核桃腐烂病又称烂皮病、黑水病等,主要为金黄壳囊孢菌Cytospora chrysosperma(有性型Valsa sordida)引发的真菌病害[1]。该病在新疆栽培核桃园中发生较为普遍,主要危害枝干的皮层,当发现皮下出现黑色黏稠的汁液时,皮下已扩展数厘米以上的病斑,轻者主枝枯死,重者整株枯萎死亡[2-4]。对核桃腐烂病的发病情况进行调查发现,部分重病园发病率高达90%[5],已成为影响新疆核桃树健康生长发育的重要病害之一。筛选出抗病种质进而选育栽培抗病新品种,是控制植物病害发生的有效途径[6]。在以往的研究中,主要通过离体评价初步筛选抗病种质,但离体材料的抗性可能弱于完整植株,鉴定结果可能会有所偏差[7]。研究发现植物在受到病原物侵染后,膜系统发生伤害,细胞膜透性增加,丙二醛(MDA)累积,它和过氧化物酶(POD)、超氧化物歧化酶(SOD)、过氧化氢酶(CAT)等抗氧化酶均参与了植物的防御,可能与植物的抗病性有关,可以作为植物抗病性鉴定的生理指标[8]。在此基础上利用主成分分析、聚类分析相结合的方法,筛选出所需的高抗种质,有效避免了传统方法的不足[9-10]。
新疆野核桃(Juglans regia L.)是胡桃科(Juglandaceae)、胡桃属(Juglans)植物,多年生落叶乔木,王磊等[11]按种子特征将其划分有14 个类型。在我国仅成片分布于巩留野核桃林自然保护区,被认为是中亚栽培核桃(Juglans regia L.)的直系祖先[12],亦是研究栽培核桃之起源、进化以及遗传变异的重要基因库。目前,对新疆野核桃的地理分布[13]、抗性生理[14-15]、遗传多样性[16-17]、营养品质[18]、年龄结构及生长特性[19]、核心种质的构建[16,20]以及遗传图谱[21]等方面进行了研究。王肇延[16]调查发现,该地区野核桃不同种质间的抗核桃腐烂病能力存在明显差别,根据患病差异可划分为4个等级。野核桃树种质资源丰富,但与其他林果种质资源相比,野核桃树种质资源抗病资源研究较少,尚需进一步挖掘。本研究以新疆野核桃7 个类型的28 份种质为材料,通过人工接种,记录不同发病情况,测定过氧化物酶(POD)、超氧化物歧化酶(SOD)、过氧化氢酶(CAT)活性和丙二醛含量(MDA)等生理生化指标,采用主成分分析、系统聚类分析对新疆野核桃抗病综合评价,筛选抗性较强的种质,以期为后续野核桃种质资源的合理开发利用提供科学依据。
1 材料和方法
1.1 试验材料
1.1.1 采样 以已构建的新疆野核桃种质资源基础数据库为采样依据[16],2021 年5 月下旬在新疆伊犁巩留县野核桃林(82°17′56″E,43°27′21″N)选取28份树势良好的种质,采摘中部外围无病害且粗细长短、含水量一致的1 年生枝条,将采集的枝条剪成20 cm小段,每份资源取5根枝段,切面用乳胶封口,记好编号放入编织袋中,带回实验室,用无菌水擦拭并用75%乙醇消毒备用。
1.1.2 培养基 PDA 培养基:马铃薯(切块去皮)200 g,琼脂13 g,葡萄糖15 g,无菌水1000 mL,用于菌株纯化[4]。致病菌株由新疆农业大学林木病理实验室提供。
菌饼:本试验所用核桃腐烂病菌株为新疆农业大学马荣研究组分离鉴定的金黄壳囊孢(Cytospora chrysosperma)(编号XJAU-936)[1],该菌株置于斜面PDA 培养基4 ℃冰箱保存,保存于新疆农业大学林木病理实验室。试验时挑取菌丝接种于PDA 培养基上,在恒温培养箱中25 ℃黑暗培养6 d,在菌落边缘打取直径5 mm的菌饼作为接种体。
1.2 试验处理
将28 份新疆野核桃枝条去掉梢端和末端,每份样本3 个重复,采用枝条打孔法[4],每根枝条用灭菌的打孔器制造3 个深达木质部的伤口,约5 mm大小,随后接种纯化6 d 的PDA 菌饼于伤口处,纱布保湿并用保鲜膜二次固定,将枝条插入浸有无菌水的花泥中,置于恒温25 ℃保湿培养。隔天取下接种体,每天早晚喷雾保湿各1 次,第12 天通过十字交叉法使用软皮尺记录病斑长度和宽度[22],并计算发病程度(公式1)[22-23]。取枝条病斑外围的韧皮部,液氮速冻后-40 ℃冰箱保存,用于测量生理指标。
1.3 实验方法
测定方法参考刘家尧等[24]编著的《植物生理学实验教程》。POD 活性采用愈创木酚显色法测定,SOD 活性采用氮蓝四唑法测定,MDA 含量采用硫代巴比妥酸法测定。CAT 活性采用北京Solarbio(CAT)ELISA试剂盒说明测定。
1.4 分析方法
主成分分析法:使用SPSS 统计分析软件,通过描述统计的方法将数据进行标准化,将标准化的数据通过降维的方式进行主成分分析[25]。在主成分提取表中,根据特征根大于1确定主成分的数量,特征根用λ表示。主成分系数等于各变量因子载荷向量除以各自主成分的特征根的算术平方根,见公式(2):
因子载荷值是指确定各个指标对各成分的影响,在成分矩阵中表示。计算各品种的综合得分,见公式(3):
Q 为综合得分,Wi 为主成分提取表中的方差贡献率,Fi 表示主成分的特征根对应的特征向量的和。利用Between-groups linkage(组间距离)法对所得结果进行系统聚类评价[26],比较新疆野核桃种质抗病能力强弱,对生理指标与发病程度进行Pearson相关性分析[27]。
1.5 数据处理
采用Excel 2016 对数据进行统计,采用SPSS 22.0数据分析软件进行多重比较分析、相关性分析、主成分分析及聚类分析。
2 结果与分析
2.1 新疆野核桃种质资源离体枝条接种后发病情况
测定结果表明,将培养6 d的核桃腐烂病菌PDA菌饼接种于枝条韧皮部,25 ℃保温保湿培养12 d,能够有效地侵染1年生枝条,且显现出不同程度的差异(图1)。

图1 新疆野核桃种质资源感病情况
Fig.1 The level of infection and canker disease of Xinjiang wild walnuts
图中为部分抗病性差异明显的野核桃资源枝条感染腐烂病的图片,从左到右依次为高抗、中抗、中感、高感。
The picture shows some wild walnut resource branches infected with canker disease with obvious difference in disease resistance,from left to right:highly resistant,mediumly resistant,mediumly sensitive,and highly sensitive.
