果实大小是非常重要的农艺性状,果实大的品种在市场上更受欢迎。遗传背景、栽培措施和化学物质喷施都能决定果实大小[1]。在相同生长条件下,有的梨属种果实非常小。如野生梨中的川梨、豆梨和杜梨等,它们的果实直径为1~2 cm,质量不到10 g[2]。这些野生梨的果实发育期非常短暂,果实膨大仅发生在花后2周内,随后果实生长异常缓慢,而栽培砂梨大多数品种的果实发育期长,单果质量超过200 g。在相同梨属种内,通常砂梨晚熟品种的果实比早熟品种的果实大,差异主要取决于果实中的细胞数量,而不是细胞大小[3]。在苹果[4]、甜瓜[5]和甜樱桃[6]中,果实大小不同的品种之间细胞数量存在显著差异。还有一些研究表明,细胞数量和大小共同决定果实大小[7]。由于细胞增殖减少和细胞变小,海棠的果实比栽培苹果小[8]。Grand Gala 是从Gala 苹果突变而来的大果品种,其果实大小与细胞数量和细胞大小有关[9]。栽培措施和化学品也可以调控果实大小。疏果施肥可以有效增大果个[10-11]。细胞分裂素和赤霉素等激素的喷施也可以显著增大果个[12]。
很多基因参与了果实大小的调控。在番茄中,果实大小相关基因已有大量的研究[13]。fw2.2、fw3.2和fw11.3被鉴定为与单果质量相关的数量性状基因座(quantitative trait locus,QTL)[14]。fw2.2 负调控细胞分裂,fas 能调控心室的数量,进而影响果实大小[15]。除果实大小直接相关基因外,还有很多基因参与调节细胞分裂和增大[16],如控制细胞分裂的细胞周期蛋白基因[17]。细胞周期蛋白激酶抑制剂基因KRP2 可以降低细胞数量、减少叶面积和改变叶形[18]。在拟南芥中,转录因子ANT[19]、生长素诱导基因ARGOS[20]和BIG BROTHER[21]可以调节器官生长和器官大小的变化。番茄中赤霉素信号转导相关基因DELLA1的沉默和GA20ox1的过表达会产生小而长的果实[22]。这些大量参与调节果实大小的基因表明,许多途径通过影响细胞数量和大小进而影响果实的大小。
笔者课题组前期发现了1个翠冠梨(Pyrus pyrifolia)的芽变材料,其成熟果实大小比普通翠冠大50%以上。研究翠冠梨的大果芽变材料的生化特性和基因表达情况将有助于深入了解调控砂梨果实大小的分子机制。
1 材料和方法
1.1 试验材料
研究使用的翠冠梨大果芽变(简称大翠冠)和正常翠冠梨材料生长于上海市农业科学院金山果树试验站。每个材料各10 株嫁接树,嫁接后树龄为6 a(年)。分别在开花后0、10、20、30、40 d 取样。0 d 的果实为去除花瓣后的花托。在每个取样点,均设置3 个生物学重复,每个生物学重复采集5个果实。
1.2 幼果组织切片
在每个采样点分别采集翠冠和大翠冠3 个果实,从中间进行横切,选择横切面的果肉组织进行石蜡切片,采样后立即用甲醛-乙酸-乙醇固定液固定,4 ℃保存。将果肉组织在分级乙醇(φ)系列中脱水(70%、80%、90%和100%,每级45 min),并用二甲苯清洗1 h,然后包埋在石蜡中。使用Leica RM2265 切片机获取薄切片。将切片在玻璃基板上干燥并再水化,然后用番红(1%,w)染色1 h。用水和乙醇洗涤后,切片用固绿(1%,w)染色。使用Eclipse Ti-S 显微成像系统观察解剖图像。使用Image-pro Plus 软件对细胞横切面面积进行测量,每个果实测量30 个细胞,每个处理共计90 个细胞。
