由解淀粉欧文氏杆菌(Erwinia amylovora)引起的梨火疫病是严重危害梨、苹果、山楂、海棠等仁果类果树生产的毁灭性细菌病害,被我国列为一类农作物病害和农业植物检疫性有害生物[1]。梨火疫病菌传播极为迅速,流行严重时在短短几周内就可从发病的花、嫩梢、叶片和幼果扩展到主枝和主干上,直至根部,短期内整株死亡、果园被毁,造成严重的经济损失[2]。该病害1780 年首次发生于美国纽约州,现已扩散传播到近60个国家和地区[3-5]。我国过去一直没有梨火疫病发生的报道,直至2016、2017年相继在新疆伊犁州、巴州首次发生并迅速在全疆蔓延,目前已传至甘肃省的张掖和武威地区,对新疆乃至全国林果业产生了巨大威胁[6]。
目前梨火疫病的防治主要有加强检疫、化学防治、果园清园、修剪和铲除发病植株、生物防治、抗病品种选育等措施[7]。但该病害防治难度大,至今仍未得到很好的控制。使用化学药剂是目前最为普遍的防治手段。用于梨火疫病防治的化学药剂主要有铜制剂、抗生素、氨基甲酸酯等,其中在果树开花期喷施农用链霉素保护花器,有效防止病原细菌的侵染和传播扩散是防效最显著、最经济的措施,在病害防治中具有重要作用[8]。从20世纪60年代末起,北美、新西兰和欧洲等许多国家和地区即开始在火疫病防治中施用农用链霉素,但它的频繁、大量和持续使用导致了病原菌抗药性菌系的产生。70 年代初初次在美国加州出现抗性菌系,随后在多地陆续发现[9-10]。病原菌抗药性种群的不断增长、抗性水平提升大大降低了农用链霉素的防效,使得病害防治更加困难[11]。铜制剂及铜制剂和其他化学药剂的混合物对梨火疫病也具有一定的防效,但对发育中的果实和叶片会产生药害[12]。总之,当前缺乏针对梨火疫病菌药效显著、无系统性药害、对环境安全的商业化杀菌剂来替代农用链霉素等药剂,急需筛选和研发新型的高效防病药剂[13]。
梨火疫病是我国梨产区近年突发的重要的国际检疫性林果病害。为有效控制病害的传播蔓延,笔者所在的研究团队通过室内毒力测定、离体组织、温室幼苗和果园试验,从多种化学药剂中筛选出了噻霉酮等防效较好的药剂应急用于生产中。噻霉酮为国内研发的有机杂环类新型广谱杀菌剂,能防治多种真菌和细菌性病害,应用于梨火疫病的防治尚未见报道。笔者在本研究中以从新疆不同地区、不同年份和寄主植物上分离获得的梨火疫病菌菌株为材料,测定病原菌对噻霉酮的敏感性,建立敏感基线,比较不同菌株的抗性差异,对于田间药剂的合理使用、监测病原菌抗药性的发生发展与治理,以及病害的安全、高效防控具有重要的科学指导意义和应用价值。
1 材料和方法
1.1 供试材料
1.1.1 供试药剂 84%噻霉酮原药,陕西西大华特制药厂。噻霉酮原药用无水乙醇溶解配制成8000µg·mL-1的母液,过滤除菌后贮存于4 ℃备用。使用时用无菌水依次将母液稀释成1600、800、400、300、200和100µg·mL-1的药液。
1.1.2 供试梨火疫病菌菌株 供试菌株为本实验室分离、鉴定并保存的梨火疫病菌(Erwinia amylovora)菌株。
1.1.3 培养基 营养琼脂培养基(NA):牛肉浸膏(ρ,后同)3.0 g·L-1,蛋白胨10.0 g·L-1,氯化钠5.0 g·L-1,蔗糖5.0 g·L-1,琼脂粉18.0 g·L-1,pH 7.2。
营养肉汤培养基(NB):牛肉浸膏3.0 g·L-1,蛋白胨10.0 g·L-1,氯化钠5.0 g·L-1,蔗糖5.0 g·L-1,pH 7.2。
1.2 供试梨火疫病菌菌株的分离、鉴定和保存
2016—2020 年从新疆巴州(库尔勒市、轮台县)、伊犁州(霍尔果斯市、巩留县)、昌吉州(昌吉市、呼图壁县)、哈密地区、阿克苏地区、喀什地区、和田地区、塔城地区、阿勒泰地区和乌鲁木齐市(三坪农场、五一农场)10个地州(区/市)的苹果、香梨、山楂、杜梨和榅桲树上采集的具有梨火疫病典型症状的花、枝条、叶片和果实疑似新鲜病样,带回实验室。通过显微镜进行细菌溢检查,有明显细菌溢产生的病样,将病健交界组织剪成5 mm×5 mm的小块置于小烧杯中,经表面灭菌后在NA 培养基上用稀释平板法分离,挑取单菌落反复划线、纯化后,获得菌株接种于斜面培养基,4 ℃低温保存。将分离获得细菌菌株通过烟草过敏反应测定致病力,依据科赫氏法则回接香梨枝条(或幼果)确定为致病菌株。提取分离致病菌株的总DNA,采用梨火疫病菌特异性引物P29TF/P29TR 和P29A/P29B 进行PCR 扩增、测序和系统发育分析[14],并结合形态及生理生化特性分析鉴定为梨火疫病菌(E.amylovora),在15%甘油管中-40 ℃冰箱保存。
1.3 噻霉酮对梨火疫病菌的毒力测定
采用滤纸片-抑菌圈法测定[15]。将供试病原菌菌株在NA培养基上活化培养,挑取单菌落至NB培养液中,28 ℃、160 r·min-1摇床震荡培养12~14 h 至培养液OD600值为0.8~1.0,无菌水稀释成108 cfu·mL-1菌悬液。吸取100µL的菌悬液,用灭菌的玻璃涂布棒均匀涂布在NA 培养基平板上。待平板晾干后,将灭菌滤纸片(直径6 mm)等距离放置在含菌平板培养基上,每皿3 片。分别吸取不同浓度梯度的噻霉酮药剂稀释液6 μL滴加于滤纸片上,以在滤纸片上加等量无菌水的含菌平板作为对照,每个药剂浓度和对照组各重复3 皿。将平板置于28 ℃培养箱中培养36 h后,采用游标卡尺十字交叉法测量抑菌圈直径,计算抑菌率。
相对抑菌率(%)=(处理抑菌圈直径-滤纸片直径)/处理抑菌圈直径×100。