由表1可见,28份材料离体枝条接种核桃腐烂病病原菌后发病程度呈明显差异,其中枝条病斑最小值为5 mm,最大值为100 mm,枝条表面病斑明显,病部枝条颜色变黑失水。J-1、J-2、XT-3、XT-4、XT-5、XY-1、XY-2、XY-4、X-5、T-2、J-3、P-1 的病斑长度均小于15 mm,其中除T-2、J-3、P-1外,其余9份材料均未形成病斑;L-3、XT-2、P-2、T-3、XY-3、J-4形成的病斑长度在15~35 mm 之间;X-4、X-3、X-2、XY-5、T-1 形成的病斑长度在35~55 mm之间;P-3、L-2、XT-1、X-1、L-1形成的病斑长度均超过55 mm。多重比较结果显示,枝条病斑最长的L-1 与未发病的9 份材料之间差异显著(p<0.05),其中侵染病斑长度小于15 mm的单株占供试单株的比例较大,占总供试单株的42.9%,而侵染病斑长度超过55 mm 抗病能力弱的单株,仅有5 株(17.9%)。由核桃腐烂病病原菌形成的病斑长度差异,反映出野核桃种质间的抗病能力存在差异。
表1 核桃腐烂病菌侵染后野核桃枝条发病程度
Table 1 The severity of disease of walnut branches infected by walnut canker

注:同列不同小写字母表示不同菌株间差异显著(p <0.05),表内数据为(平均值±标准误差)。下同。
Note: Different small letters in the same column indicate significant difference between different strains at p <0.05,the data in the Table are the mean±standard error.The same below.
2.2 核桃腐烂病菌对新疆野核桃种质生理指标的影响
接种核桃腐烂病菌12 d 后,不同新疆野核桃种质的抗病生理响应表现出差异。表2 显示,28 份野核桃材料中SOD 酶活性最高为X-4、XT-2、XY-5、T-1,且都表现为感病材料,病斑的最长的L-1、XT-1、X-1的SOD酶活性最低,可能随着病原菌侵染,已达到生理阈值;POD酶活性最高的为T-1、XT-1、X-3,3个种质均为感病材料,POD 酶活性最低的为X-5、XT-4、J-1,且3个材料均未发病;CAT酶活性最高的为XT-2、L-2、X-1,其中L-2、X-1 为重度患病材料;CAT 酶活性最低的为J-3、J-1、XT-5,其中J-1、XT-5均未发病,J-3患病较轻;MDA含量最高的为XY-3、XT-1、L-1、X-1,均为感病材料,且XT-1、L-1、X-1 3个材料的病斑长度都表现为较高,MDA含量最低的为XT-4、T-2、XY-1、XY-4、XY-2,均为未发病材料。发病材料枝条的平均POD 酶活性、SOD 酶活性、CAT酶活性、MDA含量相比未发病枝条均有不同程度上升,分别为未发病枝条(平均)的260.6%、118.9%、283.6%和192.6%,其中POD 酶活性、CAT酶活性、MDA 含量与枝条病斑长度的相关系数达0.765、0.567、0.614(表3),呈极显著正相关(p<0.01),枝条发病越严重,韧皮部POD 活性、CAT 活性、MDA 含量越高,抗性越差;野核桃发病材料SOD活性相比未发病虽有不同程度提高,但未发现与枝条病斑长度呈显著线性关系。
表2 生理指标测定结果
Table 2 The results of physiological indicators

注:表内数据为平均值±标准误差。
Note:Data in the Table are means±standard error.
表3 新疆野核桃生理指标的相关性分析
Table 3 Correlation analysis of physiological indexes of Xinjiang wild walnuts
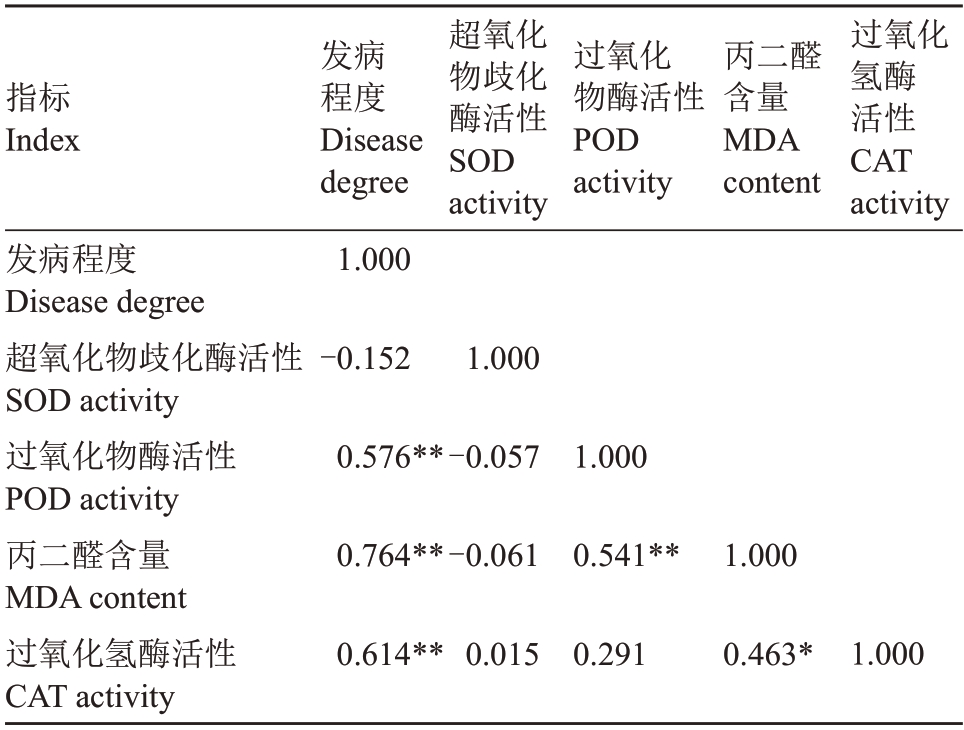
注:相关系数为Pearson 相关性分析所得,* p <0.05;** p <0.01。下同。
Note: The correlation coefficient is obtained by Pearson correlation analysis,*p <0.05;**p <0.01.The same below.