1.3 植物激素测定
在10 mL 离心管中用4 mL 乙酸乙酯萃取总共100 mg 样品,将离心管涡旋1 min,然后在4 ℃的黑暗中浸泡过夜(12 h)。将样品在4 ℃、10 000 r·min-1下离心10 min。将上清液转移到新的10 mL离心管中,然后将3 mL乙酸乙酯加入到残留物中。将试管震荡1 min 并在4 ℃、10 000 r·min-1下离心10 min。合并上清液,30 ℃真空离心浓缩至1 mL。剩余液体用0.22 μm 有机滤膜过滤,转移至1.5 mL 离心管中,30 ℃真空离心浓缩器继续蒸干至无液体。用250 μL 50%甲醇(φ)溶解管底的干物质。用0.22 μm有机滤膜过滤上清液,转移至250 μL衬管中,装入棕色样品瓶中。将10 μL上清液注入液相色谱-质谱仪(LC-MS,AB Qtrap5500)。对反式玉米素(trans-zeatin,tZT,母离子:m/z=218.0;定量离子:m/z=134.0)和赤霉素A3(gibberellin A3,GA3,母离子:m/z =345.2;定量离子:m/z =239.1)的含量分别进行分析。流动相由溶解在再蒸馏水中的甲醇和0.5%(φ)乙酸组成。标准品为tZT(Z0876)和GA3(48880)(Sigma)。使用0、2.5、5.0、10、12.5、25.0 和50.0 ng·mL-1的标准品绘制标准曲线。
1.4 RNA提取和转录组测序
使用十六烷基三甲基溴化铵法(Cetyltrimethylammonium Bromide,CTAB)分别提取30 个样本的总RNA。使用NanoDrop 2000 评估RNA 纯度和浓度。使用NEBNext Ultra RNA Library Prep Kit 构建cDNA 文库。通过Oligo(dT)磁珠富集总RNA 中带有polyA 结构的mRNA,采用离子打断的方式,将RNA打断到长度为300 bp左右的片段,纯化长度为250~300 bp 的cDNA 片段。以mRNA 为模板,用六碱基随机引物合成第一条cDNA 链,然后加入缓冲液、dNTPs、RNase H和DNA polymerase Ⅰ合成第二条cDNA链,利用AMPure XP beads纯化cDNA。纯化的双链cDNA 再进行末端修复、加A 尾并连接测序接头,然后用AMPure XP beads 进行片段大小选择。最后通过PCR 富集得到cDNA 文库并在Agilent Bioanalyzer 2100系统上评估文库质量。每个文库(约10 ng)用于使用Illumina HiSeq™4000(Illumina,San Diego,CA,USA)进行双末端测序。过滤原始序列数据以去除低质量reads。转录组的SRA 数据保存在国家基因组科学数据中心(https://ngdc.cncb.ac.cn/,登录号:CRR327161~CRR327190)。
1.5 差异表达基因的鉴定
