新疆野苹果[Malus sieversii(Ledeb.)Rome.]又称塞威士苹果,是现代栽培苹果的祖先,种群遗传多样性丰富,具有抗寒、抗旱、抗盐碱等优良特性[1]。西北地区是苹果产区之一,然而由于春季气温波动大,西北部产区易遭遇异常低温、雨雪和霜冻等不良天气而影响苹果产业[2],因此,研究新疆野苹果抗寒性对苹果产业的可持续发展具有重要的现实意义。
低温胁迫分为冷害与冻害,当植物短时间遭受低温(非冰冻温度)胁迫时,植物自身会发生一系列适应机制来响应低温,提高抗冻性[3]。低温时间延长或温度持续降低会导致植物光合作用的下降、活性氧平衡被打破,严重时会导致植物死亡[4-5]。光合作用下降是植物受到冻害时最敏感的生理反应之一[6],植物遭受冻害时,类囊体结构发生变化,PSⅡ受损,光化学效率降低,产生过多的光能,严重影响植物的光合作用[7]。近年来,低温胁迫对植物光合作用的影响已经在苹果[8]、葡萄[9]、番茄[10]等多种植物中被研究,随着转录组测序技术应用,在油菜与茶树中得到了Fd1、CP26、PsbS[11-12]等多个光合相关基因。
目前关于新疆野苹果的抗寒性主要有不同居群[13-14]、不同类型[15]、不同株系[16]、MDA含量、SOD活性[17]及与多胺[18]等生理相关研究。Zhou等[19]通过转录组测序分析发现,新疆野苹果经过冻害后,编码超氧化物歧化酶、蔗糖合酶、植物激素信号转导激酶和过氧化物酶等通路的基因发生了差异变化,其中转录因子DREB1/CBF、MYC2、WRKY71、MYB4 等在冻害胁迫中被诱导表达。新疆野苹果的遗传背景复杂,关于冻害对其光合性能指标与相关分子机理研究较少。笔者在本研究中以单株系的新疆野苹果组培苗为材料,在课题组前期研究的基础上,研究冻害对新疆野苹果组培苗光合特性的影响,以期为解析其响应冻害的分子机制提供参考。
1 材料和方法
1.1 试验材料及处理
新疆野苹果种子来自新疆伊犁哈萨克自治州霍城县。种子经层积处理,种植在5 cm×10 cm 的穴盘中,当实生苗长至6~8枚叶片时,以顶端2~3 cm茎段为外植体进行组织培养。参考何晨晨等[20]的方法进行培养基配制,每隔1个月继代1次,选取增殖较好的单株系丛生芽进行生根培养,培养60 d后选择生长基本一致的组培苗进行试验处理。实生苗与组培苗均在人工气候培养箱(RXZ 智能型,宁波江南仪器厂)中培养,培养条件为:光强5000 lx、昼25 ℃/14 h、夜23 ℃/10 h,相对湿度75%。-3 ℃模拟冻害试验处理参考范宗民等[21]的方法,在人工改造的冰箱(容声BD/BC-310MS)中进行处理,冰箱内的条件为:光强5000 lx、昼25 ℃/14 h、夜23 ℃/10 h,相对湿度75%。温度从25 ℃开始以4 ℃·h-1降温至-3 ℃,在-3 ℃模拟冻害条件下处理0、3、6、12、24 h 的组培苗为处理组,以25 ℃下培养的材料为对照,处理后材料部分用于测定叶绿素含量与荧光参数,其余经液氮冷冻后存于-80℃用于生理指标检测与转录组测序。处理组与对照均设置3次生物学重复。
1.2 叶绿素含量与荧光参数测定
采用分光光度仪测定叶绿素含量;利用慢速荧光成像系统(MAX-Imaging-PAM,WALZ,德国)测定光系统Ⅱ的最大量子产量(Fv/Fm);通过多通道植物效率仪M-PEA(Hansatech Instru-ments,Norfolk,英国)测定组培苗以吸收光能为基础的光系统Ⅱ的性能指数(PIabs)。
1.3 RNA的提取及转录组测序
将对照、0、3、6、12 h 处理的新疆野苹果组培苗进行转录组测序,分别记为:对照、T0 h、T3 h、T6 h、T12 h,每个处理3 次重复,共15 个样本进行转录组测序,转录组测序工作由杭州景杰生物科技有限公司完成。测序后使用过滤软件SOAPnuke进行数据过滤,将接头污染、未知碱基N 含量大于5%的reads、质量值低于15 的碱基占该reads 总碱基数的比例大于20%的reads去除。得到clean reads之后,使用HISAT[22] 将clean reads 与新疆野苹果双倍体参考基因组(Malus sieversii Diploid Consensus Whole Genome v1.0 Assembly&Annotation)进行序列比对。通过Bowtie2[23]将clean reads 比对到新疆野苹果基因序列中,采用RSEM[24]计算基因和转录本的表达水平。
1.4 差异表达基因的筛选及功能分析
基于DEseq2[25]方法进行DEGs 的检测,将Qvalue ≤0.05(adjusted P-value ≤0.05)的基因定义为显著差异表达基因;将每个时间差异倍数为两倍以上(log2fold change,log2FC ≥1)且FPKM ≥10 的DEGs 定义为极显著差异表达基因。根据KEGG与GO 注释结果,使用R 软件中的phyper 函数进行KEGG与GO富集分析。
