根际微生物生活在植物根系周围狭小土壤区域内[1],在植物-土壤之间的相互反馈机制中发挥着重要作用。一方面,根际土壤中存在大量的有益真菌[2],这些根际微生物可以通过养分竞争、拮抗作用和诱导系统抗性等机制对病原菌产生抑制作用,进而促进植物生长[3],另一方面则作为病原菌不断积累生态位,导致作物病害大面积发生[4]。随着菠萝连作年限增加,加之不科学的施肥管理模式,土壤微生物群落结构失衡和功能紊乱逐年加剧[5-7],菠萝心腐病(Pineapple heart rot disease)成为威胁菠萝植株健康的土传病害之一[8]。菠萝心腐病类型多样、致病菌复杂,世界菠萝主产国均有报道[9-10],分布在广东、海南地区的心腐病病原菌包括烟草疫霉菌等多种真菌[11-12],但根据地理环境和土壤理化性质呈现不同差异[13]。菠萝发生心腐病后,会导致菠萝产量和品质大幅度下降,严重制约了国内外菠萝产业的稳定发展[14-15]。因此,对土壤根际微生物群落进行调控是防控菠萝心腐病的关键。
目前,对菠萝土壤根际微生物群落的调控手段主要有选育抗性品种、改变种植方式、土壤改良和喷施化学药剂[16-18],但防控效果并不理想,甚至引发环境安全问题。随着生防菌资源的收集和有机肥料产业的发展,越来越多的研究表明[19-21],将具有生防功能的特定菌株接种到适宜的有机载体中,并与有机肥料二次发酵制成生物有机肥,一方面可以实现有机载体材料的废物再利用,另一方面有机肥为土壤微生物供应了充足养分,提高生防菌的抑菌活性,促进土壤中有益微生物竞争生态位,从而获得更好的生物防治效果。因此,笔者利用生防菌(枯草芽孢杆菌HL2 和链霉菌HL3)、载体(椰糠,菜籽饼,泥炭土)和普通有机肥进行二次发酵技术制成生物有机肥,来研究其对菠萝心腐病的防控效果,并结合高通量测序技术,真实全面地反映根际土壤微生物区系的特殊变化。
前期研究表明,在盆栽条件下,相比三元复合肥(HF)处理和普通有机肥(YJ)处理,生物有机肥处理可显著降低心腐病发病率,并改变土壤细菌群落结构,激发土壤中有益微生物的活性,但不同处理对真菌群落结构和功能的影响尚不清楚。笔者基于此盆栽试验,通过高通量测序结果的分析,进一步研究不同处理中根际土壤真菌群落结构的变化,旨在探明根际土壤真菌群落结构的调控与菠萝心腐病防控间的关系,为利用根际微生物防控菠萝心腐病提供指导。
1 材料和方法
1.1 供试材料与温室试验设计
于2017 年12 月到2018 年5 月在海南乐东黎族自治县香蕉枯萎病及热带经济作物土传病防控研究所进行温室盆栽试验,试验土壤采自多年连作菠萝园,土壤的基本理化性质如下:pH 5.31,有机质含量(w,后同)0.73%,土壤碱解氮73.01 mg·kg-1,速效磷89.06 mg·kg-1,速效钾21.55 mg·kg-1,菠萝心腐病病原菌数量为5×103 CFU·g-1。如表1所示,试验共设8个处理:三元复合肥(HF);普通有机肥(YJ);生物有机肥(KC、KN、KY、LC、LN、LY),每个处理3次重复,每次重复10 盆。生物有机肥堆制方案、盆栽试验具体设置可参见文献[22]。
表1 不同施肥处理及其发病率
Table 1 Treatments in different fertilizing methods and disease incidence
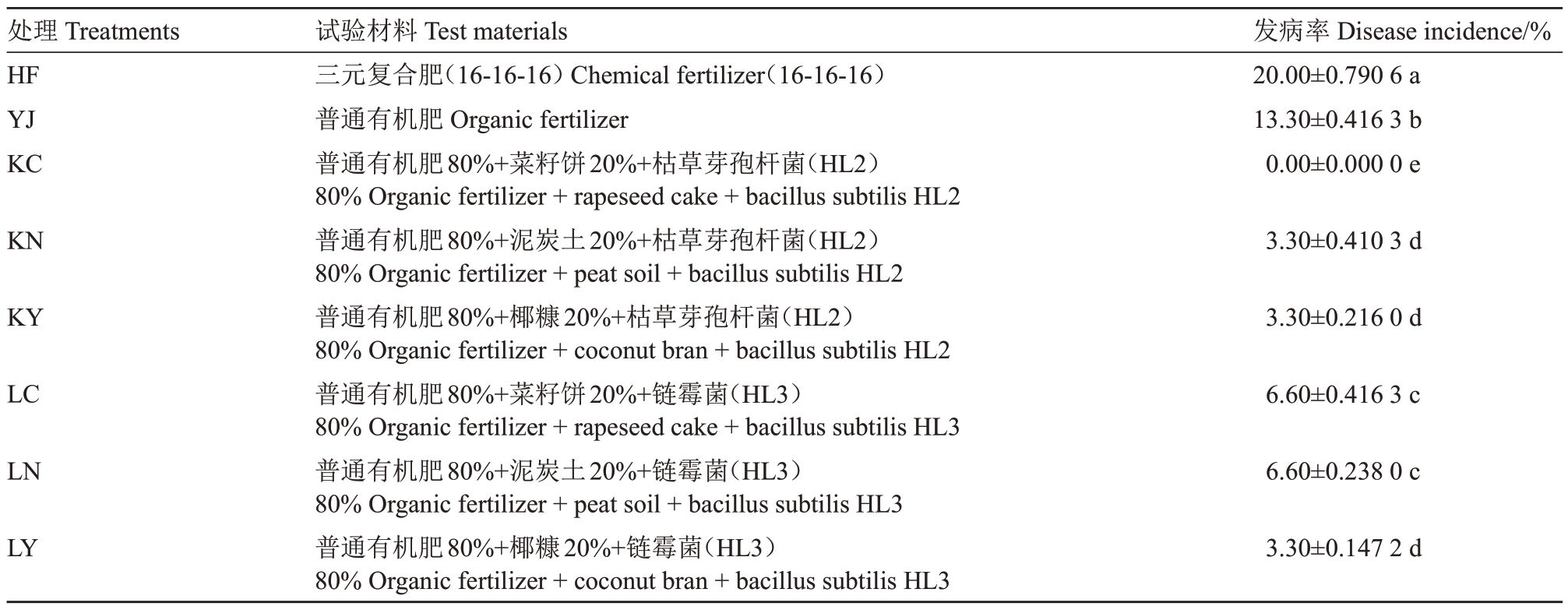
处理Treatments HF YJ KC发病率Disease incidence/%20.00±0.790 6 a 13.30±0.416 3 b 0.00±0.000 0 e KN 3.30±0.410 3 d KY 3.30±0.216 0 d LC 6.60±0.416 3 c LN 6.60±0.238 0 c LY试验材料Test materials三元复合肥(16-16-16)Chemical fertilizer(16-16-16)普通有机肥Organic fertilizer普通有机肥80%+菜籽饼20%+枯草芽孢杆菌(HL2)80%Organic fertilizer+rapeseed cake+bacillus subtilis HL2普通有机肥80%+泥炭土20%+枯草芽孢杆菌(HL2)80%Organic fertilizer+peat soil+bacillus subtilis HL2普通有机肥80%+椰糠20%+枯草芽孢杆菌(HL2)80%Organic fertilizer+coconut bran+bacillus subtilis HL2普通有机肥80%+菜籽饼20%+链霉菌(HL3)80%Organic fertilizer+rapeseed cake+bacillus subtilis HL3普通有机肥80%+泥炭土20%+链霉菌(HL3)80%Organic fertilizer+peat soil+bacillus subtilis HL3普通有机肥80%+椰糠20%+链霉菌(HL3)80%Organic fertilizer+coconut bran+bacillus subtilis HL3 3.30±0.147 2 d
1.2 项目测定与方法