使用Hisat2 软件将过滤后的Reads 比对到梨参考基因组(NCBI,GCF_000315295.1),允许不超过2个碱基的错配。使用RSEM软件运用每千个碱基转录每百万映射读取片段数(FPKM)的方法计算基因表达水平。使用基于负二项分布的DESeq2 软件对raw counts 进行分析,设置p-adjust≤0.05,|Log2Foldchange|≥1 的筛选条件比较组间表达差异的基因/转录本。不同阶段共有的差异基因数量用维恩图表示。利用京都基因与基因组百科全书(kyoto encyclopedia of genes and genomes,KEGG)通路分析将所有差异基因映射到KEGG 数据库,计算每个代谢通路的基因数。
2 结果与分析
2.1 翠冠及其芽变体开花后40 d果实发育状况
在花后0、10、20、30和40 d,翠冠和大翠冠单果质量逐渐增加(图1-A)。从花后10 d 开始,两者之间的果实大小存在显著差异,在各时期,大翠冠的果实均更大(图1-A~B)。花后120 d 的成熟果实,大翠冠的横向直径和纵向直径都大于翠冠(图1-C)。两者成熟果实单果质量的中位数分别为425.8和269.0 g(图1-D),大翠冠单果质量比翠冠果实大59.4%。石蜡切片结果显示,大翠冠果实细胞的横截面积大于翠冠(图2)。在5 个阶段中,大翠冠和翠冠的细胞大小均增加(图3),芽变后果实的细胞更大。

图1 翠冠和大翠冠开花后40 d 内果实发育情况
Fig.1 Development of the fruit of Cuiguan and Big Cuiguan at 40 days after blossom
A.幼果;B.果实发育;C.成熟果实;D.成熟果实。*表示差异显著(t 检验,p<0.05),误差线表示标准差。下同。
A.Young fruit weight; B.The fruit development; C.The mature fruit; D.The mature fruit weight.* indicates significance at p<0.05 using a student’s t-test.Bars indicate standard error.The same below.
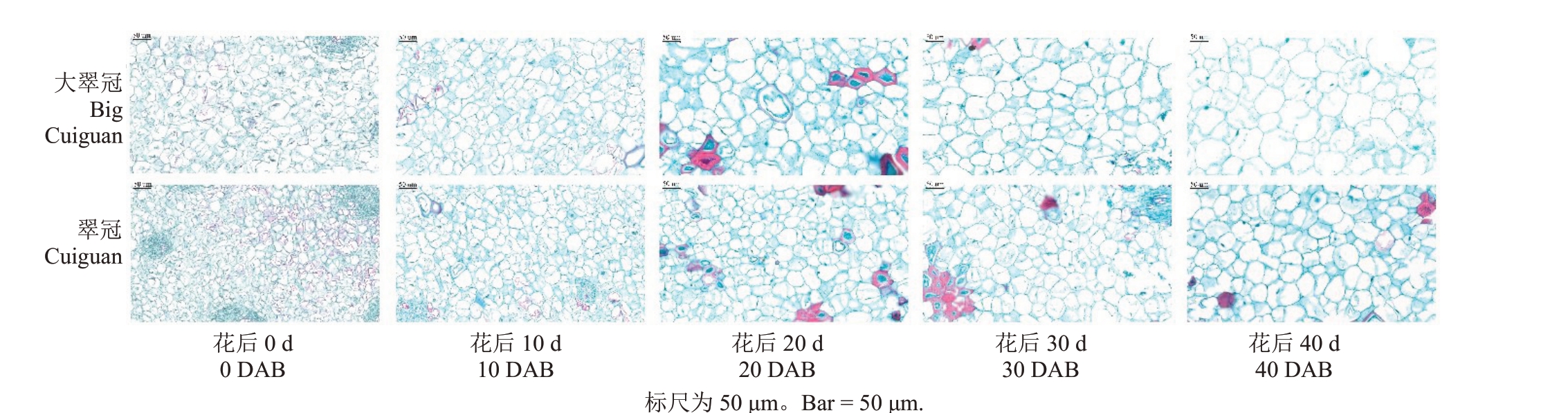
图2 翠冠和大翠冠开花后40 d 内果肉细胞横切面
Fig.2 Histological sections of young fruit cell of Cuiguan and Big Cuiguan in 40 days after blossom(DAB)
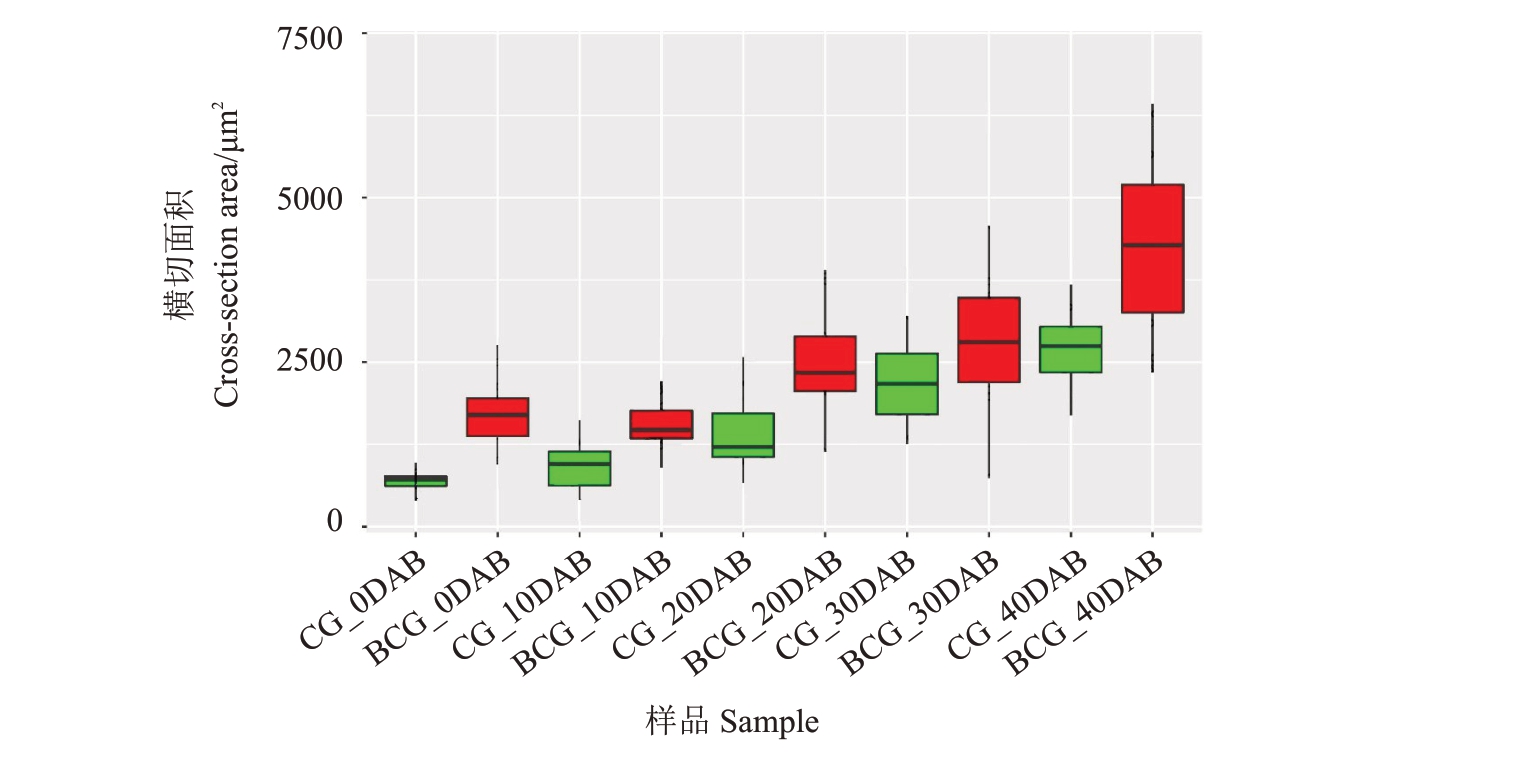
图3 翠冠(CG)和大翠冠(BCG)开花后40 d 内果肉细胞横切面积
Fig.3 The cross-section area of cell size in Cuiguan(CG)and Big Cuiguan(BCG)in 40 days after blossom
CG_0DAB 表示花后0 d 的翠冠,BCG_0DAB 表示花后0 d 的大翠冠,以此类推。
CG_0DAB represents the fruits of Cuiguan at 0 days after blossom,BCG_0DAB represents the fruits of Big Cuiguan at 0 days after flowering,and so on.
