杨梅(Morella rubra Sieb.et Zucc.)是杨梅科杨梅属常绿乔木,是我国特产水果之一,栽培历史悠久,广泛分布于长江以南地区,被吴耕民先生赞为“中国特产的初夏江南珍果”[1]。新鲜的杨梅果实香气宜人,风味浓郁,酸甜多汁,富含多种维生素和有机酸,深受广大消费者青睐。但杨梅成熟与采后贮运期间呼吸剧烈[2],常因“酒味”重而影响消费者品鉴。
“酒味”主要来源于乙醇发酵途径,丙酮酸脱羧酶(pyruvate decarboxylase,PDC;EC 4.1.1.1)和乙醇脱氢酶(alcohol dehydrogenase,ADH;EC 1.1.1.1)是该途径中的两个关键酶,前者催化丙酮酸生成乙醛和CO2,后者则催化乙醛生成乙醇[3]。前人对PDC在酵母和细菌乙醇发酵中的功能研究较为广泛,而在植物中研究较少。PDC 通常在低氧或缺氧条件下通过发酵代谢为植物提供能量[4],但涉及PDC 参与果实香气物质形成的研究鲜有报道[5]。与PDC相比,前人对植物ADH的研究较为广泛而深入。大量研究表明,ADH 参与植物低氧胁迫反应,且在调控果实生长发育和成熟衰老过程中发挥了重要作用。在番茄、苹果、甜瓜、葡萄、杧果、桃、杏等的果实研究中发现,乙醇含量在果实成熟期间不断积累,ADH基因的表达量逐渐升高[6-12]。
乙醛和乙醇等风味物质在果实成熟期间和采后贮运过程中的含量和种类发生动态变化,这通常是种类品种(遗传因素)、成熟度与采后贮藏条件等多因素综合作用的结果[13]。随种类品种不同,果实风味物质种类与含量也各不相同;果实风味物质组分及含量在品种间也存在差异,如Wang 等[14]研究发现,国内外50 个桃品种果实的乙醛、乙醇等挥发性物质组分和含量主要取决于遗传背景。果实发育和成熟阶段对乙醛和乙醇等芳香物质的合成也有影响,如在番茄、草莓等果实上,乙醛和乙醇随着果实成熟而不断积累[7,15-17]。在采后贮藏中,多数呼吸跃变型和部分非呼吸跃变型果实在不适宜的贮藏环境下积累较大量的乙醛和乙醇,从而造成异味,异味轻重程度受贮藏温度、湿度、气体条件等多因素影响;研究发现,低温贮藏和适宜的透气条件可以显著延缓桃、猕猴桃、柑橘、梨、甜瓜、葡萄等果实乙醛和乙醇积累,防止果实产生“酒味”而影响品鉴[18-25]。
在许多大宗水果中已发现PDC 和ADH 基因参与了果实成熟期间香气物质合成和采后贮藏过程异味物质产生,而在杨梅这一特色小众水果上仍缺乏深入研究。Zhu 等[26]发现随着杨梅果实成熟,乙醛和乙醇逐渐积累,Chen 等[27]发现杨梅PDC和ADH 蛋白丰度随着果实成熟而增加,但这些研究均未进一步分析基因表达与乙醛和乙醇含量间的相关性。同时,对于贮运期间杨梅果实乙醇和乙醛积累以及PDC 和ADH 基因表达的模式和关系也未见报道。本研究旨在鉴定在杨梅中高表达的PDC 和ADH 基因家族成员,探究其在不同杨梅品种果实成熟期间和贮运过程中的表达模式及其与乙醛和乙醇积累的关系,以期为杨梅果实风味形成机制以及果实异味减轻措施研发等相关研究提供参考。
1 材料和方法
1.1 试验材料与处理
用于果实发育与成熟阶段分析的12 个杨梅品种为荸荠、东魁、水晶、粉红、特早梅、浮宫1 号、黑晶、落子、八贤道、软丝安海变、乌梅和龙海水晶,其中荸荠和东魁采摘于浙江省金华市兰溪市,水晶采摘于浙江省宁波市余姚市,其余品种采摘于福建省福州市福建省农业科学院。每个品种在绿果期(S1)、转色期(S2)和成熟期(S3)分别采集。采后不同包装处理的研究试材为荸荠和东魁成熟果实,采摘于浙江省台州市仙居县,挑选大小均一、成熟度(果实色泽)一致、无明显外伤和病虫害的果实用于试验。包装处理分为3种:减压包装、普通包装和单果包装。其中减压包装用厚为0.2 mm 的聚乙烯(PE)包装袋对装有果实的篮筐封口后进行抽气至真空度为0.07 MPa;在普通包装中,装有果实的篮筐套上包装袋但不封口;在单果包装中,在透气性良好的颗粒包装盒中放入果实。果实经不同包装后放冷库预冷至10 ℃,然后放入泡沫箱中,在泡沫箱中两个篮筐之间放入经-18 ℃预先冰冻的蓄冷冰袋,封箱,于6 h 内运达实验室。抵达实验室后拆开泡沫箱,果实随包装置于冷库贮藏(温度0 ℃,湿度90%),分别于0、2、4、6、8、10 d取样。试验设置3个生物学重复,每个生物学重复包含10 个杨梅果实。杨梅果实果肉切碎后用液氮冷冻,存于-80 ℃超低温冰箱中备用。
1.2 杨梅PDC和ADH基因家族成员鉴别
从NCBI 数据库(https://www.ncbi.nlm.nih.gov/)下载杨梅基因组注释文件和基因组序列拼接文件。从NCBI数据库查找并获得拟南芥、烟草、番茄等其他植物PDC、ADH 蛋白序列,通过BLASTP(E-value<1E-20)在杨梅基因组中搜索同源基因,鉴定同源性。进一步从Pfam数据库(http://pfam.xfam.org/)下载PDC和ADH蛋白保守结构域的隐马尔可夫模型(HMM model)文 件(PDC: PF00205,ADH:PF08240),在杨梅基因组中检索杨梅PDC 和ADH基因。将2次鉴定结果并集后,使用NCBI保守结构域数据库(https://www.ncbi.nlm.nih.gov/cdd/)确认所有候选PDC 和ADH 蛋白的结构域同源性,剔除没有相应PDC 和ADH 保守结构域的蛋白,仅保留与其他物种中具有相同保守域序列的PDC 和ADH 蛋白;通过ExPASy 在线网站(https://web.expasy.org/compute_pi/)获得杨梅PDC、ADH 蛋白分子质量并预测蛋白等电点。
1.3 RNA提取与cDNA合成
杨梅果肉总RNA 提取采用Shan 等[28]描述的十六烷基三甲基溴化铵(CTAB)法。测定总RNA浓度后,参照HiScript®ⅡQ RT SuperMix for qPCR(+gDNA wiper)说明书(Vazyme),吸取1 μg 总RNA,去除基因组DNA后进行逆转录反应合成cDNA。
1.4 实时荧光定量PCR(RT-qPCR)
