葡萄(Vitis vinifera L.)为葡萄科葡萄属木质藤本植物,是世界上最古老的果树树种之一,世界各地均有栽培。近年来,无论是栽培面积和产量,还是优质高效标准化的栽培模式及管理技术,我国葡萄种植业都取得了迅猛发展,葡萄产业已成为许多果农的致富产业和经济支柱[1]。目前,栽培葡萄品种多数是欧洲葡萄,产量高,品质好,但抗逆性差。近年来一些非生物胁迫如干旱胁迫以及土壤盐渍化等严重影响了葡萄生产过程中的产量和品质,造成葡萄种植面积减少[2-4]。
类钙调磷酸酶B 互作蛋白激酶(CBL-interacting protein kinase,CIPK)是植物特有的蛋白激酶,属于SnRK3家族[5]。近年来研究发现,CIPK基因主要参与到植物逆境响应中,如干旱、高盐以及低温等,CIPK 基因功能的研究主要包括调节植物生长发育以及应答环境胁迫等方面[6]。Kim 等[7]研究ATCIPK3 转基因拟南芥植株在外源脱落酸、低温、高盐及干旱处理下的生长发育情况后发现,ATCIPK3 基因主要参与外源脱落酸和低温处理过程,相对表达量变化较大。Tripathi 等[8]研究发现,CIPK6 基因参与调节植物生长发育过程;Huertas等[9]研究发现,过表达CIPK24基因的番茄增加了耐盐性。He等[10]研究发现,CIPK6转基因棉花植株表现出对多种非生物胁迫的耐性。由此可见,CIPK基因在植物应对外界非生物胁迫方面扮演重要角色[11]。目前,关于CIPK 基因的研究主要集中在水稻、棉花和玉米等大田作物上[12-14],关于果树方面特别是葡萄CIPK 基因功能的报道还比较少[15-16]。原产我国的中国野生葡萄种质资源燕山葡萄(Vitis yeshanensis‘Yanshan’),具有极强的抗寒、抗盐和抗旱性[4]。笔者前期从燕山葡萄中筛选到1个CIPK转录因子,暂命名为VyCIPK9(登录号:MK942080)。笔者在本试验中主要构建VyCIPK9基因植物表达载体,利用叶盘法转化本氏烟,获得VyCIPK9转基因烟草,对盐处理下VyCIPK9转基因烟草种子、幼苗和植株萌发、生长发育和生理机制开展研究,解析Vy-CIPK9转基因烟草植株耐盐性的生理机制。本研究为今后挖掘葡萄抗性基因资源和培育具有耐盐性的优良葡萄新品种提供了一定的理论依据。
1 材料和方法
1.1 材料
中国野生燕山葡萄(Vitis yeshanensis‘Yanshan’)保存于河南科技学院葡萄种质资源圃。燕山葡萄为当年生扦插苗,移栽于20 cm×30 cm 的花盆中,于2021年7月中旬选取长势一致、无病虫害盆栽燕山葡萄苗,进行不同植物生长调节剂及非生物胁迫处理。烟草材料为本氏烟(Nicotiana benthamiana)。
不同植物生长调节剂处理:0.5 mmol·L-1吲哚乙酸(indole acetic acid,IAA)、0.1 mmol·L-1 6-苄氨基腺嘌呤(6-benzylaminopurine,6-BA)、0.1 mmol·L-1赤霉素(gibberellin acid 3,GA3)、3 mmol·L-1乙烯利(ethephon,Eth)、0.1 mmol·L-1水杨酸(salicylic acid,SA)、0.05 mmol·L-1茉莉酸甲酯(methyl jasmonate,MeJA)和0.1 mmol·L-1脱落酸(abscisic acid,ABA),共计7种植物生长调节剂,喷施燕山葡萄植株叶片,于处理0、3、6、9、12、24、48和72 h后收集样品,每个处理设置相应的喷无菌水为对照[4]。
不同非生物胁迫处理:材料为燕山葡萄植株,(1)低温处理:4 ℃低温(光照培养箱16 h光照/8 h黑暗,光照度36 μmol·m-2·s-1)处理,处理0、1、3、5、7、9、11、13和15 d后采集葡萄植株中部成熟叶片;(2)干旱胁迫处理:燕山葡萄盆栽土壤含水量为50%(w)时进行干旱处理,处理0、1、3、5、7、9、11、13 和15 d 后采集葡萄植株中部成熟叶片;(3)盐胁迫处理:200 mmol·L-1 NaCl 浇灌燕山葡萄植株,以盆底有溶液流出为度,处理0、1、3、5、7、9、11、13 和15 d后采集葡萄植株中部成熟叶片[3-4]。每组处理均随机选取不同盆栽苗采样,采集后的叶样品迅速放入液氮速冻后置于-80 ℃超低温冰箱保存。