2.2 翠冠和大翠冠中的反式玉米素tZT 和赤霉素A3含量测定
对果肉中tZT和GA3的含量进行分析,结果(图4)显示大部分阶段tZT 和GA3的含量差异不显著。在翠冠和大翠冠中,随着果实发育tZT 含量均逐渐降低。在花后10 d 时,大翠冠中的tZT 含量显著高于翠冠。而在花后0、20 和30 d 时,虽然翠冠中的tZT含量略高于大翠冠,但差异并不显著。GA3的含量在2个样本中均呈现下降-上升-下降的趋势。在花后20、30和40 d时,大翠冠中GA3含量高于翠冠,但仅在花后20 d时差异显著。
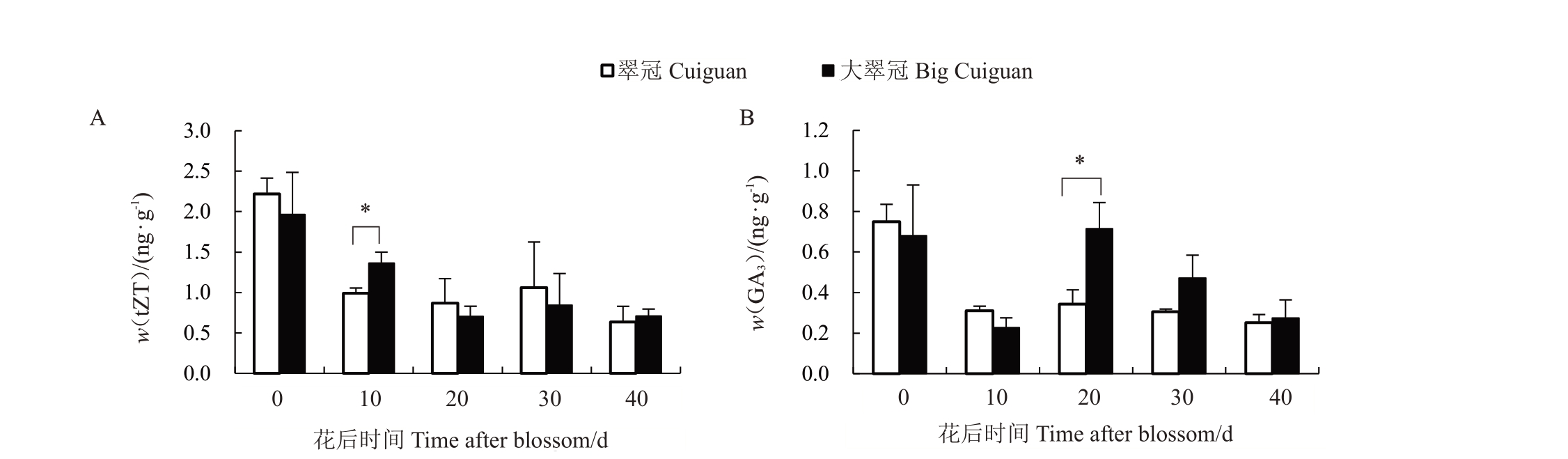
图4 翠冠和大翠冠果实发育过程中tZT 和GA3的含量
Fig.4 Content of trans-zeatin and gibberellin A3 during fruit developmentof Cuiguan and Big Cuiguan
2.3 果实发育中差异表达基因分离
为了进一步分析大翠冠和翠冠果实发育之间的分子差异,对两者花后5个时间点进行转录组分析。在30个文库中,获得双末端raw reads 42.7万~61.1万条。经过严格的质量过滤过程后,每个文库的clean reads 数范围为42.33~60.52 M,Q30 百分比≥93.4%。在每个采样时间点对翠冠和大翠冠果实内基因表达进行差异分析(图5),在花后10 d 时发现了大量的差异基因(264个上调和345个下调基因),随后花后20 d时差异基因数量下降,分别有52个上调和62个下调的差异基因。在花后0 和30 d 中分别发现了609 和639 个差异基因。综合所有5 个阶段的差异基因,在梨果实发育早期共发现了2015个差异表达基因。维恩图显示大量的差异基因为1 个时期特有。302个基因在2个以上的时期发生差异表达,51个基因在3个以上的时期发生差异表达。
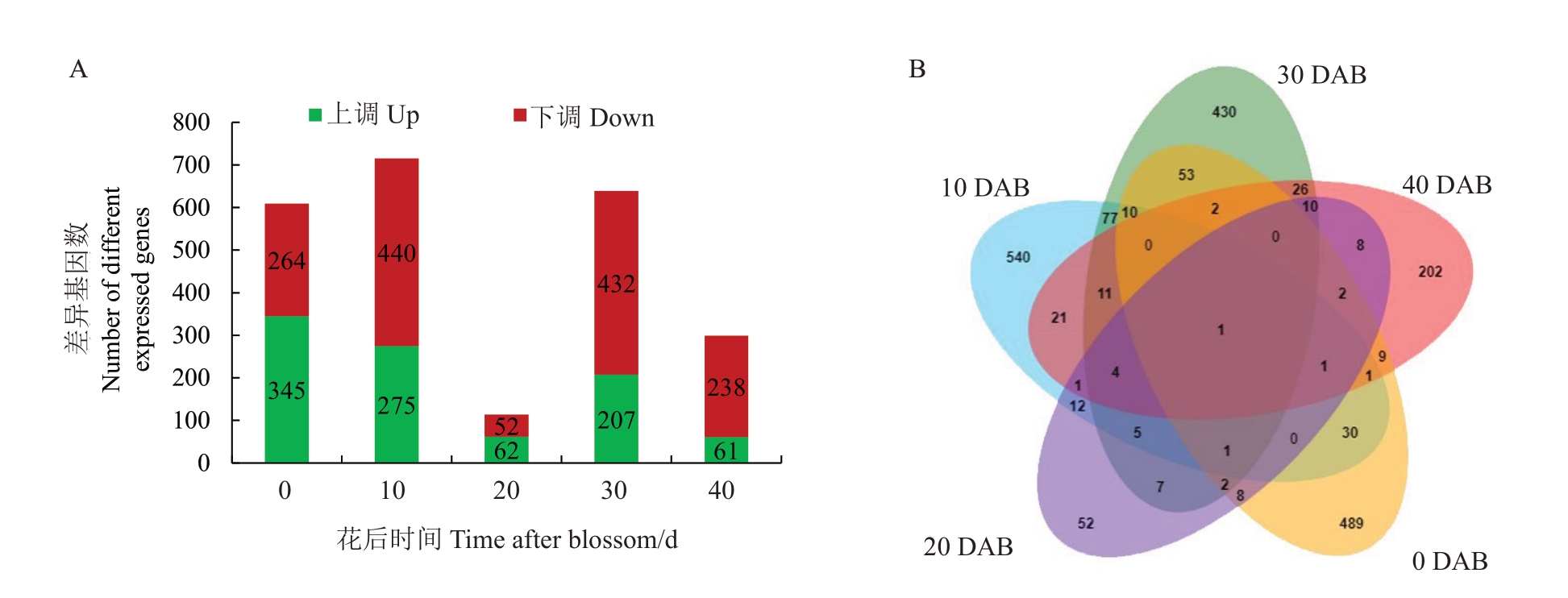
图5 翠冠和大翠冠开花后40 d 内差异基因数量
Fig.5 The different expressed genes between Cuiguan and Big Cuiguan in 40 days after blossom(DAB)
A.差异基因数量;B.5 个时期差异基因维恩图。
A.The number of differentially expressed genes;B.The Venn diagram of differentially expressed genes.