实时荧光定量PCR(real-time quantitative PCR,RT-qPCR)用于测定MrPDC1、MrPDC2、MrADH1、MrADH2 和MrADH3 的表达量。5 个基因的引物均由DNAMAN设计(表1),并通过熔解曲线和PCR产物测序对引物的特异性进行验证。以杨梅MrActin为内参基因(表1,GenBank GQ340770[29]);qPCR 体系与程序参照ChamQ Universal SYBR®qPCR Master Mix 说明书(Vazyme)。每个样品设置2 次技术重复。利用2-ΔCT法计算MrPDCs 和MrADHs 基因的相对表达量。
表1 杨梅PDC 和ADH 基因RT-qPCR 引物
Table 1 RT-qPCR primers of PDC and ADH genes in Chinese bayberry
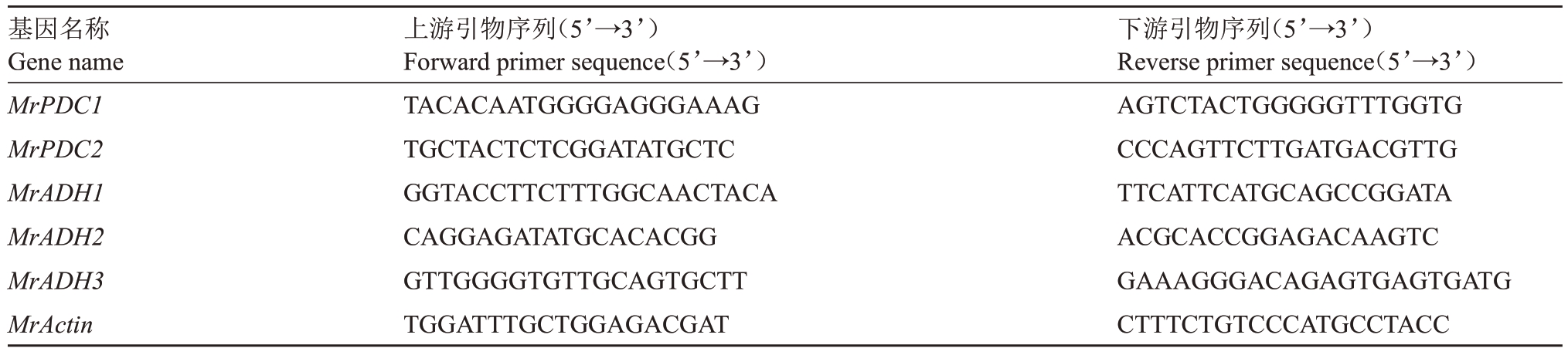
基因名称Gene name MrPDC1 MrPDC2 MrADH1 MrADH2 MrADH3 MrActin上游引物序列(5’→3’)Forward primer sequence(5’→3’)TACACAATGGGGAGGGAAAG TGCTACTCTCGGATATGCTC GGTACCTTCTTTGGCAACTACA CAGGAGATATGCACACGG GTTGGGGTGTTGCAGTGCTT TGGATTTGCTGGAGACGAT下游引物序列(5’→3’)Reverse primer sequence(5’→3’)AGTCTACTGGGGGTTTGGTG CCCAGTTCTTGATGACGTTG TTCATTCATGCAGCCGGATA ACGCACCGGAGACAAGTC GAAAGGGACAGAGTGAGTGATG CTTTCTGTCCCATGCCTACC
1.5 乙醛和乙醇含量测定
乙醛和乙醇含量测定参照Min等[30]的方法。称取3 g 经液氮充分研磨的杨梅果肉粉末于10 mL 离心管,加入4 mL 饱和NaCl 溶液,涡旋混匀;从中吸取3 mL匀浆至顶空萃取瓶,加入10 μL 1%(φ)仲丁醇,混匀。60 ℃水浴加热1 h后在气相色谱仪(Agilent 7890A)上进行静态顶空气相色谱(GC)检测,每个样品进行3次技术重复。配制1%乙醛、乙醇和仲丁醇(内标)溶液制作标准曲线,标准曲线制作以乙醛(或乙醇)浓度为横坐标,乙醛(或乙醇)与仲丁醇的峰面积之比为纵坐标。依据标准曲线计算萃取瓶中的乙醛(或乙醇)质量(μg),样品中的乙醛(或乙醇)含量计算如下:
其中,B为萃取瓶中的乙醛(或乙醇)质量(μg),V0为提取液体积(7 mL),V1为萃取瓶中提取液体积(3 mL),m为样品质量(3 g)。
1.6 果实异味评价
分别在包装处理4 d和8 d时对杨梅异味程度(1无、2轻、3中、4重、5非常重)进行赋分评价,参评人员为10人。
1.7 数据分析
试验数据使用Excel 2019进行统计、制图,应用SPSS 23 软件进行差异显著性检验(p<0.05)和Pearson相关性分析。
2 结果与分析
2.1 杨梅高表达PDC和ADH基因家族成员鉴别
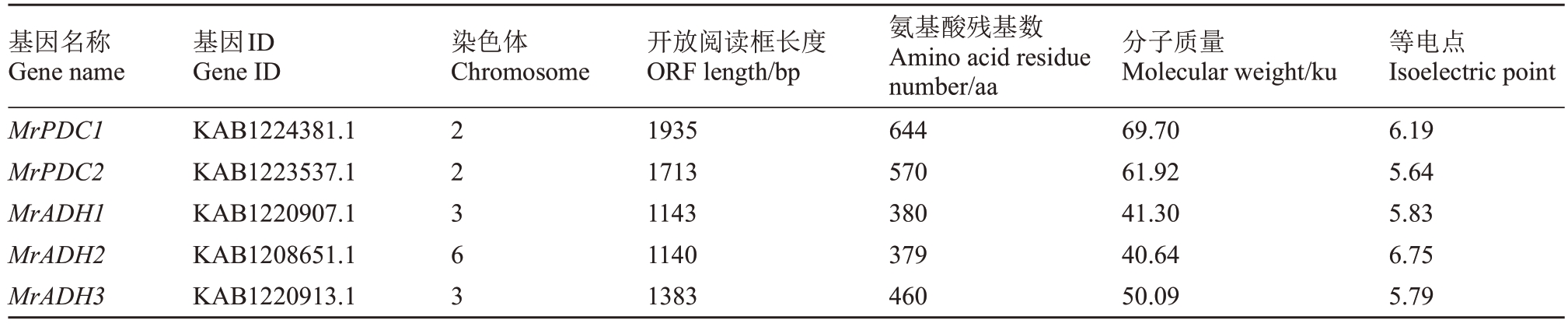
通过HMM和BLASTP检索并剔除相似度过高序列,在杨梅基因组中共鉴定出8 个PDC 和22 个ADH 基因。在项目组先前已有的荸荠和东魁杨梅转录组中分析这些基因的表达丰度,发现MrPDC1和MrPDC2 表达量之和占MrPDCs 的90%以上,MrADH1、MrADH2 和MrADH3 的 表 达 量 之 和 占MrADHs的80%以上。这些基因在基因组中的编号和染色体定位、开放阅读框(open reading frame,ORF)长度、编码蛋白的氨基酸残基数、分子质量以及等电点等信息如表2所示。经过与其他植物PDC和ADH基因进行比对,发现这5个基因编码完整的蛋白。
表2 杨梅2 个PDC 和3 个ADH 基因家族成员信息
Table 2 The information of two MrPDCs and three MrADHs in Chinese bayberry
基因名称Gene name MrPDC1 MrPDC2 MrADH1 MrADH2 MrADH3基因ID Gene ID KAB1224381.1 KAB1223537.1 KAB1220907.1 KAB1208651.1 KAB1220913.1染色体Chromosome 22363开放阅读框长度ORF length/bp 1935 1713 1143 1140 1383氨基酸残基数Amino acid residue number/aa 644 570 380 379 460分子质量Molecular weight/ku 69.70 61.92 41.30 40.64 50.09等电点Isoelectric point 6.19 5.64 5.83 6.75 5.79
2.2 杨梅果实成熟期间PDC和ADH基因表达分析
RT-qPCR 定量分析结果显示,MrPDC1 和MrPDC2 在12 个杨梅品种3 个果实发育阶段的表达模式存在差异。在9 个品种中,MrPDC1 的表达量在S3最高,其中MrPDC1在龙海水晶S3的表达量分别是S1和S2的12.30和5.70倍。而MrPDC2在8个品种中在S1 表达量最高,其中MrPDC2 在乌梅S1 的表达量分别是S2和S3的2.92和2.73倍(图1)。
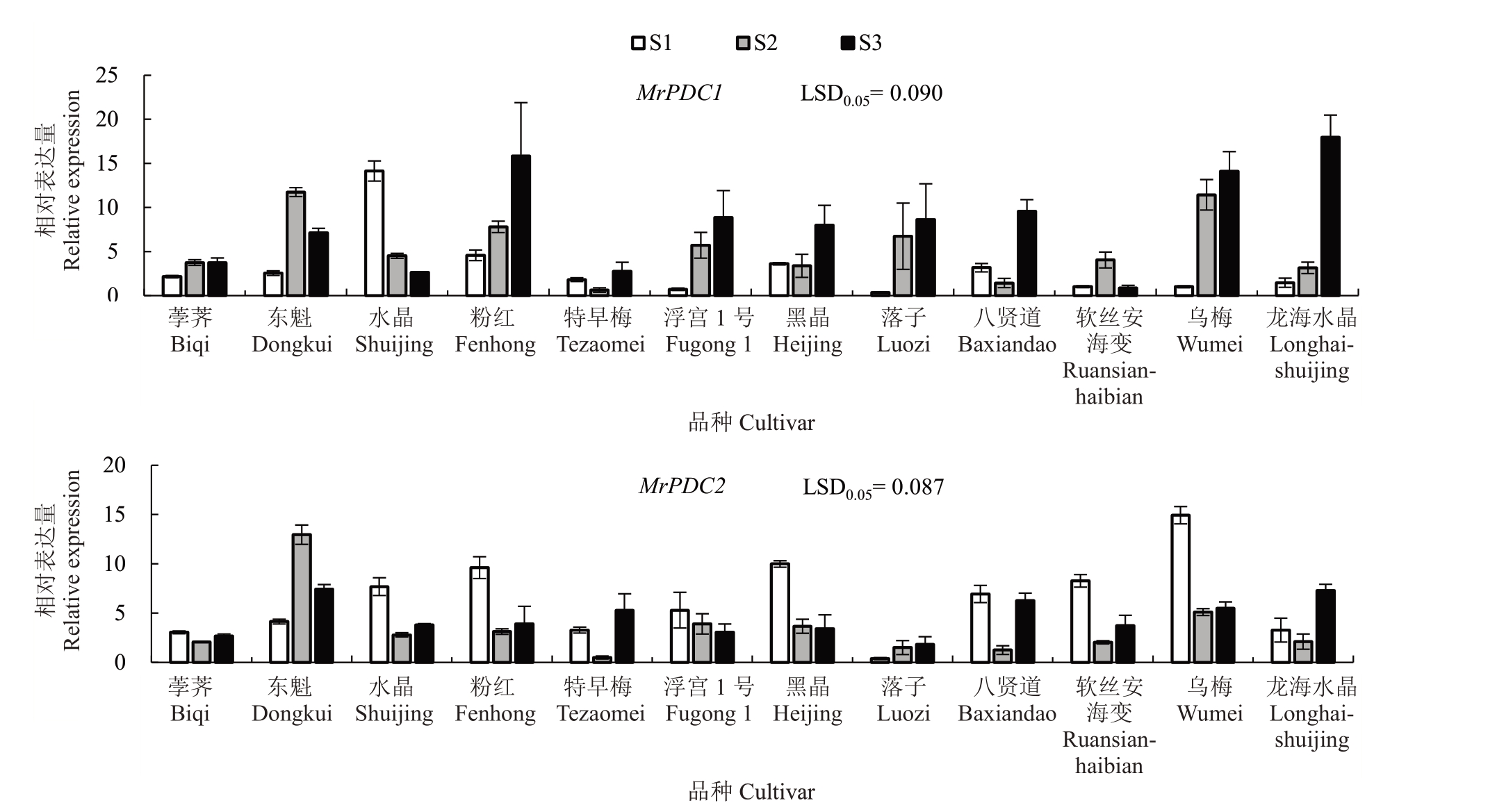
图1 12 个杨梅品种果实成熟期间MrPDC1 和MrPDC2 的表达分析
Fig.1 Expression of MrPDC1 and MrPDC2 in 12 Chinese bayberry cultivars during fruit ripening
误差线代表3 个生物学重复的SE 值,LSD 值代表不同处理间在p<0.05 水平的显著差异。下同。
Error bars indicate SE from three biological replicates.LSD indicates least-significant difference at p<0.05.The same below.