2.3 主成分分析法综合评价
2.3.1 新疆野核桃生理指标的主成分分析 对新疆野核桃抗病生理指标进行主成分分析,得到5 个主成分集合(表4),由表4 所示,前2 个主成分集合特征值都大于1,且方差贡献率累计达73.42%,对原始数据解释率较高,可作为评价新疆野核桃抗病性的综合指标。选出前两个主要成分给出的负载矩阵(表5),根据1.4计算公式,利用2个主成分特征值与表5中的相关性负荷量构建评价模型如下:
表4 新疆野核桃抗病生理指标的特征值和贡献率
Table 4 Eigenvalues and contribution rates of disease resistance related physiological indexes of Xinjiang wild walnut

表5 新疆野核桃抗病生理指标的负荷量
Table 5 Loads of physiological indexes for disease resistance of Xinjiang wild walnut
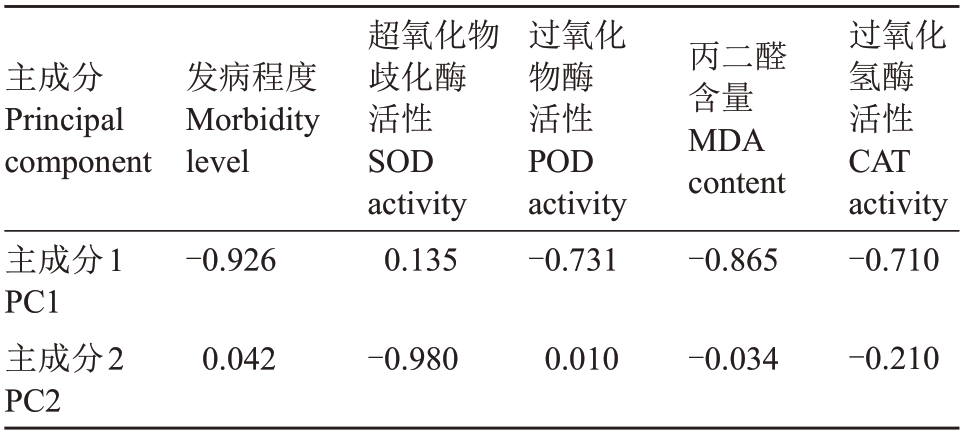
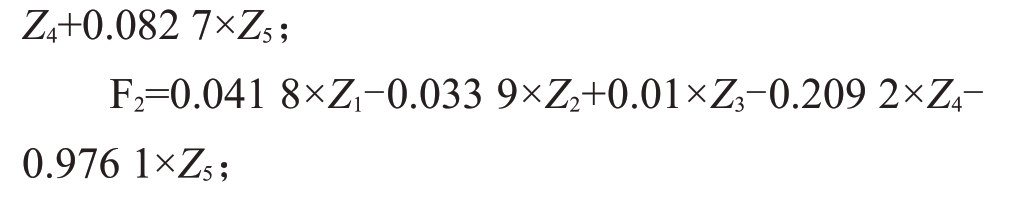
Z1~Z5为原始数据标准化处理,以主成分对应贡献率作为权重,算出综合得分:
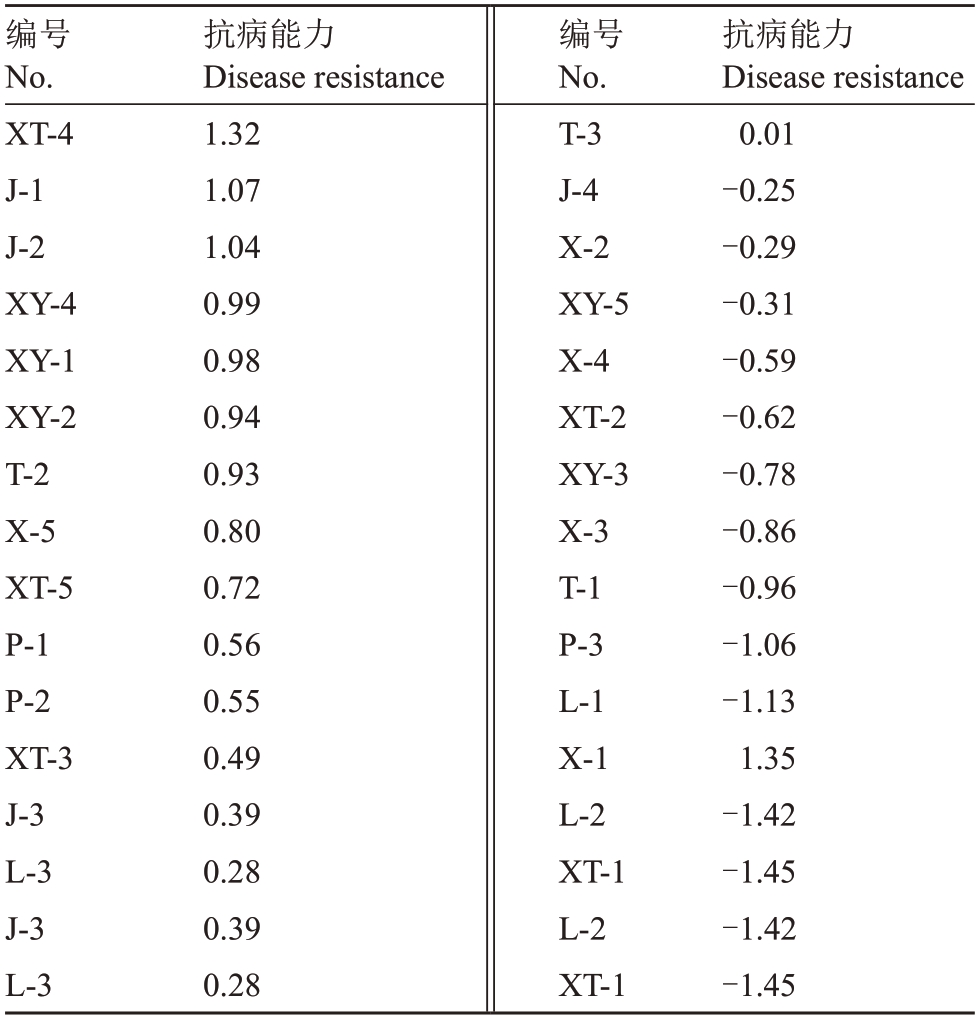
其中Z1~Z5依次代表发病程度、MDA含量、POD酶活性、CAT酶活性、SOD酶活性,F、F1、F2表示主成分的特征根对应的特征向量的和,通过计算得到不同种质新疆野核桃得分,综合得分值越大,抗病能力越好。表6显示,XT-1的抗病能力最低,达-1.45;为低抗病性。XT-4 的抗病能力最高,达1.32,为高抗病单株,其中得分为0.93~1.32的新疆野核桃种质抗病能力较好,为XT-4、J-1、J-2、XY-4、XY-1、XY-2、T-2。
表6 28 份新疆野核桃材料抗病能力比较
Table 6 Comparison of disease resistance among 28 Xinjiang wild walnut materials
2.3.2 对新疆野核桃聚类评价 根据表6野核桃抗病能力得分,算出不同类型核桃的综合得分,表7显示,7 种类型新疆野核桃抗病能力依次为尖果形>小圆形>小椭圆形>平底圆形>椭圆形>心形>卵圆形。图2 显示,28 个样本的综合抗病能力在欧式距离4 处可分为3 类,A 类包括XY-5、X-2 等4 个种质,B类包括XT-4、J-1、J-2等14个种质,C类包括L-2、XT-1等10个种质。结合表1结果可知,其中B类中的野核桃抗病能力最强,相比抗病能力最差的C类,各单株受病理侵染胁迫程度较小,且多数未发病,属高抗病类型,A类核桃则属于中间类型。此外由主成分分析综合筛选的7个高抗种质XT-4、J-1、J-2、XY-4、XY-1、XY-2、T-2 以及低发病水平的尖果型种质、小圆型种质也多属于B类,显示抗病性较强。
表7 新疆野核桃不同类型综合抗病能力分析
Table 7 Analysis on comprehensive disease resistance of different types of Xinjiang wild walnut
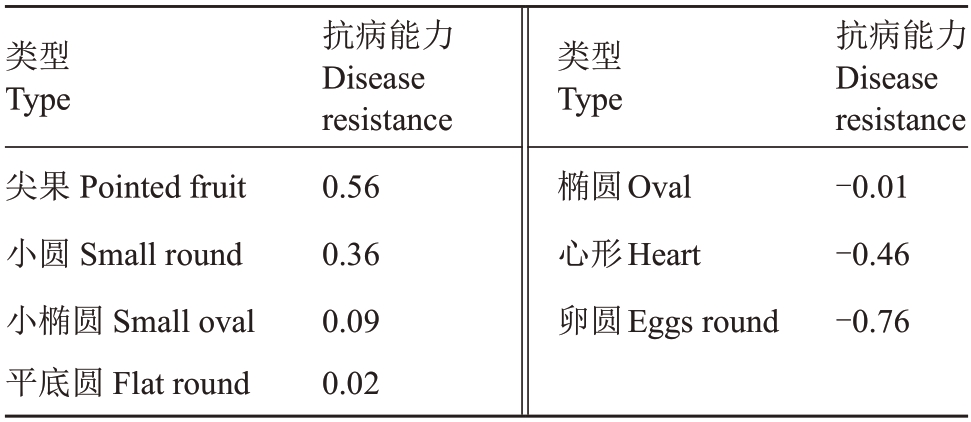
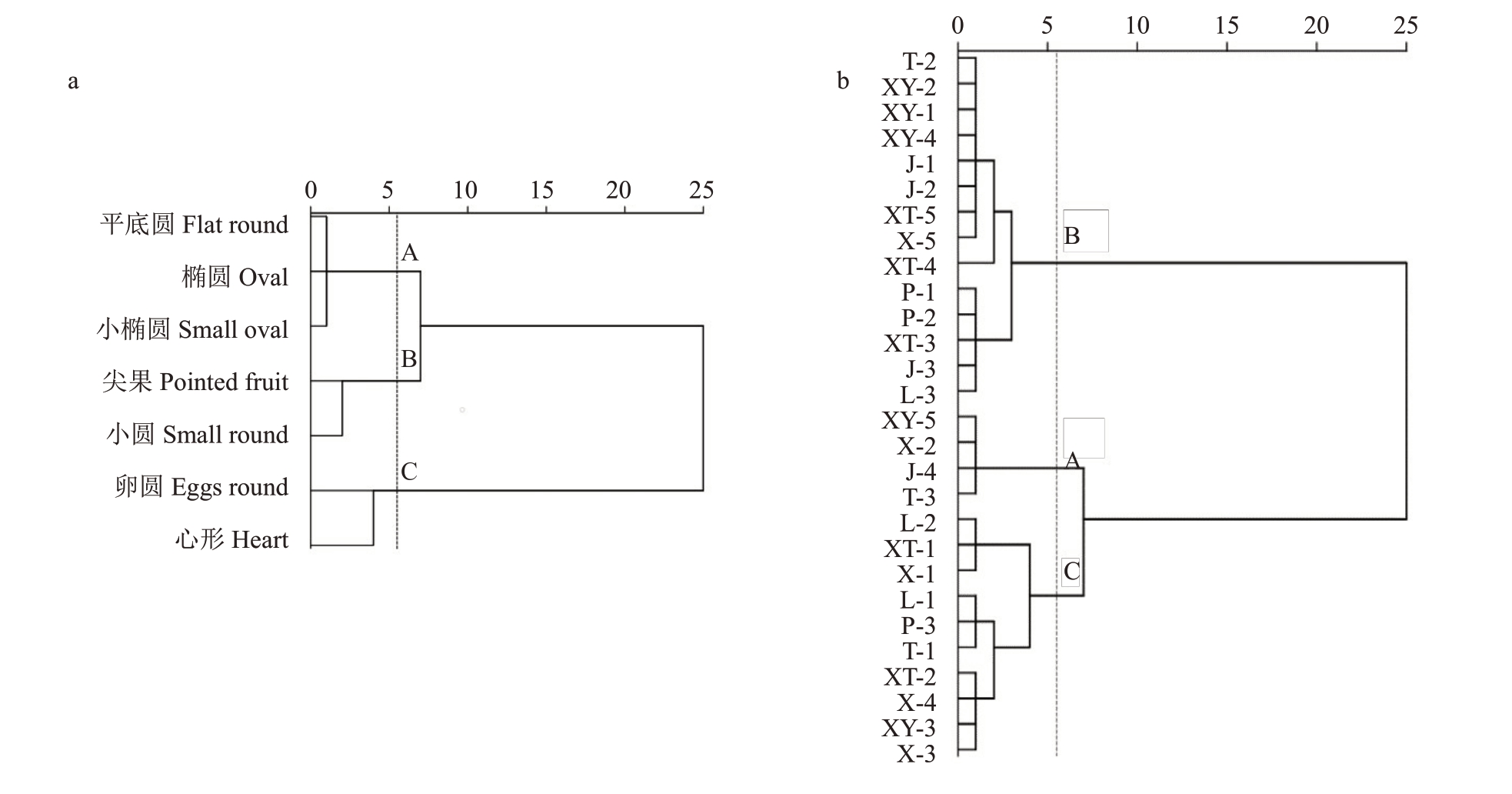
图2 新疆野核桃腐烂病抗性聚类分析
Fig.2 Cluster analysis of Xinjiang wild disease resistance among