2.4 各时期差异基因注释和表达分析
一般认为不同时期共有的差异基因可能为关键基因。在302个差异表达基因中,只发现了1个基因在所有5个时期均发生差异表达。此基因为1个长链非编码RNA(lncRNA,LOC103927682),在大翠冠中发生下调表达,推测为负调控基因。6个基因在4个时期显著差异表达,均为晚期胚胎富集蛋白基因D-29(LOC103944693),在最后4个时期在大翠冠中显著上调表达。3 个基因与金属元素有关,包括金属硫蛋白基因1(LOC103944922)、金属烟胺转运蛋白基因YSL2(LOC103951610)和铁超氧化物歧化酶基因(LOC103953698),最后4个阶段在大翠冠中下调表达。RNA 依赖性RNA 聚合酶1 的1 个基因(LOC103943088)大多数时期在大翠冠中上调表达,并且发现抗病反应蛋白206 基因(LOC103927296)在翠冠中上调表达。在2~3个时期中共有的差异基因中,1个液泡铁转运蛋白基因(LOC103935577)和1个锌指蛋白基因dof2.1(LOC103943779)在大翠冠中显著上调表达。WAT1 相关蛋白的2 个基因(LOC103939120和LOC103948386)在花后0和10 d时在大翠冠中显著上调表达。1 个extensin 基因(LOC103937946)在花后10和20 d时在大翠冠中显著上调表达。
大翠冠和翠冠之间的果实大小差异起始于花后10 d,所以除了分析不同时期共有的差异基因外,对花后0和10 d这2个时期的差异基因进行了分析(表1)。根据表达量差异倍数和基因注释挑选出部分可能与果实大小相关的基因。在花后0 d,生长素结合蛋白基因ABP19a、KLUH 和赤霉素调控蛋白6 基因在大翠冠中显著上调表达,而赤霉素3-β-双加氧酶基因下调表达。在花后10 d,扩展蛋白expansin 基因在大翠冠中显著上调表达。赤霉素2-β-双加氧酶基因的2个成员显著下调表达。细胞分裂素相关的3 个基因(ARR17、ORR10 和AHP1)在大翠冠中均显著上调表达。
表1 翠冠和大翠冠梨果实在花后0 d 和10 d 时果实大小相关的差异表达基因
Table 1 The differentially expressed genes related to fruit size between Cuiguan and Big Cuiguan at 0 and 10 days after blossom

花后时间Time after blossom/d 0基因登录号Gene accession number NW_008988303.1 Log2差异倍数(大翠冠/翠冠)Log2FC(Big Cuiguan/Cuiguan)1.04 NW_008988249.1 1.68 NW_008988744.1-1.37 NW_008988051.1 1.16 10 NW_008988145.1 2.45 NW_008988189.1-1.17 NW_008988339.1-1.14 NW_008988145.1 1.32 NW_008988583.1 1.32 NW_008988277.1基因注释Gene description生长素结合蛋白基因ABP19a Auxin-binding protein 19a细胞色素CYP78A5(KLUH)Cytochrome P450 78A5(KLUH)赤霉素3-β-双加氧酶GA3ox1 Gibberellin 3-beta-dioxygenase 1赤霉素调控蛋白GRP6 Gibberellin-regulated protein 6扩展蛋白Expansin赤霉素2-β-双加氧酶GA2ox1-1 Gibberellin 2-beta-dioxygenase 1赤霉素2-β-双加氧酶GA2ox1-2 Gibberellin 2-beta-dioxygenase 1双组分响应调节基因ARR17 Two-component response regulator ARR17双组分响应调节基因ORR10 Two-component response regulator ORR10-like含组氨酸磷酸转移蛋白AHP1 Histidine-containing phosphotransfer protein 1 1.26调控Regulation上调Up上调Up下调Down上调Up上调Up下调Down下调Down上调Up上调Up上调Up
2.5 细胞分裂素和赤霉素相关基因表达分析
细胞分裂素和赤霉素能够显著影响果实的大小,通过匹配KEGG数据库,结果显示花后0和10 d有27个差异基因富集在植物激素信号转导通路中,推测细胞分裂素和赤霉素相关基因在翠冠梨大果芽变材料幼果期发挥重要作用。在花后10 d鉴定出3个细胞分裂素和4 个赤霉素相关的差异表达基因(图6)。细胞分裂素相关的差异基因分别是含组氨酸的磷酸转移蛋白1基因AHP1、双组分反应调节剂基因ORR10和ARR17。这3个基因在大翠冠中均在花后10 d时显著上调,同时在花后30 d时也显著上调表达。赤霉素相关基因共有4 个,其中赤霉素调控蛋白基因GRP6 属于赤霉素正调控基因,其在花后0和10 d在大翠冠中显著上调表达。其余3个基因属于赤霉素氧化酶基因,都是赤霉素信号过程中的负调控因子。其中GA3ox1 在花后0 d 时在翠冠梨中显著上调表达,而GA2ox1 的2 个成员在花后10 d时在翠冠梨中上调表达。