MrADHs的表达量在不同杨梅品种果实成熟过程中差异较大,MrADH3 的表达量普遍高于MrADH1 和MrADH2。除东魁、落子与水晶S1 外,MrADH1 在其余9 个品种上在S3 表达量最高,其中在特早梅S3 的表达量分别是S1 和S2 的44.77 倍和5.12 倍。除落子外,MrADH2 在其余11 个品种上在S1 表达量最高,其中在乌梅S1 的表达量分别是S2和S3 的3.59 倍和7.30 倍。除水晶和浮宫1 号外,MrADH3 在其余10 个品种上在S3 表达量最高,MrADH3 在特早梅S3 的表达量分别是S1 和S2 的10.87倍和51.64倍(图2)。
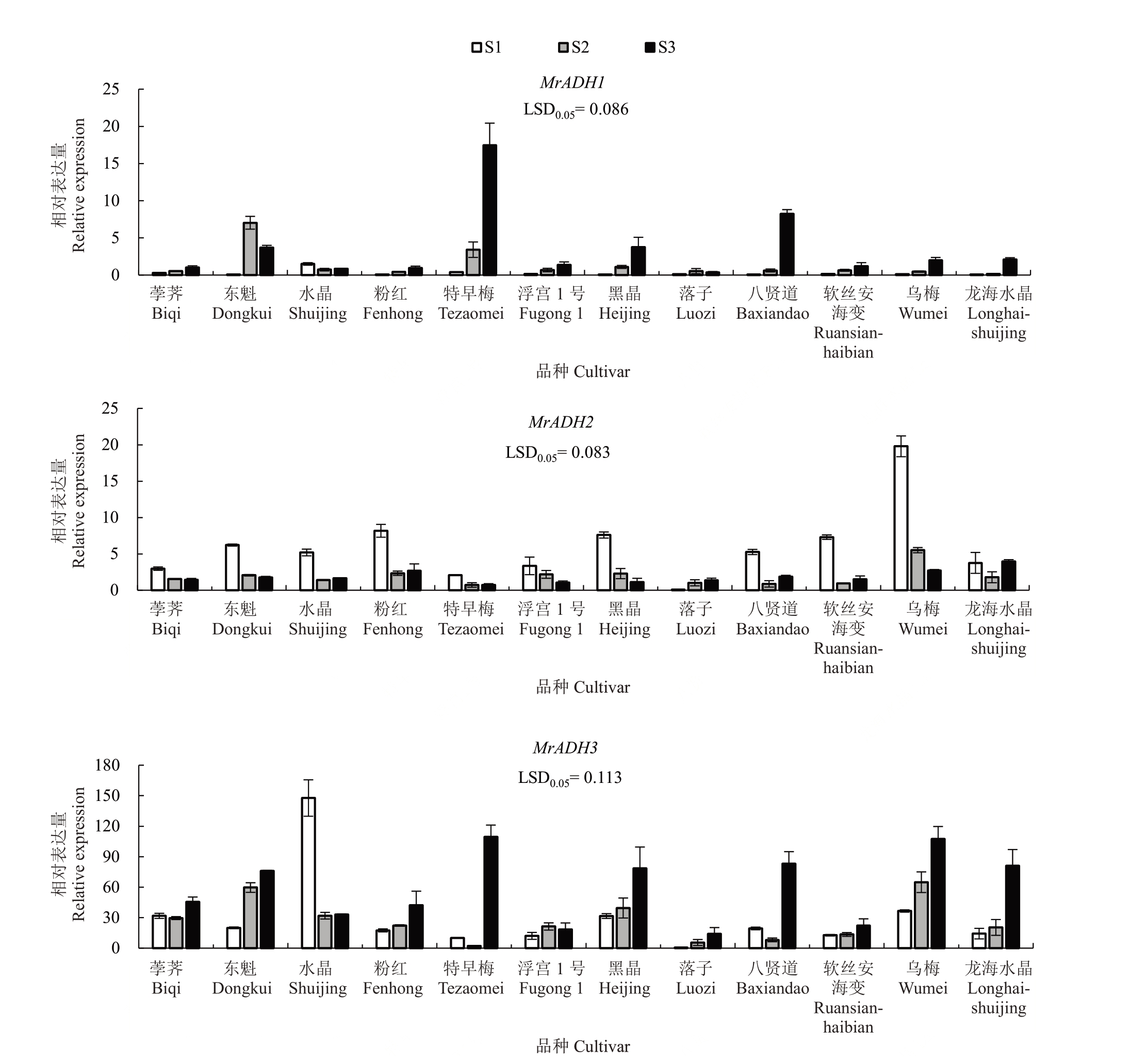
图2 12 个杨梅品种果实成熟期间MrADH1、MrADH2 和MrADH3 的表达分析
Fig.2 Expression of MrADH1,MrADH2 and MrADH3 in 12 Chinese bayberry cultivars during fruit ripening
2.3 杨梅果实成熟期间乙醛、乙醇含量与醇醛比值的变化
12个杨梅品种果实在3个发育阶段的乙醛含量(w,含量)在10.14~21.76 μg·g-1之间,其中以东魁S3果实中的乙醛含量最高。在10个品种中,果实乙醛含量随成熟而不断增加,但粉红果实中乙醛含量在S1 最高(19.22 μg·g-1),随果实成熟而不断下降;龙海水晶的乙醛含量在S2 最高。杨梅果实中的乙醇含量在12.85~307.91 μg·g-1之间,其中以特早梅S3果实中的乙醇含量最高;除落子外,其余品种的乙醇含量在果实成熟期显著升高。果实醇醛比值变化趋势与乙醇含量变化基本一致,除落子外的其余品种果实醇醛比值在S3明显升高(图3)。
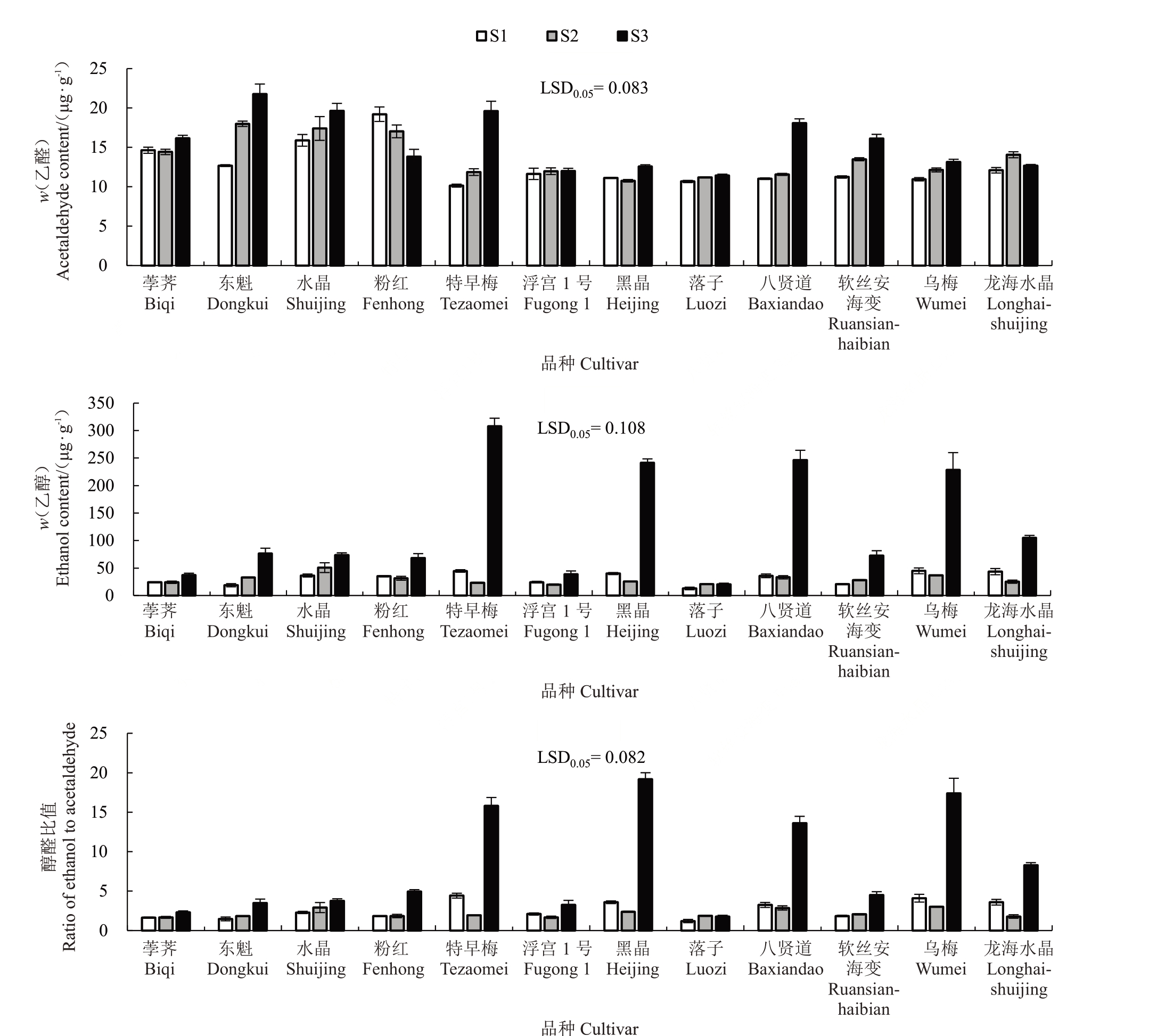
图3 12 个杨梅品种果实成熟期间乙醛、乙醇含量和醇醛比值
Fig.3 Contents of acetaldehyde and ethanol and the content ratio of ethanol to acetaldehyde in 12 Chinese bayberry cultivars during fruit ripening
Pearson 相关性分析结果表明,乙醛、乙醇含量和醇醛比值均与MrADH1 和MrADH3 的表达量呈极显著正相关,且MrPDC2与MrADH2、MrADH1与MrADH3 的表达量也呈极显著正相关(表3)。此结果与RT-qPCR 定量分析中多数品种MrADH1 和MrADH3 在果实成熟期表达量最高、MrPDC2 和MrADH2在幼果期表达量最高一致;此外,MrADH2的表达量与乙醛、乙醇含量无显著相关性。
表3 12 个品种3 个发育阶段杨梅果实乙醛、乙醇含量和醇醛比值与MrPDCs 和MrADHs 表达量的相关性
Table 3 Correlation between acetaldehyde,ethanol contents as well as the ratio of ethanol to acetaldehyde and the expression of MrPDCs and MrADHs in Chinese bayberry fruit of 12 cultivars and 3 developmental stages
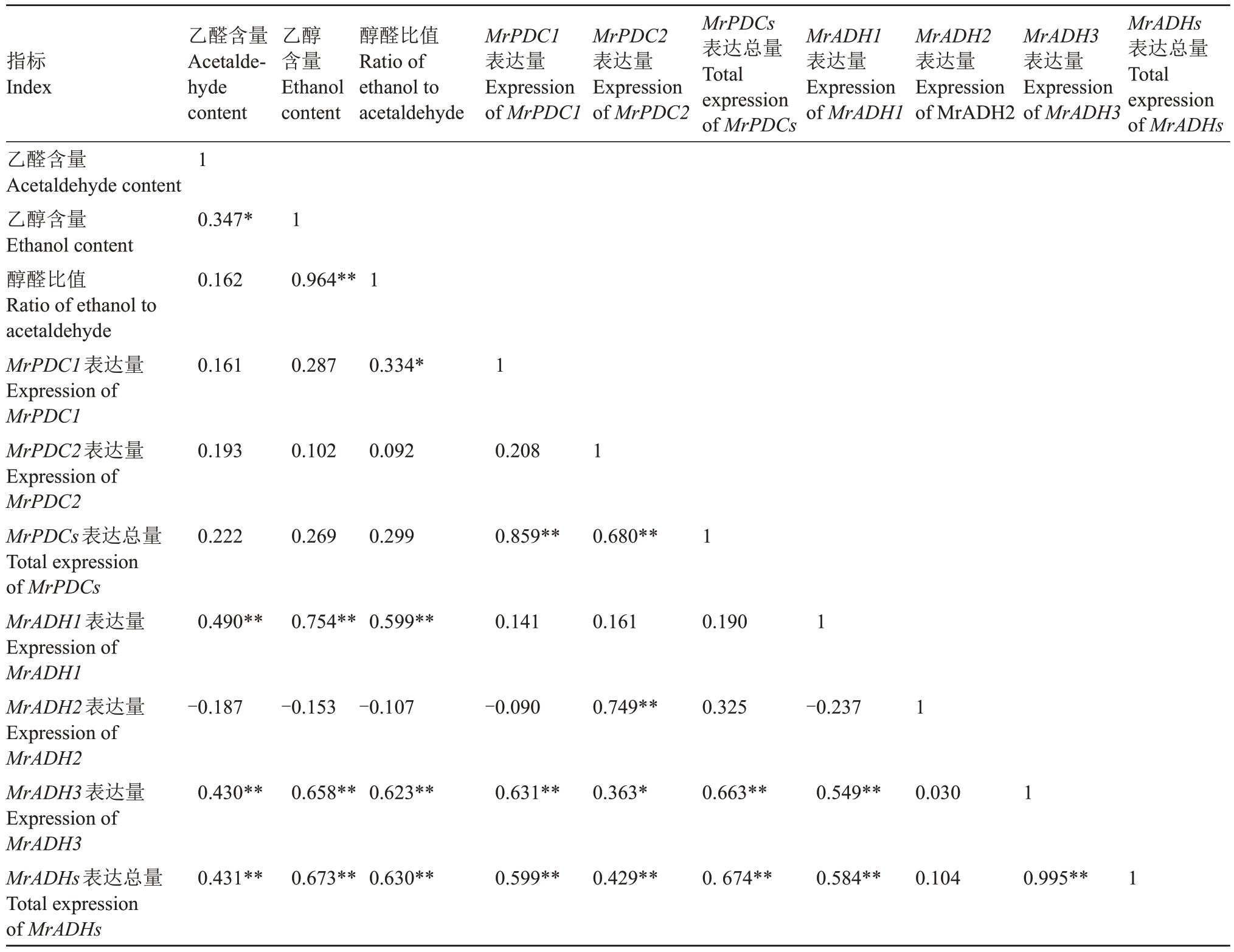
注:**表示在p<0.01 水平极显著相关,*表示在p<0.05 水平显著相关。n=10。下同。
Note:**Significant difference at p<0.01 level,*Significant difference at p<0.05 level.n=10.The same below.
指标Index乙醇含量Ethanol content醇醛比值Ratio of ethanol to acetaldehyde MrPDC1表达量Expression of MrPDC1 MrPDC2表达量Expression of MrPDC2 MrPDCs表达总量Total expression of MrPDCs MrADH1表达量Expression of MrADH1 MrADH2表达量Expression of MrADH2 MrADH3表达量Expression of MrADH3 MrADHs表达总量Total expression of MrADHs乙醛含量Acetaldehyde content乙醇含量Ethanol content醇醛比值Ratio of ethanol to acetaldehyde MrPDC1表达量Expression