a.新疆野核桃不同类型抗病能力聚类图;b.28 份新疆野核桃材料抗病能力聚类图。
a.Cluster map of different types of disease resistance of Xinjiang wild walnut;b.Cluster map of disease resistance of 28 Xinjiang wild walnut materials.
3 讨论
核桃腐烂病的发生已成为影响新疆核桃树健康生长发育的重要病害之一[28]。笔者在本研究中测定了新疆野核桃28个种质1年生离体枝条对核桃腐烂病病原菌的抗性,为抗病品种的筛选提供基础依据。结果显示核桃腐烂病菌株对各材料的致病力存在明显差异,表明野核桃单株拥有丰富的抗性变异。此外,J-1、J-2、XT-3、XT-4等12个野核桃单株在病斑表征上显示为高抗病种质,在7 个类型的新疆野核桃中,尖果型表现出比其他类型更高的抗性。
在植物与病原物长期协同进化过程中,植物形成了一系列复杂的防御机制抵御病原物的入侵。植物对病原物侵入的生理反应是以酶的催化活动实现的,体内的POD、CAT、SOD等防御酶都与抗病性有关[29]。本研究显示,POD、CAT与病斑长度呈极显著正相关,发病程度越严重,枝条韧皮部POD酶、CAT酶活性水平越高,其抗性越差。说明野核桃受到腐烂病病原菌侵染产生了大量的活性氧,高抗品种能够抵御病原菌不被侵染,因此侵入的病原物较少,活性氧产生较低,而感病品种侵入的病原菌较多,导致活性氧大量爆发。但是活性氧的爆发不仅可以抑制病原菌,还可以损伤植物本身。因此植物识别到活性氧大量爆发,反馈调节,提高了POD 和CAT 等酶的数量和活性,以抵御活性氧对树体造成的伤害[30],这与冯雷等[31]以及伏荣桃等[32]基于植物抗病响应所表现出较高酶活性水平的研究结果基本一致。其中感染严重的L-1 的POD 酶活性低于平均水平,说明寄主-病原菌互作体系已经稳定,且酶活性一般在组织坏死前增加,至后期感染严重时下降,这与刘琳等[33]研究结果一致。从植物自身的抗性而言,SOD的活性水平已被证实与机体自身的抗性密切相关,是植物细胞内抵御活性氧(ROS)伤害的主要保护性酶类,对清除和阻止ROS的产生起到重要作用[7],但也有研究认为二者没有显著线性关系。薛程[34]研究发现梨树腐烂病病害程度与枝条韧皮部SOD 活性无显著相关,蒋时姣[35]通过人工接种核桃黑斑病发现SOD活性虽有不同程度提高,但与核桃发病程度无显著相关,这与本研究结果相似。造成此结果可能是当核桃腐烂病菌侵害植株时,活性氧参与了核桃对核桃腐烂病的防御反应,抑制了植株SOD酶相关基因上调表达,造成SOD 酶系统紊乱[36],且不同的酶系在寄主-病原物互作中的作用存在差异,不同病原物与寄主的组合、同一种酶活性变化也不一致[37]。因此,一种酶在抗病中的作用不能一概而论,应根据具体情况具体分析。丙二醛是机体内脂质过氧化的最终产物之一,其含量高低可一定程度地反映植物受氧化伤害的程度[38],笔者在本研究中发现新疆野核桃腐烂病MDA 含量越高,核桃受侵染程度越严重,这与李波等[39]研究结果相同。说明野核桃离体茎段在受到病原菌侵染后,MDA 的含量增加,因而加大膜质过氧化作用对细胞的伤害,降低了植物对病害的抗逆能力[40]。