图6 7 个细胞分裂素和赤霉素相关的基因表达量
Fig.6 The transcriptional level of seven differentially expressed genes related to cytokinin and gibberellin
3 讨 论
果树芽变选种是果树培育新品种的重要途径。在梨属植物中,褐皮芽变突变体锈酥梨选自砀山酥梨[23]。在西洋梨品种La France中发现了1个大果突变体[24]。本研究中,在翠冠梨中发现了1 个大果芽变突变体,是不可多得的大果育种材料。果实大小受许多因素影响,例如遗传背景等。在果树品种驯化过程中,由于人类的选择,筛选后育成的品种比野生种要大。先前的研究报道显示,砂梨果实大小的演变主要取决于细胞分裂而不是细胞大小[3]。本研究中,大翠冠细胞的横截面积大于普通翠冠,这表明大翠冠的1个变异来源是细胞增大。由于梨果肉细胞排列无序,加上石细胞的干扰,笔者无法准确测量梨横切面上果肉细胞的层数。根据过去5 a 的物候期记录,翠冠和大翠冠的花期一致,这表明变异并非来自开花的早晚。翠冠和大翠冠的果实发育表明,花后果实大小差异逐渐变大。这表明一些重要基因可能从开花后即发挥作用。
细胞分裂素和赤霉素可以调节果实的大小和质量。细胞分裂素苄基腺嘌呤能增加Spadona 和Coscia 梨的果实大小[25]。反式玉米素是一类嘌呤类的植物细胞分裂素[26]。本研究中,在花后10 d时,大翠冠中tZT 的含量高于翠冠,暗示高含量tZT可能促进了梨果肉细胞的分裂。赤霉素的喷施可以导致Akca 梨的单果质量增加20.2%[12]。赤霉素能够控制细胞的伸长[27],其含量与最终果实大小、果皮细胞数量呈正相关[28]。本研究中在后期的3个时间点,大翠冠果肉中GA3的含量始终高于翠冠。这表明大翠冠果实大小的变异来源可能与赤霉素含量的变化相关。大翠冠和翠冠之间细胞分裂素和赤霉素含量的不同表明这2 种激素可能都参与了果实大小的控制。
芽变材料变异来源单一,可能是基因组中某个碱基或者某个小片段的插入缺失导致,这使得从DNA 层面找到变异位点比较难以实现。笔者在本研究中从基因表达入手,分析了大翠冠和翠冠之间的差异表达基因,共发现了2015 个差异表达基因。仅有1个差异基因存在于所有5个采样时期,有302个差异基因存在于2个以上采样时期。在柑橘无核突变体[29]和梨果皮突变体[30]的转录组分析中,也只发现了少量的差异表达基因。这表明芽变材料中基因表达差异不大。由于细胞分裂素和赤霉素在芽变材料中有差异,笔者重点分析了相关基因的表达情况。含组氨酸的磷酸转移蛋白基因AHP 参与细胞分裂素的信号转导机制,其功能很可能与反应调节因子基因RRs一致[31]。双组分响应调节基因ORR和ARR可由细胞分裂素诱导并调节生长发育[32]。本研究中这3个基因在花后10 d时在大翠冠中显著上调表达,而此时大翠冠中tZT 的含量也较高,表明这3个基因可能受到细胞分裂素诱导,参与果实增大调控。赤霉素双加氧酶参与了赤霉素的信号转导,能够催化赤霉素失去生物活性[33]。本研究中GA3ox1和GA2ox1 在大翠冠果实发育初期均表现出表达量下降,暗示具有生物活性的赤霉素含量上升,促进果实的增大。
除了激素相关基因外,笔者在本研究中还鉴定了一些其他基因。1个长链非编码lncRNA在所有5个时间点在大翠冠中均发生显著下调表达。该基因的功能目前尚不明确。晚期胚胎富集蛋白质基因D-29后4个时间点在大翠冠中上调。脱落酸胁迫处理后,晚期胚胎富集蛋白质在根尖分生组织和侧根原基中高度积累,导致根系扩张增强[34]。晚期胚胎富集蛋白质基因D-29 的上调可能会促进果实发育。此外,笔者鉴定出一些与金属相关的差异基因,例如金属硫蛋白样蛋白和金属-烟胺转运蛋白基因YSL2 等,在后4 个阶段在翠冠中上调表达。这些金属相关的蛋白是否参与了果实大小的调控还有待进一步研究。同时,笔者发现了1个RNA聚合酶基因RDR1和1个锌指蛋白基因dof2.1在3个以上时间点于大翠冠中上调。RDR 主要通过基因沉默调节植物的生长发育[35]。DOF 蛋白参与多种植物过程,包括发育和抗逆性[36]。大翠冠中RDR1 和dof2.1 基因的上调表达可能与果实大小相关。在花后0和10 d时,2 个WAT1 基因在大翠冠中显着上调。WAT1 基因由生长素诱导并促进细胞壁厚度增加[37],可能参与了果实发育,细胞色素P450 蛋白基因CYP78A5/KLUH主要以非细胞自主性的方式调控了拟南芥器官大小的发育,该基因在花后0 d时,在大翠冠中显著上调表达。这些基因的功能以及在果实发育中的作用还需要进一步研究。
4 结 论
对翠冠梨大果芽变进行了组织切片、激素变化研究及转录组分析,初步明确了变异来源。大翠冠在果实发育初期细胞大小更大。反式玉米素和赤霉素含量在大翠冠果实发育的一些时期高于翠冠。通过转录组分析分离了一些可能的关键差异基因,例如lncRNA、AHP1 和GA3ox1 等,为梨果实大小调控的分子机制研究提供了参考。
[1] SAN J R,SÁNCHEZ-MATA M C,CÁMARA M,PROHENS J.Eggplant fruit composition as affected by the cultivation environment and genetic constitution[J].Journal of the Science of Food and Agriculture,2014,94(13):2774-2784.