1.2 方法
1.2.1 VyCIPK9基因克隆及表达特性的分析 十二烷基磺酸钠(sodium dodecyl sulfate,SDS)/酚法提取葡萄和烟草不同组织器官RNA,用Nanadrop2000C型核酸定量仪(美国Thermal 公司)和1%(w,后同)琼脂糖凝胶电泳检测其浓度和完整性。采用Prime-ScriptTM Ⅱ1st Strand cDNA Synthesis Kit试剂盒(Ta-KaRa,日本)进行第1链cDNA的合成,以其为模板,通过特异引物进行实时荧光定量PCR(quantitative real-time PCR,qRT-PCR)反应,检测VyCIPK9 基因在燕山葡萄不同组织器官、不同植物生长调节剂和非生物胁迫下的表达水平[17]。
1.2.2 VyCIPK9 基因过表达载体的构建和转基因烟草植株的获得 利用PCR 循环系统扩增目的基因片段,并将其克隆到PMD18-T 载体中。转化大肠杆菌细胞,采用消除法筛选阳性单克隆细菌,将细菌扩增培养提取质粒。双酶切后,用1%琼脂凝胶电泳检测质粒,并进行序列确认。克隆载体经SalⅠ和BstEⅡ内切酶消化后插入到过表达载体pCAMBIA1301 中,得到最终的过表达载体pCAMBIA1301:VyCIPK9,将VyCIPK9 基因转入烟草,获得阳性VyCIPK9 转基因烟草(OE#1,OE#2,OE#3)。
1.2.3 VyCIPK9转基因烟草种子萌发和幼苗生长指标的测定 灭菌清洗VyCIPK9 转基因烟草(OE#1,OE#2,OE#3)和野生型烟草种子后,在MS培养基中进行培养,5 d后移植到含有150、250 mmol·L-1 NaCl的MS培养基中进行盐胁迫处理,在25 ℃、16 h光照和8 h 黑暗条件下发芽生长,7 d 后测定发芽率。发芽率(%)=发芽种子数/播种种子总数×100。12 d后测量幼苗根长度、株高度和鲜质量。
1.2.4 VyCIPK9 转基因烟草生理生化指标的测定VyCIPK9转基因烟草(OE#1,OE#2,OE#3)和野生型烟草在150 mmol·L-1 NaCl 处理下,在25℃、16 h 光照和8 h黑暗条件下继续生长,4周后测定其生理生化指标。
相对电导率(relative conductivity)使用电导率仪(DDS-308A 型电导仪,上海雷磁)进行测定和计算,相对电导率(%)=初电导值/终电导值×100。采用分光光度计法测定叶绿素(chlorophyll)含量、脯氨酸(Pro)含量、丙二醛(malondialdehyde,MDA)含量、超氧化物歧化酶(Superoxide dismutase,SOD)活性、过氧化物酶(peroxidase,POD)活性、过氧化氢酶(catalase,CAT)活性和过氧化氢(H2O2)含量(检测试剂 盒 编 号 分 别 为BC0990、BC0290、BC0020、BC5160、BC0090、BC0200 和BC3590;北京索莱宝科技有限公司)。
1.3 数据统计分析
借助Office Excel 2010 进行数据分析,处理均设置3 次重复,试验数据以[平均值±标准差(SD)]表示。
2 结果与分析
2.1 VyCIPK9基因的表达分析
2.1.1 VyCIPK9基因在葡萄不同组织器官中的特异表达分析 利用qrt-PCR 技术对VyCIPK9 基因在葡萄不同组织器官中特异表达进行分析,结果发现,VyCIPK9 基因在葡萄老叶中相对表达量最高,果实中最低,相对表达量大小的顺序依次为老叶>花>根>正常叶>茎>幼叶>果实(图1)。

图1 VyCIPK9 基因在葡萄不同组织器官中的特异表达分析
Fig.1 Specific expression analysis of VyCIPK9 gene in different tissues and organs of Yanshan grape
数据以平均值±SD 表示;*表示差异显著(p<0.05),**表示差异极显著(p<0.01)。下同。