菠萝苗定植后,统计菠萝心腐病发病率,测定方法可参见文献[22]。菠萝生长140 d后,各重复随机选取5 盆收集菠萝根际土作为混合样,去除植物根系和石块并过2 mm筛后,于-80 ℃保存以备提取土壤微生物总DNA。依托厦门承葛生物科技有限公司Illumina MiSeq平台对土壤DNA真菌内转录间隔区(ITS)的ITS1 区进行扩增测序,引物为ITS1F(5'-TCCTCCGCTTATTGATATGC-3')和ITS2(5'-GCTGCGTTCTTCATCGATGC-3')。根据PCR 扩增引物序列,在QIIME软件中对下机后的原始序列进行拼接和过滤后,将得到的所有样品的有效数据在USEARCH 程序中进行标准化分析,按97%的相似度将序列聚类成操作分类单元(Operational Taxonomic Unit,OTU)。将OTU 表格转换为Biom 格式,在QIIME 软件中使用BLAST 方法与UNITE FUNGAL ITS 数据库的模板序列进行比对注释,获取每个OTU所对应的真菌分类学信息,得到其代表序列的系统发育地位。
1.3 数据分析
计算各样品的Chao1 指数和Shannon 指数,比较处理间土壤真菌α 多样性的差异;基于Bray-Curits 采用主坐标分析(Principal coordinate analysis,PCoA)和相似度分析(ANOSIM)比较样品间土壤真菌群落组成的β 多样性的差异。基于p<0.05 和LDA>4.0,在https://www.bioincloud.tech 进行线性判别效应分析(LEfSe),以研究不同施肥处理中富集的土壤微生物的潜在生物标志。利用R语言软件的psych 包对真菌OTUs 进行分析,最后在Gephi 软件中实现OTU网络的可视化。在IBM SPSS 26.0中进行单因素方差分析(p<0.05),采用Origin 2021软件作图。将关键真菌OTUs 与发病率进行Pearson相关性分析,利用FUNGuild 对这些土壤真菌OTUs进行功能注释。
2 结果与分析
2.1 土壤样品测序深度和质量
通过对原始的测序序列进行处理和质控,从24个样本序列中共获得2 117 269 个ITS rRNA 序列,在97%相似度水平上分别被划分为1043 个真菌OTU。从稀释曲线(图1)来看,OTU 数量随着测序数量的增加而迅速增加,但随着测序数量的进一步增加,不同处理样本的稀释曲线均趋于平缓,表明样品测序深度合理。对测序结果进行物种注释,所有真菌ITS 基因序列分类共产生5 个门和302 个属。由图2-A 可以看出,优势真菌门为子囊菌门(80.96%),其次为担子菌门(14.78%)。由图2-B 可以看出,所有样本中丰度最高的5 个属分别为黑团球壳腔菌属(Massariosphaeria,15.02%)、青霉菌属(Penicillium,5.67%)、镰孢菌属(Fusarium,4.19%)、可可球二孢菌属(Lasiodiplodia,1.92%)、踝节菌属(Talaromyces,1.59%)。测序结果中主要优势微生物已得到分析,能够反映土壤样本中真实的真菌群落多样性。
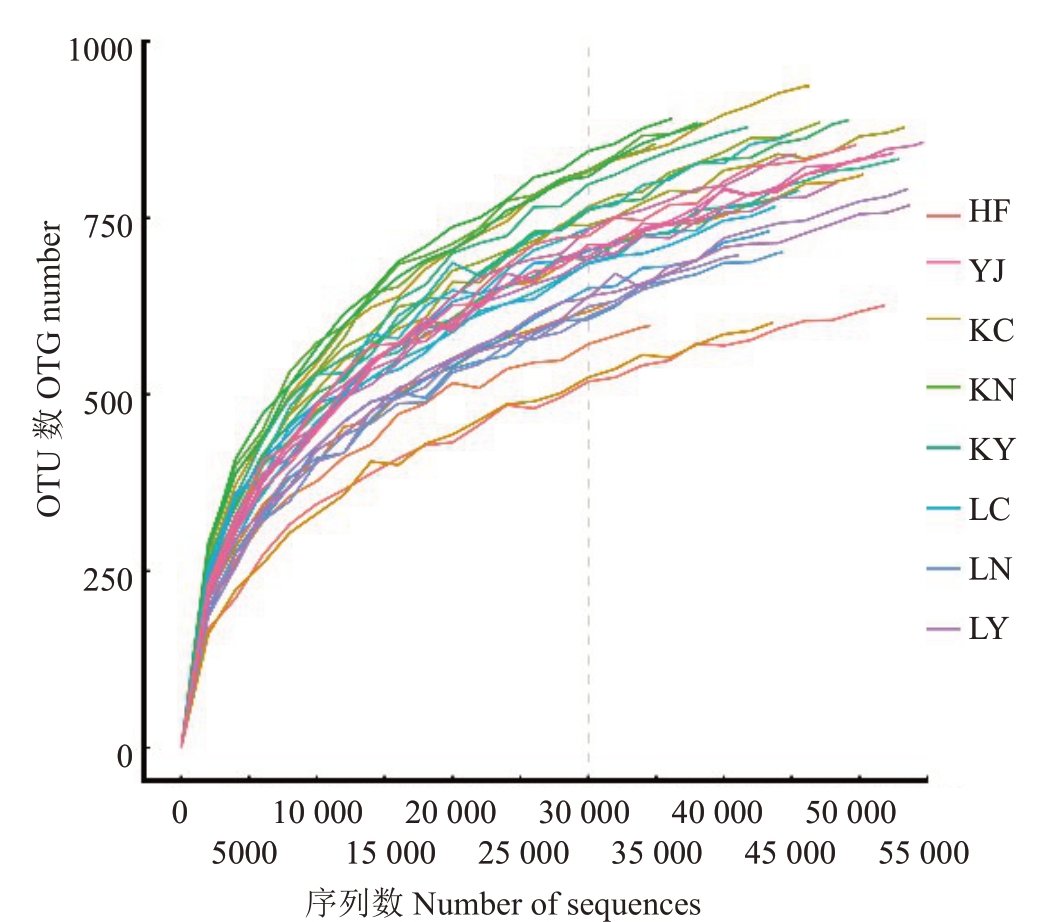
图1 不同处理样品的稀释曲线
Fig.1 OTU Rank curves and rare faction curves of each soil sample in different methods

图2 土壤真菌门水平(A)和属水平(B)的测序分布
Fig.2 Overall taxonomic sequence analyses for fungal ITS sequences at the phyla level(A)and genera level(B)
2.2 生物有机肥对土壤真菌α-多样性的影响
采用Chao 指数和Shannon 指数分别表征土壤样本微生物群落的物种丰富度和多样性,指数越大,表示物种丰富度和多样性越高。由图3-A和3-B可知,与对照HF处理相比,YJ处理和生物有机肥处理(KC、KN、KY、LC、LN、LY)的物种丰富度均显著增加(p<0.05),而LN处理多样性与HF处理相比无显著差异。与YJ处理相比,除LN处理外,生物有机肥处理(KC、KN、KY、LC、LY)的Chao 指数没有显著差异。由图3-B可看出,相比于YJ处理,KC处理Shannon 指数显著增高(p<0.05),而其他生物有机肥处理的Shannon指数均显著减低。
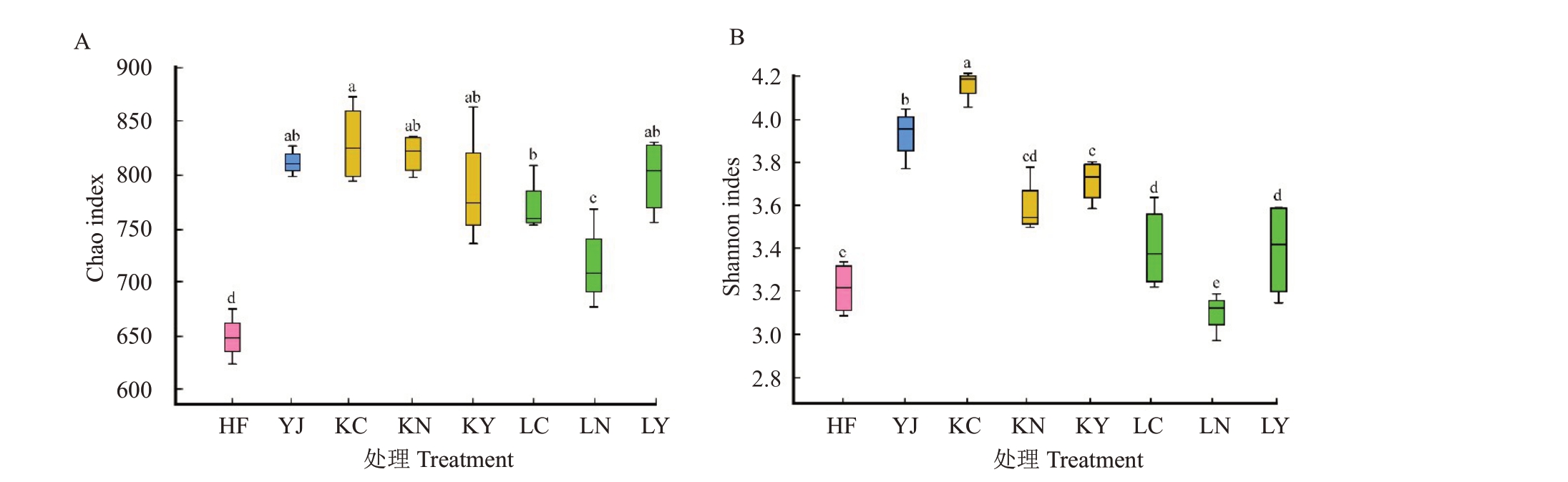
图3 不同处理土壤真菌多样性指数
Fig.3 Fungal alpha diversity in different samples
HF.三元复合肥;YJ.普通有机肥;KC、KN、KY.HL2 生物有机肥;LC、LN、LY.HL3 生物有机肥。不同字母表示差异显著(p<0.05)。下同。HF.Chemical fertilizer(16-16-16);YJ.Organic fertilizer;KC,KN,KY.Bio-organic fertilizers with Bacillus HL2;LC,LN,LY.Bio-organic fertilizers with Streptomyces HL3.Different letters indicate significant differences at p<0.05 based on the analysis of variance.The same below.