1.4 噻霉酮对梨火疫病菌的最低抑制浓度(MIC)的测定
制备含噻霉酮不同浓度梯度(0.5、1.0、1.5、2.0、2.5、3.0、4.0 和5.0µg·mL-1培养基)的NA 培养基含药平板,取2 μL梨火疫病菌菌悬液(108 cfu·mL-1)用微量移液器枪头分别滴加在药剂平板上,待菌液被培养基吸干后,置于28 ℃下培养2~3 d 后观察菌落的生长情况,以不加药剂的培养基平板为对照。使梨火疫病菌完全不生长的最低药剂浓度即为最低抑制浓度(MIC值)。
1.5 梨火疫病菌对噻霉酮的敏感基线的建立
使用DPS v9.01 软件进行数据处理。以药剂有效成分浓度对数值为自变量(x),相对抑菌率为因变量(y),建立药剂对各供试菌株的毒力回归方程,计算决定系数R2和抑制中浓度(EC50)。对各菌株EC50值进行正态分布检验(Kolmogorov-Smirnov),依据野生型敏感病原菌群体对药剂的敏感性为正态分布的原理,在SPSS 22.0数据处理系统软件中以EC50等分值为横坐标,菌株分布频率为纵坐标,绘制敏感性频率分布图,确定敏感性基线。
1.6 梨火疫病菌对噻霉酮的抗药性水平
参照FRAC制定的植物病原菌对杀菌剂敏感性的划分方法,依据噻霉酮药剂对梨火疫病菌的毒力测定结果,计算供试菌株的抗性水平。抗性水平(Rf)=供试地方菌株EC50/敏感性基线值,Rf<5 为敏感菌株;5≤Rf<10 为低抗菌株;10≤Rf<40 为中抗菌株;Rf≥40为高抗菌株[16]。
2 结果与分析
2.1 梨火疫病菌的分离
从新疆10个地州(区/市)的苹果、香梨、山楂、杜梨和榅桲树上采集的具有梨火疫病典型症状的花、枝条、叶片和果实病样中共分离获得E. amylovora菌株87个,选取具有代表性的50个分离菌株作为供试菌株,详见表1。
表1 供试E.amylovora 菌株及来源
Table 1 E.amylovora strains tested and their source

菌株编号Strain code Ea01寄主Hosts苹果Malus pumila分离年份Year isolated 2016菌株编号Strain code Y101来源Source of strain巴州轮台县Luntai County,Bazhou寄主Hosts香梨P.sinkiangensis分离年份Year isolated 2019 Ea15杜梨Pyrus betulaefolia 2016 Y99阿克苏地区Aksu area榅桲Cydonia oblonga 2019 Ea17 2016 Y120 2019 Ea18 2016 Y125 2019 Ea20 2017 Y126 2019 Ea21 2017 Y112 2019 Ea23 2017 Y81 2019 Ea32香梨P.sinkiangensis香梨P.sinkiangensis香梨P.sinkiangensis苹果M.pumila香梨P.sinkiangensis苹果M.pumila 2017 X12巴州轮台县Luntai County,Bazhou巴州轮台县Luntai County,Bazhou巴州轮台县Luntai County,Bazhou乌鲁木齐市Urumqi巴州库尔勒市Korla City,Bazhou巴州库尔勒市Korla City,Bazhou榅桲C.oblonga苹果M.pumila山楂Crataegus pinnatifida山楂C.pinnatifida香梨P.sinkiangensis香梨P.sinkiangensis 2020 Ea22 2017 X17 2020 H17 2018 X4 2020 Y6 2018 X5 2020 GX 2018 X7 2020 L苹果M.pumila苹果M.pumila香梨P.sinkiangensis山楂C.pinnatifida山楂C.pinnatifida 2018 X10巴州库尔勒市Korla City,Bazhou巴州库尔勒市Korla City,Bazhou巴州库尔勒市Korla City,Bazhou巴州库尔勒市Korla City,Bazhou巴州库尔勒市Korla City,Bazhou香梨P.sinkiangensis香梨P.sinkiangensis香梨P.sinkiangensis香梨P.sinkiangensis香梨P.sinkiangensis 2020 Z1 2018 X11 2020 H14 2018 X13 2020 H13 2018 X15 2020 J 2018 X25 2020 H10 2018 X26 2020 Z4山楂C.pinnatifida山楂C.pinnatifida榅桲C.oblonga山楂C.pinnatifida杜梨P.betulaefolia山楂C.pinnatifida 2018 X30巴州库尔勒市Korla City,Bazhou巴州库尔勒市Korla City,Bazhou巴州库尔勒市Korla City,Bazhou巴州轮台县Luntai County,Bazhou巴州轮台县Luntai County,Bazhou巴州轮台县Luntai County,Bazhou香梨P.sinkiangensis香梨P.sinkiangensis香梨P.sinkiangensis苹果M.pumila香梨P.sinkiangensis苹果M.pumila 2020 Y114 2019 