[2] CHALLICE J S,WESTWOOD M N.Numerical taxonomic studies of the genus Pyrus using both chemical and botanical characters[J].Botanical Journal of the Linnean Society,2008,67(2):121-148.
[3] ZHANG C X,TANABE K,WANG S P,TAMURA F,YOSHIDA A,MATSUMOTO K.The impact of cell division and cell enlargement on the evolution of fruit size in Pyrus pyrifolia[J].Annals of Botany,2006,98(3):537-543.
[4] GOFFINET M C,ROBINSON T L,LAKSO A N.A comparison of‘Empire’apple fruit size and anatomy in unthinned and handthinned trees[J].Journal of Horticultural Science,1995,70(3):375-387.
[5] HIGASHI K,HOSOYA K,EZURA H.Histological analysis of fruit development between two melon (Cucumis melo L. reticulatus)genotypes setting a different size of fruit[J].Journal of Experimental Botany,1999,50(339):1593-1597.
[6] OLMSTEAD J W,IEZZONI A F,WHITING M D.Genetic differences in sweet cherry fruit size are determined by cell number and not cell size[J].HortScience,2005,40(4):1008.
[7] LI Y H,ZHANG Z,SUN G M.Changes in cell number and cell size during pineapple (Ananas comosus L.) fruit development and their relationship with fruit size[J].Australian Journal of Botany,2010,58(8):673-678.
[8] HARADA T,KURAHASHI W,YANAI M,WAKASA Y,SATOH T.Involvement of cell proliferation and cell enlargement in increasing the fruit size of Malus species[J].Scientia Horticulturae,2005,105(4):447-456.
[9] MALLADI A,HIRST P M.Increase in fruit size of a spontaneous mutant of‘Gala’apple(Malus×domestica Borkh.)is facilitated by altered cell production and enhanced cell size[J].Journal of Experimental Botany,2010,61(11):3003-3013.
[10] HAMPSON C,BEDFORD K.Efficacy of blossom thinning treatments to reduce fruit set and increase fruit size of Ambrosia and Aurora Golden Gala apples[J].Canadian Journal of Plant Science,2011,91(6):983-990.
[11] OOSTHUYSE S A.Spray application of KNO3,low biuret urea,and growth regulators and hormones during and after flowering on fruit retention,fruit size and yield of mango[J].Acta Horticulturae,2015,1075:135-141.
[12] CANLI F A,PEKTAS M.Improving fruit size and quality of low yielding and small fruited pear cultivars with benzyladenine and gibberellin applications[J].European Journal of Horticultural Science,2015,80(3):103-108.
[13] AZZI L,DELUCHE C,GÉVAUDANT F,FRANGNE N,DELMAS F,HERNOULD M,CHEVALIER C.Fruit growth-related genes in tomato[J].Journal of Experimental Botany,2015,66(4):1075-1086.
[14] VAN DER KNAAP E,TANKSLEY S D.The making of a bell pepper-shaped tomato fruit:Identification of loci controlling fruit morphology in Yellow Stuffer tomato[J].Theoretical and Applied Genetics,2003,107(1):139-147.
[15] CONG B,BARRERO L S,TANKSLEY S D.Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication[J].Nature Genetics,2008,40(6):800-804.
[16] KRIZEK B A.Making bigger plants:Key regulators of final organ size[J].Current Opinion in Plant Biology,2009,12(1):17-22.
[17] JONES A R,FORERO-VARGAS M,WITHERS S P,SMITH R S,TRAAS J,DEWITTE W,MURRAY J A H.Cell-size dependent progression of the cell cycle creates homeostasis and flexibility of plant cell size[J].Nature Communications,2017,8:15060.
[18] DE VEYLDER L,BEECKMAN T,BEEMSTER G T,KROLS L,TERRAS F,LANDRIEU I,VAN DER SCHUEREN E,MAES S,NAUDTS M,INZE D.Functional analysis of cyclindependent kinase inhibitors of Arabidopsis[J].The Plant Cell,2001,13(7):1653-1668.
[19] MIZUKAMI Y,FISCHER R L.Plant organ size control:AINTEGUMENTA regulates growth and cell numbers during organogenesis[J].Proceedings of the National Academy of Sciences of the United States of America,2000,97(2):942-947.