2.3 生物有机肥对土壤真菌β-多样性的影响
采用基于Bray-curits 距离的主坐标分析(PCoA),进一步阐明不同生物有机肥对土壤样本真菌群落结构的影响。由图4 可以看出,主坐标分析(PCoA)表明不同处理土壤样本彼此分开,真菌群落结构差异显著(ANOSIM,p=0.001),而相同处理的不同样本距离相近,聚集在一起。第1和第2坐标轴分别解释了总体群落差异的27.95%和19.43%,其中,生物有机肥处理和三元复合肥(HF)处理在第1坐标轴上明显分开,HL2生物有机肥(KC、KN、KY)处理和HL3生物有机肥(LC、LN、LY)处理在第2坐标轴上明显分开。
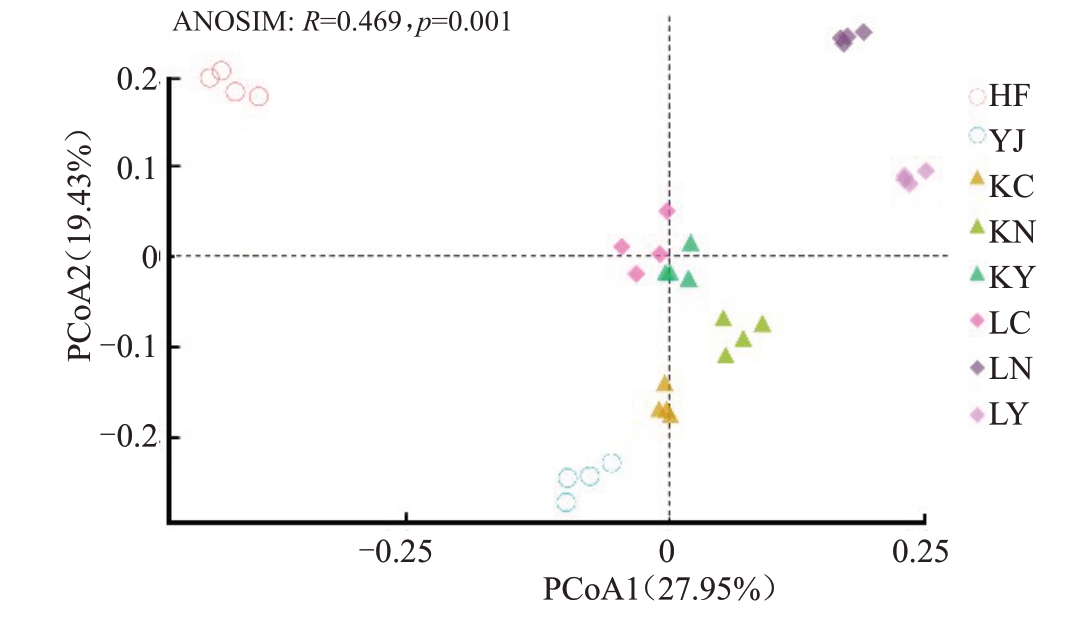
图4 基于Bray-Curtis 距离的细菌群落主坐标分析(PCoA)
Fig.4 Principal coordinate analysis(PCoA)of total soil microbial community based on the Bray-Curtis distance
2.4 生物有机肥对土壤真菌群落组成的影响
采用聚类热图和LEfSe分析进一步探索施肥方式驱动的菠萝土壤中真菌群落组成的特殊变化(图5,图6)。真菌在纲水平上的分类主要来自子囊菌门,其中,丰度较高的是座囊菌纲Dothideomycetes(30.18%)、散囊菌纲Eurotiomycetes(23.09%)、粪壳菌纲Sordariomycetes(22.21%)、未定义真菌纲Fungi_unidentified_1(14.78%),而未定义子囊菌纲Ascomycota_unidentified 和Incertae_sedis_14 的丰度不到3%。HF 中丰度较高的真菌纲是Sordariomycetes,其次是Incertae_sedis_14 和Eurotiomycetes。其中,HF 中Sordariomycetes 纲相对丰度分别是YJ、HL2 生物有机肥(KC、KN、KY)和HL3 生物有机肥(LC、LN、LY)的1.22 倍、1.71 倍和2.64 倍。Eurotiomycetes纲与HL2生物有机肥、HL3生物有机肥的丰度相比HF 分别下降0.38%、0.72%,但相对YJ 则分别提高0.11%、0.08%。相比于HF,Dothideomycetes纲在LN 中的相对丰度达到最大,而KC 与YJ 无明显差异。
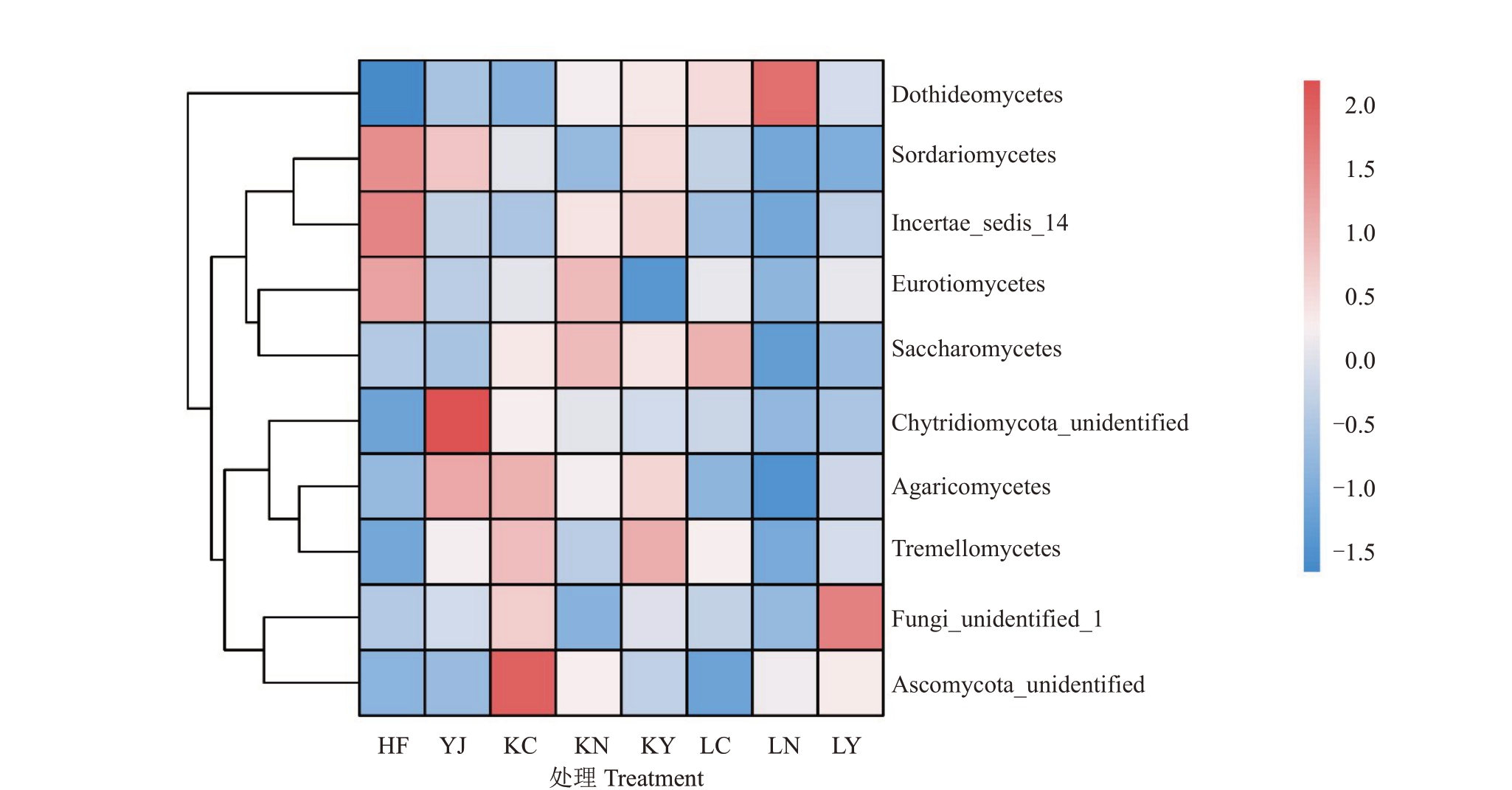
图5 不同处理真菌群落在纲水平上的分布
Fig.5 Distribution of fungal communities in different treatments at the class level
图中方块代表真菌在纲水平上的富集程度,红色和蓝色分别代表富集程度高和低。
The squares in the figure represent the level of fungal enrichment at the class level,with red and blue representing high and low levels,respectively.