X31 2020 Y107 2019 X32 2020 Y94 2019 X27 2020 Y91 2019 X34 2020 Y83 2019 X19 2020 Y109来源Source of strain伊犁州霍尔果斯市Horgos City,Ili prefecture伊犁州巩留县Gongliu County,Yili prefecture巴州库尔勒市Korla City,Bazhou哈密地区Hami area阿克苏地区Aksu area阿克苏地区Aksu area巴州库尔勒市Korla City,Bazhou昌吉州呼图壁县Hutubi County,Changji Prefecture阿克苏地区Aksu area伊犁州Yili Prefecture巴州库尔勒市Korla City,Bazhou阿勒泰市Altay City昌吉州昌吉市Changji City,Changji Prefecture伊犁州Yili Prefecture巴州库尔勒市Korla City,Bazhou和田地区Hotan area塔城地区Tacheng area阿克苏阿瓦提县Awati County,Aksu area昌吉州呼图壁县Hutubi County,Changji Prefecture阿克苏地区Aksu area乌鲁木齐三坪农场Sanping farm,Urumqi喀什市Kashgar City喀什市Kashgar City库尔勒仁和农场Renhe farm,Korla乌鲁木齐五一农场Wuyi farm,Urumqi香梨P.sinkiangensis苹果M.pumila香梨P.sinkiangensis香梨P.sinkiangensis香梨P.sinkiangensis山楂C.pinnatifida 2019 X16巴州轮台县Luntai County,Bazhou巴州轮台县Luntai County,Bazhou巴州库尔勒市Korla City,Bazhou巴州库尔勒市Korla City,Bazhou巴州库尔勒市Korla City,Bazhou巴州库尔勒市Korla City,Bazhou苹果M.pumila山楂C.pinnatifida杜梨P.betulaefolia香梨P.sinkiangensis香梨P.sinkiangensis香梨P.sinkiangensis 2020
2.2 噻霉酮对梨火疫病菌的毒力测定结果
噻霉酮对50 个供试梨火疫病菌分离菌株的室内毒力测定结果表明,EC50 值范围为12.40~813.13µg·mL-1,均值为304.61±133.45µg·mL-1。不同菌株之间的EC50存在明显差异,其中EC50值在100~300µg·mL-1之间的菌株有32个,占供试菌株的64%;EC50值>300µg·mL-1的菌株有17个,占供试菌株的34%;EC50 值<100 µg·mL-1 的菌株只有1 个。Y112(来自乌鲁木齐的山楂分离物)为最敏感的菌株(EC50=12.40µg·mL-1),Y125 菌株(来自轮台的苹果分离物)的敏感性最低,EC50(813.31 µg·mL-1)是最敏感菌株的65.59倍(表2)。
表2 噻霉酮对梨火疫病菌的室内毒力测定结果
Table 2 Indoor virulence test results of benziothiazolinone against E.amylovora strains

菌株编号Strain code Y112 X26 Y6 H10 X15 X25 X30 Ea01 Z1 Y114 X12 Ea20 Y107 Ea22 Ea21 H14 X11 X4 X17 H17 Y83 GX Y126 Z4 Ea15毒力回归方程Regression equation y=2.348 0 x+2.425 8 y=3.412 7 x+0.771 9 y=3.124 8 x+0.876 4 y=3.177 7 x+0.847 5 y=2.658 2 x+1.049 9 y=3.188 8 x+0.811 0 y=3.138 5 x+0.831 1 y=2.862 8 x+0.943 2 y=3.087 0 x+0.828 7 y=2.746 7 x+0.975 1 y=3.070 4 x+0.834 6 y=2.845 8 x+0.921 7 y=3.043 7 x+0.835 6 y=2.956 0 x+0.870 9 y=2.602 5 x+1.015 8 y=2.664 6 x+0.987 7 y=3.296 3 x+0.719 5 y=2.500 2 x+1.053 9 y=2.842 6 x+0.899 2 y=2.442 6 x+1.064 1 y=2.717 7 x+0.941 1 y=2.716 7x+0.937 6 y=2.289 8 x+1.111 9 y=2.354 4 x+1.082 8 y=2.710 0 x+0.932 4抑制中浓度EC50(95%置信区间)Half effective concentration(95%confidence