of MrPDC1 MrPDC2表达量Expression of MrPDC2 MrPDCs表达总量Total expression of MrPDCs MrADH1表达量Expression of MrADH1 MrADH2表达量Expression of MrADH2 MrADH3表达量Expression of MrADH3 MrADHs表达总量Total expression of MrADHs乙醛含量Acetaldehyde content 1 0.347*1 0.162 0.964**1 0.161 0.287 0.334*1 0.193 0.102 0.092 0.208 1 0.222 0.269 0.299 0.859**0.680**1 0.490**0.754**0.599**0.141 0.161 0.190 1-0.187-0.153-0.107-0.090 0.749**0.325-0.237 1 0.430**0.658**0.623**0.631**0.363*0.663**0.549**0.030 1 0.431**0.673**0.630**0.599**0.429**0.674**0.584**0.104 0.995**1
2.4 杨梅不同包装处理期间果实异味、乙醛含量、乙醇含量和醇醛比值的变化
为防止果实运输期间振动,生产上常采用减压包装处理。对减压包装内气体成分进行了分析,发现在0 d(减压包装后6 h)时荸荠(图4-A)和东魁(图4-B)杨梅减压包装内O2含量(φ,后同)分别为1.83%和3.20%,在0 ℃贮藏期间O2含量不断下降,到贮藏期结束时包装内O2含量几乎为0。荸荠和东魁在0 d时包装内CO2含量分别为18.07%和24.00%,而后随着贮藏期延长CO2 含量不断上升,10 d 时分别为32.60%和46.63%。
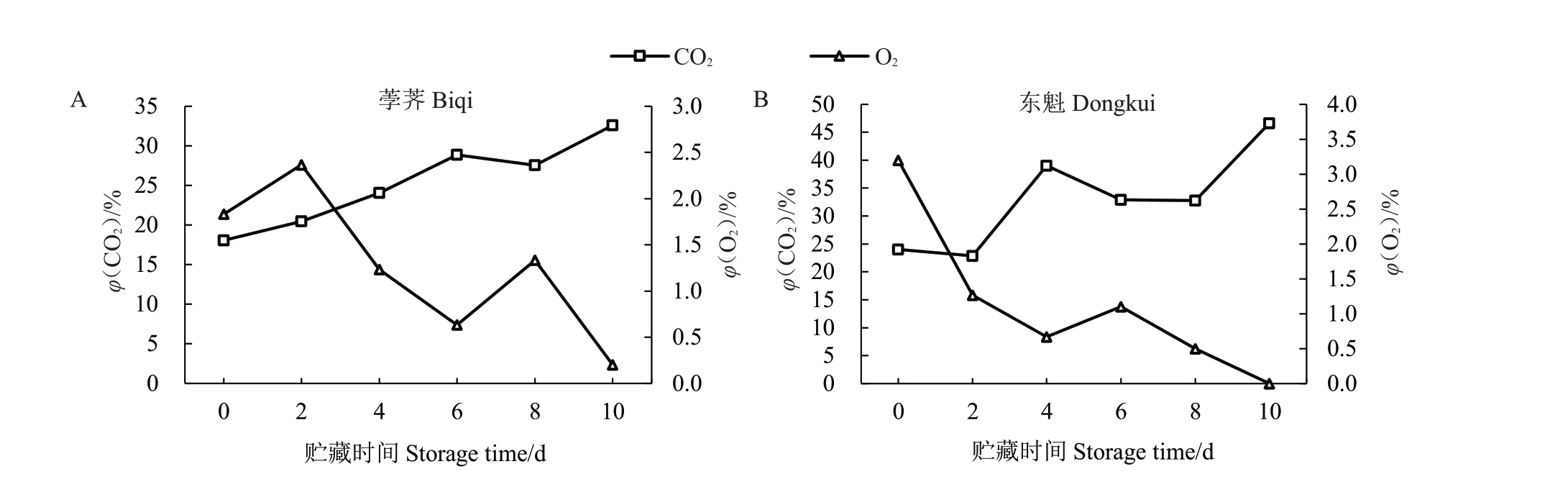
图4 荸荠和东魁杨梅减压包装处理期间CO2和O2含量
Fig.4 Contents of CO2 and O2 in Biqi and Dongkui fruit during storage following hypobaric packaging
经过果实异味评价,发现荸荠和东魁杨梅在处理4 d 和8 d 时均表现为经减压包装的果实异味最重,其次是普通包装,单果包装的果实异味最轻。就相同包装和相同贮藏时间进行对比,总体而言东魁比荸荠果实异味重(表4)。
表4 荸荠和东魁杨梅不同包装处理4 d 和8 d 的果实异味评价
Table 4 Evaluation of off-flavor in Biqi and Dongkui fruits at 4 days and 8 days from different packaging treatments
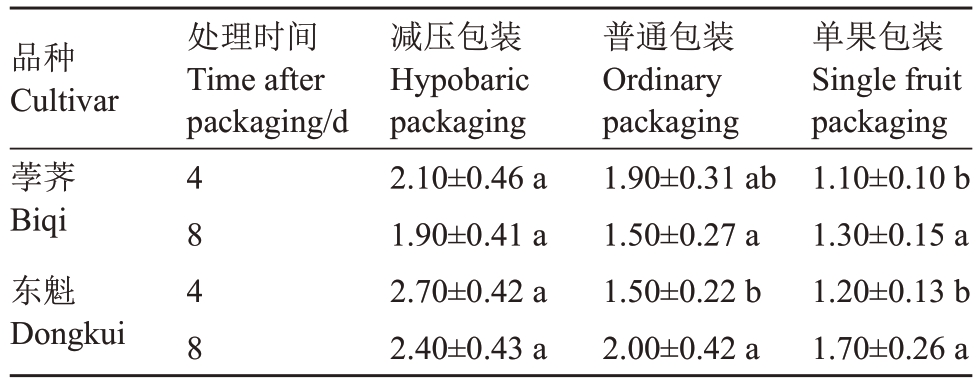
注:同列不同小字字母表示在0.05 水平存在显著差异。
Note:The different small letters in the same column indicate significant differente at 0.05 level.
品种Cultivar荸荠Biqi东魁Dongkui处理时间Time after packaging/d 4848减压包装Hypobaric packaging 2.10±0.46 a 1.90±0.41 a 2.70±0.42 a 2.40±0.43 a普通包装Ordinary packaging 1.90±0.31 ab 1.50±0.27 a 1.50±0.22 b 2.00±0.42 a单果包装Single fruit packaging 1.10±0.10 b 1.30±0.15 a 1.20±0.13 b 1.70±0.26 a
经过减压包装的荸荠和东魁果实中积累了大量的乙醛和乙醇。减压包装的荸荠果实的乙醛含量(w,后同)在0~10 d 不断上升,在10 d 时高达82.30 μg·g-1,是0 d的4.73倍;乙醇含量随着贮藏期延长逐渐上升,在6 d时达到1 091.10 μg·g-1,到10 d时仍维持在1 044.10 μg·g-1(图5-A,C)。减压包装的东魁果实的乙醛和乙醇含量均在4 d达到最高,分别为71.80 μg·g-1和1 683.60 μg·g-1(图5-B,D)。对于普通篮筐和单果包装的杨梅果实来说,两品种果实乙醛、乙醇含量和醇醛比值在贮藏期间均维持在较低水平。在10 d时,减压包装果实中乙醛、乙醇含量和醇醛比值分别为其他两种包装的2.31~10.29倍、13.68~27.79倍以及1.53~9.63倍(图5)。
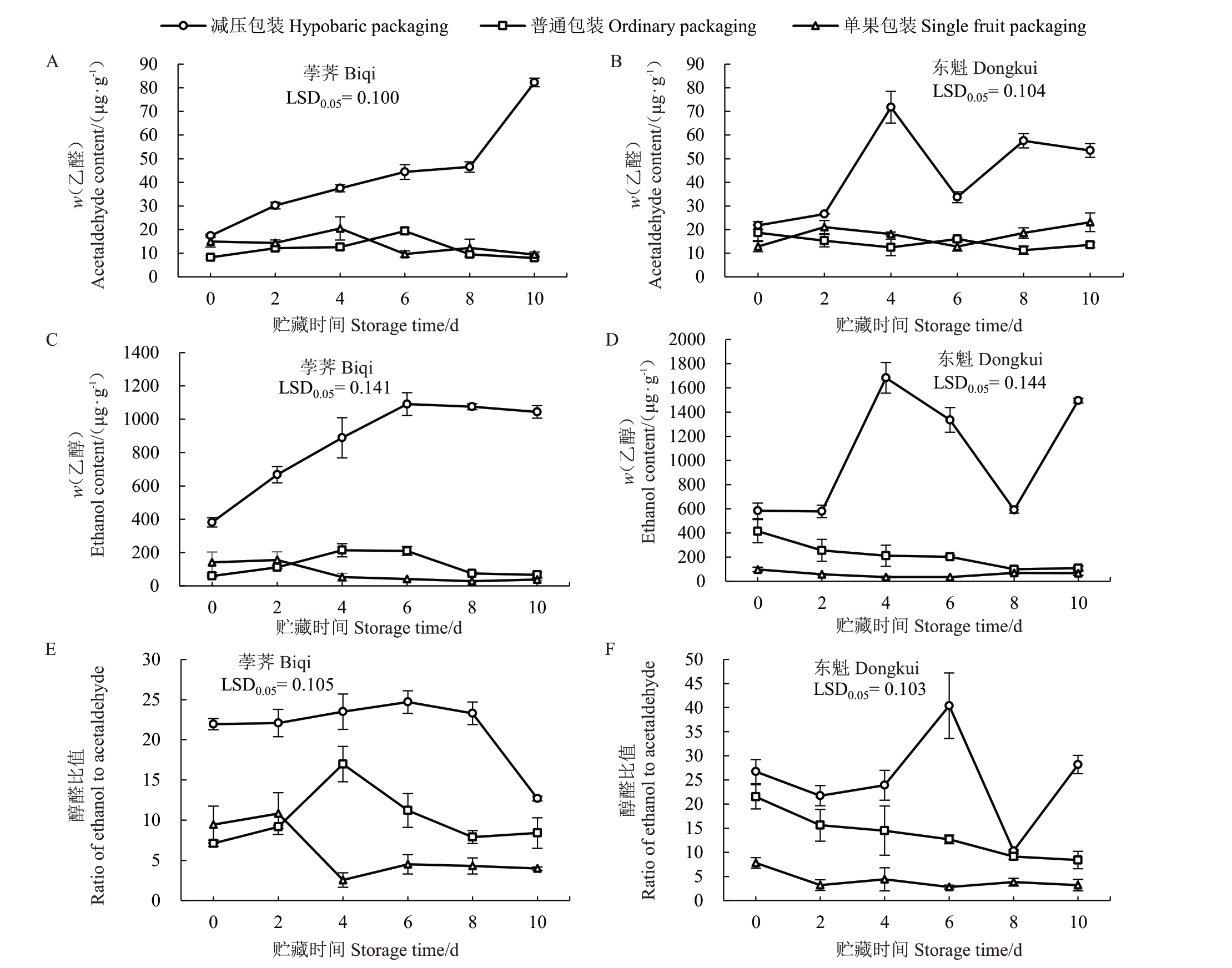