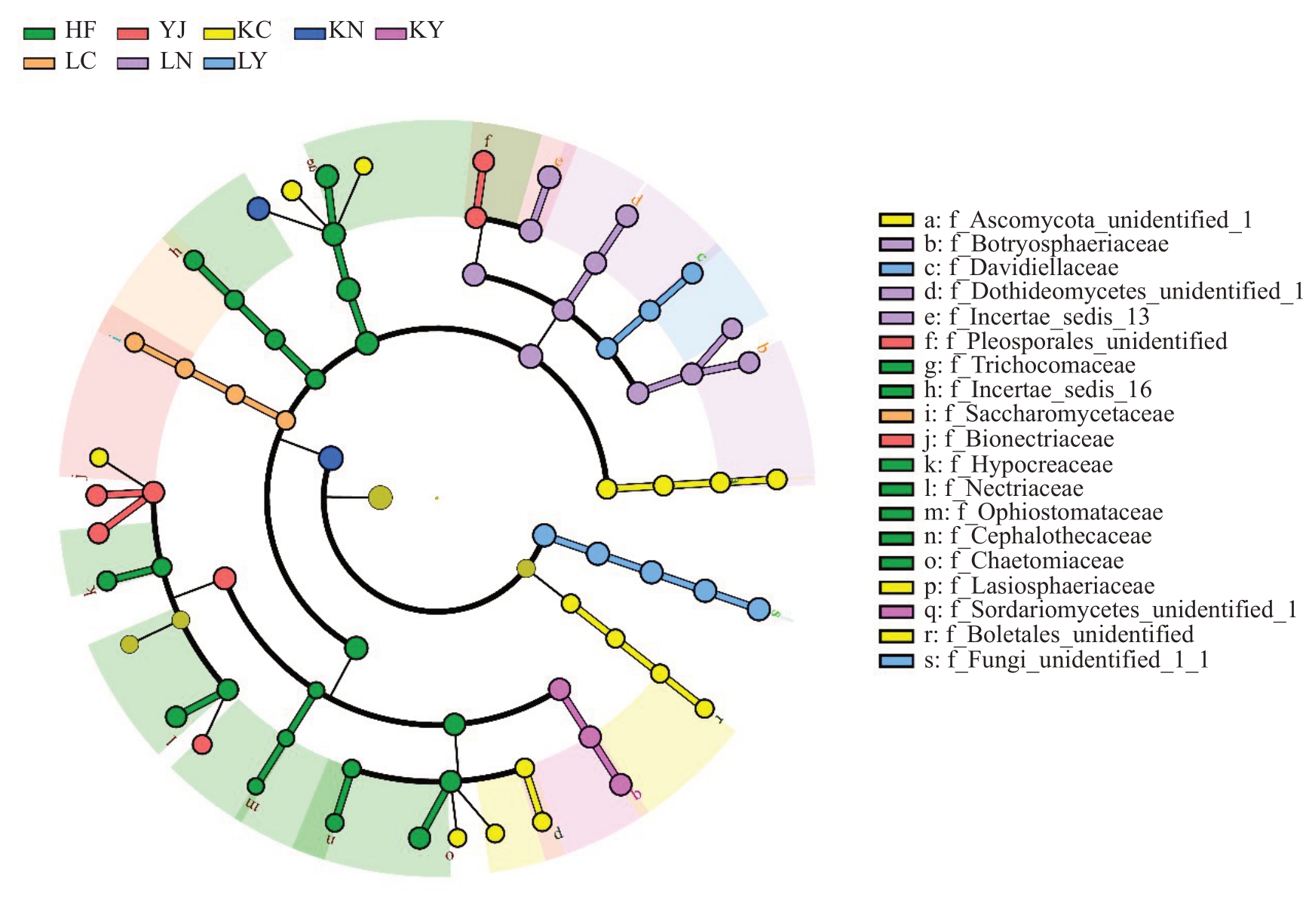
图6 基于线性判别效应分析(LEfSe)的差异菌鉴别
Fig.6 Identification of differential fungi based on the linear discriminant analysis(LDA)and effect size(LEfSe)pipeline
分支图显示了不同处理科水平土壤真菌群落的系统发育分布。
Cladogram using the LEfSe method indicating the phylogenetic distribution of the soil fungal community associated with various treatments on the level of family.
在科水平上,HF富集的差异真菌类群主要包括Chaetomiaceae、Nectriaceae、Hypocreaceae、Cephalothecaceae、Ophiostomataceae(Sordariomycetes 纲),以 及Trichocomaceae(Eurotiomycetes 纲)和Incertae_sedis_16(Incertae_sedis_14纲)。YJ中Bionectriaceae 得到富集,而Lasiosphaeriaceae、Davidiellaceae、Sordariomycetes_unidentified_1 在HL2 生物有机肥中得到富集,这些真菌类群均来自Sordariomycetes 纲。而HL3 生物有机肥富集的差异微生物在科水平上包括Incertae_sedis_13、Dothideomycetes_unidentified_1、Botryosphaeriaceae和Davidiellaceae,属于Dothideomycetes纲真菌类群。
为探究不同处理和真菌群落组成之间的关系,将KC、KN、KY 合 并 为HL2 生 物 有 机 肥 处 理(BOF_HL2),将LC、LN、LY合并为HL3生物有机肥(BOF_HL3),对其真菌Top 50 分类单元(OTUs)进行网络分析(p<0.05),在属水平上进行注释。由图7可看出,不同施肥处理后,土壤真菌网络结构发生明显变化。对比HF 和YJ 处理,HL2 和HL3 生物有机肥处理呈现出相关性更强、连接更多的复杂网络。其中,HL2和HL3生物有机肥处理的负相关的连接数分别为199、150,而HF和YJ处理是60和51,表明不同施肥处理的微生物组成存在显著差异。HF处理的真菌网络中心点为OTU5、OTU7、OTU9、OTU47、OTU83,分 别 为Penicillium、Fusarium、Chaetomiaceae_unidentified、Pulchromyces、Phialemonium;YJ 处理的网络中心点为OTU4、OTU12、OTU13、OTU23,分别为Dothideomycetes_unidentified、Bionectriaceae_unidentified、Penicillium、Clonostachys;HL2 生物有机肥处理网络中心点为OTU1、OTU2、OTU15,分 别 为Massariosphaeria、Trichocomaceae_unidentified、Talaromyces;HL3 生物有机肥处理网络中心点为OTU1、OTU8、OTU20、OTU26,分 别 为Massariosphaeria、Lasiodiplodia、Chaetothyriales_unidentified、Davidiella。
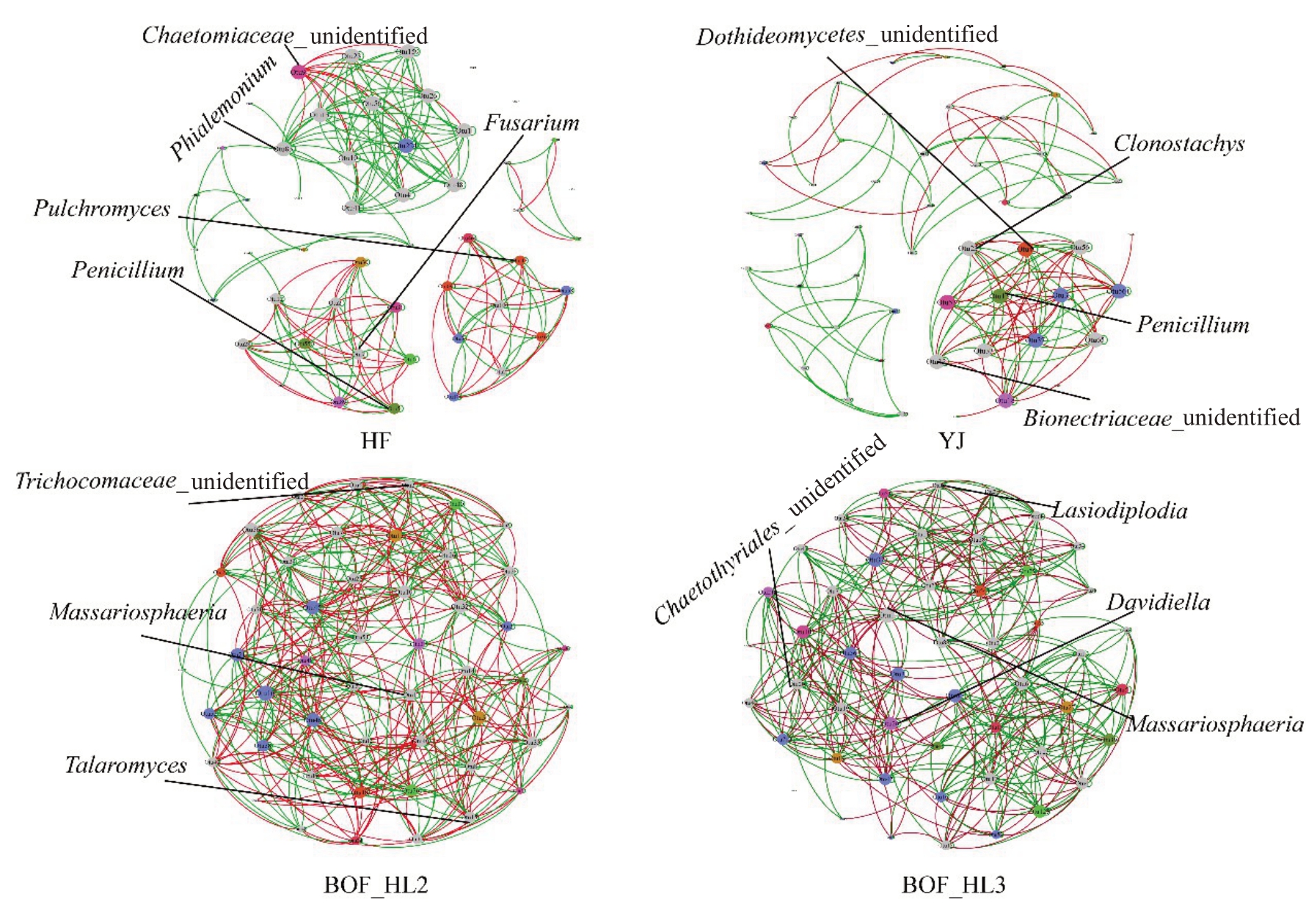
图7 不同施肥处理的真菌共生网络分析
Fig.7 Network analysis revealing the associations among fungal Top 50 OTUs in different treatments