interval)/(µg·mL-1)12.40(11.84~12.97)113.82(70.86~182.86)137.92(96.59~196.93)141.32(95.82~208.41)170.03(133.83~216.03)171.11(146.93~199.27)173.69(129.22~233.47)184.46(137.03~248.28)203.46(163.07~25.48)204.51(155.92~270.02)205.19(184.22~261.34)217.30(182.77~258.36)219.42(184.22~261.34)222.31(187.73~263.27)229.19(192.46~272.93)231.44(188.06~284.83)233.20(193.86~280.54)235.48(174.45~317.86)250.82(210.44~298.95)253.13(197.06~325.15)266.17(234.98~301.58)272.40(242.49~305.99)273.75(237.31~315.79)277.50(217.33~354.33)285.77(258.10~316.40)菌株编号Strain code Ea32 Y109 J Y91 X7 Ea23 Ea17 X19 Y120 X5 X31 Ea18 Y101 L Y99 X10 X32 Y81 X27 H13 X16 Y94 X34 X13 Y125毒力回归方程Regression equation y=2.336 2 x+1.084 3 y=2.564 7 x+0.990 4 y=2.155 2 x+1.156 5 y=2.393 6 x+1.056 9 y=2.741 4 x+0.915 8 y=2.592 0 x+0.975 9 y=2.233 6 x+1.120 4 y=2.136 2 x+1.159 0 y=2.182 5 x+1.124 4 y=2.289 9 x+1.077 9 y=1.613 4 x+1.318 5 y=1.859 6 x+1.219 6 y=1.929 4 x+1.176 6 y=2.158 4 x+1.087 6 y=2.095 1 x+1.110 1 y=1.504 8 x+1.335 7 y=1.860 8 x+1.190 6 y=1.001 6 x+1.515 4 y=1.986 9 x+1.140 0 y=2.347 4 x+0.996 0 y=0.785 2 x+1.576 2 y=2.514 6 x+0.921 7 y=1.224 3 x+1.389 1 y=1.236 4 x+1.367 2 y=0.993 9 x+1.376 5抑制中浓度EC50(95%置信区间)Half effective concentration(95%confidence interval)/(µg·mL-1)286.21(233.42~350.93)287.73(231.95~356.94)288.23(252.66~328.81)292.45(266.16~321.33)292.48(257.73~331.73)293.48(260.42~330.73)294.51(238.79~363.23)295.71(238.42~366.76)320.50(248.59~413.20)326.80(279.79~381.71)370.30(331.16~414.07)375.85(337.90~418.06)407.11(302.49~547.70)410.05(363.14~463.03)413.70(367.62~465.56)413.81(380.14~450.46)433.14(403.98~464.41)435.09(294.48~642.82)439.53(405.45~476.47)460.51(356.64~594.62)472.14(382.03~583.49)497.27(377.70~654.71)522.61(450.89~605.73)565.79(475.50~673.21)813.31(590.53~1120.15)
2.3 梨火疫病菌对噻霉酮的敏感性基线
检测结果显示,供试的50个梨火疫病菌菌株的EC50值呈现连续性变化,通过正态分布检验,Kolmogorov-Smimov Z=1.318,渐进连续性p=0.062(>0.05),符合正态分布。在敏感性范围内,绘制梨火疫病菌株对噻霉酮敏感性分布的频率图(图1)。由图1 可见,梨火疫病菌株对噻霉酮敏感性分布频率呈单峰曲线,EC50值为表现连续的正态分布,故以EC50平均值(304.61±133.45)µg·mL-1作为新疆梨火疫病菌对噻霉酮的敏感基线。
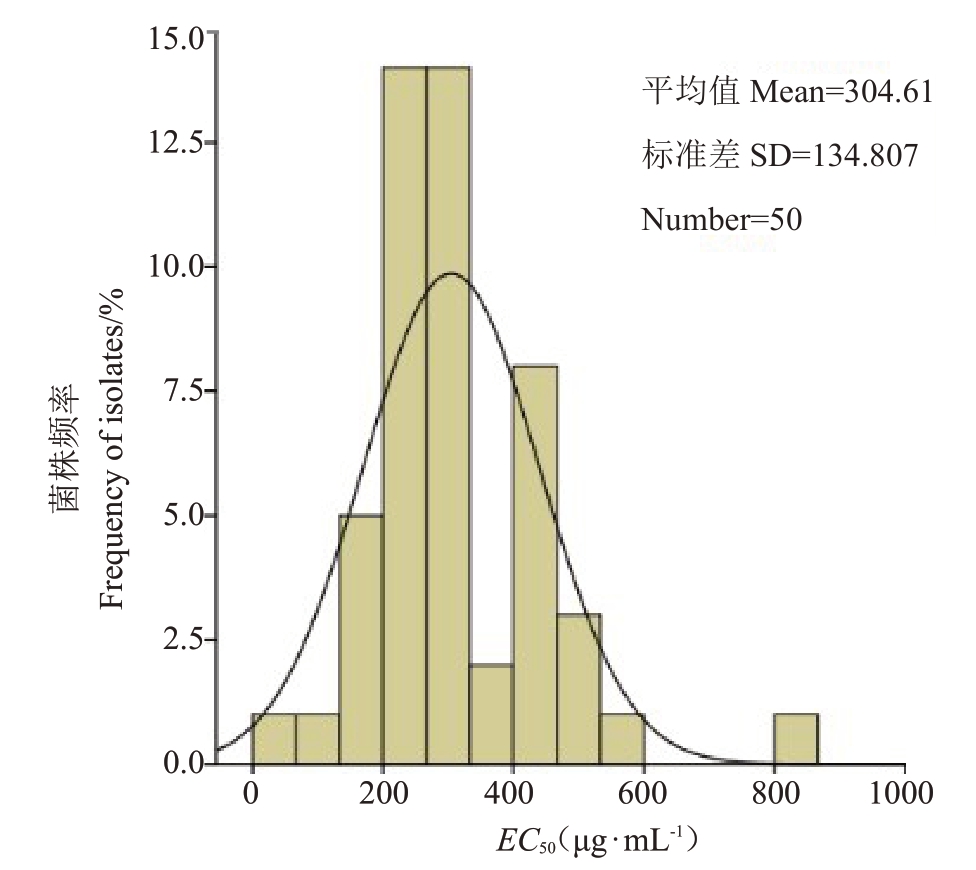
图1 梨火疫病菌对噻霉酮的敏感性频率分布
Fig.1 The frequency distribution of sensitivity of E.amylovora strains to benziothiazolinone in Xinjiang
2.4 不同地理来源、不同寄主和年份的梨火疫病菌分离菌株对噻霉酮的敏感性
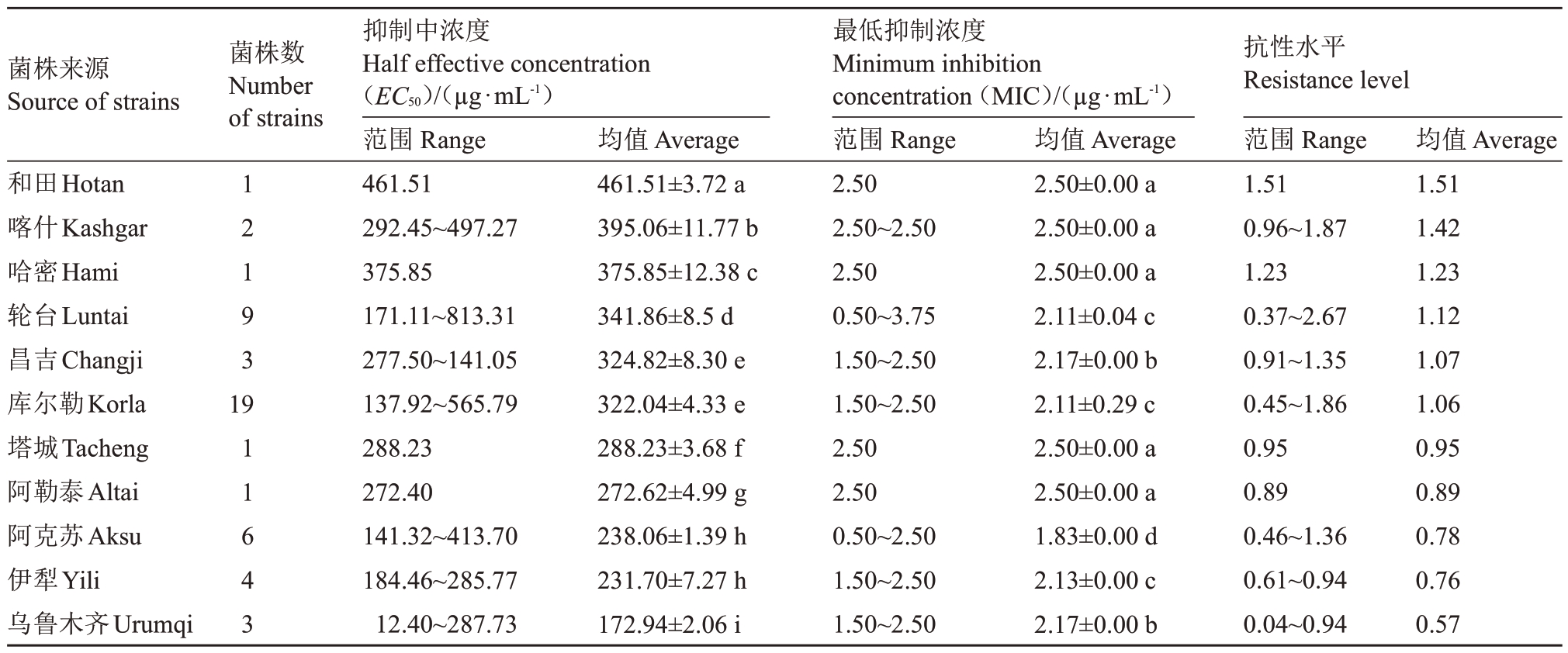