The data were expressed as mean ± SD; * indicates the difference was significant (p<0.05), ** indicates the difference was extremely significant(p<0.01).The same below.
2.1.2 不同植物生长调节剂处理下VyCIPK9基因在葡萄中的表达分析 VyCIPK9基因的相对表达量随时间变化呈现不同表达模式。VyCIPK9基因的相对表达量在7 种植物生长调节剂诱导下,在0~48 h 均呈现先升高后下降的趋势。在6-BA、GA3诱导下,VyCIPK9 基因相对表达量在6 h 最大;在MeJA 诱导下,VyCIPK9 基因相对表达量在9 h 最大;Eth、IAAhe SA 诱导12 h,相对表达量最大;ABA 诱导下,Vy-CIPK9基因相对表达量在24 h最大;其中,VyCIPK9基因对乙烯和茉莉酸甲酯的响应最敏感,最高相对表达量约为对照的11.84倍和9.86倍(图2)。

图2 不同植物生长调节剂处理下VyCIPK9 基因在葡萄中相对表达量的变化
Fig.2 Changes of VyCIPK9 gene expression in Yanshan grape treated with different plant growth regulators
2.1.3 不同非生物胁迫处理下VyCIPK9基因在葡萄中的表达分析 为进一步明确VyCIPK9基因对逆境的响应模式,对4 ℃低温、干旱胁迫和200 mmol·L-1 NaCl 盐胁迫下基因在葡萄中的相对表达量进行分析。由图3可以看出,随着低温处理时间的延长,Vy-CIPK9基因相对表达量受低温影响而出现变化,与对照(0 d)相比,低温处理后不同时间内VyCIPK9基因相对表达量均低于对照,特别是在15 d,VyCIPK9基因相对表达量仅为对照相对表达量的0.03 倍;随着干旱处理时间的延长,VyCIPK9 基因相对表达量受干旱影响而出现变化,与对照(0 d)相比,干旱处理5~15 d,VyCIPK9基因相对表达量明显低于对照,特别是在11 d,VyCIPK9 基因相对表达量仅为对照相对表达量的0.2倍;随着盐胁迫处理时间的延长,Vy-CIPK9 基因相对表达量受盐胁迫影响而出现变化,与对照(0 d)相比,盐胁迫处理下,VyCIPK9 基因相对表达量逐渐上升,盐胁迫处理3~9 d,VyCIPK9 基因相对表达量均高于对照,在处理9 d 达到最高峰,约为对照相对表达量的4.96 倍,而后逐渐降低。不同非生物胁迫处理后,VyCIPK9 基因相对表达量受盐胁迫响应变化较大。
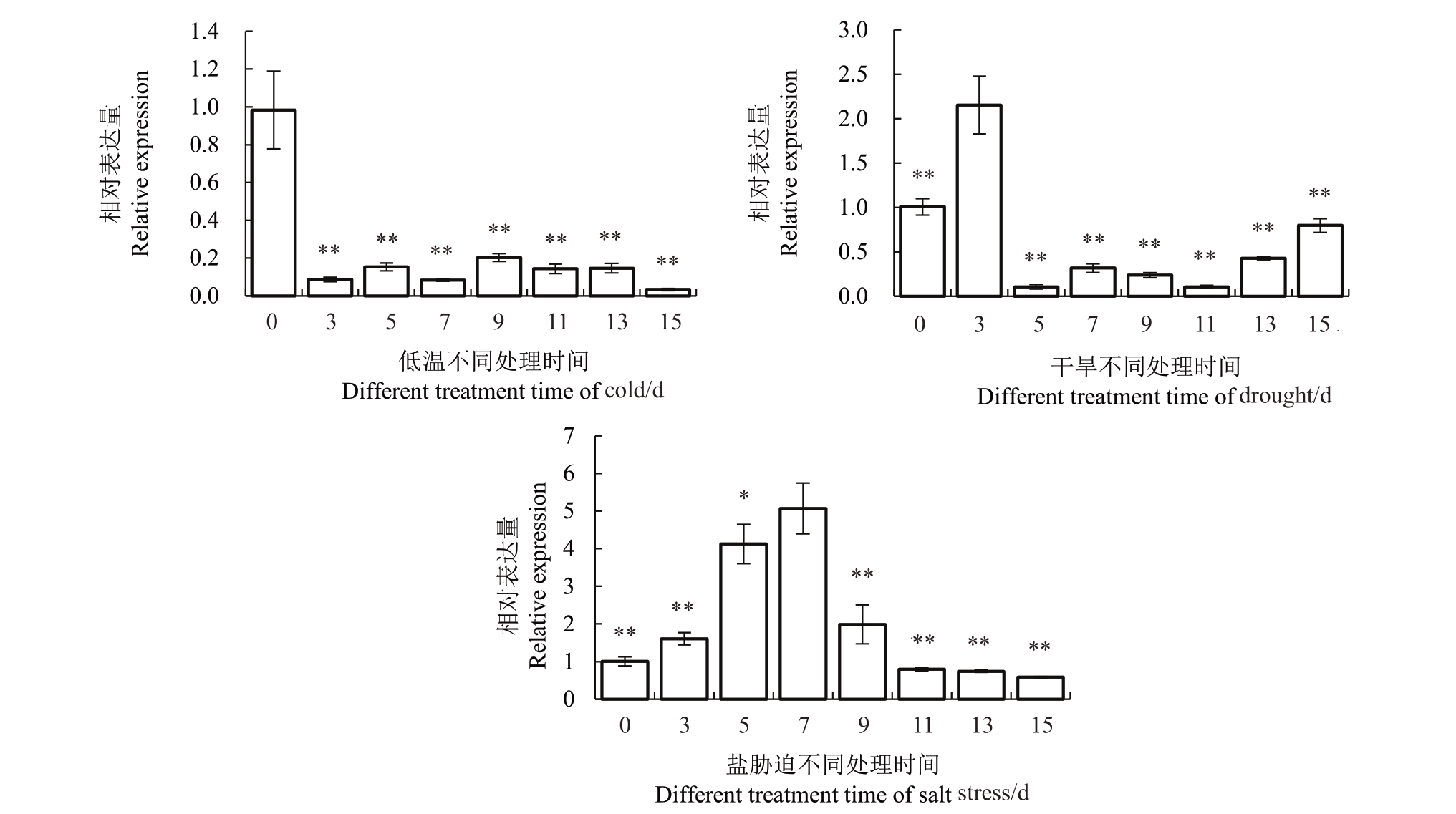
图3 不同非生物胁迫处理下VyCIPK9 基因在葡萄中相对表达量的变化
Fig.3 Changes of VyCIPK9 gene expression in Yanshan grape treated with different abiotic stress
2.2 盐胁迫处理下VyCIPK9 基因过表达对转基因烟草种子萌发和植株生长发育的影响
2.2.1 VyCIPK9基因过表达对盐胁迫下转基因烟草种子萌发的影响 将野生型烟草和VyCIPK9转基因烟草进行盐胁迫处理,观察并统计烟草种子发芽率。从图4-A可以看出,在空白MS培养基(CK)上,野生型烟草和VyCIPK9转基因烟草种子发芽情况没有明显的差别。而盐胁迫处理下,VyCIPK9 转基因烟草(OE#1,OE#2,OE#3)种子发芽率均明显高于野生型烟草。从图4-B 可以看出,在空白MS 培养基(CK)上,野生型烟草和VyCIPK9 转基因烟草(OE#1,OE#2,OE#3)种子的发芽率均超过95%,差异不明显。VyCIPK9 转基因烟草(OE#1,OE#2,OE#3)种子在含150 mmol·L-1 NaCl 盐胁迫MS 培养基上生长7 d 后,发芽率明显高于野生型烟草种子发芽率(23.33%),分别达到51.67%、53.33%和50.11%;VyCIPK9 转基因烟草(OE#1,OE#2,OE#3)种子在含250 mmol·L-1 NaCl 的MS 培养基上生长7 d 后,发芽率明显高于野生型烟草,但是总体均低于150 mmol·L-1 NaCl 盐胁迫处理下种子的发芽率。上述结果说明,在盐胁迫处理下,过表达VyCIPK9基因可以提高烟草种子的萌发率。