HF.三元复合肥;YJ.普通有机肥;BOF_HL2.HL2 生物有机肥;BOF_HL2.HL3 生物有机肥。绿线和红线分别代表强正线性(r>0.8)和强负线性(r<-0.8)相关关系。不同颜色的节点分别对应真菌属的相应OTU,每个节点的大小与连接数(即程度)成正比。
HF.Chemical fertilizer(16-16-16);YJ.Organic fertilizer;BOF_HL2.Bio-organic fertilizers with Bacillus HL2;BOF_HL3.Bio-organic fertilizers with Streptomyces HL3.Green line and red line represent strong positive linear(r>0.8)and strong negative linear(r<-0.8)and relationships,respectively.Colored nodes signify corresponding OTUs assigned to major phyla.The size of each node is proportional to the number of connections(that is,degree).
2.5 生物有机肥对土壤真菌关键微生物丰度和功能的影响
将真菌群落组成分析中出现的16 个核心真菌类群作为不同处理的差异标记,基于Tukey 检验,对其进行单因素方差分析(ANOVA)。结合图8 和表2,HF 处理中OTU5(Penicillium)和OTU9(Chaetomiaceae_unidentified)相对丰度更高,其次是OTU7(Fusarium)。与其他处理相比,OTU43(Trichoderma)、OTU47(Pulchromyces)、OTU83(Phialemonium)HF 处理得到最大丰度。根据Pearson 相关性分析,除OTU47(Pulchromyces)为显著正相关外(Pearson correlation,p<0.05),OTU5、OTU7、OTU9、OTU43、OTU9、OTU83这些微生物类群的相对丰度均与菠萝心腐病发病率呈极显著正相关(p<0.01)。与其他处理相比,YJ 处理中OTU12(Bionectriaceae_unidentified)和 OTU23(Clonostachys)丰度较高,且与发病率呈正相关,但未达到显著水平。另外,与HF处理和YJ处理相比,生物有机 肥 处 理OTU1(Massariosphaeria)、OTU4(Dothideomycetes_unidentified)丰度相对更高,其中,OTU2(Trichocomaceae_unidentified)、OTU15(Talaromyces)、OTU20(Chaetothyriales_unidentified)则分别在KN、KC、LY 处理中取到最大丰度值,这些OTU 对应的真菌类群的相对丰度与发病率呈显著负相关,Massariosphaeria、Talaromyces 和Chaetothyriales_unidentified与发病率的相关性则达到极显著水平。
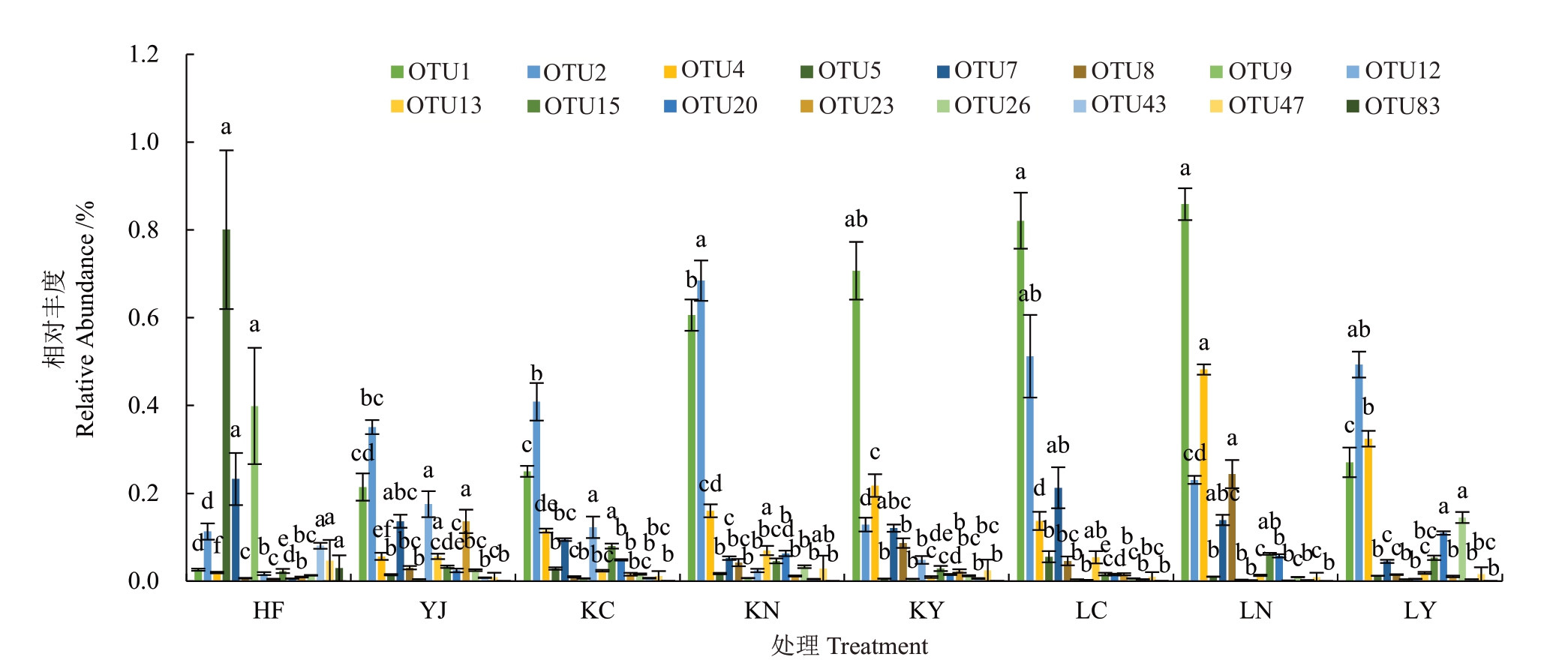
图8 特征OTUs 在不同处理中的相对丰度值
Fig.8 Dominant fungal OTUs with the relative abundance values in various samples
不同小写字母代表在p<0.05 差异显著。
Different lowercase letters indicate significant difference at p<0.05 by Tukey test.