比较新疆不同地州(区/市)来源的梨火疫病菌分离菌株对噻霉酮的的敏感性,不同地理来源菌株的EC50有较大差异(表3)。和田地区的菌株对噻霉酮的敏感性最低,EC50值为461.51µg·mL-1,MIC 为2.50µg·mL-1,抗性水平为1.51;其次是喀什、哈密、轮台和昌吉的菌株,EC50平均值在324.82~395.06µg·mL-1之间,MIC平均值为2.11~2.50µg·mL-1,抗性水平为1.07~1.42;再次是库尔勒、塔城、阿勒泰、阿克苏和伊犁的菌株,EC50平均值在231.70~322.04 µg·mL-1之间,MIC平均值在1.83~2.50µg·mL-1之间,平均抗性水平为0.76~1.06;乌鲁木齐市的菌株对噻霉酮的敏感性最高,EC50均值最低,为172.94µg·mL-1,MIC平均值为2.17µg·mL-1,抗性水平为0.57。敏感性最低的和田和最高的乌鲁木齐二地菌株的EC50值相差2.34倍,抗性水平相差2.67倍。同一地区的菌株的敏感性也存在较大差异,如轮台县的菌株EC50和MIC值差异最大,EC50最低值(171.11µg·mL-1)和最高值(609.12 µg·mL-1)二者相差3.56 倍,MIC 最低值(0.50 µg·mL-1)和最高值(3.75µg·mL-1)相差7.5倍,最低抗性水平(0.37)和最高抗性水平(2.67)相差7.22倍。
表3 不同地理来源的梨火疫病菌对噻霉酮的EC50值比较
Table 3 Comparison of EC50values of E.amylovora strains isolated to benziothiazolinone from different areas
注:同列不同小写字母表示不同菌株差异在p<0.05 水平显著,下同。
Note:Different lowercase letters in same column indicate significant differences between different strains at p<0.05.The same below.
菌株来源Source of strains菌株数Number of strains和田Hotan喀什Kashgar哈密Hami轮台Luntai昌吉Changji库尔勒Korla塔城Tacheng阿勒泰Altai阿克苏Aksu伊犁Yili乌鲁木齐Urumqi抑制中浓度Half effective concentration(EC50)/(µg·mL-1)范围Range 461.51 292.45~497.27 375.85 171.11~813.31 277.50~141.05 137.92~565.79 288.23 272.40 141.32~413.70 184.46~285.77 12.40~287.73最低抑制浓度Minimum inhibition concentration(MIC)/(µg·mL-1)范围Range 2.50 2.50~2.50 2.50 0.50~3.75 1.50~2.50 1.50~2.50 2.50 2.50 0.50~2.50 1.50~2.50 1.50~2.50抗性水平Resistance level范围Range 1.51 0.96~1.87 1.23 0.37~2.67 0.91~1.35 0.45~1.86 0.95 0.89 0.46~1.36 0.61~0.94 0.04~0.94均值Average 1.51 1.42 1.23 1.12 1.07 1.06 0.95 0.89 0.78 0.76 0.57 12193 19 11643均值Average 461.51±3.72 a 395.06±11.77 b 375.85±12.38 c 341.86±8.5 d 324.82±8.30 e 322.04±4.33 e 288.23±3.68 f 272.62±4.99 g 238.06±1.39 h 231.70±7.27 h 172.94±2.06 i均值Average 2.50±0.00 a 2.50±0.00 a 2.50±0.00 a 2.11±0.04 c 2.17±0.00 b 2.11±0.29 c 2.50±0.00 a 2.50±0.00 a 1.83±0.00 d 2.13±0.00 c 2.17±0.00 b
从不同寄主上分离的梨火疫病菌株对噻霉酮的敏感性具有一定差异(表4),榅桲和香梨上分离菌株的敏感性最低,EC50分别为398.24µg·mL-1和314.84µg·mL-1,MIC 分别为1.56µg·mL-1和2.18µg·mL-1,其次是苹果、杜梨和山楂上的分离菌株,其敏感性差异不大。2016—2020 年分离的梨火疫病菌株,EC50和抗性倍数都随年份增加而呈逐渐增大的趋势(表5)。
表4 不同寄主分离的梨火疫病菌对噻霉酮的EC50值比较
Table 4 Comparison of EC50 values of E.amylovara strains isolated to benziothiazolinone from different hosts