1.5 光合特性相关差异表达基因qRT-PCR验证
为了检测转录组测序数据的准确性,从光合特性相关的差异表达基因中选取了7 个表达量较高的差异基因进行qRT-PCR 验证,根据课题组前期试验选择UBQ 为内参基因[20]。从NCBI 中下载基因序列,利用Primer3 设计qRT-PCR 引物,引物合成由上海生物工程公司完成(表1)。以不同处理的新疆野苹果组培苗叶片RNA 为模板,参照abm 公司反转试剂盒说明书合成cDNA。qRT-PCR 按照Green Real time PCR Master MIX 试剂盒(TOYOBO,日本)进行,基因表达量采用相对定量2-ΔΔCT法,即log2(T/对照)。
表1 光合特性相关差异表达基因qRT-PCR 引物序列
Table 1 qRT-PCR primer sequences of differentially expressed genes related to photosynthetic characteristics
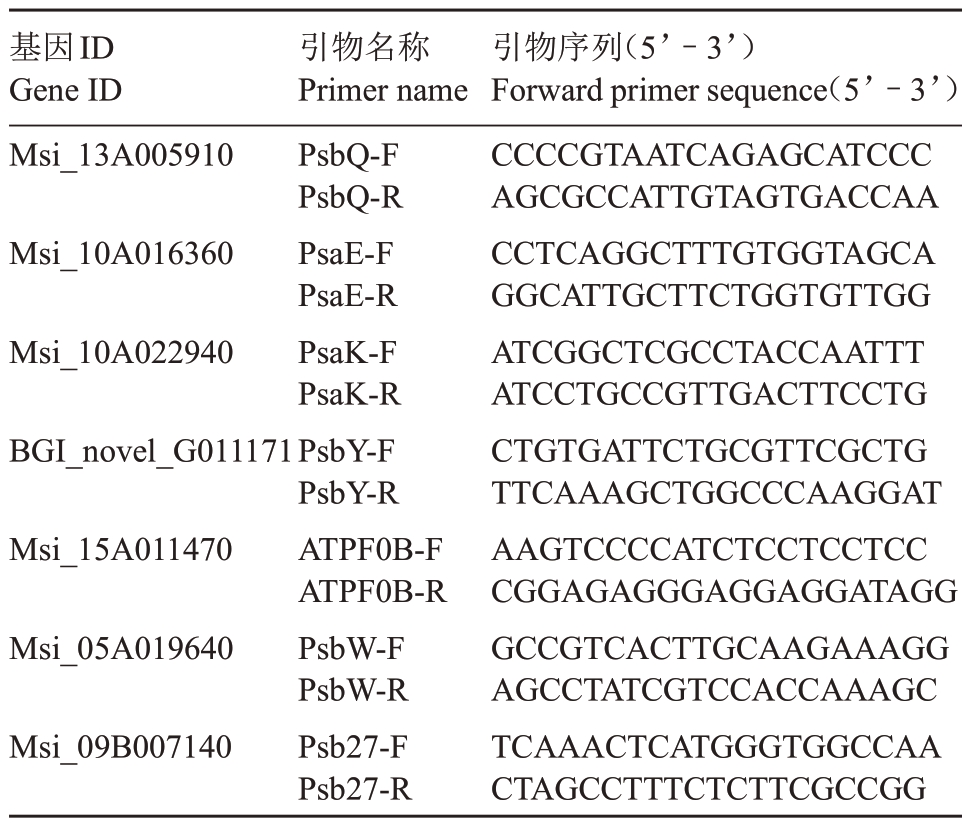
基因ID Gene ID Msi_13A005910 Msi_10A016360 Msi_10A022940 BGI_novel_G011171 Msi_15A011470 Msi_05A019640 Msi_09B007140引物名称Primer name PsbQ-F PsbQ-R PsaE-F PsaE-R PsaK-F PsaK-R PsbY-F PsbY-R ATPF0B-F ATPF0B-R PsbW-F PsbW-R Psb27-F Psb27-R引物序列(5’-3’)Forward primer sequence(5’-3’)CCCCGTAATCAGAGCATCCC AGCGCCATTGTAGTGACCAA CCTCAGGCTTTGTGGTAGCA GGCATTGCTTCTGGTGTTGG ATCGGCTCGCCTACCAATTT ATCCTGCCGTTGACTTCCTG CTGTGATTCTGCGTTCGCTG TTCAAAGCTGGCCCAAGGAT AAGTCCCCATCTCCTCCTCC CGGAGAGGGAGGAGGATAGG GCCGTCACTTGCAAGAAAGG AGCCTATCGTCCACCAAAGC TCAAACTCATGGGTGGCCAA CTAGCCTTTCTCTTCGCCGG
1.6 数据统计与分析
采用Excel 2010、SPSS 和Origin 2021 软件进行数据统计并绘制图表,利用TBtools[26]进行数据归一化热图绘制。
2 结果与分析
2.1 冻害对新疆野苹果组培苗形态及叶绿素含量的影响
新疆野苹果组培苗在-3℃模拟冻害条件下叶片形态发生明显变化,由图1-A可见,在12 h时组培苗叶片边缘及顶部叶片开始出现卷缩,在24 h时,叶片发生明显卷缩。光合作用是植物遭受冻害后的最敏感反应之一,而叶绿素是植物进行光合作用的重要色素,冻害能够抑制叶绿素的合成,加快叶绿素的降解[27],因此,叶绿素含量较高能够保证光合作用的进行。由图1-B 可见,与对照相比,叶绿素a 含量在处理24 h 时显著下降,下降16.2%,其他4 个时间点均无显著差异;叶绿素b 含量在0 h 时显著增加,增加52%,3 h 没有显著变化,6、12、24 h 叶绿素b 含量均显著下降,分别下降12.3%、28.6%、36.7%。总叶绿素含量变化趋势与叶绿素b变化趋势完全一致。类胡萝卜素含量均显著低于对照,0、3、6、12、24 h分别下降23.5%、23.5%、25.5%、19.6%、29.4%。与对照相比,0、3 h时叶绿素a/b显著下降,6 h时叶绿素a/b没有显著变化,而12 h 与24 h 时叶绿素a/b 显著增加,分别增加14.9%与14.1%。
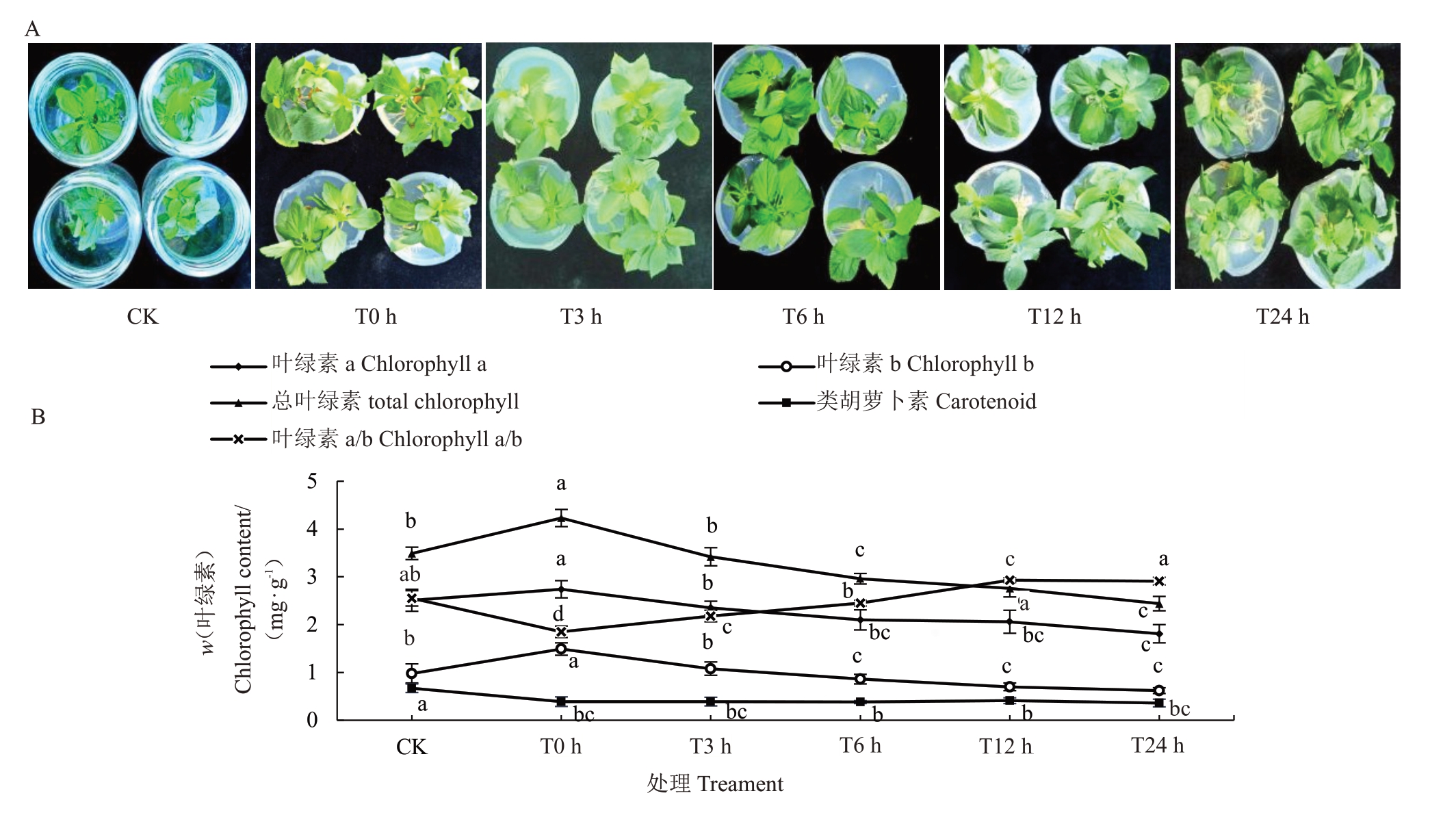
图1 冻害胁迫后新疆野苹果组培苗形态及叶绿素含量的变化
Fig.1 Changes in morphology and chlorophyll content of in vitro seedlings of Malus sieversii under freezing stress
A.形态变化;B.叶绿素含量变化。不同小写字母表示不同处理间的差异(p<0.05)。下同。
A.Morphological changes; B.Changes in chlorophyll content.Different lowercase letters indicate differences among different treatments (p<0.05).The same below.