主成分分析法可以将有限信息的利用发挥到最大化,避免重复信息的干扰,聚类分析的结果满足相同组中数据对象的差距小,不同组中的数据差距大,两种方法结合可以尽可能避免传统人为方法的不足[41]。早期菌丝在植物细胞壁间蔓延过程中,肉眼观察难以判断,如XT-4和XT-3的病斑大小一样,但主成分分析的结果中,两个数据却有相差,这可能是由于菌丝已经在XT-3 韧皮部蔓延,但表征并未显现,以及综合评价中发病单株T-2 却比未发病单株X-5 得分高,这可能是由于核桃体内酚醌类物质含量较高,接种后在伤口处上形成生理性的黑斑,与核桃腐烂病病斑混淆而影响抗性评价[42]。通过主成分分析利用多个指标综合评价,所得结果可靠性较高,可有效避免人为主观判断的误差。通过核桃腐烂病病原菌侵染不同核桃种质指标的变化,初步确定部分抗病种质,与主成分分析、聚类分析的结果较为一致,其中XT-1的抗病能力最低,XT-4的抗病能力最高。综合分析显示,得分为0.93~1.32的新疆野核桃种质抗病能力较好,分别为XT-4、J-1、J-2、XY-4、XY-1、XY-2、T-2。28 份野核桃材料抗病能力可分为三类,其中B 类核桃抗病能力最强,C 类抗病能力最差,A 类则属于中间类型。初步筛选出的7 个高抗种质可作为未来高抗单株选育的优良亲本的备选材料。
4 结论
28份新疆野核桃材料1年生枝条接种核桃腐烂病后显示出不同的抗病力,通过自然发病情况调查、离体枝条接种、生理指标POD 和CAT 酶活性以及MDA 含量测定对新疆野核桃对核桃腐烂病抗性进行了初步鉴定,筛选出的7 个抗病种质可为新疆野核桃抗病种质的进一步筛选及抗病基因鉴定打下一定的基础。
致谢:感谢巩留县林草局、野核桃沟自然保护区对本实验工作的支持。
[1] 马荣.新疆壳囊孢属真菌的分类及系统学研究[D].北京:北京林业大学,2017.MA Rong.Taxonomy and phylogeny of Cytospora in XinJiang northwest of China[D].Beijing:Beijing Forestry University,2017.
[2] 岳朝阳,孔婷婷,阿衣夏木·亚库甫,焦淑萍,张新平.核桃腐烂病主要发病因子研究[J].西北林学院学报,2015,30(1):154-157.YUE Chaoyang,KONG Tingting,Ayixiamu · Yakufu,JIAO Shuping,ZHANG Xinping.Main factors affecting walnut rot disease[J].Journal of Northwest Forestry University,2015,30(1):154-157.
[3] FAN X L,HYDE K D,LIU M,LIANG Y M,TIAN C M.Cytospora species associated with walnut canker disease in China,with description of a new species C.gigalocus[J].Fungal biology,2015,119(5):310-319.
[4] 尹永香,刘应敏,马荣,赵丽,何美茹,蔡桂芳.新疆核桃树腐烂病致病力室内测定方法的研究[J].中国农学通报,2016,32(34):108-112.YIN Yongxiang,LIU Yingmin,MA Rong,ZHAO Li,HE Meiru,CAI Guifang.Laboratory evaluation method of walnut canker pathogenicity[J].Chinese Agricultural Science Bulletin,2016,32(34):108-112.
[5] 郭开发,王刚,吴彩兰,包亚洲,赵思峰.新疆南疆核桃树腐烂病菌鉴定[J].新疆农业科学,2016,53(3):496-501.GUO Kaifa,WANG Gang,WU Cailan,BAO Yazhou,ZHAO Sifeng.Identification of canker pathogen of juglans in Southern Xinjiang[J].Xinjiang Agricultural Sciences,2016,53(3):496-501.