表2 土壤中特征微生物与菠萝心腐病发病率之间的Pearson 相关性
Table 2 Pearson correlation coefficients between the soil dominant fungi and the disease incidence

注:*p<0.05 显著相关,**p<0.01 极显著相关。
Note:*p<0.05 mean significant correlation,**p<0.01 extremely significant correlation.
OTU ID 功能类型Gulid OTU7分类Taxon属Genus镰孢菌属Fusarium科Family Nectriaceae纲Class Sordariomycetes相关系数Correlation coefficient 0.601**OTU9 Chaetomiaceae Sordariomycetes动物病原-内生-地衣寄生-植物病原-土壤腐生-木材腐生真菌Animal Pathogen-Endophyte-Lichen Parasite-Plant Pathogen-Soil Saprotroph-Wood Saprotroph未定义真菌Undefined 0.714**OTU12未定义毛壳科菌属Chaetomiaceae_unidentified生赤壳科菌属Bionectriaceae_unidentified Bionectriaceae Sordariomycetes 0.058 OTU23 OTU43 Clonostachys木霉属Trichoderma Bionectriaceae Hypocreaceae Sordariomycetes Sordariomycetes 0.324 0.783**OTU83 OTU47 OTU5 OTU15 OTU2 Cephalothecaceae Incertae_sedis_16 Trichocomaceae Trichocomaceae Trichocomaceae Sordariomycetes Incertae_sedis_14 Eurotiomycetes Eurotiomycetes Eurotiomycetes藻类寄生-藓类寄生-真菌寄生-未定义腐生真菌Algal Parasite-Bryophyte Parasite-Fungal Parasite-Undefined Saprotroph植物病原真菌Plant Pathogen动物病原-内生-体表寄生-真菌寄生-植物病原-木材腐生真菌Animal Pathogen-Endophyte-Epiphyte-Fungal Parasite-Plant Pathogen-Wood Saprotroph未定义腐生真菌Undefined Saprotroph未定义真菌Undefined未定义真菌Undefined未定义腐生真菌Undefined Saprotroph未定义真菌Undefined 0.731**0.448*0.702**-0.547**-0.444*OTU13 OTU1 Trichocomaceae Incertae_sedis_13 Eurotiomycetes Dothideomycetes未定义真菌Undefined未定义腐生真菌Undefined Saprotroph-0.105-0.457**OTU20 Incertae_sedis_13 Dothideomycetes 未定义真菌Undefined -0.535**OTU4 Dothideomycetes 未定义真菌Undefined -0.447*OTU8 OTU26单胞瓶霉属Phialemonium Pulchromyces青霉菌属Penicillium踝节菌属Talaromyces未定义发菌科菌属Trichocomaceae_unidentified青霉菌属Penicillium黑团球壳腔菌属Massariosphaeria未定义刺盾炱目菌属Chaetothyriales_unidentified未定义座囊菌纲菌属Dothideomycetes_unidentified毛色二孢菌属Lasiodiplodia Davidiella Dothideomyce -tes_unidentified_1 Botryosphaeriaceae Davidiellaceae-0.113-0.233 Dothideomycetes Dothideomycetes植物病原真菌Plant Pathogen未定义腐生真菌Undefined Saprotroph
由表2 可看出,对不同处理的土壤关键真菌微生物按照其功能进行划分,主要包括未定义真菌、未定义腐生真菌、动物病原真菌和植物病原真菌。其中,与发病率呈极显著正相关的真菌功能类型分别为:动物病原-内生-体表寄生-真菌寄生-植物病原-木材腐生菌(Fusarium)、未定义腐生真菌(Phialemonium)、未定义真菌(Penicillium、Chaetomiaceae_unidentified)和动物病原-内生-地衣寄生-植物病原-土壤腐生-木材腐生真菌(Trichoderma),另外,Pulchromyces 的功能为未定义真菌类型,与发病率显著正相关。而与发病率呈极显著负相关的真菌功能类型分别为:未定义腐生真菌(Massariosphaeria、Talaromyces)和未定义真菌(Chaetothyriales_unidentified_1)。
3 讨 论
菠萝心腐病是一种危害极大的土传病害,土壤中微生物群落多样性降低、结构失衡可导致其大面积暴发。调节土壤微生物群落结构、减少土壤中的有害微生物是防控菠萝心腐病的重要措施。本研究表明,以生防菌和有机物料制成的生物有机肥能显著降低菠萝心腐病的发病率,增加有益微生物的比例,从而改变真菌群落结构和功能,使土壤真菌群落向健康稳定的方向转变。其中,以菜籽饼为有机载体、接种芽孢杆菌二次发酵而成的生物有机肥(KC)处理后的菠萝土壤发病率最低,生防效果最好。因此,接种生防菌的生物有机肥可成为防控菠萝心腐病的重要手段,对不同处理土壤真菌群落的α-多样性分析发现,相比于三元复合肥处理,除LN 处理外,有机肥处理和生物有机肥处理显著增加了土壤真菌丰富度和物种多样性。这一结果与Xiong等[23]对香蕉枯萎病的盆栽试验相似,可能是有机肥料的加入相比常规化肥更能激发土壤微生物和活性,从而增加土壤真菌群落丰富度和多样性[24]。此外,Shen等[25]研究结果显示,施用生物有机肥两年后,真菌的α-多样性会显著减少。因此,化学合成肥料和生物有机肥对土壤微生物的作用机制可能受到试验时间和条件的影响,需要对其进一步研究。本研究主坐标分析结果显示,生物有机肥施入土壤后,土壤真菌群落结构明显不同于三元复合肥处理和普通有机肥处理。三元复合肥处理与普通有机肥处理、生物有机肥处理的土壤真菌群落多样性有显著差异,而Zhang等[26]研究也指出无机肥料和有机肥料可直接导致不同的土壤微生物群落结构,为这一研究结果提供了有效依据。本研究将接种Streptomyces HL3 制成的生物有机肥施入土壤后,HL3 生物有机肥处理中,LN 处理的α-多样性达到最小,心腐病发病率却表现为LY<LC=LN,可能是生物有机肥载体材料的不同物质组成激发了土壤真菌群落组成发生特殊变化,这可能进一步影响土壤中微生物之间的相互作用,从而发挥出不同程度的拮抗能力,减少了心腐病发生的频率[27]。
真菌微生物中的座囊菌纲、散囊菌纲和粪壳菌纲在菠萝根际土壤微生物群落体系中占有很大比重。其中,粪壳菌纲几乎在各处理中均有存在,但三元复合肥居多,有机肥次之。这可能是由于两种施肥方式人为添加了大量的化学肥料和有机物质,原生微生物群落遭到破坏,新生的微生物群落结构稳定性差,营造了利于大量粪壳菌纲真菌繁殖的生存环境[28]。将不同处理土壤真菌群落的差异微生物与心腐病发病率综合分析,具有明显丰度差异的微生物类群与发病率的相关性各不相同,这些真菌可能通过复杂的相互作用网络影响菠萝植株发病。镰孢菌属是真菌病原菌属,会侵染植物,引起多种常见的真菌病害,其相对丰度与作物连作年限有关[29]。本研究中该属在发病率最高的三元复合肥处理中丰度最大,推测其存在可能会增加连作菠萝心腐病的发病率。已有研究表明毛壳菌科真菌具有广泛的拮抗活性和促生作用,能有效降解纤维素和有机质,对多种病原菌的生长均有一定的抑制作用[30],而本研究中未定义毛壳科菌属与心腐病发病率表现为极显著正相关关系。木霉属和单胞瓶霉属分别属于Hypocreaceae科、Cephalothecaceae科,均属于粪壳菌纲分类。研究报道[31]木霉属可产生生物活性物质使香蕉枯萎病的发病率降低,但在本研究中与之不同,可能是地理环境和作物差异的原因。
OTU5 和OTU13 均为青霉菌属,但与发病率关系不一致,可能是青霉菌属的不同菌株所致,有研究表明青霉菌属对减轻尖镰孢菌侵染危害芝麻植株有一定作用[32]。本研究结果显示,踝节菌属与心腐病发病率呈极显著负相关,在发病率最低的KC 处理中丰度最大,这说明其可能在一定程度上抑制菠萝心腐病的发生。有相关研究表明,踝节菌属菌株Q2对苦瓜枯萎病、烟草黑胫病、烟草根黑腐病和马铃薯茎基腐病等多种土传病害具有明显的预防效果[33],可在土壤中稳定定殖,并直接抑制土壤中尖镰孢菌种群的恢复趋势[34]。未定义刺盾炱目菌属、黑团球壳腔菌属、未定义座囊菌纲菌属隶属于座囊菌纲,未定义发菌科菌属隶属于散囊菌纲。Martínez-Arias[35]的研究中发现,接种从散囊菌纲和座囊菌纲中分离得到的内生菌株有利于抑制榆树内生真菌病害,减少40%叶片萎蔫,提高榆树的防御代谢机制。KC处理真菌群落的差异微生物踝节菌属,和未定义刺盾炱目菌属、黑团球壳腔菌属、未定义座囊菌纲菌,以及未定义发菌科菌属,有望成为菠萝生物菌剂的备选生防资源。
在生态学角度上,降低土壤中腐生真菌的相对丰度,使得土壤中共生真菌与腐生真菌的丰度比增大,这有利于土壤生态系统的恢复和作物生长[36-37]。类似地,本研究中关键真菌类群可被划分为不同的功能类型,与发病率呈显著正相关的真菌属较多为腐生真菌;而与发病率呈显著负相关的真菌功能类型属于未定义,需要进一步探索,在以后的研究中可根据这些未定义真菌的营养方式有针对性地生产生防菌剂[38]。
4 结 论