2.2 冻害对新疆野苹果组培苗叶绿素荧光的影响
叶绿素荧光技术能够快速无损地检测作物叶片对环境胁迫的敏感性。Fv/Fm反映了光系统II 反应中心的最大光化学效率,PIabs代表以吸收光能为基础的性能指数。由图2-A可见,与对照相比,新疆野苹果组培苗在-3 ℃模拟冻害条件下,从对照到处理24 h组培苗叶片的荧光图片从深蓝色逐渐变为浅蓝色到蓝绿色,表明光化学效率逐渐下降。同时Fv/Fm数值的下降也表明光化学效率的降低。与对照相比,各处理下Fv/Fm均显著下降,0、3、6、12、24 h分别下降7.9%、12.3%、12.8%、19.9%、29.4%(图2-B)。与对照相比,各处理PIabs均显著下降,0、3、6、12与24 h 分别下降61.2%、63.7%、78.3%、77.2%与87.7%(图2-C)。而0 h 时的PIabs 与0 h 时的Fv/Fm相比,PIabs 下降更加明显。说明冻害影响了组培苗的光化学效率与光合性能指数,且光合性能指数对低温更加敏感。
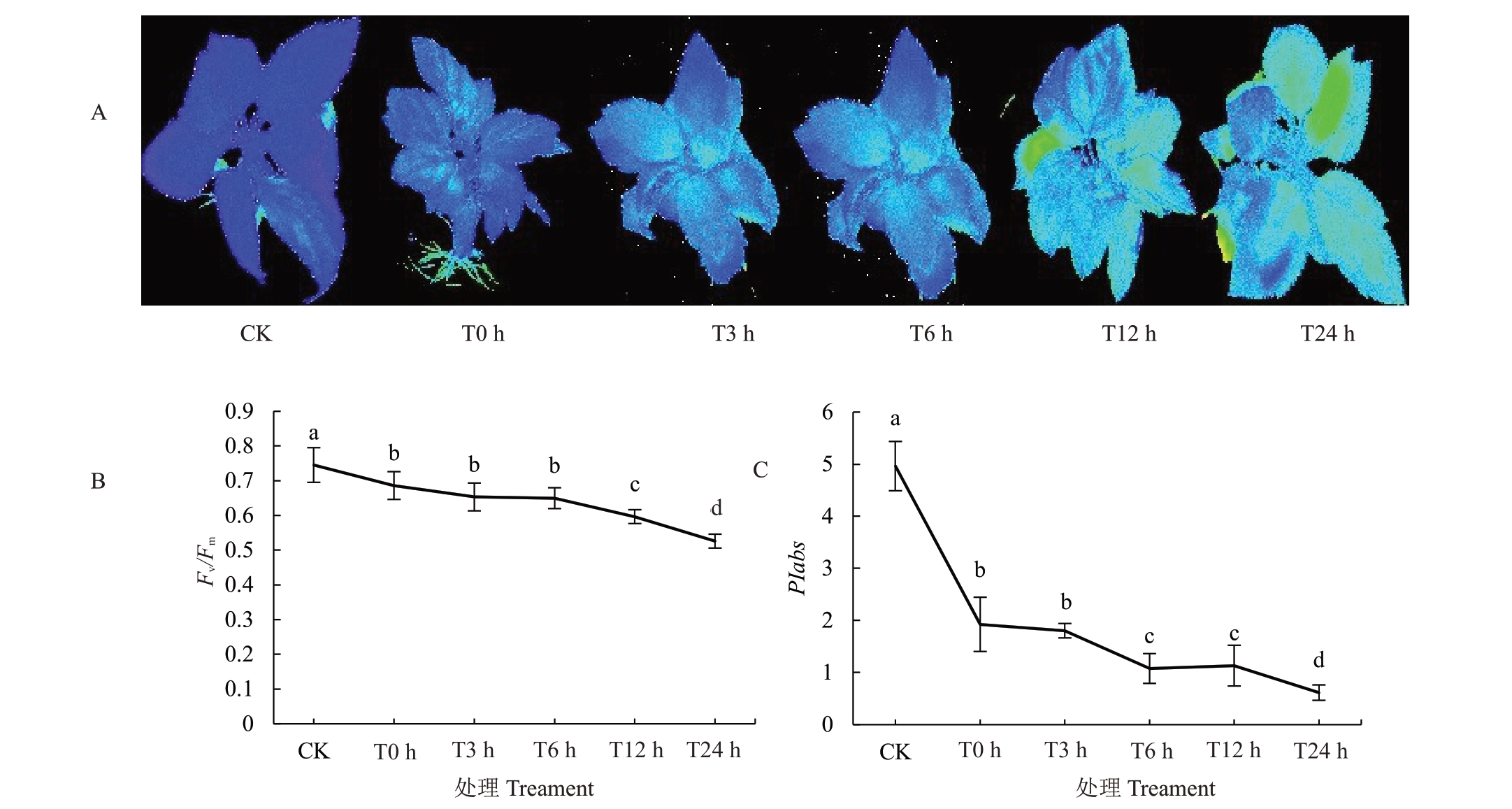
图2 冻害胁迫后新疆野苹果组培苗叶绿素荧光的变化
Fig.2 Changes in chlorophyll fluorescence of in vitro seedlings of Malus sieversii under freezing stress
A.Fv/Fm 的荧光图片;B.Fv/Fm 数值变化;C.PIabs 的数值变化。
A.Fluorescence picture of Fv/Fm;B.Numerical change of Fv/Fm;C.Numerical change of PIabs.
2.3 RNA测序数据质量评估
新疆野苹果组培苗经-3 ℃模拟冻害后样品的cDNA文库进行RNA测序,从表2看出,每个样品过滤后的reads数在42.48 M~43.31 M之间,占总reads数的95.33%~97.35%之间,每个样本过滤后的碱基总数平均在6.37 Gb~6.50 Gb 之间,其中过滤后的reads 数质量大于30 的碱基数占总碱基的比例平均在90.97%~91.67%之间,比对到新疆野苹果参考基因组的reads 平均在86.75%~87.50%之间。说明RNA测序结果较好,能够进行后续的生物学分析。
表2 冻害胁迫后RNA 测序质量评估
Table 2 Quality assessment of RNA-seq after freezing stress
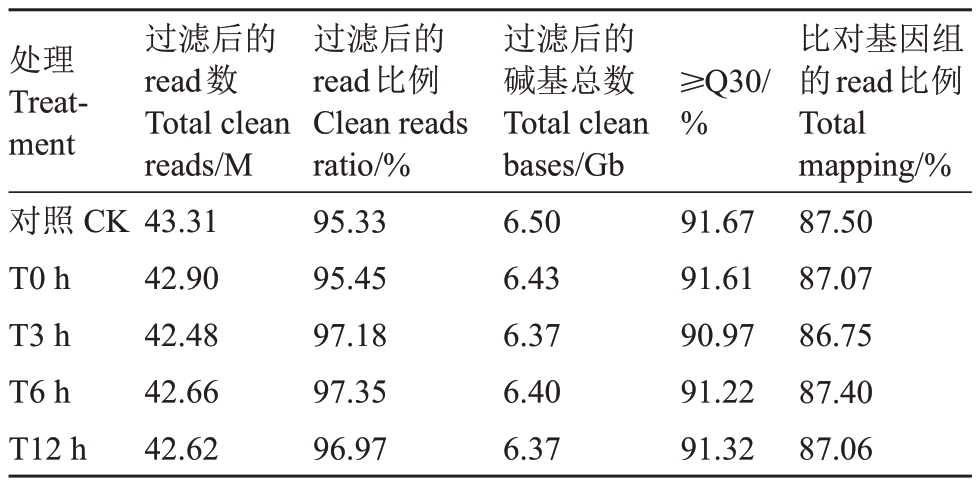
注:≥Q30 表示过滤后read 数质量大于30 的碱基数占总碱基数的比例。
Note: ≥Q30 means percentage of quality values of bases involved clean reads is equal or greater than 30.