[6] 袁汇涛,张云霞,向梅梅,罗梅,董章勇.花生黑腐病抗病品种筛选及抗病相关酶活性测定[J].核农学报,2021,35(10):2258-2266.YUAN Huitao,ZHANG Yunxia,XIANG Meimei,LUO Mei,DONG Zhangyong.Screening of peanut black rot disease-resistant varieties and determination of defense-related enzyme activities[J].Journal of Nuclear Agricultural Sciences,2021,35(10):2258-2266.
[7] PRASAD M S L,SUJATHA M,ALIVELU K,SUJATHA K.Sources of resistance to Alternariaster leaf blight in sunflower pre-breeding lines derived from interspecific crosses and wild Helianthus species[J].Crop Protection,2017,92:70-78.
[8] PETERS L P,CARVALHO G,VILHENA M B,CRESTE S,AZEVEDO R A,MONTEIRO-VITORELLO C B.Functional analysis of oxidative burst in sugarcane smut-resistant and-susceptible genotypes[J].Planta,2017,245(4):749-764.
[9] 张敏欢,王建成,杨红兰,张道远,邓鹏程,崔志军.新疆野核桃种质对低温胁迫的生理响应[J].应用生态学报,2020,31(8):2558-2566.ZHANG Minhuan,WANG Jiancheng,YANG Honglan,ZHANG Daoyuan,DENG Pengcheng,CUI Zhijun.Physiological response of Xinjiang wild walnut germplasm to low temperature stress[J].Chinese Journal of Applied Ecology,2020,31(8):2558-2566.
[10] 张朝明,赵坤,唐胜,黎兆山,陈梅,周作高,周生茂.6 个豇豆品种农艺性状的相关性、主成分及聚类分析[J].西南农业学报,2021,34(3):501-507.ZHANG Chaoming,ZHAO Kun,TANG Sheng,LI Zhaoshan,CHEN Mei,ZHOU Zuogao,ZHOU Shengmao.Correlation,principal component and cluster analysis of agronomic traits of six cowpea varieties[J].Southwest China Journal of Agricultural Sciences,2021,34(3):501-507.
[11] 王磊,崔乃然,张汉斐.新疆野核桃的研究[J].干旱区研究,1997,14(1):17-27.WANG Lei,CUI Nairan,ZHANG Hanfei.Research report of Xinjiang wild walnuts[J].Arid Zone Research,1997,14(1):17-27.
