Yuanyuan,JIANG Biao,BAO Shengyou,LOU Qunfeng,CHEN Jinfeng.Induction of autotetraploid cucumbers by unpollinated ovary culture and their characterization[J].Acta Horticulturae Sinica,2008,35(12):1781-1786.
[36] 高宁宁,李晓慧,康利允,梁慎,常高正,李海伦,王慧颖,王琰,徐小利,赵卫星.单倍体甜瓜染色体加倍技术研究[J].中国瓜菜,2021,34(6):28-32.GAO Ningning,LI Xiaohui,KANG Liyun,LIANG Shen,CHANG Gaozheng,LI Hailun,WANG Huiying,WANG Yan,XU Xiaoli,ZHAO Weixing.Study on chromosome doubling technique of haploid muskmelon[J].China Cucurbits and Vegetables,2021,34(6):28-32.
[37] EBRAHIMZADEH H,SOLTANLOO H,SHARIATPANAHI M E,ESKANDARI A,RAMEZANPOUR S S.Improved chromosome doubling of parthenogenetic haploid plants of cucumber(Cucumis sativus L.)using colchicine,trifluralin,and oryzalin[J].Plant Cell,Tissue and Organ Culture,2018,135(3):407-417.
[38] 周六千,李群,鲁思梦,宁雪飞,王贤磊.甜瓜果实糖含量性状的遗传规律与基因定位[J].分子植物育种,2021,19(18):6096-6104.ZHOU Liuqian,LI Qun,LU Simeng,NING Xuefei,WANG Xianlei.Genetic law and gene mapping of sugar content traits in melon[J].Molecular Plant Breeding,2021,19(18):6096-6104..
[39] PEREIRA L,RUGGIERI V,PEREZ S,ALEXIOU K G,FERNANDEZ M,JAHRMANN T,PUJOL M,GARCIA-MAS J.QTL mapping of melon fruit quality traits using a high-density GBS-based genetic map[J].BMC Plant Biology,2018,18(1):324.
[40] CHEN J F,CUI L,MALIK A A,MBIRA K G. In vitro haploid and dihaploid production via unfertilized ovule culture[J].Plant Cell,Tissue and Organ Culture,2011,104(3):311-319.
[41] 柯思佳,钱春桃,包卫红,倪维晨,李瑞霞.甜瓜离体雌核发育诱导单倍体研究进展[J].中国瓜菜,2018,31(9):1-5.KE Sijia,QIAN Chuntao,BAO Weihong,NI Weichen,LI Ruixia.Research progress of in vitro gynogenesis induction haploid in melon[J].China Cucurbits and Vegetables,2018,31(9):1-5.
[42] HOOGHVORST I,NOGUÉS S.Opportunities and challenges in doubled haploids and haploid inducer-mediated genome-editing systems in cucurbits[J].Agronomy,2020,10(9):1141.