比对基因组的read比例Total mapping/%87.50 87.07 86.75 87.40 87.06处理Treatment对照CK T0 h T3 h T6 h T12 h过滤后的read数Total clean reads/M 43.31 42.90 42.48 42.66 42.62过滤后的read比例Clean reads ratio/%95.33 95.45 97.18 97.35 96.97过滤后的碱基总数Total clean bases/Gb 6.50 6.43 6.37 6.40 6.37≥Q30/%91.67 91.61 90.97 91.22 91.32
2.4 新疆野苹果响应冻害光合特性相关基因筛选
根据KEGG富集分析与Q-value ≤0.05筛选新疆野苹果组培苗经过冻害后的光合相关差异表达基因,共得到了58个差异基因。由图3可见,与对照相比,0、3、6、12 h 4个处理总的差异基因数分别有13个、22个、26个、46个,上调基因分别为10个、15个、15个、28个,下调基因分别为3个、7个、11个、18个,各比较组共有的基因数有5 个,分别为BGI_novel_G001106、BGI_novel_G001286、BGI_novel_G006388、BGI_novel_G011171、Msi_13A005910。随着冻害时间的延长,差异表达基因的数量逐渐增多,其中上调的基因数始终多于下调的基因数(图3-B)。
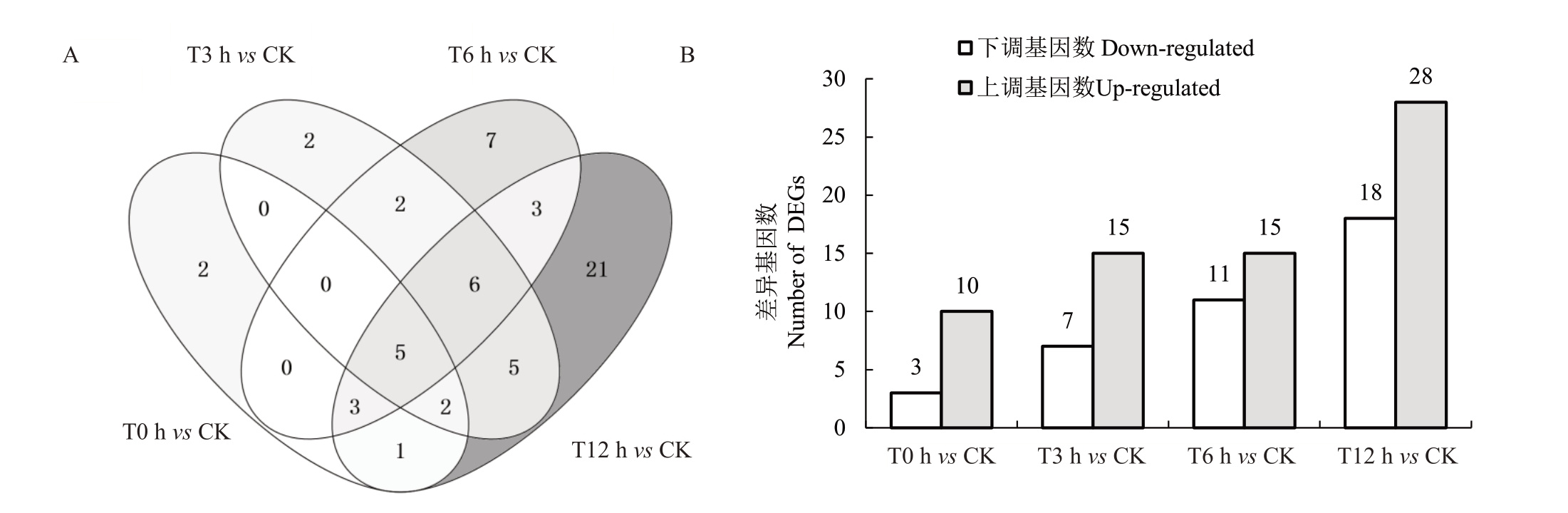
图3 4 个比较组间光合差异表达基因数
Fig.3 The numbers of differential expressed genes(DEGs)related to photosynthesis across different comparisons
A.Venn 图;B.上下调基因数柱状图。
A.Venn diagram;B.Histogram of the number of up and down genes.
2.5 新疆野苹果响应冻害光合调控相关基因的表达分析
利用TBtools 将光合调控相关的58 个差异表达基因进行归一化处理绘制热图,由图4 可见,在-3 ℃模拟冻害下,经过不同时间后与对照相比,新疆野苹果组培苗光合相关调控差异基因在处理下均表现为上调基因数多于下调基因数,其中0 h与对照相比上调基因数达到40 个,随着处理时间的延长,上调基因数逐渐减少。当处理12 h 时,上调基因有35 个,其中参与光系统Ⅱ相关基因较多,有8 个下调基因与10 个上调基因;细胞色素b6/f 相关基因有4 个上调基因与8 个下调基因;光系统I相关基因有1 个下调基因与11 个上调基因;ATP 合成酶相关基因只有3 个上调基因;光合电子传递相关基因有6 个下调与7 个上调基因;处理组与对照相比,冻害下调控光系统Ⅱ差异基因比光系统I 差异基因多,同时ATP 合成酶相关基因全部上调。根据极显著差异基因筛选条件,将4 个比较组的所有满足log2FC ≥1、Q-value ≤0.05 且表达量FPKM ≥10 进 行 筛 选,得 到Msi_13A005910(PsbQ)与BGI_novel_G011171(PsbY)两个基因,且这两个基因呈极显著上调表达。
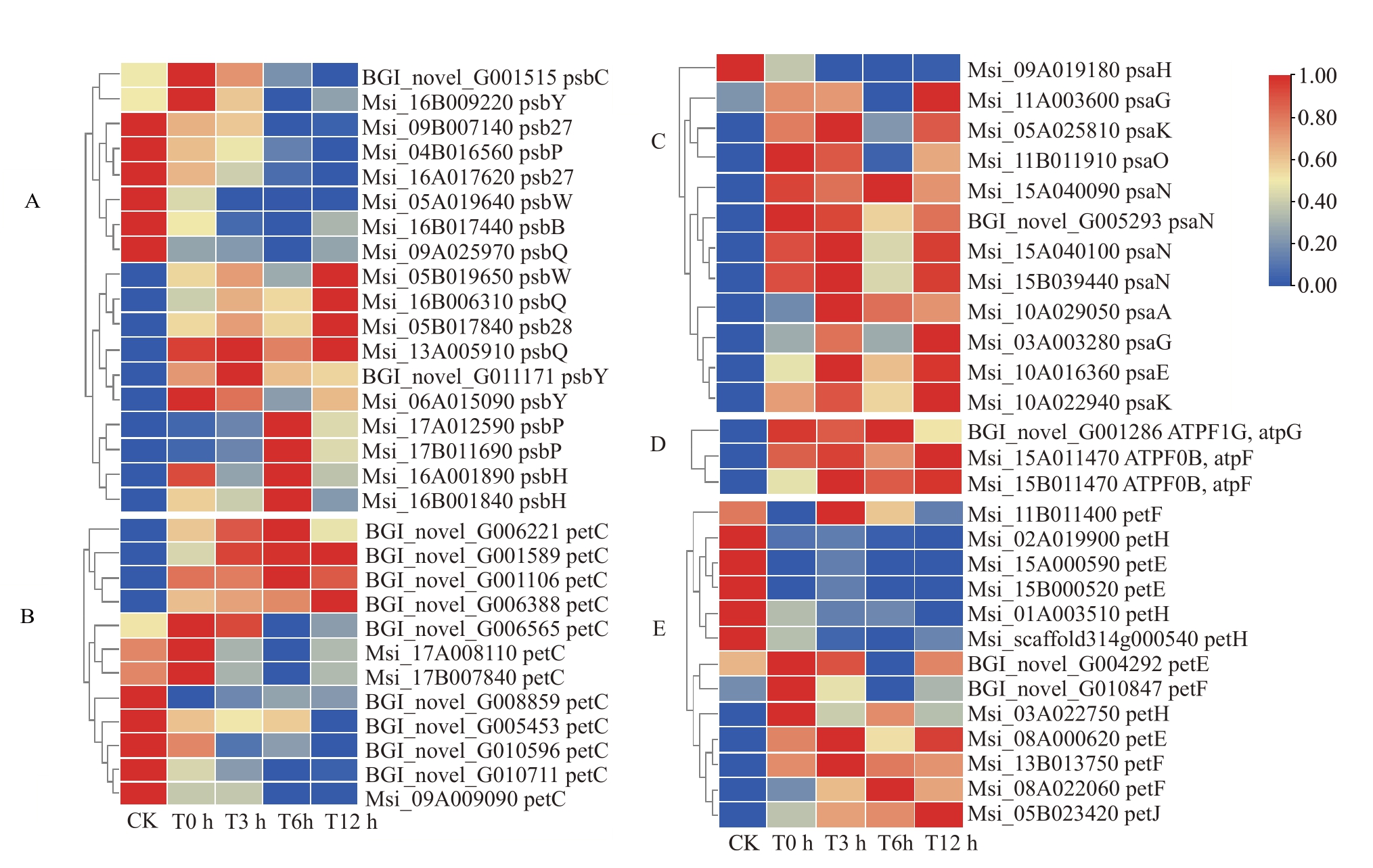
图4 参与光合作用的相关基因表达热图
Fig.4 Heat map of gene expression related to photosynthesis
A.光系统Ⅱ;B.细胞色素b6/f;C.光系统Ⅰ;D.ATP 合成酶;E.光合电子传递;红色表示高表达水平,蓝色表示低表达水平。
A.Photosystem Ⅱ;B.Cytochrome b6/f;C.Photosystem Ⅰ;D.ATP synthase;E.Photosynthetic electron transport;Red indicates high expression level,blue indicates low expression level.