寄主Hosts菌株数Number of strains榅桲C.oblonga香梨P.sinkiangensis苹果M.pumila杜梨P.betulaefolia山楂C.pinnatifida 3 24 10 3 10抑制中浓度Half effective concentration(EC50)/(µg·mL-1)范围Range 320.50~460.51 113.82~565.79 171.11~813.11 141.32~439.53 12.40~433.14均值Average 398.24±0.98 a 314.84±4.99 b 292.67±1.52 c 288.73±5.45 c 269.05±0.99 d最低抑制浓度Minimum inhibition concentration(MIC)/(µg·mL-1)范围Range 0.50~2.50 1.50~2.50 1.00~3.75 0.50~2.00 1.50~2.50均值Average 1.56±0.98 c 2.18±0.23 b 2.47±0.00 a 1.33±0.00 d 2.15±0.00 b抗性水平Resistance level范围Range 1.05~1.51 0.45~1.87 0.56~2.67 0.46~1.44 0.04~1.42均值Average 1.31 1.04 0.96 0.95 0.88
表5 不同年份分离的梨火疫病菌对噻霉酮的EC50值比较
Table 5 Comparison of EC50 values of E.amylovara strains isolated in different years to benziothiazolinone
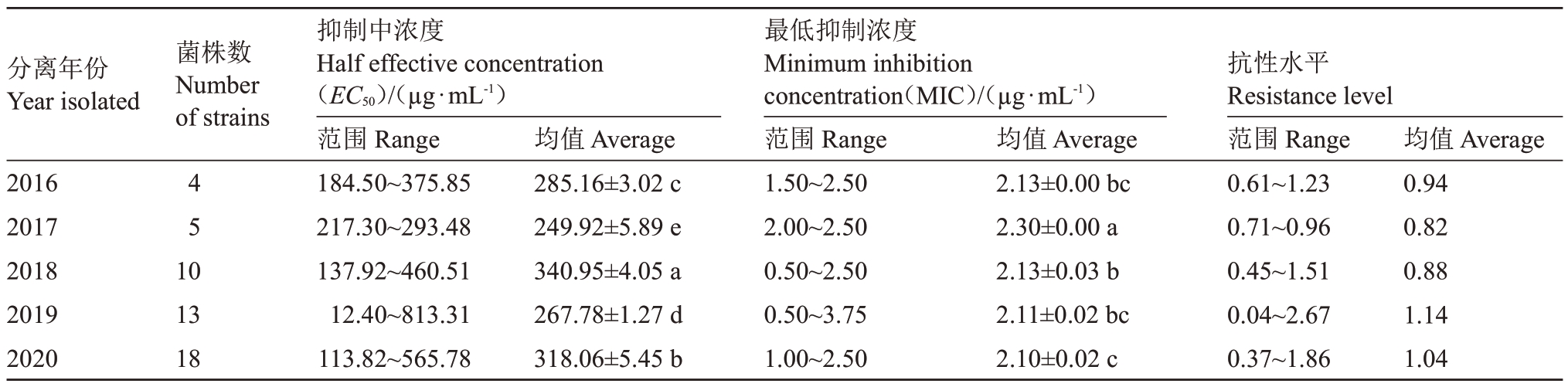
分离年份Year isolated菌株数Number of strains 2016 2017 2018 2019 2020 45 10 13 18抑制中浓度Half effective concentration(EC50)/(µg·mL-1)范围Range 184.50~375.85 217.30~293.48 137.92~460.51 12.40~813.31 113.82~565.78均值Average 285.16±3.02 c 249.92±5.89 e 340.95±4.05 a 267.78±1.27 d 318.06±5.45 b最低抑制浓度Minimum inhibition concentration(MIC)/(µg·mL-1)范围Range 1.50~2.50 2.00~2.50 0.50~2.50 0.50~3.75 1.00~2.50均值Average 2.13±0.00 bc 2.30±0.00 a 2.13±0.03 b 2.11±0.02 bc 2.10±0.02 c抗性水平Resistance level范围Range 0.61~1.23 0.71~0.96 0.45~1.51 0.04~2.67 0.37~1.86均值Average 0.94 0.82 0.88 1.14 1.04
3 讨 论
国外对梨火疫病的研究已经有200 多年的历史,很多化学药剂都尝试用于该病害的防治,但至今仍缺乏田间防效显著、不产生药害、抗药性风险低及对环境安全的细菌杀菌剂。针对在我国新发、突发的梨火疫病,目前登记用于该病害防治的药剂极为有限,筛选和研发高效安全的药剂对病害的安全防控,阻截病害的传播和扩散蔓延具有重要意义。噻霉酮是我国自主研发、作用机制独特的一种高效、低毒、广谱、安全、低残留的新型杀菌剂,已在黄瓜霜霉病、苹果腐烂病、梨黑星病、稻瘟病等多种真菌性病害防治中得到应用[17]。近些年噻霉酮在辣椒细菌性角斑病、番茄斑疹病、青枯病、柑橘溃疡病等一些细菌性病害的防治试验中也取得了较好的防效,成为具有兼防真菌和细菌病害的农药。从2017年开始,噻霉酮在新疆梨火疫病防治中试验和应用,最近已登记为该病害的防治药剂。
研究采用滤纸片-抑菌圈法测定了2016—2020年新疆不同地区分离的50个梨火疫病菌对噻霉酮的敏感性,建立了梨火疫病菌对噻霉酮的敏感基线。研究结果表明,不同梨火疫病菌株对噻霉酮的敏感程度差异较大,EC50 值在12.40~813.13 µg·mL-1。供试梨火疫病菌对噻霉酮的敏感性为正态分布的单峰曲线,未出现敏感性下降的亚群体,因此其EC50均值304.61±134.82µg·mL-1可作为梨火疫病菌对噻霉酮的敏感基线值,适用于评价当前田间菌株对噻霉酮药剂的敏感性和抗药性菌株的检测和监测。EC50值的大小是衡量药剂毒力大小的重要指标。苗则彦等[18]通过平板菌落计数法测定了噻霉酮对沈阳地区保护地番茄上番茄斑疹病菌(Pseudomonas syringae pv.tomato)分离菌株的毒力,EC50值为2.66µg·mL-1。姚廷山等[19]采用喷雾法进行室内抑菌试验,确定了噻霉酮、农用链霉素、溴菌腈对柑橘溃疡病菌(Xanthomonas axonopodis pv. citri)的EC50分别为46.59、86.18 和102.91 mg·kg-1,噻霉酮的毒力最强。可见噻霉酮对不同植物病原细菌的毒力不同,其EC50值有明显差异。其原因一方面可能与病原菌本身的生理差异有关;另一方面药剂的物理性能、毒力测定的不同方法都可能是影响因素。本研究采用的滤纸片-抑菌圈法是细菌杀菌剂毒力测定常用的方法,该法常因药剂在培养基中的扩散程度、纸碟浸渍药液的时间、抑菌圈测量的偏差等而影响测定结果的准确性。有研究认为,平板菌落法测定细菌杀菌剂毒力具有变异系数小、可重复性好的优点[20]。因此,后续将考虑采用平板菌落法进一步证实本研究结果,以保证结果的准确性。