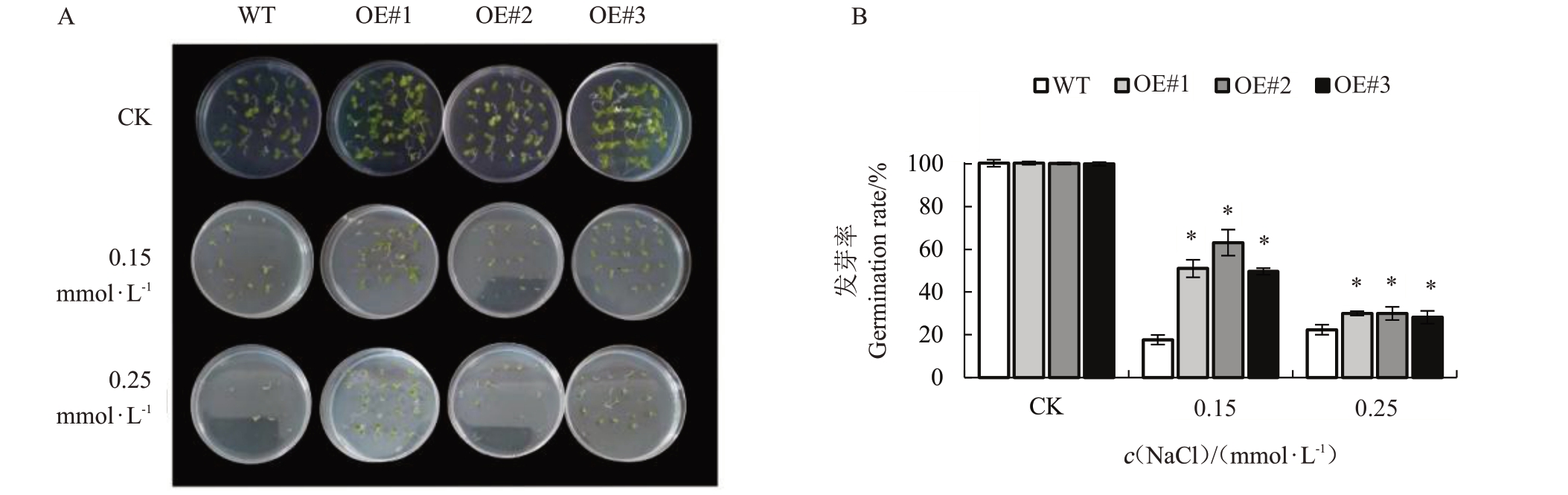
图4 不同浓度NaCl 处理对烟草种子萌发的影响
Fig.4 Effects of NaCl at different concentrations on tobacco seed germination
A.VyCIPK9 转基因烟草和野生型烟草在对照和不同浓度NaCl 胁迫下种子的萌发情况;B.VyCIPK9 转基因烟草和野生型烟草在对照和不同浓度NaCl 胁迫下种子的发芽率。
A.Seed germination of VyCIPK9 transgenic tobacco and wild-type tobacco under control and different concentrations of NaCl stress;B.The seed germination rate of VyCIPK9 transgenic tobacco and wild-type tobacco under control and different concentrations of NaCl stress.
2.2.2 VyCIPK9 基因过表达对盐胁迫下转基因烟草幼苗根长度、苗高和鲜质量变化的影响 将野生型烟草和转基因烟草幼苗进行盐胁迫处理,观察烟草幼苗的生长状况,在空白MS 培养基(CK)上野生型烟草和转基因烟草幼苗生长的状况基本一致(图5-A)。在空白MS 培养基(CK)上,VyCIPK9 转基因烟草(OE#1,OE#2,OE#3)幼苗根长度、株高度和鲜质量变化不大,差异不显著(图5-B~D)。施加不同浓度NaCl 盐胁迫处理会不同程度影响烟草幼苗根长度。以野生型烟草幼苗为对照,Vy-CIPK9 转基因烟草(OE#1,OE#2,OE#3)幼苗在含150 mmol·L-1 NaCl 盐胁迫的MS培养基上生长12 d后,根长度分别为23.13、22.72 和22.10 mm,均显著高于野生型烟草根长度(16.30 mm,p<0.05);鲜质量则分别为0.035、0.038 和0.035 g,均高于野生型烟草幼苗鲜质量(0.023 g);而VyCIPK9 转基因烟草(OE#1,OE#2,OE#3)株高度却显著低于野生型烟草(p<0.05);幼苗在含250 mmol·L-1 NaCl 盐胁迫的MS 培养基上生长12 d 后,根长度、鲜质量和株高度与野生型烟草相比,变化趋势一致,但是均低于150 mmol·L-1 NaCl处理。上述结果说明,在盐胁迫处理下过表达VyCIPK9 基因可以改变烟草幼苗的生长发育。

图5 不同浓度NaCl 处理对烟草幼苗生长的影响
Fig.5 Effects of NaCl at different concentrations on tobacco growth
A.VyCIPK9 转基因烟草和野生型烟草在不同浓度NaCl 盐胁迫下幼苗根和植株生长发育情况;B~D.VyCIPK9 转基因烟草和野生型烟草在不同浓度NaCl 盐胁迫下幼苗根长度、株高度和鲜质量。
A.The growth and development of seedling roots and plants of VyCIPK9 transgenic tobacco and wild-type tobacco under control and different concentrations of NaCl; B-D.Root length, plant height and fresh weight of VyCIPK9 transgenic tobacco and wild-type tobacco seedlings under control and different concentrations of NaCl stress.