2.6 新疆野苹果响应冻害光合调控相关基因GO功能分析
对58个参与光合作用的差异表达基因进行GO富集分析,由图5可见,参与光合作用的58个基因同时在多个通路上表达,这些基因具有多个不同功能。富集的前20 个条目中,细胞组成条目有15 个,基因数较多的为光合膜、类囊体、膜蛋白复合物、光系统、叶绿素体、质体,候选基因数分别为:30 个、30 个、25 个、24 个、16 个、16 个;生物过程相关条目只有光合作用1个,候选基因有24个;分子功能相关条目有电子传递活性、2Fe-2s簇结合、电子传递器与质体喹啉-质体蓝素还原酶活性4个,候选基因数分别为15个、8个、4个与7个。
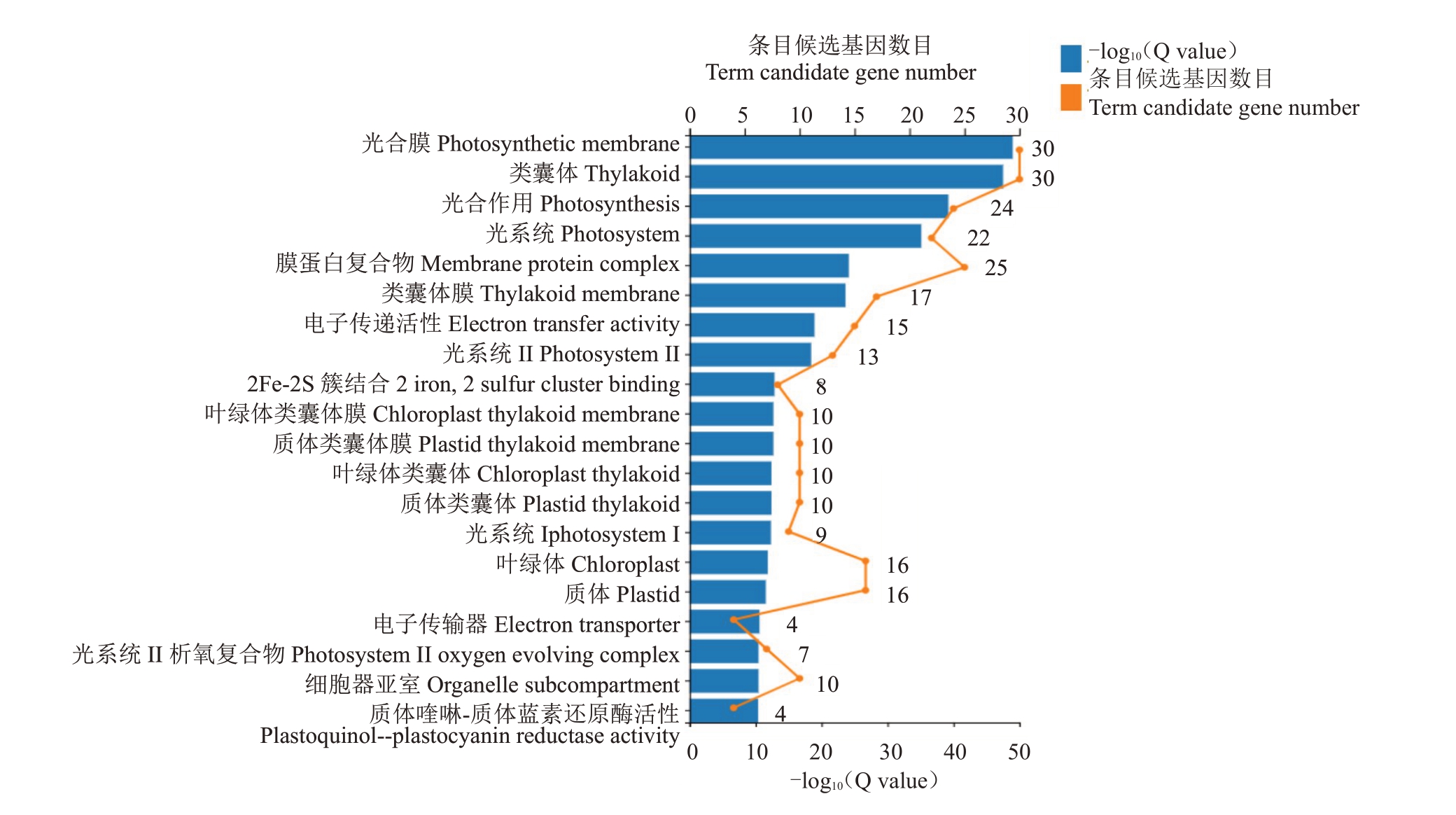
图5 光合相关差异表达基因的GO 富集图
Fig.5 GO enrichment map of photosynthesis-related differentially expressed genes
2.7 新疆野苹果响应冻害光合特性差异表达基因qRT-PCR验证
从转录组分析结果中选出PsbQ、PsaE、PsaK、PsbY、ATPF0B、Psb27 和PsbW 7 个光合作用相关的差异表达基因进行qRT-PCR 验证。结果(图6)表明,在0、3、6、12 h冻害处理下PsbQ、PsaE、PsaK、PsbY 与ATPF0B 5 个基因的表达量均上调,Psb27 和PsbW 2个基因的表达量均下调,与转录组测序结果表达趋势一致。
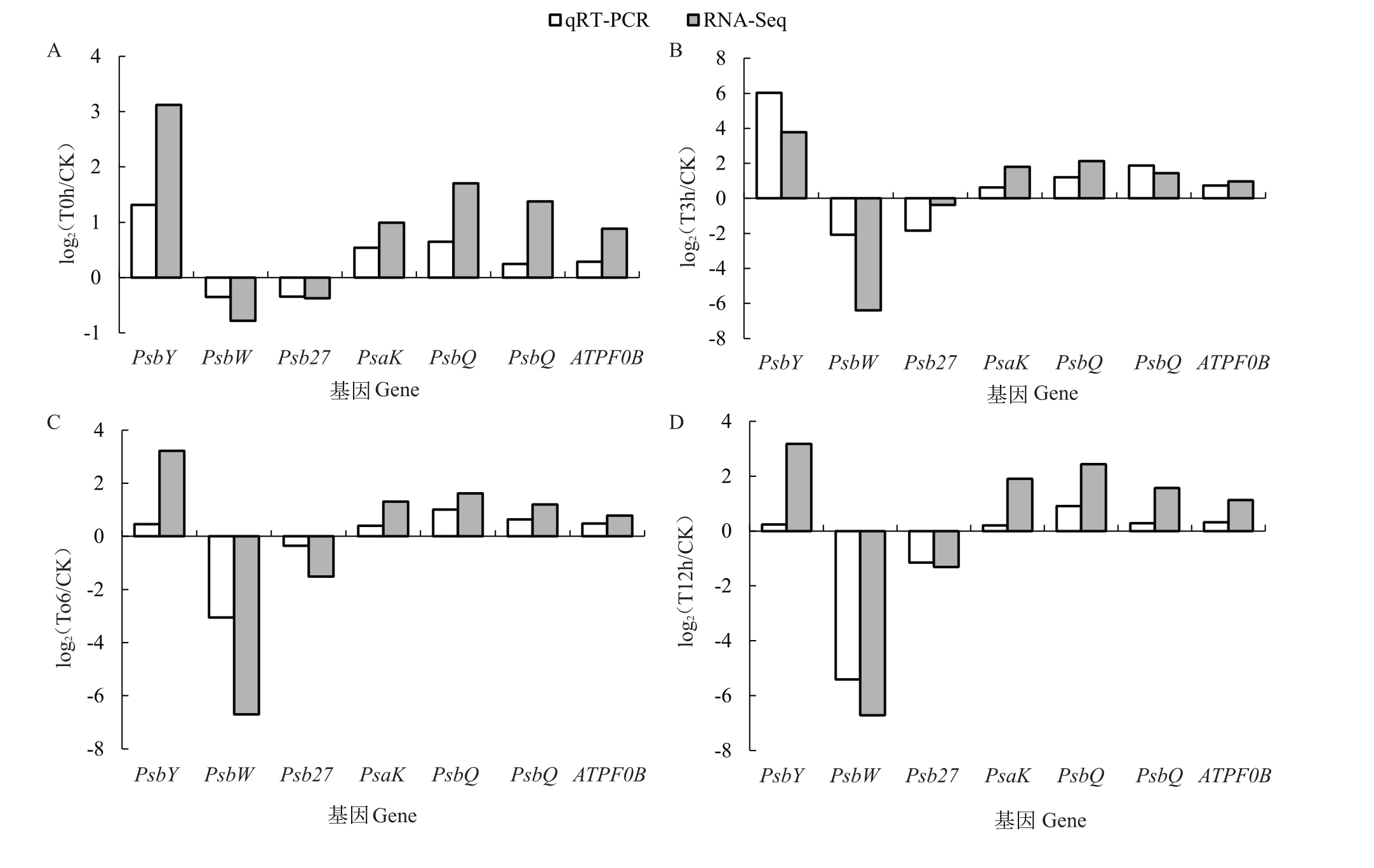
图6 差异基因的转录组表达量与qRT-PCR 验证
Fig.6 Transcriptome expression and qRT-PCR validation of differential gene
A.0 h 处理与对照的差异基因表达量;B.3 h 处理与对照的差异基因表达量;C.6 h 处理与对照下的差异基因表达量;D.12 h 处理与对照的差异基因表达量。
A.Expression of DEGs between 0 h treatment and CK;B.Expression of DEGs between 3 h treatment and CK;C.Expression of DEGs between 6 h treatment and CK;D.Expression of DEGs between 12 h treatment and CK.