图5 荸荠(A、C、E)和东魁(B、D、F)杨梅果实包装处理期间乙醛、乙醇含量与醇醛比值
Fig.5 Contents of acetaldehyde and ethanol and the ratio of ethanol to acetaldehyde in Biqi(A,C,E)and Dongkui(B,D,F)fruit during packaging treatments
2.5 杨梅果实不同包装处理期间PDC和ADH基因表达分析
由图6 可见,PDC 和ADH 基因在3 种包装的荸荠和东魁杨梅果实中表达各异,MrPDC1、MrPDC2、MrADH1和MrADH3在减压包装杨梅果实中的表达量明显高于普通篮筐和单果包装。在荸荠杨梅中,MrPDCs 和MrADHs 在减压包装果实贮藏2 d 时表达量最高,随后保持稳定或下降;各基因表达水平在普通篮筐和单果包装果实中差异较小(图6-A)。对于东魁杨梅,各基因在减压包装果实中的表达量总体呈现下降趋势,其中MrPDCs 在0~8 d 表达量较高,MrADH1 表达量在6 d 达到峰值;MrADH3 在2~6 d先降后升,呈现“V”形变化,而后不断下降;在贮藏期间,各基因在普通篮筐和单果包装果实中的表达量无明显差异(图6-B)。在2 d时,减压包装果实中MrPDC1、MrPDC2、MrADH1 和MrADH3 分别为其他两种包装的2.50~10.32倍、5.97~21.74倍、4.40~33.19倍和2.46~19.26倍。
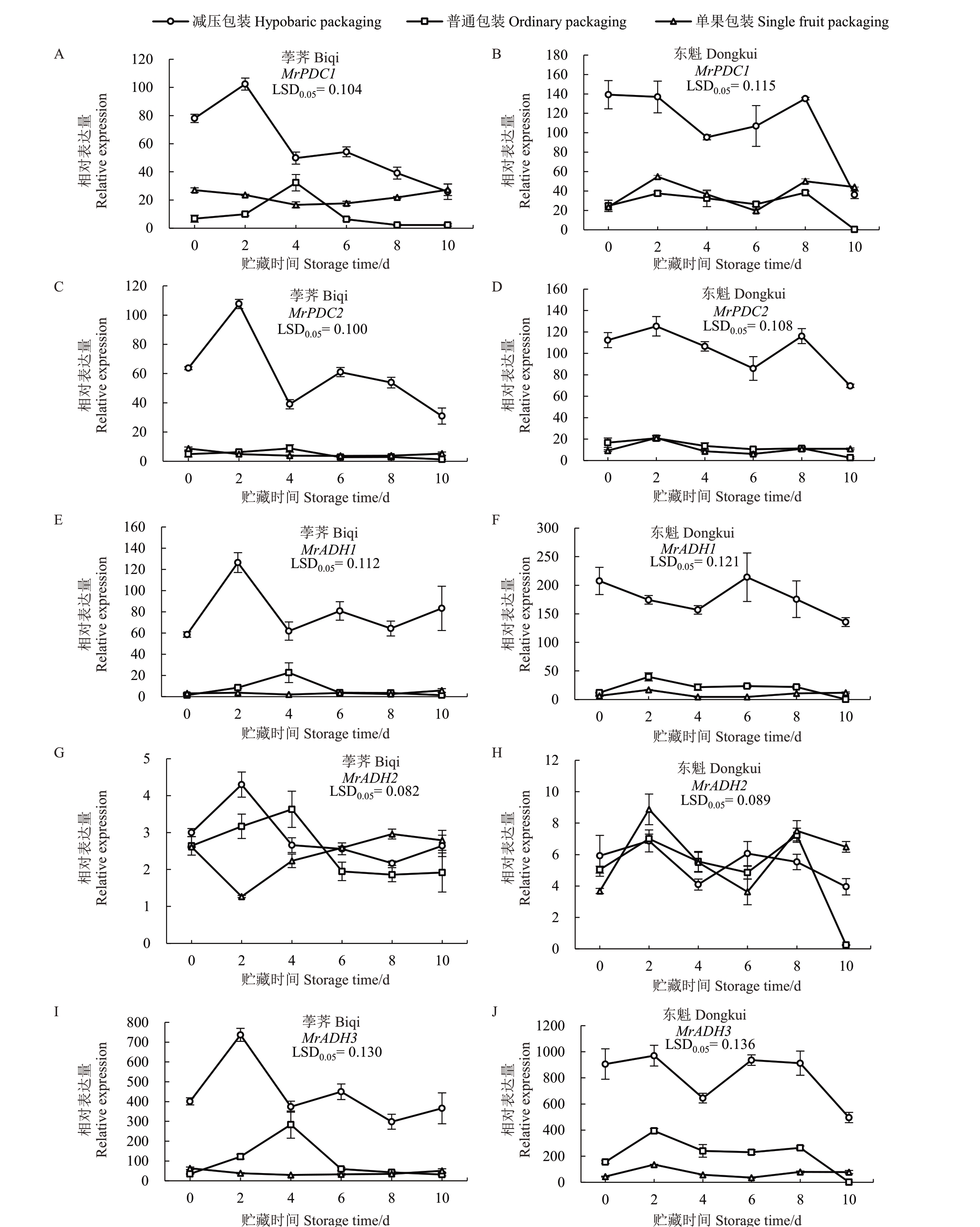
图6 荸荠(A、C、E、G、I)和东魁(B、D、F、H、J)杨梅果实包装处理期间MrPDCs 和MrADHs 的表达
Fig.6 Expression of MrPDCs and MrADHs in Biqi(A,C,E,G,I)and Dongkui(B,D,F,H,J)fruit during packaging treatments
结合Pearson相关性分析,发现杨梅不同包装处理过程中乙醛、乙醇含量和醇醛比值与MrPDC1、MrPDC2、MrADH1 和MrADH3 表达量之间均呈极显著正相关,而与MrADH2 的表达量无显著相关性。其中乙醛含量与乙醇含量相关系数达0.847(p<0.01),乙醇含量与醇醛比值相关系数达0.808(p<0.01),PDC 和ADH 表达总量相关系数高达0.953(p<0.01),MrADH1与MrADH3相关系数高达0.969(p<0.01)(表5),表 明MrPDC1、MrPDC2、MrADH1和MrADH3在乙醛和乙醇积累中发挥重要作用。
表5 荸荠和东魁果实包装处理期间乙醛、乙醇含量和醇醛比值与MrPDCs 和MrADHs 表达量的相关性
Table 5 Correlation between acetaldehyde,ethanol contents as well as the ratio of ethanol to acetaldehyde and the expression of MrPDCs and MrADHs in Biqi and Dongkui fruit from different packaging treatments
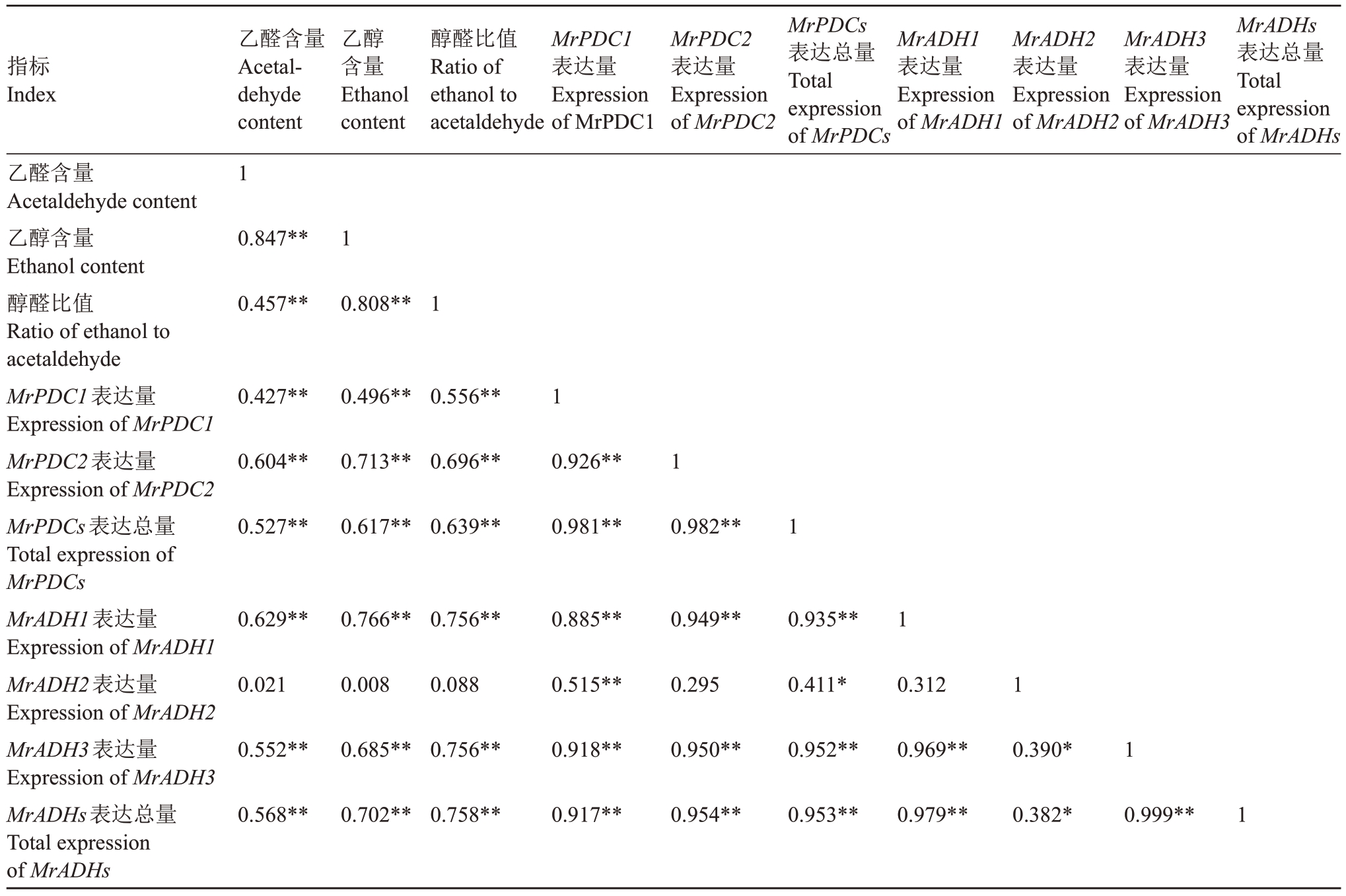
指标Index乙醇含量Ethanol content醇醛比值Ratio of ethanol to acetaldehyde MrPDC1表达量Expression of MrPDC1 MrPDC2表达量Expression of MrPDC2 MrPDCs表达总量Total expression of MrPDCs MrADH1表达量Expression of MrADH1 MrADH2表达量Expression of MrADH2 MrADH3表达量Expression of MrADH3 MrADHs表达总量Total expression of MrADHs乙醛含量Acetaldehyde content乙醇含量Ethanol content醇醛比值Ratio of ethanol to acetaldehyde MrPDC1表达量Expression of MrPDC1 MrPDC2表达量Expression of MrPDC2 MrPDCs表达总量Total expression of MrPDCs MrADH1表达量Expression of MrADH1 MrADH2表达量Expression of MrADH2 MrADH3表达量Expression of MrADH3 MrADHs表达总量Total expression of