[12] 白玲,阎国荣,许正.伊犁野果林植物多样性及其保护[J].干旱区研究,1998,15(3):10-13.BAI Ling,YAN Guorong,XU Zheng.Study on phyto-diversity and conservation of the wild fruit forest of Tianshan Mts in Xinjiang[J].Arid Zone Research,1998,15(3):10-13.
[13] 董玉芝,朱小虎,陈虹,梁风丽,叶尔江,王肇延.新疆巩留野核桃林调查及其分析[J].植物遗传资源学报,2012,13(3):386-392.DONG Yuzhi,ZHU Xiaohu,CHEN Hong,LIANG Fengli,YE Erjiang,WANG Zhaoyan.Investigation and analysis on the wild walnut in Gongliu,Xinjiang[J].Journal of Plant Genetic Resources,2012,13(3):386-392.
[14] 余甜,张萍,陈韦多.干旱胁迫下3 种类型新疆野核桃保护酶和丙二醛含量的变化[J].江苏农业科学,2018,46(16):119-121.YU Tian,ZHANG Ping,CHEN Weiduo.Changes of protective enzymes and malondialdehyde contents of three types of walnut in Xinjiang under drought stress[J].Jiangsu Agricultural Sciences,2018,46(16):119-121.
[15] 韩冷,徐敏,张萍.干旱胁迫下新疆野核桃幼苗叶片渗透调节物质变化研究[J].天津农业科学,2018,24(8):1-3.HAN Leng,XU Min,ZHANG Ping.Changes of osmoregulation in Xinjiang wild walnut seedlings under drought stress[J].Tianjin Agricultural Sciences,2018,24(8):1-3.
[16] 王肇延.新疆野核桃资源及遗传多样性的分析[D].乌鲁木齐:新疆农业大学,2011.WANG Zhaoyan.Analysis of resource and genetic diversity of wild walnut in Xinjiang[D].Urumqi:Xinjiang Agricultural University,2011.
[17] 张捷,张萍,李勤霞.新疆野核桃核心种质的构建[J].果树学报,2018,35(2):168-176.ZHANG Jie,ZHANG Ping,LI Qinxia.Construction of core germplasm of Xinjiang wild walnut[J].Journal of Fruit Science,2018,35(2):168-176.
[18] 周红,张萍,李彦荣.新疆野核桃坚果营养成分测定及分析[J].果树学报,2019,36(5):621-628.ZHOU Hong,ZHANG Ping,LI Yanrong.Analysis of nutritive components of different types of Xinjiang wild walnuts[J].Journal of Fruit Science,2019,36(5):621-628.
[19] 张维,李海燕,崔东,杨允菲.新疆野核桃自然保护区不同坡向野核桃幼苗种群年龄结构及生长特征[J].应用生态学报,2017,28(2):382-390.ZHANG Wei,LI Haiyan,CUI Dong,YANG Yunfei.Age structure and growth characteristics of Juglans cathayensis seedling populations at different slope aspects in Wild Walnut Natural Conservation Area of Xinjiang,China[J].Chinese Journal of Applied Ecology,2017,28(2):382-390.
[20] 袁海涛.新疆野核桃种质资源基础数据库的建立与核心种质构建方法研究[D].乌鲁木齐:新疆农业大学,2012.YUAN Haitao.Construction of Xinjiang wild walnut resource database and research on methods of building core collection[D].Urumqi:Xinjiang Agricultural University,2012.
[21] 李勤霞.新疆野核桃遗传连锁图谱的构建[D].乌鲁木齐:新疆农业大学,2016.LI Qinxia.Construction of genetic linkage map in Juglans regia[D].Urumqi:Xinjiang Agricultural University,2016.
[22] 周玉霞.我国梨树腐烂病病原菌的初步鉴定及两种培养表型菌株的致病性分析[D].武汉:华中农业大学,2013.ZHOU Yuxia.The Preliminary Identification for the pathogens and the pathogenicity of two different culturing phenotypes strains of the pear Valsa canker in China[D].Wuhan:Huazhong Agricultural University,2013.
[23] 张美鑫,翟立峰,周玉霞,陈晓忍,贾娜娜,洪霓,王国平.梨腐烂病致病力的室内快速测定方法研究[J].果树学报,2013,30(2):317-322.ZHANG Meixin,ZHAI Lifeng,ZHOU Yuxia,CHEN Xiaoren,JIA Nana,HONG Ni,WANG Guoping.Laboratory determining methods of the pathogenicity of Valsa canker in pear[J].Journal of Fruit Science,2013,30(2):317-322.
[24] 刘家尧,刘新.植物生理学实验教程[M].北京:高等教育出版社,2010:72-81.LIUJiayao,LIUXin.Experimentalcourseinplantphysiology[M].Beijing:Higher Education Press,2010:72-81.
[25] 葛帅,王蓉蓉,王颖瑞,叶美玲,覃业优,丁胜华,周辉,蒋立文,邓放明.湖南常见辣椒品种游离氨基酸主成分分析及综合评价[J].食品科学技术学报,2021,39(2):91-102.GE Shuai,WANG Rongrong,WANG Yingrui,YE Meiling,QIN Yeyou,DING Shenghua,ZHOU Hui,JIANG Liwen,DENG Fangming.Principal component analysis and comprehensive evaluation of free amino acids of different peppers in Hunan[J].Journal of Food Science and Technology,2021,39(2):91-102.
[26] 井津.聚类分析在煤矿安全事故特征分析的应用[J].陕西煤炭,2015,34(1):95-98.JING Jin.Application of cluster analysis in the research of mine safety accident characteristics[J].Shaanxi Meitan,2015,34(1):95-98.
[27] BARLOW A L,MACLEOD A,NOPPEN S,SANDERSON J,GUÉRIN C J.Colocalization analysis in fluorescence micrographs:Verification of a more accurate calculation of pearson’s correlation coefficient[J].Microscopy and Microanalysis,2010,16(6):710-724.
[28] 阿布都如苏里·阿不来提.核桃腐烂病发生规律及药剂防治技术研究[D].乌鲁木齐:新疆农业大学,2014.Abudurusuli·Abulaiti.Walnut stalk canker disease occurrence law and chemistry preventing measures research[D].Urumqi:Xinjiang Agricultural University,2014.