3 讨 论
叶绿素是光合作用重要的光合色素,冻害条件下会使叶绿素含量降低,细胞器膜发生变化;严重时会出现质壁分离、淀粉粒消失和细胞受损等现象,从而对植物产生伤害[28]。在本研究中,-3 ℃冻害处理24 h 后组培苗叶片的叶绿素a、叶绿素b、叶绿素总含量、类胡萝卜素含量整体呈下降趋势,原因可能是低温处理对其体内代谢减弱,养分吸收速度下降,抑制叶绿素合成,产生超氧自由基积累并引发叶绿素的分解。这与葡萄在低温胁迫下的研究结果相一致[29]。此外,叶绿素a/b 出现升高的趋势,表明捕光色素含量降低,这与耿东梅等[30]的研究结果相一致。
低温胁迫下,光系统Ⅱ是植物光合结构中敏感的部位之一[31]。光系统Ⅱ作为光化学反应中心涉及了光能的吸收、转换与电子传递等光合过程[32],而PIabs作为捕光色素光能的吸收、光系统Ⅱ对光能的捕获和电子传递三方面的综合指标[33]。本实验中,-3 ℃冻害模拟条件下,新疆野苹果组培苗的光化学效率和光合性能指数受到了显著的抑制。叶绿素荧光图像从深蓝变为蓝绿色,表明组培苗的光化学效率受到影响,组培苗在温度降低的过程中光化学效率已经开始受到影响。处理0 h 时,组培苗的Fv/Fm与PIabs 分别下降了7.9%和61.2%,且随着冻害时间的延长,PIabs的下降程度始终大于Fv/Fm,表明PIabs对冻害更加敏感。胡文海等[34]研究发现,低温会使番茄叶片的天线色素对光能的吸收能力下降,光系统Ⅱ对光能的捕获能力降低,电子传递受阻等光合性能下降。
基于Illumina HiSeq 技术已经证明转录组学是帮助真核生物的基因注释与基因表达研究的有效方法[35]。在本研究中,根据转录组测序及KEGG 富集筛选得到58 个光合调控相关的差异基因,对58个差异基因进行GO 富集发现,这些基因主要发生在细胞组成中。笔者发现组培苗光合作用中的光系统Ⅱ、光系统Ⅰ、光合电子传递链、细胞色素、ATP合成酶等相关基因在冻害胁迫的各时间段表达情况不一致,其中光系统Ⅱ差异基因比光系统Ⅰ多,可能是冻害对组培苗的光系统Ⅱ的影响大于光系统Ⅰ,这与PIabs 的下降有一定的关系。在光系统Ⅱ中,下调的基因主要为氧增强蛋白基因PsbC、Psb27、PsbB、PsbW,上 调 的 基 因 主 要 为PsbP、PsbH、PsbY、PsbQ、Psb28。研究表明,psb27 可以增强非光化学猝灭和对波动光条件的耐受性[36],同时PsbW 下降也会使PQ 池氧化还原状态发生变化[37]。而Psb28 与PsbH 结合过程又可以对光系统Ⅱ起到保护作用[38]。在光系统Ⅰ中,差异基因主要为光系统Ⅰ亚基基因,且PsbB 与光系统Ⅰ亚基相互作用为铁氧还蛋白提供停靠点[39],同时PsaN、PsaG 与PsaK 是编码PEP 羧激酶的主要基因,促进光合作用氧耗散[40]。细胞色素b6/f 复合体在生氧的光合作用中连接光系统Ⅱ与光系统Ⅰ的电子传递[41]。笔者在本实验中发现细胞色素b6f 前体编码基因PetC在冻害胁迫下显著下调,郑清雷等[42]研究发现,在激素逆境胁迫前期PetC 基因上调,但随着时间的延长,PetC 基因开始下调,同时发现在其他金属胁迫方面,PetC 基因始终呈下调表达。在电子传递链中,差异基因主要为光系统Ⅰ受体侧铁氧还蛋白PetE、PetF、PetH 基因。在ATP 合成酶中,差异基因主要为AtpF 与AtpG,这两个基因是ATP合酶CF0 亚基的编码基因[43],推测CF0 亚基容易受到冻害胁迫的影响。
极显著差异表达基因PsbQ(Msi_13A005910)属于光系统Ⅱ氧释放增强蛋白3。PsbQ的主要功能有:与PsbP 的结合,维持高等植物光系统Ⅱ中水氧化机械的催化Mn簇[44],对光系统Ⅱ的水氧化与氧气的释放起着重要作用,它们的缺失也会导致光系统氧化侧和还原侧的电子传递缺陷[45]。同时PsbQ 与绿色植物的光系统Ⅱ管腔表面结合,稳定放氧复合体中心构象并维持整体酶促功能[46],也有人提出PsbQ 对叶绿体类囊体的稳定性起着重要作用[47]。本研究中-3 ℃模拟冻害条件下PsbQ 基因上调,这与在海棠与木薯研究中的结果相一致[48-49],而在低温和短光周期对笃斯越橘光合作用研究中发现PsbQ显著下调表达[50],可能是不同树种的作用方式不完全一致。此外,BGI_novel_G011171(PsbY)也呈显著上调。研究发现,PsbY 与细胞色素b559 紧密接触或直接接触,PsbY 较少的植物更容易受到光抑制[51],这与Zhou 等[19]的研究结果一致,这表明PsbY在新疆野苹果组培苗冻害中起着光保护作用。PsbQ与PsbY在新疆野苹果响应冻害胁迫中的功能与作用需进一步深入研究。
4 结 论
新疆野苹果组培苗在-3 ℃模拟冻害条件下,与对照相比,Fv/Fm与PIabs均下降,叶片从处理12 h开始卷缩,处理24 h 时叶绿素含量均下降,叶绿素a/b上升,通过转录组测序筛选到58个光合调控相关显著差异表达基因,其中Msi_13A005910(PsbQ)与BGI_novel_G011171(PsbY)发生极显著上调表达。PsbQ与PsbY可能会通过光氧化与光保护缓解冻害对组培苗叶绿体及光合作用产生的影响。
[1] 陈学森,韩明玉,苏桂林,刘凤之,过国南,姜远茂,毛志泉,彭福田,束怀瑞.当今世界苹果产业发展趋势及我国苹果产业优质高效发展意见[J].果树学报,2010,27(4):598-604.CHEN Xuesen,HAN Mingyu,SU Guilin,LIU Fengzhi,GUO Guonan,JINAG Yuanmao,MAO Zhiquan,PENG Futian,SHU Huairui.Discussion on today’s world apple industry trends and the suggestions on sustainable and efficient development of apple industry in China[J].Journal of Fruit Science,2010,27(4):598-604.
[2] 韩立新,王红艳·苹果花期低温冻害防御措施[J].现代园艺,2019(1):72-73.HAN Lixin,WANG Hongyan.Defensive measures of low temperature and frost injury during apple flowering period [J].Contemporary Horticulture,2019(1):72-73.
[3] WU J K,DUAN X J,ZHU Z L,SANG Z Y,DUAN J,JIA Z K,MA L Y.Physiological and transcriptome analysis of Magnolia denudata leaf buds during long-term cold acclimation[J/OL].BMC Plant Biology,2021,21(1):460.https://bmcplantbiol.biomedcentral.com/articles/10.1186/s12870-021-03181-5.