研究所测定的梨火疫病菌50个菌株中,MIC在0.50~3.75µg·mL-1之间,未发现敏感性显著降低的菌株。33个菌株对噻霉酮的EC50小于200µg·mL-1,占供试菌株的66%,17个菌株的EC50大于300µg·mL-1,占供试菌株的34%。菌株的抗性水平为0.041~2.67倍之间(均值为1.00),只有1 个菌株抗性倍数大于2。结果表明,目前新疆分离的梨火疫病菌对噻霉酮均为敏感菌株,尚未发现抗性菌株。研究还发现,来自不同地区、不同寄主和年份的梨火疫病菌分离菌株对噻霉酮敏感性、抗性水平都存在一定差异。从苹果和香梨寄主上分离的菌株对噻霉酮敏感性水平最低,EC50均值高于山楂、杜梨和榅桲上的分离菌株,这可能与梨火疫病在苹果、香梨种植区发现早、危害重、用药普遍有关。2016—2020 年分离菌株的EC50值表现出随年份逐渐增大、敏感性下降、抗性水平略有增加的趋势,这可能与用药时间的长短相关。和田地区种植的果树上用药很少,但分离菌株H13 的EC50值最大、对噻霉酮敏感性水平最低。该地区分离的菌株仅有一个,不能代表该地区菌株的敏感性水平,还需在后续工作中收集更多菌株,进一步测定比较。因此,影响菌株的敏感性和抗药性产生的因素是多方面的,与施药剂量、使用频率和使用时间造成的选择压力、药剂的作用机制密切相关。此外还可能与病原菌自身的生理差异、抗药性突变以及与生产中使用的其他药剂之间的交互抗性有关[21-22]。
有研究表明,水稻白叶枯病菌在离体和活体上对噻枯唑的敏感性表现不一致,活体上表现抗药性的菌株,在离体上不表现抗药性[23]。噻霉酮作为梨火疫病防治的新登记药剂,试验应用只有几年的时间。通过测定田间分离的梨火疫病菌菌株对噻霉酮的敏感性评价菌株的抗药性水平,目前尚未发现在离体条件下的抗性菌株。但在田间活体上是否存在抗药性菌株还需测定证实。在今后的应用中,应避免加大使用剂量和频繁重复用药,减少药剂的选择压力;将不同杀菌剂混合使用或交替使用,防止或延缓药性的产生。同时要持续监测梨火疫病菌在活体寄主上对药剂的敏感性的变化,并对田间药效验证,进一步评估其抗性风险,为噻霉酮科学使用、持久发挥良好的防病效果提供参考。
4 结 论
测定2016—2020 年50 个梨火疫病菌新疆分离菌株对噻霉酮药剂的EC50 均值为(304.61±134.82)µg·mL-1,确定为梨火疫病菌对噻霉酮的敏感基线值,适用于对噻霉酮药剂敏感性和抗药性菌株的检测和监测。未发现抗药性菌株,对噻霉酮的抗性风险低,在生产上可与其他药剂交替使用。
[1] 胡白石,许志刚,周国梁,易建平,焦国尧.梨火疫病的进境风险分析[J].植物保护学报,2001,28(4):303-308.HU Baishi,XU Zhigang,ZHOU Guoliang,YI Jianping,JIAO Guoyao.Primary risk analyses of import of Erwinia amylovora to China[J].Acta Phytophylacica Sinica,2001,28(4):303-308.
[2] DJAIMURZINA A,UMIRALIEVA Z,ZHARMUKHAMEDOVA G,BORN Y,BÜHLMANN A,REZZONICO F.Detection of the causative agent of fire blight- Erwinia amylovora (Burrill)Winslow et al.in the Southeast of Kazakhstan[J].Acta Horticulturae,2014,18(1056):129-132.
[3] MARINOVA-TODOROVA M,RANTA J,HANNUNEN S.The suitability of finnish climate for fire blight (Erwinia amylovora)epidemics on apple[J].Agricultural & Food Science,2015,24(1):59-66.
[4] DOOLOTKELDIEVA T,BOBUSHEVA S.Fire blight disease caused by Erwinia amylovora on rosaceae plants in Kyrgyzstan and biological agents to control this disease[J].Advances in Microbiology,2016,6(11):831-851.
[5] ZHAO Y Q,TIAN Y L,WANG L M,GENG G M,ZHAO W J,HU B S,ZHAO Y F.Fire blight disease,a fast-approaching threat to apple and pear production in China[J].Journal of Integrative Agriculture,2019,18(4):815-820.
[6] 王俊,高建诚,巴音克西克,木也沙·买买提,张军恒,田艳丽,胡白石.利用电加热自动消毒修枝剪阻断梨火疫病田间传播[J].植物检疫,2022,36(2):25-28.WANG Jun,GAO Jiancheng,Bayinkexike,Muyassar·Mamat,ZHANG Junheng,TIAN Yanli,HU Baishi.Blocking field spread of fire blight electric heating automatic disinfection prunning scissors[J].Plant Quarantine,2022,36(2):25-28.