2.2.3 VyCIPK9基因过表达对盐胁迫下转基因烟草植株生理指标的影响 从图6 可以看出,在正常生长条件下(0 d),VyCIPK9转基因烟草(OE#1,OE#2,OE#3)植株和野生型烟草植株叶片的叶绿素含量差异不大;盐胁迫处理10 d后,转基因烟草和野生型烟

图6 VyCIPK9 基因过表达对盐胁迫(150 mmol·L-1)下烟草植株生理指标的影响
Fig.6 Effects of 150 mmol·L-1 NaCl treatment on tobacco plant physiological indexes
草植株叶片叶绿素含量均下降,VyCIPK9 转基因烟草(OE#1,OE#2,OE#3)植株叶片叶绿素含量(w,后同)分别为1.313、1.427 和1.267 mg·g-1,显著高于野生型烟草叶片叶绿素含量(0.983 mg·g-1)(图6-A)。从图6-B可以看出,在对照(0 d)条件下,VyCIPK9转基因烟草(OE#1,OE#2,OE#3)和野生型烟草植株叶片的Pro 含量差异不大;盐胁迫处理10 d 后,Vy-CIPK9转基因烟草(OE#1,OE#2,OE#3)和野生型烟草植株叶片Pro 含量均升高,VyCIPK9 转基因烟草(OE#1,OE#2,OE#3)植株叶片Pro 含量分别为598.847、611.897和598.940 μg·g-1,显著高于野生型烟草植株叶片Pro 含量(301.397 μg·g-1)。从图6-C和图6-D 均可以看出,在正常生长条件下(0 d)下,VyCIPK9转基因烟草(OE#1,OE#2,OE#3)和野生型烟草植株叶片的相对电导率、MDA 含量差异均不大;盐胁迫处理10 d 后,VyCIPK9 转基因烟草(OE#1,OE#2,OE#3)植株叶片相对电导率、MDA含量均显著低于野生型烟草。
2.2.4 VyCIPK9 过表达对盐胁迫下转基因烟草植株保护酶活性的影响 从图7-A 可以看出,在正常生长条件下(0 d),VyCIPK9 转基因烟草(OE#1,OE#2,OE#3)和野生型烟草植株SOD 酶活性差异不大;盐胁迫处理10 d 后,VyCIPK9 转基因烟草(OE#1,OE#2,OE#3)和野生型烟草植株SOD 酶活性均下降,VyCIPK9 转基因烟草(OE#1,OE#2,OE#3)植 株 的SOD 酶 活 性 分 别 为656.46、670.527、632.447 U·g-1,显著高于野生型烟草植株的SOD酶活性(461.65 U·g-1)(p<0.05)。从图7-B 和图7-C 均可以看出,在正常生长条件下(0 d),VyCIPK9转基因烟草(OE#1,OE#2,OE#3)和野生型烟草植株的POD 酶活性、CAT 酶活性差异均不大;盐胁迫处理10 d 后,VyCIPK9 转基因烟草(OE#1,OE#2,OE#3)POD 酶 活 性、CAT 酶 活 性 与 之 前SOD 酶活性出现相同的变化趋势,均显著高于野生型烟草植株POD 酶活性、CAT 酶活性(p<0.05)。从图7-D 可以看出,在正常生长条件下(0 d),Vy-CIPK9 转基因烟草(OE#1,OE#2,OE#3)和野生型烟草植株H2O2含量差异不大;盐胁迫处理10 d 后,转基因烟草和野生型烟草植株H2O2含量均升高,Vy-CIPK9转基因烟草(OE#1,OE#2,OE#3)植株H2O2含量(b,后同)分别为20.880、20.727和20.250 mmol·g-1,与野生型烟草植株H2O2含量(28.420 mmol·g-1)相比显著下降。这说明VyCIPK9 基因过表达提高了盐胁迫下转基因烟草植株保护酶活性,降低了H2O2 含量,VyCIPK9 转基因烟草植株受到的伤害较轻。
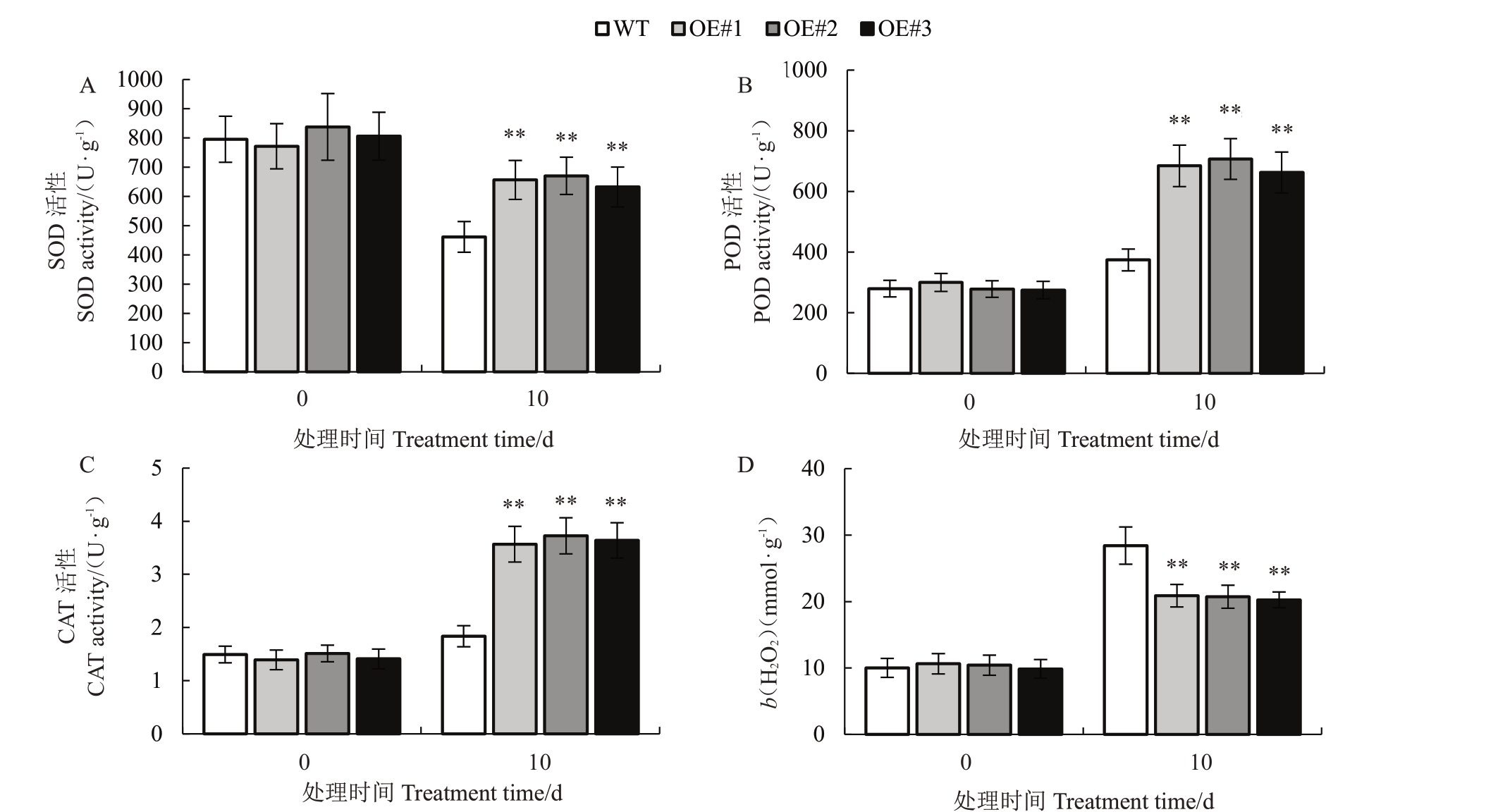
图7 VyCIPK9 基因过表达对盐胁迫(150 mmol·L-1)下烟草植株保护酶活性的影响
Fig.7 Effect of 150 mmol·L-1 NaCl treatment on tobacco plant biochemical indexes
3 讨 论