[4] AN J P,WANG X F,ZHANG X W,XU H F,BI S Q,YOU C X,HAO Y J.An apple MYB transcription factor regulates cold tolerance and anthocyanin accumulation and undergoes MIEL1-mediated degradation[J].Plant Biotechnology Journal,2020,18(2):337-353.
[5] AGARWAL M,HAO Y J,KAPOOR A,DONG C H,FUJII H,ZHENG X W,ZHU J K.A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance[J].The Journal of Biological Chemistry,2006,281(49):37636-37645.
[6] BILSKA A,SOWINSKI P.Closure of plasmodesmata in maize(Zea mays) at low temperature:A new mechanism for inhibition of photosynthesis[J].Annals of Botany,2010,106(5):675-686.
[7] HAO J H,GU F Y,ZHU J,LU S W,LIU Y F,LI Y F,CHEN W Z,WANG L P,FAN S X,XIAN C J.Low night temperature affects the phloem ultrastructure of lateral branches and raffinose family oligosaccharide (RFO) accumulation in RFO-transporting plant melon (Cucumis melo L.) during fruit expansion[J].PLoS One,2017,11(8):e0160909.
[8] 向春燕·苹果果实叶绿素荧光参数与低温胁迫关系的研究[D].杨凌:西北农林科技大学,2014.XIANGChunyan.Studyontherelationshipbetweenchlorophyllfluorescence parameters and low temperature stress of apple fruit[D].Yangling:Northwest A&F University,2014.
[9] 孙鲁龙,耿庆伟,邢浩,杜远鹏,翟衡.低温处理葡萄根系对叶片PSII 活性的影响[J].植物学报,2017,52(2):159-166.SUN Lulong,GENG Qingwei,XING Hao,DU Yuanpeng,ZHAI Heng.Effect of low temperature treatments in root of grapevine on PSII activity in leaves[J].Chinese Bulletin of Botany,2017,52(2):159-166.
[10] WANG M L,ZHANG S X,DING F.Melatonin mitigates chilling-induced oxidative stress and photosynthesis inhibition in tomato plants[J/OL].Antioxidants(Basel),2020,9(3):218.https://doi.org/10.3390/antiox9030218.
[11] 金姣姣,刘自刚,米文博,徐明霞,邹娅,徐春梅,赵彩霞.利用RNA-Seq 鉴定调控甘蓝型油菜叶片光合特性的低温胁迫应答基因[J].生物技术通报,2022,38(4):1-17.JIN Jiaojiao,LIU Zigang,MI Wenbo,XU Mingxia,ZOU Ya,XU Chunmei,ZHAO Caixia.Identification of low temperature stress-responsive genes that regulate photosynthetic characteristics in the leaves of Brassica napus by RNA-sequencing[J].Biotechnology Bulletin,2022,38(4):1-17.
[12] 王君雅,陈玮,刘丁丁,陈亮,姚明哲,马春雷.不同品种茶树新梢响应“倒春寒”的转录组分析[J].茶叶科学,2019,39(2):181-192.WANG Junya,CHEN Wei,LIU Dingding,CHEN Liang,YAO Mingzhe,MA Chunlei.The transcriptome analysis of different tea cultivars in response to the spring cold spells[J].Journal of Tea Science,2019,39(2):181-192.
[13] 张军,杨敏生,张钢,郭仲军,臧润国.新疆野苹果天然群体抗寒性的电阻抗图谱分析[J].林业科学,2011,47(4):20-26.ZHANG Jun,YANG Minsheng,ZHANG Gang,GUO Zhongjun,ZANG Runguo.Assessing frost hardiness of Malus sieversii natural populations by electrical impedance spectroscopy[J].Scientia Silvae Sinicae,2011,47(4):20-26.
[14] 王泽华.不同居群新疆野苹果抗寒性研究[D].乌鲁木齐:新疆农业大学,2017.WANG Zehua.Study on cold resistance of Malus sieversii in different populations[D].Urumqi:Xinjiang Agricultural University,2017.
[15] 闫娟娟,秦伟,肖运强,马依努尔姑.新疆野苹果28 个种下类型叶片解剖结构对比研究[J].新疆农业大学学报,2014,37(4):298-305.YAN Juanjuan,QIN Wei,XIAO Yunqiang,Mnyinuergu.Comparative study on anatomical structure of 28 types of Malus sieversii leaves[J].Journal of Xinjiang Agricultural University,2014,37(4):298-305.
[16] 于立洋,李政,韩佩尧,张静,汪敏骅,田晓晓,张军.8 个新疆野苹果优良无性系抗寒性比较[J].核农学报,2017,31(9):1827-1835.YU Liyang,LI Zheng,HAN Peiyao,ZHANG Jing,WANG Minhua,TIAN Xiaoxiao,ZHANG Jun.Comparison of cold hardiness of 8 Malus sieversii clones[J].Journal of Nuclear Agricultural Sciences,2017,31(9):1827-1835.
[17] 文玉珍,徐晖,于玮玮,杨美玲,龙鸿,阎国荣.低温胁迫下新疆野苹果离体叶片生理特性分析[J].天津农学院学报,2014,21(1):39-42.WEN Yuzhen,XU Hui,YU Weiwei,YANG Meiling,LONG Hong,YAN Guorong.Physiological analysis of leaves in vitro of Malus sieversi under low temperature stress[J].Journal of Tianjin Agricultural University,2014,21(1):39-42.
[18] 赵玲玲,宋来庆,刘志,谢兴斌,翟衡,郝玉金·低温胁迫下4 种苹果砧木叶片多胺的变化[J].果树学报,2008,25(2):151-156.ZHAO Lingling,SONG Laiqing,LIU Zhi,XIE Xingbin,ZHAI Heng,HAO Yujin.Changes of polyamines in the leaves of 4 apple rootstocks during low temperature stress[J].Journal of Fruit Science,2008,25(2):151-156.
[19] ZHOU P,LI X,LIU X S,LIU X J,WEN X J,ZHANG Y,ZHANG D Y.Transcriptome profiling of Malus sieversii under freezing stress after being cold- acclimated[J/OL].BMC Genomics,2021,22(1):681.https://bmcgenomics.biomedcentral.com/articles/10.1186/s12864-021-07998-0.
[20] 何晨晨,刘俐君,鲁晓燕.基于转录组测序分析NaCl 胁迫下新疆野苹果叶和根糖酵解相关基因的表达[J].果树学报,2020,37(7):951-961.HE Chenchen,LIU Lijun,LU Xiaoyan.Analysis of genes related to glycolysis in the leaves and roots of Malus sieversii under NaCl stress based on transcriptome sequencing[J].Journal of Fruit Science,2020,37(7):951-961.
[21] 范宗民,孙军利,赵宝龙,刘怀锋,于坤,章智钧,刘晶晶.不同砧木‘赤霞珠’葡萄枝条抗寒性比较[J].果树学报,2020,37(2):215-225.FAN Zongmin,SUN Junli,ZHAO Baolong,LIU Huaifeng,YU Kun,ZHANG Zhijun,LIU Jingjing.Evaluation of cold resistance of one-year shoots from‘Cabernet Sauvignon’grape vine grafted on different rootstocks[J].Journal of Fruit Science,2020,37(2):215-225.
[22] KIM D,LANGMEAD B,SALZBERG S L.HISAT:A fast spliced aligner with low memory requirements[J].Nature Methods,2015,12(4):357-360.
[23] LANGMEAD B,SALZBERG S L.Fast gapped-read alignment with Bowtie 2[J].Nature Methods,2012,9(4):357-359.
[24] LI B,COLIN N D.RSEM:Accurate transcript quantification from RNA-Seq data with or without a reference genome[J/OL].BMC Bioinformatics,2011,12(1):323.https://bmcbioinformatics.biomedcentral.com/articles/10.1186/1471-2105-12-323.
[25] LOVE M I,HUBER W,ANDERS S.Moderated estimation of foldchange and dispersion for RNA-seq data with DESeq2[J].Genome Biology,2014,15(12):550.
[26] CHEN C J,CHEN H,ZHANG Y,HANNAH R T,MARGARET H F,HE Y H,XIA R.TBtools:An integrative toolkit developed for interactive analyses of big biological data[J].Molecular Plant,2020,13(8):1194-1202.