MrADHs乙醛含量Acetaldehyde content 1 0.847**1 0.457**0.808**1 0.427**0.496**0.556**1 0.604**0.713**0.696**0.926**1 0.527**0.617**0.639**0.981**0.982**1 0.629**0.766**0.756**0.885**0.949**0.935**1 0.021 0.008 0.088 0.515**0.295 0.411*0.312 1 0.552**0.685**0.756**0.918**0.950**0.952**0.969**0.390*1 0.568**0.702**0.758**0.917**0.954**0.953**0.979**0.382*0.999**1
3 讨 论
随着果实成熟,醛醇类物质在果实中积累使得果实呈现浓郁风味,其中乙醇和乙醛起着重要作用。乙醛和乙醇积累期间PDC 和ADH 基因的表达情况因种类、品种和成熟度而异[15]。前人研究发现,FaPDC1在草莓果实成熟和香气形成中发挥着重要作用[17];CmPDC1、CmADH1和CmADH2的表达量随着甜瓜果实成熟不断上升[5-6];VvADH2 表达量在葡萄果实成熟后期明显增加[31];然而在部分物种中,ADH 基因表达量和醛醇类物质含量随果实成熟而下降;白梨中有2个ADH基因表达量随着果实成熟度升高而下降[32];陈美霞[33]对杏的研究发现,不同发育期的杏果实香气物质积累不一,果实绿果期的醛类和醇类含量最高,成熟果实在最佳采收期前后乙醛和乙醇含量降低。本研究发现,2 个PDC 基因在不同杨梅品种果实成熟期间呈现出不同的表达模式,MrPDC1 在多数品种成熟期的表达量最高,而MrPDC2在绿果期的表达量最高。不同杨梅品种中MrADH1和MrADH3的表达量呈现与成熟度一致的趋势,MrADH2的表达量呈现与成熟度相反的趋势,且果实中MrADH3 的表达量明显高于MrADH1 和MrADH2,MrADH3 在10 个杨梅品种中的表达量在成熟期达到最高,表明MrADH3 在调节杨梅果实成熟期间乙醛和乙醇积累中发挥着重要作用。
多数杨梅品种果实乙醛和乙醇含量总体随着果实成熟度增加而不断升高,结合基因表达分析发现,MrADH1和MrADH3高表达的杨梅品种所积累的乙醛和乙醇较多。Zhu 等[26]研究发现,荸荠杨梅中乙醛和乙醇含量随着果实成熟呈现上升趋势,在成熟期的含量显著高于绿果期、转色期和红熟期,且在红熟期和完熟期中ADH 的增强表达与乙醛和乙醇含量的上升存在密切关系;另一研究结果显示,随着杨梅转色成熟,PDC和ADH蛋白丰度增加[27]。本研究发现MrPDCs和MrADHs基因在果实成熟期表达增强,与笔者先前研究结果[26-27]相印证,表明PDC 和ADH在醛醇转化中发挥重要作用,参与杨梅成熟过程中乙醛和乙醇的合成。
普通篮筐包装是杨梅生产上常用的传统包装形式,后来发展出减压包装,其目的主要是为了防止果实运输期间振动,单果包装是近年来为实现杨梅精品化贮运而不断改良出的新型包装。由于果实呼吸消耗氧气,减压包装造成了低O2和高CO2环境。减压或低氧通过PDC和ADH介导对果实风味产生影响,这在其他果实上已有报道。草莓、番荔枝、葡萄等果实在高CO2条件下乙醛和乙醇含量显著提高,ADH、PDC酶活性明显增强,PDC和ADH基因表达不断上调[25,34-35];但在冬枣上,研究表明减压处理反而对抑制乙醛、乙醇积累有显著效果,研究者认为减压处理可以降低枣果的呼吸强度和乙烯释放量,调节枣果的生理代谢,从而抑制酒化现象的发生[36-37]。本研究表明杨梅减压包装急剧诱导乙醛和乙醇积累,在两个品种上表现一致。乙醛和乙醇是果实采后重要挥发性芳香物质,较低浓度的乙醛和乙醇可以促进果实风味形成并维持果实品质,而高浓度则使果实进行有害代谢而产生异味[38]。李红卫[39]对冬枣乙醇积累机制的研究表明,乙醇含量超过一定阈值则使冬枣果实品质劣化。在本研究中,减压包装明显促进杨梅果实乙醛和乙醇积累的同时也使果实呈现严重酒味和异味,而乙醛和乙醇的积累是由于多个MrADHs和MrPDCs表达受到强烈诱导。相比之下,普通包装和单果包装的杨梅果实中这些基因表达量以及乙醛和乙醇含量远低于减压包装,果实异味不明显。因此,杨梅生产上如采用减压包装,宜在低温下进行贮运并尽可能缩短处于包装中的时间,果实运达目的地后需及时拆开减压包装袋以避免果实长时间处于密闭环境。本研究表明MrADHs和MrPDCs 表达可受低O2和/或高CO2诱导,但是所涉及的具体机制等有待进一步研究。
4 结 论
研究结果表明,MrADH1 和MrADH3 在果实成熟期间乙醛和乙醇积累中起着重要作用;减压包装处 理 诱 导 了MrPDC1、MrPDC2、MrADH1 和MrADH3 表达,导致乙醛和乙醇过度积累以及果实异味产生。研究结果对指导杨梅果实采后保鲜及异味控制具有重要意义,杨梅果实采后贮运需避免长时间密闭环境,以防止果实产生异味。