时空表达特异性即基因在发育时间和组织空间上的表达特异性可以为探究一个基因的功能提供初步线索。余义和等[18]研究发现VvCIPK10 基因在京秀葡萄根和叶中均高表达。闫朝辉等[19]研究发现VvCIPK13 基因和VvCIPK14 基因在佳丽酿葡萄卷须中相对表达量最高。路志浩等[20]研究发现VvCIPK15 基因在红宝石无核葡萄根中相对表达量最高。这些有关CIPK 基因在植物器官、组织或细胞中表达的研究,有助于进一步了解CIPK 基因自身的功能以及作为优异基因资源应用到其他相关研究当中。本试验也通过实时定量PCR 技术对其组织特异性进行分析,发现VyCIPK9 基因在葡萄根、茎、叶、花、果实中均有表达,在老叶中表达最高。
植物生长发育过程中会受到激素和各种环境的调控[21-22]。前人研究发现不同植物的CIPK 基因易受到不同植物生长调节剂和逆境的调控,相对表达量出现较大变化,如余义和等[18]研究发现,葡萄的VvCIPK10基因能够响应干旱、低温和盐胁迫;闫朝辉等[19]研究发现,葡萄CIPK13 基因和CIPK14 基因均响应多种激素、高盐和干旱胁迫;路志浩等[20]研究发现,葡萄VvCIPK15基因在脱落酸、盐和低温处理下相对表达量均升高。本试验研究发现,Vy-CIPK9 基因受到乙烯、茉莉酸甲酯和高盐胁迫处理后,相对表达量均升高。本试验研究结果和前人研究发现的CIPK 基因受到生物生长调节剂和环境刺激后相对表达量出现变化且变化趋势为升高的结果一致。
CIPK基因普遍存在于各类植物中,在植物多种非生物胁迫抗性中起着重要作用[23-25]。魏国强等[26]用25、50 mmol·L-1 NaCl处理黄瓜幼苗,发现植株高度、鲜质量和干质量均降低。刘萌等[27]盐胁迫试验表明,野生型大豆的生长显著受到盐胁迫的抑制,而转基因大豆幼苗的生长状况优于野生型大豆。当细胞受到氧化伤害时,植物体通过抗氧化酶系统(如SOD酶、POD酶、CAT酶等)和其他物质清除植物体内累积的活性氧物质,降低自由基对生物高分子的破坏,从而保证植物的正常生长。Essa[28]研究发现,200 mmol·L-1 NaCl 水培处理下,烟草植株中SOD酶、POD 酶、CAT 酶含量在处理1 d 时显著增加;这说明在盐胁迫下SOD酶活性增强,有助于清除多余的活性氧,保证植物正常的生长发育;同时,这也可能是烟草对盐逆境的一种防御机制,以减少自身受到的伤害。Deng 等[29]研究发现,小麦TaCIPK29 基因在烟草植株中过量表达,提高了转基因烟草过氧化物酶活性,增强了转基因植株的耐盐性。Niu等[30]研究发现,苹果MdCIPK6 基因在拟南芥中过量表达,提高了转基因烟草活性氧含量等相关指标,也增强了转基因植株的耐盐性。本试验为了进一步验证VyCIPK9 基因的功能,构建了VyCIPK9 基因的表达载体,将VyCIPK9基因转化烟草,测定幼苗和成苗植株的相关指标。结果表明,在盐胁迫条件下转基因烟草种子发芽率较高和幼苗生长效果较好,生长4周后的植株,随着盐胁迫处理时间的延长,VyCIPK9转基因烟草植株叶片的叶绿素、Pro 含量和氧化酶活性均高于野生型烟草植株,相对电导率、MDA 含量和H2O2含量均低于野生型烟草植株(p<0.05)。这说明转基因烟草植株光合特性和抗逆能力均强于野生型烟草植株,受盐胁迫伤害低于野生型烟草植株,验证了CIPK基因有助于提高植株的耐盐性,本试验研究结果与前人研究结果均一致[26-30]。
4 结 论
VyCIPK9基因在燕山葡萄各个组织器官中均有表达,主要在葡萄老叶中大量表达。在不同植物生长调节剂和非生物胁迫处理后,VyCIPK9 基因相对表达量均发生变化。通过转基因烟草技术,本研究验证了VyCIPK9基因有助于提高转基因烟草植株的耐盐性。本研究为进一步研究葡萄CIPK基因参与逆境胁迫的分子功能,以及为探讨葡萄抗逆分子机制提供了一定的参考。
[1] 杨盛迪,郭大龙,裴茂松,刘海楠,韦同路,余义和.干旱胁迫下葡萄AQP 基因家族的鉴定及转录调控网络预测[J].果树学报,2021,38(10):1638-1652.YANG Shengdi,GUO Dalong,PEI Maosong,LIU Hainan,WEI Tonglu,YU Yihe.Identification of grapevine AQP family and prediction for transcriptional regulatory network under drought stress[J].Journal of Fruit Science,2021,38(10):1638-1652.