[27] 岳俊芹,张素瑜,李向东,邵运辉,方保停,葛胜修,王汉芳,张德奇,杨程,时艳华,秦峰.低温胁迫对小麦叶绿素荧光参数及产量的响应[J].麦类作物学报,2021,41(1):105-110.YUE Junqin,ZHANG Suyu,LI Xiangdong,SHAO Yunhui,FANG Baoting,GE Shengxiu,WANG Hanfang,ZHANG Deqi,YANG Cheng,SHI Yanhua,QIN Feng.Effect of low temperature stress on chlorophyll fluorescence parameters and yield of wheat[J].Acta Cropica Sinica,2021,41(1):105-110.
[28] 李晓靖,崔海军.低温胁迫下植物光合生理研究进展[J].山东林业科技,2018,48(6):90-94.LI Xiaojing,CUI Haijun.Research progress on the physiological response of plants to environmental stress[J].Journal of Shandong Forestry Science and Technology,2018,48(6):90-94.
[29] 赵滢,王振兴,许培磊,杨义明,刘迎雪,刘海双,艾军.山葡萄‘双丰’和‘左优红’叶绿素荧光特性及活性氧代谢与低温伤害的关系[J].园艺学报,2018,45(4):650-658.ZHAO Ying,WANG Zhenxing,XU Peilei,YANG Yiming,LIU Yingxue,LIU Haishuang,AI Jun.The characteristics of chlorophyll fluorescence and metabolism of reactive oxygen species in relation to the cold injury of vitis amurensis‘Shuangfeng’and‘Zuoyouhong’[J].ActaHorticulturaeSinica,2018,45(4):650-658.
[30] 耿东梅,单立山,李毅,Жигyнoв Aнaтoлий Bacильeвич.土壤水分胁迫对红砂幼苗叶绿素荧光和抗氧化酶活性的影响[J].植物学报,2014,49(3):282-291.GENG Dongmei,SHAN Lishan,LI Yi,Жигyнoв Aнaтoлий Bacильeвич.Effect of soil water stress on chlorophyll fluorescence and antioxidant enzyme activity in reaumuria soongorica seedlings[J].Chinese Bulletin of Botany,2014,49(3):282-291.
[31] MURATA N,TAKAHASHI S,NISHIYAMA Y,ALLAKHVERDIEV S I.Photoinhibition of photosystem II under environmental stress[J].Biochimica et Biophysica Acta-Bioenergetics,2007,1767(6):414-421.
[32] LIU X J,AN B Y,GU N,GUO C N,SUN X G,WANG H.Response of leaf photosynthetic characteristics of Syringa oblata and Syringa reticulata var. mandshurica to chilling stress[J].Journal of Forestry Research,2020,31(4):521-530.
[33] PISANI P L.Probing photosynthesis:Mechanisms,regulation,and adaptation[M].London,Taylor&Francis,2000:445-483.
[34] 胡文海,胡雪华,闫小红,周升团.低温胁迫及恢复对番茄快速叶绿素荧光诱导动力学特征的影响[J].中国农业气象,2021,42(10):859-869.HU Wenhai,HU Xuehua,YAN Xiaohong,ZHOU Shengtuan.Response of chlorophyll fluorescence transient in leaves of tomato under chilling stress and subsequent recovery[J].Chinese Journal of Agrometeorology,2021,42(10):859-869.
[35] CROUCHER N J,FOOKES M C,PERKINS T T,TURNER D J,MARGUERAT S B,KEANE T,QUAIL M A,HE M,ASSEFA S,BÄHLER J,KINGSLEY R A,PARKHILL J,BENTLEY S D,DOUGAN G,THOMSON N R.A simple method for directional transcriptome sequencing using Illumina technology[J/OL].Nucleic Acids Research,2009,37(22):e148.Doi:10.1093/NAR/GKP811
[36] JOHNSON V M,BISWAS S,ROOSE J L,PAKRASI H B,LIU H J.Psb27.A photosystem II assembly protein,enables quenching of excess light energy during its participation in the PSII lifecycle[J/OL].Photosynthesis Research,2022:1-8.DOI:10.1007/s11120-021-00895-3.
[37] JOSÉ G,GARCÍA C,LASZLO K,TÜNDE T,SAMI K,ELENA A,EGBERT J B,FIKRET M,CHRISTIANE F,WOLFGANG P S.The PsbW protein stabilizes the supramolecular organization of photosystem II in higher plants[J].The Plant Journal:For Cell and Molecular Biology,2011,65(3):368-381.
[38] XIAO Y N,HUANG G Q,YOU X,ZHU Q J,WANG W D,KUANG T Y,HAN G Y,SUI S F,SHEN J R.Structural insights into cyanobacterial photosystem II intermediates associated with Psb28 and Tsl0063[J].Nature Plants,2021,7(8):1132-1142.
[39] CHITNIS V P,KE A,CHITNIS P R.The PsaD subunit of photosystem I.Mutations in the basic domain reduce the level of PsaD in the membranes[J].Plant Physiology,1997,115(4):1699-1705.
[40] FURUMOTO T,HATA S,IZUI K.Isolation and characterization of cDNAs for differentially accumulated transcripts between mesophyll cells and bundle sheath strands of maize leaves[J].Plant&Cell Physiology,2000,41(11):1200-1209.
[41] MALONE L A,QIAN P,MAYNEORD G E,HIT CH COCK A,JOHNSON M P.Cryo-em structure of the spinach cytochrome b6fcomplex at 3.6 resolution[J].Nature,2009,575(7783):535-539.
[42] 郑清雷,余陈静,姚坤存,黄宁,阙友雄,凌辉,许莉萍.甘蔗Rieske Fe/S 蛋白前体基因ScPetC 的克隆及表达分析[J].作物学报,2020,46(6):844-857.ZHENG Qinglei,YU Chenjing,YAO Kuncun,HUANG Ning,QUE Youxiong,LING Hui,XU Liping.Cloning and expression analysis of sugarcane Fe/S precursor protein gene ScPetC[J].Acta Agronomica Sinica,2020,46(6):844-857.
[43] HÖGLUND A S,PLANT A L,GRAY J C.Expression of the wheat chloroplast gene for CF0 subunit IV of ATP synthase[J].Current Genetics,1990,18(5):471-476.
[44] KAKIUCHI S,UNO C,IDO K,NISHIMURA T,NOGUCHI T,IFUKU K,SATO F.The PsbQ protein stabilizes the functional binding of the PsbP protein to photosystem II in higher plants[J].Biochimical Biophysica Acta,2012,1817(8):1346-1351.
[45] BRICKER T M,FRANKEL L K.Auxiliary functions of the PsbO,PsbP and PsbQ proteins of higher plant Photosystem II:A critical analysis[J].Journal of Photochemistry and Photobiology B Biology,2011,104(1/2):165-178.
[46] IDO K,NIELD J,FUKAO Y,NISHIMURA T,SATO F,IFUKU K.Cross-linking evidence for multiple interactions of the PsbP and PsbQ proteins in a higher plant photosystem II supercomplex[J].The Journal of Biological Chemistry,2014,289(29):20150-20157.
[47] ANDERSON J M,CHOW W S,DE L R J.Dynamic flexibility in the structure and function of photosystem II in higher plant thylakoid membranes:The grana enigma[J].Photosynthesis Research,2008,98(1/3):575-587.
[48] JIAXM,ZHUYF,HUY,ZHANGR,CHENGL,ZHUZL,ZHAO T,ZHANG X Y,WANG Y X.Integrated physiologic,proteomic,and metabolomic analyses of Malus halliana adaptation to salinealkali stress[J].Horticulture Research,2019,6(1):1-19.
[49] 庄盈婷.木薯叶片和叶绿体应答盐胁迫的比较蛋白质组学研究[D].海口:海南大学,2014.ZHUANG Yingting.Comparative proteomics of Manihot esculenta leaves and chloroplasts under salt stress[D].Haikou:Hainan University,2014.
[50] 乌凤章.低温和短光周期对笃斯越橘叶绿素荧光特性和光合作用相关蛋白的影响[J].东北农业大学学报2018,49(12):18-26.WU Fengzhang.Effect of low temperature and short photoperiod on photosynthetic related proteins and chlorophyll fluorescence characteristics of Vaccinium uligiuosum L.[J].Journal of Northeast Agricultural University,2018,49(12):18-26.
[51] LOTTA V S,SERENA S,JÖRG M,CHRISTIANE F,FIKRET M,WOLFGANG P S.The PsbY protein of arabidopsis photosystem II is important for the redox control of cytochrome b559[J].Biochimica et Biophysica Acta-Bioenergetics,2016,1857(9):1524-1533.