[1] 李兴军,吕均良,李三玉.中国杨梅研究进展[J].四川农业大学学报,1999,17(2):224-229.LI Xingjun,LÜ Junliang,LI Sanyu.Advances in bayberry of China[J].Journal of Sichuan Agricultural University,1999,17(2):224-229.
[2] 席玙芳,郑永华,应铁进,应金法,陈宗良.杨梅果实采后的衰老生理[J].园艺学报,1994,21(3):213-216.XI Yufang,ZHENG Yonghua,YING Tiejin,YING Jinfa,CHEN Zongliang.Senescence phyisology of chinese bayberry fruit during storage[J].Acta Horticulturae Sinica,1994,21(3):213-216.
[3] TADEGE M,DUPUIS I,KUHLEMEIER C.Ethanolic fermentation:New functions for an old pathway[J].Trends in Plant Science,1999,4(8):320-325.
[4] WATERS I,MORRELL S,GREENWAY H,COLMER T D.Effects of anoxia on wheat seedlings II.Influence of O2 supply prior to anoxia on tolerance to anoxia,alcoholic fermentation,and sugar levels[J].Journal of Experimental Botany,1991,42(11):1437-1447.
[5] WANG M M,ZHANG L,BOO K H,PARK E,DRAKAKAKI G,ZAKHAROV F.PDC1,a pyruvate/α-ketoacid decarboxylase,is involved in acetaldehyde,propanal and pentanal biosynthesis in melon (Cucumis melo L.) fruit[J].The Plant Journal,2019,98(1):112-125.
[6] MANRÍQUEZ D,EL-SHARKAWY I,FLORES F B,EL-YAHYAOUI F,REGAD F,BOUZAYEN M,LATCHÉ A,PECH J C.Two highly divergent alcohol dehydrogenases of melon exhibit fruit ripening- specific expression and distinct biochemical characteristics[J].Plant Molecular Biology,2006,61(4):675-685.
[7] LONGHURST T,LEE E,HINDE R,BRADY C,SPEIRS J.Structure of the tomato Adh2 gene and Adh2 pseudogenes,and a study of Adh2 gene expression in fruit[J].Plant Molecular Biology,1994,26(4):1073-1084.
[8] DEFILIPPI B G,KADER A A,DANDEKAR A M.Apple aroma:Alcohol acyltransferase,a rate limiting step for ester biosynthesis,is regulated by ethylene[J].Plant Science,2005,168(5):1199-1210.
[9] TESNIÈRE C,TORREGROSA L,PRADAL M,SOUQUET J M,GILLES C,SANTOS K D,CHATELET P,GUNATA Z.Effects of genetic manipulation of alcohol dehydrogenase levels on the response to stress and the synthesis of secondary metabolites in grapevine leaves[J].Journal of Experimental Botany,2006,57(1):91-99.