[2] CHONG J L,LE HENANFF G,BERTSCH C,BERNARD W.Identification,expression analysis and characterization of defense and signaling genes in Vitis vinifera[J].Plant Physiology and Biochemistry,2008,46(4):469-481.
[3] LI G R,XU W W,JING P W,HOU X J,FAN X C.Overexpression of VyDOF8,a Chinese wild grapevine transcription factor gene,enhances drought tolerance in transgenic tobacco[J].Environmental and Experimental Botany,2021,190:104592.
[4] 张庆田,韩真,李秀杰,朱自果,李勃.燕山葡萄VyMAPK 基因的克隆及表达分析[J].植物生理学报,2020,56(10):2211-2220.ZHANG Qingtian,HAN Zhen,LI Xiujie,ZHU Ziguo,LI Bo.Cloning and expression analysis of MAPK genes in Vitis yeshanensis[J].Plant Physiology Journal,2020,56(10):2211-2220.
[5] XIANG Y,HUANG Y M,XIONG L Z.Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement[J].Plant Physiology,2007,144(3):1416-1428.
[6] LUAN S.The CBL-CIPK network in plant calcium signaling[J].Trends in Plant Science,2009,14(1):37-42.
[7] KIM K N,CHEONG Y H,GRANT J J,PANDEY G K,LUAN S.CIPK3,a calcium sensor-associated protein kinase that regulates abscisic acid and cold signal transduction in Arabidopsis[J].The Plant Cell,2003,15(2):411-423.
[8] TRIPATHI V,PARASURAMAN B,LAXMI A,DEBASIS C.CIPK6,a CBL-interacting protein kinase is required for development and salt tolerance in plants[J].The Plant Journal,2009,58(5):778-790.
[9] HUERTAS R,OLÍAS R,ELJAKAOUI Z,FRANCISCO J G,JUN L,PAZ A D M,ANDRES B,MARIA P R.Overexpression of SlSOS2 (SlCIPK24) confers salt tolerance to transgenic tomato[J].Plant,Cell&Environment,2012,35(8):1467-1482.
[10] HE L R,YANG X Y,WANG L C,ZHU L F,ZHOU T,DENG J W,ZHANG X L.Molecular cloning and functional characterization of a novel cotton CBL- interacting protein kinase gene(GhCIPK6)reveals its involvement in multiple abiotic stress tolerance in transgenic plants[J].Biochemical and Biophysical Research Communications,2013,435(2):209-215.
[11] PANDEY G K,KANWAR P,PANDEY A.Functional role of CBL-CIPK in abiotic stresses[M]// Global comparative analysis of CBL-CIPK gene families in plants.Switzerland:Springer,2014:65-77.
[12] SHABALA S,ALNAYEF M,BOSE J,CHEN Z H,GAYATRI V,ZHOU M X,LANA S,YU M.Revealing the role of the calcineurin B-like protein-interacting protein kinase 9 (CIPK9) in rice adaptive responses to salinity,osmotic stress,and K+ deficiency[J].Plants,2021,10(8):1513.
[13] SUN W N,ZHANG B,DENG J W,CHEN L,ULLAH A,YANG X Y.Genome-wide analysis of CBL and CIPK family genes in cotton:Conserved structures with divergent interactions and expression[J].Physiology and Molecular Biology of Plants,2021,27(2):359-368.
[14] 袁志恒,申腾飞,王琦,薛瑞丽,邰付菊.玉米CIPK3 蛋白激酶的克隆及表达分析[J].河南农业大学学报,2012,46(4):359-363,369.YUAN Zhiheng,SHEN Tengfei,WANG Qi,XUE Ruili,TAI Fuju.Cloning and expression analysis of a CBL-interacting protein kinase of maize[J].Journal of Henan Agricultural University,2012,46(4):359-363.
[15] YU Y H,XIA X L,YIN W L,ZHANG H Z.Comparative genomic analysis of CIPK gene family in Arabidopsis and Populus[J].Plant Growth Regulation,2007,52(2):101-110.
[16] LI G R,QUAN R,CHENG S S,HUO X J,HU H L.Cloning and activity analysis of grape VvCIPK10 promote[J].International Journal of Agriculture&Biology,2020,24(2):299-303.
[17] LIVAK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method[J].Methods,2001,25(4):402-408.