在菠萝发病土壤中施入具有生防功能的生物有机肥,可以改变由菠萝长期连作形成的土壤真菌群落区系,从而激发土壤生态系统中本土有益真菌的群落和功能多样性。以菜籽饼、有机肥为有机材料接种枯草芽孢杆菌(HL2)的生物有机肥施入土壤后,有效减少了连作菠萝心腐病发生,可能是枯草芽孢杆菌(HL2)与土壤中的有益真菌类群通过综合作用的方式重塑了土壤真菌群落,占据了更多有利的生态位,达到防控菠萝心腐病的目的。本研究中发现根际土壤中踝节菌属,和未定义刺盾炱目菌属、黑团球壳腔菌属、未定义座囊菌纲菌,以及未定义发菌科菌属真菌抑制了心腐病发生,但具体作用机制仍需要进一步探究。
[1] 杨潇湘,张蕾,黄小琴,伍文宪,周西全,杜磊,黎怀忠,刘勇.基于高通量测序分析大豆和油菜根际微生物群落结构的差异[J].应用生态学报,2019,30(7):2345-2351.YANG Xiaoxiang,ZHANG Lei,HUANG Xiaoqin,WU Wenxian,ZHOU Xiquan,DU Lei,LI Huaizhong,LIU Yong.Difference of the microbial community structure in the rhizosphere of soybean and oilseed rape based on high-throughput pyrosequencing analysis[J].Chinese Journal of Applied Ecology,2019,30(7):2345-2351.
[2] 杨永,张学军,李寐华,王登明,王广智,张永兵,伊鸿平.微生物肥料对设施长期连作哈密瓜根际土壤真菌群落结构的影响[J].应用与环境生物学报,2018,24(1):68-74.YANG Yong,ZHANG Xuejun,LI Meihua,WANG Dengming,WANG Guangzhi,ZHANG Yongbing,YI Hongping.Effects of microbiological fertilizer on rhizosphere soil fungus communities under long-term continuous cropping of protected Hami melon[J].Chinese Journal of Applied and Environmental Biology,2018,24(1):68-74.
[3] BAKKER P A H M,DOORNBOS R F,ZAMIOUDIS C,BERENDSEN R L,PIETERSE C M J.Induced systemic resistance and the rhizosphere microbiome[J].Plant Pathology Journal,2013,29(2):136-143.
[4] SANTHANAM R,LUU V T,WEINHOLD A,GOLDBERG J,OH Y,BALDWIN I T.Native root-associated bacteria rescue plant from a sudden-wilt disease that emerged during continuous cropping[J].Proceedings of the National Academy of Sciences,2015,112(36):E5013-E5020.
[5] RATTI M F,ASCUNCE M S,LANDIVAR J J,GOSS E M.Pineapple heart rot isolates from ecuador reveal a new genotype of phytophthora nicotianae[J].Plant Patholology,2018,67(8):1803-1813.
[6] DEVA-AZIZ N M,ALI R.Relationship of soil physicochemical properties and existence of Phytophthora sp.in pineapple plantations[J].Indonesian Journal of Science and Technology,2017,2(1):81-86.
[7] SHE S Y,NIU J J,ZHANG C,XIAO Y H,CHEN W,DAI L J,LIU X D,YIN H Q.Significant relationship between soil bacterial community structure and incidence of bacterial wilt disease under continuous cropping system[J].Archives of Microbiology,2017,199(2):267-275.
[8] 唐浩真,胡英宏,任泽广,杨姝钰,赵艳,王蓓蓓,张晓波,阮云泽.不同生物有机肥对连作菠萝生长及防控心腐病效果[J].微生物学通报,2021,48(11):4156-4166.TANG Haozhen,HU Yinghong,REN Zeguang,YANG Shuyu,ZHAO Yan,WANG Beibei,ZHANG Xiaobo,RUAN Yunze.Effect of different biological organic fertilizers on the growth of pineapple under continuous cropping and the heart rot[J].Microbiology,2021,48(11):4156-4166.
[9] OCULI J,BUA B,OCWA A.Reactions of pineapple cultivars to pineapple heart rot disease in central Uganda[J].Crop Protection,2020,135:105213.
[10] SIDIK S,SAPAK Z.Evaluation of selected chemical pesticides for controlling bacterial heart rot disease in pineapples variety MD2[J].Earth and Environmental Science,2021,757(1):12072.
[11] 罗建军,刘琼光,何衍彪,李志斌,魏楚丹.广东菠萝心腐病病原鉴定[J].广东农业科学,2012,39(13):90-92.LUO Jianjun,LIU Qiongguang,HE Yanbiao,LI Zhibin,WEI Chudan.Identification of the pathogen causing pineapple heartrot disease[J].Guangdong Agricultural Sciences,2012,39(13):90-92.
[12] 张开明,黎乙东,郑服丛,李锐.海南岛菠萝疫霉种及交配型的研究[J].热带作物学报,1992,13(2):89-92.ZHANG Kaiming,LI Yidong,ZHEN Fucong,LI Rui.Identification and mating type of phytophthora spp.from pineapple in Hainan[J].Chinese Journal of Tropical Crops,1992,13(2):89-92.
[13] 何衍彪,谢云巧,吴婧波,苏雨.土壤酸碱度、空气湿度对菠萝心腐病发生的影响[J].中国植保导刊,2020,40(8):14-18.HE Yanbiao,XIE Yunqiao,WU Jingbo,SU Yu.Influence of soil pH and air humidity on the occurrence of Phytophthora nicotianae[J].China Plant Protection,2020,40(8):14-18.
[14] ESPINOSA- RODRÍGUEZ C J,NIETO- ANGEL D,LEÓNGARCÍA DE A C D,VILLEGAS-MONTER Á,AGUILARPÉREZ L A,AYALA-ESCOBAR V.Etiology of the heart rot of pineapple [Ananas comosus (L.) Merril] MD2 cultivar in Isla,Veracruz,México[J].Revista Mexicana de Fitopatología,2015,33(1):104-115.
[15] 沈会芳,林壁润,孙光明,蒲小明,陆新华,张景欣,何衍彪,詹儒林.海南菠萝心腐病菌烟草疫霉的生物学特性研究[J].广东农业科学,2014,41(2):92-94.SHEN Huifang,LIN Birun,SUN Guangming,PU Xiaoming,LU Xinhua,ZHANG Jingxin,HE Yanbiao,ZHAN Rulin.Research on the biological characteristics of Phytophthora nicotianae,a pathogen of heart rot of pineapple in Hainan[J].Guangdong Agricultural Sciences,2014,41(2):92-94.
[16] CHEN J,GONG J L,XU M G.Implications of continuous and rotational cropping practices on soil bacterial communities in pineapple cultivation[J].European Journal of Soil Biology,2020,97:103172.
[17] 杨祁云,沈会芳,刘平平,孙大元,蒲小明,张景欣,林壁润.我国菠萝使用农药登记现状及对策[J].中国南方果树,2021,50(3):172-179.YANG Qiyun,SHEN Huifang,LIU Pingping,SUN Dayuan,PU Xiaoming,ZHANG Jingxin,LIN Birun.Current situation and countermeasures of Pineapple pesticide registration in China[J].South China Fruits,2021,50(3):172-179.