[20] HU Y X,XIE Q,CHUA N H.The Arabidopsis auxin-inducible gene ARGOS controls lateral organ size[J].The Plant Cell,2003,15(9):1951-1961.
[21] DISCH S,ANASTASIOU E,SHARMA V K,LAUX T,FLETCHER J C,LENHARD M.The E3 ubiquitin ligase BIG BROTHER controls Arabidopsis organ size in a dosage-dependent manner[J].Current Biology,2006,16(3):272-279.
[22] MARTÍ C,ORZÁEZ D,ELLUL P,MORENO V,CARBONELL J,GRANELL A.Silencing of DELLA induces facultative parthenocarpy in tomato fruits[J].The Plant Journal,2007,52(5):865-876.
[23] HENG W,JIA B,HUANG H N,YANG J Y,WANG Z T,LIU P,LIU L,YE Z F,ZHU L W.Identification of russet-associated microRNAs in the exocarp of a Dangshansuli pear mutant (Pyrus bretschneideri Rehd.) by high- throughput sequencing[J].Tree Genetics&Genomes,2016,12(6):1-7.
[24] REUSCHER S,ISUZUGAWA K,KAWACHI M,OIKAWA A,SHIRATAKE K.Comprehensive elemental analysis of fruit flesh from European pear‘La France’and its giant fruit bud mutant indicates specific roles for B and Ca in fruit development[J].Scientia Horticulturae,2014,176:255-260.
[25] STERN R A,FLAISHMAN M A.Benzyladenine effects on fruit size,fruit thinning and return yield of‘Spadona’and‘Coscia’pear[J].Scientia Horticulturae,2003,98(4):499-504.
[26] OSUGI A,KOJIMA M,TAKEBAYASHI Y,UEDA N,KIBA T,SAKAKIBARA H.Systemic transport of trans-zeatin and its precursor have differing roles in Arabidopsis shoots[J].Nature Plant,2017,3:17112.
[27] UBEDA-TOMÁS S,FEDERICI F,CASIMIRO I,BEEMSTER G T S,BHALERAO R,SWARUP R,DOERNER P,HASELOFF J,BENNETT M J.Gibberellin signaling in the endodermis controls Arabidopsis root meristem size[J].Current Biology,2009,19(14):1194-1199.
[28] ZHANG C X,TATEISHI N,TANABE K.Pollen density on the stigma affects endogenous gibberellin metabolism,seed and fruit set,and fruit quality in Pyrus pyrifolia[J].Journal of Experimental Botany,2010,61(15):4291-4302.
[29] XIAO J P,ZHANG L L,FAN F J,LIU X B.Comparative transcript profiling reveals the mechanism of female sterility associated with seedless ponkan mandarin (Citrus reticulata Blanco)[J].Genome,2018,61(8):595-604.
[30] WANG Y Z,ZHANG S J,DAI M S,SHI Z B.Pigmentation in sand pear (Pyrus pyrifolia) fruit:Biochemical characterization,gene discovery and expression analysis with exocarp pigmentation mutant[J].Plant Molecular Biology,2014,85(1/2):123-134.
[31] MA Q H,TIAN B.Characterization of a wheat histidine-containing phosphotransfer protein (HP) that is regulated by cytokininmediated inhibition of leaf senescence[J].Plant Science,2005,168(6):1507-1514.
[32] WANG W C,LIN T C,KIEBER J,TSAI Y C.Response regulators 9 and 10 negatively regulate salinity tolerance in rice[J].Plant&Cell Physiology,2019,60(11):2549-2563.
[33] 岳川,曾建明,曹红利,王新超,章志芳.高等植物赤霉素代谢及其信号转导通路[J].植物生理学报,2012,48(2):118-128.YUE Chuan,ZENG Jianming,CAO Hongli,WANG Xinchao,ZHANG Zhifang.Gibberellins metabolism and signaling pathway in higher plant[J].Plant Physiology Communications,2012,48(2):118-128.
[34] CHEN Y S,LO S F,SUN P K,LU C A,HO T H D,YU S M.A late embryogenesis abundant protein HVA1 regulated by an inducible promoter enhances root growth and abiotic stress tolerance in rice without yield penalty[J].Plant Biotechnology Journal,2015,13(1):105-116.
[35] TANTIKANJANA T,RIZVI N,NASRALLAH M E,NASRALLAH J B.A dual role for the S-locus receptor kinase in self-incompatibility and pistil development revealed by an Arabidopsis rdr6 mutation[J].The Plant Cell,2009,21(9):2642-2654.
[36] CHEN P X,YAN M J,LI L,HE J Q,ZHOU S X,LI Z X,NIU C D,BAO C N,ZHI F,MA F W,GUAN Q M.The apple DNAbinding one zinc-finger protein MdDof54 promotes drought resistance[J].Horticulture Research,2020,7(1):195.
[37] RANOCHA P,DIMA O,NAGY R,FELTEN J,CORRATGÉFAILLIE C,NOVÁK O,MORREEL K,LACOMBE B,MARTINEZ Y,PFRUNDER S,JIN X,RENOU J P,THIBAUD J B,LJUNG K,FISCHER U,MARTINOIA E,BOERJAN W,GOFFNER D. Arabidopsis WAT1 is a vacuolar auxin transport facilitator required for auxin homoeostasis[J].Nature Communications,2013,4:2625.