[18] 余义和,李秀珍,郭大龙,张会灵,杨英军,李学强,张国海.葡萄类钙调磷酸酶B 亚基互作蛋白激酶VvCIPK10 的特性与表达[J].中国农业科学,2016,49(19):3798-3806.YU Yihe,LI Xiuzhen,GUO Dalong,ZHANG Huiling,YANG Yingjun,LI Xueqiang,ZHANG Guohai.Characteristics and expression of calmodulin like B subunit interaction protein VvCIPK10 in grapevine[J].Scientia Agricultura Sinica,2016,49(19):3798-3806.
[19] 闫朝辉,李桂荣,扈岩松,周瑞金,扈惠灵,苗卫东,朱自果.欧洲葡萄中CIPK 基因的克隆及表达分析[J].园艺学报,2017,44(8):1463-1476.YAN Chaohui,LI Guirong,HU Yansong,ZHOU Ruijin,HU Huiling,MIAO Weidong,ZHU Ziguo.Cloning and expression analysis of CIPK genes in grapevine[J].Acta Horticulturae Sinica,2017,44(8):1463-1476.
[20] 路志浩,霍建强,马钰,胡炜,毛娟.葡萄CIPK 基因家族的鉴定表达分析[J].西北农业学报,2017,26(11):1619-1630.LU Zhihao,HUO Jianqiang,MA Yu,HU Wei,MAO Juan.Genome-wide identification and expression analysis of the CIPK gene family in grape[J].Acta Agriculturae Boreali-Occidentalis Sinica,2017,26(11):1619-1630.
[21] 邵云.苹果蔗糖非发酵相关蛋白激酶基因MpSnRK2.10 和Md-CIPK03 在非生物逆境应答中的功能研究[D].杨凌:西北农林科技大学,2019.SHAO Yun.Functional analysis of two apple sucrose nonfermenting-1-related kinase genes MpSnRK2.10 and MdCIPK03 in response to abiotic stress[D].Yangling:Northwest A & F University,2019.
[22] 闫亚飞.烟草NtCIPK23 基因的克隆、表达分析及转基因过量表达研究[D].重庆:重庆大学,2012.YAN Yafei.Clone,expression characterization and over-expressing transgenic study of tobacco NtCIOK23 gene[D].Chongqing:Chongqing University,2012.
[23] 陈小晶,王冬梅,关红辉,郭剑,沙小茜,李永祥,张登峰,刘旭洋,何冠华,石云素,宋燕春,王天宇,黎裕,刘颖慧,李春辉.玉米CIPK 基因家族的鉴定及ZmCIPK3 的抗旱性功能研究[J/OL].植物遗传资源学报:1-15[2022-03-29].https://doi.org/10.13430/j.cnki.jpgr.20220107006.CHEN Xiaojing,WANG Dongmei,GUAN Honghui,GUO Jian,SHA Xiaoqian,LI Yongxiang,ZHANG Dengfeng,LIU Xuyang,HE Guanhua,SHI Yunsu,SONG Yanchun,WANG Tianyu,LI Yu,LIU Yinghui,LI Chunhui.Identification of CIPK gene family members and investigation of the drought tolerance of Zm-CIPK3 in maize[J/OL].Journal of Plant Genetic Resources:1-15[2022-03-29].https://doi.org/10.13430/j.cnki.jpgr.20220107006.
[24] SU W H,REN Y J,WANG D J,HUANG L,FU X Q,LING H,SU Y C,HUANG N,TANG H C,XU L P,QUE Y X.New insights into the evolution and functional divergence of the CIPK gene family in Saccharum[J].BMC Genomics,2020,21:868.
[25] XI Y,LIU J Y,DONG C,CHENG Z M.The CBL and CIPK gene family in grapevine (Vitis vinifera):Genome-wide analysis and expression profiles in response to various abiotic stresses[J].Frontiers in Plant Science,2017,8:978.
[26] 魏国强,朱祝军,方学智,李娟,程俊.NaCl 胁迫对不同品种黄瓜幼苗生长、叶绿素荧光特性和活性氧代谢的影响[J].中国农业科学,2004,37(11):1754-1759.WEI Guoqiang,ZHU Zhujun,FANG Xuezhi,LI Juan,CHENG Jun.The effects of NaCl stress on plant growth,chlorophyll fluorescence characteristics and active oxygen metabolism in seedlings of two cucumber cultivars[J].Scientia Agricultura Sinica,2004,37(11):1754-1759.
[27] 刘萌,陈鹏,王洪芹,王红艳.盐胁迫对野生和栽培大豆幼苗生长和抗氧化酶活性及膜脂过氧化的影响[J].山东农业科学,2014,46(5):38-41.LIU Meng,CHEN Peng,WANG Hongqin,WANG Hongyan.Effects of salt stress on growth,antioxidant enzyme activity and membrane lipid peroxidation of wild and cultivated soybean seedlings[J].Shandong Agricultural Sciences,2014,46(5):38-41.
[28] ESSA T A.Effect of salinity stress on growth and nutrient composition of three soybean[Glycine max(L.)Merrill]cultivars[J].Journal of Agronomy and Crop Science,2002,188(2):86-93.
[29] DENG X M,HU W,WEI S Y,ZHOU S Y,ZHANG F,HAN J P,CHEN L H,LI Y,FENGJ L,FANG B.TaCIPK29,a CBL-interacting protein kinase gene from wheat,confers salt stress tolerance in transgenic tobacco[J].PLoS One,2013,8(7):e69881.
[30] NIU L L,DONG B Y,SONG Z H,ZHOU S Y,ZHANG F,HAN J P,CHEN L H,LI Y,FENG J L,FANG B.Genome-wide identification and characterization of CIPK family and analysis responses to various stresses in apple (Malus domestica)[J].International Journal of Molecular Sciences,2018,19(7):2131.