[18] 谷会,贾志伟,侯晓婉,张鲁斌.氯化钙处理对菠萝黑腐病的防控效果及机制分析[J].热带作物学报,2019,40(12):2481-2488.GU Hui,JIA Zhiwei,HOU Xiaowan,ZHANG Lubin.Effects and mechanism of calcium chloride treatment on reducing pineapple black rot[J].Chinese Journal of Tropical Crops,2019,40(12):2481-2488.
[19] 曲成闯,陈效民,张志龙,王诺,闾婧妍,张俊,黄春燕.施用生物有机肥对黄瓜连作土壤有机碳库和酶活性的持续影响[J].应用生态学报,2019,30(9):3147-3154.QU Chengchuang,CHEN Xiaomin,ZHANG Zhilong,WANG Nuo,LÜ Jingyan,ZHANG Jun,HUANG Chunyan.Long-term effects of bio-organic fertilizer application on soil organic carbon pool and enzyme activity of cucumber continuous cropping[J].Chinese Journal of Applied Ecology,2019,30(9):3147-3154.
[20] SHARMA K,AKHTAR N,UPADHYAY A K,MANNAN M A.Efficacy of Trichoderma harzianum,a biocontrol agent for controlling opportunistic fungal pathogens[J].Journal of Pharmaceutical Sciences and Research,2020,12(2):282-285.
[21] 刘珊珊,夏萌,胡夏茹,王云舟,李春雨,陶成圆,沈宗专,张楠,李荣,沈其荣.石灰碳铵熏蒸联合生物有机肥对香蕉枯萎病和细菌群落的影响[J].应用生态学报,2020,31(12):4189-4196.LIU Shanshan,XIA Meng,HU Xiaru,WANG Yunzhou,LI Chunyu,TAO Chengyuan,SHEN Zongzhuan,ZHANG Nan,LI Rong,SHEN Qirong.Effects of lime and ammonium carbonate fumigation coupled with bio-organic fertilizer application on banana fusarium wilt and bacterial community[J].Chinese Journal of Applied Ecology,2020,31(12):4189-4196.
[22] 胡英宏,任泽广,杨姝钰,赵艳,王蓓蓓,张晓波,阮云泽.生物有机肥对菠萝心腐病发生和土壤细菌群落结构的影响[J].应用与环境生物学报,2022,28(4):1-10.HU Yinghong,REN Zeguang,YANG Shuyu,ZHAO Yan,WANG Beibei,ZHANG Xiaobo,RUAN Yunze.Effects of bioorganic fertilizers on pineapple heart rot and bacterial community structure[J].Chinese Journal of Applied and Environmental Biology,2022,28(4):1-10.
[23] XIONG W,GUO S,JOUSSET A,ZHAO Q Y,WU H S,LI R,KOWALCHUK G A,SHEN Q R.Bio-fertilizer application induces soil suppressiveness against Fusarium wilt disease by reshaping the soil microbiome[J].Soil Biology and Biochemistry,2017,114:238-247.
[24] JANNOURA R,BRUNS C,JOERGENSEN R G.Organic fertilizer effects on pea yield,nutrient uptake,microbial root colonization and soil microbial biomass indices in organic farming systems[J].European Journal of Agronomy,2013,49:32-41.
[25] SHEN Z Z,RUAN Y Z,CHAO X,ZHANG J,LI R,SHEN Q R.Rhizosphere microbial community manipulated by 2 years of consecutive biofertilizer application associated with banana Fusarium wilt disease suppression[J].Biology and Fertility of Soils,2015,51(5):553-562.
[26] ZHANG Q C,IMRAN H S,XU D T,WANG G H,LIN X Y,GHULAM J,NAZIM H,ARSHAD N C.Chemical fertilizer and organic manure inputs in soil exhibit a vice versa pattern of microbial community structure[J].Applied Soil Ecology,2012,57:1-8.
[27] MOHAMMAD A,ABDUL M.Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes:A review[J].Bioresource Technology,2000,74(1):35-47.
[28] 卢宝慧,高成林,赵玥,刘晨阳,杨鹤,唐玲玲,许永华.运用高通量测序技术分析人参不同栽培模式根际土壤微生物多样性[J].东北林业大学学报,2021,49(3):113-119.LU Baohui,GAO Chenglin,ZHAO Yue,LIU Chenyang,YANG He,TANG Lingling,XU Yonghua.Panax ginseng rhizosphere microorganism diversity in different cultivation modes by highthroughput sequencing technology[J].Journal of Northeast Forestry University,2021,49(3):113-119.
[29] 聂园军,李瑞珍,赵佳,薄晓峰.西瓜连作对根际微生物群落的影响[J].中国瓜菜,2019,32(1):6-11.NIE Yuanjun,LI Ruizhen,ZHAO Jia,BO Xiaofeng.Relationship between watermelon’s rhizosphere microbial communities and continuous cropping obstacle[J].China Cucurbits and Vegetables,2019,32(1):6-11.
[30] 李金花,高克祥,万利,曹国玉,焦方园,王延平,田叶韩,赵炳杰,李传荣.微生物菌剂对楸树幼苗生长及根际土细菌群落结构的影响[J].生态学报,2020,40(21):7588-7601.LI Jinhua,GAO Kexiang,WAN Li,CAO Guoyu,JIAO Fangyuan,WANG Yanping,TIAN Yehan,ZHAO Bingjie,LI Chuanrong.Effects of microbial agent on the growth of Catalpa bungei seedlings and the diversity of bacterial community in rhizosphere soil[J].Acta Ecologica Sinica,2020,40(21):7588-7601.
[31] THANGAVELU R,GOPI M.Combined application of native Trichoderma isolates possessing multiple functions for the control of Fusarium wilt disease in banana cv.Grand Naine[J].Biocontrol Science and Technology,2015,25(10):1147-1164.
[32] RADHAKRISHNAN R,PAE S B,SHIM K B,BAEK I Y.Penicillium sp.mitigates Fusarium-induced biotic stress in sesame plants[J].Biotechnology Letters,2013,35(7):1073-1078.
[33] 田叶韩,彭海莹,王德浩,李晓芳,何邦令,高克祥.产紫篮状菌的生防潜力及其对土壤微生物群落的调控[J].应用生态学报,2020,31(10):3255-3266.TIAN Yehan,PENG Haiying,WANG Dehao,LI Xiaofang,HE Bangling,GAO Kexiang.Biocontrol potential of Talaromyces purpurogenus and its regulation on soil microbial community[J].Chinese Journal of Applied Ecology,2020,31(10):3255-3266.
[34] 赵洋,田叶韩,高克祥,付学松.转录组分析产紫篮状菌Q2 菌株抑制尖镰孢菌生长的潜在机制[J].山东农业科学,2021,53(8):94-101.ZHAO Yang,TIAN Yehan,GAO Kexiang,FU Xuesong.Transcriptome analysis of potential inhibitory mechanisms of Talaromyces purpureogenus strain Q2 against Fusarium oxysporum[J].Shandong Agricultural Sciences,2021,53(8):94-101.
[35] MARTÍNEZ-ARIAS C,SOBRINO-PLATA J,ORMEÑO-MONCALVILLO S,GIL L,RODRÍGUEZ- CALCERRADA J,MARTÍN J A.Endophyte inoculation enhances Ulmus minor resistance to Dutch elm disease[J].Fungal Ecology,2021,50:101024.
[36] SCHMIDT R,MITCHELL J,SCOW K.Cover cropping and notill increase diversity and symbiotroph:Saprotroph ratios of soil fungal communities[J].Soil Biology and Biochemistry,2019,129:99-109.
[37] 刘珊珊,胡夏茹,王云舟,李春雨,陶成圆,李荣,沈其荣.石灰碳铵熏蒸联合生物有机肥施用对香蕉土壤真菌群落的影响[J].应用与环境生物学报,2021,27(5):1326-1333.LIU Shanshan,HU Xiaru,WANG Yunzhou,LI Chunyu,TAO Chengyuan,LI Rong,SHEN Qirong.Effects of lime and ammonium carbonate fumigation coupled with bio-organic fertilizer application on banana fungal community[J].Chinese Journal of Applied and Environmental Biology,2021,27(5):1326-1333.
[38] KIM S H,VUJANOVIC V.Relationship between mycoparasites lifestyles and biocontrol behaviors against Fusarium spp.and mycotoxins production[J].Applied Microbiology and Biotechnology,2016,100(12):5257-5272.