[10] SINGH R K,SANE V A,MISRA A,ALI S A,NATH P.Differential expression of the mango alcohol dehydrogenase gene family during ripening[J].Phytochemistry,2010,71(13):1485-1494.
[11] ZHANG B,SHEN J Y,WEI W W,XI W P,XU C J,FERGUSON I,CHEN K S.Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening[J].Journal of Agricultural and Food Chemistry,2010,58(10):6157-6165.
[12] GONZÁLEZ- AGÜERO M,TRONCOSO S,GUDENSCHWAGER O,CAMPOS-VARGAS R,MOYA-LEÓN M A,DEFILIPPI B G.Differential expression levels of aromarelated genes during ripening of apricot (Prunus armeniaca L.)[J].Plant Physiology and Biochemistry,2009,47(5):435-440.
[13] 张海英,韩涛,许丽,刘晓伟.果实的风味构成及其调控[J].食品科学,2008,29(4):464-469.ZHANG Haiying,HAN Tao,XU Li,LIU Xiaowei.Flavor constituents of fruits and its regulation[J].Food Science,2008,29(4):464-469.
[14] WANG Y J,YANG C X,LI S H,YANG L,WANG Y N,ZHAO J B,JIANG Q.Volatile characteristics of 50 peaches and nectarines evaluated by HP-SPME with GC-MS[J].Food Chemistry,2009,116:356-364.
[15] SPEIRS J,LEE E,HOLT K,YONG-DUK K,STEELE SCOTT N,LOVEYS B,SCHUCH W.Genetic manipulation of alcohol dehydrogenase levels in ripening tomato fruit affects the balance of some flavor aldehydes and alcohols[J].Plant Physiology,1998,117(3):1047-1058.
[16] MOUMMOU H,TONFACK L B,CHERVIN C,BENICHOU M,YOUMBI E,GINIES C,LATCHÉ A,PECH J C,VAN DER REST B.Functional characterization of SlscADH1,a fruit-ripening-associated short-chain alcohol dehydrogenase of tomato[J].Journal of Plant Physiology,2012,169(15):1435-1444.
[17] MOYANO E,ENCINAS-VILLAREJO S,LÓPEZ-RÁEZ J A,REDONDO-NEVADO J,BLANCO-PORTALES R,BELLIDO M L,SANZ C,CABALLERO J L,MUÑOZ-BLANCO J.Comparative study between two strawberry pyruvate decarboxylase genes along fruit development and ripening,post-harvest and stress conditions[J].Plant Science,2004,166(4):835-845.
[18] ZHANG B,XI W P,WEI W W,SHEN J Y,FERGUSON I,CHEN K S.Changes in aroma-related volatiles and gene expression during low temperature storage and subsequent shelf-life of peach fruit[J].Postharvest Biology and Technology,2011,60:7-16.
[19] BOTONDI R,RUSSO V,MENCARELLI F.Anaerobic metabolism during short and long term storage of kiwifruit[J].Postharvest Biology and Technology,2012,64:83-90.
[20] 李盼盼,钟雨,戚雯烨,宋亦超,郑小林.美味猕猴桃‘布鲁诺’果实贮藏过程中乙醇代谢与挥发性成分的变化[J].果树学报,2016,33(7):865-873.LI Panpan,ZHONG Yu,QI Wenye,SONG Yichao,ZHENG Xiaolin.Changes in ethanol fermentation metabolism and volatile metabolites in kiwifruit‘Bruno’during storage at room and low temperature[J].Journal of Fruit Science,2016,33(7):865-873.
[21] CAO J P,WANG C Y,XU S T,CHEN Y Z,WANG Y,LI X,SUN C D.The effects of transportation temperature on the decay rate and quality of postharvest ponkan (Citrus reticulata Blanco) fruit in different storage periods[J].Scientia Horticulturae,2019,247:42-48.
[22] 王志华,高剑利,王文辉,贾朝爽,姜云斌.不同贮藏温度对‘红香酥’梨果实品质和相关生理指标的影响[J].中国果树,2020(5):13-19.WANG Zhihua,GAO Jianli,WANG Wenhui,JIA Chaoshuang,JIANG Yunbin.Effects of different storage temperature on fruit quality and related physiological indexes of‘Hongxiangsu’pear[J].China Fruits,2020(5):13-19.
[23] 邵青旭,高湘荃,时晓雪,齐红岩.低温贮藏对薄皮甜瓜果实风味品质的影响[J].沈阳农业大学学报,2020,51(1):62-69.SHAO Qingxu,GAO Xiangquan,SHI Xiaoxue,QI Hongyan.The effects of low temperature storage on the flavor qualities of oriental melon[J].Journal of Shenyang Agricultural University,2020,51(1):62-69.
[24] CIRILLI M,BELLINCONTRO A,DE SANTIS D,BOTONDI R,COLAO M C,MULEO R,MENCARELLI F.Temperature and water loss affect ADH activity and gene expression in grape berry during postharvest dehydration[J].Food Chemistry,2012,132:447-454.
[25] MAOZ I,DE ROSSO M,KAPLUNOV T,VEDOVA A D,SELA N,FLAMINI R,LEWINSOHN E,LICHTER A.Metabolomic and transcriptomic changes underlying cold and anaerobic stresses after storage of table grapes[J].Scientific Reports,2019,9:1-14.
[26] ZHU C Q,FENG C,LI X,XU C J,SUN C D,CHEN K S.Analysis of expressed sequence tags from Chinese bayberry fruit(Myrica rubra Sieb.and Zucc.) at different ripening stages and their association with fruit quality development[J].International Journal of Molecular Sciences,2013,14(2):3110-3123.
[27] CHEN Y Y,ZHANG Z H,ZHONG C Y,SONG X M,LIN Q H,HUANG C M,HUANG R H,CHEN Wei.Functional analysis of differentially expressed proteins bayberry (Myrica rubra Sieb.et Zucc.) fruits during ripening[J].Food Chemistry,2016,190:763-770.
[28] SHAN L L,LI X,WANG P,CAI C,ZHANG B,SUN C D,ZHANG W S,XU C J,FERGUSON I,CHEN K S.Characterization of cDNAs associated with lignification and their expression profiles in loquat fruit with different lignin accumulation[J].Planta,2008,227(6):1243-1254.
[29] NIU S S,XU C J,ZHANG W S,ZHANG B,LI X,WANG K L,FERGUSON I B,ALLAN A C,CHEN K S.Coordinated regulation of anthocyanin biosynthesis in Chinese bayberry (Myrica rubra) fruit by a R2R3 MYB transcription factor[J].Planta,2010,231(4):887-899.
[30] MIN T,YIN X R,SHI Y N,LUO Z R,YAO Y C,GRIERSON D,FERGUSON I B,CHEN K S.Ethylene-responsive transcription factors interact with promoters of ADH and PDC involved in persimmon (Diospyros kaki) fruit de-astringency[J].Journal of Experimental Botany,2012,63(18):6393-6405.
[31] TESNIÈRE C,VERRIÈS C.Molecular cloning and expression of cDNAs encoding alcohol dehydrogenases from Vitis vinifera L.during berry development[J].Plant Science,2000,157(1):77-88.
[32] ZENG W W,QIAO X,LI Q H,LIU C X,WU J,YIN H,ZHANG S L.Genome-wide identification and comparative analysis of the ADH gene family white pear (Pyrus bretschneideri)and other Rosaceae species[J].Genomics,2020,112(5):3484-3496.
[33] 陈美霞.杏果实风味物质的组成及其遗传特性的研究[D].泰安:山东农业大学,2005.CHEN Meixia.Studies of the on heritance and constituents of flavor in apricot (Armeniaca vulgaris L.) [D].Tai’an:Shandong Agricultural University,2005.
[34] MUÑOZ M T,ESCRIBANO M I,MERODIO C.Ethanolic metabolism in cherimoya fruit during storage at ambient and under high CO2 atmospheres[J].Journal of Horticultural Science,1997,72(3):363-370.
[35] KE D Y,ZHOU L L,KADER A A.Mode of oxygen and carbon dioxide action on strawberry ester biosynthesis[J].Journal of American Society for Horticultural Science,1994,119(5):971-975.
[36] 薛梦林,张继澍,张平,王莉.减压对冬枣采后生理生化变化的影响[J].中国农业科学,2003,36(2):196-200.XUE Menglin,ZHANG Jishu,ZHANG Ping,WANG Li.Effect of hypobaric storage on physiological and biochemical changes of Dong jujube fruit during cold storage[J].Scientia Agricultura Sinica,2003,36(2):196-200.
[37] 曹志敏,张平,王莉,高飞.减压对冬枣生理生化变化的研究[J].食品科学,2005,26(10):250-252.CAO Zhimin,ZHANG Ping,WANG Li,GAO Fei.Effect of hypobaric storage on physiological and biochemical changes of Dong jujube[J].Food Science,2005,26(10):250-252.
[38] PESIS E.The role of the anaerobic metabolites,acetaldehyde and ethanol,in fruit ripening,enhancement of fruit quality and fruit deterioration[J].Postharvest Biology and Technology,2005,37:1-19.
[39] 李红卫.冬枣采后衰老调控与乙醇积累机理的研究[D].北京:中国农业大学,2003.LI Hongwei.Studies on the senescence regulation and mechanism of ethanol accumulation of harvested“Brumal jujube”[D].Beijing:China Agricultural University,2003.