[7] 杨金花,徐叶挺,张校立.梨火疫病研究进展[J].分子植物育种,2022,20(3):1003-1013.YANG Jinhua,XU Yeting,ZHANG Xiaoli.Advances of fire blight in pear[J].Molecular Plant Breeding,2022,20(3):1003-1013.
[8] VAN DER ZWET T,OROLAZA-HALBRENDT N,ZELLER W.Fire blight history,biology,and management[M].Texas,USA:The American Phytopathological Society Press,2012.
[9] FÖRSTER H,MCGHEE G C,SUNDIN G W,ADASKAVEG J E.Characterization of streptomycin resistance in isolates of Erwinia amylovora in California[J].Phytopathology,2015,105(10):1302-1310.
[10] RUSSO N L,BURR T J,BRETH D I,ALDWINCKLE H S.Isolation of streptomycin-resistant isolates of Erwinia amylovora in New York[J].Plant Disease,2008,92(5):714-718.
[11] SUNDIN G W,WANG N.Antibiotic resistance in plant-pathogenic bacteria[J].Annual Review of Phytopathology,2018,56:161-180.
[12] AĆIMOVIĆ S G,ZENG Q,MCGHEE G C,SUNDIN G W,WISE J C.Control of fire blight (Erwinia amylovora) on apple trees with trunk-injected plant resistance inducers and antibiotics and assessment of induction of pathogenesis-related protein genes[J].Frontiers in Plant Science,2015,6:16.
[13] NORELLI J L,JONES A L,ALDWINCKLE H S.Fire blight management in the twenty-first century:using new technologies that enhance host resistance in apple[J].Plant Disease,2003,87(7):756-765.
[14] 袁英哲,韩剑,王岩,罗明,包慧芳,张春竹,黄伟.梨火疫病菌活菌快速定量检测方法的建立[J].果树学报,2020,37(9):1425-1433.YUAN Yingzhe,HAN Jian,WANG Yan,LUO Ming,BAO Huifang,ZHANG Chunzhu,HUANG Wei.Establishment of rapid quantitative detection of viable Erwinia amylovora[J].Journal of Fruit Science,2020,37(9):1425-1433.
[15] 陈长卿,隋原,杨丽娜,周洪波,张文中,高洁.烟草野火病菌对细菌杀星的敏感性测定及敏感基线建立[J].吉林农业大学学报,2011,33(6):600-603.CHEN Changqing,SUI Yuan,YANG Lina,ZHOU Hongbo,ZHANG Wenzhong,GAO Jie.Sensitivity determination and sensitivity baseline establishment of Pseudomonas syringae pv.tabaci to streptomycin saikuzuo[J].Journal of Jilin Agricultural University,2011,33(6):600-603.
[16] 毕秋艳,马志强,韩秀英,张小风,王文桥,赵建江.不同机制杀菌剂对小麦白粉病的敏感性及与三唑酮的交互抗性[J].植物保护学报,2017,44(2):331-336.BI Qiuyan,MA Zhiqiang,HAN Xiuying,ZHANG Xiaofeng,WANG Wenqiao,ZHAO Jianjiang.Sensitivity of diverse fungicides on powdery mildew of wheat and cross resistance with triadimefon[J].Acta Phytophylacica Sinica,2017,44(2):331-336.
[17] 宋根苗,蒋家珍,邱立红.噻霉酮和苯醚甲环唑混配对4 种不同病原菌的增效作用[J].植物保护,2012,38(4):171-174.SONG Genmiao,JIANG Jiazhen,QIU Lihong.Synergistic activity of benziothiazoline/difenoconazole complex against four different fungi[J].Plant Protection,2012,38(4):171-174.
[18] 苗则彦,赵杨,李颖,寇永春,白元俊.春雷霉素和噻霉酮对番茄斑疹病菌联合毒力及防病效果[J].植物保护,2015,41(2):216-219.MIAO Zeyan,ZHAO Yang,LI Ying,KOU Yongchun,BAI Yuanjun.Co-toxicity of kasugamycin and benziothiazolinone to Pseudomonas syringae pv.tomato and its control effect[J].Plant Protection,2015,41(2):216-219.
[19] 姚廷山,周常勇,胡军华,冉春,李鸿筠,刘浩强,肖田.3 种杀菌剂对柑橘溃疡病菌的室内毒力测定[J].江西农业大学学报(自然科学版),2009,31(6):1026-1029.YAO Tingshan,ZHOU Changyong,HU Junhua,RAN Chun,LI Hongjun,LIU Haoqiang,XIAO Tian.Toxicity of three fungicides to Xanthomonas axonopodis pv. citri[J].Acta Agriculturae Universitatis Jiangxiensis(Natural Sciences Edition),2009,31(6):1026-1029.
[20] 游文莉,许文耀.杀细菌剂毒力测定方法的研究[J].农药科学与管理,2002,23(2):21-22.YOU Wenli,XU Wenyao.Studies on the testing methods of toxicity for bactericide[J].Pesticide Science and Administration,2002,23(2):21-22.
[21] 李洁,钟杰,黄军,赵晓,朱宏建.植物病原细菌链霉素抗性研究进展[J].生物技术通报,2013,(9):18-26.LI Jie,ZHONG Jie,HUANG Jun,ZHAO Xiao,ZHU Hongjian.Progress of streptomycin-resistance of pathogenic bacteria of plant[J].Biotechnology Bulletin,2013,(9):18-26.
[22] 郑肖兰,傅帅,郑服丛,李锐,吴伟怀,贺春萍.植物病原菌抗药性研究进展[J].热带农业科学,2011,31(1):86-90.ZHENG Xiaolan,FU Shuai,ZHENG Fucong,LI Rui,WU Weihuai,HE Chunping.Research advances on fungicide resistance in plant pathogens[J].Chinese Journal of Tropical Agriculture,2011,31(1):86-90.
[23] 沈光斌,周明国.水稻白叶枯病菌对噻枯唑的抗药性监测[J].植物保护,2002,28(1):9-11.SHEN Guangbin,ZHOU Mingguo.Resistance monitoring of Xanthomonas oryzae pv.Oxyzae to saikuzuo[J].Plant Protection,2002,28(1):9-11.