[29] 王桥美,范静华,果志华,陈建斌.烟草黑胫病菌毒素对烟草防御性相关酶的诱导作用[J].云南农业大学学报(自然科学),2011,26(1):20-25.WANG Qiaomei,FAN Jinghua,GUO Zhihua,CHEN Jianbin.Induction effect of toxin extracts of Phytophthora nicotianae on tobacco defense-related enzyme activities[J].Journal of Yunnan Agricultural University(Natural Science),2011,26(1):20-25.
[30] 张志伟,李月月,何杜鹃,周化斌,杨海龙.高氧条件下灵芝子实体活性氧含量及其代谢相关酶活性[J].菌物学报,2020,39(1):99-109.ZHANG Zhiwei,LI Yueyue,HE Dujuan,ZHOU Huabin,YANG Hailong.Changes of reactive oxygen species content and related metabolic enzyme activities in the fruiting body of Ganoderma lingzhi under high oxygen condition[J].Mycosystema,2020,39(1):99-109.
[31] 冯雷,耿增超,徐万里,薛泉宏,孙宁川,雇玉忠,唐光木.几种酶活及核桃长势对腐烂病胁迫的响应[J].华北农学报,2019,34(4):217-222.FENG Lei,GENG Zengchao,XU Wanli,XUE Quanhong,SUN Ningchuan,GU Yuzhong,TANG Guangmu.Response of enzyme activities and growth vigoron of walnut to Jugladis canker stress[J].Acta Agriculturae Boreali-Sinica,2019,34(4):217-222.
[32] 伏荣桃,王剑,陈诚,赵黎宇,陈雪娟,卢代华.稻曲病菌毒素对不同抗病水稻品种生理生化特性的影响[J].生态学杂志,2021,40(9):2793-2801.FU Rongtao,WANG Jian,CHEN Cheng,ZHAO Liyu,CHEN Xuejuan,LU Daihua.Effects of mycotoxins of Ustilaginoidea virens on physiological-biochemical characteristicsof different resistant rice varieties[J].Chinese Journal of Ecology,2021,40(9):2793-2801.
[33] 刘琳,侯喜林,王利英,陈晓峰.不结球白菜感染芜菁花叶病毒后4 种防御酶活性变化及其抗病相关性[J].南京农业大学学报,2009,32(3):14-18.LIU Lin,HOU Xilin,WANG Liying,CHEN Xiaofeng.Changes of four protective enzyme activities and relationships to resistance in non-heading Chinese cabbage after infection of Turnip mosaic virus[J].Journal of Nanjing Agricultural University,2009,32(3):14-18.
[34] 薛程.梨树腐烂病抗性种质资源筛选及相关生理特性研究[D].合肥:安徽农业大学,2014.XUE Cheng.Screening of accession resistant to pear Valsa canker and studying of their physiological characters in Pyrus germplasm resources[D].Hefei:Anhui Agricultural University,2014.
[35] 蒋时姣.核桃黑斑病抗性评价及抗病机理研究[D].雅安:四川农业大学,2019.JIANG Shijiao.Sensitivity evaluation and disease resistance mechanism to walnut blight[D].Yaan:Sichuan Agricultural University,2019.
[36] LIU H X,XIN Z Y,ZHANG Z Y.Changes in activities of antioxidant-related enzymes in leaves of resistant and susceptible wheat inoculated with Rhizoctonia cerealis[J].Agricultural Sciences in China,2011,10(4):526-533.
[37] 杜蕙,王春明,郭建国,漆永红,蒋晶晶.葡萄生单轴霉菌对葡萄几种防御酶活性的影响[J].江苏农业科学,2019,47(15):151-154.DU Hui,WANG Chunming,GUO Jianguo,QI Yonghong,JIANG Jingjing.Effects of grape uniaxial fungi on the activities of several defense enzymes in grapes[J].Jiangsu Agricultural Sciences,2019,47(15):151-154.
[38] TATAR O,GEVREK M N.Influence of water stress on proline accumulation,lipid peroxidation and water content of wheat[J].Asian Journal of Plant Sciences,2008,7(4):409-412.
[39] 李波,祝一鸣,单体江,伍慧雄,孙思,王军.AXY22 和苯并噻二唑的组合诱导对桉树抗病相关酶活性和丙二醛的影响[J].中国森林病虫,2016,35(6):1-5.LI Bo,ZHU Yiming,SHAN Tijiang,WU Huixiong,SUN Si,WANG Jun.Effects of combined induction by AXY22 and BTH on disease-resistant enzymes and MDA of eucalyptus[J].Forest Pest and Disease,2016,35(6):1-5.
[40] 商闯,马春红,翟彩霞,李运朝,董文琦,崔四平,侯立白,贾银锁.丙二醛(MDA)含量在玉米诱导抗病过程中的变化[J].华北农学报,2007,22(S2):29-32.SHANG Chuang,MA Chunhong,ZHAI Caixia,LI Yunchao,DONG Wenqi,CUI Siping,HOU Libai,JIA Yinsuo.The changes of malondialdenyde content on course of the induced diseaseresistance[J].Acta Agriculturae Boreali-Sinica,2007,22(S2):29-32.
[41] 苏欣欣,肖洋,胡晓航,马亚怀,李彦丽.基于灰色关联度分析和主成分分析法评估糖用甜菜品种的适应性[J].中国农学通报,2021,37(30):39-46.SU Xinxin,XIAO Yang,HU Xiaohang,MA Yahuai,LI Yanli.The adaptability evaluation of sugar beet varieties based on grey relational analysis and principal component analysis[J].Chinese Agricultural Science Bulletin,2021,37(30):39-46.
[42] ARAJI S,GRAMMER T A,GERTZEN R,ANDERSON S D,MIKULIC-PETKOVSEK M,VEBERIC R,PHU M L,SOLAR A,LESLIE C A,DANDEKAR A M,ESCOBAR M A.Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut[J].Plant Physiology,2014,164(3):1191-1203.